Collection
Microbial endocrinology
- Submission status
- Open
- Submission deadline
 This Collection supports and amplifies research related to SDG 3.
This Collection supports and amplifies research related to SDG 3.
Microbial endocrinology, by definition, represents the integration of the fields of microbiology and neurobiology. It is based on the use of neurochemistry as a shared evolutionary language linking microorganisms, including infectious bacteria, with elements of the host. Many of the neuroactive compounds that are integral to communication within the host, such as between neural and immune components, first evolved in plants and then prokaryotes. The recognition that microorganisms can not only produce, but also recognize, many of these same neuroactive compounds utilized by the host, such as the catecholamines, means that there is the opportunity for bi-directional communication between host and the microbiota. It has now been over 30 years since the first report that neurochemicals associated with the stress response could influence the growth and production of virulence factors by pathogenic bacteria. Since then, the ability of microorganisms to produce and respond to neuroactive chemicals that can influence host physiological and behavioral processes largely through the microbiome-gut-brain axis have been increasingly reported.
The purpose of this Collection is to explore the multi-faceted application of microbial endocrinology, and by extension bi-directional communication between microbiota and host, to various aspects of health and disease, including behavioural aspects. In the past, microbial endocrinology-related research has centered mainly on the infectious disease component with numerous reports demonstrating the role of microbial recognition of host-produced neurochemicals, principally the stress-related catecholamines, as being integral to the development of pathogenesis. More recently, the utilization of a microbial endocrinology-based bi-directional communication approach has also provided critical insights into how the microbiome-gut-brain axis may influence health and behaviour as well. Reports have shown that production of microbial production of neurochemicals by gut microbiota may serve as a primary mechanism by which the bacteria interact with the host through interaction with the enteric nervous system, or by uptake into the portal circulation, ultimately influencing the brain with consequent effects on behaviour. The microbial endocrinology-based discovery that PMAT- and OCT-like uptake systems in a gut bacterium may interact with not only host signals but also with selective serotonin reuptake inhibitor antidepressants has provided a new mechanism by which to examine the well-known ability of antipsychotics to influence gut microbial diversity often leading to untoward side effects. Further evidence of the application of microbial endocrinology to the potential modulation of the microbiome-gut-brain axis can be seen in the use of probiotics that can convert neurochemical precursors found in food into bioactive compounds such as dopamine.
The scope of this Collection is envisioned to also include the application of a microbial endocrinology-based approach to aspects of nutrition. Leveraging the microbiome has been proposed as a possible means to impact human nutrition practices to mitigate disease risk and promote health. Analogously, modulation of the gut microbiome is also envisioned as a means to increase the efficiency of animal food production. Although not intensively studied to date, microbial endocrinology may have a role to play in nutrition since food-based neurochemical substrates can alter neurochemical production by the gut microbiota and thereby influence host physiology. For example, plants such as mucuna, are known to contain extremely high amounts of the dopamine precursor L-dopa.
In conclusion, the purpose of this Collection is to publish a wide spectrum of studies that further our understanding, as well as engender new studies, into the role that the evolutionary, neurochemistry-based bi-directional communication between host and microbiota, which is the basis of microbial endocrinology, carry for improving health and the treatment of disease.
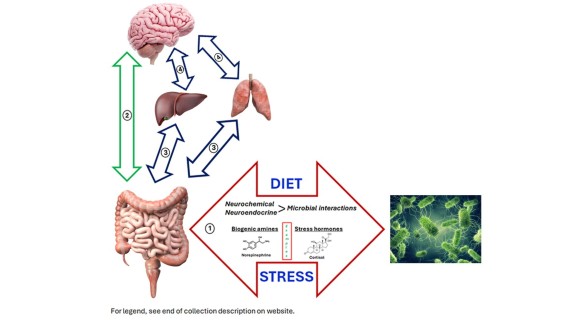
Legend of the hero image figure:
Schematic outlining the bi-directional pathways by which the gut microbiota can potentially influence the brain.
①: In the gastrointestinal tract, neurochemicals, or their precursors (e.g. L-dopa), may be present in the diet and/or produced directly by the gut microbiota through the utilization of host- or diet-derived sources. Host stress will also result in the alteration of the gut luminal environment and may influence the diversity and function of the gut microbiota and/or the ability of infectious agents to establish a productive infection through, for example, attachment to the mucosa.
②: The microbiome-gut-brain pathway is comprised of elements of the enteric nervous system that innervate the intestinal villi and are enmeshed with other neural pathways (e.g. the vagus nerve) to provide a direct communication pathway to the brain. Neurochemicals may also enter the portal circulation where they can directly influence brain function as long as they are able to cross the blood brain barrier.
③ and ④: The microbiome-gut-liver-brain axis, in addition to the microbiome-gut-respiratory-brain axis are two routes which may ultimately influence brain behavior and function that have been more recently theorized and investigated as compared to the “traditional” microbiome-gut-brain axis as shown in ②. It should be noted that aspects of each of these bi-directional axes, specifically that occurring between the brain and various organs, have long been published before the inclusion of the microbiome was recognized (see text for references). Further, as shown in the illustration, it should be understood that it is explicitly not the case that the microbiome is necessarily involved in all communication between the brain and various organ systems. Further, while the preponderance of publications has addressed the role of the microbiome in influencing brain function through these axes, far less is known about the ability of the brain to influence the microbiome. Thus, the extent to which the use of bi-directional arrows is appropriate for each of these axes (with the possible exception of the microbiome-gut-brain axis) should be considered as an area of investigation in its infancy.
Editors
-
Mark Lyte, PhD, MS, MT(ASCP)
Iowa State University, United States
