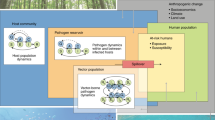
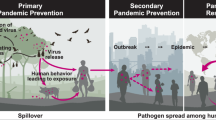
Abstract
Emerging infectious diseases, biodiversity loss, and anthropogenic environmental change are interconnected crises with massive social and ecological costs. In this Review, we discuss how pathogens and parasites are responding to global change, and the implications for pandemic prevention and biodiversity conservation. Ecological and evolutionary principles help to explain why both pandemics and wildlife die-offs are becoming more common; why land-use change and biodiversity loss are often followed by an increase in zoonotic and vector-borne diseases; and why some species, such as bats, host so many emerging pathogens. To prevent the next pandemic, scientists should focus on monitoring and limiting the spread of a handful of high-risk viruses, especially at key interfaces such as farms and live-animal markets. But to address the much broader set of infectious disease risks associated with the Anthropocene, decision-makers will need to develop comprehensive strategies that include pathogen surveillance across species and ecosystems; conservation-based interventions to reduce human–animal contact and protect wildlife health; health system strengthening; and global improvements in epidemic preparedness and response. Scientists can contribute to these efforts by filling global gaps in disease data, and by expanding the evidence base for disease–driver relationships and ecological interventions.
Key points
-
Human activities have created a planetary polycrisis that includes pandemics, climate change and the sixth mass extinction.
-
Climate change, land change, agriculture and wildlife use — the major threats to biodiversity — are also driving a global rise in infectious diseases.
-
Biodiversity loss is generally harmful to human health.
-
Interventions that target spillover interfaces for high-risk pathogens, such as avian influenza or coronaviruses, could prevent some future pandemics.
-
Even with these interventions, investments in health systems and pandemic preparedness will be an important part of living in the Anthropocene.
-
The world needs better real-time biosurveillance infrastructure to track pathogens across species and ecosystems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
No original data were generated during the course of this study, but all data required to reproduce the figures here can be found at: https://github.com/viralemergence/pnpc.
Code availability
All code developed in this study can be found at: https://github.com/viralemergence/pnpc. The minimal statistical analysis and all other data and plotting related analysis were conducted in R version 4.3.2 (2023-10-31) (R Core Team 2023) using the rstanarm package (R Core Team 2023; Goodrich et al. 2024). All other packages we used are referenced in the repository’s README file.
References
Daszak, P., Cunningham, A. A. & Hyatt, A. D. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 287, 443–449 (2000).
Wolfe, N. D., Dunavan, C. P. & Diamond, J. Origins of major human infectious diseases. Nature 447, 279–283 (2007).
Woolhouse, M., Scott, F., Hudson, Z., Howey, R. & Chase-Topping, M. Human viruses: discovery and emergence. Phil. Trans. R. Soc. Lond. B 367, 2864–2871 (2012).
Meadows, A. J., Stephenson, N., Madhav, N. K. & Oppenheim, B. Historical trends demonstrate a pattern of increasingly frequent and severe spillover events of high-consequence zoonotic viruses. BMJ Glob. Health 8, e012026 (2023).
Keesing, F. et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652 (2010).
Baker, R. E. et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 20, 193–205 (2022).
Allen, T. et al. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8, 1124 (2017).
Lawler, O. K. et al. The COVID-19 pandemic is intricately linked to biodiversity loss and ecosystem health. Lancet Planet. Health 5, e840–e850 (2021).
Glidden, C. K. et al. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr. Biol. 31, R1342–R1361 (2021).
Kock, R. A. & Caceres-Escobar, H. Situation Analysis on the Roles and Risks of Wildlife in the Emergence of Human Infectious Diseases (IUCN, 2022).
Stephens, P. R. et al. The macroecology of infectious diseases: a new perspective on global-scale drivers of pathogen distributions and impacts. Ecol. Lett. 19, 1159–1171 (2016).
Huang, S., Farrell, M. & Stephens, P. R. Infectious disease macroecology: parasite diversity and dynamics across the globe. Phil. Trans. R. Soc. Lond. B 376, 20200350 (2021).
Johnson, P. T. J., de Roode, J. C. & Fenton, A. Why infectious disease research needs community ecology. Science 349, 1259504 (2015).
Carlson, C. J. et al. A global parasite conservation plan. Biol. Conserv. 250, 108596 (2020).
Rubio‐Godoy, M. & de León, G. P. Equal rights for parasites: Windsor 1995, revisited after ecological parasitology has come of age. Biol. Conserv. https://doi.org/10.1016/j.biocon.2023.110174 (2023).
Weinstein, S. B. & Kuris, A. M. Independent origins of parasitism in Animalia. Biol. Lett. 12, 20160324 (2016).
Dolphin, K. & Quicke, D. L. J. Estimating the global species richness of an incompletely described taxon: an example using parasitoid wasps (Hymenoptera: Braconidae). Biol. J. Linn. Soc. Lond. 73, 279–286 (2008).
Carlson, C. J., Dallas, T. A., Alexander, L. W., Phelan, A. L. & Phillips, A. J. What would it take to describe the global diversity of parasites? Proc. R. Soc. B 287, 20201841 (2020).
Dobson, A. P., Lafferty, K. D., Kuris, A. M., Hechinger, R. F. & Jetz, W. Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl Acad. Sci. USA 105, 11482–11489 (2008).
Larsen, B. B., Miller, E. C., Rhodes, M. K. & Wiens, J. J. Inordinate fondness multiplied and redistributed: the number of species on Earth and the new pie of life. Q. Rev. Biol. 92, 229–265 (2017).
Dallas, T. A. et al. Gauging support for macroecological patterns in helminth parasites. Glob. Ecol. Biogeogr. 27, 1437–1447 (2018).
Mollentze, N. & Streicker, D. G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl Acad. Sci. USA. 117, 9423–9430 (2020).
Geoghegan, J. L., Duchêne, S. & Holmes, E. C. Comparative analysis estimates the relative frequencies of co-divergence and cross-species transmission within viral families. PLoS Pathog. 13, e1006215 (2017).
Hoberg, E. P. & Brooks, D. R. A macroevolutionary mosaic: episodic host‐switching, geographical colonization and diversification in complex host–parasite systems. J. Biogeogr. 35, 1533–1550 (2008).
Wallace, M. et al. Making sense of the virome in light of evolution and ecology. Preprint at EcoEvoRxiv https://doi.org/10.32942/X21K8T (2024).
Kamiya, T., O’Dwyer, K., Nakagawa, S. & Poulin, R. Host diversity drives parasite diversity: meta‐analytical insights into patterns and causal mechanisms. Ecography 37, 689–697 (2014).
Poulin, R. & Jorge, F. The geography of parasite discovery across taxa and over time. Parasitology 146, 168–175 (2019).
Jorge, F. & Poulin, R. Poor geographical match between the distributions of host diversity and parasite discovery effort. Proc. Biol. Sci. 285, 20180072 (2018).
Quicke, D. L. J. We know too little about parasitoid wasp distributions to draw any conclusions about latitudinal trends in species richness, body size and biology. PLoS ONE 7, e32101 (2012).
Rosenberg, R., Johansson, M. A., Powers, A. M. & Miller, B. R. Search strategy has influenced the discovery rate of human viruses. Proc. Natl. Acad. Sci. USA 110, 13961–13964 (2013).
Gibb, R. et al. Mammal virus diversity estimates are unstable due to accelerating discovery effort. Biol. Lett. 18, 20210427 (2022).
Lu, L. et al. Temporal dynamics, discovery, and emergence of human-transmissible RNA viruses. Mol. Biol. Evol. 41, msad272 (2024).
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
Carlson, C. J. et al. The Global Virome in One Network (VIRION): an atlas of vertebrate–virus associations. mBio 13, e0298521 (2022).
Carroll, D. et al. The global virome project. Science 359, 872–874 (2018).
Wille, M., Geoghegan, J. L. & Holmes, E. C. How accurately can we assess zoonotic risk? PLoS Biol. 19, e3001135 (2021).
Olival, K. J. et al. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650 (2017).
Albery, G. F. et al. Urban-adapted mammal species have more known pathogens. Nat. Ecol. Evol. 6, 794–801 (2022).
Zhou, P. et al. Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. Proc. Natl. Acad. Sci. USA 113, 2696–2701 (2016).
Irving, A. T., Ahn, M., Goh, G., Anderson, D. E. & Wang, L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature 589, 363–370 (2021).
Brook, C. E., Rozins, C., Guth, S. & Boots, M. Reservoir host immunology and life history shape virulence evolution in zoonotic viruses. PLoS Biol. 21, e3002268 (2023).
Guth, S. et al. Bats host the most virulent—but not the most dangerous—zoonotic viruses. Proc. Natl Acad. Sci. USA 119, e2113628119 (2022).
Guth, S., Visher, E., Boots, M. & Brook, C. E. Host phylogenetic distance drives trends in virus virulence and transmissibility across the animal–human interface. Phil. Trans. R. Soc. Lond. B 374, 20190296 (2019).
Walker, J. W., Han, B. A., Ott, I. M. & Drake, J. M. Transmissibility of emerging viral zoonoses. PLoS ONE 13, e0206926 (2018).
Cincotta, R. P., Wisnewski, J. & Engelman, R. Human population in the biodiversity hotspots. Nature 404, 990–992 (2000).
de Thoisy, B., Gräf, T., Mansur, D. S., Delfraro, A. & Dos Santos, C. N. D. The risk of virus emergence in South America: a subtle balance between increasingly favorable conditions and a protective environment. Annu. Rev. Virol. 11, 43–65 (2024).
Brierley, L., Vonhof, M. J., Olival, K. J., Daszak, P. & Jones, K. E. Quantifying global drivers of zoonotic bat viruses: a process-based perspective. Am. Nat. 187, E53–E64 (2016).
Han, B. A. et al. Undiscovered bat hosts of filoviruses. PLoS Negl. Trop. Dis. 10, e0004815 (2016).
Becker, D. J. et al. Optimising predictive models to prioritise viral discovery in zoonotic reservoirs. Lancet Microbe 3, e625–e637 (2022).
Schulz, J. E. et al. Serological evidence for Henipa-like and Filo-like viruses in Trinidad bats. J. Infect. Dis. 221, S375–S382 (2020).
Hernández, L. H. A. et al. First genomic evidence of a Henipa-like virus in Brazil. Viruses 14, 2167 (2022).
Halsey, S. Defuse the dilution effect debate. Nat. Ecol. Evol. 3, 145–146 (2019).
Halliday, F. W., Rohr, J. R. & Laine, A.-L. Biodiversity loss underlies the dilution effect of biodiversity. Ecol. Lett. 23, 1611–1622 (2020).
Mahon, M. B. et al. A meta-analysis on global change drivers and the risk of infectious disease. Nature 629, 830–836 (2024).
Huang, Z. Y. X., Yu, Y., VAN Langevelde, F. & DE Boer, W. F. Does the dilution effect generally occur in animal diseases? Parasitology 144, 823–826 (2017).
Gallagher, S. J. et al. Healthy but smaller herds: predators reduce pathogen transmission in an amphibian assemblage. J. Anim. Ecol. 88, 1613–1624 (2019).
Lopez, L. K. et al. A healthy but depleted herd: predators decrease prey disease and density. Ecology 104, e4063 (2023).
Levi, T., Kilpatrick, A. M., Mangel, M. & Wilmers, C. C. Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. USA 109, 10942–10947 (2012).
Johnson, P. T. J., Preston, D. L., Hoverman, J. T. & Richgels, K. L. D. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233 (2013).
Huang, Z. Y. X., VAN Langevelde, F., Estrada-Peña, A., Suzán, G. & DE Boer, W. F. The diversity–disease relationship: evidence for and criticisms of the dilution effect. Parasitology 143, 1075–1086 (2016).
Allan, B. F., Keesing, F. & Ostfeld, R. S. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 17, 267–272 (2003).
Keesing, F. et al. Hosts as ecological traps for the vector of Lyme disease. Proc. Biol. Sci. 276, 3911–3919 (2009).
Gibb, R. et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402 (2020).
Ecke, F. et al. Population fluctuations and synanthropy explain transmission risk in rodent-borne zoonoses. Nat. Commun. 13, 7532 (2022).
Previtali, M. A. et al. Relationship between pace of life and immune responses in wild rodents. Oikos 121, 1483–1492 (2012).
Albery, G. F. & Becker, D. J. Fast-lived hosts and zoonotic risk. Trends Parasitol. 37, 117–129 (2021).
Plourde, B. T. et al. Are disease reservoirs special? Taxonomic and life history characteristics. PLoS ONE 12, e0180716 (2017).
Farrell, M. J., Stephens, P. R., Berrang-Ford, L., Gittleman, J. L. & Davies, T. J. The path to host extinction can lead to loss of generalist parasites. J. Anim. Ecol. 84, 978–984 (2015).
Esser, H. J., Herre, E. A., Kays, R., Liefting, Y. & Jansen, P. A. Local host–tick coextinction in neotropical forest fragments. Int. J. Parasitol. 49, 225–233 (2019).
Sitko, J. & Heneberg, P. Systemic collapse of a host–parasite trematode network associated with wetland birds in Europe. Parasitol. Res. 119, 935–945 (2020).
Wood, C. L. et al. A reconstruction of parasite burden reveals one century of climate-associated parasite decline. Proc. Natl. Acad. Sci. USA 120, e2211903120 (2023).
Carlson, C. J. et al. Parasite biodiversity faces extinction and redistribution in a changing climate. Sci. Adv. 3, e1602422 (2017).
Neely, W. J. et al. Host-associated helminth diversity and microbiome composition contribute to anti-pathogen defences in tropical frogs impacted by forest fragmentation. R. Soc. Open Sci. 11, 240530 (2024).
Johnson, P. T. J., Preston, D. L., Hoverman, J. T. & LaFonte, B. E. Host and parasite diversity jointly control disease risk in complex communities. Proc. Natl. Acad. Sci. USA 110, 16916–16921 (2013).
Ezenwa, V. O. & Jolles, A. E. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science 347, 175–177 (2015).
Sweeny, A. R. et al. Experimental parasite community perturbation reveals associations between Sin Nombre virus and gastrointestinal nematodes in a rodent reservoir host. Biol. Lett. 16, 20200604 (2020).
Dougherty, E. R. et al. Paradigms for parasite conservation. Conserv. Biol. 30, 724–733 (2016).
Tompkins, D. M., Carver, S., Jones, M. E., Krkošek, M. & Skerratt, L. F. Emerging infectious diseases of wildlife: a critical perspective. Trends Parasitol. 31, 149–159 (2015).
Sanderson, C. E. & Alexander, K. A. Unchartered waters: climate change likely to intensify infectious disease outbreaks causing mass mortality events in marine mammals. Glob. Chang. Biol. 26, 4284–4301 (2020).
Kock, R. A. et al. Saigas on the brink: multidisciplinary analysis of the factors influencing mass mortality events. Sci. Adv. 4, eaao2314 (2018).
Fereidouni, S. et al. Mass die-off of saiga antelopes, Kazakhstan, 2015. Emerg. Infect. Dis. 25, 1169–1176 (2019).
Pruvot, M. et al. Outbreak of peste des petits ruminants among critically endangered Mongolian saiga and other wild ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 26, 51–62 (2020).
De Castro, F. & Bolker, B. Mechanisms of disease‐induced extinction. Ecol. Lett. 8, 117–126 (2005).
Bonhoeffer, S., Lenski, R. E. & Ebert, D. The curse of the pharaoh: the evolution of virulence in pathogens with long living propagules. Proc. Biol. Sci. 263, 715–721 (1996).
Fisher, M. C. & Garner, T. W. J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 18, 332–343 (2020).
Stegen, G. et al. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544, 353–356 (2017).
Castro Monzon, F., Rödel, M.-O., Ruland, F., Parra-Olea, G. & Jeschke, J. M. Batrachochytrium salamandrivorans’ amphibian host species and invasion range. Ecohealth 19, 475–486 (2022).
Hoyt, J. R., Kilpatrick, A. M. & Langwig, K. E. Ecology and impacts of white-nose syndrome on bats. Nat. Rev. Microbiol. 19, 196–210 (2021).
Springborn, M. R., Weill, J. A., Lips, K. R., Ibáñez, R. & Ghosh, A. Amphibian collapses increased malaria incidence in Central America. Environ. Res. Lett. 17, 104012 (2022).
Frank, E. G. The economic impacts of ecosystem disruptions: costs from substituting biological pest control. Science 385, eadg0344 (2024).
Xie, R. et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 622, 810–817 (2023).
Muñoz, G. et al. Stranding and mass mortality in Humboldt penguins (Spheniscus humboldti), associated to HPAIV H5N1 outbreak in Chile. Prev. Vet. Med. 227, 106206 (2024).
Nerpel, A. et al. SARS-ANI: a global open access dataset of reported SARS-CoV-2 events in animals. Sci. Data 9, 438 (2022).
Fagre, A. C. et al. Assessing the risk of human-to-wildlife pathogen transmission for conservation and public health. Ecol. Lett. 25, 1534–1549 (2022).
Weary, T. E. et al. Common cold viruses circulating in children threaten wild chimpanzees through asymptomatic adult carriers. Sci. Rep. 14, 10431 (2024).
Köndgen, S. et al. Pandemic human viruses cause decline of endangered great apes. Curr. Biol. 18, 260–264 (2008).
Hopkins, S. R. et al. Editorial essay: protected areas and One Health. Park. Recreat. 30, 6–13 (2024).
Bernstein, A. S. et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci. Adv. 8, eabl4183 (2022).
Dobson, A. P. et al. Ecology and economics for pandemic prevention. Science 369, 379–381 (2020).
Vora, N. M. et al. Want to prevent pandemics? Stop spillovers. Nature 605, 419–422 (2022).
Vora, N. M. et al. Interventions to reduce risk for pathogen spillover and early disease spread to prevent outbreaks, epidemics, and pandemics. Emerg. Infect. Dis. 29, 1–9 (2023).
Winkler, K., Fuchs, R., Rounsevell, M. & Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 12, 2501 (2021).
IPBES. Summary for Policymakers of The Global Assessment Report on Biodiversity and Ecosystem Services (IPBES, 2019).
Plowright, R. K. et al. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet. Health 5, e237–e245 (2021).
Becker, D. J. et al. Macroimmunology: the drivers and consequences of spatial patterns in wildlife immune defence. J. Anim. Ecol. 89, 972–995 (2020).
Eby, P. et al. Pathogen spillover driven by rapid changes in bat ecology. Nature 613, 340–344 (2023).
Gibb, R. et al. The anthropogenic fingerprint on emerging infectious diseases. Preprint at bioRxiv https://doi.org/10.1101/2024.05.22.24307684 (2024).
Eskew, E. A. et al. Reservoir displacement by an invasive rodent reduces Lassa virus zoonotic spillover risk. Nat. Commun. 15, 3589 (2024).
Skinner, E. B., Glidden, C. K., MacDonald, A. J. & Mordecai, E. A. Human footprint is associated with shifts in the assemblages of major vector-borne diseases. Nat. Sustain. 6, 652–661 (2023).
Morand, S., McIntyre, K. M. & Baylis, M. Domesticated animals and human infectious diseases of zoonotic origins: domestication time matters. Infect. Genet. Evol. 24, 76–81 (2014).
Greenspoon, L. et al. The global biomass of wild mammals. Proc. Natl. Acad. Sci. USA 120, e2204892120 (2023).
Johnson, C. K. et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 5, 14830 (2015).
Jones, B. A. et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 110, 8399–8404 (2013).
Hayek, M. N. The infectious disease trap of animal agriculture. Sci. Adv. 8, eadd6681 (2022).
Jori, F., Hernandez-Jover, M., Magouras, I., Dürr, S. & Brookes, V. J. Wildlife–livestock interactions in animal production systems: what are the biosecurity and health implications? Animal Front. 11, 8–19 (2021).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 12, 869–875 (2022).
Mordecai, E. A. et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690–1708 (2019).
Carlson, C. J., Carleton, T. A., Odoulami, R. C. & Trisos, C. H. The historical fingerprint and future impact of climate change on childhood malaria in Africa. Preprint at bioRxiv https://doi.org/10.1101/2023.07.16.23292713 (2023).
Childs, M. L., Lyberger, K., Harris, M., Burke, M. & Mordecai, E. A. Climate warming is expanding dengue burden in the Americas and Asia. Preprint at medRxiv https://doi.org/10.1101/2024.01.08.24301015 (2024).
Carlson, C. J., Bannon, E., Mendenhall, E., Newfield, T. & Bansal, S. Rapid range shifts in African Anopheles mosquitoes over the last century. Biol. Lett. 19, 20220365 (2023).
Kraemer, M. U. G. et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4, 854–863 (2019).
Eisen, R. J. & Eisen, L. Evaluation of the association between climate warming and the spread and proliferation of Ixodes scapularis in northern states in the eastern United States. Ticks Tick-borne Dis. 15, 102286 (2024).
McCoy, K. D. et al. Climate change in the Arctic: testing the poleward expansion of ticks and tick-borne diseases. Glob. Change Biol. 29, 1729–1740 (2023).
VanWormer, E. et al. Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Sci. Rep. 9, 15569 (2019).
Morales-Castilla, I. et al. Forecasting parasite sharing under climate change. Phil. Trans. R. Soc. Lond. B 376, 20200360 (2021).
Carlson, C. J. et al. Climate change increases cross-species viral transmission risk. Nature 607, 555–562 (2022).
Trisos, C. H., Merow, C. & Pigot, A. L. The projected timing of abrupt ecological disruption from climate change. Nature 580, 496–501 (2020).
Pigot, A. L., Merow, C., Wilson, A. & Trisos, C. H. Abrupt expansion of climate change risks for species globally. Nat. Ecol. Evol. 7, 1060–1071 (2023).
Worobey, M. et al. The Huanan seafood wholesale market in Wuhan was the early epicenter of the COVID-19 pandemic. Science 377, 951–959 (2022).
Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278 (2003).
Wang, M. et al. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 11, 1860–1865 (2005).
Xiao, K. et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature 583, 286–289 (2020).
Hughes, L. J., Morton, O., Scheffers, B. R. & Edwards, D. P. The ecological drivers and consequences of wildlife trade. Biol. Rev. Camb. Phil. Soc. 98, 775–791 (2023).
Huong, N. Q. et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013–2014. PLoS ONE 15, e0237129 (2020).
Anthony, S. J. et al. Global patterns in coronavirus diversity. Virus Evol. 3, vex012 (2017).
He, W.-T. et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell 185, 1117–1129.e8 (2022).
Brook, C. E. et al. Population trends for two Malagasy fruit bats. Biol. Conserv. 234, 165–171 (2019).
Ripple, W. J. et al. Bushmeat hunting and extinction risk to the world’s mammals. R. Soc. Open. Sci. 3, 160498 (2016).
Roy, H. E. et al. (eds) Thematic Assessment Report on Invasive Alien Species and Their Control (IPBES Secretariat, 2023).
Bryant, J. E., Holmes, E. C. & Barrett, A. D. T. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 3, e75 (2007).
Mnzava, A., Monroe, A. C. & Okumu, F. Anopheles stephensi in Africa requires a more integrated response. Malar. J. 21, 156 (2022).
Ryan, S. J. et al. Mapping current and future thermal limits to suitability for malaria transmission by the invasive mosquito Anopheles stephensi. Malar. J. 22, 104 (2023).
Rohr, J. R. et al. A planetary health innovation for disease, food and water challenges in Africa. Nature 619, 782–787 (2023).
Weets, C. M. & Katz, R. Global approaches to tackling antimicrobial resistance: a comprehensive analysis of water, sanitation and hygiene policies. BMJ Glob. Health 9, e013855 (2024).
Bürgmann, H. et al. Water and sanitation: an essential battlefront in the war on antimicrobial resistance. FEMS Microbiol. Ecol. 94, https://doi.org/10.1093/femsec/fiy101 (2018).
Orozovic, G., Orozovic, K., Lennerstrand, J. & Olsen, B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS ONE 6, e16028 (2011).
Rosi, E. J., Fick, J. B. & Han, B. A. Are animal disease reservoirs at risk of human antiviral exposure? Environ. Sci. Technol. Lett. 10, 439–445 (2023).
Ostfeld, R. S. & Keesing, F. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 14, 722–728 (2000).
Couper, L. I., MacDonald, A. J. & Mordecai, E. A. Impact of prior and projected climate change on US Lyme disease incidence. Glob. Change Biol. 27, 738–754 (2021).
Plowright, R. K. et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. Biol. Sci. 278, 3703–3712 (2011).
Becker, D. J., Eby, P., Madden, W., Peel, A. J. & Plowright, R. K. Ecological conditions predict the intensity of Hendra virus excretion over space and time from bat reservoir hosts. Ecol. Lett. 26, 23–36 (2023).
Baranowski, K., Faust, C. L., Eby, P. & Bharti, N. Quantifying the impacts of Australian bushfires on native forests and gray-headed flying foxes. Glob. Ecol. Conserv. 27, e01566 (2021).
Mo, M. et al. Estimating flying-fox mortality associated with abandonments of pups and extreme heat events during the austral summer of 2019–20. Pac. Conserv. Biol. 28, 124–139 (2021).
Plaza, P. I., Gamarra-Toledo, V., Euguí, J. R. & Lambertucci, S. A. Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerg. Infect. Dis. 30, 444–452 (2024).
Guinat, C. et al. Bayesian phylodynamics reveals the transmission dynamics of avian influenza A(H7N9) virus at the human–live bird market interface in China. Proc. Natl Acad. Sci. USA 120, e2215610120 (2023).
Offeddu, V., Cowling, B. J. & Peiris, J. S. M. Interventions in live poultry markets for the control of avian influenza: a systematic review. One Health 2, 55–64 (2016).
Yin, S. et al. Habitat loss exacerbates pathogen spread: an agent-based model of avian influenza infection in migratory waterfowl. PLoS Comput. Biol. 18, e1009577 (2022).
Vandegrift, K. J., Sokolow, S. H., Daszak, P. & Kilpatrick, A. M. Ecology of avian influenza viruses in a changing world. Ann. NY Acad. Sci. 1195, 113–128 (2010).
Prosser, D. J., Teitelbaum, C. S., Yin, S., Hill, N. J. & Xiao, X. Climate change impacts on bird migration and highly pathogenic avian influenza. Nat. Microbiol. 8, 2223–2225 (2023).
Crits-Christoph, A. et al. Genetic tracing of market wildlife and viruses at the epicenter of the COVID-19 pandemic. Cell 187, 5468–5482.e11 (2024).
Pekar, J. E. et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science 377, 960–966 (2022).
Cui, J., Li, F. & Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192 (2019).
Lednicky, J. A. et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature 600, 133–137 (2021).
Vlasova, A. N. et al. Novel canine coronavirus isolated from a hospitalized patient with pneumonia in East Malaysia. Clin. Infect. Dis. 74, 446–454 (2022).
Rulli, M. C., D’Odorico, P., Galli, N. & Hayman, D. T. S. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat. Food 2, 409–416 (2021).
Beyer, R. M., Manica, A. & Mora, C. Shifts in global bat diversity suggest a possible role of climate change in the emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total. Environ. 767, 145413 (2021).
Warmuth, V. M., Metzler, D. & Zamora-Gutierrez, V. Human disturbance increases coronavirus prevalence in bats. Sci. Adv. 9, eadd0688 (2023).
Meyer, M. et al. Bat species assemblage predicts coronavirus prevalence. Nat. Commun. 15, 2887 (2024).
Busch, J. & Engelmann, J. Cost-effectiveness of reducing emissions from tropical deforestation, 2016–2050. Environ. Res. Lett. 13, 015001 (2017).
Jones, I. J. et al. Improving rural health care reduces illegal logging and conserves carbon in a tropical forest. Proc. Natl. Acad. Sci. USA 117, 28515–28524 (2020).
Rockström, J. et al. The planetary commons: a new paradigm for safeguarding Earth-regulating systems in the anthropocene. Proc. Natl. Acad. Sci. USA 121, e2301531121 (2024).
Kashwan, P., Biermann, F., Gupta, A. & Okereke, C. Planetary justice: prioritizing the poor in earth system governance. Earth Syst. Gov. 6, 100075 (2020).
Albery, G. F. et al. The science of the host–virus network. Nat. Microbiol. 6, 1483–1492 (2021).
Tseng, K. K. et al. Viral genomic features predict orthopoxvirus reservoir hosts. Preprint at bioRxiv https://doi.org/10.1101/2023.10.26.564211 (2023).
Fischhoff, I. R., Castellanos, A. A., Rodrigues, J. P. G. L. M., Varsani, A. & Han, B. A. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proc. R. Soc. B 288, 20211651 (2021).
Betke, B. A., Gottdenker, N. L., Meyers, L. A. & Becker, D. J. Ecological and evolutionary characteristics of anthropogenic roosting ability in bats of the world. iScience 27, 110369 (2024).
Pruvot, M. et al. WildHealthNet: supporting the development of sustainable wildlife health surveillance networks in Southeast Asia. Sci. Total. Environ. 863, 160748 (2023).
Stevens, T. et al. A minimum data standard for wildlife disease studies. Preprint at EcoEvoRxiv https://doi.org/10.32942/x2tw4j (2024).
Richards, B. J., Miller, K. J. & White, C. L. WHISPers—Providing Situational Awareness of Wildlife Disease Threats to the Nation—A Fact Sheet for the Biosurveillance Community (USGS, 2022).
Geoghegan, J. L. et al. Virological sampling of inaccessible wildlife with drones. Viruses 10, 300 (2018).
Sievers, B. L. et al. Smart markets: harnessing the potential of new technologies for endemic and emerging infectious disease surveillance in traditional food markets. J. Virol. 98, e0168323 (2024).
Wägele, J. W. et al. Towards a multisensor station for automated biodiversity monitoring. Basic. Appl. Ecol. 59, 105–138 (2022).
Ärje, J. et al. Automatic image‐based identification and biomass estimation of invertebrates. Methods Ecol. Evol. 11, 922–931 (2020).
Baird, D. J. & Hajibabaei, M. Biomonitoring 2.0: a new paradigm in ecosystem assessment made possible by next-generation DNA sequencing. Mol. Ecol. 21, 2039–2044 (2012).
Heberling, J. M., Miller, J. T., Noesgaard, D., Weingart, S. B. & Schigel, D. Data integration enables global biodiversity synthesis. Proc. Natl Acad. Sci. USA 118, e2018093118 (2021).
Li, S. L. et al. Mapping environmental suitability of Haemagogus and Sabethes spp. mosquitoes to understand sylvatic transmission risk of yellow fever virus in Brazil. PLoS Negl. Trop. Dis. 16, e0010019 (2022).
Gippet, J. M. W., Bates, O. K., Moulin, J. & Bertelsmeier, C. The global risk of infectious disease emergence from giant land snail invasion and pet trade. Parasit. Vectors 16, 363 (2023).
Simons, D., Attfield, L. A., Jones, K. E., Watson-Jones, D. & Kock, R. Rodent trapping studies as an overlooked information source for understanding endemic and novel zoonotic spillover. PLoS Negl. Trop. Dis. 17, e0010772 (2023).
Redding, D. W., Gibb, R. & Jones, K. E. Ecological impacts of climate change will transform public health priorities for zoonotic and vector-borne disease. Preprint at bioRxiv https://doi.org/10.1101/2024.02.09.24302575 (2024).
García-Peña, G. E. et al. Land-use change and rodent-borne diseases: hazards on the shared socioeconomic pathways. Phil. Trans. R. Soc. Lond. B 376, 20200362 (2021).
Astorga, F. et al. Biodiversity data supports research on human infectious diseases: global trends, challenges, and opportunities. One Health 16, 100484 (2023).
Edmunds, S. C. et al. Publishing data to support the fight against human vector-borne diseases. Gigascience 11, giac114 (2022).
Shimabukuro, P. et al. Bridging biodiversity and health: the Global Biodiversity Information Facility’s initiative on open data on vectors of human diseases. GigaByte 2024, gigabyte117 (2024).
Južnič-Zonta, Ž. et al. Mosquito Alert: leveraging citizen science to create a GBIF mosquito occurrence dataset. GigaByte 2022, gigabyte54 (2022).
Carney, R. M. et al. Integrating global citizen science platforms to enable next-generation surveillance of invasive and vector mosquitoes. Insects 13, 675 (2022).
Rund, S. S. C., Moise, I. K., Beier, J. C. & Martinez, M. E. Rescuing troves of hidden ecological data to tackle emerging mosquito-borne diseases. J. Am. Mosq. Control. Assoc. 35, 75–83 (2019).
DiEuliis, D., Johnson, K. R., Morse, S. S. & Schindel, D. E. Specimen collections should have a much bigger role in infectious disease research and response. Proc. Natl. Acad. Sci. USA 113, 4–7 (2016).
Thompson, C. W. et al. Preserve a voucher specimen! The critical need for integrating natural history collections in infectious disease studies. mBio 12, e02698-20 (2021).
Colella, J. P. et al. Leveraging natural history biorepositories as a global, decentralized, pathogen surveillance network. PLoS Pathog. 17, e1009583 (2021).
Wood, C. L. & Vanhove, M. P. M. Is the world wormier than it used to be? We’ll never know without natural history collections. J. Anim. Ecol. 92, 250–262 (2023).
Bell, K. C., Carlson, C. J. & Phillips, A. J. Parasite collections: overlooked resources for integrative research and conservation. Trends Parasitol. 34, 637–639 (2018).
Hämmerle, M. et al. Screening great ape museum specimens for DNA viruses. Sci. Rep. 14, 29806 (2024).
Speer, K. A. et al. A comparative study of RNA yields from museum specimens, including an optimized protocol for extracting RNA from formalin-fixed specimens. Front. Ecol. Evol. 10, 953131 (2022).
Martinez, S. et al. Living safely with bats: lessons in developing and sharing a global One Health educational resource. Glob. Health Sci. Pract. 10, e2200106 (2022).
Amman, B. R. et al. Marburgvirus resurgence in Kitaka Mine bat population after extermination attempts, Uganda. Emerg. Infect. Dis. 20, 1761–1764 (2014).
Viana, M. et al. Effects of culling vampire bats on the spatial spread and spillover of rabies virus. Sci. Adv. 9, eadd7437 (2023).
Jenkins, H. E. et al. Effects of culling on spatial associations of Mycobacterium bovis infections in badgers and cattle. J. Appl. Ecol. 44, 897–908 (2007).
Hopkins, S. R. et al. Evidence gaps and diversity among potential win–win solutions for conservation and human infectious disease control. Lancet Planet. Health 6, e694–e705 (2022).
Reaser, J. K., Witt, A., Tabor, G. M., Hudson, P. J. & Plowright, R. K. Ecological countermeasures for preventing zoonotic disease outbreaks: when ecological restoration is a human health imperative. Restor. Ecol. 29, e13357 (2021).
Prist, P. R. et al. Promoting landscapes with a low zoonotic disease risk through forest restoration: the need for comprehensive guidelines. J. Appl. Ecol. 60, 1510–1521 (2023).
Gallagher, M. R. et al. Can restoration of fire-dependent ecosystems reduce ticks and tick-borne disease prevalence in the eastern United States? Ecol. Appl. 32, e2637 (2022).
Pattanayak, S. K., Kramer, R. A. & Vincent, J. R. Ecosystem change and human health: implementation economics and policy. Phil. Trans. R. Soc. Lond. B 372, 20160130 (2017).
Hopkins, S. R. et al. How to identify win–win interventions that benefit human health and conservation. Nat. Sustain. 4, 298–304 (2020).
Trisos, C. H. et al. Mosquito net fishing exemplifies conflict among Sustainable Development Goals. Nat. Sustain. 2, 5–7 (2019).
Bonwitt, J. et al. Unintended consequences of the ‘bushmeat ban’ in West Africa during the 2013–2016 Ebola virus disease epidemic. Soc. Sci. Med. 200, 166–173 (2018).
Li, Y. et al. Closure of live bird markets leads to the spread of H7N9 influenza in China. PLoS ONE 13, e0208884 (2018).
Quammen, D. Spillover: Animal Infections and the Next Human Pandemic (W. W. Norton, 2012).
Osofsky, S. A., Lieberman, S., Walzer, C., Lee, H. L. & Neme, L. A. An immediate way to lower pandemic risk: (not) seizing the low-hanging fruit (bat). Lancet Planet. Health 7, e518–e526 (2023).
Dzingirai, V. et al. Structural drivers of vulnerability to zoonotic disease in Africa. Phil. Trans. R. Soc. Lond. B 372, 20160169 (2017).
Magalhães, A. R. et al. Neglected tropical diseases risk correlates with poverty and early ecosystem destruction. Infect. Dis. Poverty 12, 32 (2023).
Sokolow, S. H. et al. Ecological and socioeconomic factors associated with the human burden of environmentally mediated pathogens: a global analysis. Lancet Planet. Health 6, e870–e879 (2022).
Farmer, P. Social inequalities and emerging infectious diseases. Emerg. Infect. Dis. 2, 259–269 (1996).
Fiorella, K. J. et al. Human health alters the sustainability of fishing practices in east Africa. Proc. Natl. Acad. Sci. USA 114, 4171–4176 (2017).
Bonds, M. H., Keenan, D. C., Rohani, P. & Sachs, J. D. Poverty trap formed by the ecology of infectious diseases. Proc. Biol. Sci. 277, 1185–1192 (2010).
Worsley-Tonks, K. E. L. et al. Strengthening global health security by improving disease surveillance in remote rural areas of low-income and middle-income countries. Lancet Glob. Health 10, e579–e584 (2022).
Wenham, C. et al. Global health security and universal health coverage: from a marriage of convenience to a strategic, effective partnership. BMJ Glob. Health 4, e001145 (2019).
Moore, M. et al. Core components of infectious disease outbreak response. SSM - Health Syst. 3, 100030 (2024).
Pike, J., Bogich, T., Elwood, S., Finnoff, D. C. & Daszak, P. Economic optimization of a global strategy to address the pandemic threat. Proc. Natl. Acad. Sci. USA 111, 18519–18523 (2014).
Galaz, V. et al. Financial influence on global risks of zoonotic emerging and re-emerging diseases: an integrative analysis. Lancet Planet. Health 7, e951–e962 (2023).
Hoffman, S. J. et al. International treaties have mostly failed to produce their intended effects. Proc. Natl. Acad. Sci. USA 119, e2122854119 (2022).
Liew, J. H. et al. International socioeconomic inequality drives trade patterns in the global wildlife market. Sci. Adv. 7, eabf7679 (2021).
Gallo-Cajiao, E. et al. Global governance for pandemic prevention and the wildlife trade. Lancet Planet. Health 7, e336–e345 (2023).
Heckley, A. M., Lock, L. R. & Becker, D. J. A meta‐analysis exploring associations between habitat degradation and Neotropical bat virus prevalence and seroprevalence. Ecography 2024, e07041 (2024).
Cohen, L. E., Fagre, A. C., Chen, B., Carlson, C. J. & Becker, D. J. Coronavirus sampling and surveillance in bats from 1996–2019: a systematic review and meta-analysis. Nat. Microbiol. 8, 1176–1186 (2023).
Vicente-Santos, A. et al. Serum proteomics reveals a tolerant immune phenotype across multiple pathogen taxa in wild vampire bats. Front. Immunol. 14, 1281732 (2023).
Albery, G. F., Sweeny, A. R., Becker, D. J. & Bansal, S. Fine‐scale spatial patterns of wildlife disease are common and understudied. Funct. Ecol. 36, 214–225 (2022).
Civitello, D. J. et al. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 112, 8667–8671 (2015).
Di Bari, C. et al. The global burden of neglected zoonotic diseases: current state of evidence. One Health 17, 100595 (2023).
Benavides, J. A. et al. Defining new pathways to manage the ongoing emergence of bat rabies in Latin America. Viruses 12, 1002 (2020).
Standley, C. J., Fogarty, A. S., Miller, L. N. & Sorrell, E. M. One Health systems assessments for sustainable capacity strengthening to control priority zoonotic diseases within and between countries. Risk Manag. Healthc. Policy 16, 2497–2504 (2023).
Ceballos, G. et al. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015).
Van Kerkhove, M. D., Ryan, M. J. & Ghebreyesus, T. A. Preparing for ‘Disease X’. Science 374, 377 (2021).
Morse, S. S. et al. Prediction and prevention of the next pandemic zoonosis. Lancet 380, 1956–1965 (2012).
Wolfe, N. D., Daszak, P., Kilpatrick, A. M. & Burke, D. S. Bushmeat hunting, deforestation, and prediction of zoonoses emergence. Emerg. Infect. Dis. 11, 1822–1827 (2005).
Wolfe, N. D. et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363, 932–937 (2004).
Dai, L. et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell 182, 722–733.e11 (2020).
Nachbagauer, R. et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021).
Gibb, R. et al. Data proliferation, reconciliation, and synthesis in viral ecology. Bioscience 71, 1148–1156 (2021).
Stephens, P. R. et al. Global Mammal Parasite Database version 2.0. Ecology 98, 1476 (2017).
Bensch, S., Hellgren, O. & Pérez-Tris, J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353–1358 (2009).
Luis, A. D. et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Biol. Sci. 280, 20122753 (2013).
Heckley, A. M. & Becker, D. J. Tropical bat ectoparasitism in continuous versus fragmented forests: a gap analysis and preliminary meta-analysis. Ecol. Evol. 13, e9784 (2023).
Murray, M. H. et al. City sicker? A meta‐analysis of wildlife health and urbanization. Front. Ecol. Environ. 17, 575–583 (2019).
Fanelli, D. Negative results are disappearing from most disciplines and countries. Scientometrics 90, 891–904 (2012).
Winands-Kalkuhl, S. & Holm-Müller, K. Bilateral vs. multilateral? On the economics and politics of a global mechanism for genetic resource use. J. Nat. Resour. Policy Res. 7, 305–322 (2015).
Deplazes-Zemp, A. et al. The Nagoya protocol could backfire on the Global South. Nat. Ecol. Evol. 2, 917–919 (2018).
Lajaunie, C. & Morand, S. Nagoya protocol and infectious diseases: hindrance or opportunity? Front. Public. Health 8, 238 (2020).
Laird, S. et al. Rethink the expansion of access and benefit sharing. Science 367, 1200–1202 (2020).
Richter, C. D. European Patent Office grants controversial patent protecting virus: lessons from the Middle East respiratory syndrome coronavirus outbreak. Nat. Biotechnol. 39, 287–290 (2021).
Moore, S., Hill, E. M., Dyson, L., Tildesley, M. J. & Keeling, M. J. Retrospectively modeling the effects of increased global vaccine sharing on the COVID-19 pandemic. Nat. Med. 28, 2416–2423 (2022).
Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 22, 1293–1302 (2022).
Sedyaningsih, E. R., Isfandari, S., Soendoro, T. & Supari, S. F. Towards mutual trust, transparency and equity in virus sharing mechanism: the avian influenza case of Indonesia. Ann. Acad. Med. Singap. 37, 482–488 (2008).
Carlson, C. J. et al. Save lives in the next pandemic: ensure vaccine equity now. Nature 626, 952–953 (2024).
Carlson, C. et al. Engineering data equity: the LISTEN principles. Preprint at SSRN https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5022896 (2024).
Acknowledgements
The authors thank S. Hopkins, F. Keesing, R. Ostfeld, A. Phelan, Z. O’Donoghue, A. Sweeny, L. Damodaran and S. Seifert for helpful conversations and edits during the preparation of the manuscript. This work was supported by an National Science Foundation (NSF) Biology Integration Institute grant (DBI 2213854) to the Verena programme (viralemergence.org). C.J.C. was additionally supported by the NSF (SES 2314520).
Author information
Authors and Affiliations
Contributions
C.J.C., C.B.B., D.J.B., C.A.W. and T.P. researched data for the article. All authors contributed substantially to discussion of the content, wrote the article, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.J.C. has previously received funding support from the Coalition for Epidemic Preparedness Innovations; has been a consultant for the US Department of State on Global Health issues; and receives funding from the Carnegie Corporation of New York and the US National Science Foundation for research related to the Pandemic Agreement. D.J.B. is a member of the Lancet-PPATS Commission on Prevention of Viral Spillover. The authors declare no other competing interests.
Peer review
Peer review information
Nature Reviews Biodiversity thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GBIF: www.gbif.org
One Health High-Level Expert Panel: https://www.who.int/groups/one-health-high-level-expert-panel
One Health Joint Plan of Action (2022-2026): https://www.who.int/publications/i/item/9789240059139
The Pathogen Harmonized Observatory (PHAROS) database: www.pharos.viralemergence.org
Verena: www.viralemergence.org
Supplementary information
Glossary
- Arbovirus
-
A shorthand for arthropod-borne (such as mosquito-borne or tick-borne) virus.
- Emerging infectious disease
-
An infectious disease that has recently undergone an expansion of host range, geographic range, impact or even just attention from scientific research or public health; this term is subjective, and is frequently used interchangeably with the terms zoonotic, vector-borne and environmentally transmitted diseases.
- Epidemic
-
An infectious disease outbreak in humans, with a significant duration, size or impact.
- Epizootic
-
An infectious disease outbreak in non-human animals, with a significant duration, size or impact.
- Host competence
-
The ability of a host to amplify and transmit infection to another susceptible host or vector.
- Macroparasite
-
Parasites that can be observed with the naked eye, such as ticks, fleas and some worms; the term is sometimes used interchangeably with the term parasites.
- Microparasite
-
Microscopic parasites such as bacteria, viruses and some worms (for example, schistosomes); the term is sometimes used interchangeably with the term pathogens.
- One Health
-
A principle that emphasizes the connections between human health, animal health and the environment, as well as the importance of solutions that benefit all three.
- Pandemic
-
A high-impact global outbreak of a human infectious disease. This term is subjective; some sources use the term to capture any global outbreak, and others limit their definition based on the degree of circulation or mortality (see Supplementary Note 3 for all criteria we use).
- Panzootic
-
A high-impact global outbreak of an infectious disease of animals.
- Parasite
-
An organism that exists in an adversarial symbiotic relationship with a host.
- Parasitoid
-
An organism that must kill a host to complete its life cycle (for example, braconid wasps).
- Pathogen
-
An infectious microorganism that causes disease in a host.
- Reservoir
-
Competent hosts that sustain pathogen transmission at the population or community level.
- Spillback
-
Human-to-animal pathogen transmission (also called reverse zoonosis).
- Spillover
-
Animal-to-human pathogen transmission.
- Synanthropic
-
Living alongside humans (for example, in cities or human-built structures).
- Vector-borne disease
-
A disease caused by a pathogen that is transmitted by an arthropod vector, such as a mosquito, tick or flea.
- Zoonotic disease
-
A human infectious disease caused by a pathogen of animal origin.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carlson, C.J., Brookson, C.B., Becker, D.J. et al. Pathogens and planetary change. Nat. Rev. Biodivers. 1, 32–49 (2025). https://doi.org/10.1038/s44358-024-00005-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44358-024-00005-w
This article is cited by
-
Adaptive ecosystem restoration to mitigate zoonotic risks
Nature Ecology & Evolution (2025)
-
Assessing the spatiotemporal distribution of bufonid herpesvirus 1 (BfHV1) in Europe
Scientific Reports (2025)
-
Upscaling effects on infectious disease emergence risk emphasize the need for local planning in primary prevention within biodiversity hotspots
Scientific Reports (2025)
-
Viral epidemic potential is not uniformly distributed across the bat phylogeny
Communications Biology (2025)
-
Public trust in science has declined since COVID — virologists need to unite around safety standards
Nature (2025)