Abstract
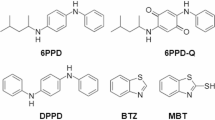
N-(1,3-Dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) is a ubiquitous rubber antioxidant and antiozonant that extends the lifetime of common rubber products, such as those found in tires. It transforms into a quinone derivative following certain environmental conditions. 6PPD and the quinone can leach into the environment and cause severe morbidity to aquatic life at diminutive concentrations, with health effects on humans still not fully understood. With legislation on the horizon to ban 6PPD entirely, developing effective methods for its removal and conversion to safe compounds is essential. Here we show that 6PPD survives microwave-assisted pyrolysis and escapes in the oil product, rendering decontamination essential. We introduce a decontamination strategy that removes 6PPD from end-of-life tires before it enters the broader ecosystem. We demonstrate the catalytic upgrade of 6PPD to safe chemicals and the valorization of crumb rubber to aromatics and carbon black using microwave-assisted pyrolysis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or Supplementary Information. Source data are provided with this paper.
References
Wagner, S. et al. Tire wear particles in the aquatic environment—a review on generation, analysis, occurrence, fate and effects. Water Res. 139, 83–100 (2018).
Tian, Z. et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 371, 185–189 (2021).
Brinkmann, M. et al. Acute toxicity of the tire rubber-derived chemical 6PPD-quinone to four fishes of commercial, cultural, and ecological importance. Environ. Sci. Technol. Lett. 9, 333–338 (2022).
Hiki, K. et al. Acute toxicity of a tire rubber-derived chemical, 6PPD quinone, to freshwater fish and crustacean species. Environ. Sci. Technol. Lett. 8, 779–784 (2021).
Du, B. et al. First report on the occurrence of N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) and 6PPD-quinone as pervasive pollutants in human urine from south China. Environ. Sci. Technol. Lett. 9, 1056–1062 (2022).
Chen, X. et al. Analysis, environmental occurrence, fate and potential toxicity of tire wear compounds 6PPD and 6PPD-quinone. J. Hazard. Mater. 452, 131245 (2023).
Grynkiewicz-Bylina, B., Rakwic, B. & Słomka-Słupik, B. Tests of rubber granules used as artificial turf for football fields in terms of toxicity to human health and the environment. Sci. Rep. 12, 6683 (2022).
2021 US Scrap Tire Management Summary (US Tire Manufacturers Association, 2021).
Thomas, B. S. & Gupta, R. C. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew. Sustain. Energy Rev. 54, 1323–1333 (2016).
Dabic-Miletic, S., Simic, V. & Karagoz, S. End-of-life tire management: a critical review. Environ. Sci. Pollution Res. 28, 68053–68070 (2021).
Baker-Fales, M., Chen, T.-Y. & Vlachos, D. G. Scale-up of microwave-assisted, continuous flow, liquid phase reactors: application to 5-hydroxymethylfurfural production. Chem. Eng. J. 454, 139985 (2023).
Osorio-Vargas, P. et al. Catalytic pyrolysis of used tires on noble-metal-based catalysts to obtain high-value chemicals: reaction pathways. Catal. Today 394–396, 475–485 (2022).
Undri, A., Meini, S., Rosi, L., Frediani, M. & Frediani, P. Microwave pyrolysis of polymeric materials: waste tires treatment and characterization of the value-added products. J. Anal. Appl. Pyrolysis 103, 149–158 (2013).
Song, Z. et al. Microwave pyrolysis of tire powders: evolution of yields and composition of products. J. Anal. Appl. Pyrolysis 123, 152–159 (2017).
Bing, W. et al. Microwave fast pyrolysis of waste tires: effect of microwave power on product composition and quality. J. Anal. Appl. Pyrolysis 155, 104979 (2021).
Luo, Y., Selvam, E., Vlachos, D. G. & Ierapetritou, M. Economic and environmental benefits of modular microwave-assisted polyethylene terephthalate depolymerization. ACS Sustain. Chem. Eng. 11, 4209–4218 (2023).
Rajasekhar Reddy, B. et al. Microwave assisted heating of plastic waste: effect of plastic/susceptor (SiC) contacting patterns. Chem. Eng. Process. - Proc. Inten. 182, 109202 (2022).
Kim, T., Lee, J. & Lee, K. H. Full graphitization of amorphous carbon by microwave heating. RSC Adv. 6, 24667–24674 (2016).
Xu, J. et al. High-value utilization of waste tires: a review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 742, 140235 (2020).
Boucher, O. & Reddy, M. S. Climate trade-off between black carbon and carbon dioxide emissions. Energy Policy 36, 193–200 (2008).
Osorio-Vargas, P. et al. Valorization of waste tires via catalytic fast pyrolysis using palladium supported on natural halloysite. Ind. Eng. Chem. Res. 60, 18806–18816 (2021).
COSMO-RS (SCM, 2023).
Walker, T. W. et al. Recycling of multilayer plastic packaging materials by solvent-targeted recovery and precipitation. Sci. Adv. 6, eaba7599 (2020).
Wojeicchowski, J. P., Ferreira, A. M., Abranches, D. O., Mafra, M. R. & Coutinho, J. A. P. Using COSMO-RS in the design of deep eutectic solvents for the extraction of antioxidants from Rosemary. ACS Sustain. Chem. Eng. 8, 12132–12141 (2020).
Remler, R. F. The solvent properties of acetone. Ind. Eng. Chem. 15, 717–720 (1923).
Giakoumakis, N. S. et al. Total revalorization of high impact polystyrene (HIPS): enhancing styrene recovery and upcycling of the rubber phase. Green Chem. 26, 340–352 (2024).
Cheng, H., Hu, Y. & Reinhard, M. Environmental and health impacts of artificial turf: a review. Environ. Sci. Technol. 48, 2114–2129 (2014).
Challis, J. K. et al. Occurrences of tire rubber-derived contaminants in cold-climate urban runoff. Environ. Sci. Technol. Lett. 8, 961–967 (2021).
Baker-Fales, M., Gutiérrez-Cano, J. D., Catalá-Civera, J. M. & Vlachos, D. G. Temperature-dependent complex dielectric permittivity: a simple measurement strategy for liquid-phase samples. Sci. Rep. 13, 18171 (2023).
How Much Carbon Dioxide is Produced per Kilowatthour of U.S. Electricity Generation? (US Energy Information Administration, 2022).
Chen, L. et al. Solar-light-activated periodate for degradation and detoxification of highly toxic 6PPD-quinone at environmental levels. Nat. Water 2, 453–463 (2024).
Liu, J. et al. Reductive defluorination of branched per- and polyfluoroalkyl substances with cobalt complex catalysts. Environ. Sci. Technol. Lett. 5, 289–294 (2018).
Toppinen, S., Rantakyla, T.-K., Salmi, T. & Aittamaa, J. Kinetics of the liquid-phase hydrogenation of benzene and some monosubstituted alkylbenzenes over a nickel catalyst. Ind. Eng. Chem. Res. 35, 1824–1833 (1996).
Sinfelt, J. H. Catalytic hydrogenolysis on metals. Catal. Letters 9, 159–172 (1991).
Mitra, J., Zhou, X. & Rauchfuss, T. Pd/C-catalyzed reactions of HMF: decarbonylation, hydrogenation, and hydrogenolysis. Green Chem. 17, 307–313 (2015).
Pelckmans, M., Renders, T., Van De Vyver, S. & Sels, B. F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 19, 5303–5331 (2017).
Benbrook, D. M. et al. Biologically active heteroarotinoids exhibiting anticancer activity and decreased toxicity. J. Med. Chem. 40, 3567–3583 (1997).
Gutierrez-Cano, J. D. et al. A new stand-alone microwave instrument for measuring the complex permittivity of materials at microwave frequencies. IEEE Trans. Instrum. Meas. 69, 3595–3605 (2020).
Pye, C. C., Ziegler, T., Lenthe, E. V. & Louwen, J. N. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package—Part II. COSMO for real solvents. Can. J. Chem. 87, 790–797 (2009).
Omnic 8.2 (Thermo Fisher Scientific, 2010).
TRIOS 5.1.1 (TA Instruments, 2024).
Omega TRH Central 1.3 (OMEGA USB Products, 2013).
ASPEN Suite (AspenTech, 2024).
Acknowledgements
This work was supported by the National Science Foundation (grant no. OIA – 2119754). B.C.V. acknowledges a Graduate Research Fellowship through the National Science Foundation (grant no. 1940700). Crumb rubber was generously supplied by Liberty Tire Recycling. This research used beamline 7-BM (QAS) of the National Synchrotron Light Source II, a US DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory (contract no. DE-SC0012704). Beamline operations were supported in part by the Synchrotron Catalysis Consortium (US DOE, Office of Basic Energy Sciences; grant no. DE-SC0012335). We thank L. Ma, N. Marinkovic and S. N. Ehrlich at the National Synchrotron Light Source II for their assistance with the XAS measurements. The TGA–MS used here is part of the Center for Plastics Innovation, an Energy Frontier Research Center funded by the US DOE, Office of Science, Basic Energy Sciences (grant no. DE-SC0021166).
Author information
Authors and Affiliations
Contributions
S.N. conceptualized the work and performed a formal analysis. S.N., P.B., M.B.-F., B.C.V., E.S., K.Y. and W.Z. performed the investigations. S.N., P.B., M.B.-F., B.C.V., E.S., K.Y. and W.Z. created the methodology. S.N., P.B., B.C.V., E.S., K.Y., W.Z. and D.G.V. wrote the original draft, whereas S.N. reviewed and edited it. D.G.V. administered the project and acquired funding.
Corresponding author
Ethics declarations
Competing interests
S.N. and D.G.V. are inventors on a patent application related to this work filed by the University of Delaware. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Ning Yan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26 and Tables 1–6.
Source data
Source Data Fig. 1
Temperature data, pyrolysis oil yields.
Source Data Fig. 2
Extraction data and COSMO solubility.
Source Data Fig. 3
Temperature data, extraction yields.
Source Data Fig. 4
XAS data, conversions and product yields.
Source Data Fig. 5
Costs and profitability index values.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Najmi, S., Bhalode, P., Baker-Fales, M. et al. End-of-life tire decontamination from 6PPD and upcycling. Nat Chem Eng 1, 597–607 (2024). https://doi.org/10.1038/s44286-024-00110-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-024-00110-9
This article is cited by
-
Fractionated degradation and valorization of polypropylene waste into sulfonate surfactants
Nature Communications (2025)
-
Microwave-heated solvent extraction and catalysis for end-of-life tire decontamination
Nature Chemical Engineering (2024)