Abstract
While migratory cells can quickly change their mode of migration in complex three-dimensional environments, it is not clear why. Understanding the dynamic and reciprocal relationship migrating cells have with their microenvironments may help reveal why migratory plasticity, or mode-switching, is a common feature of eukaryotic cell motility. In this review, we discuss the physical and mechanical properties of cells and the environments they move through, and how those properties can influence each other. Given the dual role of the cytoskeleton in cell migration and cellular mechanics, we suggest that migratory plasticity derives from the necessity for the cell to maintain mechanical homeostasis in diverse physical environments.
Similar content being viewed by others
Introduction
Cells migrating within complex three-dimensional (3D) matrices are highly adaptable to their environment. Motile cells can undergo migratory plasticity, adjusting their own physical properties and structures in response to physical changes in the surrounding extracellular matrix (ECM). These changes can culminate in a switch in the fundamental mechanisms the cells use to propel themselves through 3D ECMs1. Classically, it was understood that cells could switch between a small number of discrete migration modes, such as the lamellipodia-based migration used for mesenchymal migration, and small blebs for ameboid forms of movement2. More recently, the identification of a cell’s migration mode based on the type of protrusion used has been challenged by the discovery that there is a continuum of migration modes, including cells that blend both lamellipodia and bleb-based protrusions3. Further, defining the state of a cell based on a single criterion, such as protrusion identity, may not be sufficient to fully describe the molecular basis of discrete modes of migration and how they change over time4. Thus, it remains a major challenge within the field of cell migration to understand why cells engage in migratory plasticity and how many different modes of 3D cell migration exist.
In response to cues from their physical environment, migratory cells can change the physical mechanisms they use to move, which is reminiscent of dynamic reciprocity. Coined by Dr. Mina Bissell and colleagues5, dynamic reciprocity predicts that there is a continuous, bi-directional crosstalk between the physical and chemical structure of the ECM and cellular gene expression. For example, mouse mammary epithelial cells grown on a rigid 2D surface do not readily express and secrete milk proteins. When the same cells are cultured on a soft 3D collagen matrix, they upregulate milk protein secretion, which is disrupted if the collagen matrix is stiffened by covalent crosslinking6. Further, malignant breast cancer cells revert to a more normal phenotype when cultured in 3D collagen gels by downregulating Raf/MEK/ERK signaling to reduce matrix metalloproteinase (MMP)-9 expression7. This downregulation likely prevents the cells from remodeling their physical environment via protease activity. Finally, the secretion of MMP-1 to degrade the ECM is essential during amphibian arm joint regeneration by enabling cell migration, proliferation, and differentiation8. Thus, dynamic reciprocity, or the cell’s ability to influence and be influenced by the ECM, is an essential component of normal cell and tissue function.
In this review, we first summarize the mechanical properties of extracellular matrices that can modulate the mode of 3D cell migration. Next, we will discuss the mechanical properties of migrating cells, which can be modified in response to the structure of the ECM. Finally, we highlight how the mechanical properties of the ECM and cells can crosstalk via dynamic reciprocity to affect migration mode decisions.
Mechanical properties of the ECM shaping 3D motility
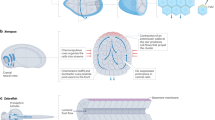
Several physical properties of the ECM can govern cell dynamics (Fig. 1). Namely, the simple confinement of a cell when constrained by two or more surfaces, complex confinement in fibrillar matrices, spatial organization of fibrillar matrices, matrix stiffness or rigidity, elastic or viscoelastic behavior, and the hydrostatic pressure of extracellular fluid.
Simple and complex confinement is the basis of 3D environments. Confinement can be induced by 2 or more surfaces and by the pores formed in a fibrillar matrix. Spatial organization, or the physical properties, of a fibrillar matrix adds more complexity for migrating cells. Alignment of fibers, thickness, and pore size are all important factors that affect migration in a fibrillar environment. The most elusive properties of a 3D environment are the mechanical properties. Mechanical properties are present at all levels of 3D environments.
Simple confinement
Historically, cell motility has been studied using two-dimensional (2D) plastic or glass surfaces, where the bottom of the cell is attached to matrix proteins coating the 2D surface, while the top and sides of the cell are surrounded by liquid9. Placing cells in 3D microenvironments that contact two or more of the cell’s surfaces is referred to as confined cell migration10.
Simple confinement occurs when an upper surface is present so that the top and bottom of the cell are in physical contact with the opposing surfaces11. A channel represents a slightly more complicated version of simple confinement, when the sides of the cells are similarly confined by two additional confining surfaces, orthogonal to the top and bottom planes. Simple confinement is commonly used to test how squeezing the cell top, bottom, and/or sides can change the migration mechanisms cells use to move12,13.
Complex confinement
In contrast to simple confinement, cells moving through a fibrillar protein matrix, such as type I collagen gels or cell-derived matrices (CDMs), experience complex confinement14. During complex confinement, the diverse geometry and mechanics of the fibrillar matrix can direct migratory plasticity with inputs that are not present in simple confinement. For example, a cell migrating through complex confinement will encounter a variety of pore sizes and channel diameters, thicker (bundled) and thinner matrices, aligned or unaligned matrix fibers, and covalently cross-linked fibrillar matrix proteins15. Together, these properties can also affect higher-order structural characteristics of the bulk material like rigidity and elasticity (Fig. 1). These interdependent physical characteristics of the fibrillar matrix can make it more difficult to understand the precise cause of the change in cell behavior. Confining fibrous matrices have been shown to trigger unique migration modes such as nuclear piston migration16,17 and unstable bleb migration18,19.
Hydrostatic pressure
The hydrostatic pressure of the extracellular fluid can affect cell migration in confined environments. As the cell moves through small channels, where water flow can be reduced or stopped, pressure from the fluid environment can push against the plasma membrane20. Cells can respond to hydrostatic pressure through at least two mechanisms. First, when presented with a choice between two paths of differential hydrostatic pressure, cells move towards the low-pressure environment via barotaxis21,22. Here, cells use protrusions to probe potential paths of migration21. During barotaxis, hydrostatic pressure is sensed by the mechanosensitive calcium channel TRPM7, which regulates cortical non-muscle myosin II (NMII) activity to direct the cell towards the path of least resistance22. Second, motile cells can adapt to elevated hydrostatic pressure by moving water from the front to the back of the cell to propel themselves through simple confinement13,23. While dendritic cells use macropinocytosis to transport water from the leading edge to the back of the cell24, sarcoma cells coordinate sodium/proton pumps such as NHE1, the SWELL1 anion channel, and the aquaporin 5 water channel to direct water from the front to the back of the cell25. The result of either mechanism is likely to reduce hydrostatic pressure on the cell membrane to allow for more efficient migration through confined channels and tissues.
Matrix rigidity
The relative stiffness or rigidity of the bulk ECM fibrillar network is a measure of the material’s ability to deform in response to an applied force. Rigid materials are less likely to deform in response to a force compared to a softer material. ECM rigidity can significantly impact cellular mechanics and behavior26,27, including during cancer progression28,29,30. Classically, a rigid matrix will activate actomyosin contractility within adhesive cells, leading to larger and stronger adhesions and more robust actin stress fibers31,32. A gradient of increasing rigidity within an ECM will trigger durotaxis, along with larger and stronger adhesions. Together, these responses can give rise to larger-scale changes in tissue behavior. Specifically, cancer progression and malignancy are often associated with stiffer, more cross-linked ECMs33, which promotes cancer cell invasion and tumor progression28,34,35. We speculate that a more flexible substrate may be harder to move across because the kinetic energy produced by the cell is spent on deforming the ECM instead of propelling the cell forward. In support of this speculation, breast cancer cells exhibit faster migration speeds on stiffer substrates, independent of the width of the channel28.
Matrix elasticity and viscoelasticity
Elasticity and viscoelasticity are an object’s time-dependent response to an applied force. Highly elastic materials will immediately return to their original shapes after a force is applied. Viscoelastic materials will return to their original shape over a longer period or will only partially return to their original shape36,37. How the ECM responds to cellular forces will likely affect how cells move through the material.
The viscoelastic nature of tissues varies across the human body. All tissues, including reconstituted ECM, soft, and stiff tissues, are viscoelastic. However, the stiffer skeletal tissues are more viscoelastic than the reconstituted ECM components by as much as a factor of ten37. Four mechanisms exist which influence a fibrous matrix’s viscoelasticity37,38,39: (1) fiber-fiber crosslinking38,39, (2) fiber entanglement, (3) protein denaturation, (4) fiber type37,38,39. We speculate that cells function optimally in an ECM with a specific range of viscoelasticity. For example, slow relaxing (more viscoelastic) hydrogels decreased mesenchymal stem cell migration speeds, but fast-relaxing (more elastic) hydrogels caused the cells to move faster40. Additionally, monocytes have improved migratory abilities when placed in stiff, fast-relaxing microenvironments41 and fibrosarcoma cells migrate faster when placed in more elastic microenvironments42. Viscoelasticity also influences migration mode decisions. In linearly elastic 3D matrices, fibroblasts use the lobopodial mode of migration; while in nonlinearly elastic matrices, fibroblasts switch to the lamellipodial mode of migration14,16.
Physical and mechanical properties of the cell
Like the ECM, cells can have unique and diverse physical properties. All the following are likely cell-type-dependent responses. The stiffness, elastic, and viscoelastic behavior of cells is diverse and depends on the type of cell and the type of environment that the cell is in36,37. Optimal cellular function is dependent on these mechanical properties staying within an ideal range26,36. Therefore, cells are likely managing mechanical homeostasis. Mechanical property changes are evident in pathological settings such as cancer progression43,44.
Cellular stiffness
Stiffness is a well-characterized mechanical property of a cell, which is mainly regulated by the cytoskeleton36. Vimentin intermediate filaments can regulate cellular stiffness and protect the cell from physical damage45,46. One way vimentin protects migratory cells is by shielding the nucleus from mechanical stress during 3D migration, which reduces nuclear rupture and the resulting DNA damage47. Interestingly, when vimentin expression is reduced, perinuclear stiffness decreases in fibroblasts47 and reduced vimentin expression in epithelial cells corresponds to a switch to ameboid migration48. The actin cytoskeleton is also a major contributor to cellular stiffness due to its numerous indirect connections to the ECM and the strain stiffening induced by actin motor proteins49,50,51,52. For example, cellular strain due to interactions with the physical environment, including matrix stiffness, compression, and stretching, can also make a cell stiffer by increasing the tension within the actin cytoskeleton49,50,53. However, stretching a whole cell, such as human airway smooth muscle cells, induces cellular softening54. Since each mode of migration uses a unique sub-population of NMII55, these findings suggest that certain modes of migration can stiffen specific regions of the actin cytoskeleton.
Cellular viscoelasticity
The viscoelastic nature of the cell is important to study because it affects many cellular functions, including mitosis, stem cell differentiation, and cancer progression50,56,57,58. At the cellular scale, changes to viscoelasticity, like strain stiffening, can occur when the cell is stretched or compressed53,59. These changes may partially be driven by physical changes within the cytoplasm since it has been shown to behave like a viscoelastic liquid60. This cytoplasmic viscoelasticity can help maintain organelle positioning, like in the mitotic spindle of dividing cells56. These findings suggest that at the cytoplasmic level, changes to viscoelasticity can passively govern cellular organization, which could be an important factor in how motile cells adjust to their physical environment.
The cytoskeleton also has viscoelastic properties. The actin cytoskeleton exhibits stress softening and stiffening behaviors when stretched and compressed61. These mechanical properties contribute to the cell’s mechanical features, which have been shown to have similar properties to glassy material when under strain62. Due to their viscoelasticity, intermediate filaments like vimentin and keratin maintain cellular mechanical homeostasis during stretching and compression46,47,63,64. As individual filaments, vimentin can dissipate up to 70% of large and fast deformations65, which is achieved through conformational changes to vimentin’s molecular structure as it is stretched66. Vimentin’s recovery post-stretch is not immediate and can result in viscoelastic strain stiffening behaviors in response to repeated stretching65,66. Individual filaments are organized as networks inside the cell to regulate the cell’s elasticity and are a major contributor to the cytoplasm’s toughness and resilience to deformations67. Cell-based experiments show that the keratin networks are resilient to large forces because of their extensibility and slow recovery to force loading68. Together, these findings suggest that the viscoelastic behavior of intermediate filament networks can be partially attributed to the viscoelasticity of individual filaments.
Importantly, the viscoelasticity of cells can drastically shift in disease. Some cancers become more viscous, while others become more elastic compared to their normal counterparts43,44,62,69. We speculate that these types of changes could correspond with cells using specific modes of 3D migration.
A cell’s viscoelasticity is influenced by the physical organization of the microenvironment and the mechanical cues it provides. Adenocarcinoma cells have reduced creep compliance and increased elasticity when plated in 3D collagen gels compared to 2D surfaces27. Blocking β1 integrin binding reverted the elasticity of cells in 3D back to the 2D phenotype. Further, when the concentration of collagen was increased, breast adenocarcinoma cells showed increases in stiffness and intracellular fluidization49. Together, these results indicate that the microenvironment can be a regulator of cellular mechanical properties by modulating the viscoelastic properties of the cytoskeleton.
Migratory modes in confinement
Cell migration is a complex function that requires adaptability to overcome physical difficulties within the microenvironment12,16,70. By using different modes of migration, called migratory plasticity, cells can achieve migration despite the difficult physical properties of the microenvironment. Migratory cells interact with their microenvironments in different ways depending on the mode of migration. Important factors for migration mode decisions are how adhesive a cell is to the ECM, determining where in the cell forces are produced and how much force is produced55, and how it navigates through an ECM. This can be categorized as path-creating, when the cell makes its own channels using proteolytic enzymes, or path finding, when the cell probes the porous ECM and chooses a permissible path71,72.
In this section, we will discuss migration modes, the types of microenvironments they are observed in, and which mechanical properties change when cells use those modes of migration (Table 1).
Lamellipodial migration, sometimes called mesenchymal, is powered by actin polymerization at the leading edge of the cell, which drives the membrane forward. The lamellipodium is made of a dense, branched network of actin filaments, which relies on actin organizing proteins like CDC42, Arp2/3, and Rac173. The trailing edge of the cell uses cortical actomyosin to aid in contraction. The rear retraction both pulls the cell membrane to the rest of the cell body and pushes the nucleus forward72,74. This mode of migration is often observed in cells migrating on flat, 2D surfaces, which are very stiff and inelastic42. Lamellipodial migration is also supported in 3D microenvironments such as type II collagen, cell-derived matrix, dermal explants, hydrogels, and confined microchannels14,18,75,76. Lamellipodial migration is supported by nonlinearly elastic matrices such as reconstituted collagen or cell-derived matrix14. Additionally, lamellipodial migration is only supported if the following conditions are met. (1) The cell must be able to adhere to the microenvironment74. (2) The nucleus is not excessively confined16. (3) The microenvironment is modifiable through degradation or deformation. Disruption to any of these parameters has been shown to reduce migratory ability but, in some cases, can induce migration mode changes18,70.
Lobopodial migration is characterized by blunt, cylindrical protrusions called lobopodia and a generally uniaxial cell body14,16. Lobopodia are formed by increased cytoplasmic pressure due to at least two distinct mechanisms: nuclear pulling and nuclear pushing16,17. The pulling mechanism uses the nucleus like a cork to create a leaky seal between the anterior and posterior of the cell16. The nucleus is pulled forward by a complex network of actomyosin, vimentin, tropomyosins, and plectin16,31,70,77,78. Pulling the nucleus forward then increases hydraulic pressure, which drives the protrusion forward. Due to the increased actomyosin contractility required for lobopodial migration, we speculate that lobopodial cells will exhibit increased stiffness within the actin and vimentin cytoskeletons, especially in the anterior of the cell. This mechanism is triggered by linearly elastic materials like cell-derived matrix and mammalian dermis14. Interestingly, the nuclear pulling mechanism is triggered in nonlinearly elastic materials like type I collagen if matrix metalloproteinase activity is inhibited16,70.
Stable bleb ameboid migration is characterized by a large leader bleb at the cell’s anterior, followed by a constricted bottle neck, and a rounded rear containing most of the cell’s organelles12,79. This mode of migration is powered by the retrograde flow of cortical F-actin in the leader bleb79,80. The retrograde flow generates friction between the cell and microenvironment to power cell movement80. Actomyosin contractility is necessary to create the retrograde flow of F-actin towards the rear of the cell79,80. Fascinatingly, this mode of migration is only observed in cells placed in highly constrictive environments with minimal cell-matrix adhesion12,48,79,80. The nucleus serves as a mechanosensor for constriction of the cell and can activate cortical actomyosin contractility to promote stable bleb migration79,81.
Unstable bleb ameboid is characterized by a rounded cell body with rapid plasma membrane blebbing at the periphery of the cell18,82. Cortical actomyosin contractility downstream of RhoA and ROCK activity is essential for this mode of migration19. This contractility increases the hydraulic pressure of the cytoplasm to create the small blebs and drive the leading edge of the cell forward. At the base of each bleb, adhesion-forming and mechanosensitive proteins like talin, vinculin, and integrins are enriched83. This mode of migration does not require pericellular proteolytic activity18,19,83. The unstable bleb ameboid has been observed in type I collagen and the Matrigel basement membrane extract18,19,82,84.
The osmotic engine is a unique mode of migration powered by water flow from the front of the cell through the back. The directional flow of water is controlled by polarized localization of ion channels, water channels, RhoA, and CDC4213,25,85. Active RhoA allows NHE1 and AQP5 at the front of the cell to bring ions into the cell, which are followed by water13. SWELL1 and AQP4 are localized to the back of the cell by CDC42 to help direct water out the back of the cell13,25. The osmotic engine still operates even when the actin cytoskeleton is completely depolymerized and NMII activity is inhibited13. These findings suggest that cells using the osmotic engine will be quite soft compared to other motile cells. This mode of migration has mainly been observed in microchannels coated with adhesive molecules like type I collagen and fibronectin13. Activation of the osmotic engine is possible using osmotic shock or potentially by increasing the viscosity of the extracellular fluid13,25,85.
Dynamic reciprocity and the mode of cell migration
Cells are in a dynamic and reciprocal relationship with their microenvironments. In this section, we will discuss how the microenvironment (Fig. 1) can regulate migratory behaviors. Then we will discuss how migratory cells remodel their microenvironments (Fig. 2A) and provide two specific examples of how this can impact the mode of cell migration (Fig. 2B).
A Modes of migration are plotted by how they change their microenvironments to the degree of substrate complexity. Cells can permanently digest their ECM, semi-permanently deform it, or leave no changes. Substrates increase in complexity as more features are compiled to support a certain mode of migration. Stable bleb ameboid requires a confined environment, while the osmotic engine also requires confinement and increased hydrostatic pressure. The lamellipodial mode of migration requires moderate confinement and fibrillar matrices, while the nuclear piston requires the same conditions and a linearly elastic matrix. Lamellipodial cells can digest their ECM and deform it through contractility. The front-pulling nuclear piston mode of migration does pull on its matrix, but changes are not permanent to the elasticity of the fibers. B Displaying migration mode changes with examples 1 and 2. Green boxes describe the physical and mechanical properties of the microenvironment that support the mode of migration. Red boxes describe how that migration mode changes its microenvironment.
The physical microenvironment can govern migration mode decisions
The least complex microenvironment able to support 3D migration is simple confinement (Fig. 1). Migration mode switches are often associated with changes to the ECM that make it more difficult to move the bulky nucleus through it, such as modifying fibrous matrix crosslinking or the concentration of fiber components14,35,74. Cells confined between two solid surfaces support stable bleb ameboid migration12 while confinement between four solid surfaces supports the osmotic engine modes of migration13. Fibrillar matrices are also confining cells but possess more physical arrangements, which makes migration more difficult. The next layer of the hierarchy is the physical arrangement of the substrate. Pore size likely varies, which defines the ease of passage, but alignment and thickness of fibers can also make directionality decisions and integrin binding much more difficult, leading to migration mode switches74,76. At the top of the hierarchy are the mechanical properties of the substrate because their modulation is a deciding factor in migration mode decisions. Some ECM modifications can reduce movement of the nucleus by making pores and channels smaller, but they can also modify matrix stiffness30,35,86,87 and viscoelasticity14. Further, changing the ECM’s mechanical properties can also affect the cell’s mechanical properties and its migratory behavior35,40,88. For example, a cell confined by four surfaces can still migrate and will choose paths of least resistance21,22. However, when faced with changes to the microenvironment’s mechanical properties, like increased osmotic pressure of the cell media, the osmotic engine can be activated13. Another example comes from fibroblasts, which will migrate through fibrillar matrices with small pore sizes. They will only activate the nuclear piston when those matrices are covalently crosslinked and possess linearly elastic properties14.
Migratory cells remodel their microenvironment
Migratory cells change the physical properties of the matrices they move through. Two methods of matrix remodeling have been associated with cell migration. The first method is digestion: cells break down matrix fibers into smaller segments to create a path for migration. Collagenase-driven degradation can increase pore sizes, increase fluidity, and reduce the stiffness of fibers that make up a pore89. Lamellipodial migration and some forms of ameboid migration are associated with high amounts of collagenase activity71,90. Also, an ameboid-based “worrying” mechanism relies on persistent frictional forces between the cell membrane and the ECM to fragment ECM fibers and create a path independent of collagenase activity84. These newer findings challenge conventional thinking on ameboid migration, having a low impact on ECM structure18,19. Paths for cell migration can also be created by cells using physical forces to move matrix fibers, as an alternative to cleaving the matrix proteins. While elastic matrices will return to their original configuration after the deforming forces are removed, viscoelastic matrices may be permanently or semi-permanently restructured in response to cellular force. During unstable ameboid migration, MDA-MB-231 and MDA-MB-468 cells showed increased collagen density and alignment at the site of bleb-ECM contact83. Another type of ameboid migration, the “chimney method”, uses a low amount of adhesions in favor of frictional forces between the cell membrane and ECM to achieve locomotion18. Since this method uses small amounts of proteases and cannot apply strong forces to the ECM, the cell must become much more deformable to fit through small pores. Based on these findings, we expect that the environmental impact of cells using ameboid migration modes will depend on the cell type and the mechanical properties of the ECM the cells are moving. Linearly elastic matrices trigger the front-pulling nuclear piston mode of migration, which suggests that any pulling of fibers from this mechanism is unlikely to be permanent14. Cells using the lamellipodial mode of migration can also align the fibers of collagen matrices using contractile forces and have been shown to require this prestrain before migration can be achieved74. Matrix alignment has been shown to decrease pore size and increase stiffness of collagen matrices91. Alignment also affects the efficacy of proteolytic enzymes on the ECM fibers92. Considering the viscoelastic nature of collagen matrices37, the fibers are likely to at least partially return to their former arrangement once the forces are removed. Finally, the rear pushing version of the nuclear piston uses the anterior protrusion as a wedge to spread fibers apart and make a path17. This mode of migration is observed in viscoelastic hydrogels and may only produce a semi-permanent restructuring of the substrate.
Dynamic reciprocity in migration mode decisions
The two major factors that activate ameboid migration are confinement and protease inhibition12,19,48. Protease inhibition may activate ameboid migration because it leads to increased confinement of the nucleus due to increased compression from the extracellular environment. In response to confinement, the nucleus can become softer to achieve collagenase-independent ameboid migration93. This can be accomplished through disassembly of the vimentin intermediate filament network48. Nucleoskeleton proteins, like lamins, are also critical regulators of nuclear mechanics. Modulation of lamin A:B ratios94 and post-translational modifications of lamin A/C change the stiffness of the nucleus93,95. Higher lamin A:B ratios have been shown to stiffen the nucleus so much that migration is slowed or stopped in favor of protecting the nucleus from rupture94. Inhibition of collagenase activity leads to phosphorylation of lamin A/C, resulting in the necessary softening of the nucleus to support ameboid migration93. These findings suggest that lamins are downstream of mechanical signals originating from the ECM. Indeed, the phosphorylation status of lamins is controlled by the stiffness and elasticity of the extracellular environment through actomyosin contractility96. In summary, mechanical signals from the ECM can reduce the stiffness of the nucleus through nuclear lamins, which allows for ameboid migration. In return, an ameboid cell will use the chimney form of ameboid migration, which does not significantly remodel its microenvironment18. We speculate that once the nucleus is relieved of its confinement, the cell will switch back to the mesenchymal (lamellipodial) mode of migration.
Primary fibroblasts have been shown to use the lamellipodial and lobopodial modes of migration14. The protein plectin is expressed by fibroblasts and serves as a linker between actin and vimentin97. This interaction must occur for cells to be able to pull the nucleus forward like a piston to achieve lobopodial migration77. Interestingly, plectin’s interaction with vimentin is dependent on NMII activity and on the stiffness of the substrate77. Therefore, a substrate that is too stiff will inhibit lobopodial migration by discouraging the plectin-vimentin interaction. This will force the migratory cell into using lamellipodia, which is associated with high levels of proteolytic activity, leading to larger amounts of collagen digestion70. Lamellipodial cells will also produce a prestrain on the matrix before moving the nucleus forward74. This will align the matrix fibers and potentially increase the degradability of the matrix by proteolysis, while also decreasing pore size and increasing stiffness74,91,92. A softer substrate will allow for the vimentin-plectin interaction, which is a critical step for the nuclear piston to form and achieve lobopodial migration, where matrix fibers are pushed and pulled instead of digested. Newer findings have indicated that plectin increases fibroblast stiffness and viscoelasticity through its linking of vimentin and actin98. This adds a new layer of complexity to plectin’s role in regulating fibroblast migration mode by suggesting that a certain mechanical state must be maintained to achieve lobopodial migration.
In summary, the mechanical properties of the matrix can govern the physical structure and mechanical properties of the cell moving through it. This dynamic reciprocity can then lead to different modifications to the structure of the ECM depending on the mode of migration used by the cell.
Conclusions
Not all modes of migration have been associated with specific mechanical properties of the physical environment that the cells are moving through. Developing a clearer understanding of this cause-and-effect relationship will be critical to understanding if a particular mode of migration is important for cell functions and understanding how cells affect their microenvironments. Future studies of the cell’s intracellular mechanical properties during migration mode switches could help reveal why migratory plasticity is so prevalent in eukaryotic cells.
Data availability
No datasets were generated or analyzed during the current study.
References
Yamada, K. M. & Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 (2019).
Petrie, R. J. & Yamada, K. M. At the leading edge of three-dimensional cell migration. J. Cell Sci. 125, 5917–5926 (2012).
Garcia-Arcos, J. M., Jha, A., Waterman, C. M. & Piel, M. Blebology: principles of bleb-based migration. Trends Cell Biol. 34, 838–853 (2024).
Rafelski, S. M. & Theriot, J. A. Establishing a conceptual framework for holistic cell states and state transitions. Cell 187, 2633–2651 (2024).
Bissell, M. J., Hall, H. G. & Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 (1982).
Lee, E. Y., Parry, G. & Bissell, M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J. Cell Biol. 98, 146–155 (1984).
Beliveau, A. et al. Raf-induced MMP9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vivo. Genes Dev. 24, 2800–2811 (2010).
Matsubara, H., Inoue, T. & Agata, K. Reintegration of blastema and stump by reciprocal interaction for functional joint regeneration in frogs. Dev. Biol. 525, 282–293 (2025).
Abercrombie, M., Dunn, G. A. & Heath, J. P. The shape and movement of fibroblasts in culture. Soc. Gen. Physiol. Ser. 32, 57–70 (1977).
Paul, C. D., Hung, W. C., Wirtz, D. & Konstantopoulos, K. Engineered models of confined cell migration. Annu. Rev. Biomed. Eng. 18, 159–180 (2016).
Bergert, M., Chandradoss, S. D., Desai, R. A. & Paluch, E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc. Natl. Acad. Sci. USA 109, 14434–14439 (2012).
Liu, Y. J. et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015).
Stroka, K. M. et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014).
Petrie, R. J., Gavara, N., Chadwick, R. S. & Yamada, K. M. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 197, 439–455 (2012).
Doyle, A. D. Generation of 3D collagen gels with controlled diverse architectures. Curr. Protoc. Cell Biol. 72, 10 20 11–10 20 16 (2016).
Petrie, R. J., Koo, H. & Yamada, K. M. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014).
Lee, H. P. et al. The nuclear piston activates mechanosensitive ion channels to generate cell migration paths in confining microenvironments. Sci. Adv. https://doi.org/10.1126/sciadv.abd4058 (2021).
Wolf, K. et al. Compensation mechanism in tumor cell migration : mesenchymal–amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160, 267–277 (2003).
Sahai, E. & Marshall, C. J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 5, 711–719 (2003).
Lennon-Duménil, A. M. & Moreau, H. D. Barotaxis: how cells live and move under pressure. Curr. Opin. Cell Biol. 72, 131–136 (2021).
Prentice-Mott, H. V. et al. Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments. Proc. Natl. Acad. Sci. USA 110, 21006–21011 (2013).
Zhao, R. et al. Cell sensing and decision-making in confinement: the role of TRPM7 in a tug of war between hydraulic pressure and cross-sectional area. Sci. Adv. https://doi.org/10.1126/sciadv.aaw7243 (2019).
Li, Y. & Sun, S. X. Transition from actin-driven to water-driven cell migration depends on external hydraulic resistance. Biophys. J. 114, 2965–2973 (2018).
Moreau, H. D. et al. Macropinocytosis overcomes directional bias in dendritic cells due to hydraulic resistance and facilitates space exploration. Dev. Cell 49, 171–188.e175 (2019).
Zhang, Y. et al. Polarized NHE1 and SWELL1 regulate migration direction, efficiency and metastasis. Nat. Commun. 13, 6128 (2022).
Staunton, J. R., So, W. Y., Paul, C. D. & Tanner, K. High-frequency microrheology in 3D reveals mismatch between cytoskeletal and extracellular matrix mechanics. Proc. Natl. Acad. Sci. USA 116, 14448–14455 (2019).
Baker, E. L., Bonnecaze, R. T. & Zaman, M. H. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys. J. 97, 1013–1021 (2009).
Wang, M. et al. Microchannel stiffness and confinement jointly induce the mesenchymal-amoeboid transition of cancer cell migration. Nano Lett. 19, 5949–5958 (2019).
Mancini, A. et al. Multiple aspects of matrix stiffness in cancer progression. Front. Oncol. 14, 1406644 (2024).
Khoo, A. S. et al. Breast cancer cells transition from mesenchymal to amoeboid migration in tunable three-dimensional silk-collagen hydrogels. ACS Biomater. Sci. Eng. 5, 4341–4354 (2019).
Sao, K. et al. Myosin II governs intracellular pressure and traction by distinct tropomyosin-dependent mechanisms. Mol. Biol. Cell 30, 1170–1181 (2019).
Sridharan, R., Cavanagh, B., Cameron, A. R., Kelly, D. J. & O’Brien, F. J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 89, 47–59 (2019).
Najafi, M., Farhood, B. & Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 120, 2782–2790 (2019).
Alonso-Nocelo, M. et al. Matrix stiffness and tumor-associated macrophages modulate epithelial to mesenchymal transition of human adenocarcinoma cells. Biofabrication 10, 035004 (2018).
Hiraki, H. L. et al. Fiber density and matrix stiffness modulate distinct cell migration modes in a 3D stroma mimetic composite hydrogel. Acta Biomater. 163, 378–391 (2023).
Jung, W., Li, J., Chaudhuri, O. & Kim, T. Nonlinear Elastic and Inelastic Properties of Cells. J. Biomech. Eng. https://doi.org/10.1115/1.4046863 (2020).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Nam, S., Hu, K. H., Butte, M. J. & Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl. Acad. Sci. USA 113, 5492–5497 (2016).
Nam, S., Lee, J., Brownfield, D. G. & Chaudhuri, O. Viscoplasticity enables mechanical remodeling of matrix by cells. Biophys. J. 111, 2296–2308 (2016).
Wu, D. T. et al. Hydrogel viscoelasticity modulates migration and fusion of mesenchymal stem cell spheroids. Bioeng. Transl. Med. 8, e10464 (2023).
Adebowale, K. et al. Monocytes use protrusive forces to generate migration paths in viscoelastic collagen-based extracellular matrices. Proc. Natl. Acad. Sci. USA 122, e2309772122 (2023).
Adebowale, K. et al. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nat. Mater. 20, 1290–1299 (2021).
Ketene, A. N., Schmelz, E. M., Roberts, P. C. & Agah, M. The effects of cancer progression on the viscoelasticity of ovarian cell cytoskeleton structures. Nanomedicine 8, 93–102 (2012).
Wu, Y. et al. BRMS1 expression alters the ultrastructural, biomechanical and biochemical properties of MDA-MB-435 human breast carcinoma cells: an AFM and Raman microspectroscopy study. Cancer Lett. 293, 82–91 (2010).
Grolleman, J. et al. Environmental stiffness restores mechanical homeostasis in vimentin-depleted cells. Sci. Rep. 13, 18374 (2023).
Mendez, M. G., Restle, D. & Janmey, P. A. Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys. J. 107, 314–323 (2014).
Patteson, A. E. et al. Vimentin protects cells against nuclear rupture and DNA damage during migration. J. Cell Biol. 218, 4079–4092 (2019).
Wang, Y.-J. et al. Lamin A/C and vimentin as a coordinated regulator during amoeboid migration in microscale confined microenvironments. Nano Lett. 23, 6727–6735 (2023).
Kim, J. E., Reynolds, D. S., Zaman, M. H. & Mak, M. Characterization of the mechanical properties of cancer cells in 3D matrices in response to collagen concentration and cytoskeletal inhibitors. Integr. Biol.10, 232–241 (2018).
Wullkopf, L. et al. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell 29, 2378–2385 (2018).
Lu, L., Oswald, S. J., Ngu, H. & Yin, F. C. Mechanical properties of actin stress fibers in living cells. Biophys. J. 95, 6060–6071 (2008).
Fischer, R. S., Gardel, M., Ma, X., Adelstein, R. S. & Waterman, C. M. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr. Biol. 19, 260–265 (2009).
Fernández, P. & Ott, A. Single cell mechanics: stress stiffening and kinematic hardening. Phys. Rev. Lett. 100, 238102 (2008).
Krishnan, R. et al. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE 4, e5486 (2009).
Cowan, J. M., Duggan, J. J., Hewitt, B. R. & Petrie, R. J. Non-muscle myosin II and the plasticity of 3D cell migration. Front. Cell Dev. Biol. 10, 1047256 (2022).
Xie, J. et al. Contribution of cytoplasm viscoelastic properties to mitotic spindle positioning. Proc. Natl. Acad. Sci. USA 119, e2115593119 (2022).
Han, S. J., Kwon, S. & Kim, K. S. Contribution of mechanical homeostasis to epithelial-mesenchymal transition. Cell Oncol.45, 1119–1136 (2022).
Sliogeryte, K., Botto, L., Lee, D. A. & Knight, M. M. Chondrocyte dedifferentiation increases cell stiffness by strengthening membrane-actin adhesion. Osteoarthr. Cartil. 24, 912–920 (2016).
Fernández, P., Pullarkat, P. A. & Ott, A. A master relation defines the nonlinear viscoelasticity of single fibroblasts. Biophys. J. 90, 3796–3805 (2006).
Berret, J. F. Local viscoelasticity of living cells measured by rotational magnetic spectroscopy. Nat. Commun. 7, 10134 (2016).
Chaudhuri, O., Parekh, S. H. & Fletcher, D. A. Reversible stress softening of actin networks. Nature 445, 295–298 (2007).
Fabry, B. et al. Scaling the microrheology of living cells. Phys. Rev. Lett. 87, 148102 (2001).
Melissa, G. M., Shin-Ichiro, K. & Robert, D. G. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–1851 (2010).
Patteson, A. E., Vahabikashi, A., Goldman, R. D. & Janmey, P. A. Mechanical and non-mechanical functions of filamentous and non-filamentous vimentin. Bioessays 42, e2000078 (2020).
Block, J. et al. Nonlinear loading-rate-dependent force response of individual vimentin intermediate filaments to applied strain. Phys. Rev. Lett. 118, 048101 (2017).
Block, J. et al. Viscoelastic properties of vimentin originate from nonequilibrium conformational changes. Sci. Adv. 4, eaat1161 (2018).
Hu, J. et al. High stretchability, strength, and toughness of living cells enabled by hyperelastic vimentin intermediate filaments. Proc. Natl. Acad. Sci. USA 116, 17175–17180 (2019).
Fudge, D. et al. The intermediate filament network in cultured human keratinocytes is remarkably extensible and resilient. PLoS ONE 3, e2327 (2008).
Hu, S., Yang, C., Hu, D. & Lam, R. H. W. Microfluidic biosensing of viscoelastic properties of normal and cancerous human breastcells. IEEE 12, 90–95 (2017).
Petrie, R. J., Harlin, H. M., Korsak, L. I. & Yamada, K. M. Activating the nuclear piston mechanism of 3D migration in tumor cells. J. Cell Biol. 216, 93–100 (2017).
Infante, E. et al. LINC complex-Lis1 interplay controls MT1-MMP matrix digest-on-demand response for confined tumor cell migration. Nat. Commun. 9, 2443 (2018).
Lomakin, A. J. et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science https://doi.org/10.1126/science.aba2894 (2020).
Petrie, R. J. & Yamada, K. M. Multiple mechanisms of 3D migration: the origins of plasticity. Curr. Opin. Cell Biol. 42, 7–12 (2016).
Doyle, A. D., Sykora, D. J., Pacheco, G. G., Kutys, M. L. & Yamada, K. M. 3D mesenchymal cell migration is driven by anterior cellular contraction that generates an extracellular matrix prestrain. Dev. Cell 56, 826–841.e824 (2021).
Oyanagi, J., Ogawa, T., Sato, H., Higashi, S. & Miyazaki, K. Epithelial-mesenchymal transition stimulates human cancer cells to extend microtubule-based invasive protrusions and suppresses cell growth in collagen gel. PLoS ONE 7, e53209 (2013).
Doyle, A. D., Carvajal, N., Jin, A., Matsumoto, K. & Yamada, K. M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 6, 8720 (2015).
Marks, P. C., Hewitt, B. R., Baird, M. A., Wiche, G. & Petrie, R. J. Plectin linkages are mechanosensitive and required for the nuclear piston mechanism of three-dimensional cell migration. Mol. Biol. Cell 33, ar104 (2022).
Newman, D. et al. 3D matrix adhesion feedback controls nuclear force coupling to drive invasive cell migration. Cell Rep. 42, 113554 (2023).
Ruprecht, V. et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160, 673–685 (2015).
Hawkins, R. J. et al. Spontaneous contractility-mediated cortical flow generates cell migration in three-dimensional environments. Biophys. J. 101, 1041–1045 (2011).
Venturini, V. et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370, eaba2644 (2020).
Ferrari, R. et al. MT1-MMP directs force-producing proteolytic contacts that drive tumor cell invasion. Nat. Commun. 10, 4886 (2019).
Guzman, A., Avard, R. C., Devanny, A. J., Kweon, O. S. & Kaufman, L. J. Delineating the role of membrane blebs in a hybrid mode of cancer cell invasion in three-dimensional environments. J. Cell Sci. 133, jcs236778 (2020).
Driscoll, M. K. et al. Proteolysis-free amoeboid migration of melanoma cells through crowded environments via bleb-driven worrying. Dev. Cell 59, 2414–2428.e2418 (2024).
Bera, K. et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature 611, 365–373 (2022).
Berger, A. J., Linsmeier, K. M., Kreeger, P. K. & Masters, K. S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 141, 125–135 (2017).
Chepizhko, O. et al. Confined cell migration along extracellular matrix space in vivo. Proc. Natl. Acad. Sci. USA 122, e2414009121 (2025).
Barriga, E. H. & Mayor, R. Adjustable viscoelasticity allows for efficient collective cell migration. Semin. Cell Dev. Biol. 93, 55–68 (2019).
Schultz, K. M., Kyburz, K. A. & Anseth, K. S. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl. Acad. Sci. USA 112, E3757–3764 (2015).
Orgaz, J. L. et al. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat. Commun. 5, 4255 (2014).
Taufalele, P. V., VanderBurgh, J. A., Munoz, A., Zanotelli, M. R. & Reinhart-King, C. A. Fiber alignment drives changes in architectural and mechanical features in collagen matrices. PLoS ONE 14, e0216537 (2019).
Yeganegi, A., Whitehead, K., de Castro Brás, L. E. & Richardson, W. J. Mechanical strain modulates extracellular matrix degradation and byproducts in an isoform-specific manner. Biochim. Biophys. Acta Gen. Subj. 1867, 130286 (2023).
Das, A., Barai, A., Monteiro, M., Kumar, S. & Sen, S. Nuclear softening is essential for protease-independent migration. Matrix Biol. 82, 4–19 (2019).
Harada, T. et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 204, 669–682 (2014).
Karling, T. & Weavers, H. Immune cells adapt to confined environments in vivo to optimise nuclear plasticity for migration. EMBO Rep. 26, 1238–1268 (2025).
Buxboim, A. et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 24, 1909–1917 (2014).
Jiu, Y. et al. Bidirectional interplay between vimentin intermediate filaments and contractile actin stress fibers. Cell Rep. 11, 1511–1518 (2015).
Conboy, J. P. et al. Plectin affects cell viscoelasticity at small and large deformations. Preprint at bioRxiv https://doi.org/10.1101/2025.05.28.656588 (2025).
Gonzalez-Molina, J. et al. Extracellular fluid viscosity enhances liver cancer cell mechanosensing and migration. Biomaterials 177, 113–124 (2018).
Acknowledgements
This work was funded by a National Institute of General Medical Sciences of the National Institutes of Health grant (R35GM156335).
Author information
Authors and Affiliations
Contributions
R.J.P. and J.J.D. developed the focus of the review. J.J.D. wrote the main manuscript text and prepared the figures and tables. R.J.P. and J.J.D. both edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duggan, J.J., Petrie, R.J. The role of dynamic reciprocity in 3D cell migration: connecting cell and matrix mechanics to migratory plasticity. npj Biol. Phys. Mech. 2, 21 (2025). https://doi.org/10.1038/s44341-025-00027-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44341-025-00027-1
This article is cited by
-
‘Memory foam’ skeleton in cells helps them to navigate
Nature (2025)