Abstract
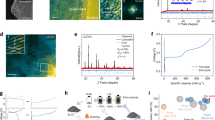
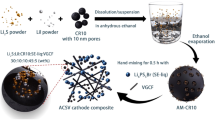
Existing lithium-ion battery recycling methods often involve energy-, chemical- and/or waste-intensive processes. Here we demonstrated a self-looped electrochemical battery recycling approach that enables efficient recycling of lithium and transition metals from spent cathode materials. These recycled materials can be directly applied to manufacture new batteries without further treatment. By operating electrochemical hydrogen evolution and oxidation reactions in a three-chamber porous solid electrolyte reactor, input Li2SO4 solution can be converted into lithium hydroxide and sulfuric acid with a Li+ transport efficiency of around 90%, at current densities of 100 mA cm−2 and low energy consumption (starting from 0.36 V). This is followed by a stoichiometric acid leaching and alkaline precipitation process that separates spent lithium metal oxides into high-purity (>99.7%) lithium and transition metal hydroxide products. The Li2SO4 solution can be successfully restored at the end of each recycling cycle, enabling a sustainable process that requires only H2O2 as an external input. This approach avoids external cation contamination and eliminates the need for waste stream treatments.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated during this study are included in the Article and its Supplementary Information. Source data are provided with this paper.
References
Crabtree, G. The coming electric vehicle transformation. Science 366, 422–424 (2019).
Harper, G. et al. Recycling lithium-ion batteries from electric vehicles. Nature 575, 75–86 (2019).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Wang, X. et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte. Nano Energy 60, 205–212 (2019).
Neumann, J. et al. Recycling of lithium-ion batteries—current state of the art, circular economy, and next generation recycling. Adv. Energy Mater. 12, 2102917 (2022).
Chandran, V. et al. Comprehensive review on recycling of spent lithium-ion batteries. Mater. Today Proc. 47, 167–180 (2021).
Bibra, E. M. et al. Global EV Outlook 2022: Securing Supplies for an Electric Future (International Energy Agency, 2022).
Ma, X., Azhari, L. & Wang, Y. Li-ion battery recycling challenges. Chem 7, 2843–2847 (2021).
Baum, Z. J., Bird, R. E., Yu, X. & Ma, J. Lithium-ion battery recycling─overview of techniques and trends. ACS Energy Lett. 7, 712–719 (2022).
Zeng, A. et al. Battery technology and recycling alone will not save the electric mobility transition from future cobalt shortages. Nat. Commun. 13, 1341 (2022).
Lou, S. et al. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 11, 5700 (2020).
Stinn, C. & Allanore, A. Selective sulfidation of metal compounds. Nature 602, 78–83 (2022).
Tran, M. K., Rodrigues, M.-T. F., Kato, K., Babu, G. & Ajayan, P. M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 4, 339–345 (2019).
Kim, D. K. et al. Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett. 8, 3948–3952 (2008).
Xu, P., Tan, D. H. S., Gao, H., Rose, S. & Chen, Z. in Encyclopedia of Energy Storage (ed. Cabeza, L. F.) 98–107 (Elsevier, 2022).
Xu, P. et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 4, 2609–2626 (2020).
Lin, J. et al. Sustainable upcycling of spent lithium-ion batteries cathode materials: stabilization by in situ Li/Mn disorder. Adv. Energy Mater. 12, 2201174 (2022).
Gupta, V. et al. Scalable direct recycling of cathode black mass from spent lithium-ion batteries. Adv. Energy Mater. 13, 2203093 (2023).
Ma, X. et al. Recycled cathode materials enabled superior performance for lithium-ion batteries. Joule 5, 2955–2970 (2021).
Wang, H. et al. LiMn1−xFexPO4 nanorods grown on graphene sheets for ultrahigh-rate-performance lithium ion batteries. Angew. Chem. Int. Ed. 50, 7364–7368 (2011).
Piątek, J. et al. Sustainable Li-ion batteries: chemistry and recycling. Adv. Energy Mater. 11, 2003456 (2021).
Tan, J. et al. Recycling-oriented cathode materials design for lithium-ion batteries: elegant structures versus complicated compositions. Energy Stor. Mater. 41, 380–394 (2021).
Wang, J. et al. Direct conversion of degraded LiCoO2 cathode materials into high-performance LiCoO2: a closed-loop green recycling strategy for spent lithium-ion batteries. Energy Stor. Mater. 45, 768–776 (2022).
Li, L. et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 5, 5224–5233 (2017).
Montoya, A. T. et al. Direct recycling of lithium-ion battery cathodes: a multi-stage annealing process to recover the pristine structure and performance. ACS Sustain. Chem. Eng. 10, 13319–13324 (2022).
Zhu, X.-H. et al. Recycling valuable metals from spent lithium-ion batteries using carbothermal shock method. Angew. Chem. Int. Ed. 62, e202300074 (2023).
Arshad, F. et al. A comprehensive review of the advancement in recycling the anode and electrolyte from spent lithium ion batteries. ACS Sustain. Chem. Eng. 8, 13527–13554 (2020).
Zheng, R. et al. A closed-loop process for recycling Li/NixCoyMn(1−x−y)O2 from mixed cathode materials of lithium-ion batteries. Green Energy Environ. 2, 42–50 (2017).
Lai, X. et al. Turning waste into wealth: a systematic review on echelon utilization and material recycling of retired lithium-ion batteries. Energy Stor. Mater. 40, 96–123 (2021).
Gao, W. et al. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: a closed-loop process. Environ. Sci. Technol. 51, 1662–1669 (2017).
Meng, F., McNeice, J., Zadeh, S. S. & Ghahreman, A. Review of lithium production and recovery from minerals, brines, and lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 42, 123–141 (2021).
Xiao, J., Shi, F., Glossmann, T., Burnett, C. & Liu, Z. From laboratory innovations to materials manufacturing for lithium-based batteries. Nat. Energy 8, 329–339 (2023).
Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Zhang, X. et al. Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 13, 2880 (2022).
Zhang, P., Yokoyama, T., Itabashi, O., Suzuki, T. M. & Inoue, K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47, 259–271 (1998).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Fan, E. et al. Sustainable recycling technology for li-ion batteries and beyond: challenges and future prospects. Chem. Rev. 120, 7020–7063 (2020).
Liu, Z., Yu, A. & Lee, J. Y. Synthesis and characterization of LiNi1−x−yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 81–82, 416–419 (1999).
Qian, G. et al. Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture. Energy Stor. Mater. 27, 140–149 (2020).
Sa, Q. et al. Synthesis of diverse linixmnycozo2 cathode materials from lithium ion battery recovery stream. J. Sustain. Metall. 2, 248–256 (2016).
Daily Lithium Battery Cathode Precursor and Material Prices. SMM https://www.metal.com/Lithium%20Battery%20Cathode%20Precursor%20and%20Material (accessed March 2024).
Sim, S.-J., Lee, S.-H., Jin, B.-S. & Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. 10, 11114 (2020).
Yao, Y. et al. Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review. ACS Sustain. Chem. Eng. 6, 13611–13627 (2018).
Wang, J. et al. Sustainable upcycling of spent LiCoO2 to an ultra-stable battery cathode at high voltage. Nat. Sustain. 6, 797–805 (2023).
Xu, J. et al. A green and sustainable strategy toward lithium resources recycling from spent batteries. Sci. Adv. 8, eabq7948 (2022).
EverBatt: Argonne’s closed-loop battery life-cyle model. Argonne National Laboratory https://www.anl.gov/amd/everbatt (2018).
H2a: hydrogen analysis production models. National Renewable Energy Laboratory https://www2.nrel.gov/hydrogen/h2a-production-models (2018).
US Energy Information Administration. How much carbon dioxide is produced per kilowatthour of U.S. electricity generation? Independent Statistics and Analysis https://www.eia.gov/tools/faqs/faq.php?id=74&t=11 (2024).
Lander, L. et al. Financial viability of electric vehicle lithium-ion battery recycling. iScience 24, 102787 (2021).
Lyu, Y. et al. Selective extraction of critical metals from spent Li-ion battery cathode: cation–anion coordination and anti-solvent crystallization. Adv. Mater. 36, 2312551 (2024).
Strauss, M. L., Diaz, L. A., McNally, J., Klaehn, J. & Lister, T. E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex m4195 ion exchange resin. Hydrometallurgy 206, 105757 (2021).
Acknowledgements
This work was supported by the David and Lucile Packard Foundation (grant no. 2020-71371) and the Alfred P. Sloan Foundation (grant no. FG-2021-15638).
Author information
Authors and Affiliations
Contributions
Z.F., X.Z. and H.W. conceived the project and designed the experiments. X.Z., Z.F. and Y. F. perform the experimental study. P.Z. performed the TEA study. Z.F. and H.W. wrote the manuscript with support from all authors.
Corresponding author
Ethics declarations
Competing interests
Z.F. and H.W. are listed as inventors on a patent application filed by Rice University that pertains to this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Zhi Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Information: Methods, Figs. 1–38, Tables 1–7 and references.
Supplementary Data 1
Source data for supplementary figures.
Source data
Source Data Fig. 2
Statistical source data for Fig. 2 (including Li concentration data from chromatography, electrolyte pH and electrochemistry data).
Source Data Fig. 3
Statistical source data for Fig. 3 (XRD data, electrolyte pH and electrochemistry data).
Source Data Fig. 4
Statistical source data for Fig. 4 (XRD data, electrochemistry data and preliminary TEA data).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, Z., Zhu, P., Zhang, X. et al. Self-looped electrochemical recycling of lithium-ion battery cathode materials to manufacturing feedstocks. Nat Chem Eng 2, 142–151 (2025). https://doi.org/10.1038/s44286-025-00186-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00186-x
This article is cited by
-
Recovery of Li, Ni, Co, and Mn from Cathode Mass of Spent Lithium-Ion Batteries by Deep Eutectic Solvent-Based Pressurized Leaching
Journal of Sustainable Metallurgy (2025)
-
A review for high-value utilization of retired spent electrolyte for lithium-ion battery: how to reduce the waste of resources?
Ionics (2025)
-
Electrochemical Solid-State Electrolyte Reactors: Configurations, Applications, and Future Prospects
Nano-Micro Letters (2025)