Abstract
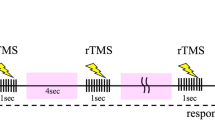
Binding multisensory information into episodic memory depends partly on the timing of the hippocampal theta rhythm which provides time windows for synaptic modification. In humans, theta rhythmic sensory stimulation (RSS) enhances episodic memory when the stimuli are synchronised across the visual and auditory domain compared to when they are out-of-synchrony. However, recent studies show mixed evidence if the improvement in episodic memory is the result of modulating hippocampal theta activity. In the current study, we investigated whether pre-stimulus brain state could explain part of this variance in the neural and behavioural effects induced by the RSS, via recording 24 participants’ brain activity with MEG during a multisensory theta RSS memory paradigm. Our findings suggest that pre-stimulus alpha power modulates entrainment strength in sensory regions, which in turn predicts subsequent memory formation. These findings suggest that for non-invasive brain stimulation tools to be effective it is crucial to consider brain-state dependent effects.
Similar content being viewed by others
Data availability
Preprocessed data are available from https://doi.org/10.6084/m9.figshare.29468597. Any additional information required to reanalyse the data reported in this paper is available upon request.
Code availability
All original code has been deposited at https://osf.io/skm4q/.
References
Tulving, E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 53, 1–25 (2002).
Moscovitch, M. The hippocampus as a ‘stupid,’ domain-specific module: Implications for theories of recent and remote memory, and of imagination. Can. J. exp. Psychol 62, 62–79 (2008).
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957).
Bliss, T. V. P. & Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356 (1973).
Neves, G., Cooke, S. F. & Bliss, T. V. P. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 9, 65–75 (2008).
Hölscher, C., Anwyl, R. & Rowan, M. J. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. J. Neurosci. 17, 6470–6477 (1997).
Huerta, P. T. & Lisman, J. E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15, 1053–1063 (1995).
Hyman, J. M., Wyble, B. P., Goyal, V., Rossi, C. A. & Hasselmo, M. E. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J. Neurosci. 23, 11725–11731 (2003).
Clouter, A., Shapiro, K. L. & Hanslmayr, S. Theta phase synchronization is the glue that binds human associative memory. Curr. Biol. 27, 3143–3148.e6 (2017).
Hanslmayr, S., Axmacher, N. & Inman, C. S. Modulating human memory via entrainment of brain oscillations. Trends Neurosci. 42, 485–499 (2019).
Wang, D., Clouter, A., Chen, Q., Shapiro, K. L. & Hanslmayr, S. Single-trial phase entrainment of theta oscillations in sensory regions predicts human associative memory performance. J. Neurosci. 38, 6299–6309 (2018).
Plog, E., Antov, M. I., Bierwirth, P., Keil, A. & Stockhorst, U. Phase-synchronized stimulus presentation augments contingency knowledge and affective evaluation in a fear-conditioning task. eNeuro 9, ENEURO.0538–20.2021 (2022).
Haegens, S., Cousijn, H., Wallis, G., Harrison, P. J. & Nobre, A. C. Inter- and intra-individual variability in alpha peak frequency. NeuroImage 92, 46–55 (2014).
Aton, S. J. Set and setting: how behavioral state regulates sensory function and plasticity. Neurobiol. Learn. Mem. 106, 1–10 (2013).
Bergmann, T. O. Brain state-dependent brain stimulation. Front. Psychol. 9, 2108 (2018).
Rutishauser, U., Kotowicz, A. & Laurent, G. A method for closed-loop presentation of sensory stimuli conditional on the internal brain-state of awake animals. J. Neurosci. Methods 215, 139–155 (2013).
Tang, Y.-Y., Rothbart, M. K. & Posner, M. I. Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn. Sci. 16, 330–337 (2012).
Serin, F., Wang, D., Davis, M. H. & Henson, R. Does theta synchronicity of sensory information enhance associative memory? Replicating the theta-induced memory effect. Brain Neurosci. Adv. 8, 23982128241255798 (2024).
Kahn, M. et al. Gamma sensory stimulation and effects on the brain. Preprint at https://doi.org/10.1101/2023.10.30.564197 (2023).
Adaikkan, C. & Tsai, L.-H. Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci. 43, 24–41 (2020).
Martorell, A. J. et al. Multi-sensory gamma stimulation ameliorates alzheimer’s-associated pathology and improves cognition. Cell 177, 256–271.e22 (2019).
Schneider, M., Tzanou, A., Uran, C. & Vinck, M. Cell-type-specific propagation of visual flicker. Cell Reports 42, 5 (2023).
Soula, M. et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat. Neurosci. 26, 570–578 (2023).
Wang, D., Shapiro, K. L. & Hanslmayr, S. Altering stimulus timing via fast rhythmic sensory stimulation induces STDP-like recall performance in human episodic memory. Curr. Biol. 33, 3279–3288.e7 (2023).
Müller, M. M. et al. Feature-selective attention enhances color signals in early visual areas of the human brain. Proc. Natl. Acad. Sci. USA 103, 14250–14254 (2006).
Obleser, J. & Kayser, C. Neural entrainment and attentional selection in the listening brain. Trends Cogn. Sci. 23, 913–926 (2019).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A.-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009).
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Kleiner, M., Brainard, D. & Pelli, D. G. What’s new in Psychtoolbox-3? 14, (2007).
Pelli, D. G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Gramfort, A. et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 7, 267 (2013).
Ferrante, O. et al. FLUX: a pipeline for MEG analysis. NeuroImage 253, 119047 (2022).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Murzin, V., Fuchs, A. & Scott Kelso, J. A. Detection of correlated sources in EEG using combination of beamforming and surface Laplacian methods. J. Neurosci. Methods 218, 96–102 (2013).
JASP Team. JASP. (2025).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48 (2015).
Klimesch, W., Sauseng, P. & Hanslmayr, S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 53, 63–88 (2007).
Jensen, O. & Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Human Neurosci. 4, 186 (2010).
Hanslmayr, S., Staudigl, T. & Fellner, M.-C. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Human Neurosci. 6, 74 (2012).
Popov, T. Misinterpreting electrophysiology in human cognitive neuroscience. 2025.06.25.661032 Preprint at https://doi.org/10.1101/2025.06.25.661032 (2025).
Popov, T. & Staudigl, T. Cortico-ocular coupling in the service of episodic memory formation. Prog. Neurobiol. 227, 102476 (2023).
Lachaux, J.-P. et al. Studying single-trials of phase synchronous activity in the brain. Int. J. Bifurc. Chaos 10, 2429–2439 (2000).
Van Veen, B. D., Van Drongelen, W., Yuchtman, M. & Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44, 867–880 (1997).
Händel, B. F., Haarmeier, T. & Jensen, O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J. Cogn. Neurosci. 23, 2494–2502 (2011).
Kelly, S. P., Gomez-Ramirez, M. & Foxe, J. J. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur. J. Neurosci. 30, 2224–2234 (2009).
Sauseng, P. et al. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur. J. Neurosci. 22, 2917–2926 (2005).
Shapiro, K. & Hanslmayr, S. The role of brain oscillations in the temporal limits of attention. in The Oxford Handbook of Attention 620–651 (Oxford University Press, 2014).
Snyder, A. C. & Foxe, J. J. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: a high-density electrical mapping study. J. Neurosci. 30, 4024–4032 (2010).
Dijk, H. van, Schoffelen, J.-M., Oostenveld, R. & Jensen, O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci. 28, 1816–1823 (2008).
Benwell, C. S. Y. et al. Frequency and power of human alpha oscillations drift systematically with time-on-task. NeuroImage 192, 101–114 (2019).
Behrmann, M., Geng, J. J. & Shomstein, S. Parietal cortex and attention. Curr. Opin. Neurobiol. 14, 212–217 (2004).
Simeonov, L. & Das, R. The rhythm of memory. Does theta frequency audio/visual flicker improve recall? Front. Behav. Neurosci. 19, 1555081 (2025).
Blanpain, L. T. et al. Multisensory flicker modulates widespread brain networks and reduces interictal epileptiform discharges. Nat. Commun. 15, 3156 (2024).
Biau, E., Wang, D., Park, H., Jensen, O. & Hanslmayr, S. Neocortical and hippocampal theta oscillations track audiovisual integration and replay of speech memories. J. Neurosci. 45, 21 (2025).
Gould, I. C., Rushworth, M. F. & Nobre, A. C. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J. Neurophysiol. 105, 1318–1326 (2011).
Hanslmayr, S. et al. Prestimulus oscillations predict visual perception performance between and within subjects. NeuroImage 37, 1465–1473 (2007).
Rohenkohl, G. & Nobre, A. C. Alpha oscillations related to anticipatory attention follow temporal expectations. J. Neurosci. 31, 14076–14084 (2011).
Thut, G., Nietzel, A., Brandt, S. A. & Pascual-Leone, A. α-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 26, 9494–9502 (2006).
Park, H. et al. Blocking of irrelevant memories by posterior alpha activity boosts memory encoding. Hum. Brain Mapp. 35, 3972–3987 (2014).
Jensen, O. Distractor inhibition by alpha oscillations is controlled by an indirect mechanism governed by goal-relevant information. Commun. Psychol. 2, 36 (2024).
Zhigalov, A. & Jensen, O. Alpha oscillations do not implement gain control in early visual cortex but rather gating in parieto-occipital regions. Hum. Brain Mapp. 41, 5176–5186 (2020).
Griffiths, B. J., Martín-Buro, M. C., Staresina, B. P., Hanslmayr, S. & Staudigl, T. Alpha/beta power decreases during episodic memory formation predict the magnitude of alpha/beta power decreases during subsequent retrieval. Neuropsychologia 153, 107755 (2021).
Griffiths, B. J., Martín-Buro, M. C., Staresina, B. P. & Hanslmayr, S. Disentangling neocortical alpha/beta and hippocampal theta/gamma oscillations in human episodic memory formation. NeuroImage 242, 118454 (2021).
Antony, J. W., Ngo, H.-V. V., Bergmann, T. O. & Rasch, B. Real-time, closed-loop, or open-loop stimulation? Navigating a terminological jungle. J. Sleep. Res. 31, e13755 (2022).
Marguet, S. L. & Harris, K. D. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J. Neurosci. 31, 6414–6420 (2011).
Lakatos, P. et al. Global dynamics of selective attention and its lapses in primary auditory cortex. Nat. Neurosci. 19, 1707–1717 (2016).
Chun, M. M. & Turk-Browne, N. B. Interactions between attention and memory. Curr. Opin. Neurobiol. 17, 177–184 (2007).
Zoefel, B. & VanRullen, R. Oscillatory mechanisms of stimulus processing and selection in the visual and auditory systems: state-of-the-art, speculations and suggestions. Front. Neurosci. 11, 296 (2017).
Hsu, T.-Y., Tseng, P., Liang, W.-K., Cheng, S.-K. & Juan, C.-H. Transcranial direct current stimulation over right posterior parietal cortex changes prestimulus alpha oscillation in visual short-term memory task. NeuroImage 98, 306–313 (2014).
Silvanto, J., Muggleton, N. & Walsh, V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 12, 447–454 (2008).
Acknowledgements
This research was supported by grants from the Economic Social Sciences Research Council (https://esrc.ukri.org/, ES/R010072/1 to S.H. and K.L.S.) and the Medical Research Council Doctoral Training Program in Precision Medicine (MR/W006804/1 to E.M.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors would like to thank Gabriela Cruz, Máté Gyurkovics, Hamed Haque, and Felix Siebenhühner from the Palva lab for their help on the MEG preprocessing pipeline, and Frances Crabbe, Xuan Cui, Jacqueline McDiarmid, and Gavin Paterson for their help on MEG and MRI data acquisition, and everyone from the Neurotechnology, Cognition and Oscillations Lab and Professor Maria Wimber’s lab at the University of Glasgow for their helpful inputs. The authors would also like to thank Tzvetan Popov for his suggestions and for sharing code on the eye tracking data analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.H., K.L.S. and D.W.; investigation, D.W. and E. M.; formal analysis, and writing—original draft, D.W.; writing—review and editing, D.W., E.M., K.L.S. and S.H.; funding acquisition, and supervision, S.H. and K.L.S.
Corresponding authors
Ethics declarations
Competing interests
S.H. acts as scientific adviser to Clarity Technologies Inc. All other authors declare no competing interests.
Peer review
Peer review information
Communications Psychology thanks Roxane S. Hoyer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Troby Ka-Yan Lui. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Marcantoni, E., Shapiro, K.L. et al. Pre-stimulus alpha power modulates trial-by-trial variability in theta rhythmic multisensory entrainment strength and theta-induced memory effect. Commun Psychol (2026). https://doi.org/10.1038/s44271-026-00406-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44271-026-00406-x