Abstract
Background
Malignant mesothelioma is a tumour that is strongly associated with a history of asbestos exposure, and which derives from mesothelial cells that line the serous cavities of the body. The tumour most commonly arises in the pleural cavity, but can also arise in the pericardium, peritoneum, and tunica vaginalis. At present the lesion has a very poor prognosis and is an incurable form of cancer with median survival times of up to 19 months being quoted for some histological subtypes. A large proportion of mesotheliomas have been shown to be arginine auxotrophic, leading to new research for therapeutics which might exploit this potential vulnerability.
Methods
We measured the levels of General Control Non-derepressible 2 (GCN2) protein in malignant mesothelioma tumour samples and determined whether these levels correlate with clinical outcomes.
Results
We observed that the expression levels of GCN2 correlated with patient survival and was an independent prognostic variable in pairwise comparisons with all available clinical data.
Conclusion
These findings suggest that GCN2 levels provides prognostic information and may allow for stratification of care pathways. It may suggest that targeting GCN2 is a viable strategy for mesothelioma therapy development.
Similar content being viewed by others
Background
Malignant mesothelioma is a treatment-limited cancer derived from mesothelial cells that form the surface level of the protective serous membrane coating the serous cavities of the body, namely, the pleural, pericardial and peritoneal cavities as well as the tunica vaginalis, an embryological invagination of the peritoneum into the scrotum that encloses the testis and paratesticular tissues [1]. Asbestos exposure is the predominant cause of malignant mesothelioma [1], while most cases (90%) occur in the pleura (Malignant Pleural Mesothelioma (MPM)), with the peritoneum lining the abdominopelvic cavity contributing 7–10% of malignant mesothelioma cases [2]. Without treatment, median survival is 7–10 months, reaching 19 months for some histological subtypes [3], which increases to a maximum of 22 months with treatment [4]. The most effective chemotherapeutic strategy for MPM is a combinatorial treatment using cisplatin-pemetrexed, however, this yields a poor response rate of only 41.3% [5]. This poor response rate, coupled with a 5-year survival rate of only 5% [6], underscores the need for greater understanding of the cellular processes underpinning MPM pathogenesis to develop more effective, targeted, therapies and improve patient outlook.
Recent randomised clinical trials have demonstrated the potential value of immune checkpoint inhibitors (nivolumab plus ipilimumab) [7] and arginine depletion strategies [8] in treating MPM—although doubts have been raised over the efficacy of an immune checkpoint inhibition approach [9]. One potential avenue for therapeutic development may be found in the observation that around 60% of MPM tumours do not express asparagine synthetase (ASS-1) [10]. ASS-1 catalyses the rate limiting step in arginine biosynthesis, and its loss, a common occurrence in multiple cancers, causes tumours to become reliant on extracellular arginine to survive [11]. The arginine auxotrophic phenotype is currently being investigated therapeutically through administration of a pegylated arginine deiminase (ADI-PEG-20) in the Arginine Deiminase and Mesothelioma (ADAM) study [8], along with the standard combination of pemetrexed (Pem) and cisplatin [12].
Analogously, the molecular basis of ADI-PEG-20 treatment has been demonstrated to depend on the GCN2-eIF2α-ATF4 pathway in arginine-dependent invasive bladder cancer [13]. General Control Non-derepressible 2 (GCN2) is a protein kinase that is activated through amino-acid starvation stress, which has been implicated in multiple cancers [14, 15]. GCN2 activation may be dependent on ribosomal stalling [16, 17], deacylated tRNA levels increasing [18, 19], or P-stalk proteins associating with the ribosome [18, 20, 21], all of which result in autophosphorylation and subsequent phosphorylation of the serine 51 residue of the eukaryotic initiation factor eIF2α, which prevents the formation of translational pre-initiation complexes. This results in the Integrated Stress Response (ISR)—a global repression of mRNA translation and the upregulation of the transcription factor activating transcription factor 4 (ATF4)—a transcription factor which promotes cell survival, unless under chronic or acute stress levels, where instead apoptosis is activated [14]. There are ongoing trials GCN2-targetting therapeutics [22] and several pre-clinical inhibitors in development [23,24,25].
ASS-1 deficient bladder cancer cells are ADI-PEG-20 sensitive, activating the GCN2-eIF2α-ATF4 pathway and inducing C/EBP homologous protein (CHOP) expression. This resulted in the selective apoptosis in the ASS-1-deficient bladder cancer cells. Furthermore, it has been previously noted through the work of Dalton et al. that CHOP expression is a biomarker of malignant mesothelioma [26]. We hypothesised that GCN2 levels would be elevated in malignant mesothelioma biopsy samples, and this may be a prognostic biomarker for patient outcomes. We measured GCN2 levels in formalin-fixed paraffin-embedded samples using immunohistochemical means. Here we report that GCN2 levels are a strong prognostic marker for mesothelioma patient outcomes.
Methods
Tissue microarrays
Tissue microarrays (TMAs) were obtained from Mesobank tissue bank based at the NHS Royal Papworth Hospital, the largest UK pleural mesothelioma biobank [27]. TMAs were prepared by the biobank for 204 patients with cores of 0.6 mm diameter, with between four and five replicates for each patient included on concurrent locations within the set. Demographic data was provided for each patient, including age at diagnosis, time to death, gender, TNM (tumour, node, metastasis) classification, and histopathology (Table 1). Normal kidney, heart, and liver tissue was dispersed throughout the slide sets for the purpose of microscope orientation, and these also underwent staining. Consistency in staining was observed for these tissues within and between slides, indicating that staining conditions were consistent and not a source of error.
Immunohistochemistry
Antigen retrieval and de-paraffinisation was performed using DAKO EnVision™ FLEX Target Retrieval solution (high pH) buffer in a DAKO PT Link. Sections were blocked in EnVision™ Flex Peroxidase-Blocking Reagent and incubated overnight at 4 °C with anti-GCN2 rabbit monoclonal antibody (Invitrogen MA5-32704) at a dilution of 1:100. Immunostaining using DAKO EnVision™ FLEX system (Agilent Technologies) on a DAKO Autostainer Link48 was carried out according to manufacturer’s protocol. DAKO substrate working solution was used as a chromogenic agent for 2 × 5 mins and sections were counterstained with EnVision™ FLEX haematoxylin. Sections known to stain positively were included in each batch and negative controls were prepared by replacing the primary antibody with DAKO antibody diluent.
Scoring
The methodology was based on previous mesothelioma immunohistochemical investigations [26]. To quantify GCN2 levels, an image library compiling each tumour section was captured using LEICA ICC50 HD microscope at 10x magnification, using the Leica LAS EZ Application Suite Version 3.4.0. Individual sections were then scored on two scales. First, staining intensity scores from 0 to 5 were assigned independently by three persons and averaged. Second, proportion staining scores from 0 to 100 were assigned, representing the percentage of all cells in the section stained. These scores were multiplied for each section to give a value from 0 to 500 and an average of all replicates calculated for each patient.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 9 (GraphPad Prism version 9.5.1 for Windows, San Diego, California USA) and RStudio. Survival was assessed from date of histological diagnosis to death. Patients that were still alive were excluded from the survival analysis. Univariate survival analysis for demographic data and GCN2 scores used the log-rank (LR) test and was visualised as Kaplan–Meier (KM) survival curves, both in GraphPad Prism. The pROC package [28] in RStudio [29] was used to generate ROC (receiver operator characteristic) curves to determine whether GCN2 was a prognostic marker in malignant pleural mesothelioma (MPM) patients. C-statistics (also referred to as area under curve/AUC) were estimated by the package for logistic regression models fitted to 1-year survival outcomes. Based on previous work in MPM [26, 30], a significance cut-off of 0.65 for the C-statistic was used, where a value of 0.5 indicates a relationship governed by chance. Differences in GCN2 scores between histological subtypes (epithelioid, biphasic, sarcomatoid) were determined in GraphPad Prism using an ordinary one-way ANOVA with Bonferroni post hoc test to correct for family-wise error.
Results
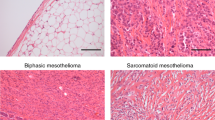
Patient cohort characteristics
Five tissue microarray (TMA) slides were obtained from the Mesobank [27] containing malignant pleural mesothelioma (MPM) samples from a total of 204 patients. Accompanying patient demographic data are summarised in Table 1 and Fig. 1. Clinical data were complete to January 2022. Most patients were male (90.2%) with a median age of 69 years. Epithelioid histology was the most prevalent (126 of 204, 61.8%), followed by biphasic (47 of 204, 23.0%), and sarcomatoid histological subtype (sarcomatoid 29 of 204, 14.2%, desmoplastic 2 of 204, 1.0%). TNM (tumour, node, metastasis) data was provided partially or entirely for 65 of 204 patients. For patients with these data available, 56.9% (T3-T4, 37 of 65) had more extensive tumours that covered all pleural surfaces in one side of the body. 41.3% showed some degree of lymph node involvement (N1-N3, 26 of 63), and 35.5% of cancers showed evidence of distant metastasis (M1/1a, 22 of 62). Four patients (1.9%) were alive at time of data analysis, and the living status data were missing for three patients (1.5%). Three patients (1.5%) were excluded due to no tissue sections were present for these patients within the slide set.
Kaplan–Meier curves corresponding to patients captured in TMA demographic data. Statistical significance of figures a–d show in the top right, referring to univariate survival analysis using the log-rank test. a Histological subtype with epithelioid, biphasic and sarcomatoid. b Metastasis state. c Tumour score. d Node score.
Determination of GCN2 levels
The scoring of the TMA for GCN2 was performed in parallel by three persons (LTG, NH, and MN) all of whom where blinded to the clinical data. Intensity of GCN2 staining was scaled from 0 to 5 (0 for no discernible staining, 1 being little staining, 3 for moderate staining, 5 for intense staining) (see Fig. 2a). Each section was then given a percentage value for the extent of malignant cells that were stained. The staining score for GCN2 levels was then calculated through multiplying the staining score with the extent percentage giving a range of 0 to 500 for each section. GCN2 staining showed no correlation with histological subtype, metastasis score or tumour score (see Fig. 2b–d).
a Representative microarrays of intensity scores 1–5. b GCN2 Scores in different histological subtype. c GCN2 scores compared with metastasis scores. d GCN2 score in the different tumour score subtypes. Note there was only one instance of Tumour Score “0” in the dataset. In b and d, statistical differences were calculated by an ANOVA with Bonferroni post hoc test, while for c, a non-paired t-test was used.
Univariate analysis
Histological subtype (p < 0.0001) and metastasis scores (p = 0.0049) were prognostic with univariate analysis (see Fig. 1), although neither tumour nor node score had any significant impact on survival. GCN2 scores (see Fig. 3) had a significant association with prognosis (p = 0.0009). Those cases scoring in the upper two quintiles (“High GCN2”) had a significantly shorter survival than those in the lower two quintiles (“Low GCN2”) or the rest of the cohort (“Remainder”) (High GCN2—181.5 Days, Low GCN2—461.5 Days, Remainder 382.0 Days).
a, b Kaplan–Meier curve of GCN2 score in all mesothelioma cases, and box-whisker plot of survival in high (upper two quintiles) and low (lower two quintiles) GCN2 scores. Median survivals: GCN2 High 181.5 Days, GCN2 Low 461.5 Days. c High GCN2 group vs. all other cases. Median survivals: GCN2 High 181.5 Days, Remainder 382.0 Days. d High GCN2 score vs. Low GCN2 in epithelioid histology only. Median survival High 217.0 Days, Low 484.0 Days.
Multivariate analysis
Using Cox regression in multivariate analysis, GCN2 was a significant indicator of survival, independent histology (see Table 2). However, GCN2 scoring was not a significant predictor of survival independent of metastasis score (p = 0.4643). The C-statistic value for GCN2 score was 0.690 (see Table 3), with histopathology (epithelial vs. non-epithelial) performing marginally worse with a value of 0.657. Addition of the GCN2 score to histopathology logistic regression did not improve the C-statistic significantly.
Discussion
In this study, we have examined the expression levels of GCN2 in malignant pleural mesothelioma (MPM) samples and correlated these levels with patient survival. Previous work had shown the CHOP levels predicted patient survival in MPM [26], however, the work of Dalton et al. had not determined the role of upstream signalling, which may have contributed to those elevated CHOP levels. GCN2, being part of the Integrated Stress Response (ISR), may influence CHOP levels as much as either PERK and/or endoplasmic reticulum stress. Furthermore, recent progression in pharmaceutical trials targeting ASS-1 dependency in MPM cases suggest a role for GCN2 in MPM.
One weakness of the study presented here is that we are unaware of the activation status of the GCN2 which have identified. Determining whether the higher expression of GCN2 correlates to increases in eIF2α levels, or a higher proportion of GCN2-autophosphorylation levels, as previously observed [31], would strengthen the association between GCN2 levels and CHOP. Additionally, it remains to be seen whether GCN2 overexpression is also responsible for ASS-1 level expression increases within this dataset.
In invasive bladder cancers, it has been demonstrated that ASS-1 loss promotes cancer cell survival through GCN2 expression [13], and that administration of ADI-PEG-20 causes apoptosis through GCN2/ATF4 mediated CHOP activation. Interestingly, ASS-1 protein and mRNA levels have recently been shown to be dependent on BRCA-associated protein 1 (BAP1) levels in MPM cells and tumours [32]. Although exact percentages vary between 23% [33] and 49% [34], it has been shown in several studies that a significant proportion of MPMs have lower BAP1 levels, and germline mutations in the BAP1 gene, which lower protein levels and reduce nuclear localisation are associated with a predisposition to MPM [35]. Through engineering MeT5A-BAP1w-/KO cell lines, which phenocopy BAP1-deficient cancers, Barnett et al. also showed that the BAP1 mediated increase in ASS-1 levels conferred greater resistance to ADI-PEG-20 [32]—which may in turn mean that these tumours do not have elevated GCN2 levels.
In summary, we have shown that GCN2 levels are a useful prognostic biomarker for MPM survival. This may suggest that targeting GCN2 through small molecules, such as GCN2iB and others [24, 36], may be a fruitful avenue for future therapeutic development.
Data availability
Blinded IHC data are available on request to the corresponding author.
Change history
05 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s44276-024-00040-2
References
Rudd RM. Malignant mesothelioma. Brit Med Bull. 2010;93:105–23.
Senek M, Robertson S, Darlison L, Creech L, Tod A. Malignant pleural mesothelioma patients’ experience by gender: findings from a cross-sectional UK-national questionnaire. BMJ Open Respir Res. 2022;9:e001050.
Sauter JL, Dacic S, Galateau-Salle F, Attanoos RL, Butnor KJ, Churg A, et al. The 2021 WHO classification of tumors of the pleura: advances since the 2015 classification. J Thorac Oncol. 2022;17:608–22.
Opitz I, Friess M, Kestenholz P, Schneiter D, Frauenfelder T, Nguyen-Kim TDL, et al. A new prognostic score supporting treatment allocation for multimodality therapy for malignant pleural mesothelioma a review of 12 years’ experience. J Thorac Oncol. 2015;10:1634–41.
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44.
Broggio J, John S, Wong K, Gildea C, Emmett M, Finnigan S, et al. Cancer survival in England: adult, stage at diagnosis and childhood-patients followed up to 2018. Office for National Statistics;2019
Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–86.
Szlosarek PW, Steele JP, Nolan L, Gilligan D, Taylor P, Spicer J, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1–deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2016;3:58.
Meirson T, Pentimalli F, Cerza F, Baglio G, Gray SG, Correale P, et al. Comparison of 3 randomized clinical trials of frontline therapies for malignant pleural mesothelioma. JAMA Netw Open. 2022;5:e221490.
Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12:7126–31.
Long Y, Tsai WB, Wang D, Hawke DH, Savaraj N, Feun LG, et al. Argininosuccinate synthetase 1 (ASS1) is a common metabolic marker of chemosensitivity for targeted arginine- and glutamine-starvation therapy. Cancer Lett. 2017;388:54–63.
Szlosarek PW, Phillips MM, Pavlyk I, Steele J, Shamash J, Spicer J, et al. Expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1–deficient mesothelioma: safety, efficacy, and resistance mechanisms. JTO Clin Res Reports. 2020;1:100093.
Sahu D, Gupta S, Hau AM, Nakashima K, Leivo MZ, Searles SC, et al. Argininosuccinate synthetase 1 loss in invasive bladder cancer regulates survival through general control nonderepressible 2 kinase–mediated eukaryotic initiation factor 2α activity and is targetable by pegylated arginine deiminase. Am J Pathol. 2017;187:200–13.
Neill G, Masson GR. A stay of execution: ATF4 regulation and potential outcomes for the integrated stress response. Front Mol Neurosci. 2023;16:1112253.
Gold LT, Masson GR. GCN2: roles in tumour development and progression. Biochem Soc Trans. 2022;50:737–45.
Ishimura R, Nagy G, Dotu I, Chuang JH, Ackerman SL. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. eLife. 2016;5:568.
Pochopien AA, Beckert B, Kasvandik S, Berninghausen O, Beckmann R, Tenson T, et al. Structure of Gcn1 bound to stalled and colliding 80S ribosomes. Proc Natl Acad Sci USA. 2021;118:e2022756118.
Inglis AJ, Masson GR, Shao S, Perisic O, McLaughlin SH, Hegde RS, et al. Activation of GCN2 by the ribosomal P-stalk. Proc Natl Acad Sci USA. 2019;116:201813352. Mar 12
Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell. 2000;6:269–79.
Harding HP, Ordonez A, Allen F, Parts L, Inglis AJ, Williams RL, et al. The ribosomal P-stalk couples amino acid starvation to GCN2 activation in mammalian cells. eLife. 2019;8:1.
Masson GR. Towards a model of GCN2 activation. Biochem Soc Trans. 2019;47:1481–8.
Drees J, Chan AS, Li Y, Kangas T, Zhang W, Fumagalli M, et al. 1327 Novel GCN2 modulator HC-7366 decreases pulmonary metastases and reduces myeloid-derived suppressor cells. J Immunother Cancer 2022;10. https://doi.org/10.1136/jitc-2022-SITC2022.1327.
Kato Y, Kunimasa K, Takahashi M, Harada A, Nagasawa I, Osawa M, et al. GZD824 inhibits GCN2 and sensitizes cancer cells to amino acid starvation stress. Mol Pharmacol. 2020;98:669–76.
Fujimoto J, Kurasawa O, Takagi T, Liu X, Banno H, Kojima T, et al. Identification of novel, potent, and orally available GCN2 inhibitors with type I half binding mode. ACS Med Chem Lett. 2019;10:1498–503.
Nakamura A, Nambu T, Ebara S, Hasegawa Y, Toyoshima K, Tsuchiya Y, et al. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci USA. 2018;115:E7776–85.
Dalton LE, Clarke HJ, Knight J, Lawson MH, Wason J, Lomas DA, et al. The endoplasmic reticulum stress marker CHOP predicts survival in malignant mesothelioma. Br J Cancer. 2013;108:1340–7.
Rintoul RC, Rassl DM, Gittins J, Marciniak SJ, collaborators M. MesobanK UK: an international mesothelioma bioresource. Thorax. 2016;71:380.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77.
R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013.
Nowak AK, Francis RJ, Phillips MJ, Millward MJ, Schaaf AAvander, Boucek J, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res. 2010;16:2409–17.
Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, Panis DND, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–96.
Barnett SE, Kenyani J, Tripari M, Butt Z, Grosman R, Querques F, et al. BAP1 loss is associated with higher ASS1 expression in epithelioid mesothelioma: implications for therapeutic stratification. Mol Cancer Res. 2023;21:411–27.
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–72.
McGregor SM, Dunning R, Hyjek E, Vigneswaran W, Husain AN, Krausz T. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol. 2015;46:1670–8.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5.
Lough L, Sherman D, Becerra-Flores M, Vasudevan D, Lavinda O, Ni E, et al. Triazolo[4,5-d]pyrimidines as validated general control nonderepressible 2 (GCN2) protein kinase inhibitors reduce growth of leukemia cells. Comput Struct Biotechnol J. 2018;16:350–60.
Acknowledgements
We thank the Dundee Imaging Facility and the Data Analysis Group at the University of Dundee and those at the Mesobank for their help in this work. We thank the Tayside Biorepository for useful conversations and help with ethical and legal requirements. We thank Professor Stefan Marciniak for helpful discussion and feedback on the study. Material used in the Research Programme was obtained from Mesobank, a Research Tissue Bank supported by Asthma and Lung UK and The Victor Dahdaleh Foundation.
Funding
LTG is funded through the Ninewells Cancer Campaign. GRM is funded by a Baxter Fellowship from the University of Dundee. Part of this work was supported by TENOVUS grant number 119615.
Author information
Authors and Affiliations
Contributions
LTG conducted image scoring, data analysis and manuscript preparation. SB and NMK conducted slide staining and provided advice and training to LTG on scoring. NH and MN provided additional scoring data. GRM devised the experiments, conducted data analysis and aided in manuscript and figure preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All samples were collected and used under ethic and consented approval to participate in the study. All tumour samples obtained from MesobanK UK, Cambridge. Ethical approval for MesobanK was granted by the East of England—Cambridge Central Research Ethics Committee (18/EE/0161) on 9th August 2018. This work uses data that has been provided by patients and collected by the NHS as part of their care and support. The data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of NHS Digital. Access to the data was facilitated by the UK Health Security Agency Office for Data Release.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gold, L.T., Bray, S.E., Kernohan, N.M. et al. The amino-acid stress sensing eIF2α kinase GCN2 is a survival biomarker for malignant mesothelioma. BJC Rep 1, 4 (2023). https://doi.org/10.1038/s44276-023-00004-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-023-00004-y