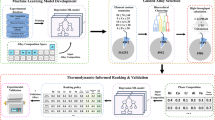
Abstract
Low-carbon ammonia decomposition via nonthermal plasma is a promising method for on-site hydrogen production, but finding optimal catalysts is challenging. Here we use multiscale simulations to link catalytic activity to nitrogen adsorption energy (EN) and identify the best catalysts for conventional heating and nonthermal plasma: Ru and Co, respectively. With an ideal EN of −0.51 eV for plasma catalysis, we applied machine learning to screen 3,300+ catalysts and designed efficient, earth-abundant alloys such as Fe3Cu, Ni3Mo, Ni7Cu and Fe15Ni. Plasma catalytic experiments at 400 °C further validated that the above alloys achieved higher conversions than the individual metals, and they also have comparable performance to Co. Our techno-economic analysis demonstrated potential economic benefits of plasma catalytic ammonia decomposition over Ni3Mo, highlighting a H2 production cost below the US$1 per kg H2 target and a low carbon footprint of ~0.91 kg of CO2 per kg H2.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. Data used for developing MKM, machine learning models, experimental work and TEA are available in Supplementary Data 1.
Code availability
The microkinetic models and the machine learning algorithms are included as Python modules, and data files used to create all the plots are provided in Supplementary Data 1.
References
Global CO2 Emissions from Transport by Sub-sector in the Net Zero Scenario, 2000–2030 (International Energy Agency, 2022); https://www.iea.org/data-and-statistics
Anika, O. C. et al. Prospects of low and zero-carbon renewable fuels in 1.5-degree net zero emission actualisation by 2050: a critical review. Carbon Capture Sci. Technol. 5, 100072 (2022).
Griffiths, S. & Uratani, J. Zero and low-carbon ammonia shipping fuel. Glob. Energy 28, 46–58 (2022).
Bagheri, S., Bagheri, H. & Rahimpour, M. R. in Progresses in Ammonia: Science, Technology and Membranes (eds Basile, A. & Rahimpour, M.R.) 281–305 (Elsevier, 2024).
Cheddie, D. in Hydrogen Energy-Challenges and Perspectives (ed. Minić, D.) Ch. 13 (IntechOpen, 2012).
Lin, L. et al. Techno-economic analysis and comprehensive optimization of an on-site hydrogen refuelling station system using ammonia: hybrid hydrogen purification with both high H2 purity and high recovery. Sustain. Energy Fuels 4, 3006–3017 (2020).
Panić, I., Cuculić, A. & Ćelić, J. Color-coded hydrogen: production and storage in maritime sector. J. Mar. Sci. Eng. 10, 1995 (2022).
Hansgen, D. A., Vlachos, D. G. & Chen, J. G. Using first principles to predict bimetallic catalysts for the ammonia decomposition reaction. Nat. Chem. 2, 484–489 (2010).
Yin, S.-F., Xu, B.-Q., Ng, C.-F. & Au, C.-T. Nano Ru/CNTs: a highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Appl. Catal. B 48, 237–241 (2004).
Su, T. et al. Review on Ru-based and Ni-based catalysts for ammonia decomposition: research status, reaction mechanism, and perspectives. Energy Fuels 37, 8099–8127 (2023).
Verschoor, J. C., de Jongh, P. E. & Ngene, P. Recent advances in thermocatalytic ammonia synthesis & decomposition. Curr. Opin. Green Sustain. Chem. 50, 100965 (2024).
Bayer, B. N., Bhan, A. & Bruggeman, P. J. Reaction pathways and energy consumption in NH3 decomposition for H2 production by low temperature, atmospheric pressure plasma. Plasma Chem. Plasma Process. 44, 1–18 (2024).
Salehabadi, A., Zanganeh, J. & Moghtaderi, B. Mixed metal oxides in catalytic ammonia cracking process for green hydrogen production: a review. Int. J. Hydrogen Energy 63, 828–843 (2024).
Kidnay, A. J., Parrish, W. R. & McCartney, D. G. in Fundamentals of Natural Gas Processing (eds Kidnay, A. J. et al.) Ch. 6 (CRC Press, 2019).
Andersen, J. et al. Ammonia decomposition in a dielectric barrier discharge plasma: insights from experiments and kinetic modeling. Chem. Eng. Sci. 271, 118550 (2023).
Neyts, E. C., Ostrikov, K., Sunkara, M. K. & Bogaerts, A. Plasma catalysis: synergistic effects at the nanoscale. Chem. Rev. 115, 13408–13446 (2015).
Lefferts, L. Leveraging expertise in thermal catalysis to understand plasma catalysis. Angew. Chem. Int. Ed. 136, e202305322 (2024).
Gao, H. et al. Plasma-activated mist: continuous-flow, scalable nitrogen fixation, and aeroponics. ACS Sustain. Chem. Eng. 11, 4420–4429 (2023).
Zhu, N., Hong, Y., Qian, F. & Liang, J. Research progress on plasma-catalytic hydrogen production from ammonia: Influencing factors and reaction mechanism. Int. J. Hydrogen Energy 59, 791–807 (2024).
Mehta, P. et al. Plasma-catalytic ammonia synthesis beyond the equilibrium limit. ACS Catal. 10, 6726–6734 (2020).
Wang, L., Zhao, Y., Liu, C., Gong, W. & Guo, H. Plasma driven ammonia decomposition on a Fe-catalyst: eliminating surface nitrogen poisoning. Chem. Commun. 49, 3787–3789 (2013).
El-Shafie, M., Kambara, S. & Hayakawa, Y. Plasma-enhanced catalytic ammonia decomposition over ruthenium (Ru/Al2O3) and soda glass (SiO2) materials. J. Energy Inst. 99, 145–153 (2021).
Meng, S. et al. NH3 decomposition for H2 production by thermal and plasma catalysis using bimetallic catalysts. Chem. Eng. Sci. 283, 119449 (2024).
Katebah, M. & Linke, P. Analysis of hydrogen production costs in Steam-Methane Reforming considering integration with electrolysis and CO2 capture. Cleaner Eng. Technol. 10, 100552 (2022).
Andersen, J., Christensen, J., Østberg, M., Bogaerts, A. & Jensen, A. Plasma-catalytic ammonia decomposition using a packed-bed dielectric barrier discharge reactor. Int. J. Hydrogen Energy 47, 32081–32091 (2022).
Mehta, P. et al. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 1, 269–275 (2018).
Laidler, K. J. & King, M. C. The development of transition-state theory. J. Phys. Chem. 87, 2657–2664 (1983).
Truhlar, D. G., Garrett, B. C. & Klippenstein, S. J. Current status of transition-state theory. J. Phys. Chem. 100, 12771–12800 (1996).
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys. Rev. Lett. 99, 016105 (2007).
Guo, W. & Vlachos, D. G. Patched bimetallic surfaces are active catalysts for ammonia decomposition. Nat. Commun. 6, 8619 (2015).
Wolcott, C. A., Medford, A. J., Studt, F. & Campbell, C. T. Degree of rate control approach to computational catalyst screening. J. Catal. 330, 197–207 (2015).
Yang, Y., Achar, S. K. & Kitchin, J. R. Evaluation of the degree of rate control via automatic differentiation. AIChE J. 68, e17653 (2022).
Winter, L. R. & Chen, J. G. N2 fixation by plasma-activated processes. Joule 5, 300–315 (2021).
Fridman, A. & Kennedy, L. A. in Plasma Physics and Engineering (eds Fridman, A. & Kennedy, L. A.) Ch. 3 (CRC Press, 2004).
Bayer, B. N., Bruggeman, P. J. & Bhan, A. Species, pathways, and timescales for NH3 formation by low-temperature atmospheric pressure plasma catalysis. ACS Catal 13, 2619–2630 (2023).
Ma, H. et al. Observation and rationalization of nitrogen oxidation enabled only by coupled plasma and catalyst. Nat. Commun. 13, 402 (2022).
Hammer, B. & Nørskov, J. K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 343, 211–220 (1995).
Artrith, N., Lin, Z. & Chen, J. G. Predicting the activity and selectivity of bimetallic metal catalysts for ethanol reforming using machine learning. ACS Catal 10, 9438–9444 (2020).
Lundberg, S. M. & Lee, S.-I. A unified approach to interpreting model predictions. In Proc. Advances in Neural Information Processing Systems Vol. 30 4765–4774 (Curran Associates, 2017).
Bonizzoni, G. & Vassallo, E. Plasma physics and technology; industrial applications. Vacuum 64, 327–336 (2002).
Mayyas, A. T., Ruth, M. F., Pivovar, B. S., Bender, G. & Wipke, K. B. Manufacturing Cost Analysis for Proton Exchange Membrane Water Electrolyzers (National Renewable Energy Laboratory, 2019).
Renewable Power Generation Costs in 2022 (International Renewable Energy Agency, 2022); https://www.irena.org/Publications/2023/Aug/Renewable-Power-Generation-Costs-in-2022
McNaul, S. et al. Strategies for Achieving the DOE Hydrogen Shot Goal: Thermal Conversion Approaches (National Energy Technology Laboratory, 2023); https://www.netl.doe.gov
Bogaerts, A. & Neyts, E. C. Plasma technology: an emerging technology for energy storage. ACS Energy Lett. 3, 1013–1027 (2018).
Araújo, K., Koerner, C., Carcas, F., Hampshire, J. & Peterson, K. An Assessment of Policies and Regional Diversification with Energy, Critical Minerals and Economic Development in Emerging Markets (US Department of Energy, 2023).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 6, 8245 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Wan, M., Yue, H., Notarangelo, J., Liu, H. & Che, F. Deep learning-assisted investigation of electric field–dipole effects on catalytic ammonia synthesis. JACS Au 2, 1338–1349 (2022).
Wittreich, G. R., Alexopoulos, K. & Vlachos, D. G. in Handbook of Materials Modeling: Applications: Current and Emerging Materials (eds Andreoni, W. & Yip, S.) 1377–1404 (Springer, 2020).
Prats, H., Illas, F. & Sayos, R. General concepts, assumptions, drawbacks, and misuses in kinetic Monte Carlo and microkinetic modeling simulations applied to computational heterogeneous catalysis. Int. J. Quantum Chem. 118, e25518 (2018).
Biondo, O. et al. Insights into the limitations to vibrational excitation of CO2: validation of a kinetic model with pulsed glow discharge experiments. Plasma Sources Sci. Technol. 31, 074003 (2022).
Bron, J. & Wolfsberg, M. Effect of vibrational anharmonicity on hydrogen-deuterium exchange equilibria involving ammonia molecules. J. Chem. Phys 57, 2862–2869 (1972).
Fridman, A. in Plasma Chemistry (ed. Fridman, A.) Ch. 3 (Cambridge Univ. Press, 2008).
Hamilton, R. I. & Papadopoulos, P. N. Using SHAP values and machine learning to understand trends in the transient stability limit. IEEE Trans. Power Syst. 39, 1384–1397 (2023).
Mastropietro, A., Feldmann, C. & Bajorath, J. Calculation of exact Shapley values for explaining support vector machine models using the radial basis function kernel. Sci. Rep. 13, 19561 (2023).
Winther, K. T. et al. Catalysis-Hub.org, an open electronic structure database for surface reactions. Sci. Data 6, 75 (2019).
Acknowledgements
F.C., S.A.I. and C.M. acknowledge the support provided by the Department of Energy, Basic Energy Science, Catalysis Science, Early Career Research Program under award number DE-SC0024553. F.C., Q.L., Y.L. and J.Y. acknowledge the support by the Department of Energy, Fusion Energy Science, under award number DE-SC0025553 for developing the mechanism of plasma-induced radical–surface interactions and DFT-free machine learning workflow. F.C., S.A.I. and C.M. extend their thanks for the computational resources provided by Unity at Massachusetts Green High Performance Computing Center (MGHPCC), as well as the San Diego Supercomputer Center at University of California San Diego, and the Texas A&M High Performance Research Computing FASTER cluster, through allocation numbers CHM220074 and CHE200076 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program. This research used the resources of the National Energy Research Scientific Computing Center (NERSC), a US Department of Energy Office of Science User Facility supported by the Office of Science of the US Department of Energy under contract number DE-AC02-05CH11231 using NERSC awards BES-ERCAP0027465 and BES-ERCAP0028368. Y.Y. acknowledges financially support by the National Natural Science Foundation of China (grant numbers 22472018, 22272015 and 21503032), Y.Y., S.M. and Y.S. acknowledge support from Frontier Science Center for Smart Materials and assistance from Instrumental Analysis Center (X. Gao), Dalian University of Technology for characterization of the catalysts. We acknowledge H. Liu for helpful discussions.
Author information
Authors and Affiliations
Contributions
S.A.I., Y.L. and Q.L. performed the multiscale simulations and developed the MKM. S.M. and Y.S. conducted catalyst synthesis, characterization and ammonia decomposition experiments. C.M. and J.Y. developed machine learning models. M.H.B. carried out the TEA and helped to analyze the data. Y.Y. directed the experimental validation project and assisted with analysis of both the experimental and theoretical data. F.C. developed the idea of the project, supervised the study, contributed to theory and machine learning model development, helped to analyze both the theoretical and experimental data, and managed the project. All authors contributed to writing and editing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Machine learning model and screening workflow for bimetallic surface design.
(a) Schematic of the Random Forest (RF) model to predict EN, utilizing d-band information and solid-state properties of bimetallic surfaces. Key features include d-band descriptors (filling, center, width, skewness, and kurtosis up to the Fermi level), local Pauling electronegativity (reflecting delocalized sp-states), and metal-specific physical constants (spatial extent of d-orbitals, squared adsorbate-metal d coupling matrix element, work function, atomic radius, ionization potential, electron affinity, and Pauling electronegativity). (b) Screening workflow used to evaluate over 3,300 bimetallic alloy compositions based on ML-predicted d-band fillings and nitrogen adsorption energy.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43, Tables 1–38 and Notes 1–10.
Supplementary Data 1
Atomic coordinates of all the DFT models.
Supplementary Code 2
Python scripts and input files for machine learning-based screening and MKM.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmat Ibrahim, S., Meng, S., Milhans, C. et al. Interpretable machine learning-guided plasma catalysis for hydrogen production. Nat Chem Eng 2, 699–710 (2025). https://doi.org/10.1038/s44286-025-00287-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00287-7
This article is cited by
-
Plasma catalysis research for sustainability
Frontiers of Chemical Science and Engineering (2025)