Abstract
The learning health system (LHS) offers a framework to accelerate evidence generation and care improvement, yet widespread adoption remains limited. In this perspective, we explore strategies to operationalize the LHS in the era of artificial intelligence, including biomedical informatics and health information technology integration, workforce development, quality improvement, and data governance. We highlight promising institutional models and propose policy, educational, and financial reforms to support scalable, value-driven innovation in increasingly complex and resource-constrained health systems.
Similar content being viewed by others
Introduction
The learning health system (LHS) can be defined as the infrastructure, diverse stakeholders, and culture required to integrate continuous cycles of evidence-based care model improvement and innovation1. This cyclical process consists of data collection and analysis, generation of new knowledge, and subsequent translation of this knowledge into interventions designed to improve healthcare quality and value2. The LHS concept has been endorsed by the Joint Commission, Agency for Healthcare Research and Quality (AHRQ), the National Academy of Medicine, and the World Health Organization3,4,5. Consensus of the model’s potential to transform health systems has been accelerated by mandates for greater efficiency and higher-quality care in the setting of growing fiscal constraints. But while the LHS ecosystem and its requisite competencies have been well described1,6,7,8,9,10, multidimensional limitations to cultivating this complex adaptive system persist, including siloed data, lack of adaptive governance, misaligned incentives, underdeveloped workforce competencies and cultural resistance to change11. This Perspective argues that to fully realize the transformative potential of artificial intelligence (AI) in health care, and for the long-standing translational research gap to be closed, LHS principles must evolve from aspirational goals to a consensus strategy in US health systems. We describe how the challenges facing AI implementation and LHS development frequently overlap and explore how AI may serve to accelerate the LHS, offer examples of health systems that have successfully adopted LHS principles, highlight strategies to address common challenges in the era of AI, review current and future approaches to workforce education, and conclude with recommendations for how the LHS might successfully evolve in the AI era despite increasing fiscal constraints.
Ambidextrous health information technology, biomedical informatics leadership
Widespread adoption of the electronic health record (EHR) marked the beginning of the digital age of health care. Subsequent advances in big data12, digital care13, and AI14 have created opportunities for alignment between health information technology (HIT) and biomedical informatics (BMI). HIT encompasses the hardware, software, infrastructure design, and implementation expertise necessary to manage and protect clinical and administrative data across a health system. This includes software selection, contract negotiation, systems integration and testing, and user training—all aimed at delivering enterprise-wide, reliable, cost-effective, and secure digital solutions in support of operational stability and strategic goals. In contrast, BMI is an interdisciplinary scientific field focused on the effective use of biomedical data, information, and technology to improve human health. BMI includes disciplines such as data science, natural language processing, predictive modeling, and implementation science, and is often directed at solving complex problems across people, processes, and technologies15. When aligned, HIT and BMI enable organizations to balance the demands of operational reliability with the need for continuous learning and innovation. When aligned, HIT and BMI enable organizations to balance the requirements for operational reliability and effectiveness with needs for change, growth, and discovery16,17. Such ambidextrous leadership—the optimization of current operations while simultaneously driving novel solutions to challenges and innovation—has been shown to improve organizational performance in both healthcare and technology industries18,19.
The COVID-19 pandemic accelerated many health systems’ adoption of LHS values and demonstrated the impact from strategic allocation of cross-disciplinary organizational resources to support time-sensitive quality and operational priorities, as well as high-value research20,21,22,23,24,25,26,27. Productive collaboration between HIT and BMI efficiently generated knowledge for time-sensitive clinical and operations decision-making; and strategic implementation of digital health innovations at scale. Examples included the rapid development of capacity-demand prediction dashboards through HIT–BMI collaboration, telehealth-supported direct and remote patient monitoring care models in a New York City health system challenged with inpatient capacity; basic laboratory test result-based predictions of Covid infection, pneumonia, and clinical deterioration; and the rapid implementation of a EHR-integrated employee vaccination superstation28,29,30,31. Academic institutions reporting maturation in this operationalized synergy of HIT and BMI describe the stakeholder engagement, relationship building, and collaborative, matrixed leadership required to achieve significant gains, including the pragmatic timelines for such transformation8,15,27,28,32. Another urgent priority was the democratization of data access for rapid-cycle testing of quality improvement processes by limiting regulatory barriers without compromising research ethics, patient privacy, and consent standards, which a few academic health systems have described28,33.
Limitations to high-performing routine HIT systems, such as issues with data quality, missingness, and bias; data access and siloed data from disparate sources; errors in data production; lack of standardized data production processes; and inadequate interoperability, all continue to conspire against the broad implementation of an LHS model34,35. Data harmonization techniques have been described in detail, as it has the importance of universally recognized data standards34,36,37. Recent studies have demonstrated that large language models (LLMs) are capable of executing data extraction, preprocessing, and foundational analytic tasks with increasing reliability38,39,40,41. A future with automated or semi-automated data harmonization could be transformative, significantly reducing barriers to data acquisition and standardization and enabling researchers to devote greater effort towards higher-order analytics and model development. However, the implementation of LLMs for such foundational tasks will require strategies to mitigate hallucinations, ensure pipeline transparency and auditability, and address challenges related to reproducibility and regulatory compliance42. While such foundational progress continues, rapid growth in digital health innovation and the increasing demand for health data have brought new challenges. These include capacity limitations and high costs for data storage and analysis; gaps in the translational science expertise needed to ensure diverse BMI clinical interventions (e.g., AI-based clinical decision support, guideline delivery systems, population health management platforms and dashboards) are effectively integrated into clinical workflows and clinician practice to yield the desired impact43,44,45,46,47, as well as the capacity and leadership for functional governance of these complex systems48,49,50.
Research and quality in the AI era of the LHS: two sides of the same coin?
To date the explosion of healthcare AI research has predominantly centered on the development and validation of models using curated data sets, efforts that have demonstrated the potential of AI rather than its real-world impact46,51,52,53. In contrast, relatively few AI tools have been implemented into clinical workflows and rigorously evaluated for their effect on patient-centered outcomes or cost-effectiveness. Such implementation efforts are substantially more ambitious endeavors, requiring high-quality, interoperable real-time data, substantial computational power, and the technical expertise to enable seamless EHR integration. Moreover, the risk of distributional shift and data drift poses a threat to the sustained performance of deployed models, necessitating adaptive or continuous learning systems. These capabilities further increase complexity to the informatics infrastructure and technical expertise required to support them54,55,56. The need for rapidly accessible, interoperable data, alongside robust data management and governance frameworks, has long been championed by healthcare researchers and research ethicists, yet remains largely aspirational across most health systems37. However, growing recognition that high-performing HIT–BMI capabilities are foundational to the safe and effective clinical implementation of adaptive AI algorithms underscores a critical insight: data readiness is the essential first step toward AI readiness, closely followed by clinician readiness57,58,59. The promise of AI enhancing care quality and operational efficiency may be the tipping point for health systems to regard investments in data infrastructure and specialized workforce development as strategic imperatives, particularly for the deployment of locally developed algorithms in academic medical centers60,61.
The benefits afforded to the LHS are self-evident: the rapid acquisition of data and prompt analysis of care delivery gaps, along with the evaluation of interventions designed to close them. Notable examples of centralized health system network data repositories—Patient-Centered Outcomes Research Institute’s PCORnet and INSIGHT clinical research network, and University of California’s Center for Data-driven Insights and Innovation (CDI2)—demonstrate the potential to advance care quality, enhance health system operations, and accelerate clinical research62,63,64. Yet, despite the downstream opportunities such investments present, many health systems face persistent challenges in generating meaningful translational research. Contributing factors include prohibitive costs for the requisite infrastructure (particularly local development of AI models), concerns regarding data privacy and sharing, inflexible institutional review (IRBs) processes, and competing organizational priorities. The traditional model of research within most health systems has been heavily dependent on funding from large federal agencies (e.g., the National Institutes of Health), with a predominant focus on basic science65. While this emphasis has advanced critical foundational knowledge, it may have constrained the translation of discoveries into clinical impact. This paradigm is now under pressure from shifting federal research priorities away from the historic investments in basic science, including reductions in both direct funding and indirect costs support65,66,67. Amid these challenges may emerge the opportunity for health systems to realign their strategic research capabilities with evolving funding priorities, potentially strengthening capacity for translational and clinical research that advances LHS objectives.
Pre-clinical silent trials of clinical decision support (CDS) tools, run in the background without influencing clinical care, have been endorsed by ethical AI implementation frameworks. These trials have been shown to improve model performance and may assist with successful downstream implementation68,69. Integration of CDS tools into the EHR enables prospective, quality improvement-focused validation, including assessments of data drift, bias, and feasibility through mixed-methods studies, the results of which can inform downstream CDS design refinements prior to the safe execution of clinical studies69. Similarly, rapid cycle comparative effectiveness trials of quality improvement interventions have been facilitated through total EHR-integration of automated A/B and randomized testing methods70,71,72. These approaches can guide critical health system decisions regarding intervention impact and support iterative user-centered design, closing the traditional gap between clinical operations and the cost and expediency of rigorous scientific evaluation.
The principles of the LHS not only provide a foundation for generating high-quality research but also offer a natural pathway to an alternative approach to rapidly testing interventions: quality improvement. The National Academy of Medicine defines Quality as the “degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge”. Continual quality improvement is a core concept of the LHS. A foundational pillar in optimizing QI outcomes is the democratization of data access for rapid cycle testing of quality improvement processes by limiting regulatory barriers without compromising research ethics, patient privacy, and consent standards. The UC San Diego Health System developed a multidisciplinary stakeholder group approach (Align and Coordinate Quality Improvement, Research, and Evaluation–ACQUIRE), including members of the IRB, to expedite quality improvement and health sciences research that is aligned with health system priorities33. This approach provides the foundation for meaningful quality initiatives, some of which have had a profound impact on patient-centered outcomes73,74. Other health systems have described similar approaches with meaningful improvements in quality, for example, the impact of large-scale, randomized patient notification promoting influenza vaccination compliance to optimize messaging strategies75,76. New York University Langone Health has also championed randomized quality initiatives (e.g., strategies to improve smoking cession, minimizing missed appointments) and reports that the savings from these interventions more than paid for the operational costs for QI programmatic oversight needed to run the program overseeing these quality initiatives70.
Education and workforce development
Novel frameworks and tools have been used to identify common barriers to implementing the LHS77, including inadequate stakeholder understanding of LHS principles and processes, and insufficient training for healthcare professionals (HCP) to effectively fulfill their collaborative roles within the model11,78. The proliferation of Master of Business Administration (MBA) programs in healthcare, often linked to the recognition of systemic challenges in U.S. healthcare, emphasizes management training79. The value of MBA education in advancing population health outcomes remains unclear, with studies demonstrating diverse motivations among healthcare MBA students80,81,82,83. Similarly, traditional graduate and post-graduate research training programs have not been primarily designed to develop the attributes and skills required for research within an LHS framework. Despite the absence of an established standard for US healthcare workforce education in health system improvement science (or their roles in it), a diverse range of educational programs serve to cultivate expertise aligned with LMH principles.
Since 2018, the Patient Centered Outcomes Research Institute and the AHRQ have jointly supported the development of LHS Researcher Training Programs and LHS Centers of Excellence. Their explicit goal has been to train researchers in the core competencies necessary for the collaborative generation of new knowledge that can be rapidly implemented to improve the quality of care, patient outcomes, and health system performance84. In addition to traditional research methods, these competencies include systems science; ethics of research and implementation in health systems; improvement and implementation science; engagement, leadership, and research management6,85. Similarly, curricula of Accreditation Council for Graduate Medical Education (ACGME)—accredited clinical fellowship programs in both Clinical Informatics (a subspecialty recognized by ABMS) and Healthcare Administration, Leadership, and Management have included many comparable competencies aligned with LHS concepts86,87. Further, some contemporary clinical informatics master’s programs have begun to incorporate focused, multidisciplinary management training, aimed to equip BMI leaders with the requisite leadership skills to successfully design and implement novel healthcare technologies into clinical practice88,89,90.
Other LHS educational programs have been described for PhD students91, clinician trainees and clinicians92,93,94,95, as well as for interdisciplinary professionals96,97; including LHS postgraduate certificate and degree programs at US centers of excellence98,99. Importantly, such changes can be easily added within existing training programs with minimal additional cost. For example, surgical residents at the University of California, San Diego may gain experience with LHS principles during protected research time. Skills acquired through this training have been instrumental in improving completed operative consent documentation, adherence to duty hour regulations, and crisis management25,100,101. However, formal evaluation of these training programs is limited. Future research is needed to examine their long-term impact on trainee career trajectories, to develop training strategies that engage non-specialist frontline HCPs, and to evaluate the broader effects of these interventions on staff engagement, wellbeing, and workforce retention96,102,103,104,105.
The digital age of healthcare has introduced new challenges well beyond the longstanding fragmentation and operational complexity of the U.S. health system. Clinicians face a growing crisis of information overload, compounded by traditional educational models that emphasize biological knowledge mastery, clinical information acquisition, and task completion106. The anticipated disruption of knowledge-based tasks by AI presents an opportunity to evolve medical training by integrating traditional clinical reasoning with computational expertise. Recent proposals have called for a radical redesign of medical curricula, prioritizing higher-order meta-cognitive skills such as knowledge management and contextualized synthesis, as well as problem solving, and discovery107. Curricular reform should also address the practical application of digital technologies; foundational training in BMI and data analytics to work in the era of big data; and education in health system improvement science. Learners should be equipped to understand, interpret, and manage both the capabilities and limitations of AI107; as well as to implement and evaluate constantly evolving, diverse technology-based interventions108,109.
In recognition of the evolving competencies required in digital health care models, the American Medical Association and the Association of American Medical Colleges have advocated for the integration of data science education into the training of HCPs and health administrators109,110,111. While framed within the context of modern digital health, this advocacy is not new112. Calls for clinician education to expand beyond it’s bioscience foundation and include systems engineering and information science to mitigate against technical obsolescence dates back over half a century113,114. Despite continued expert recommendations, consensus curriculum requirements at the level of the ACGME, Liaison Committee on Medical Education, or National Board of Medical Examiners have yet to reflect significant change115,116. More significant progress has been achieved within individual medical schools and health systems, with programmatic development to integrate foundational clinical informatics and digital health knowledge into medical school117,118 and residency curriculums119, as well as nursing120 and healthcare executive continued education121,122. Notable institutions, such as McWilliams School of Biomedical Informatics at UT Houston and Jacobs Technion-Cornell Institute at Cornell Tech, have introduced distinct but equally broad masters curricula aimed at cultivating the diverse expertise required for healthcare innovation and transformation107,123. In acknowledgement of current variability, the creation of a Master of Digital Health, analogous to the masters in public health, has recently been proposed124. This degress would offer a standardized core curriculum spanning foundational competencies across requisite domains, with optional specialized tracks tailored to specific areas of practice and expertise.
Towards financial viability—strategies to operationalize the LHS
Wide-spread adoption of the LHS has been constrained, in part by the perceived imbalance between cost and benefit within a predominantly fee-for-service U.S. healthcare model. This challenge is further compounded by dramatically increasing administrative and traditional research costs over recent decades125,126,127. The recent announcement to immediately standardize indirect cost rates of federal research grants will likely compound these challenges for academic institutions, at least in the short- to medium-term66,67. Some sociotechnical elements of the LHS, such as HIT–BMI integration, interdisciplinary alignment, workforce training curriculum changes, and QI culture refinement of IRB boundaries, are relatively cost-neutral, primarily requiring continuous cultural prioritization and incentive restructuring to yield long-term impact. In contrast, investments in digital health innovations and the expansion of HIT/BMI infrastructure necessary to support both the LHS and the AI-enabled future of healthcare are substantially cost-additive, particularly when viewed through the lens of traditional research funding models.
At a meso-level, increased internal front-end investments in pragmatic translational, clinical, health services, and health economics research infrastructure may yield downstream economic returns for individual health systems. Such infrastructure enables the evaluations of digital care innovations through rapid-cycle science, generating time-sensitive knowledge to inform superior investment strategy in a competitive environment characterized by rapidly evolving and high-cost health technologies47. Organizations with the capabilities and capacity for continuous, data-driven decisions regarding investment in care model innovation, grounded in locally curated knowledge of real-world impact, may gain a meaningful market advantage. This includes confirming that technology-based interventions produce sustained improvements in patient-centered outcomes, determining cost effectiveness at scale, and verifying that AI systems improve operational process efficiency as intended. Examples of health systems deliberately integrating LHS and executive strategy include Penn Medicine’s Center for Health Care Innovation and Transformation, University of Colorado’s Health Innovation Center, UC San Diego’s Jacobs Center for Health Innovation, and Stanford University’s Clinical Excellence Research Center128,129,130,131,132,133. These institutions leverage strategic leadership roles, academic infrastructure, multidisciplinary research expertise, and large, high-quality datasets to rapidly identify, evaluate, iterate, and prioritize promising care model interventions and facilitate the implementation and dissemination of these interventions at scale for improved healthcare outcomes, efficiency, and value. Should federal oversight of healthcare AI adopt a decentralized governance model, the burden of infrastructure would fall to individual health systems, effectively creating a new mandatory cost center. This shift could incentivize systems to creatively extract additional local value through investments in LHS infrastructure, as described above. Similarly, thoughtful reallocation of resources from low-value clinical programs towards comparative effectiveness research capabilities could support the development of scalable, evidence-based AI strategies and broader LHS functionality134,135.
At a macro-level, planned reductions of indirect cost support from the NIH may increase the financial burden of traditional research, potentially motivating federal and private foundations to expand investments in health services research. Prioritizing scientific inquiry focused on improving care delivery efficiency may serve to accelerate both digital health innovation and the reengineering of care delivery, ultimately enhancing cost effectiveness136. There is a growing need for the Centers for Medicare and Medicaid Services to further develop financing and reimbursement models for emerging digital and innovative care models that have yet to be established as standard care137. Academic health systems that cultivate the LHS functionality to generate new evidence on the efficacy and cost-effectiveness of novel clinical interventions could be eligible for targeted support. Such support may be especially crucial for publicly funded health systems striving towards value-based care. This may be partly justified if centralization of AI oversight and governance can be achieved at LHS-designated centers and demonstrates lower cumulative costs than similar fragmented processes within every single health system138.
Some drivers of rising healthcare costs (e.g., aging population, increasing prevalence of chronic and complex conditions, growing cybersecurity demands) cannot be easily reversed for the subsequent reallocation of capital towards fixed LHS costs, such as HIT–BMI expansion and research infrastructure. However, other causes, such as the dramatic increases in healthcare administration expenses over the past 30 years, warrant targeted interruption to improve system efficiency125,139,140. Continued advocacy for insurance regulation reform, including the simplification of prior authorization and related administrative requirements, may help offset the burden of increasingly complex commercial insurer policies141. Advances in AI present a pivotal opportunity to reduce costs by semi-automating numerous labor-intensive administrative tasks. Applications include medical coding and billing, insurance authorizations, denial appeals, and other claims processes, and regulatory reporting of quality measures. Early evaluations of such AI-enabled automation are promising, suggesting the potential for significant cost savings through operational efficiency142,143,144.
Conclusion
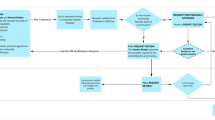
The LHS affords a compelling model for advancing health care delivery in an era shaped by digital innovation and AI. Although the core principles of the LHS have garnered broad support, realizing their full potential and overcoming challenges requires deliberate investment in data infrastructure, integration of HIT and BMI, workforce development, and adaptive governance (Fig. 1). Foreword thinking academic health systems have demonstrated that meaningful progress is achievable when clinical, operational, and research priorities are aligned around continuous learning. While many health systems face different barriers, creative solutions may enable motivated ones to move forward with this approach (Table 1). Looking ahead, the convergence of AI, digital infrastructure, and operational analytics may enable health systems not only to justify investments in LHS infrastructure but to restructure the cost model of care delivery itself. If AI can meaningfully reduce healthcare’s exorbitant administrative expenses, it may become the first innovation capable of offsetting the cost of its own adoption. Realizing the LHS as a strategic imperative demands more than technical progress—it will require the coordinated redesign of health system infrastructure to enable continuous learning, adaptation, and innovation at scale. Institutions that succeed in this may not only gain a durable competitive advantage but also chart a path toward long-term sustainability in academic health care.
By resolving barriers to LHS adoption—ranging from governance inefficiencies to interdisciplinary silos—health systems can unlock transformative returns. These include not only AI-readiness and digital innovation capacity, but also measurable improvements in care quality, operational efficiency, and staff retention.
Data availability
No datasets were generated or analysed during the current study.
References
Friedman, C. P., Lomotan, E. A., Richardson, J. E. & Ridgeway, J. L. Socio-technical infrastructure for a learning health system. Learn Health Syst. 8, e10405 (2024).
Friedman, C. P., Wong, A. K. & Blumenthal, D. Achieving a nationwide learning health system. Sci. Transl. Med. 2, 57cm29 (2010).
AHRQ Research Summit on Learning Health Systems: Executive Summary. Agency for Healthcare Research and Quality https://www.ahrq.gov/news/events/ahrq-research-summit-learning-health-system-summary.html (2017).
Smith, M., Saunders, R., Stuckhardt, L., McGinnis, J. M., eds. Best Care at lower cost: the path to continuously learning health care in America (National Academies Press, 2013).
World Health Organization. Learning health systems: pathways to progress. Flagship report of the Alliance for Health Policy and Systems Research (World Health Organization, 2022).
Forrest, C. B., Chesley, F. D. Jr., Tregear, M. L. & Mistry, K. B. Development of the learning health system researcher core competencies. Health Serv. Res. 53, 2615–2632 (2018).
Franklin, P. D. et al. Development of a learning health system science competency assessment to guide training and proficiency assessment. Learn Health Syst. 6, e10343 (2022).
McDonald, P. L., Phillips, J., Harwood, K., Maring, J. & van der Wees, P. J. Identifying requisite learning health system competencies: a scoping review. BMJ Open 12, e061124 (2022).
Rosenthal, G. E. et al. The academic learning health system: a framework for integrating the multiple missions of academic medical centers. Acad. Med. 98, 1002–1007 (2023).
Lindsell, C. J. et al. Learning from what we do, and doing what we learn: a learning health care system in action. Acad. Med. 96, 1291–1299 (2021).
Shaw, L. et al. Developing a codebook to characterize barriers, enablers, and strategies for implementing learning health systems from a multilevel perspective. Learn Health Syst. 9, e10452 (2025).
Mallappallil, M., Sabu, J., Gruessner, A. & Salifu, M. A review of big data and medical research. SAGE Open Med. 8, 2050312120934839 (2020).
Burde, H. Health law the HITECH act-an overview. Virtual Mentor 13, 172–175 (2011).
Haug, C. J. & Drazen, J. M. Artificial intelligence and machine learning in clinical medicine, 2023. N. Engl. J. Med. 388, 1201–1208 (2023).
Mann, D. M., Stevens, E. R., Testa, P. & Mherabi, N. From silos to synergy: integrating academic health informatics with operational IT for healthcare transformation. NPJ Digit. Med. 7, 185 (2024).
Sood, H., NcNeil, K. & Keogh, B. Chief clinical information officers: clinical leadership for a digital age. BMJ 358, j3295 (2017).
Sanchez-Pinto, L. N. et al. The emerging role of the chief research informatics officer in academic health centers. Appl. Clin. Inform. 8, 845–853 (2017).
Dinesh Babu, M., Bijay Prasad, K. & Tara Prasad, U. Impact of ambidextrous leadership on innovative work behaviour and employee performance in the IT sector. Heliyon 10, e33124 (2024).
Mutonyi, B. R., Gonzalez-Pinero, M., Slatten, T. & Lien, G. Driving innovation in health care: exploring the impact of ambidextrous leadership on creative performance among frontline health professionals in Norway. BMC Health Serv. Res. 24, 268 (2024).
Mauer, E. et al. A predictive model of clinical deterioration among hospitalized COVID-19 patients by harnessing hospital course trajectories. J. Biomed. Inform. 118, 103794 (2021).
Steel, P. A. D. et al. Telehealth follow up in emergency department patients discharged with COVID-like illness and exertional hypoxia. Am. J. Emerg. Med. 49, 426–430 (2021).
Goyal, P. et al. Clinical characteristics of COVID-19 in New York City. N. Engl. J. Med. 382, 2372–2374 (2020).
Wulff, R. T. et al. Laboratory interventions to eliminate unnecessary rapid COVID-19 testing during a reagent shortage. Am. J. Clin. Pathol. 158, 401–408 (2022).
Carlile, M. et al. Deployment of artificial intelligence for radiographic diagnosis of COVID-19 pneumonia in the emergency department. J. Am. Coll. Emerg. Physicians Open 1, 1459–1464 (2020).
Reeves, J. J. et al. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J. Am. Med. Inform. Assoc. 27, 853–859 (2020).
Yang, H. S. et al. Routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. Clin. Chem. 66, 1396–1404 (2020).
Hsu, H. et al. Clinical informatics during the COVID-19 pandemic: lessons learned and implications for emergency department and inpatient operations. J. Am. Med. Inform. Assoc. 28, 879–889 (2021).
Payne, P. R. O., Wilcox, A. B., Embi, P. J. & Longhurst, C. A. Better together: integrating biomedical informatics and healthcare IT operations to create a learning health system during the COVID-19 pandemic. Learn Health Syst. 6, e10309 (2022).
Douthit, B. J. The influence of the learning health system to address the COVID-19 pandemic: an examination of early literature. Int. J. Health Plan. Manag. 36, 244–251 (2021).
Bains, J. et al. Utilizing telemedicine in a novel approach to COVID-19 management and patient experience in the emergency department. Telemed. e-Health 27, 254–260 (2021).
Longhurst, C. A., Kremer, B. & Maysent, P. S. Rapid implementation of a vaccination superstation. JAMA 325, 931–932 (2021).
Johnson, K. B. & Patel, N. R. Biomedical informatics and health information technology: a critical, pragmatic collaboration for clinical transformation. J. Gen. Intern. Med. 36, 530–532 (2021).
El-Kareh, R., Brenner, D. A. & Longhurst, C. A. Developing a highly-reliable learning health system. Learn Health Syst. 7, e10351 (2023).
Schmidt, B. M., Colvin, C. J., Hohlfeld, A. & Leon, N. Definitions, components and processes of data harmonisation in healthcare: a scoping review. BMC Med. Inform. Decis. Mak. 20, 222 (2020).
Kush, R. D. et al. FAIR data sharing: the roles of common data elements and harmonization. J. Biomed. Inform. 107, 103421 (2020).
Gazzarata, R. et al. HL7 fast healthcare interoperability resources (HL7 FHIR) in digital healthcare ecosystems for chronic disease management: scoping review. Int J. Med Inform. 189, 105507 (2024).
Wilkinson, M. D. et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Huang, J. et al. A critical assessment of using ChatGPT for extracting structured data from clinical notes. NPJ Digit. Med. 7, 106 (2024).
Konet, A. et al. Performance of two large language models for data extraction in evidence synthesis. Res. Synth. Methods 15, 818–824 (2024).
Ralevski, A. et al. Using large language models to abstract complex social determinants of health from original and deidentified medical notes: development and validation study. J. Med. Internet Res. 26, e63445 (2024).
Park, S., Choi, W. & Choi, I. Enhancing clinical data extraction from pathology reports: a comparative analysis of large language models. Stud. Health Technol. Inform. 316, 756–760 (2024).
Gilbert, S., Kather, J. N. & Hogan, A. Augmented non-hallucinating large language models as medical information curators. NPJ Digit. Med. 7, 100 (2024).
Hartman, V. et al. Developing and evaluating large language model-generated emergency medicine handoff notes. JAMA Netw. Open 7, e2448723 (2024).
Steel, P. A. D. et al. MyEDCare: evaluation of a smartphone-based emergency department discharge process. Appl. Clin. Inform. 12, 362–371 (2021).
Masterson Creber, R. M. et al. Using mobile integrated health and telehealth to support transitions of care among patients with heart failure (MIGHTy-Heart): protocol for a pragmatic randomised controlled trial. BMJ Open 12, e054956 (2022).
Seneviratne, M. G., Shah, N. H. & Chu, L. Bridging the implementation gap of machine learning in healthcare. BMJ Innov. 6, 45–47 (2020).
Christopher, A., Longhurst, K. S., Chopra, A., Atreja, A. & Brownstein, J. S. A call for artificial intelligence implementation science centers to evaluate clinical effectiveness. NEJM AI 1 https://doi.org/10.1056/AIp2400223 (2024).
Lim, H. C. et al. Toward a learning health care system: a systematic review and evidence-based conceptual framework for implementation of clinical analytics in a digital hospital. Appl. Clin. Inf. 13, 339–354 (2022).
Reddy, S. Generative AI in healthcare: an implementation science informed translational path on application, integration and governance. Implement Sci. 19, 27 (2024).
Dowding, D. et al. Dashboards for improving patient care: review of the literature. Int. J. Med. Inform. 84, 87–100 (2015).
Wardi, G. et al. Bringing the promise of artificial intelligence to critical care: what the experience with sepsis analytics can teach us. Crit. Care Med. 51, 985–991 (2023).
Bedi, S. et al. Testing and evaluation of health care applications of large language models: a systematic review. JAMA 333, 319–328 (2025).
Goldberg, C. B. et al. To do no harm-and the most good-with AI in health care. Nat. Med. 30, 623–627 (2024).
Kamel Rahimi, A. et al. Implementing AI in hospitals to achieve a learning health system: systematic review of current enablers and barriers. J. Med. Internet Res. 26, e49655 (2024).
Challen, R. et al. Artificial intelligence, bias and clinical safety. BMJ Qual. Saf. 28, 231–237 (2019).
Beecy, A. N., Longhurst, C. A., Singh, K., Wachter, R. M. & Murray, S. G. The chief health AI officer-an emerging role for an emerging technology. NEJM AI 1, AIp2400109 (2024)..
Ranjbar, A. et al. Managing risk and quality of AI in healthcare: are hospitals ready for implementation?. Risk Manag. Health. Policy 17, 877–882 (2024).
Thomas, D. M. et al. Transforming big data into AI-ready data for nutrition and obesity research. Obesity 32, 857–870 (2024).
Scott, I. A., van der Vegt, A., Lane, P., McPhail, S. & Magrabi, F. Achieving large-scale clinician adoption of AI-enabled decision support. BMJ Health Care Inform. 31, https://doi.org/10.1136/bmjhci-2023-100971 (2024).
Malhan, A. S., Sadeghi, R. K., Pavur, R. & Pelton, L. Healthcare information management and operational cost performance: empirical evidence. Eur. J. Health Econ. 25, 963–977 (2024).
Meyer, R. & Degoulet, P. Assessing the capital efficiency of healthcare information technologies investments: an econometric perspective. Yearb Med. Inform. 114–127 https://www.ncbi.nlm.nih.gov/pubmed/18660886 (2008).
Butte, A. & Han, C. Annual report center for data-driven insights and innovation 2022–2023 https://www.ucop.edu/uc-health/_files/cdi2_2022-2023_annual_report.pdf (University of California Health, 2023).
The national patient-centered clinical research network https://pcornet.org/.
PCORI INSIGHT clinical research network https://www.pcori.org/research-results/2021/insight-clinical-research-network.
Trends in NIH-supported basic, translational, and clinical research: FYs 2009-2022 https://nexus.od.nih.gov/all/2023/10/31/trends-in-nih-supported-basic-translational-and-clinical-research-fys-2009-2022/.
Supplemental guidance to the 2024 NIH grants policy statement: indirect cost rates. Notice No. NOT-OD-25-068 (National Institute of Health Office of Policy for Extramural Research Administration (OPERA), 2025).
Thorp, H. H. A direct hit. Science 387, 807 (2025).
McCradden, M. D. et al. A Research ethics framework for the clinical translation of healthcare machine learning. Am. J. Bioeth. 22, 8–22 (2022).
Kwong, J. C. C. et al. The silent trial–the bridge between bench-to-bedside clinical AI applications. Front. Digit. Health 4, 929508 (2022).
Horwitz, L. I., Kuznetsova, M. & Jones, S. A. Creating a learning health system through rapid-cycle, randomized testing. N. Engl. J. Med. 381, 1175–1179 (2019).
Wilson, M. G., Palmer, E., Asselbergs, F. W. & Harris, S. K. Integrated rapid-cycle comparative effectiveness trials using flexible point of care randomisation in electronic health record systems. J. Biomed. Inform. 137, 104273 (2023).
Austrian, J. et al. Applying A/B testing to clinical decision support: rapid randomized controlled trials. J. Med. Internet Res. 23, e16651 (2021).
Boussina, A. et al. Impact of a deep learning sepsis prediction model on quality of care and survival. NPJ Digit. Med. 7, 14 (2024).
Tai-Seale, M. et al. AI-generated draft replies integrated into health records and physicians’ electronic communication. JAMA Netw. Open 7, e246565 (2024).
Lee, W. N. et al. Large-scale influenza vaccination promotion on a mobile app platform: a randomized controlled trial. Vaccine 38, 3508–3514 (2020).
Wijesundara, J. G. et al. Electronic health record portal messages and interactive voice response calls to improve rates of early season influenza vaccination: randomized controlled trial. J. Med. Internet Res. 22, e16373 (2020).
Greenberg-Worisek, A. J. et al. The learning health system competency appraisal inventory (LHS-CAI): a novel tool for assessing LHS-focused education needs. Learn Health Syst. 5, https://doi.org/10.1002/lrh2.10218 (2021).
Collard, H. R. & Grumbach, K. A call to improve health by achieving the learning health care system. Acad. Med. 98, 29–35 (2023).
Larson, D. B., Chandler, M. & Forman, H. P. MD/MBA programs in the United States: evidence of a change in health care leadership. Acad. Med. 78, 335–341 (2003).
Hollis, R. J., Pockros, B. M. & Chen, L. The MBA in medical education: current MD/MBA student aspirations, perceptions, and motivations. J. Surg. Res. 259, 305–312 (2021).
Krupat, E., Dienstag, J. L., Kester, W. C. & Finkelstein, S. N. Medical students who pursue a joint MD/MBA degree: who are they and where are they heading?. Eval. Health Prof. 40, 203–218 (2017).
Turner, A. D., Stawicki, S. P. & Guo, W. A. Competitive advantage of MBA for physician executives: a systematic literature review. World J. Surg. 42, 1655–1665 (2018).
Lee, C. S., Ooi, A. S. H., Zenn, M. R. & Song, D. H. The utility of a master of business administration degree in plastic surgery: determining motivations and outcomes of a formal business education among plastic surgeons. Plast. Reconstr. Surg. Glob. Open 6, e1796 (2018).
Building the workforce. Agency for Healthcare Research and Quality https://www.ahrq.gov/learning-health-systems/building-workforce.html (2022).
Learning health system competencies: training the next generation of researchers. Agency for Healthcare Research and Quality https://www.ahrq.gov/funding/training-grants/summary.html (2021).
Lingham, V. et al. A systematic approach to the design and implementation of clinical informatics fellowship programs. Appl. Clin. Inform. 14, 951–960 (2023).
Patel, T. N. et al. Structure and funding of clinical informatics fellowships: a national survey of program directors. Appl. Clin. Inform. 15, 155–163 (2024).
Stanford Medicine Master of Science in Clinical Informatics Management https://med.stanford.edu/master-clinical-informatics-management.html.
Informatics DUsMoMiC https://medschool.duke.edu/education/health-professions-education-programs/master-management-clinical-informatics.
Weill Cornell Medicine Population Health Sciences. Masters in Health Informatics https://phs.weill.cornell.edu/graduate-education-clinical-training/masters-track/health-informatics.
McMahon, M., Bornstein, S., Brown, A. & Tamblyn, R. Training for impact: PhD modernization as a key resource for learning health systems. Health Policy 15, 10–15 (2019).
Wysham, N. G. et al. Development and refinement of a learning health systems training program. EGEMS 4, 1236 (2016).
Kalra, A., Adusumalli, S. & Sinha, S. S. Cultivating skills for success in learning health systems: learning to lead. J. Am. Coll. Cardiol. 70, 2450–2454 (2017).
Kohn, M. S. et al. Creating learning health systems and the emerging role of biomedical informatics. Learn Health Syst. 6, e10259 (2022).
Duke learning health system training program https://sites.duke.edu/learninghealth/.
Dushyanthen, S., Perrier, M., Chapman, W., Layton, M. & Lyons, K. Fostering the use of learning health systems through a fellowship program for interprofessional clinicians. Learn Health Syst. 6, e10340 (2022).
Dushyanthen, S. et al. Designing an interprofessional online course to foster learning health systems. Stud. Health Technol. Inform. 310, 1241–1245 (2024).
Harvard Medical School Safety, Quality, Informatics, and Leadership Certificate Program https://postgraduateeducation.hms.harvard.edu/certificate-programs/safety-quality-informatics-leadership.
University of Michigan Medical School Department of Learning Health Science Education https://medschool.umich.edu/departments/learning-health-sciences/education.
Reeves J. J. et al. Leveraging lean methodology to improve compliance with work-hour restrictions. JAMA Surg. https://doi.org/10.1001/jamasurg.2024.5518 (2024).
Reeves, J. J. et al. Association of electronic surgical consent forms with entry error rates. JAMA Surg. 155, 777–778 (2020).
Yano, E. M. Training an embedded workforce to realize health system impacts and the promise of learning health systems comment on “early career outcomes of embedded research fellows: an analysis of the health system impact fellowship program. Int. J. Health Policy Manag. 13, 8667 (2024).
Kasaai, B., Thompson, E., Glazier, R. H. & McMahon, M. Early career outcomes of embedded research fellows: an analysis of the health system impact fellowship program. Int. J. Health Policy Manag. 12, 7333 (2023).
Lopatina, E., Singal, D. & Manhas, K. P. Reflections on the health system impact fellowship and the future of embedded research comment on “early career outcomes of embedded research fellows: an analysis of the health system impact fellowship program. Int. J. Health Policy Manag. 13, 8615 (2024).
Petrie, S., Cheng, I., McMahon, M. & Lavis, J. N. Future leaders in a learning health system: exploring the health system impact fellowship. Health. Manag. Forum 37, 151–155 (2024).
Wartman, S. A. & Combs, C. D. Reimagining medical education in the age of AI. AMA J. Ethics 21, E146–E152 (2019).
Zhang, J. & Fenton, S. H. Preparing healthcare education for an AI-augmented future. NPJ Health Syst. 1, 4 (2024).
Berwick, D. M. & Finkelstein, J. A. Preparing medical students for the continual improvement of health and health care: Abraham Flexner and the new “public interest”. Acad. Med. 85, S56–S65 (2010).
REPORT 4 OF THE COUNCIL ON MEDICAL EDUCATION (A-19) Augmented Intelligence in Medical Education (Resolution 317-A-18) American Medical Association https://www.ama-assn.org/system/files/cme-report-4-a19-annotated.pdf (2019).
Policy H-295.857 Augmented Intelligence In Medical Education American Medical Association, Medical Education (2019).
Artificial Intelligence and Academic Medicine. Association of American Medical Colleges. https://www.aamc.org/about-us/mission-areas/medical-education/artificial-intelligence-and-academic-medicine#jump-link-1.
Car, J. et al. The digital health competencies in medical education framework: an international consensus statement based on a Delphi study. JAMA Netw. Open 8, e2453131 (2025).
Stead, E. A. Jr. Medical education and practice. Ann. Intern. Med. 72, 271–274 (1970).
Stead, E. A. Jr. Creation of personnel at the medical/computer science interface: should it be a specialty?. J. Med. Syst. 8, 3–6 (1984).
Skochelak, S. E. A decade of reports calling for change in medical education: what do they say?. Acad. Med. 85, S26–S33 (2010).
Lehmann, C. U. et al. Clinical informatics fellowship programs: in search of a viable financial model: an open letter to the centers for medicare and medicaid services. Appl. Clin. Inform. 6, 267–270 (2015).
Zainal, H., Tan, J. K., Xiaohui, X., Thumboo, J. & Yong, F. K. Clinical informatics training in medical school education curricula: a scoping review. J. Am. Med. Inform Soc. 30, 604–616 (2023).
Hersh W. et al. From competencies to competence: model, approach, and lessons learned from implementing a clinical informatics curriculum for medical students. In Health professionals’ education in the age of clinical information systems, mobile computing and social networks (Elsevier Inc., 2017).
Baker, C. K. et al. A model curriculum for an emergency medicine residency rotation in clinical informatics. J. Educ. Teach. Emerg. Med. 7, C1–C50 (2022).
Hajizadeh, A., Khodavandi, M., Eslami, Z., Irannejad, B. & Monaghesh, E. A systematic review of informatics competencies: requirements for nurse managers in healthcare organisations. J. Res. Nurs. 28, 301–311 (2023).
McWilliams School of Biomedical Informatics at UTHealth Houston, master of science in biomedical informatics https://sbmi.uth.edu/prospective-students/academics/masters.htm (2025).
Leading digital transformation in health care, Harvard Medical School Executive Education https://execonline.hms.harvard.edu/leading-digital-transformation-in-healthcare.
Jacobs Technion-Cornell Dual Master of Science Degrees with a Concentration in Health Tech, Cornell Tech. https://tech.cornell.edu/programs/masters-programs/jacobs-technion-cornell-dual-ms-health-tech/.
Car, J. & Topol, E. J. Advocating for a master of digital health degree. JAMA 333, 753–754 (2025).
Woolhandler, S. & Himmelstein, D. U. The deteriorating administrative efficiency of the U.S. health care system. N. Engl. J. Med. 324, 1253–1258 (1991).
McDonough, J. E. Our greedy health care system. Am. J. Public Health 107, 1744–1745 (2017).
Cantlupe J. The rise (and rise) of the healthcare administrator. Athena Health. https://www.athenahealth.com/knowledge-hub/sites/insight/files/The%20rise%20(and%20rise)%20of%20the%20healthcare%20administrator.pdf.
University of Colorado Health Care Innovation Center https://www.uchealth.org/innovation/about-us/.
Faruki, A. A., Zane, R. D. & Wiler, J. L. The role of academic health systems in leading the “third wave” of digital health innovation. JMIR Med. Educ. 8, e32679 (2022).
Penn Medicine’s Center for Health Care Innovation and Transformation. https://chti.upenn.edu/.
Mahoney, K. B., Merchant, R. M. & Schnall, M. D. Build or buy? Managing the new technology decision tree. Front Health Serv. Manag. 41, 21–25 (2024).
Stanford Health Care appoints inaugural chief data scientist. https://med.stanford.edu/news/all-news/2022/03/nigam-shah-inaugural-chief-data-scientist-stanford-health-care.html.
Joan & Irwin Jacobs Center for Health Innovation at UC San Diego Health (JCHI) https://healthinnovation.ucsd.edu/about/our-team.
Sox, H. C. Defining comparative effectiveness research: the importance of getting it right. Med. Care 48, S7–S8 (2010).
Sox, H. C. & Goodman, S. N. The methods of comparative effectiveness research. Annu. Rev. Public Health 33, 425–445 (2012).
Thomas, H., Davenport, M. H. & Dan, J. How AI is helping companies redesign processes. Harv. Bus. Rev. (2023).
The Advanced Research Projects Agency for Health (ARPA-H), open funding opportunities. https://arpa-h.gov/explore-funding/open-funding-opportunities.
Rogers, P. et al. Optimizing the implementation of clinical predictive models to minimize national costs: sepsis case study. J. Med. Internet Res. 25, e43486 (2023).
Jiwani, A., Himmelstein, D., Woolhandler, S. & Kahn, J. G. Billing and insurance-related administrative costs in United States’ health care: synthesis of micro-costing evidence. BMC Health Serv. Res. 14, 556 (2014).
Chernew, M. & Mintz, H. Administrative expenses in the US health care system: why so high? JAMA 326, 1679–1680 (2021).
America’s hospitals and health systems continue to face escalating operational costs and economic pressures as they care for patients and communities. American Hospital Association https://www.aha.org/system/files/media/file/2024/05/Americas-Hospitals-and-Health-Systems-Continue-to-Face-Escalating-Operational-Costs-and-Economic-Pressures.pdf (2024).
Kim, J. S. et al. Can natural language processing and artificial intelligence automate the generation of billing codes from operative note dictations? Glob. Spine J. 13, 1946–1955 (2023).
Boussina, A. et al. Large language models for more efficient reporting of hospital quality measures. NEJM AI 1, https://doi.org/10.1056/aics2400420. (2024).
De Barros, A. et al. Determining prior authorization approval for lumbar stenosis surgery with machine learning. Glob. Spine J. 14, 1753–1759 (2024).
Author information
Authors and Affiliations
Contributions
P.A.D.S., G.W., and C.A.L. conceptualized the idea and developed the outline. P.A.D.S. and G.W. prepared the initial paper text, and P.A.D.S. prepared Fig. 1. All authors (P.A.D.S., G.W., C.A.L., and R.A.H.) reviewed, revised, and contributed to the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steel, P.A.D., Wardi, G., Harrington, R.A. et al. Learning health system strategies in the AI era. npj Health Syst. 2, 21 (2025). https://doi.org/10.1038/s44401-025-00029-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44401-025-00029-0
This article is cited by
-
Student mental health is in crisis — here’s how to help
Nature (2026)