Abstract
Background
Breast cancer is the most common cancer in women, and the first-line treatment for patients with hormone-receptor positive/HER2-negative metastatic breast cancer is CDK4/6 inhibitor plus endocrine therapy. Understanding the impact of CDK4/6 inhibitor dose reduction, which occurs in about half of the patients, is important.
Methods
This real-world cohort study is based on electronic health records from Capital Region of Denmark. All women with metastatic breast cancer initiating first-line treatment with CDK4/6 inhibitor between May 2017 and October 2022 were included.
Results
A total of 546 patients were eligible for inclusion in the 12-week landmark analysis and 192 (35%) experienced dose reduction. These patients were older, had worse ECOG PS, more received prior adjuvant endocrine treatment, and more received fulvestrant as the endocrine backbone. Dose reduction was associated with reduced overall survival (39.9 vs. 54.3 months) and shorter treatment duration (18.0 vs. 26.9 months). Adjusted hazard ratio for death was 1.38 (95% CI: 1.01–1.89).
Conclusions
Dose reduction of CDK4/6 inhibitors within the first 12 weeks of treatment was associated with significantly higher mortality and shorter treatment duration. These findings contrast with previous analyses showing no effect of dose reduction, likely due to considering immortal time bias in this study.
Similar content being viewed by others
Background
With approximately 5000 new registered cases in 2020, breast cancer is the most common cancer among women in Denmark [1]. In high income countries, approximately 3–6% of new breast cancer diagnoses present with distant metastases [2]. Although the far majority have early-stage breast cancer at the time of diagnosis, the risk of distant recurrence of hormone-receptor (HR) positive breast cancer is high, with a 20-year risk ranging from 13% to 41% depending on initial tumour size and lymph node involvement [3].
Breast cancers are classified by the expression status of hormone-receptor and human epidermal growth factor receptor 2 (HER2). Dysregulation of the cyclin D-CDK4/6-pRb pathway has been identified as a key mediator of endocrine resistance in HR-positive, HER2-negative breast cancer. Randomised trials and a pooled analysis demonstrated that the addition of CDK4/6 inhibitors to endocrine therapy is beneficial in terms of progression free survival (PFS) and that ribociclib lead to a significant overall survival (OS) improvement [4,5,6,7,8,9,10,11].
In November 2016, palbociclib was the first CDK4/6 inhibitor to obtain approval from the European Medicines Agency (EMA). Subsequently, the Danish Medicines Council approved palbociclib in February 2017, followed by the approval of ribociclib in 2018 and abemaciclib in 2019. Since then, the combination of an aromatase inhibitor with a CDK4/6 inhibitor has become the established standard of care in the first-line treatment of patients diagnosed with HR-positive, HER2-negative metastatic breast cancer. In Denmark, the recommended choice of CDK4/6 inhibitor has changed over time due to cost considerations in the publicly funded healthcare system.
CDK4/6 inhibitors are associated with limited toxicity. Palbociclib and ribociclib may lead to haematological toxicity in the form of neutropenia, while abemaciclib primarily causes gastrointestinal toxicity in the form of diarrhoea. When managed through dose reductions and treatment interruptions, side effects are often reversible and short-lived [12,13,14,15,16].
Previous studies have examined the impact of dose reduction on the efficacy of the three CDK4/6 inhibitors, with the majority of studies showing no significant loss of efficacy [13,14,15, 17,18,19,20,21]. It is generally considered safe to reduce the dosage of any CDK4/6 inhibitor without compromising its efficacy. But analysis of the effect of dose reduction can be susceptible to immortal time bias, and most of these studies have not taken this into account [14, 15, 18,19,20,21].
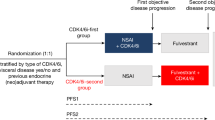
Immortal time refers to a span of time in the observation period before the subject is exposed, and during which the outcome under study could not have occurred [22]. Although the patient is not immortal during this time, they necessarily must remain alive until the first dose-reduction to be categorised into the dose-reduction group. When traditional survival analysis methods are used there is a biased favour towards the group of exposed patients, which in this case would favour the dose-reduction group [23]. Statistical techniques can handle changes in exposure over the course of follow-up time, including landmark analysis and the use of time-dependent covariates (Fig. 1) [24].
Illustration of a naive misclassification and b landmark analysis to address immortal-time (adapted from Jackson et al. [31]).
Examining the impact of dose reduction on treatment outcomes through an experimental design is not feasible. Therefore, the adoption of an observational study design is deemed more appropriate, provided that statistical biases are carefully considered and addressed.
In this study, we utilised a real-world cohort of Danish women diagnosed with HR-positive, HER2-negative metastatic breast cancer. Our aim was to examine the association between dose reduction of CDK4/6 inhibitors and overall survival (OS). To address the potential influence of immortal time bias, we used landmark analyses, which we then compared to traditional, naive survival analyses that did not explicitly consider the presence of immortal time bias.
Methods
Study population
This real-world cohort study utilised electronic health records (EHR) from all women, who initiated first-line treatment with a CDK4/6 inhibitor in the Capitol Region of Denmark for HR-positive, HER2-negative metastatic breast cancer between 1 May 2017 and 9 September 2022. Patients were excluded if they moved outside the Capitol Region of Denmark and Region Zealand after the diagnosis of metastatic disease.
Data sources
The EHR (Sundhedsplatformen, EPIC Systems Corporation) provided data extracts concerning patient characteristics, survival status, co-morbidities, along with information regarding medications, including their duration and dosages throughout the treatment period. EHR data were extracted from a central repository that contains data for all hospitals treating metastatic breast cancer in Capital Region of Denmark and Region Zealand. Information about the presence of visceral metastases and ECOG performance status was obtained through a manual review of the EHR for a randomly selected sample of 80 patients from the dose reduction group and 80 patients from the full dose group. The findings obtained through the manual record review were subsequently imputed to their respective groups using multiple imputation. Furthermore, we performed manual record reviews for all patients who discontinued CDK4/6 inhibitor treatment, with a focus on the most recent evaluation scan prior to discontinuation. This allowed us to categorise the reasons for discontinuation as either progression, toxicity, or other factors.
Information about hormone receptor and HER-2 status was obtained from the regional copy of the National Pathology Registry, which has maintained records dating back to 2009 and information about clinical biochemical samples were collected from the LABKA database. Information about treatments and diagnosis prior to the implementation of current EHR in May 2016 to March 2017 was collected from the prior regional system (SDB) feeding data to The Danish National Patient Register that contains data dating back to 2007.
Both the EHR, SDB, LABKA database and the Pathology Registry are publicly maintained and contain identifiable data. Data were linked using the unique Danish personal identification number.
The EHR data extract was retrieved on 11 November 2023 with data restricted to 6 September 2023. Manual reviews of records were performed from January to November 2023. During the manual review, misclassified end dates of treatments were corrected.
Exposure–dose reduction
The exposure of interest was dose reduction. We defined dose reduction as a prescribed daily dose below 300 mg for abemaciclib, 125 mg for palbociclib and 600 mg for ribociclib. Patients who initiated CDK 4/6 inhibitor treatment at a reduced dose were categorised as dose reduced. We did not classify a treatment interruption as a dose reduction if the treatment was subsequently resumed at the full recommended dosage.
Outcomes
The primary outcome of interest was overall survival defined as time from the end of the 12-week landmark to death of any cause. Vital status was obtained from the EHR, which is continuously updated from the Danish Population Register.
Secondary outcomes were treatment duration and chemotherapy-free survival. Treatment duration was defined as time from first dose CDK4/6 inhibitor to the end date of last dose period or death, whichever occurred first. Chemotherapy-free survival was defined as time from the end of the 12-week landmark time to initiation of chemotherapy or death, whichever occurred first.
We performed post hoc analysis of the reasons for discontinuation CDK4/6 inhibitor treatment.
Landmark analysis and immortal time bias
In the landmark analysis, we included patients who received a combination of a CDK4/6 inhibitor and endocrine therapy as their first-line treatment regimen and had a treatment duration of no less than 12 weeks.
The selection of the 12-week landmark time was based on the median duration of 12 weeks from the initiation of treatment at recommended dose level to the occurrence of first dose reduction (Fig. 2). To assess the impact of the chosen landmark time on the results, we conducted additional sensitivity analyses using alternative landmark times at 6 and 9 months.
In addition to the landmark analysis, we performed a naive survival analysis that did not attempt to address immortal time bias. The first dose reduction at any point in time reclassified a patient from the full dose group to the dose reduction group, and time that had passed until dose reduction was assigned as exposed time. To allow comparison of our results with the MONALEESA-2, -3 and -7 safety and impact analysis, we calculated relative dose intensities (RDI) and grouped patients by RDI percentile that align to those reported at ≤71%, 72–96% and ≥97% relative DI [14]. We estimated the hazard ratio using the Cox regression model and adjusted for the same baseline covariates applied in the landmark analyses.
Statistics
Patient demographics and disease characteristics were described with numbers and percentages for categorical variables and median with interquartile ranges for age and BMI. Characteristics are documented at the time of treatment initiation. Any difference was examined with t-test for age and BMI and Chi2 or Fisher’s exact test for categorical variables. Patients alive were censored by 9th September 2023. Pre-planned analyses included overall survival, treatment duration and chemotherapy-free survival estimated using the Kaplan–Meier method. Post hoc analysis included time from CDK4/6 inhibitor discontinuation to death and reasons for discontinuation. Multivariable cox proportional hazards regression models were applied to assess hazard ratio for the outcomes of interest and adjusted for ECOG performance status, presence of visceral metastases, primary metastatic breast cancer, age as continuous variable, Charlson’s Comorbidity Index, BMI, LDH, endocrine therapy backbone (fulvestrant vs. other) and CDK4/6 inhibitor agent. The variables included in the multivariate cox analysis were selected based on their clinical relevance. Median follow-up time was calculated by the reverse Kaplan–Meier estimator. The proportional hazard assumption was tested by Schoenfeld residuals and no deviations from the assumption were found.
Statistical analyses were carried out using the R statistical software (version 4.2.2 R Foundation for Statistical Computing, Vienna, Austria). Multiple Imputation was performed using the mice package with 500 imputations [25].
The statistical analysis plan is provided in Supplementary Material.
Results
In this study, 674 patients initiated first-line treatment with a CDK4/6 inhibitor in combination with endocrine therapy for HR-positive and HER2-negative metastatic breast cancer. Of these, 52 patients (7.7%) started treatment at a lower than recommended dose, and an additional 365 patients (54%) had their dose reduced at least once during the follow-up period, with a median time of 12 weeks to the first dose reduction (Fig. 2). The median dose intensity was 83% (IQR: 66–100%).
In total, 546 patients (81%) remained on treatment until the 12-week landmark time and were included in the landmark analysis. Within the first 12 weeks, 192 patients (35%) were prescribed a reduced dose of CDK4/6 inhibitor and were categorised into the dose reduction group. The remaining 354 patients were categorised into the full dose group.
Patients who underwent dose reduction were significantly older, more had received prior adjuvant endocrine treatment, and more received fulvestrant as the endocrine backbone compared to individuals in the full dose group (Table 1). There were no significant differences observed in terms of co-morbidity, or the presence of visceral metastases between the two groups. However, the ECOG performance score was significantly lower in the full dose group.
In the 12-week landmark analysis, with a median follow-up time of 39.1 months (IQR: 26.7–55.3 months), dose reduction was significantly associated with shorter median overall survival of 39.9 months (95% CI: 35.2–50.7) vs. 54.3 months (95% CI: 49.7–NE) (Fig. 3a) corresponding to a crude hazard ratio (HR) of 1.61 (95% CI: 1.22–2.13) and an adjusted HR of 1.38 (95% CI: 1.01–1.89). Cox model results for OS are provided in Supplementary Table S1. Treatment duration was significantly shorter in the group of patients with dose reductions compared to the full dose group, 18.0 months (95% CI: 11.7–26.3) vs. 26.9 months (95% CI: 23.9–31.5), and the adjusted HR for treatment discontinuation was 1.34 (95% CI: 1.04–1.73) (Fig. 3b). Moreover, chemotherapy-free survival was shorter in the dose-reduction group at 30.1 months (95% CI: 25.5–38.3) vs. 38.8 months (95% CI: 33.3–43.3), adjusted HR of 1.31 (95% CI: 0.98–1.74) (Fig. 3c).
In the naive analysis, the median overall survival for the dose-reduction group was 53.6 months (95% CI: 47.2–60.7), while for the full-dose group it was 39.2 months (95% CI: 32.6–45.2). The adjusted HR for death was 0.53 (95% CI: 0.40–0.70), indicating a favourable outcome for individuals who received a CDK4/6 inhibitor in reduced dosage (Fig. 4a). When examining the analysis of relative dose intensities, the adjusted HR for the RDI < 72% group compared to the RDI ≥ 97% group was 0.42 (95% CI: 0.29–0.59) (Fig. 4b).
The results of the analyses at 6- and 9-month landmark times were comparable, yet less pronounced, to the 12-week landmark analysis. The median overall survival was 47.4 months (95% CI: 38.6–NE) vs. 52.5 months (95% CI: 46.9–NE) and 45.7 months (95% CI: 36.2–NE) vs. 48.2 months (95% CI: 43.9–NE) for the group with dose reduction and full dose group, respectively, at the two landmark times. The adjusted HR for death were 1.28 (95% CI: 0.89–1.84) and 1.18 (95% CI: 0.80–1.76) for 6- and 9-month landmarks times. The same tendencies were observed for treatment duration and chemotherapy-free survival (Supplementary Tables S2 and S3 and Supplementary Figs. S1 and S2).
Within the dose-reduction group, 44.3% discontinued treatment due to disease progression, 17.7% due to adverse effects, and 5.2% for other reasons. In the full-dose group, the respective percentages were 43.8, 11.0, and 4.8%. The adjusted HR for treatment discontinuation due to disease progression for the dose-reduction group was 1.35 (95% CI: 0.99–1.85) compared to the full-dose group, and for toxicity, it was 1.20 (95% CI: 0.76–1.89) (Supplementary Fig. S3).
Overall survival measured from the time of discontinuation of CDK4/6 inhibitor did not differ between the dose-reduction compared to the full-dose group with an adjusted HR of 0.98 (95% CI: 0.71–1.36) (Supplementary Fig. S4).
Discussion
In this study real-world cohort study, we found that dose reduction of a CDK4/6 inhibitor within the first 12 weeks of treatment was associated with an increased risk of death with a HR of 1.38 (95% CI: 1.01–1.89) when adjusted for ECOG performance status, presence of visceral metastases, age, comorbidity, BMI, LDH > ULN, endocrine backbone, primary metastatic disease, and CDK4/6 inhibitor agent.
To assess the potential influence of immortal time bias, we carried out a naive survival analyses for comparison. These analyses led to results that are in direct contradiction to the outcomes of our landmark analysis showing a reversal of the hazard ratio for death. This immortal time bias is due to the necessity of a longer survival period for a dose reduction to be documented. Our results highlight the importance of explicitly detailing methodological choices for addressing immortal time bias when analysing data that are vulnerable to this bias.
We found that the time from CDK4/6 inhibitor discontinuation to death did not differ between dose-reduced and full-dose patients, but those in the dose-reduction group had a significantly shorter treatment duration. We assessed whether patients in the dose-reduction group discontinued treatment more often due to disease progression, suggesting sub-therapeutic dose level, or due to intolerable toxicity, which may warrant further dose reduction attempts. The reasons for discontinuing treatment with CDK4/6 inhibitor did not differ significantly between the two groups, although there was a tendency towards both earlier disease progression and more toxicity in the dose-reduction group. These analyses were not pre-planned, and additional research designed to assess reasons for treatment discontinuation and approaches to maintain patients on CDK4/6 inhibitor treatment is warranted.
Our study has several limitations that need to be considered. Being a retrospective study, the accuracy of information relied upon electronic health records, and the lack of randomisation introduces the possibility of confounding factors affecting the results. The baseline characteristics between the dose-reduction and full-dose cohorts were imbalanced, with the dose-reduction cohort having more previous endocrine treatments, potential endocrine resistance, higher age, and higher ECOG performance status. These factors may affect both treatment tolerance and efficacy, potentially acting as confounders if not appropriately accounted for in the analysis. While we adjusted our analysis, the actual health status of the included patients may be inaccurately documented leading to residual confounding. Missing data and the need for multiple imputations may lead to further bias. The study cohort is relatively homogeneous in terms of ethnicity and race, so the results may not be generalisable to other populations. Independent validation in other population-based cohorts should be performed. Changing costs for the three approved CDK4/6 inhibitors resulted in shifts in drug choices during the study period. We attempted to mitigate this issue by adjusting for the CDK4/6 inhibitor agent in the Cox regression analysis. Landmark analysis introduces a selection bias due to the exclusion of patients who discontinued due to early intolerable toxicity or progressive disease. Hence, the results may not be generalisable to all patients who start treatment with a CDK4/6 inhibitor.
We identified three studies that examined the effect of reducing CDK4/6 inhibitor dosing and that utilised either landmark analysis or considered dose reduction as a time-dependent covariate [13, 16, 17]. One study with only 56 patients found that patients treated with palbociclib at a reduced dose intensity (RDI < 80%) at the 12 week landmark experienced significantly shorter PFS compared to those treated with RDI ≥ 80% [17], while another based on the PALOMA-2 trial found no significant impact of dose reduction on PFS among patients treated with palbociclib and letrozole at 3, 6 and 9 months landmark times [13]. A safety analysis of MONARCH-2 and -3 data similarly found that abemaciclib dose reduction, when considered as a time-dependent covariate, had no statistically significant impact on PFS [16]. All three studies focused on PFS as the primary endpoint.
Several other studies have found no negative impact of CDK4/6 inhibitor dose reductions on PFS or OS [14, 15, 18,19,20]. These studies, however, did not outline measures to mitigate the potential risk of immortal time bias.
Our findings add to the growing body of research that advocates for the utilisation of analytical approaches explicitly designed to prevent biases, including immortal time bias, when conducting comparative efficacy research using observational data [27,28,29,30].
In conclusion, we found a detrimental effect on overall survival of CDK4/6 inhibitor dose reduction. To our knowledge, this study is the first to present data on the effect of CDK4/6 inhibitor dose reduction on overall survival while considering immortal time bias. While our findings should not prompt a change in the current practice of dose reductions for managing CDK4/6 inhibitor-related toxicities we hope it might cause reevaluation of previous studies, including those based on the PALOMA-3 and MONALEESA-2, -3 and -7 trials, to validate our findings.
Data availability
Data may be provided upon approved request.
References
NORDCAN 2.0. Association of the Nordic Cancer Registries. 2024. https://nordcan.iarc.fr/en/dataviz/tables?mode=cancer&group_populations=1&sexes=2&populations=208&years=2021.
Daily K, Douglas E, Romitti PA, Thomas A. Epidemiology of de novo metastatic breast cancer. Clin Breast Cancer. 2021;21:302–8.
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–46.
Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: a systematic review and meta-analysis. Int J Mol Sci. 2020;21:6400
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–36.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–7.
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–16.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–24.
Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116.
Finn RS, Boer K, Bondarenko I, Patel R, Pinter T, Schmidt M, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183:419–28.
Goetz MP, Toi M, Huober J, Sohn J, Trédan O, Park IH, et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2− advanced breast cancer: final overall survival results of MONARCH 3. Ann Oncol. 2024;35:718–27.
Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018;10:175883591879332.
Diéras V, Harbeck N, Joy AA, Gelmon K, Ettl J, Verma S, et al. Palbociclib with letrozole in postmenopausal women with ER+/HER2− advanced breast cancer: hematologic safety analysis of the randomized PALOMA-2 trial. Oncologist. 2019;24:1514–25.
Burris HA, Chan A, Bardia A, Thaddeus Beck J, Sohn J, Neven P, et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br J Cancer. 2021;125:679–86.
Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21:1165–75.
Rugo HS, Huober J, García-Sáenz JA, Masuda N, Sohn JH, Andre VAM, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26:e53–65.
Moftakhar B, Lekkala M, Strawderman M, Smith TC, Meacham P, Fitzgerald B, et al. Impact of early dose intensity reduction of Palbociclib on clinical outcomes in patients with hormone-receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183:411–8.
Ismail RK, van Breeschoten J, Wouters MWJM, van Dartel M, van der Flier S, Reyners AKL, et al. Palbociclib dose reductions and the effect on clinical outcomes in patients with advanced breast cancer. Breast. 2021;60:263–71.
Kristensen KB, Thomsen IMN, Berg T, Kodahl AR, Jensen AB. Dose modifications of ribociclib and endocrine therapy for treatment of ER+ HER2− metastatic breast cancer. Breast Cancer Res Treat. 2021;188:799–809.
Wilkie J, Schickli MA, Berger MJ, Lustberg M, Reinbolt R, Noonan A, et al. Progression-free survival for real-world use of palbociclib in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer. 2020;20:33–40.
Low JL, Lim E, Bharwani L, Wong A, Wong K, Ow S, et al. Real-world outcomes from use of CDK4/6 inhibitors in the management of advanced/metastatic breast cancer in Asia. Ther Adv Med Oncol. 2022;14:175883592211396.
Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167:492–9.
Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–9.
Zabor EC, Assel M. On the need for landmark analysis or time-dependent covariates. J Urol. 2023;209:1060–2.
van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45. http://www.jstatsoft.org/v45/i03/.
Castelo-Branco L, Pellat A, Martins-Branco D, Valachis A, Derksen JWG, Suijkerbuijk KPM, et al. ESMO guidance for reporting oncology real-world evidence (GROW). Ann Oncol. 2023;34:1097–112.
Boyne DJ, Cheung WY, Hilsden RJ, Sajobi TT, Batra A, Friedenreich CM, et al. Association of a shortened duration of adjuvant chemotherapy with overall survival among individuals with stage III colon cancer. JAMA Netw Open. 2021;4:e213587.
Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–5.
Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4:63.
García-Albéniz X, Hsu J, Hernán MA. The value of explicitly emulating a target trial when using real world evidence: an application to colorectal cancer screening. Eur J Epidemiol. 2017;32:495–500.
Jackson BE, Greenup RA, Strassle PD, Deal AM, Baggett CD, Lund JL, et al. Understanding and identifying immortal-time bias in surgical health services research: an example using surgical resection of stage IV breast cancer. Surg Oncol. 2021;37:101539.
Author information
Authors and Affiliations
Contributions
AB: conceived and designed the study, performed manual patient record reviews and drafted the manuscript. AH: performed manual patient record reviews and helped drafting the manuscript. AK: played an important role in interpreting the results and revised the manuscript. TB: played an important role in interpreting the results and revised the manuscript. IT: performed manual patient record reviews and revised the manuscript. UL: played an important role in interpreting the results and revised the manuscript. TP: conceived and designed the study, performed statistical analyses and helped drafting the manuscript. All authors approve the final version of the manuscript, and all authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
AB received reimbursement for attending symposia from Gilead. AFH received reimbursement for attending symposia from Daiichi Sankyo. ASK received institutional grants from Roche and Pfizer; and received compensation as a member of the scientific advisory board from Novartis, Astra Zeneca, MDS, Roche and Eli Lilly Danmark A/S (all outside the submitted work). TB received reimbursement for attending symposia from Daiichi Sankyo; received institutional grants from Pfizer, Astra Zeneca, Novartis, Samsung Bioepis, Seattle Genetics, Merck, Eli Lilly, and Daiichi Sankyo; and received compensation as a member of the scientific advisory board from Novartis (outside the submitted work). UL received institutional grants from Pfizer, Eli Lilly, BMS, GSK, Incyte, and Roche; and received compensation as a member of the scientific advisory board from Bayer, Novartis and Pfizer (all outside the submitted work). IT and TSP declare no potential competing interests.
Ethics approval and consent to participate
The study was approved by The Research Ethics Committee Secretary in the Capital Region (approval number R-22033077) and filed to the Danish Data Protection Agency (approval number P-2022-287). The study was conducted by a multidisciplinary team comprising specialists in clinical oncology, clinical pharmacology, and public health. We followed the ESMO Guidance for Reporting Oncology real-World evidence (GROW) [26].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bjerrum, A., Henriksen, A.F., Knoop, A.S. et al. Overall survival after CDK4/6 inhibitor dose reduction in women with metastatic breast cancer. BJC Rep 2, 82 (2024). https://doi.org/10.1038/s44276-024-00108-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-024-00108-z
This article is cited by
-
Impact of adverse events on survival outcomes in patients treated with CDK4/6 inhibitors for advanced breast cancer
Cancer Chemotherapy and Pharmacology (2025)