Abstract
Global outbreaks of human immunodeficiency virus (HIV) and respiratory viruses - severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza, accounted for ~50 million infections in 2024. Prenatal exposure to these viruses poses substantial risks to maternal and fetal health, yet the underlying immunological mechanisms remain incompletely understood. Despite differences in viral biology and transmission, mounting evidence reveals a convergent theme of maternal immune activation during pregnancy. Even without vertical transmission, virus-elicted maternal immune responses alter the maternal-fetal interface and gut microbiome, reshaping fetal immunity and birth outcomes. These immune perturbations increase susceptibility to infections, neurodevelopmental disorders, and immune-mediated diseases later in life. Here, we discuss viral immune evasion strategies that modulate maternal immunity and review current clinical and emerging therapeutic approaches aimed at mitigating long-term consequences in exposed children. Understanding how prenatal viral exposure shapes lifelong health is critical for developing targeted interventions and reducing postnatal disease burden.
Similar content being viewed by others
Introduction
Viral infections during pregnancy present a unique clinical challenge to maternal and child health, often leading to adverse outcomes across both immediate and long-term development windows. Historically, clinical concerns over prenatal viral infections have centered on vertical transmission and congenital infection1,2. However, growing evidence now indicates that even in the absence of direct fetal infection, maternal immune responses can negatively impact fetal immunity and development through epigenetic, non-genomic, and inflammatory pathways3,4,5. These indirect, immune-mediated mechanisms remain underexplored, despite their potential to alter lifelong health trajectories.
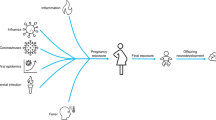
Globally, prenatal exposure to viral infections represent a substantial yet underrecognized contributor to adverse pregnancy outcomes and pediatric vulnerability. Among these, three pandemic-potential viruses - HIV, SARS-CoV-2, and influenza virus are held accountable for millions of infections worldwide, especially in pregnant individuals. HIV infections affect over 38 million people worldwide, including approximately 1.3 million pregnant women annually6,7. Over the past decade alone, over 15 million children have been born to mothers living with HIV, many of whom are HIV-exposed but uninfected (HEU), and remain at elevated risk for impaired neurodevelopmental, immunologic, and metabolic outcomes8,9. Meanwhile, the recent COVID-19 pandemic caused by SARS-CoV-2, introduced novel challenges to maternal-fetal medicine, affecting at least 116 million pregnancies worldwide and raising concerns about placental inflammation, maternal immune activation (MIA), and the potential for long-term sequelae in exposed offspring10,11,12,13,14. In parallel, seasonal and pandemic influenza continues to cause substantial maternal morbidity and mortality each year, with infection during pregnancy linked to fetal growth restriction, prematurity, and neurodevelopmental impairment15,16. Altogether, these viruses remain globally prevalent and continue to affect large numbers of pregnancies through chronic infection or recurrent and pandemic-scale outbreaks, making them central to contemporary maternal-fetal care.
Despite their differences in structure, tissue tropism, and mechanisms of transmission, these viruses also share a common capacity to disrupt the maternal-fetal interface. HIV can cross the placenta through cell-associated and receptor-mediated routes17,18,19, whereas SARS-CoV-2 and influenza virus typically do not breach the placental barrier20,21,22. Despite divergent transmission patterns, all three viruses induce systemic maternal inflammation, disturb placental architecture, and reformat fetal immunity through indirect immunopathogenic mechanisms. Notably, MIA, often in the absence of infection, emerges as a central determinant of long-term immune and developmental outcomes5,11,14,22,23,24. This shared feature allows a comparative framework to examine convergent mechanisms driven by maternal and placental inflammation and altered intrauterine signaling.
In this review, we synthesize current knowledge on how prenatal exposure to HIV, SARS-CoV-2, and influenza modulates maternal immunity, alters placental function, and imprints the developing fetal immune system25,26,27,28. Indeed, these three viruses are paradigmatic examples of globally prevalent viral exposures, where we leveraged on their distinct virological and clinical characteristics (i.e., chronic versus acute infection, persistent versus episodic MIA, and differing tissue tropism) to identify both convergent and virus-specific effects on offspring immunity. We also outline shared and virus-specific mechanistic framework for maternal immunopathogenesis, discuss how early-life immune perturbations may predispose exposed offspring to increased infection risk, allergic inflammation, neurodevelopmental disorders, and other immune-mediated conditions. Finally, we review emerging therapeutics and preventive strategies aimed at mitigating these long-term risks, and identify research gaps that must be addressed to improve outcomes for future generations.
Clinical Outcomes of Prenatal Viral Infections
Prenatal viral infections can disrupt maternal and fetal health, specifically the finely tuned, trimester-specific immunological balance, compromise placental integrity, and induce inflammatory cascades affecting pregnancy outcomes and fetal development. The clinical manifestations, likelihood of transmission, and severity of outcomes of these exposures are virus-specific and are influenced by various factors, such as gestational age at infection, viral tropism29, strain-specific differences30, and the route of transmission29. Despite HIV’s high risk of vertical transmission, congenital anomalies are not due to direct fetal infection in HIV-exposed pregnancies25,26. In contrast, influenza virus and SARS-CoV-2 rarely cross the placenta, yet both are associated with increased maternal mortality and impacts on fetal neurodevelopment27,28. Here, we examine the maternal and fetal outcomes associated with these globally-relevant viral pathogens to elucidate both shared and distinct consequences of prenatal viral exposure extending beyond birth, predisposing offspring to long-term neurodevelopmental and psychiatric disorders3, autoimmune diseases, and metabolic conditions later in life31.
Prenatal HIV infection: sustained maternal immune activation and altered development in offspring
HIV infection during pregnancy drives sustained maternal immune activation, disrupting immune tolerance and altering intrauterine environment. This chronic inflammation promotes T cell exhaustion, and is associated with poor pregnancy outcomes, including preterm labor, chorioamnionitis, and fetal growth restriction32,33,34,35. Vertical transmission occurs via three main routes: transplacental microtransfusion during pregnancy, direct exposure during delivery, and postnatally through breastfeeding6,36,37,38, with approximately one-third of infected neonates had detectable virus at birth and the remainder infected perinatally or postnatally, indicating independent cycles of viral replication37.
Two major viral strains are known to differ in pathogenicity and transmission: HIV-1, the predominant global strain, is more transmissible, virulent, and pathogenic than HIV-239, which is largely confined to West Africa36,40. In eastern and southern Africa, where 17 million women living with HIV (WLH) reside, maternal mortality is 2–10 times higher among pregnant WLH41 compared to pregnant women negative for HIV. Without intervention, vertical transmission rates range from 15% to 45%7,37, but can be reduced to < 1% with integrated strategies involving early maternal HIV testing, prophylaxis during delivery, and sustained antiretroviral therapy (ART)6,26. ART similarly reduces HIV-2 transmission rates to 0.2–5%39,42, significantly lower than the 25–40% observed for HIV-1 pregnancies26.
Large cohort studies confirm that ART throughout pregnancy mitigates maternal morbidity and adverse outcomes43,44 but introduces complexity to pregnancy outcomes. Protease-inhibitor-based regimens have been linked to increased risks of preterm birth, low birthweight, and small-for-gestational age infants43,45,46. Inflammatory mediators, particularly maternal cytokines, remain elevated even with effective ART and are associated with impaired fetal gut development and immune dysfunction24,47. Placental pathology in ART-treated WLH often reveals vascular malperfusion48, chorioamnionitis, villitis, and inflammation, factors compromising fetal development18,49,50 and enhancing vertical transmission51. These abnormalities persist despite viral suppression, suggesting that HIV-driven inflammation or immune dysregulation is irreversible despite ART use24,50.
Recent data support the use of integrase inhibitor-based regimens52 over older protease inhibitors and non-nucleoside reverse-transcriptase inhibitor (NNRTI) regimens53 due to their improved tolerability and reduced adverse pregnancy outcomes43. Nevertheless, ART does not fully reverse HIV-associated pregnancy complications24,36,47, as HIV-mediated chronic inflammation and immune activation persist even in the absence of maternal viremia43,45,54. HIV infection reduces the overall thymic output48,55,56,57, alters peripheral tolerance, and increases miscarriage risk56. Infection during early gestation is often associated with fetal demise49.
HIV infection severity is shaped less by acute symptoms and more by viral load, duration of exposure, and ART58,59,60, with chronic immune activation and placental inflammation occurring even in clinically asymptomatic pregnant WLH and contributing to immune reprogramming in exposed but uninfected infants61. Even in the absence of fetal infection, in utero exposure causes sustained fetal inflammation and impaired placental function, leading to long-term growth deficits to the offspring62. Postnatally, HIV-infected infants, particularly under 6 months, remain vulnerable to severe infections such as pneumonia (Pneumocystis carinii pneumonia)63. By age two, HIV-exposed but uninfected (HEU) infants showed lower growth metrics64 and increased rates of neurodevelopmental disorders8,9. Though these HEU infants remained uninfected due to effective maternal ART, they also exhibited altered T cell and innate immune responses8,65 and differences in early neurodevelopmental outcomes8,9. Despite advances in ART and effective prevention strategies, over one million infants are born annually to pregnant WLH. Most HIV-exposed children remain uninfected postnatally37, but the long-term effects of prenatal HIV and ART exposure on neurodevelopment, immune regulation, and metabolic programming remain ill-defined and warrants continued longitudinal investigation. These outcomes may result from indirect effects of maternal inflammation, and could potentially influence long-term immune competence and susceptibility to infections66,67.
Prenatal SARS-CoV-2 infection: Placental pathology and neurodevelopmental risk in offspring
SARS-CoV-2, a positive-sense RNA virus of the Coronaviridae family68 and a causative agent of coronavirus disease (COVID-19), emerged as a significant threat to maternal and fetal health in 2019. Prenatal exposure has been associated with increased risks of pre-eclampsia, pre-eclampsia-like endothelial injury, postpartum hemorrhage, severe maternal infections, preterm birth, intrauterine growth restriction (IUGR), and maternal death69,70,71,72,73. In pregnant individuals with COVID-19 and comorbidities, such as obesity, maternal mortality rates reached 8.7%74. Although SARS-CoV-2 spreads primarily via respiratory droplets and physical contact, alternative ocular and transplacental transmission routes are documented in < 2% of cases21,75,76,77. Viral RNA has been detected in umbilical cord blood, placental tissue, breast milk, and vaginal swabs20,78. The presence of SARS-CoV-2-specific IgM and IgG antibodies in breastmilk and cord blood further suggests possibility of in utero immune priming, though its frequency remained low but occurred most efficiently during first and second trimesters20,21,79. Nevertheless, vertical transmission remains rare, and instead of fetal infection, COVID-19 pregnancies are characterized by pronounced maternal and placental inflammation largely governed by maternal T cells and fetal stromal cells14,80. Histopathological studies consistently report abnormal placental angiogenesis and overt intervillous inflammation during maternal infection81. Despite these placental abnormalities, confirmed fetal infection remains infrequent20, suggesting that SARS-CoV-2 primarily affects pregnancy outcomes through indirect maternal immune mechanisms. Further, the nasal and gut microbiomes of pregnant women with SARS-CoV-2 infection showed increased abundance of Bacteroidales and members of the Firmicutes phylum82,83, previously associated with anti-inflammatory effects, providing insights on the gut microbial changes during gestational SARS-CoV-2 infections.
Disease severity appears to be a key modifier of prenatal immune outcomes, as symptomatic and severe maternal SARS-CoV-2 infections are associated with heightened systemic inflammation, respiratory distress syndrome, interferon dysregulation, and placental vascular inflammatory changes84,85,86,87. Severe maternal COVID-19 is further associated with elevated IFN-λ levels, a cytokine classically involved in mucosal antiviral defense14,87. Interestingly, asymptomatic or mild infections generally induce subtle immune perturbations yet still trigger rare and detectable changes in fetal immunity79,88,89,90.
The clinical consequences of prenatal SARS-CoV-2 exposure extend beyond delivery. In a 2020 retrospective cohort study of 7,772 infants, 3% were born to mothers with SARS-CoV-2 infection during pregnancy91. In a separate cohort, two neonates displayed rare but severe neonatal outcomes, including microcephaly, seizures, severe parenchymal atrophy, encephalomalacia, and elevated systemic inflammation at birth, despite negative PCR results for SARS-CoV-284. In another US cohort, 72% of neonates (or 10 of 14) born to SARS-CoV-2 infected mothers during gestation showed increased hospitalization due to respiratory distress syndrome (RDS)92. These prenatally-exposed neonates had sub-optimal neurodevelopment93 and a higher incidence of adverse neurodevelopmental diagnoses in the first year of life compared with unexposed newborns91,94. One case of second-trimester maternal infection was linked to placentitis-induced fetal death and oxidative damage to the fetoplacental unit84. Long-term follow-up studies conducted on prenatal SARS-CoV-2-exposed children reveal increased association with respiratory distress95, developmental delays96, and sub-optimal neuromotor development in early life93. Moreover, prenatal exposure has been associated with multisystem inflammatory syndrome in children (MIS-C) and post-infectious encephalitis and chronic neurologic impairment, underscoring the potential for long-term neurologic and immunologic sequelae84,97.
Prenatal influenza virus infection: a threat to maternal well-being
Influenza virus infection during pregnancy significantly increases the risk of hospitalization27, respiratory failure, acute respiratory distress syndrome (ARDS), secondary bacterial pneumonia, preterm birth, low birth weight, and maternal death compared to infected non-pregnant individuals98,99. Although vertical transmission during active influenza infection appears unlikely100, maternal infection contributes to adverse outcomes primarily through chronic inflammation, such as pre-eclampsia and placental dysfunction99,100. Symptomatic maternal influenza infections, particularly when accompanied by fever and systemic infection, is linked to robust maternal cytokine responses, maternal gut microbial dysregulation, and placental vascular inflammation22,101,102. While some studies reported associations between mild or subclinical influenza infections during pregnancy and pre-eclampsia, especially in unvaccinated cohort103, findings remain inconsistent. Variability in clinical outcomes likely reflects differences in cohort size, infection timing, severity, and circulating influenza strains104,105.
Maternal inflammation plays a central role in influenza-associated pregnancy and developmental complications in offspring, including behavioral, cognitive, and histopathological abnormalities100. Although large-scale meta-analyses of the 1957 influenza pandemic found no association between prenatal infection and schizophrenia risk across trimesters16, more recent epidemiological studies suggest that prenatal influenza exposure may increase the risks of neurodevelopmental disorders, such as cognitive impairment and behavioral abnormalities22,100, schizophrenia, bipolar disorder, and autism, later in life5,106. Experimental animal models further support potential long-term neurological consequences of prenatal influenza exposure, despite the absence of direct fetal infection. In a pregnant rhesus macaque model infected during the third trimester, neonates displayed significant loss in bilateral gray matter volumes in the cingulate and parietal lobes and localized reductions in white matter volume107. Similarly, murine models show that offspring of influenza-infected dams exhibit stunted growth and heightened vulnerability to unrelated infections, suggesting impaired immune development and altered immune programming in early life108. Despite these findings, contemporary data on fetal and infant outcomes following prenatal influenza exposure from more recent influenza seasons are still lacking109.
Viral Strategies in Breaching the Placental Barrier
The maternal-fetal interface is fortified with a network of robust physical, cellular, and immunological barriers that protect the developing fetus while supporting tolerance. However, viruses can disrupt these defenses by infecting placental cells directly or by triggering systemic and localized immunopathology, leading to a weakened maternal immunity, increased fetal exposure, and adverse pregnancy outcomes. Key viral strategies include: (i) direct infection of macrophages and trophoblasts56,84, (ii) immune modulation62, (iii) molecular mimicry78,110,111, (iv) exploitation of placental transport system78,112,113, and (v) hijacking pregnancy-induced immune tolerance30,113,114. This section examines the mechanisms of vertical transmission in viruses with established transplacental routes, such as HIV, as well as viruses like SARS-CoV-2 and influenza that primarily cause maternal pulmonary infections without confirmed placental tropism.
Multimodal entry and immune exploitation of HIV
HIV, unlike many acute infections, establishes persistent reservoirs in maternal, placental, and fetal tissues early in pregnancy, often by 8 weeks of gestation17,18. HIV has been detected in decidual leukocytes, villous trophoblasts, and Hofbauer cells, suggesting that infection is not confined to a single cellular compartment17,18. In utero transmission occurs primarily in the third trimester and proceeds via three primary routes: (i) fetal exposure to infected amniotic fluid115,116, (ii) direct trophoblast infection, and (iii) receptor-mediated transcytosis117,118 (Fig. 1). Cell-free and cell-associated HIV cross the placenta by exploiting multiple receptors, CD4, C-X-C motif chemokine receptor 4 (CXCR4), dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN), and C-C motif chemokine receptor 5 (CCR5)19, with R5-tropic variants (macrophage-tropic, non-syncytium-inducing) mostly transmitted via CCR5 in infants19,119,120. Although cell-free HIV is detectable in amniotic fluid58 potentially interacting with fetal oronasal and gastrointestinal mucosa via CD4+CCR5+ T cells, it is not necessarily correlated with vertical transmission, suggesting that its presence alone is not predictive of fetal infection115,116. Some studies propose that the amniotic fluid may even be protective, when pregnant WLH with high plasma viral loads go on to deliver uninfected neonates58,59. Interestingly, in utero HIV transmission also occurs via immune cells with low-CD4 expression (e.g. macrophages, dendritic cells) and CD4-negative trophoblasts113, challenging the assumption that HIV entry strictly depends on CD4 expression.
HIV-1 crosses the placental barrier primarily in the third trimester through three major routes: (i) exposure to infected amniotic fluid, (ii) direct infection of trophoblasts, and (iii) transcytosis across distinct anatomical sites. a Placental villous tree: HIV-1 infects the placental villous tree by targeting Hofbauer cells (fetal macrophages), villous cytotrophoblasts, and decidual leukocytes. Both cell-free and cell-associated HIV traverse the villi via receptors including CD4, CCR5, CXCR4, and DC-SIGN, with CCR5-tropic R5 strains being preferentially transmitted. HIV-infected PBMCs utilize syncytin-mediated fusion to enter villous tissues, establishing early viral reservoirs detectable as early as 8 weeks gestation. b Fetal intestinal mucosa: HIV particles present in infected amniotic fluid access the fetal gut, where they interact with CD4⁺CCR5⁺ T cells in the intestinal mucosa, leading to activation of Th1 and Th17 responses. However, amniotic fluid is not always infectious, even in mothers with high plasma viral loads, suggesting a context-dependent protective role of the amniotic environment. c Layers of the feto-maternal interface: At the feto-maternal interface, HIV crosses through cell-associated and antibody-mediated transcytosis and direct trophoblast infection, exploiting Fc receptors such as FcRn and Fcγ-RI/III for entry. HIV-infected immune cells, including macrophages and dendritic cells with low or absent CD4 expression, mediate cell-associated transmission, which is more efficient than free-virus transmission. Hofbauer cells can transfer antibody-bound virus to fetal tissues via FcRn, a process blocked by FcRn inhibition. The gestational upregulation of FcRn and viral heterogeneity likely contribute to the increased risk of transmission in late pregnancy and delivery.
Finally, placental infection by HIV occurs most efficiently through parallel strategies: cell-association, antibody-mediated transcytosis, and trophoblast infection117,118. In vivo models mimicking placental architecture demonstrated that PBMC-associated HIV readily crosses the placenta and establishes productive fetal infection, whereas cell-free virus does not generally traverse118. HIV-infected T cells can also undergo syncytin-mediated fusion with placental trophoblasts to establish latent viral reservoirs into fetal tissues113,121. Viral latency and these layered compartments render the virus inaccessible to the external environment unless targeted by small molecules or broadly neutralizing antibodies122. Importantly, HIV exploits the neonatal Fc receptor (FcRn) or non-canonical Fc receptors (Fcγ-RI and Fcγ-RIII)117 for transcytosis, with Hofbauer cells mediating maternal antibody-bound HIV transmission112. Neutralizing HIV-1 antibodies reduced infectivity and were more effectively favored than non-neutralizing IgG for transcytosis, which was abrogated by blocking or knocking down the FcRn receptor117. The increased cell-surface expression of FcRn with gestational age, combined with the high viral heterogeneity of HIV strains, likely explain why most of the in utero HIV transmission events occur late in pregnancy or during delivery38,123.
Pregnancy alters systemic immunity in complex, trimester-specific ways. These shifts are further exaggerated in pregnant WLH, influencing both vertical transmission and fetal development24. Pregnant WLH exhibit distinct cytokine signatures across gestation24,47, including elevated IFNγ-induced protein 10 (IP-10/CXCL10), antiviral cytokine (IFN-λ1), Th1 cytokines (IL-8, IL-12, IL-12p70), Th2 cytokine (IL-5), and Th17 cytokine (IL-17A)47,124,125. First-trimester plasma often contains high IL-6, IL-9, and TNF-α levels24,47,61. CD4+ T cell proliferation is also suppressed, while CD8+ T cells are expanded, mirroring the systemic immune activation seen in non-pregnant HIV infection57. Circulating markers of immune activation and disease progression (β2-microglobulin, neopterin, and TNF-α)48,55,56 are also elevated, including IL-10 levels57. Paradoxically, IL-10, a cytokine generally linked to immune suppression appears to dampen TNF-α release and may indirectly reduce vertical transmission risk57. Finally, the placenta modulates fetal exposure to maternal inflammation through selective cytokine transfer. Comparative analyses of maternal and umbilical cord plasma show elevated IL-5 and IL-10 but reduced IL-8 and macrophage inflammatory protein-1α (MIP-1α) in maternal plasma, while cord blood is enriched in RANTES and E-selectin125. These compartmentalized cytokine patterns highlight the placenta’s regulatory role in filtering and reshaping the fetal cytokine milieu, influencing local inflammatory response and controlling disease progression in exposed but uninfected neonates47,125.
Immune-driven fetal effects of SARS-CoV-2 without evidence of direct transmission
Unlike HIV, SARS-CoV-2 is not consistently detected in fetal tissues or placental compartments80, and direct vertical transmission is considered rare20,21. However, pregnancies complicated by SARS-CoV-2 infection exhibit pronounced immunological alterations at the maternal-fetal interface, with implications for placental function and fetal development70. Proteomic profiling of maternal-fetal dyads has shown that in utero SARS-CoV-2 exposure activates robust maternal pro-inflammatory responses, largely driven by the NF-κB pathway14,87. Cord blood samples from exposed but uninfected neonates had increased levels of IFN-α, EGF, FGF, IL-17, and IL-15, while chemokines such as RANTES are reduced87. IFN-λ is hypothesized to be protective by restricting fetal viral dissemination20. Treatment with pegylated IFN-λ in COVID-19 patients similarly enhanced interferon-stimulated gene (ISG) expression in dendritic and B cells, promoting viral clearance without impairing adaptive responses126.
Maternal antibody responses also contribute to fetal protection. Passive transfer of SARS-CoV-2-specific IgG to newborn has been well documented95,105,127,128. In a study of over 4600 pregnant mother-infant dyads, maternal antibodies correlated with detectable neonatal immunity129, reinforcing the importance of maternal vaccination and booster immunizations in conferring passive protection. Importantly, vaccination against SARS-CoV-2 early in pregnancy has not been associated with increased risk of structural birth defects, and several studies demonstrated maternal immunization enhances antibody transfer, reduces maternal morbidity, and improves pregnancy outcomes95,127,128.
Systemic immune activation and vascular pathology without vertical infection by influenza virus
Like SARS-CoV-2, influenza virus infection during pregnancy is associated with increased fetal risk despite limited evidence for direct transplacental infection22. Maternal influenza infection triggers a hyperactive innate and adaptive immune responses, leading to widespread and sustained vascular inflammation99, paralleling immunopathological features in SARS-CoV-2 infection. In pregnant mice, IAV infection triggers systemic “vascular storm”, marked by neutrophil and T cell influx, upregulation of adhesion molecules (ICAM-I and VCAM-I) and heightened expression of pattern-recognition receptors (TLR7 and TLR9), leading to placental vascular dysfunction and impaired blood flow to the fetus22. Notably, while certain H1N1 strains can infect fetal trophoblast cells in vitro130, there is little in vivo evidence for productive vertical transmission. IAV RNA has not been detected in fetal tissues during maternal infection100, suggesting that fetal complications are primarily mediated through maternal immune dysregulation rather than direct infection. Cytokine-driven placental dysfunction, systemic inflammation, and vascular injury are thought to underlie the developmental effects observed in animal models and epidemiological studies of prenatal IAV exposure22,99,108.
Prenatal IAV infection can also induce long-lasting immune imprinting in offspring. Fetal exposure to maternal IAV during early gestation elicits prolonged immune activation and links to resistance to secondary infections in offspring, highlighting the enduring consequences of gestational immune perturbation108.
Influence of Viral Factors on Disrupted Maternal Immunity during Prenatal Infections
Prenatal viral infections impact maternal health and fetal development not only from direct cytopathic effects but also by sophisticated viral immune evasive strategies. HIV, SARS-CoV-2, and influenza viruses exploit host immune pathways in distinct ways, including hijacking innate antiviral signaling131, subverting interferon responses132,133, and reprogramming cytokine networks35,84,134 at the maternal-fetal interface. Below, we highlight viral mechanisms that alter cellular receptor engagement73,135,136, evade antiviral pathways137, and promote pro-inflammatory cascades32,33,34,138,139,140,141, integrating evidences from clinical cohorts, in vivo models, and in vitro systems.
HIV
HIV leverages both its genetic diversity and accessory protein function to evade maternal immune responses. A member of the Retroviridae family, its ~9.8 kb RNA genome encodes structural (Gag, Pol, and Env), regulatory (Tat and Rev), and accessory proteins (Vif, Vpr, Vpu, and Nef)36,142. Mutations in Env gp41, Gag NC, Pol RT, and Rev drive viral heterogeneity and emergence of escape mutants, leading to impaired maternal cytotoxic T lymphocyte (CTL) responses and enhanced vertical transmission36,119,143. This increased viral heterogeneity among pregnant WLH contributes persistent reservoirs of transmissible variants that evade early infant immune responses120. Notably, CTL escape mutants remain stable post-transmission and contain subdominant epitopes, contributing to uncontrolled HIV replication in infants65,143,144. Neutralizing maternal antibodies targeting Env, particularly V3 loop of gp120 protein, are associated with reduced vertical transmission risk123, highlighting the protective potential of maternal humoral responses and supports the immunotherapeutic strategies that enhance Env-specific immunity145,146.
While most HIV genes contribute to pathogenesis, maternal immune dysregulation is mainly mediated by regulatory and accessory proteins. Surface-expressed Env (gp120), the trans-activator of transcription III (Tat-III), and negative regulatory factor (Nef), are potent inflammatory activators through NF-κB signaling, leading to the overexpression of IL-1β, TNF-α, and IL-632,33,34. In pregnant WLH, Nef-specific immune responses are characterized by diminished IL-10, while Gag-specific T cells exhibit Granzyme B expression, resulting in enhanced viral replication and impaired viral control35. Nef further suppresses antigen presentation by downregulating CD4, MHC-I, and MHC-II expression135,136, while impairing thymopoeisis and peripheral T cell development through altered surface marker trafficking and oligomer formation147. Tat, Rev, and Vpr enhance viral transcription and amplify maternal viral load148, whereas Vpu enhances virion release and immune evasion by degrading tetherin and CD4, thus downregulating MHC expression149. Tat also mimics host angiogenic proteins. Its arginine- and lysine-rich domain shares sequence homology with vascular endothelial growth factor-A (VEGF-A), promoting extracellular adhesion and integrin expression111,148. These integrins interact with osteopontin, a key regulator of decidual angiogenesis, linking Tat-induced angiogenesis to vascular complications like pre-eclampsia73. Additionally, Tat acts as a neurotoxin, activating microglia and inducing oxidative stress-mediated apoptosis in dopaminergic neurons via mitogen-activated protein kinase (MAPK) and caspase signaling cascades. These neuroinflammatory processes contribute to neurocognitive and behavioral disorders observed in prenatally-exposed infants150,151,152,153. While HIV proteins such as Tat can contribute to long-term aging and neurodevelopmental impairments including memory recognition in children154 and preclinical models155,156, infants who are exposed but uninfected (HEU) in the current ART era are unlikely to experience direct exposure to these proteins; observed developmental delays were more likely mediated by maternal immune activation and placental inflammation during gestation8,9.
SARS-CoV-2
Although vertical transmission of SARS-CoV-2 is rare80, the virus significantly alters maternal immune signaling at the placental interface78,157, mediated by the angiotensin-converting enzyme 2 (ACE2) receptor73 (Fig. 2). The secreted viral accessory protein ORF8 was detected in fetal compartments in over 60% of the maternal-fetal tissues from infected pregnancies78. ORF has an Ig-like folding157 and shares sequence homology with the human Fc region of IgG, suggesting that it may hijack the neonatal Fc receptor (FcRn) to facilitate transplacental transfer78. ORF8 also mimics IL-17 and binds to the IL-17 receptor, triggering a proinflammatory cascade110. Additionally, ORF8 directly binds to the C1qA subunit, triggering the classical complement pathway activation and contributing to inflammation in the fetal amnion and cord blood78.
Following maternal SARS-CoV-2 infection, SARS-CoV-2 may induce fetal immune dysregulation via multiple converging pathways, potentially impacting postnatal neurodevelopment. The SARS-CoV-2 accessory protein ORF8 was detected at the maternal–fetal interface and within fetal compartments. A magnified inset of the interface highlights ORF8 binding to the globular domain of C1qA subunit, initiating classical complement pathway leading to induction of proinflammatory cytokines (IL-17A and IL-17E) and fetal inflammation. Owing to its immunoglobulin-like folding, ORF8 is hypothesized to mimic the Fc region of IgG, facilitating its translocation across the placenta via the neonatal Fc receptor (FcRn).
Placental tissues from SARS-CoV-2-infected pregnancies display widespread expression of nucleocapsid and spike (S) proteins, along with elevated levels of inflammatory and oxidative stress markers, including pyrin domain-containing 1 protein, MIP-1β (or CCL4), stromal cell-derived factor 1, IL-13, and IL-10, with notable decrease in hCG hormones indicating impaired endocrine function84. Amongst viral proteins, the S protein is a key inducer of inflammation. In vitro studies using human and murine macrophages show that S protein binds to toll-like receptors (TLR2 and TLR4), initiating NF-κB-mediated pro-inflammatory signaling138,139,140. Upregulation of TLR2 and its adaptor molecule, MyD88, has been implicated with COVID-19 severity158. SARS-CoV-2 also employs non-structural proteins (NSPs) to evade interferon-mediated antiviral responses. NSP1 blocks IRF3 phosphorylation and downregulates IFN transducers, including Tyk2 and STAT2137. NSP1 also interacts with STAT1, inhibiting types I, II, and III IFN signaling133. These findings point to potential targeted interventions, including S protein-based vaccines and NS protein-focused antiviral agents to counteract maternal immune hyperactivation during pregnancy.
Influenza virus
Influenza viruses, members of the Orthomyxoviridae family, and including both influenza A (IAV) and B (IBV) subtypes, modulate host immunity through conserved and subtype-specific mechanisms98. A key immune evasion strategy by influenza viruses is the downregulation of MHC-I surface expression on infected cells, impairing CD8+ T cell-mediated viral clearance159. While IAV broadly suppresses intracellular MHC-I expression, IBV preferentially reduces cell-surface MHC-I expression159.
During pregnancy, IAV infection exacerbates maternal immune dysregulation by activating the NLRP3 inflammasome pathway141. The viral matrix protein 2 (M2), a proton-selective ion channel, triggers inflammasome activation, resulting in IL-1β release and systemic inflammation141. While M2 is an attractive therapeutic target for universal vaccines, resistance to adamantane derivatives targeting M2 were reported in circulating influenza viruses160. Finally, prenatal IAV exposure disrupts fetal immune development and hematopoeisis. Flow cytometric analyses demonstrate that influenza infection during early gestation expands myeloid progenitors in fetal bone marrow and concurrently reduced NK and B cells in the fetal lungs and spleen108. Postnatally, alveolar macrophages in IAV-exposed offsprings exhibit reduced phagocytic function and diminished capacity to clear second-hit pathogens108, suggesting that prenatal IAV can durably reprogram fetal immunity.
Long-term Immunological Implications of Prenatal Viral Infection on the Offsprings
Prenatal viral exposure can profoundly alter fetal immune programming and disrupt immune system development, even in the absence of direct fetal infection. These early-life immune perturbations arise form maternal immune activation, transplacental cytokine transfer, and fetal exposure to viral antigens or viral induced inflammation (Fig. 3). Central to these changes is the concept of trained immunity, wherein innate immune cells undergo long-term reprogramming, resulting in dysregulated responses to subsequent environmental insults114,161. Skewed T helper (Th) cell phenotypes (Th1, Th2, and Th17 subsets), has been observed in exposed yet uninfected infants following prenatal infection with HIV, SARS-CoV-2, and influenza virus, with implications on infection susceptibility, vaccine response, allergic sensitization, and neurodevelopment11,14,162,163,164,165,166. Notably, disruptions of the gut microbiome have been reported in neonates and children prenatally exposed to HIV23,167,168,169 and SARS-CoV-290,92,170, as well as in preclinical models of gestational influenza101,102,108,159,171. These alterations contribute to impared immune ontogeny, postnatal developmental outcomes, increased susceptibility to early-life infections, and heightened morbidity in the offspring (Table 1).
Maternal infection with SARS-CoV-2, influenza virus, or HIV induces maternal immune activation characterized by interferon and cytokine signaling. These signals act on the placenta to engage antiviral defense mechanisms, including pattern recognition receptors, interferon-stimulated genes, and trophoblast-intrinsic restriction factors, while also altering placental vascular, metabolic, and barrier functions. The magnitude and duration of these responses, shaped by infection severity and chronicity of prenatal viral exposures, modify the intrauterine immune milieu and contribute to fetal immune imprinting, resulting in altered vaccine and immunologic responses, increased susceptibility to early-life infections, gut-brain microbiome dysbiosis, and long-term behavioral, neurologic, and intellectual complications in exposed but uninfected offspring.
HIV
Longitudinal studies in Kenya demonstrated that infants exposed to prenatal HIV experience a 2.5–3.0% monthly decline in perforin-expressing NK cell activity compared to healthy infants, thereby diminishing cytolytic activity, impairing viral replication control and placental vasculature development172. These immune disruptions have been associated with reduced nutrient transfer and restricted fetal growth172. Furthermore, neonates with prenatal HIV exposure without vertical transmission (HEU) showed impaired neutrophil, Th2 bias, and monocytic activation, leading to inefficient pathogen killing and sub-optimal adaptive response163. HEU neonates are also born with reduced peripheral Th1, Th2, Th17, and CD4+CD25++ regulatory T cell subsets, increasing their susceptibility to infections during early months of life164. Of concern, HEU children exhibit elevated IL-6 levels in serum and cerebrospinal fluid at birth and up to 6 months of age8,9, a cytokine implicated in wasting during chronic infections and indicative of impaired growth and failure to thrive9. Elevated IL-6, together with other inflammatory mediators (GM-CSF, IFN-γ, IL-10, IL-12p70, IL-1β, IL-2, IL-4, and neutrophil gelatinase-associated lipocalin (NGAL)) measured in serum of HEU infants at 6-10 weeks of age, was further associated with poorer motor neurodevelopment and central nervous system (CNS) dysregulation166.
Importantly, HIV exposure may shape fetal immunity in ways that influence viral evolution during pregnancy. Analysis of 721 in utero transmission dyads revealed that infant-derived HIV-1 Env gene sequences were distinct and genetically divergent from maternal variants, with unique immunodominant selection sites65,123. These immunodominant sites unique to fetal viruses suggest that fetal immune responses exert selective pressure on viral populations during gestation, contributing to the emergence of escape mutants123. Supporting this, other studies found that CTL escape mutations in the gag gene were associated with increased viral evolutionary rates in perinatally infected infants65,123, reinforcing the idea that fetal immunity is not merely passive but may actively shape HIV transmission dynamics in utero.
Prenatal HIV exposure also significantly alters the developing gut microbiome in HIV-exposed uninfected (HEU) infants, in the absence of infection, leading to reduced microbial diversity, delayed maturation, and decreased abundance of beneficial bacteria, alongside increased inflammation and levels of potentially pro-inflammatory microbes23,169,173. HEU infants frequently exhibit lower levels of commensal bacteria and less mature gut microbiomes compared to HIV unexposed neonates, even when breastfed169,174, and a shift toward taxa including Blautia, Klebsiella, and members of the Ruminococcaceae family, which may drive inflammation23,168,173. Low-birth-weight HEU infants were also associated with maternal third-trimester elevations in Spirochaetes169. Although these HEU infants appear physically healthy, these microbial interactions correlate with gut-dependent inflammation, increased morbidity, and higher rates of early-life infections compared to infants born to unexposed mothers23, suggesting a compromised immune environment.
Maternal factors, including HIV viral load, systemic inflammation, and ART exposure (i.e., lamivudine, nevirapine, and kynurenine) in breastmilk further shape the infant gut, with ART exposure linked to decreased Bifidobacterium longum167,174. Additionally, variations in human breastmilk oligosaccharide composition in WLH imprint the HEU gut microbiome, highlighting the combined roles of feeding practices and maternal gut microbiota167,168,173. Given the critical role of the gut microbiome in educating infant immunity, these disruptions likely contribute to increased infection susceptibility and immune dysregulation in HEU infants early in life. Understanding these complex interactions is essential for developing targeted interventions to support immune maturation and improve health outcomes, particularly in resource-limited setttings where HIV transmission risks and feeding practices vary.
SARS-CoV-2
Proteomic profiling of two-day-old neonates exposed to SARS-CoV-2 in utero revealed early respiratory dysregulation, including elevated inflammatory markers such as IL-1β, IL-18, and caspase 114, indicative of inflammasome activation and systemic distress shortly after birth. Concurrent upregulation of T cell-associated cytokines (IL-33, NFATC3, and CCL21) suggests early Th2-skewed T cell priming, which may predispose these infants to allergic responses following immunization with pro-Th2 vaccines14,165. IL-17 and complement activation pathway, both implicated in COVID-19-associated placental and fetal inflammation78,110, also likely contribute to fetal immune imprinting. Beyond core inflammatory signatures14, unique cytokine profiles have been associated with increased respiratory distress (RD) among SARS-CoV-2-exposed, uninfected neonates95. Distinct markers such as iduronidase (IDUA), TREM2, GHRL, anterior gradient 3, PCOLCE, and protein disulfide isomerase family members have been linked to impaired ciliary function and elevated IgE production in this population with RD95.
Notably, neonates born to SARS-CoV-2-exposed mothers with recent or ongoing infections displayed elevated IL-10 and CXCL8, consistent with prenatal immune activation11. These enhanced fetal cytokine levels yet distinct from maternal profiles after prenatal SARS-CoV-2 infection suggest early fetal priming and protection against maternal immune activation11,80,175. Further, expansion of humoral innate (NK, NK T, and innate-like Vδ2+ γδ T cells) and adaptive (CD161-expressing CD8+ T cells and CD25+FoxP3+ Treg) immune cell populations in these exposed neonates support that prenatal SARS-CoV-2 exposure may prime protective or compensatory immune pathways during development11,80.
Prenatal SARS-CoV-2 exposure, especially during early gestation, has been associated with alterations in maternal vaginal, gut, and oral microbiomes, which are reflected in the infant’s oral microbiome170. In a recent study of 94 mother-infant pairs, infants prenatally exposed to SARS-CoV-2 exhibited decreased microbial diversity and richness, especially when maternal infection occurred in the second and third trimesters, indicating destabilization of the infant gut microbiome even later in gestation90. Compared to infants of uninfected mothers, beneficial taxa, such as Bifidobacterium and Staphylococcus were significantly depleted, whereas Rothia and Enterococcus were increased and overall microbial composition showed greater individual variability90. In another cohort, prenatally exposed infants exhibited elevated fecal calprotectin170, indicative of early intestinal inflammation176. Stool homogenates from exposed infants also reported elevated inflammatory cytokines, IL-6 and IFN-γ92, reflecting microbiome-influenced immune activation, consistent with the capacity of SARS-CoV-2 to infect gut enterocytes177,178. Given that maternal microbiota strongly shapes infant microbial colonization and immune development179, disruptions caused by prenatal SARS-CoV-2 exposure may impair initial gut seeding, disturb the normal maternal-fetal microbiome crosstalk, and lead to long-term microbiota instability, potentially influencing early immune system maturation even in mild and asymptomatic maternal infections88,89,90.
Influenza virus
Prenatal influenza infection has been linked to altered neurodevelopment. In mouse models, early gestational exposure to IAV results in persistent disruption of glial fibrillary acidic protein (GFAP) expression, a critical marker for neurogenesis and injury response, suggesting long-lasting alterations in glial function in the brain180. In vitro studies reinforce influenza’s neurotropic potential. H1N1 infection induces neurotoxic protein aggregates, such as α-synuclein and Disrupted-in-Schizophrenia 1 (DISC1), impairing proteostasis and cellular signaling in neural tissues181. When combined with DISC1 mutations, MIA from influenza infection, synergistically leads to behavioral and neurodevelopmental deficits resembling childhood-onset schizophrenia4. Notably, influenza’s non-structural protein 1 (NS1) enhances neurotropism and immune evasion. Mutations in NS1 promote viral spread from the lungs to the brain182. NS1 also upregulates inducible nitric oxide synthase (iNOS) expression in neurons, impairing cell growth, accelerating premature senescence, and contributing to oxidative stress-induced neurological damage183.
Given the well-established links between gestational IAV exposure and neurodevelopmental impairment100,102,106,180, several studies have investigated its impact on offspring gut immunity via the gut-brain axis. Prenatal IAV infection perturbs the gastrointestinal immune environment, with preclinical models showing increased development of the offspring’s gut-associated lymphoid tissue (GALT)101, a key hub for modulating gastrointestinal homeostasis184. Exposed offspring also exhibit elevated neutrophils, macrophages, pro-inflammatory cytokines, such as IL-6 and IFN-γ in the gut, indicative of persistent inflammation101,102. Changes in gut microbiota composition and function have been reported in offspring exposed to prenatal IAV171, alongside fetal cortical abnormalities and alterations in the fetal brain transcriptomes102. In a recent study on neonatal piglets born to unvaccinated sows challenged with IAV, bulk RNA sequencing of hippocampal tissues identified upregulation neurogenesis-associated genes and downregulation of genes involved in vascular development, with these brain gene expression changes strongly correlating with microbial community abundances171. Notably, maternal vaccination mitigated viral pathogenesis, microbiome disruption, and brain transcriptional changes, particularly protecting against both gastrointestinal and neurological effects171. Overall, prenatal influenza infection creates an inflammatory intrauterine environment that reshapes the offspring’s gut immune system and microbiome, with consequential impacts on neurodevelopment.
Clinical management and preventive measures in children following prenatal viral exposure
Management of children exposed to prenatal viral infections, such as HIV, SARS-CoV-2, and influenza, requires a multidisciplinary, pathogen-specific, and often immunologically targeted approach. Clinical and preventive strategies typically converge on three immunological axes: (i) blocking viral entry185,186,187,188, (ii) leveraging maternal and placental immunity189,190, and (iii) transferring protective antibodies via maternal vaccination or passive immunization191,192,193. While the efficacy of these interventions varies by pathogen, they collectively aim to reduce long-term morbidity through early diagnosis, immune protection, and targeted supportive care. As understanding of fetal and neonatal immunity deepens, future interventions are likely to incorporate more precise immunologic and virologic targets for prenatal and postnatal intervention (Table 2).
HIV
Effective prevention of vertical HIV transmission begins with maternal pre-exposure prophylaxis (PrEP), particularly using nucleoside reverse transcriptase inhibitors (NRTIs) with high placental transport and protection during childbirth2. Combinatorial ART significantly reduces HIV transmission risk when initiated early; however, postnatal prophylaxis for infants should begin within six hours of birth and be tailored by maternal viral load and infant’s exposure risk60. Low-risk infants, defined as those with maternal HIV RNA < 50 copies/mL prior to delivery, typically require a 2-week zidovudine (ZDV) monotherapy, while high-risk infants (born to mothers with unsuppressed viremia or lacking ART during pregnancy), require a triple regimen (ZDV, lamivudine, and raltegravir) for 2–3 weeks2,194,195. Regular virologic testing at birth and up to 6 months is recommended to address multiple stages of viral replication, specifically during breasftfeeding when transmission risk persists, and neonatal immunity is immature2,194,195. If virologic tests remain negative, clinical and virologic monitoring continues through 18 months to confirm HIV status196. Additional management includes prophylaxis against severe pneumonia63,196 and screening for congenital co-infections such as cytomegalovirus (CMV), hepatitis B, and hepatitis C based on maternal risk factors60. Toxicity surveillance is essential due to reported raltegravir-associated hyperbilirubinemia60,197. Standard immunization should follow clinical guidelines; however, live vaccines, such as BCG, should be deferred in HIV-confirmed infants due to risk of disseminating the infection198.
Over the years, various therapeutic strategies were explored to prevent HIV reservoir establishment and enhance early viral suppression. Broadly neutralizing monoclonal antibodies (bnAb) in clinical evaluation have been engineered for increased breadth and potency against HIV strains through high-dose formulations199, prolonged serum half-life through Fc engineering200, and enhanced transplacental delivery by optimizing FcRn-IgG interactions191. For example, leronlimab (CCR5 receptor-targeting) has shown promise in FcRn-enhanced formulations191. To date, 17 bnAbs in clinical development target conserved regions of the HIV envelope, including the CD4 binding site, V1-V3 glycans, membrane-proximal external region, gp120-gp41 interface, and the “silent face” epitope201,202. Next-generation strategies include combining long-acting bnAbs with ART to sustain viral suppression and limit immune activation189,201, mRNA-based nanoparticle vaccines, with prime-boost regimens promoting maturation of bnAb precursors203, gene editing tools, such as CRISPR-Cas9, targeting viral co-receptors (CCR5, CXCR4, and HIV LTR loci) for latent reservoirs eradication204,205, and immune checkpoint inhibitors to promote immune surveillance of residual infected cells206,207. While combinatorial approaches may be advantageous than single-agent blockade, the enhanced effect may increase the toxicity of certain strategies. Hence, these approaches must balance toxicity, immune rejection, off-target effects, viral tropism switching, and economic feasibility, particularly in resource-limited settings.
SARS-CoV-2
Vertical transmission of SARS-CoV-2 is rare80, with most neonatal infections occurring postnatally. Maternal mRNA vaccination, especially after 20 weeks of gestation, significantly reduces the risk of hospitalization in infants under 6 months208,209,210 by facilitating transplacental antibody transfer during this vulnerable period211. Antibody transfer efficiency depends on the timing of maternal vaccination, antibody subclass distribution, fetal sex, and circulating variants86,87,209,212. Notably, the early third trimester represents the optimal window for antibody transfer, with declining efficacy if vaccination occurs between 32 and 36 weeks of gestation87,209. These findings underscore the importance of strategically timed maternal immunization to maximize neonatal benefit.
Postnatal management begins with reverse transcription-polymerase chain reaction (RT-PCR) testing at birth, followed by repeated testing at 7–14 days and 2–4 weeks, even for neonates born to asymptomatic mothers12. While RT-PCR is the diagnostic gold-standard, its sensitivity is reduced in asymptomatic or mildly symptomatic neonates due to potentially low viral loads213, and serologic testing lacks specificity for prenatal infection214. Consequently, management in neonates remains largely supportive79, including respiratory support (oxygen supplementation or mechanical ventilation for severe cases) and in select moderate-to-severe cases, remdesivir therapy10,79. Comprehensive neonatal care protocols evolved to include antenatal corticosteroids, delayed cord clamping, selective mother-newborn separation, rigorous monitoring for respiratory10,79,215 and neurological complications97, and infection control during breastfeeding to reduce transmission79. Long-term follow-up is critical to detect neurodevelopmental delays, cardiovascular anomalies, and immune dysfunctions that may emerge following prenatal SARS-CoV-2 exposure94.
Experimental therapeutic strategies targeting congenital SARS-CoV-2 infection under investigation include neutralizing monoclonal antibodies (bamlanivimab or casirivimab) have shown potential for use in high-risk neonates192,193, although concerns regarding immunosuppression remain216. Novel approaches targeting the ACE2 receptor, via receptor-binding domain (RBD) and non-RBD inhibitors, decoy receptors, or mRNA-based immunotherapeutics, are also under development185,186,187,188. In pregnancies complicated by maternal hyperinflammation, immunomodulatory agents like corticosteroids and IL-6 inhibitors may help mitigate fetal injury217,218. Continued research is essential to fully elucidate the spectrum of congenital SARS-CoV-2 infection and develop evidence-based strategies for the prevention and management of congenital infection.
Influenza virus
While evidence of vertical transmission of influenza virus is rare, maternal infection significantly increases risks of severe maternal morbidity and neonatal complications15. Early intervention includes diagnosis in neonates via RT-PCR for prompt confirmation of potential infection219. For newborns with confirmed influenza infection, supportive care with fluid management and respiratory support should initiate immediately, with antiviral therapy109. Oseltamivir is recommended within 48 h of symptom onset, though may be considered beyond this window in critically ill infants with severe respiratory distress or multisystem involvement98,109,219. Besides neonatal testing, seasonal influenza vaccination is recommended for all pregnant individuals regardless of gestational age since maternal vaccination reduces maternal morbidity, enhances transplacental antibody transfer, and lowers infant influenza by 63% during the first months of life15,109,211,220. However, maternal antibody titers wane by 48% after eight weeks of life220 and current inactivated vaccines are ineffective in children younger than 6 months221, highlighting the need for novel age-appropriate platforms. Promising candidates such as a recombinant M2-based vaccines, which confer maternal and neonatal protection, are under development222.
Adjunctive and investigational strategies are emerging, including TLR4 agonists demonstrating preclinical protection in trials, host-targeted drugs being repurposed as antiviral countermeasures190, and neutralizing antibodies, engineered nanobodies, and inhibitors of haemagglutinin or neuraminidase offering broad-strain therapeutic coverage223,224. Clinical data for infants under 1 year, particularly children prenatally exposed to influenza, are scarce. Future strategies must address this gap with coordinated advances in maternal immunization, infant-specific vaccines, and age-appropriate therapeutics tailored for early infancy.
Concluding remarks and outlook
Although the short-term effects of prenatal viral infections, including fetal loss, preterm birth, and congenital anomalies, are increasingly recognized, the long-term consequences for both maternal and offspring health remain poorly defined. It is becoming clear that viral exposures during gestation can alter immune programming and impact neurodevelopmental trajectories, with outcomes that may only be apparent years later. Recent work has highlighted maternal immune activation, particularly dysregulation of cytokines involving IL-6, TNF-α, and IFN-γ, as a key mechanism linking in utero infection to postnatal disorders, including autism spectrum disorder, attention-deficit/hyperactivity disorder (ADHD), and autoimmune conditions225. However, the specific pathways involved, and their developmental windows of vulnerability, remain incompletely understood. At the same, progress in identifying maternal-fetal genetic and immunological determinants of susceptibility is beginning to shed light on which pregnancies may be most at risk of vertical transmission or long-term sequelae. Variants in host genes, including HLA alleles, interferon signaling pathways, or viral entry receptors, are emerging important modifiers of host-pathogen interactions. Nevertheless, translating these insights into clinical practice will require well-powered, longitudinal cohort studies capable of integrating genetic, immunological, environmental, and behavioral data across diverse populations. Another area poised for advancement is the development of targeted therapeutics and diagnostics tailored for use in pregnancy, a context historically excluded from many clinical trials. Immune modulating therapies226, immune cell-based approaches227,228, and long-acting small-molecule antivirals218,229,230 are promising, but require rigorous evaluation for safety and efficacy. Similarly, non-invasive tools such as maternal blood-based biomarkers or fetal imaging could support early risk stratification and timely intervention. Yet, the deployment of such strategies remain limited, particularly in low-resource settings where prenatal care and viral surveillance infrastructure are often lacking195. Given the complexity of transplacental antibody transfer209, specific antibody properties, placental Fc receptor expression, and fetal sex should be integrated into the design of next-generation vaccines and immunotherapies to ensure broad and effective protection across gestational ages and populations.
As the field moves forward, key challenges remain: (i) how to disentangle the complex interplay between viral exposure, maternal immunity, and fetal development; (ii) how to design interventions that are both effective and equitable; and (iii) how to prepare for future viral threats with the potential for transplacental transmission. Ultimately, a better understanding of the virus-specific mechanisms to perturb hosts’ immunological and developmental consequences during prenatal viral infections will be essential to guide maternal-fetal care strategies and reduce the long-term burden of congenital viral infections on global health.
Data Availability
No datasets were generated or analysed in this work.
References
Hcini, N. et al. Arboviruses and pregnancy: are the threats visible or hidden? Trop. Dis. Travel Med Vaccines 10, 4 (2024).
Rogan, S. C. & Beigi, R. H. Management of Viral Complications of Pregnancy: Pharmacotherapy to Reduce Vertical Transmission. Obstet. Gynecol. Clin. North Am. 48, 53–74 (2021).
Smith, S. E., Li, J., Garbett, K., Mirnics, K. & Patterson, P. H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702 (2007).
Lipina, T. V., Zai, C., Hlousek, D., Roder, J. C. & Wong, A. H. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J. Neurosci. 33, 7654–7666 (2013).
Brown, A. S. & Meyer, U. Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. Am. J. Psychiatry 175, 1073–1083 (2018).
Dude, A. M., Jones, M. & Wilson, T. Human Immunodeficiency Virus in Pregnancy. Obstet. Gynecol. Clin. North Am. 50, 389–399 (2023).
Cerveny, L., Murthi, P. & Staud, F. HIV in pregnancy: Mother-to-child transmission, pharmacotherapy, and toxicity. Biochim Biophys. Acta Mol. Basis Dis. 1867, 166206 (2021).
Dirajlal-Fargo, S. et al. HIV-exposed-uninfected infants have increased inflammation and monocyte activation. Aids 33, 845–853 (2019).
Johann-Liang, R., Cervia, J. & Noel, G. J. Serum levels of Interleukin-6 (IL-6) and failing to thrive (FTT) in children infected with human immunodeficiency virus (HIV). ♦ 719. Pediatr. Res. 41, 122–122 (1997).
Elgin, T. G. et al. The changing landscape of SARS-CoV-2: Implications for the maternal-infant dyad. J. Neonatal Perinat. Med 13, 293–305 (2020).
Gee, S. et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 22, 1490–1502 (2021).
Olsen, E. O. et al. SARS-CoV-2 infections among neonates born to pregnant people with SARS-CoV-2 infection: Maternal, pregnancy and birth characteristics. Paediatr. Perinat. Epidemiol. 36, 476–484 (2022).
Wei, S. Q., Bilodeau-Bertrand, M., Liu, S. & Auger, N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193, E540–E548 (2021).
Foo, S. S. et al. The systemic inflammatory landscape of COVID-19 in pregnancy: Extensive serum proteomic profiling of mother-infant dyads with in utero SARS-CoV-2. Cell Rep. Med 2, 100453 (2021).
Grohskopf, L. A., Ferdinands, J. M., Blanton, L. H., Broder, K. R. & Loehr, J. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2024-25 Influenza Season. 1–17 (US Department of Health and Human Services, Centers for Disease Control and Prevention, 2024).
Selten, J. P. & Morgan, V. A. The evidence of influenza A virus infection during pregnancy as a risk factor for neuropsychiatric disorder in offspring. Mol. Psychiatry 30, 3835–3836 (2025).
Lewis, S. H., Reynolds-Kohler, C., Fox, H. E. & Nelson, J. A. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet 335, 565–568 (1990).
Johnson, E. L. & Chakraborty, R. HIV-1 at the placenta: immune correlates of protection and infection. Curr. Opin. Infect. Dis. 29, 248–255 (2016).
Johnson, E. L. & Chakraborty, R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 9, 101 (2012).
Fenizia, C. et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 11, 5128 (2020).
Lucot-Royer, L. et al. Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection. Sci. Rep. 14, 3458 (2024).
Liong, S. et al. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc. Natl. Acad. Sci. USA 117, 24964–24973 (2020).
Machiavelli, A., Duarte, R. T. D., Pires, M. M. S., Zarate-Blades, C. R. & Pinto, A. R. The impact of in utero HIV exposure on gut microbiota, inflammation, and microbial translocation. Gut Microbes 10, 599–614 (2019).
Vyas, P. et al. Impact of HIV status on systemic inflammation during pregnancy. AIDS 35, 2259–2268 (2021).
Barral, M. F. et al. Risk factors of HIV-1 vertical transmission (VT) and the influence of antiretroviral therapy (ART) in pregnancy outcome. Rev. Inst. Med Trop. Sao Paulo 56, 133–138 (2014).
Yang, L., Cambou, M. C. & Nielsen-Saines, K. The End Is in Sight: Current Strategies for the Elimination of HIV Vertical Transmission. Curr. HIV/AIDS Rep. 20, 121–130 (2023).
Mertz, D. et al. Pregnancy as a risk factor for severe outcomes from influenza virus infection: A systematic review and meta-analysis of observational studies. Vaccine 35, 521–528 (2017).
Cambou, M. C. et al. Time series analysis of comprehensive maternal deaths in Brazil during the COVID-19 pandemic. Sci. Rep. 14, 23960 (2024).
Racicot, K. & Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Invest 127, 1591–1599 (2017).
Foo, S. S. et al. Asian Zika virus strains target CD14(+) blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat. Microbiol 2, 1558–1570 (2017).
Rudolph, M. D. et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 21, 765–772 (2018).
Lv, T., Cao, W. & Li, T. HIV-related immune activation and inflammation: current understanding and strategies. J. Immunol. Res. 2021, 7316456 (2021).
Ben Haij, N. et al. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-κB Pathway. PLoS One 10, e0129425 (2015).
Nabel, G. & Baltimore, D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326, 711–713 (1987).
Cocker, A. T. H. et al. Pregnancy Gestation Impacts on HIV-1-Specific Granzyme B Response and Central Memory CD4 T Cells. Front Immunol. 11, 153 (2020).
Levy, J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 57, 183–289 (1993).
Blanche, S. Mini review: Prevention of mother-child transmission of HIV: 25 years of continuous progress toward the eradication of pediatric AIDS? Virulence 11, 14–22 (2020).
Rouzioux, C. et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. Am. J. Epidemiol. 142, 1330–1337 (1995).
Diwan, B., Saxena, R. & Tiwari, A. HIV-2 and its role in conglutinated approach towards Acquired Immunodeficiency Syndrome (AIDS) Vaccine Development. Springerplus 2, 7 (2013).
Peruski, A. H. et al. Trends in HIV-2 Diagnoses and Use of the HIV-1/HIV-2 Differentiation Test - United States, 2010-2017. MMWR Morb. Mortal. Wkly Rep. 69, 63–66 (2020).
Lathrop, E., Jamieson, D. J. & Danel, I. HIV and maternal mortality. Int J. Gynaecol. Obstet. 127, 213–215 (2014).
Ter Schiphorst, E., Hansen, K. C., Holm, M. & Honge, B. L. Mother-to-child HIV-2 transmission: comparison with HIV-1 and evaluation of factors influencing the rate of transmission. A systematic review. Trans. R. Soc. Trop. Med Hyg. 116, 399–408 (2022).
Marazzi, M. C. et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS 25, 1611–1618 (2011).
Sundar, K. G. et al. Prompt Initiation of Maternal Antiretroviral Therapy After HIV Seroconversion in Pregnancy Effectively Prevents Vertical Transmission and Other Adverse Infant Outcomes. Pediatr. Infect. Dis. J. 44, 40–43 (2025).
Shinar, S. et al. Perinatal outcomes in women living with HIV-1 and receiving antiretroviral therapy-a systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 101, 168–182 (2022).
Grosch-Woerner, I. et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1-infected women. HIV Med 9, 6–13 (2008).
Akoto, C., Norris, S. A. & Hemelaar, J. Maternal HIV infection is associated with distinct systemic cytokine profiles throughout pregnancy in South African women. Sci. Rep. 11, 10079 (2021).
Kalk, E. et al. Placental pathology in HIV infection at term: a comparison with HIV-uninfected women. Trop. Med Int Health 22, 604–613 (2017).
Maswime, S., Pule, C., Mtshali, Z., Chawana, R. & Matjila, M. HIV, Placental Lesions, and Adverse Perinatal Outcomes. J. Infect. Dis. 224, S691–S693 (2021).
Ikumi, N. M., Matjila, M., Gray, C. M., Anumba, D. & Pillay, K. Placental pathology in women with HIV. Placenta 115, 27–36 (2021).
Chi, B. H. et al. Acute and chronic chorioamnionitis and the risk of perinatal human immunodeficiency virus-1 transmission. Am. J. Obstet. Gynecol. 194, 174–181 (2006).
Goulding, A. N. et al. Antiretroviral Therapy in Pregnancy: A 2023 Review of the Literature. Curr. HIV/AIDS Rep. 21, 1–10 (2024).
Zash, R. et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N. Engl. J. Med 381, 827–840 (2019).
Shivakoti, R. et al. Inflammation and micronutrient biomarkers predict clinical HIV treatment failure and incident active TB in HIV-infected adults: a case-control study. BMC Med 16, 161 (2018).
Mikyas, Y. et al. Immunologic activation during pregnancy: serial measurement of lymphocyte phenotype and serum activation molecules in HIV-infected and uninfected women. J. Reprod. Immunol. 33, 157–170 (1997).
Kolte, L. et al. Dysregulation of CD4+CD25+CD127lowFOXP3+ regulatory T cells in HIV-infected pregnant women. Blood 117, 1861–1868 (2011).
Hygino, J. et al. The impact of pregnancy on the HIV-1-specific T cell function in infected pregnant women. Clin. Immunol. 145, 177–188 (2012).
Lobato, A. C. et al. HIV-1 RNA detection in the amniotic fluid of HIV-infected pregnant women. Mem. Inst. Oswaldo Cruz 105, 720–721 (2010).
Farzin, A., Boyer, P., Ank, B., Nielsen-Saines, K. & Bryson, Y. Amniotic fluid exhibits an innate inhibitory activity against HIV type 1 replication in vitro. AIDS Res Hum. Retroviruses 29, 77–83 (2013).
Transmission, P. o. T. o. H. D. P. a. P. o. P. Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States. (2025).
Shiau, S. et al. Unique Profile of Inflammation and Immune Activation in Pregnant People With HIV in the United States. J. Infect. Dis. 227, 720–730 (2023).
Yu, W., Hu, X. & Cao, B. Viral Infections During Pregnancy: The Big Challenge Threatening Maternal and Fetal Health. Matern Fetal Med 4, 72–86 (2022).
Graham, S. M. Prophylaxis against Pneumocystis carinii pneumonia for HIV-exposed infants in Africa. Lancet 360, 1966–1968 (2002).
le Roux, S. M. et al. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. Lancet Child Adolesc. Health 3, 234–244 (2019).
Nazziwa, J. et al. Higher HIV-1 evolutionary rate is associated with cytotoxic T lymphocyte escape mutations in infants. J. Virol. 98, e0007224 (2024).
Ruck, C., Reikie, B. A., Marchant, A., Kollmann, T. R. & Kakkar, F. Linking Susceptibility to Infectious Diseases to Immune System Abnormalities among HIV-Exposed Uninfected Infants. Front Immunol. 7, 310 (2016).
Abange, W. B. et al. Alteration of the gut fecal microbiome in children living with HIV on antiretroviral therapy in Yaounde, Cameroon. Sci. Rep. 11, 7666 (2021).
Ludwig, S. & Zarbock, A. Coronaviruses and SARS-CoV-2: A Brief Overview. Anesth. Analg. 131, 93–96 (2020).
Villar, J. et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 175, 817–826 (2021).
Metz, T. D. et al. Association of SARS-CoV-2 Infection With Serious Maternal Morbidity and Mortality From Obstetric Complications. Jama 327, 748–759 (2022).
Serrano, B. et al. Confirmation of preeclampsia-like syndrome induced by severe COVID-19: an observational study. Am. J. Obstet. Gynecol. MFM 5, 100760 (2023).
Aagaard, K. M. & Shamshirsaz, A. A. Lingering impact: Maternal SARS-CoV-2 infection during early pregnancy results in fetal situs inversus. Med 5, 1338–1339 (2024).
Yokosaki, Y., Tanaka, K., Higashikawa, F., Yamashita, K. & Eboshida, A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 24, 418–427 (2005).
de Montalvao Franca, A. P. F. et al. Clinical characteristics of pregnant women with COVID-19 and infection outcomes in one of the largest cities in the Brazilian Amazon. BMC Infect. Dis. 24, 1175 (2024).
Vivanti, A. J. et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 11, 3572 (2020).
Mukhra, R., Krishan, K. & Kanchan, T. Possible modes of transmission of Novel coronavirus SARS-CoV-2: a review. Acta Biomed. 91, e2020036 (2020).
Ocansey, S. et al. Ocular Symptoms of SARS-CoV-2: Indication of Possible Ocular Transmission or Viral Shedding. Ocul. Immunol. Inflamm. 28, 1269–1279 (2020).
Azamor, T. et al. Transplacental SARS-CoV-2 protein ORF8 binds to complement C1q to trigger fetal inflammation. EMBO J. https://doi.org/10.1038/s44318-024-00260-9 (2024).
Barrero-Castillero, A. et al. COVID-19: neonatal-perinatal perspectives. J. Perinatol. 41, 940–951 (2021).
Garcia-Flores, V. et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 13, 320 (2022).
Gao, L. et al. Single-cell analysis reveals transcriptomic and epigenomic impacts on the maternal–fetal interface following SARS-CoV-2 infection. Nat. Cell Biol. 25, 1047–1060 (2023).
Crovetto, F. et al. Nasopharyngeal microbiota profiling of pregnant women with SARS-CoV-2 infection. Sci. Rep. 12, 13404 (2022).
Juarez-Castelan, C. J. et al. The Entero-Mammary Pathway and Perinatal Transmission of Gut Microbiota and SARS-CoV-2. Int J. Mol. Sci. https://doi.org/10.3390/ijms231810306 (2022).
Benny, M. et al. Maternal SARS-CoV-2, Placental Changes and Brain Injury in 2 Neonates. Pediatrics https://doi.org/10.1542/peds.2022-058271 (2023).
Cervia, C. et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 147, 545–557 e549 (2021).
Bordt, E. A. et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med 13, eabi7428 (2021).
Rubio, R. et al. Maternal and neonatal immune response to SARS-CoV-2, IgG transplacental transfer and cytokine profile. Front Immunol. 13, 999136 (2022).
Zhang, D. et al. Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge. J. Korean Med Sci. 38, e120 (2023).
Upadhyay, V. et al. Mild SARS-CoV-2 infection results in long-lasting microbiota instability. mBio 14, e0088923 https://doi.org/10.1128/mbio.00889-23 (2023).
Ignatyeva, O. et al. The crossover effect of COVID-19 in pregnancy on the infant microbiome. Front Microbiol 16, 1569279 (2025).
Edlow, A. G., Castro, V. M., Shook, L. L., Kaimal, A. J. & Perlis, R. H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw. Open 5, e2215787 (2022).
Jin, J. C. et al. SARS CoV-2 detected in neonatal stool remote from maternal COVID-19 during pregnancy. Pediatr. Res 93, 1375–1382 (2023).
Fajardo Martinez, V. et al. Neuromotor repertoires in infants exposed to maternal COVID-19 during pregnancy: a cohort study. BMJ Open 13, e069194 (2023).
Firestein, M. R. et al. Assessment of Neurodevelopment in Infants With and Without Exposure to Asymptomatic or Mild Maternal SARS-CoV-2 Infection During Pregnancy. JAMA Netw. Open 6, e237396 (2023).
Man, O. M. et al. Respiratory distress in SARS-CoV-2 exposed uninfected neonates followed in the COVID Outcomes in Mother-Infant Pairs (COMP) Study. Nat. Commun. 15, 399 (2024).
Fajardo-Martinez, V. et al. Neurodevelopmental delay in children exposed to maternal SARS-CoV-2 in-utero. Sci. Rep. 14, 11851 (2024).
de Moraes, F. M. et al. SARS-CoV-2 Infection and Possible Neonatal Neurological Outcomes: A Literature Review. Viruses https://doi.org/10.3390/v14051037 (2022).
Uyeki, T. M., Hui, D. S., Zambon, M., Wentworth, D. E. & Monto, A. S. Influenza. Lancet 400, 693–706 (2022).
Oseghale, O. et al. Influenza Virus Infection during Pregnancy as a Trigger of Acute and Chronic Complications. Viruses https://doi.org/10.3390/v14122729 (2022).
Shi, L., Tu, N. & Patterson, P. H. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J. Dev. Neurosci. 23, 299–305 (2005).
Liong, S. et al. Influenza A virus infection during pregnancy causes immunological changes in gut-associated lymphoid tissues of offspring mice. Am. J. Physiol. Gastrointest. Liver Physiol. 325, G230–G238 (2023).
Otero, A. M. et al. Influenza A virus during pregnancy disrupts maternal intestinal immunity and fetal cortical development in a dose- and time-dependent manner. Mol. Psychiatry 30, 13–28 (2025).
Laake, I. et al. Risk of pregnancy complications and adverse birth outcomes after maternal A(H1N1)pdm09 influenza: a Norwegian population-based cohort study. BMC Infect. Dis. 18, 525 (2018).
Rose, A. M. C. et al. Vaccine effectiveness against influenza A(H3N2) and B among laboratory-confirmed, hospitalised older adults, Europe, 2017-18: A season of B lineage mismatched to the trivalent vaccine. Influenza Other Respir. Viruses 14, 302–310 (2020).
de Jong, M. D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med 12, 1203–1207 (2006).
Kepinska, A. P. et al. Schizophrenia and Influenza at the Centenary of the 1918-1919 Spanish Influenza Pandemic: Mechanisms of Psychosis Risk. Front Psychiatry 11, 72 (2020).
Short, S. J. et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol. Psychiatry 67, 965–973 (2010).
Jacobsen, H. et al. Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat. Commun. 12, 4957 (2021).
Olson, S. M., Dawood, F. S., Grohskopf, L. A. & Ellington, S. Preventing Influenza Virus Infection and Severe Influenza Among Pregnant People and Infants. J. Women’s. Health (Larchmt.) 33, 1591–1598 (2024).
Wu, X. et al. Viral Mimicry of Interleukin-17A by SARS-CoV-2 ORF8. mBio 13, e0040222. https://doi.org/10.1128/mbio.00402-22 (2022).
Albini, A. et al. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med 2, 1371–1375 (1996).
Palmeira, P., Quinello, C., Silveira-Lessa, A. L., Zago, C. A. & Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 985646 (2012).
Tang, Y. et al. Endogenous Retroviral Envelope Syncytin Induces HIV-1 Spreading and Establishes HIV Reservoirs in Placenta. Cell Rep. 30, 4528–4539 e4524 (2020).
Kinder, J. M., Stelzer, I. A., Arck, P. C. & Way, S. S. Immunological implications of pregnancy-induced microchimerism. Nat. Rev. Immunol. 17, 483–494 (2017).
Fazely, F. et al. Simian immunodeficiency virus infection via amniotic fluid: a model to study fetal immunopathogenesis and prophylaxis. J. Acquir Immune Defic. Syndr. (1988) 6, 107–114 (1993).
Bunders, M. J. et al. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood 120, 4383–4390 (2012).
Gupta, S. et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 9, e1003776 (2013).
Lagaye, S. et al. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 75, 4780–4791 (2001).
Matala, E., Hahn, T., Yedavalli, V. R. & Ahmad, N. Biological characterization of HIV type 1 envelope V3 regions from mothers and infants associated with perinatal transmission. AIDS Res Hum. Retroviruses 17, 1725–1735 (2001).
Ahmad, N. Molecular mechanisms of HIV-1 mother-to-child transmission and infection in neonatal target cells. Life Sci. 88, 980–986 (2011).
Peta, K., Matjila, M. & Ikumi, N. The Complex Role of CD8+ T Cells in Placental HIV Infection. Eur. J. Immunol. 55, e51615 (2025).
Johnson, E. L., Chu, H., Byrareddy, S. N., Spearman, P. & Chakraborty, R. Placental Hofbauer cells assemble and sequester HIV-1 in tetraspanin-positive compartments that are accessible to broadly neutralizing antibodies. J. Int AIDS Soc. 18, 19385 (2015).
Marichannegowda, M. H. et al. Different evolutionary pathways of HIV-1 between fetus and mother perinatal transmission pairs indicate unique immune selection in fetuses. Cell Rep. Med 2, 100315 (2021).
Lei, J., Yin, X., Shang, H. & Jiang, Y. IP-10 is highly involved in HIV infection. Cytokine 115, 97–103 (2019).
Bebell, L. M. et al. Distinct cytokine profiles in late pregnancy in Ugandan people with HIV. Sci. Rep. 14, 10980 (2024).
Santer, D. M. et al. Interferon-λ treatment accelerates SARS-CoV-2 clearance despite age-related delays in the induction of T cell immunity. Nat. Commun. 13, 6992 (2022).
Rowe, S. L. et al. COVID-19 Vaccination During Pregnancy and Major Structural Birth Defects. Pediatrics https://doi.org/10.1542/peds.2024-069778 (2025).
Cambou, M. C. et al. Longitudinal Evaluation of Antibody Persistence in Mother-Infant Dyads After Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy. J. Infect. Dis. 227, 236–245 (2023).
Murphy, E. A. et al. SARS-CoV-2 vaccination, booster, and infection in pregnant population enhances passive immunity in neonates. Nat. Commun. 14, 4598 (2023).
Littauer, E. Q. et al. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog. 13, e1006757 (2017).
Bollati, M. et al. Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antivir. Res 87, 125–148 (2010).
Fu, X. Y., Schindler, C., Improta, T., Aebersold, R. & Darnell, J. E. Jr. The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc. Natl. Acad. Sci. USA 89, 7840–7843 (1992).
Feng, K. et al. Interactome profiling reveals interaction of SARS-CoV-2 NSP13 with host factor STAT1 to suppress interferon signaling. J. Mol. Cell Biol. 13, 760–762 (2021).
Casazza, R. L., Philip, D. T. & Lazear, H. M. Interferon Lambda Signals in Maternal Tissues to Exert Protective and Pathogenic Effects in a Gestational Stage-Dependent Manner. mBio 13, e03857–03821 (2022).
Mann, J. K. et al. Ability of HIV-1 Nef to downregulate CD4 and HLA class I differs among viral subtypes. Retrovirology 10, 100 (2013).
Schwartz, O., Marechal, V., Le Gall, S., Lemonnier, F. & Heard, J. M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med 2, 338–342 (1996).
Kumar, A. et al. SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J. Virol. 95, e0026621 (2021).
Shirato, K. & Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 7, e06187 (2021).
Khan, S. et al. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife https://doi.org/10.7554/eLife.68563 (2021).
Walls, A. C. et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181, 281–292.e286 (2020).
Ichinohe, T., Pang, I. K. & Iwasaki, A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11, 404–410 (2010).
Human Immunodeficiency Viruses and Human T-Cell Lymphotropic Viruses. IARC Monogr. Eval. Carcinog. Risks Hum. 67, 1−27 (1996).
Goulder, P. J. et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412, 334–338 (2001).
Wellensiek, B. P., Sundaravaradan, V., Ramakrishnan, R. & Ahmad, N. Molecular characterization of the HIV-1 gag nucleocapsid gene associated with vertical transmission. Retrovirology 3, 21 (2006).
Permar, S. R. et al. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J. Clin. Invest 125, 2702–2706 (2015).
Rossi, P. et al. Presence of maternal antibodies to human immunodeficiency virus 1 envelope glycoprotein gp120 epitopes correlates with the uninfected status of children born to seropositive mothers. Proc. Natl. Acad. Sci. USA 86, 8055–8058 (1989).
Stove, V. et al. Signaling but not trafficking function of HIV-1 protein Nef is essential for Nef-induced defects in human intrathymic T-cell development. Blood 102, 2925–2932 (2003).
Debaisieux, S., Rayne, F., Yezid, H. & Beaumelle, B. The ins and outs of HIV-1 Tat. Traffic 13, 355–363 (2012).
Khan, N. & Geiger, J. D. Role of Viral Protein U (Vpu) in HIV-1 Infection and Pathogenesis. Viruses https://doi.org/10.3390/v13081466 (2021).
Marino, J. et al. Functional impact of HIV-1 Tat on cells of the CNS and its role in HAND. Cell Mol. Life Sci. 77, 5079–5099 (2020).
Borrajo, A., Spuch, C., Penedo, M. A., Olivares, J. M. & Agis-Balboa, R. C. Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann. Med 53, 43–69 (2021).
Gaskill, P. J., Miller, D. R., Gamble-George, J., Yano, H. & Khoshbouei, H. HIV, Tat and dopamine transmission. Neurobiol. Dis. 105, 51–73 (2017).
Cho, S. B. et al. Tat‑aldose reductase prevents dopaminergic neuronal cell death by inhibiting oxidative stress and MAPK activation. Int J. Mol. Med 47, 751–760 (2021).
Dara, J. et al. Multivariable analysis to determine if HIV-1 Tat dicysteine motif is associated with neurodevelopmental delay in HIV-infected children in Malawi. Behav. Brain Funct. 11, 38 (2015).
Yadav-Samudrala, B. J., Yadav, A. P., Patel, R. P. & Fitting, S. HIV-1 Tat protein alters medial prefrontal cortex neuronal activity and recognition memory. iScience 28, 112075 (2025).
Zhao, X. et al. Long-term HIV-1 Tat Expression in the Brain Led to Neurobehavioral, Pathological, and Epigenetic Changes Reminiscent of Accelerated Aging. Aging Dis. 11, 93–107 (2020).
Flower, T. G. et al. Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.2021785118 (2021).
Zheng, M. et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 22, 829–838 (2021).
Koutsakos, M. et al. Downregulation of MHC Class I Expression by Influenza A and B Viruses. Front Immunol. 10, 1158 (2019).
Krug, R. M. & Aramini, J. M. Emerging antiviral targets for influenza A virus. Trends Pharmacol. Sci. 30, 269–277 (2009).
Leveque, L. & Khosrotehrani, K. Can maternal microchimeric cells influence the fetal response toward self antigens?Chimerism 2, 71–77 (2011).
Becker, Y. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers—a review and hypothesis. Virus Genes 28, 5–18 (2004).
Djoba Siawaya, J. F. & Maloupazoa Siawaya, A. C. HIV-exposed uninfected infants immune defects: From pathogen sensing, oxidative burst to antigens responses. iScience 26, 108427 (2023).
Brito-Perez, Y. et al. Impaired T helper cell responses in human immunodeficiency virus-exposed uninfected newborns. Immun. Inflamm. Dis. 9, 1541–1553 (2021).
van der Ploeg, E. K. et al. Type-2 CD8(+) T-cell formation relies on interleukin-33 and is linked to asthma exacerbations. Nat. Commun. 14, 5137 (2023).
Sevenoaks, T. et al. Association of maternal and infant inflammation with neurodevelopment in HIV-exposed uninfected children in a South African birth cohort. Brain Behav. Immun. 91, 65–73 (2021).
Bender, J. M. et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci. Transl. Med 8, 349ra100 (2016).
McGann, C. M. et al. Effect of maternal HIV status on the early neonatal microbiome. J. Pediatr. Infect. Dis. Soc. https://doi.org/10.1093/jpids/piaf076 (2025).
Chandiwana, P. et al. Antenatal gut microbiome profiles and effect on pregnancy outcome in HIV infected and HIV uninfected women in a resource limited setting. BMC Microbiol 23, 4 (2023).
Leftwich, H. K. et al. The microbiota of pregnant women with SARS-CoV-2 and their infants. Microbiome 11, 141 (2023).
Gaulke, C. A. et al. Maternal vaccination partially protects piglets against influenza A virus associated alteration of the microbiome and hippocampal gene expression. Vet. Microbiol 306, 110544 (2025).
Slyker, J. A. et al. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in Kenyan infants during the first year of life. Front Immunol. 3, 399 (2012).
Jackson, C. L. et al. Evolution of the Gut Microbiome in HIV-Exposed Uninfected and Unexposed Infants during the First Year of Life. mBio 13, e0122922 (2022).
Grant-Beurmann, S. et al. Dynamics of the infant gut microbiota in the first 18 months of life: the impact of maternal HIV infection and breastfeeding. Microbiome 10, 61 (2022).
Packer, C. H. et al. Maternal-fetal cytokine profiles in acute SARS-CoV-2 breakthrough infection after COVID-19 vaccination. Front Immunol. 15, 1506203 (2024).
Chen, C. C., Huang, J. L., Chang, C. J. & Kong, M. S. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J. Pediatr. Gastroenterol. Nutr. 55, 541–547 (2012).
Chou, J., Thomas, P. G. & Randolph, A. G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 23, 177–185 (2022).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Sanidad, K. Z. & Zeng, M. Y. Neonatal gut microbiome and immunity. Curr. Opin. Microbiol 56, 30–37 (2020).
Fatemi, S. H. et al. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol. Psychiatry 7, 633–640 (2002).
Marreiros, R. et al. Disruption of cellular proteostasis by H1N1 influenza A virus causes alpha-synuclein aggregation. Proc. Natl. Acad. Sci. USA 117, 6741–6751 (2020).
Spesock, A. et al. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 85, 7048–7058 (2011).
Yan, Y. et al. NS1 of H7N9 Influenza A Virus Induces NO-Mediated Cellular Senescence in Neuro2a Cells. Cell Physiol. Biochem 43, 1369–1380 (2017).
Bemark, M., Pitcher, M. J., Dionisi, C. & Spencer, J. Gut-associated lymphoid tissue: a microbiota-driven hub of B cell immunity. Trends Immunol. 45, 211–223 (2024).
Kim, Y. S. et al. Mulberry Component Kuwanon C Exerts Potent Therapeutic Efficacy In Vitro against COVID-19 by Blocking the SARS-CoV-2 Spike S1 RBD:ACE2 Receptor Interaction. Int J. Mol. Sci. https://doi.org/10.3390/ijms232012516 (2022).
Mediouni, S. et al. Identification of potent small molecule inhibitors of SARS-CoV-2 entry. SLAS Discov. 27, 8–19 (2022).
Wang, L. et al. Discovery of potential small molecular SARS-CoV-2 entry blockers targeting the spike protein. Acta Pharm. Sin. 43, 788–796 (2022).
Puhl, A. C. et al. Computational and Experimental Approaches Identify Beta-Blockers as Potential SARS-CoV-2 Spike Inhibitors. ACS Omega 7, 27950–27958 (2022).
Cunningham, C. K. et al. Safety, Tolerability, and Pharmacokinetics of Long-Acting Broadly Neutralizing HIV-1 Monoclonal Antibody VRC07-523LS in Newborn Infants Exposed to HIV-1. J. Pediatr. Infect. Dis. Soc. https://doi.org/10.1093/jpids/piaf002 (2025).
Beigel, J. H. et al. Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference. Antivir. Res 167, 45–67 (2019).
Zikos, J. et al. FcRn-enhancing mutations lead to increased and prolonged levels of the HIV CCR5-blocking monoclonal antibody leronlimab in the fetuses and newborns of pregnant rhesus macaques. MAbs 16, 2406788 (2024).
Hirsch, C. et al. SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19. Cochrane Database Syst. Rev. 6, CD014945 (2022).
Rau, C. et al. Treatment of Infants and Children With SARS-CoV-2 Monoclonal Antibodies: A European Case Series. Pediatr. Infect. Dis. J. 42, 125–129 (2023).
Chadwick, E. G. & Ezeanolue, E. E. & Committee On Pediatric, A. Evaluation and Management of the Infant Exposed to HIV in the United States. Pediatrics https://doi.org/10.1542/peds.2020-029058 (2020).
Penazzato, M. et al. Antiretroviral postnatal prophylaxis to prevent HIV vertical transmission: present and future strategies. J. Int AIDS Soc. 26, e26032 (2023).
Krist, A. H. & Crawford-Faucher, A. Management of newborns exposed to maternal HIV infection. Am. Fam. Physician 65, 2049–2056 (2002).
Jacobs, T. G., van Aerde, K. J., Colbers, A. & Burger, D. M. Raltegravir-based Postnatal HIV Prophylaxis Therapy in a Neonate After in Utero Dolutegravir Exposure. Pediatr. Infect. Dis. J. 41, 131–132 (2022).
Kilapandal Venkatraman, S. M., Sivanandham, R., Pandrea, I. & Apetrei, C. BCG Vaccination and Mother-to-Infant Transmission of HIV. J. Infect. Dis. 222, 1–3 (2020).
Wang, S. S., Yan, Y. S. & Ho, K. US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives. Antib. Ther. 4, 262–272 (2021).
Ko, S., Jo, M. & Jung, S. T. Recent Achievements and Challenges in Prolonging the Serum Half-Lives of Therapeutic IgG Antibodies Through Fc Engineering. BioDrugs 35, 147–157 (2021).
Diomede, L. et al. Passively transmitted gp41 antibodies in babies born from HIV-1 subtype C-seropositive women: correlation between fine specificity and protection. J. Virol. 86, 4129–4138 (2012).
Awan, S. F., Happe, M., Hofstetter, A. R. & Gama, L. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr. Opin. HIV AIDS 17, 247–257 (2022).
Willis, J. R. et al. Vaccination with mRNA-encoded nanoparticles drives early maturation of HIV bnAb precursors in humans. Science 389, eadr8382 (2025).
Xiao, Q., Guo, D. & Chen, S. Application of CRISPR/Cas9-Based Gene Editing in HIV-1/AIDS Therapy. Front Cell Infect. Microbiol 9, 69 (2019).
Wang, J. W., Liu, J. H. & Xun, J. J. CCR5 gene editing and HIV immunotherapy: current understandings, challenges, and future directions. Front Immunol. 16, 1590690 (2025).
Castelli, V. et al. Immune Checkpoint Inhibitors in People Living with HIV/AIDS: Facts and Controversies. Cells https://doi.org/10.3390/cells10092227 (2021).
Gubser, C., Chiu, C., Lewin, S. R. & Rasmussen, T. A. Immune checkpoint blockade in HIV. EBioMedicine 76, 103840 (2022).
Halasa, N. B. et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months - 17 States, July 2021-January 2022. MMWR Morb. Mortal. Wkly Rep. 71, 264–270 (2022).
Lopez, P. A. et al. Placental transfer dynamics and durability of maternal COVID-19 vaccine-induced antibodies in infants. iScience 27, 109273 (2024).
Halasa, N. B. et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N. Engl. J. Med 387, 109–119 (2022).
Male, V. & Jones, C. E. Vaccination in pregnancy to protect the newborn. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-025-01162-5 (2025).
Wessel, R. E. & Dolatshahi, S. Quantitative mechanistic model reveals key determinants of placental IgG transfer and informs prenatal immunization strategies. PLoS Comput Biol. 19, e1011109 (2023).
Garcia, H., Allende-Lopez, A., Morales-Ruiz, P., Miranda-Novales, G. & Villasis-Keever, M. A. COVID-19 in Neonates with Positive RT-PCR Test. Systematic Review. Arch. Med Res 53, 252–262 (2022).
Whitman, J. D. et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat. Biotechnol. 38, 1174–1183 (2020).
Amatya, S. et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J. Perinatol. 40, 987–996 (2020).
Conte, E. et al. Do Anti-SARS-CoV-2 Monoclonal Antibodies Have an Impact on Pregnancy Outcome? A Systematic Review and Meta-Analysis. Vaccines (Basel) https://doi.org/10.3390/vaccines11020344 (2023).
van de Veerdonk, F. L. et al. A guide to immunotherapy for COVID-19. Nat. Med 28, 39–50 (2022).
Lei, S., Chen, X., Wu, J., Duan, X. & Men, K. Small molecules in the treatment of COVID-19. Signal Transduct. Target Ther. 7, 387 (2022).
Santos, R. P. & Tristram, D. A practical guide to the diagnosis, treatment, and prevention of neonatal infections. Pediatr. Clin. North Am. 62, 491–508 (2015).
Alexander-Miller, M. A. Challenges for the Newborn Following Influenza Virus Infection and Prospects for an Effective Vaccine. Front Immunol. 11, 568651 (2020).
Suragh, T. A., Hibbs, B., Marquez, P. & McNeil, M. M. Age inappropriate influenza vaccination in infants less than 6 months old, 2010-2018. Vaccine 38, 3747–3751 (2020).
Sakala, I. G., Honda-Okubo, Y., Li, L., Baldwin, J. & Petrovsky, N. A M2 protein-based universal influenza vaccine containing Advax-SM adjuvant provides newborn protection via maternal or neonatal immunization. Vaccine 39, 5162–5172 (2021).
Verma, V., Sinha, N. & Raja, A. Nanoscale warriors against viral invaders: a comprehensive review of Nanobodies as potential antiviral therapeutics. MAbs 17, 2486390 (2025).
Bonomini, A., Mercorelli, B. & Loregian, A. Antiviral strategies against influenza virus: an update on approved and innovative therapeutic approaches. Cell Mol. Life Sci. 82, 75 (2025).
Kwon, H.-K., Choi, G. B. & Huh, J. R. Maternal inflammation and its ramifications on fetal neurodevelopment. Trends Immunol. 43, 230–244 (2022).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Huang, N., Chi, H. & Qiao, J. Role of Regulatory T Cells in Regulating Fetal-Maternal Immune Tolerance in Healthy Pregnancies and Reproductive Diseases. Front Immunol. 11, 1023 (2020).
Sakaguchi, S., Kawakami, R. & Mikami, N. Treg-based immunotherapy for antigen-specific immune suppression and stable tolerance induction: a perspective. Immunother. Adv. 3, ltad007 (2023).
Money, D. M. Antiviral and antiretroviral use in pregnancy. Obstet. Gynecol. Clin. North Am. 30, 731–749 (2003). vii.
Wang, D., Li, Z. & Liu, Y. An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics. J. Infect. Public Health 13, 1405–1414 (2020).
Acknowledgements
S.-S.F. was supported by the research grants from the Cleveland Clinic Foundation.
Author information
Authors and Affiliations
Contributions
G.M.S., Conceptualization, Data curation, Visualization, Writing - original draft, and Writing- review and editing; T.A., D.F.-M., C.O., M.C.C., K.N.-S., Writing- review and editing; W.C., Visualization and Writing – review and editing; S.-S.F., Conceptualization, Visualization, and Writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salem, G.M., Azamor, T., Familiar-Macedo, D. et al. Mechanistic insights into the impact of prenatal viral infections on maternal and offspring immunity. npj Viruses 4, 7 (2026). https://doi.org/10.1038/s44298-026-00174-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44298-026-00174-9