Abstract
CeCl3 can reduce the damage caused by OP pesticides, in this study we used the brain of silkworms to investigate the mechanism of CeCl3 effects on pesticide resistance. The results showed that phoxim treatments led to brain damages, swelling and death of neurons, chromatin condensation and mitochondrial damage. Normal nerve conduction was severely affected by phoxim treatments, as revealed by: increases in the contents of neurotransmitters Glu, NO and ACh by 63.65%, 61.14% and 98.54%, respectively; decreases in the contents of 5-HT and DA by 53.19% and 43.71%, respectively; reductions in the activities of Na+/K+-ATPase, Ca2+/Mg2+-ATPase and AChE by 85.27%, 85.63% and 85.63%, respectively; and increase in the activity of TNOS by 22.33%. CeCl3 pretreatment can significantly reduce such damages. Results of DGE and qRT-PCR indicated that CeCl3 treatments significantly upregulated the expression levels of CYP4G23, cyt-b5, GSTs-σ1, ace1, esterase-FE4 and β-esterase 2. Overall, phoxim treatments cause nerve tissue lesions, neuron death and nerve conduction hindrance, but CeCl3 pretreatments can promote the expression of phoxim resistance-related genes in silkworm brains to reduce phoxim-induced damages. Our study provides a potential new method to improve the resistance of silkworms against OP pesticides.
Similar content being viewed by others
Introduction
Silkworm (Bombyx mori, B. mori) is an important economic insect and a model species for Lepidoptera, in China it produces more than 80% of raw silk of the world1. It has been domesticated for about 5,700 years in China. However, B. mori became very sensitive to pesticides and other chemicals because of long-term indoor breeding and limited exposure to the outside environment. Therefore, it is also used as a model insect to study the toxicology of pesticides and pest control and serves as an environmental indicator. Organophosphorus (OP) based pesticides are one of the most widely used pesticides. The main mechanism of action of OP pesticides is to irreversibly bind acetylcholinesterase (AChE) and inhibit its activity, which leads to the accumulation of neurotransmitter acetyl choline (ACh) in synaptic clefts then insect convulsion and eventual death, because nervous excitement cannot be terminated2,3. Wide use of pesticides has significantly improved agriculture productions, but the pollution from them to the environment has also become a big problem. In China, pesticide-contaminated mulberry causes up to 30% loss in silk industry every year4. Therefore, how to reduce such losses has become a popular topic in recent years. Previous studies have shown that titanium dioxide can reduce the damage caused by OP pesticide phoxim and the mechanism has been explored5.
Rare earth elements (REEs) are widely used in various industries due to their diverse physical and chemical properties6, including agriculture and pharmaceutical industry, for example Cerium can relieves the inhibition of chlorophyll biosynthesis of maize caused by magnesium deficiency7, Cerium can increase taxol accumulation of Taxus cuspidata cells8. Recent study showed that CeCl3 pretreatment could increase the growth and survival rate of B. mori under phoxim-induced toxicity by increasing antioxidant capacity and improving protein and carbohydrate metabolism9. CeCl3 pretreatment could decrease oxidative damage of B. mori caused by nucleopolyhedrovirus infection via increasing antioxidant capacity10. CeCl3 pretreatment could alter the gene expression pattern and relieves the damages in the midgut of B. mori caused by phoxim11. CeCl3 can relieves the damages caused by phoxim, protect B. mori silk gland and remit the reduction in body weight and cocooning rate through changing gene expression patterns and reducing oxidative stress12.
Brain is the main part of central nervous system and the important target of phoxim. Whether CeCl3 can relieve the brain damage in B. mori caused by phoxim is still unknown. In this study, brain was used as the study object to further explore the mechanism of relief from toxic symptoms caused by phoxim under CeCl3 pretreatment.
Results
Histopathological evaluation of brain
As shown by the histological photomicrographs of B. mori larval brain sections in Fig. 1, both control group (Fig. 1A) and CeCl3-treated group (Fig. 1B) had no abnormal pathological changes, with spherical glial cells, fusiform neurons cells and nerve fiber tracts showing clear and complete structures. In the phoxim-exposed group, we observed that glial cells and neurons were swollen, along with loss in cell contents, nucleus fragmentation, cell death, nerve fibers breakage, protein aggregation and adipose degeneration (Fig. 1C). As a contrast, the CeCl3 + phoxim-treated group did not show such pathological changes (Fig. 1D). These results demonstrated that phoxim exposure caused brain damages, while CeCl3 treatments were able to reduce them.
Brain ultrastructure evaluation
Changes in brain ultrastructure in silkworms were presented in Fig. 2. Both the control group (Fig. 2A) and CeCl3-treated group (Fig. 2B) had normal structures, along with evenly distributed nuclear chromatin, integral mitochondria structure, clear mitochondria ridges. The phoxim-treated group (Fig. 2C), on the other hand, showed karyopyknosis, chromatin marginalization, swelling mitochondria, vacuolar degeneration, rough surfaced endoplasmic reticulum and extended golgi’s apparatus, while the CeCl3 + phoxim-treated group had reduced phoxim-induced damages (Fig. 2D).
Neurotransmitter contents and enzyme activities in the brain
Contents of neurotransmitters and activities of related enzymes were analyzed in order to investigate phoxim-induced nerve damages in the brain of fifth instar larvae. As shown in Table 1, in the control group and CeCl3-treated group, the contents of neurotransmitters Glutamate (Glu), nitric oxide (NO), ACh, 5-hydroxytryptamine (5-HT) and dopamine (DA) did not change significantly. However, in the phoxim-treated group, contents of Glu, NO and ACh were all significantly increased, by 63.65%, 61.14% and 98.54%, respectively (P < 0.01), while the 5-HT and DA contents were significantly decreased by 53.19% and 43.71%, respectively (P < 0.01) (Table 1). These results indicated that CeCl3 treatments significantly alleviated phoxim-induced damages. In addition, the CeCl3-treated group did not show significant differences in the activities of Na+/K+-ATPase, Ca2+/Mg2+-ATPase, AChE and total nitric oxide synthase (TNOS) from those of the control group; as a contrast, phoxim treatments significantly reduced the activities of Na+/K+-ATPase, Ca2+/Mg2+-ATPase and AChE by 85.27%, 85.63% and 85.63%, respectively (all P < 0.01), while significantly increasing the activity of TNOS by 22.33% (P < 0.01) (Table 2); these results demonstrated that phoxim can significantly interfere with the nerve conduction in the brain of silkworm fifth instar larvae and that CeCl3 can relieve such interference.
High throughput sequencing
In order to investigate the changes of gene expression pattern in brain caused by CeCl3’s and phoxim treatment, we used the digital gene expression (DGE) method to detect the differences in gene expression. The CeCl3-treatment group (Fig. 3A), the phoxim-exposure group (Fig. 3B) and the CeCl3 + phoxim group (Fig. 3C) had 355, 282 and 422 genes that were significantly changed compared with the control group, among which 88, 69 and 120 genes were upregulated, respectively and 267, 213 and 302 genes were downregulated, respectively. According to the results from Gene Ontology (GO) function analysis, those genes are divided into the following groups: binding activity, structural molecule activity, ligase activity, transferase activity, phosphatase activity, ATPase activity, hydrolase activity, oxidoreductase activity, peptidase activity, catalytic activity, kinase activity and endopeptidase inhibitor activity. The significantly changed genes that have been known in each group were listed in Table S1.
qRT-PCR verification of gene expression changes
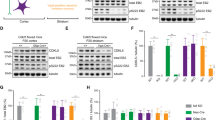
A number of genes that showed significantly different expression patterns in DGE assay were selected for qRT-PCR verification due to their functions. These genes are involved in nerve conduction, pesticide metabolism, apoptosis and oxidative stress. With CeCl3 + phoxim treatments, the expression levels of cytochrome P450 family 4G23 (CYP4G23), cytochrome b5 (cyt-b5), GSTs-σ1, acetylcholinesterase type 1 gene (ace1), esterase-FE4, β-esterase 2 and catalase (CAT) were increased 12.583, 8.623, 16.462, 5.843, 6.714, 2.583 and 2.793 fold, respectively. With CeCl3 treatment, CYP4G23 was increased by 3.472 fold, while phoxim treatment increased CYP4G23’s level by 6.363 fold, ace1’s level by 19.453 fold and esterase-FE4’s level by 3.671 fold (Table 3). The results indicated that CeCl3 changes the gene expression response of silkworm larva’s brains to phoxim, consistent with the DGE results.
Discussion
OP pesticides are widely used in China. Application of large quantity of pesticides can certainly control varied types of pests, but it is also polluting the environment. Silkworms are very sensitive to pesticides, thus pesticide pollution causes serious losses to China’s sericulture4. How to increase silkworm’s pesticide resistance to mitigate economic losses has become an urgent problem. In this study, we found that addition of an appropriate amount of CeCl3 onto mulberry leaves can reduce phoxim-induced damages. The mechanism of CeCl3’s effects on phoxim-induced damages was also investigated in order to seek for methods to better improve silkworm’s resistance to pesticides.
The major damage of phoxim comes from its destroy to nervous3. We also performed detailed investigation on silkworm brains after phoxim treatments. Phoxim caused serious brain tissue damages and cell death (Fig. 1). Analysis of ultrastructure revealed severely damaged endoplasmic reticulum, golgi apparatus and mitochondria (Fig. 2). At the same time, the contents of neurotransmitters Glu, NO and ACh were increased significantly, along with significantly decreased contents of 5-HT and DA and significantly inhibited activities of Na+/K+-ATPase, Ca2+/Mg2+-ATPase and AChE. Na+/K+-ATPase, Ca2+ and Mg2+ ion, AChE play an important role in conduction of action potential, ACh transport, ACh degradation respectively13,14. When CeCl3 was given to the larvae, such changes were significantly relieved.
Cytochrome P450, GSTs and esterase are essential parts of the detoxification system of insects15,16. In this study, we observed increased expression of CYP4G23, a member of CYP4 families which are well consistent with their predicted role in xenobiotic metabolism17, indicating that phoxim poisoning induces CYP4G23 as a response in larval brain, such response was even stronger in the CeCl3 pretreatment group (Table 3). Cyt-b5, an essential molecule in P450-mediated detoxification18, was significant increase in the group of CeCl3 + phoxim (Table 3). GSTs have multiple subunits and play important roles in the biotransformation of exogenous compounds, drug metabolism and the protection against peroxidation damages. Therefore, increases in the expression of GSTs are one of the major reasons of enhanced pesticide resistance19. In this study, we found that the subunit GSTs-σ1’s expression was significantly reduced by phoxim but significantly increased by CeCl3 pretreatments (Table 3), indicating that CeCl3 promotes the response to phoxim through increasing GSTs contents. Esterases exert their detoxification effects by direct binding to or hydrolysis of pesticides and many studies have reported that overexpression of esterases can increase insects’ resistance to pesticides20,21. The overexpression of β-esterase increase the resistance against deltamethrin in Rhipicephalus (Boophilus) microplus22. The expression quantity of esterase-FE4 is positive correlation to acephate in Myzus persicae (SULZER)23. In the present study, CeCl3 treatments increased the expression levels of AChE, β-esterase 2 and esterase-FE4 (Table 3), which may be one of the reasons for increased pesticide resistance of silkworms. We also observed a significant increase in the expression of GPI anchor after CeCl3 treatments (Table S1), AChE is anchored to neuron membrane through GPI anchor. Previous study has indicated that increased AChE anchoring leads to improved pesticide resistance24.
Mitochondria are not only the energy factories of eukaryotic cells but also the primary targets of toxicity after pesticides enter cells25,26. Pesticides destroy mitochondrial structure to release free radicals, which damage DNA, interfere signal transduction and affect the expression of apoptosis-related genes that leads to apoptosis27,28,29,30. In the present study, phoxim caused serious damages to the mitochondria of silkworm larval brains, cell abnormalities and even apoptosis and all these damages were alleviated by CeCl3 pretreatments (Figs 1 and 2). Results from high-throughput sequencing indicated that CeCl3 treatment can increase the expression of CAT, xanthine dehydrogenase (XDH) and serine-pyruvate aminotransferase (AGT) (Table 3, Table S1), these enzymes reduce mitochondrial damages through scavenging free radicals.
Cerium ion has some special features, including functioning as an antibiotic31 and cleaving the phosphodiester of DNA32. Wang et al. attempted to hydrolyze the phosphodiester of OP using Cerium complexes with saccharides and revealed that the complexes were able to degrade methamidophos, omethoate and dimethoate33. These complexes can also reduce the chlorpyrifos and parathion residue in jujube34. We speculate that absorbed Cerium ion may hydrolyze phoxim to some extent and protect the silkworm, which needs further studies to confirm.
In this study, we found that CeCl3 pretreatment could reduce phoxim-induced nerve damages. CeCl3 enhanced the expression of detoxification enzymes, esterases, P450s and GSTs, which participate in phoxim metabolism; CeCl3 could also upregualte the genes related to free radical clearance and decrease the expression of apoptosis genes to protect neurons in the brain. As a result, CeCl3 may enhance phoxim metabolism in silkworms to protect their nerve systems. This study illustrated the potential mechanisms of CeCl3’s effect to enhance phoxim metabolism in silkworm brains and provided a theoretical basis to use CeCl3 as an additive to improve silkworms’ pesticide tolerance in sericulture.
Methods
Insect and chemicals
The larvae of B. mori (strain: Qiufeng × baiyu) maintained in our laboratory were reared at 27 ± 2 °C on mulberry leaves under a 12 h light/12 h dark cycle.
Phoxim was purchased from Sigma-Aldrich (USA). CeCl3 (analytical grade, 99.99%) was purchased from Shanghai Chem. Co. (China).
Treatment and brain tissue collection
Phoxim stock solution was prepared by 10 × dilution using acetone. For the treatment, phoxim stock solution was dissolved in water to obtain a concentration of 4 μg/mL. The lethal concentration (LC50) of phoxim in B. mori was 7.86 μg/mL and at 4 μg/mL, B. mori showed poisoning symptoms without death1,4. In a pre-experiment, different concentrations (0.1, 0.2, 0.5, 1.0 and 1.5 mg/L) of CeCl3 was used, which were administered to fifth-instarlarvae, the optimum concentration of CeCl3 was 0.5 mg/L for growth of these larvae10. B. mori larvae were feed with CeCl3-treated leaves (leaves were dipped in 0.5 mg/L CeCl3 solution for 1 min and dried in the air) and normal leaves respectively three times a day until the 2nd day of fifth-instar. Then a part of the larvae of these two groups were feed with phoxim-treated leaves (leaves were dipped in 4 μg/mL phoxim for 1 min and dried in the air). Each treatment was performed three times. Forty-eight hours after phoxim treatments, 100 fifth-instar larvae was selected randomly from each group to collect brain tissues that were frozen at −80 °C for further study.
Histopathological evaluation of brain
All histopathological examinations were performed using the follow laboratory procedures. Five brains from the larvae of each group were embedded in paraffin, sliced (5 μm thickness), placed onto glass slides and stained with hematoxylin-eosin for 15 min. Stained samples were observed and photographed using an optical microscope (Nikon U-III Multi-point Sensor System, Japan).
Observation of brain ultrastructure
Five larvae’s brain tissues of each group were fixed in freshly prepared 0.1 M sodium cacodylate buffer with 2.5% glutaraldehyde and 2% formaldehyde, before being treated at 4 °C with 1% osmium tetroxide in 50 mM sodium cacodylate (pH 7.2-7.4) for 2 h. Staining was performed overnight with 0.5% aqueous uranyl acetate. After serial dehydration with ethanol (75, 85, 95 and 100%), the specimens were embedded in Epon 812 and sliced. Ultrathin sections were treated with uranyl acetate and lead citrate and observed with a HITACHI H600 TEM (HITACHI Co., Japan). The damages of brain was determined by observing the changes in nuclear morphology, (e.g., chromatin condensation and fragmentation).
Assay of enzymatic activities
To determine enzymatic activities, brain tissues were homogenized in 0.15 M NaCl. The homogenate of brains was centrifuged at 3,000 g for 15 min at 4 °C, A portion of supernatant was used to measure the activities of different enzymes. The activities of AChE, Ca2+-ATPase, Ca2+/Mg2+-ATPase, Na+/K+-ATPase and TNOS in the brain were measured spectrophotometrically with commercial kits (Nanjing Jiancheng Bioengineering Institute, China), targeting the oxidation of oxyhaemoglobin to methaemoglobin by nitric oxide.
Measurements of neurochemicals
The homogenate of brains was centrifuged at 12,000 g for 20 min at 4 °C. The concentrations of DA, 5-HT and ACh were measured spectrophotometrically with commercially kits (Nanjing Jiancheng Bioengineering Institute, China).
Glu concentrations were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) and standard curves were generated by using standard Glu stock solutions. Sample Glu levels were detected using a spectrophotometer at 340 nm and expressed as μmol/g prot. The concentration of NO in the brain was measured using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China). The OD value was determined by using a spectrophotometer (U-3010, Hitachi, Japan). NO results were read with OD values at 550 nm. The results were calculated using the following formula: NO (μmol/L) = (Asample – Ablank)/(Astandard – Ablank) × 20 (μmol/L).
Total RNA isolation
Trizol reagent was used to extract the total RNA from brain samples (Takara, Dalian China) and treated with DNase to remove potential genomic DNA contamination. The quality of the RNA was quantitated spectrophotometrically at 260 and 280 nm.
Digital gene expression library preparation and sequencing
For RNA library construction and deep sequencing, equal quantities of brain RNA samples (n = 3) were pooled for the control group and the treatment group, respectively. Approximately 6 μg of RNA representing each group was submitted to Solexa (now Illumina Inc.) for sequencing. Detailed methodology was according to the method described by Gu et al.35.
qRT-PCR analysis
The specific primers for the 7 genes of interest are listed in Table 4. The internal reference gene was actin3. qRT-PCR was performed using the 7500 Real-time PCR System (ABI) with SYBR Premix Ex TaqTM (Takara, Japan) according to the manufacturer’s instructions. The qRT-PCR analysis was carried out following the method described in the studies of Peng et al. and Wang et al.4,36.
Additional Information
How to cite this article: Wang, B. et al. Molecular Signatures of Reduced Nerve Toxicity by CeCl3 in Phoxim-exposed Silkworm Brains. Sci. Rep. 5, 12761; doi: 10.1038/srep12761 (2015).
References
Li, B. et al. Resistance comparison of domesticated silkworm (Bombyx mori L.) and wild silkworm (Bombyx mandarina M.) to phoxim insecticide. Afr J Biotechnol 9, 1771–1775 (2010).
Shang, J. Y. et al. Expression of two types of acetylcholinesterase gene from the silkworm, Bombyx mori, in insect cells. Insect Sci 14, 443–449 (2007).
Nath, B. S. & Kumar, R. P. S. Toxic impact of organophos-phorus insecticides on acetylcholinesterase activity in the silkworm, Bombyx mori L. Ecotoxicology and environmental safety 42, 157–162 (1999).
Peng, G. D. et al. Transcriptional characteristics of acetylcholinesterase genes in domestic silkworms (Bombyx mori) exposed to phoxim. Pestic Biochem Phys 101, 154–158 (2011).
Xie, Y. et al. Molecular mechanisms of reduced nerve toxicity by titanium dioxide nanoparticles in the phoxim-exposed brain of Bombyx mori. PloS one 9, e101062 (2014).
Palasz, A. & Czekaj, P. Toxicological and cytophysiological aspects of lanthanides action. Acta biochimica Polonica 47, 1107–1114 (2000).
Zhou, M. et al. Cerium relieves the inhibition of chlorophyll biosynthesis of maize caused by magnesium deficiency. Biol Trace Elem Res 143, 468–477 (2011).
Yang, S., Lu, S. H. & Yuan, Y. J. Lipidomic analysis reveals differential defense responses of Taxus cuspidata cells to two elicitors, methyl jasmonate and cerium (Ce4+). Biochimica et biophysica acta 1781, 123–134 (2008).
Li, B. et al. Cerium Chloride Improves Protein and Carbohydrate Metabolism of Fifth-Instar Larvae of Bombyx mori Under Phoxim Toxicity. Biol Trace Elem Res 150, 214–220 (2012).
Li, B. et al. Effects of Added CeCl3 on Resistance of Fifth-Instar Larvae of Silkworm to Bombyx mori Nucleopolyhedrovirus Infection. Biol Trace Elem Res 146, 318–324 (2012).
Yu, X. et al. Mechanisms of larval midgut damage following exposure to phoxim and repair of phoxim-induced damage by cerium in Bombyx mori. Environmental toxicology 30, 452–460 (2015).
Li, B. et al. Molecular mechanisms of silk gland damage caused by phoxim exposure and protection of phoxim-induced damage by cerium chloride in Bombyx mori. Environmental toxicology (2014).
Brini, M. & Carafoli, E. Calcium Pumps in Health and Disease. Physiol Rev 89, 1341–1378 (2009).
Alrajhi, D. H. Properties of Ca2+/Mg2+ ATPase from Rat-Brain and Its Inhibition by Pyrethroids. Pestic Biochem Phys 37, 116–120 (1990).
Rufingier, C., Pasteur, N., Lagnel, J., Martin, C. & Navajas, M. Mechanisms of insecticide resistance in the aphid Nasonovia ribisnigri (Mosley) (Homoptera : Aphididae) from France. Insect biochemistry and molecular biology 29, 385–391 (1999).
Fournier, D. & Mutero, A. Modification of acetylcholinesterase as s mechanism of resistance to insecticides. Comparative Biochemistry and Physiology Part C 108, 19–31 (994).
Feyereisen, R. Evolution of insect P450. Biochem Soc T 34, 1252–1255 (2006).
Kandel, S. E. & Lampe, J. N. Role of protein-protein interactions in cytochrome P450-mediated drug metabolism and toxicity. Chemical research in toxicology 27, 1474–1486 (2014).
Sonoda, S. & Igaki, C. Characterization of acephate resistance in the diamondback moth Plutella xylostella. Pestic Biochem Phys 98, 121–127 (2010).
Mouches, C. et al. Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex. Proceedings of the National Academy of Sciences of the United States of America 87, 2574–2578 (1990).
Qiao, C. L. & Raymond, M. The Same Esterase B1 Haplotype Is Amplified in Insecticide-Resistant Mosquitos of the Culex-Pipiens Complex from the America and China. Heredity 74, 339–345 (1995).
Singh, N. K. & Rath, S. S. Esterase mediated resistance against synthetic pyrethroids in field populations of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in Punjab districts of India. Vet Parasitol 204, 330–338 (2014).
Srigiriraju, L., Semtner, P. J., Anderson, T. D. & Bloomquist, J. R. Esterase-Based Resistance in the Tobacco-Adapted Form of the Green Peach Aphid, Myzus Persicae (Sulzer) (Hemiptera: Aphididae) in the Eastern United States. Arch Insect Biochem 72, 105–123 (2009).
Kakani, E. G., Bon, S., Massoulie, J. & Mathiopoulos, K. D. Altered GPI modification of insect AChE improves tolerance to organophosphate insecticides. Insect biochemistry and molecular biology 41, 150–158 (2011).
Carlson, K. & Ehrich, M. Organophosphorus compound-induced modification of SH-SY5Y human neuroblastoma mitochondrial transmembrane potential. Toxicol Appl Pharm 160, 33–42 (1999).
Sinha, K., Das, J., Pal, P. B. & Sil, P. C. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87, 1157–1180 (2013).
Carlson, K., Jortner, B. S. & Ehrich, M. Organophosphorus compound-induced apoptosis in SH-SY5Y human neuroblastoma cells. Toxicol Appl Pharmacol 168, 102–113 (2000).
Xue, X. L., Yuan, D. X. & Fan, G. F. Effects of dimethoate and triazophos on ultra-structural lesions in gill of Sinonovacula constricta. Marine environmental science 26, 568–572, 582 (2007).
Slotkin, T. A. & Seidler, F. J. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull 72, 232–274 (2007).
Wang, X. D. The expanding role of mitochondria in apoptosis. Gene Dev 15, 2922–2933 (2001).
Huang, L. F. & Shen, X. S. The biological function of rare earth element. Acta Medicinae Sinicas 15, 693–695 (2002).
Igawa, T., Sumaoka, J. & Komiyama, M. Hydrolysis of oligonucleotides by homogeneous Ce(IV)/EDTA complex. Chemistry Letters 4, 356–357 (2000).
Wang, D. F. et al. Organophosphorous Pesticide Degradation by Cerium Complexes. Periodical of Ocean University of China 34, 577–581 (2004).
Wu, H., Wang, D., Shi, J., Xue, S. & Gao, M. Effect of the Complex of Zinc(II) and Cerium(IV) with Chitosan on the Preservation Quality and Degradation of Organophosphorus Pesticides in Chinese Jujube (Zizyphus jujuba Mill. cv. Dongzao). Journal of Agricultural and Food Chemistry 58, 5757–5762 (2010).
Gu, Z. et al. The adverse effects of phoxim exposure in the midgut of silkworm, Bombyx mori. Chemosphere 96, 33–38 (2014).
Wang, Y. H. et al. Changes in the activity and the expression of detoxification enzymes in silkworms (Bombyx mori) after phoxim feeding. Pestic Biochem Physiol 105, 13–17 (2013).
Acknowledgements
This work was funded by the National High Technology Research and Development Program of China (863 Program) (Grant No. 2013AA102507), the China Agriculture Research System (CARS-22-ZJ0305), the Science & Technology support Program of Suzhou (SYN201406), the Graduate Education Innovation Project of Jiangsu Province (CXZZ15_156).
Author information
Authors and Affiliations
Contributions
Binbin Wang, Fanchi Li and Min Ni, wrote the main manuscript text, Hua Zhang, Kaizun Xu, Jianghai Tian and Jingsheng Hu prepared Table S1 and Fig. 3, Weide Shen and Bing Li designed the experiments. All authors read and approved the final manuscript.
Significantly changed genes classify.
(A) Functional categorization of 355 genes which significantly altered by CeCl3 pretreatment; (B) Functional categorization of 282 genes which significantly altered by phoxim exposure; (C) Functional categorization of 422 genes which significantly altered by CeCl3 + phoxim treatment; Genes were classified based on the GO function.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, B., Li, F., Ni, M. et al. Molecular Signatures of Reduced Nerve Toxicity by CeCl3 in Phoxim-exposed Silkworm Brains. Sci Rep 5, 12761 (2015). https://doi.org/10.1038/srep12761
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12761
This article is cited by
-
Anti-larval activity of actinobacterial extract for Helicoverpa armigera and Spodoptera litura
International Journal of Tropical Insect Science (2022)