Abstract
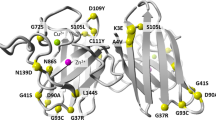
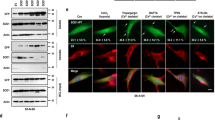
Mutations in the human copper/zinc superoxide dismutase 1 (hSOD1) gene cause familial amyotrophic lateral sclerosis (ALS). It remains unknown whether large animal models of ALS mimic more pathological events seen in ALS patients via novel mechanisms. Here, we report the generation of transgenic pigs expressing mutant G93A hSOD1 and showing hind limb motor defects, which are germline transmissible, and motor neuron degeneration in dose- and age-dependent manners. Importantly, in the early disease stage, mutant hSOD1 did not form cytoplasmic inclusions, but showed nuclear accumulation and ubiquitinated nuclear aggregates, as seen in some ALS patient brains, but not in transgenic ALS mouse models. Our findings revealed that SOD1 binds PCBP1, a nuclear poly(rC) binding protein, in pig brain, but not in mouse brain, suggesting that the SOD1-PCBP1 interaction accounts for nuclear SOD1 accumulation and that species-specific targets are key to ALS pathology in large mammals and in humans.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Dion PA, Daoud H, Rouleau GA . Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet 2009; 10:769–782.
Boillée S, Vande Velde C, Cleveland DW . ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 2006; 52:39–59.
Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 362:59–62.
Turner BJ, Talbot K . Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol 2008; 85:94–134.
Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 1994; 264:1772–1775.
Reaume AG, Elliott JL, Hoffman EK, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet 1996; 13:43–47.
Julien JP . Amyotrophic lateral sclerosis unfolding the toxicity of the misfolded. Cell 2001; 104:581–591.
Bendotti C, Carrì MT . Lessons from models of SOD1-linked familial ALS. Trends Mol Med 2004; 10:393–400.
Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ . Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol 2011; 7:616–630.
Martin LJ, Liu Z, Chen K, et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol 2007; 500:20–46.
Dal Canto MC, Gurney ME . Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res 1995; 676:25–40.
Shibata N . Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology 2001; 21:82–92.
Wong PC, Pardo CA, Borchelt DR, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 1995; 14:1105–1116.
Guegan C, Przedborski S . Programmed cell death in amyotrophic lateral sclerosis. J Clin Invest 2003; 111:153–161.
Wengenack TM, Holasek SS, Montano CM, Gregor D, Curran GL, Poduslo JF . Activation of programmed cell death markers in ventral horn motor neurons during early presymptomatic stages of amyotrophic lateral sclerosis in a transgenic mouse model. Brain Res 2004; 1027:73–86.
Reyes NA, Fisher JK, Austgen K, VandenBerg S, Huang EJ, Oakes SA . Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J Clin Invest 2010; 120:3673–3679.
Honig LS, Rosenberg RN . Apoptosis and neurologic disease. Am J Med 2000; 108:317–330.
Migheli A, Atzori C, Piva R, et al. Lack of apoptosis in mice with ALS. Nat Med 1999; 5:966–967.
He BP, Strong MJ . Motor neuronal death in sporadic amyotrophic lateral sclerosis (ALS) is not apoptotic. A comparative study of ALS and chronic aluminium chloride neurotoxicity in New Zealand white rabbits. Neuropathol Appl Neurobiol 2000; 26:150–160.
Embacher N, Kaufmann WA, Beer R, et al. Apoptosis signals in sporadic amyotrophic lateral sclerosis: an immunocytochemical study. Acta Neuropathol 2001; 102:426–434.
Kakita A, Oyanagi K, Nagai H, Takahashi H . Eosinophilic intranuclear inclusions in the hippocampal pyramidal neurons of a patient with amyotrophic lateral sclerosis. Acta Neuropathol 1997; 93:532–536.
Seilhean D, Takahashi J, El Hachimi KH, et al. Amyotrophic lateral sclerosis with neuronal intranuclear protein inclusions. Acta Neuropathol 2004; 108:81–87.
Forsberg K, Andersen PM, Marklund SL, Brännström T . Glial nuclear aggregates of superoxide dismutase-1 are regularly present in patients with amyotrophic lateral sclerosis. Acta Neuropathol 2011; 121:623–634.
Prather RS, Hawley RJ, Carter DB, Lai L, Greenstein JL . Transgenic swine for biomedicine and agriculture. Theriogenology 2003; 59:115–123.
Aigner B, Renner S, Kessler B, et al. Transgenic pigs as models for translational biomedical research. J Mol Med (Berl) 2010; 88:653–664.
Yang D, Yang H, Li W, et al. Generation of PPARγ mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res 2011; 21:979–982.
Yang D, Wang CE, Zhao B, et al. Expression of Huntington's disease protein results in apoptotic neurons in the brains of cloned transgenic pigs. Hum Mol Genet 2010; 19:3983–3994.
Gordon T, Ly V, Hegedus J, Tyreman N . Early detection of denervated muscle fibers in hindlimb muscles after sciatic nerve transection in wild type mice and in the G93A mouse model of amyotrophic lateral sclerosis. Neurol Res 2009; 31:28–42.
Gosztonyi G, Naschold U, Grozdanovic Z, Stoltenburg-Didinger G, Gossrau R . Expression of Leu-19 (CD56, N-CAM) and nitric oxide synthase (NOS) I in denervated and reinnervated human skeletal muscle. Microsc Res Tech 2001; 55:187–197.
Dupuis L, Gonzalez de Aguilar JL, di Scala F, M, et al. Nogo provides a molecular marker for diagnosis of amyotrophic lateral sclerosis. Neurobiol Dis 2002; 10:358–365.
Atkin JD, Scott RL, West JM, Lopes E, Quah AK, Cheema SS . Properties of slow- and fast-twitch muscle fibres in a mouse model of amyotrophic lateral sclerosis. Neuromuscul Disord 2005; 15:377–388.
de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 2008; 119:497–503.
Okamoto K, Hirai S, Amari M, Sakurai A . Electron micrograph of ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neuropathology 1996; 16:112–116.
Piao YS, Wakabayashi K, Kakita A, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 2003; 13:10–22.
Makeyev AV, Liebhaber SA . The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 2002; 8:265–278.
Nandal A, Ruiz JC, Subramanian P, et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab 2011; 14:647–657.
Xia M, He H, Wang Y, et al. PCBP1 is required for maintenance of the transcriptionally silent state in fully grown mouse oocytes. Cell Cycle 2012; 11:2833–2842.
Jaarsma D, Haasdijk ED, Grashorn JA, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis 2000; 7:623–643.
Barbeito LH, Pehar M, Cassina P, et al. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev 2004; 47:263–274.
He CZ, Hays AP . Expression of peripherin in ubiquinated inclusions of amyotrophic lateral sclerosis. J Neurol Sci 2004; 217:47–54.
Kawashima T, Furuta A, Doh-ura K, Kikuchi H, Iwaki T . Ubiquitin-immunoreactive skein-like inclusions in the neostriatum are not restricted to amyotrophic lateral sclerosis, but are rather aging-related structures. Acta Neuropathol 2000; 100:43–49.
Okamoto K, Hirai S, Amari M, Watanabe M, Sakurai A . Bunina bodies in amyotrophic lateral sclerosis immunostained with rabbit anti-cystatin C serum. Neurosci Lett 1993; 162:125–128.
Sasaki S, Maruyama S . Immunocytochemical and ultrastructural studies of the motor cortex in amyotrophic lateral sclerosis. Acta Neuropathol 1994; 87:578–585.
Matsumoto S, Goto S, Kusaka H, et al. Ubiquitin-positive inclusion in anterior horn cells in subgroups of motor neuron diseases: a comparative study of adult-onset amyotrophic lateral sclerosis, juvenile amyotrophic lateral sclerosis and Werdnig-Hoffmann disease. J Neurol Sci 1993; 115:208–213.
van Welsem ME, Hogenhuis JA, Meininger V, Metsaars WP, Hauw JJ, Seilhean D . The relationship between Bunina bodies, skein-like inclusions and neuronal loss in amyotrophic lateral sclerosis. Acta Neuropathol 2002; 103:583–589.
Maekawa S, Leigh PN, King A, et al. TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology 2009; 29:672–683.
Everett CM, Wood NW . Trinucleotide repeats and neurodegenerative disease. Brain 2004; 127:2385–2405.
Gertz B, Wong M, Martin LJ . Nuclear localization of human SOD1 and mutant SOD1-specific disruption of survival motor neuron protein complex in transgenic amyotrophic lateral sclerosis mice. J Neuropathol Exp Neurol 2012; 71:162–177.
Chang LY, Slot JW, Geuze HJ, Crapo JD . Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol 1988; 107:2169–2179.
Pickering BM, Mitchell SA, Spriggs KA, Stoneley M, Willis AE . Bag-1 internal ribosome entry segment activity is promoted by structural changes mediated by poly(rC) binding protein 1 and recruitment of polypyrimidine tract binding protei n 1. Mol Cell Biol 2004; 24:5595–5605.
Chen-Plotkin AS, Lee VM, Trojanowski JQ . TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol 2010; 6:211–220.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 program, 2011CB944203), the National Natural Science Foundation of China (31071293) to LL; the National Institutes of Health (NS036232, NS041669 and NS045016) to XL and SL; Talent Program of Yunnan Province, China, and The Professorial Fellowship of Monash University, Australia to ZX; the National Natural Science Foundation of China (81171179, 81272439), Key Sci-Tech Research Projects of Guangdong Province, China (2008A030201019) to XJ, and The State Key Laboratory of Molecular Developmental Biology, China. We would like to thank the staffs of the pig farm for animal husbandry and assistance in the behavioral tasks. We thank Cheryl Strauss for critically reading this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Generation of hSOD1 transgenic piglets. (PDF 166 kb)
Supplementary information, Figure S2
Muscle atrophy in hSOD1 transgenic pig. (PDF 225 kb)
Supplementary information, Figure S3
Loss of neuronal cells in the spinal cord of hSOD1 transgenic pigs. (PDF 452 kb)
Supplementary information, Figure S4
Axonal degeneration in hSOD1 transgenic pig. (PDF 509 kb)
Supplementary information, Table S1
Copy numbers of the mutant hSOD1 gene in transgenic pigs. (PDF 82 kb)
Supplementary information, Table S2
Body weight of hSOD1 transgenic and WT pigs. (PDF 112 kb)
Supplementary information, Movie S1
Treadmill running test of a WT pig at the age of 16 months using the fixed speed mode. (MOV 9357 kb)
Supplementary information, Movie S2
Treadmill running test of TG-11 pig at the age of 16 months using the fixed speed mode. (MOV 9327 kb)
Supplementary information, Movie S3
Treadmill running test of a WT pig at the age of 18 months using the accelerating speed mode. (MOV 9488 kb)
Supplementary information, Movie S4
Treadmill running test of TG-11 pig at the age of 18 months using the accelerating speed mode. (MOV 9211 kb)
Supplementary information, Data S1
Methods (PDF 473 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Wang, G., Sun, H. et al. Species-dependent neuropathology in transgenic SOD1 pigs. Cell Res 24, 464–481 (2014). https://doi.org/10.1038/cr.2014.25
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2014.25
Keywords
This article is cited by
-
Impact of Plant-Derived Compounds on Amyotrophic Lateral Sclerosis
Neurotoxicity Research (2023)
-
Double knock-in pig models with elements of binary Tet-On and phiC31 integrase systems for controllable and switchable gene expression
Science China Life Sciences (2022)
-
Differential CircRNA Expression Profiles in PK-15 Cells Infected with Pseudorabies Virus Type II
Virologica Sinica (2021)
-
Bridging the gap: large animal models in neurodegenerative research
Mammalian Genome (2017)
-
From animal models to human disease: a genetic approach for personalized medicine in ALS
Acta Neuropathologica Communications (2016)