Abstract
Background
A previously conducted study reported that insomnia rates among oncology patients in Ireland are twice that of the general population, and that a breast cancer diagnosis was an independent predictor for clinical insomnia disorder. The aim of this study is to explore this interaction further in a larger cohort of breast cancer patients.
Methods
We evaluated sleep disturbance and sleep hygiene practices among adult breast cancer patients via questionnaires. Sociodemographic data, clinical characteristics, sleep history and attitudes towards sleep assessments were collected and analysed.
Results
The comprehensive 40-item questionnaire was completed by 315 patients. Of this cohort, 56% reported a change in their sleeping patterns since their cancer diagnosis, with over 55% of the study population having sub-threshold or clinical insomnia disorder. Although 64.2% of patients believed that questions regarding sleep should be part of breast cancer assessment, only 32% recalled being asked about sleep by a healthcare worker. Moreover, only 27.1% of respondents felt their sleeping difficulties were adequately dealt with since their diagnosis.
Conclusion
In summary, sleep disturbance is prevalent among breast cancer patients. Despite a majority of breast cancer patients recognising the importance of sleep assessment, a significant gap remains in healthcare providers addressing these concerns effectively.
Similar content being viewed by others
Introduction
Sleep disorders are frequently reported among cancer patients, both during treatment, in the post-treatment phase, and subsequently in palliative care settings [1,2,3,4]. Reduced sleep duration and poor sleep quality is associated with an increased risk of cancer recurrence and decreased cancer survival [5,6,7,8]. In contrast to the general population sleep disturbance is greater in younger rather than older patients [9]. Sleep disturbance experienced by both patients and their partners are associated with increased levels of fear of cancer recurrence [10]. Additionally, there is a well-established relationship between cancer related fatigue and sleep deficiency, underscoring the importance of addressing sleep issues in this population [11, 12].
A recent evaluation of sleep patterns in an Irish oncology population demonstrated an insomnia rate twice that of the general population [13]. In that study univariate analysis demonstrated that age under 65, a breast cancer diagnosis, current chemotherapy receipt and alcohol consumption, and a previous history of anxiety were predictors of insomnia syndrome [13]. Despite the significant symptomatic burden of sleep disturbances, these issues are often unrecognised and are inadequately addressed in clinical practice, particularly when compared to other symptoms such as pain and fatigue [14,15,16]. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology advocate for specialised sleep assessment, acknowledging the increasing prevalence of related issues among cancer patients [17]. Nevertheless, a 2020 study revealed that only 34% of Irish oncology patients recalled undergoing assessment for sleep disturbance as part of their treatment [13]. As a breast cancer diagnosis was identified as an independent predictor of clinical insomnia disorder within the Irish oncology population [13], we aimed to explore this interaction further by quantifying the prevalence of sleep disturbance within a specific Irish cohort of breast cancer patients. Additionally, we aimed to examine patient’s opinions on sleep hygiene and evaluate sleep hygiene practices within this population.
This was a cross-sectional questionnaire-based study conducted between September 2023 and March 2024 in adult patients diagnosed with breast cancer at Cork University Hospital/University College Cork Cancer Centre. A comprehensive 40-item composite questionnaire was formulated from a previously established questionnaire supplemented by additional questions devised by the authors [13].
The survey was circulated to patients attending the Outpatient Department. Institutional ethical board approval was obtained (Clinical Research Ethics Committee of the Cork Teaching Hospitals, ECM 01/2025/PUB), and all participants provided informed consent. Patients’ behaviours, attitudes and perceptions regarding sleep hygiene and assessment were examined. Clinical characteristics, sociodemographic and lifestyle data, were recorded. Sleep disturbance was assessed using the Insomnia Severity Index (ISI) as a baseline [18]. The ISI was selected as it is a validated instrument for identifying cases of insomnia within a population [18,19,20]. Data analysis was conducted using IBM SPSS V26. Descriptive statistics were employed to summarise the demographic and clinical characteristics of the participants. Pearsons’s and Spearman’s rank correlations were used to determine the association of clinical, lifestyle and demographic factors with ISI scores. Statistical significance was assumed by a p-value < 0.05.
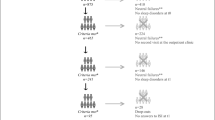
A total of 315 questionnaires were completed, with baseline demographics summarise in Table 1 and clinical characteristics summarised in Table 2.
Among all patients, 56.8% (n = 179) reported a change in their sleeping patterns since their cancer diagnosis, with 72.4% (n = 228) stating they had not experienced sleep difficulties prior to diagnosis. An analysis of ISI scores revealed that 4.8% (n = 14) of this population exhibited severe clinical insomnia, 18.2% (n = 53) demonstrated moderate clinical insomnia, 33.2% (n = 97) experienced subthreshold clinical insomnia, and 43.8% (n = 128) reported no clinically significant insomnia, as shown in Fig. 1. Only one-third of the patients expressed satisfaction with their current sleep patterns (33.3%, n = 105), while more than half indicated that their sleeping patterns caused them at least some degree of distress (54.3%, n = 171).
High caffeine intake, alcohol consumption, low levels of physical activity, and the use of electronic devices before bed are all recognised as potentially modifiable factors that adversely affect sleep [13]. At the time of the study, 87% (n = 274) of participants reported caffeine consumption, 70.5% (n = 222) reported alcohol consumption, and 96.2% (n = 303) reported they used electronic devices before bed. Just under a quarter of our patients (n = 78, 24.8%) indicated that they believed alcohol consumption could affect sleep and sleeping patterns.
In our cohort, 62.5% (n = 197) of patients believed that sleep assessment should be a component of cancer treatment; however, only 23.8% (n = 75) felt that their sleeping difficulties had been adequately addressed since diagnosis. Under one-third of the study population recalled being asked about their sleep by a healthcare worker. Only 12% (n = 8) of patients with moderate or severe insomnia felt that their sleeping difficulties had been sufficiently addressed since diagnosis.
The findings of our study both align with and diverge from those reported by Harrold and colleagues [13], who investigated sleep disturbance across a broader oncology population in Ireland. In Harrold’s study, 44% of patients met criteria for clinical insomnia (moderate or severe), while our breast cancer-specific cohort demonstrated a higher overall prevalence when including both subthreshold and clinical insomnia.
Consistent with Harrold’s findings and multiple other studies [4, 13], we observed a statistically significant association between age and insomnia severity, with patients under 65 reporting higher ISI scores (p < 0.001; V = 0.288). However, unlike Harrold’s study, which also identified alcohol consumption as a predictor of insomnia, we found no statistically significant associations between insomnia and any other clinical, lifestyle or demographic variable as presented in Table 3. In the present study 46% of patients had received chemotherapy and only 24 of 315 patients were within a year of chemotherapy receipt. In contrast, 245 of 294 patients in the study of Harrold et al. were receiving active chemotherapy. As previously reported in patients receiving chemotherapy for breast cancer sleep quality predictors improve with duration of time from chemotherapy treatment [21], which may explain the differences observed.
Our results both resonate with, and differ from those of Mercadante et al. [3]; who studied patients in palliative care settings over a 6 month period of whom 10% had breast cancer and 3.8% had prostate cancer. Over 60% of all patients had sleep disturbance, with hormone therapy and use of opioids and steroids being positively associated with sleep disturbance. Considering that the vast majority of our patients had early stage cancer with good performance state (ECOG 0-1; 90.5%), our results may not capture palliative-phase sleep burdens. This supports the view that sleep disturbance in cancer is multifactorial and may evolve and changes as the diseases progresses.
The fact that our patient population was largely composed of individuals no longer receiving active treatment may help explain the higher prevalence of chronic, rather than acute, sleep disturbances. Previous studies have linked treatment-related toxicity – particularly during chemotherapy- with significant sleep disturbance [9, 22]. In our population, only 7.6% of our patient had completed chemotherapy within the past year. This may account for the lower frequency of treatment-related sleep complaints. These contrasting findings likely reflect post-treatment survivorship sleep challenges, rather than acute treatment-related insomnia. Our findings point to persistent sleep issues in the survivorship phase. These insights highlight the need for tailored sleep management strategies across different phases of the cancer care continuum.
Our findings demonstrate that there is a high prevalence of sleep disorders among this population, with more than half of the participants reporting changes in their sleep patterns since their cancer diagnosis despite over two-thirds lacking any prior history of sleep-related issues. This high level of prevalence aligns with findings from existing literature in similar populations [13]. There were high levels of consumption of caffeine and alcohol in our population, modifiable risk factors known to negatively affect sleep hygiene. One-fifth of patients reported using alcohol as a sleeping aid, yet only one-quarter recognised the potential impact of alcohol on sleep. This gap in patient awareness of sleep hygiene mirrors findings from previous studies [14, 23] and further highlights the need for patient education, and the importance of the provision of sleep hygiene education information to this population in the clinical setting.
The strengths of this study include a substantial data collection window, its large sample size, as well as a broad selection criterion, with no exclusion of particular demographic characteristics, current disease activity, or co-morbidities. This generated a study sample more representative of the ‘real-world’. Limitations include the use of a self-reported point prevalence questionnaire without objective measures of sleep disturbance and insomnia such as wearable devices [24,25,26]. Additional limitations include a lack of ethnic diversity [27].
In conclusion, there is a high level of sleep disturbance among breast cancer patients; despite a majority of breast cancer patients recognising the importance of sleep assessment, a significant gap remains in healthcare providers’ addressing these concerns effectively. This study underscores the urgent need for integrated sleep assessment into oncology care [28]. Further steps include exploring healthcare provider attitudes and knowledge of sleep disturbance and sleep assessment in oncology care. Recommendations for sleep assessment and sleep hygiene education should be integrated into oncology patient care plans and survivorship guidelines to ensure consistent and proactive management of this issue.
The significant burden of sleep disturbance and clinical insomnia in oncology cohorts is further reflected in contemporaneous nationally conducted research; The Menopause after cancer (MAC) [29] and Sleepio after cancer (SAC) [30] studies have both examined large cohorts of women cancer patients with clinical insomnia disorder. Whilst identification and recognition of sleep disturbance amongst cancer patients remains a challenge, the provision of treatment to those diagnosed with clinical insomnia disorder also poses a problem, with limited access to cognitive behavioural therapy for insomnia being available. Various studies including both the MAC and SAC studies examined the efficacy of digital cognitive behavioural therapy for insomnia (dCBT-I), which could address current limitations in treatment access [29,30,31]. Incorporating these digital interventions into clinical pathways and survivorship guidelines may help tailor care to specific patient group by addressing individual risk profiles and the multifactorial nature of sleep disturbance. Such personalised approaches enable more targeted care and have the potential to improve treatment outcomes across diverse oncology populations.
Data availability
Data from the study available on reasonable request from corresponding author.
References
Roscoe JA, Kaufman ME, Matheson-Rusby SF, Palesh OG, Ryan JL, Kohli S, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12:35–42. https://doi.org/10.1634/theoncologist.12-S1-35.
Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med Rev. 2021;56:101409. https://doi.org/10.1016/j.smrv.2020.101409.
Mercadante S, Aielli F, Adile C, Ferrera P, Valle A, Carton C, et al. Sleep Disturbances in Patients With Advanced Cancer in Different Palliative Care Settings. J Pain Symptom Manag. 2015;50:786–92. https://doi.org/10.1016/j.jpainsymman.2015.06.018.
Hofmeister D, Schulte T, Mehnert-Theuerkauf A, Gee A, Zenger M, Essen P, et al. The association between sleep problems and general quality of life in cancer patients and in the general population. Front Psychol. 2022;13:960029. https://doi.org/10.3389/fpsyg.2022.960029.
Duzova US, Duzova M, Altinel B. The effect of sleep quality on attitudes toward death in breast cancer survivors. Support Care Cancer. 2024;32:666 https://doi.org/10.1007/s00520-024-08865-w.
Ping-Weng Y, Hong RM, Chen VC, Tsai CJ, Yeh DC, Fang YH. Sleep Quality and Related Factors in Patients with Breast Cancer: A Cross-Sectional Study in Taiwan. Cancer Manag Res. 2021;13:4725–33. https://doi.org/10.2147/CMAR.S302966.
Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–45.
Lanza G, Mogavero MP, Salemi M, Ferri R. The Triad of Sleep, Immunity, and Cancer: A Mediating Perspective. Cells 2024;13. https://doi.org/10.3390/cells13151246
Yaremchuk K. Sleep Disorders in the Elderly. Clin Geriatr Med. 2018;34:205–16. https://doi.org/10.1016/j.cger.2018.01.008.
Perndorfer C, Soriano EC, Siegel SD, Spencer RMC, Otto AK, Laurenceau JP. Fear of Cancer Recurrence and Sleep in Couples Coping With Early-Stage Breast Cancer. Ann Behav Med. 2022;56:1131–43. https://doi.org/10.1093/abm/kaac018.
Thong MSY, van Noorden CJF, Steindorf K, Arndt V. Cancer-Related Fatigue: Causes and Current Treatment Options. Curr Treat Options Oncol. 2020;21:17 https://doi.org/10.1007/s11864-020-0707-5.
Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27:2747–53. https://doi.org/10.1007/s00520-019-04746-9.
Harrold EC, Idris AF, Keegan NM, Corrigan L, Teo MY, O’Donnell M, et al. Prevalence of Insomnia in an Oncology Patient Population: An Irish Tertiary Referral Center Experience. J Natl Compr Canc Netw. 2020;18:1623–30. https://doi.org/10.6004/jnccn.2020.7611.
Fakih R, Rahal M, Hilal L, Hamieh L, Dany M, Karam S, et al. Prevalence and Severity of Sleep Disturbances among Patients with Early Breast Cancer. Indian J Palliat Care. 2018;24:35–38. https://doi.org/10.4103/IJPC.IJPC_137_17.
Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. https://doi.org/10.1200/JCO.2001.19.3.895.
Mark S, Cataldo J, Dhruva A, Paul SM, Chen L-M, Hammer MJ, et al. Modifiable and non-modifiable characteristics associated with sleep disturbance in oncology outpatients during chemotherapy. Support Care Cancer. 2017;25:2485–94. https://doi.org/10.1007/s00520-017-3655-2.
Sanft T, Denlinger CS, Armenian S, Baker KS, Broderick G, Demark-Wahnefried W, et al. NCCN Guidelines Insights: Survivorship, Version 2.2019. J Natl Compr Canc Netw. 2019;17:784–94. https://doi.org/10.6004/jnccn.2019.0034.
Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. https://doi.org/10.1093/sleep/34.5.601.
Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. https://doi.org/10.1016/s1389-9457(00)00065-4.
Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14:429–41. https://doi.org/10.1002/pon.860.
Henneghan AM, Carter P, Stuifbergan A, Parmelee B, Kesler S. Relationships between self-reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology. 2018;27:1937–43. https://doi.org/10.1002/pon.4745.
Pinucci I, Maraone A, Tarsitani L, Pasquini M. Insomnia among Cancer Patients in the Real World: Optimising Treatments and Tailored Therapies. Int J Environ Res Public Health. 2023;20. https://doi.org/10.3390/ijerph20053785.
Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. 2023;77:59–69. https://doi.org/10.1016/j.pcad.2023.02.005.
Kreutz C, Müller J, Schmidt ME, Steindorf K. Comparison of subjectively and objectively assessed sleep problems in breast cancer patients starting neoadjuvant chemotherapy. Support Care Cancer. 2021;29:1015–23. https://doi.org/10.1007/s00520-020-05580-0.
Enderlin CA, Coleman EA, Cole C, Richards KC, Hutchins LF, Sherman AC. Subjective sleep quality, objective sleep characteristics, insomnia symptom severity, and daytime sleepiness in women aged 50 and older with nonmetastatic breast cancer. Oncol Nurs Forum. 2011;38:E314–25. https://doi.org/10.1188/11.ONF.E314-E325.
Grayson S, Sereika S, Harpel C, Diego E, Steiman JG, McAuliffe PF, et al. Factors associated with sleep disturbances in women undergoing treatment for early-stage breast cancer. Support Care Cancer. 2022;30:157–66. https://doi.org/10.1007/s00520-021-06373-9.
Barber LE, McCullough LE, Johnson DA. Eyes Wide Open: Sleep as a Potential Contributor to Racial and Ethnic Disparities in Cancer. Cancer Epidemiol Biomark Prev. 2024;33:471–9. https://doi.org/10.1158/1055-9965.EPI-23-1117.
Davidson JR. Boosting access to insomnia treatment for cancer patients. Sleep. 2014;37:1277–8. https://doi.org/10.5665/sleep.3908.
Donohoe F, O’Meara Y, Roberts A, Comerford L, Valcheva I, Kearns U et al. Multimodal, Technology-Assisted Intervention for the Management of Menopause after Cancer Improves Cancer-Related Quality of Life-Results from the Menopause after Cancer (Mac) Study. Cancers. 2024;16. https://doi.org/10.3390/cancers16061127
Treacy T, O’Meara Y, Galligan MC, Henry AL, Lensen SF, Higgins MJ, et al. The Sleepio After Cancer (SAC) study. Digital cognitive behavioural therapy for insomnia (dCBT-I) in women cancer patients - Trial protocol of a randomised controlled trial. Contemp Clin Trials. 2024;136:107337. https://doi.org/10.1016/j.cct.2023.107337.
Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37:1305–14. https://doi.org/10.5665/sleep.3918.
Acknowledgements
The authors are grateful to the patients who completed the study and to our nursing and administrative colleagues for their assistance with the conduct of the study. This study was conducted as a final year medical project at the College of Medicine and Health, University College Cork, Ireland
Author information
Authors and Affiliations
Contributions
Contributions Study design: EV, SOR Data Collection: EV Data Analysis: EV, CSW, TT, SOR Manuscript writing EV, CSW, TT, SOR Final draft approval: EV, CSW, TT, SOR.
Corresponding author
Ethics declarations
Competing interests
Seamus O'Reilly is Deputy Editor of the journal BJC Reports but did not have a role in the peer review assessment of the manuscript.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Approval was obtained from a Cork Teaching Hospitals Clinical Research Ethics Committee ECM 01/2025/PUB. Informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vallely, E., Weadick, C.S., Treacy, T. et al. Sleep hygiene in patients with early-stage breast cancer: a short report. BJC Rep 3, 84 (2025). https://doi.org/10.1038/s44276-025-00196-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-025-00196-5