Abstract
Genetic defects in myelin formation and maintenance cause leukodystrophies, a group of white matter diseases whose mechanistic underpinnings are poorly understood1,2. Hypomyelination and congenital cataract (HCC), one of these disorders, is caused by mutations in FAM126A, a gene of unknown function3. We show that FAM126A, also known as hyccin, regulates the synthesis of phosphatidylinositol 4-phosphate (PtdIns(4)P), a determinant of plasma membrane identity4,5,6. HCC patient fibroblasts exhibit reduced PtdIns(4)P levels. FAM126A is an intrinsic component of the plasma membrane phosphatidylinositol 4-kinase complex that comprises PI4KIIIα and its adaptors TTC7 and EFR3 (refs 5,7). A FAM126A–TTC7 co-crystal structure reveals an all-α-helical heterodimer with a large protein–protein interface and a conserved surface that may mediate binding to PI4KIIIα. Absence of FAM126A, the predominant FAM126 isoform in oligodendrocytes, destabilizes the PI4KIIIα complex in mouse brain and patient fibroblasts. We propose that HCC pathogenesis involves defects in PtdIns(4)P production in oligodendrocytes, whose specialized function requires massive plasma membrane expansion and thus generation of PtdIns(4)P and downstream phosphoinositides8,9,10,11. Our results point to a role for FAM126A in supporting myelination, an important process in development and also following acute exacerbations in multiple sclerosis12,13,14.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aggarwal, S., Yurlova, L. & Simons, M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 21, 585–593 (2011).
Perlman, S. J. & Mar, S. Leukodystrophies. Adv. Exp. Med. Biol. 724, 154–171 (2012).
Zara, F. et al. Deficiency of hyccin, a newly identified membrane protein, causes hypomyelination and congenital cataract. Nat. Genet. 38, 1111–1113 (2006).
Tan, J. & Brill, J. A. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit. Rev. Biochem. Mol. Biol. 49, 33–58 (2014).
Nakatsu, F. et al. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell. Biol. 199, 1003–1016 (2012).
Hammond, G. R. V. et al. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337, 727–730 (2012).
Baird, D., Stefan, C., Audhya, A., Weys, S. & Emr, S. D. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J. Cell. Biol. 183, 1061–1074 (2008).
Nawaz, S. et al. Phosphatidylinositol 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J. Neurosci. 29, 4794–4807 (2009).
Musse, A. A., Gao, W., Homchaudhuri, L., Boggs, J. M. & Harauz, G. Myelin basic protein as a ‘PI(4,5)P2-modulin’: a new biological function for a major central nervous system protein. Biochemistry 47, 10372–10382 (2008).
Goebbels, S. et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 30, 8953–8964 (2010).
Snaidero, N. et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290 (2014).
Franklin, R. J. M. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Crawford, A. H., Chambers, C. & Franklin, R. J. M. Remyelination: the true regeneration of the central nervous system. J. Comp. Pathol. 149, 242–254 (2013).
Fancy, S. P. J., Chan, J. R., Baranzini, S. E., Franklin, R. J. M. & Rowitch, D. H. Myelin regeneration: a recapitulation of development? Annu. Rev. Neurosci. 34, 21–43 (2011).
Di Paolo, G. & De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 (2006).
Balla, T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 (2013).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Audhya, A. & Emr, S. D. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593–605 (2002).
Balla, A., Tuymetova, G., Tsiomenko, A., Varnai, P. & Balla, T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell 16, 1282–1295 (2005).
Wu, X. et al. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev. Cell 28, 19–29 (2014).
Biancheri, R. et al. Phenotypic characterization of hypomyelination and congenital cataract. Ann. Neurol. 62, 121–127 (2007).
Biancheri, R. et al. Hypomyelination and congenital cataract broadening the clinical phenotype. Arch Neurol-Chicago 68, 1191–1194 (2011).
Traverso, M. et al. Novel FAM126A mutations in hypomyelination and congenital cataract disease. Biochem. Biophys. Res. Commun. 439, 369–372 (2013).
Trotter, P. J., Wu, W.-I., Pedretti, J., Yates, R. & Voelker, D. R. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J. Biol. Chem. 273, 13189–13196 (1998).
Audhya, A., Foti, M. & Emr, S. D. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673–2689 (2000).
Tan, J., Oh, K., Burgess, J., Hipfner, D. R. & Brill, J. A. PI4KIIIα is required for cortical integrity and cell polarity during Drosophila oogenesis. J. Cell. Sci. 127, 954–966 (2014).
Gazzerro, E. et al. Hyccin, the molecule mutated in the leukodystrophy hypomyelination and congenital cataract (HCC), is a neuronal protein. PLoS ONE 7, e32180 (2012).
Tomasch, J. Size, distribution, and number of fibres in the human corpus callosum. Anat. Rec. 119, 119–135 (1954).
Graf von Keyserlingk, D. & Schramm, U. Diameter of axons and thickness of myelin sheaths of the pyramidal tract fibres in the adult human medullary pyramid. Anat. Anz. 157, 97–111 (1984).
Kawasoe, T. et al. Isolation and characterization of a novel human gene, DRCTNNB1A, the expression of which is down-regulated by β-catenin. Cancer Res. 60, 3354–3358 (2000).
Fancy, S. P. J. et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 (2009).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Hubner, N. C. & Mann, M. Extracting gene function from protein–protein interactions using Quantitative BAC InteraCtomics (QUBIC). Methods 53, 453–459 (2011).
Bojjireddy, N. et al. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 289, 6120–6132 (2014).
Doublié, S. Preparation of selenomethionyl proteins for phase determination. Meth. Enzymol. 276, 523–530 (1997).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Meth. Enzymol. 276, 307–326 (1997).
Hendrickson, W. A. & Ogata, C. M. in Methods in Enzymology: Macromolecular Crystallography Vol. 276 (eds Carter, C. W. & Sweet, R. M.) 494–523 (Academic Press, 1997).
Adams, P. D., Grosse-Kunstleve, R. W. & Hung, L. W. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1952 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Fitzner, D. et al. Myelin basic protein-dependent plasma membrane reorganization in the formation of myelin. EMBO J. 25, 5037–5048 (2006).
Acknowledgements
We thank F. Nakatsu for helpful discussions and J. Chung, H. Czapla, L. Lucast and F. Wilson for technical assistance. HCC patient and control fibroblasts were kindly provided by the Cell Line and DNA Biobank from Patients Affected by Genetic Diseases (Giannina Gaslini Institute), part of the Telethon Network of Genetic Biobanks (project no. GTB12001). Primate brain samples were generously provided by N. Sestan and A. Sousa (Yale University). This work was supported by the National Institutes of Health (R37NS036251, R01DK082700, P30DK45735 and P30DA018343 to P.D.C., R01GM080616 to K.M.R., R01GM095982 to T.C.W., and K99GM110121 to J.M.B.), the Simons Foundation (to P.D.C.), Telethon (GP2007250 to C.M.), the Mariani Foundation (C.M.), the San Paolo Foundation (to C.M.), and the European Leukodystrophy Association Foundation (to C.M.). J.M.B. was a recipient of a Jane Coffin Childs Memorial Fund fellowship, and C.M.S. was a recipient of a National Science Foundation Graduate Research Fellowship (DGE-1122492).
Author information
Authors and Affiliations
Contributions
J.M.B., X.W., R.C., T.C.W., K.M.R. and P.D.C. designed the experiments. J.M.B., X.W., R.C., M.S.O., C.M.S., M.M. and A.R. performed the experiments. E.G., S.A., S.B., R.B., F.Z., C.M. and M.S. generated and contributed cells, other tools, and reagents. J.M.B., K.M.R. and P.D.C. wrote the manuscript, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 FAM126A/B are identified as interaction partners of EFR3A/B, and FAM126A is expressed as two splice forms, with the 58 kD splice form the major form in mammalian brain.
(a) HeLa cells stably expressing EFR3A-GFP, EFR3B-GFP, or GFP only were subjected to GFP immunoprecipitation. The purified proteome was digested and analyzed by LC-MS/MS, and peptide and protein identities were determined using MaxQuant. Shown is the sequence coverage of FAM126A (left) and FAM126B (right) from two separate experiments: EFR3A-GFP vs. GFP IP (black bars) and EFR3B-GFP vs. GFP IP (white bars). (b) Top, Domain cartoon of the two splice forms of FAM126A (isoform 1, 58 kD corresponding to NCBI NP_115970.2; isoform 2, 47 kD, corresponding to NCBI XP_005249951.1), with regions common to both splice isoforms in cyan and regions unique to each splice isoform in yellow (isoform 1) and magenta (isoform 2). Bottom, Confirmation of the identity of the 47 kD splice form. Immunoprecipitations using an anti-FAM126A antibody raised against residues 2-308 of human FAM126A or a negative control (rabbit IgG) were performed from lysates of primary human skin fibroblasts and analyzed by SDS-PAGE followed by Coomassie staining. The band at ∼47 kD in the anti-FAM126A sample was excised and analyzed by mass spectrometry. Shown is the list of identified peptides; those colored in magenta are unique to isoform 2 (XP_005249951.1). (c) Immunoblot analysis of FAM126A from lysates from lung of wild-type (WT) and FAM126A knockout (KO) mice. (d) Immunoblot analysis of FAM126A in lysates from gray matter (G) and adjacent white matter (W) samples from human or macaque brain (parietal cortex) and control (C) or patient-derived, FAM126A-deficient (P) primary human skin fibroblasts, demonstrating that isoform 1 is the major form in brain, and isoform 2 is the major form in skin fibroblasts. Arrowheads denote background bands at ∼43 kD in mouse lung (c) and ∼57 kD in fibroblasts (d). Shown are representative immunoblots from three independent experiments.
Supplementary Figure 2 FAM126A is a soluble, cytosolic protein that is recruited to the plasma membrane by EFR3B and TTC7B.
COS-7 cells were transfected with the indicated combinations of the following plasmids: GFP-FAM126A-N, FAM126A-GFP, soluble mCherry (mCherry), a plasma membrane-targeted mCherry containing the first 11 residues of Lyn kinase (PM-mCherry) either alone (a) or in combination with EFR3B-HA and TTC7B-mTagBFP (b). The cells were imaged live by confocal microscopy; shown are single z-plane images. Scale bars, 20 μm.
Supplementary Figure 3 Purification of PI4KIIIα complexes for in vitro kinase assay and representative electron density maps for the FAM126A-N/TTC7B crystal structure.
(a) 3xFLAG-tagged PI4KIIIα (wild-type or a kinase-dead point mutant) were expressed in Expi293 cells alone or co-expressed with TTC7B or both TTC7B and FAM126A-N. The kinases (or kinase complexes) were purified by anti-FLAG affinity chromatography and analyzed by SDS-PAGE, staining with Coomassie blue. Arrowhead indicates Hsp70, which partially co-purified with all samples (and whose identity was verified by mass spectrometry). (b) Top, Experimental electron density map, contoured at 1.0σ, into which the initial model was built. The map was calculated with phases from a SAD experiment after density modification and sharpened using B-factors (−30 Å2). The initial model before refinement is shown in green. Bottom, 2Fo-Fc map of the same region, contoured at 1.0σ, with B-factor sharpening (−30 Å2). The refined model is shown in green. (c) Point mutations in FAM126A that underlie HCC are indicated in the TTC7B/FAM126A-N structure. Most of the disease-causing mutations in FAM126A result in premature termination; two known disease-causing missense mutations (L53P and C57R), indicated here, likely cause FAM126A misfolding.
Supplementary Figure 4 Biochemical evidence for defects in PI4KIIIα function in HCC fibroblasts and FAM126A KO mice.
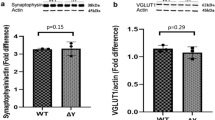
(a) Quantification of changes in protein levels shown in Fig. 5. Shown are the ratios of protein levels between the two indicated samples from immunoblots in Fig. 5, quantified using densitometry. Top left, HCC patient and control fibroblasts (Fig. 5a). ∗, PI4KIIIα, p = 0.0049; FAM126A, p = 0.0026; FAM126B, p = 0.0008; TTC7A, p = 0.0003; TTC7B, p = 0.019; EFR3A, p = 0.0011; n = 3 independent experiments (with 3 total technical replicates for all, except 4 for PI4KIIIα). Top right, Primary cultured oligodendrocytes and cortical neurons (Fig. 5d). ∗, PI4KIIIα, p = 0.019; FAM126B, p = 0.043; TTC7A, p = 0.048; EFR3B, p = 0.027; n = 2 independent experiments (with 2 total technical replicates for all, except 5 for PI4KIIIα and 4 for FAM126A). Bottom left, FAM126A KO and WT mouse brain (Fig. 5e). ∗, FAM126A, p = 0.0020; TTC7A, p = 0.0003; EFR3A, p = 0.024; n = 3 independent experiments (with 3 total technical replicates for all, except 4 for PI4KIIIα and 5 for TTC7A). Bottom right, FAM126A KO and WT mouse optic nerve (Fig. 5f). ∗, FAM126A, p = 0.0008; TTC7A, p = 0.025; EFR3A, p = 0.0056; n = 2 independent experiments (with 2 total technical replicates for all, except 3 for EFR3A, EFR3B, and FAM126A). Significance was calculated using either an unpaired two-tailed Student’s t-test with equal variance (top left) or a two-tailed, paired ratio t-test (all others). Error bars represent standard deviation. (b) Rescue of PI4KIIIα complex levels in HCC patient fibroblasts by expression of FAM126A-GFP. Immunoblot (IB) analysis of PI4KIIIα complex components in lysates from control fibroblasts or HCC patient fibroblasts transduced with either a control lentivirus (−) or lentivirus containing a C-terminally tagged FAM126A construct corresponding to isoform 2, the major form in fibroblasts (see Supplementary Fig. 1d). Shown are representative immunoblots from three independent experiments. (c) FAM126A mRNA is more abundant than FAM126B mRNA in human fibroblasts. cDNA from human fibroblasts was analyzed by qRT-PCR using primers specific to FAM126A and FAM126B. Shown are relative amounts of FAM126A to FAM126B, with FAM126B value normalized to 1 (two-tailed, Student’s t-test, unequal variance, p = 0.0004; n = 3 independent experiments). Error bars represent standard deviation. (d) Plasma membrane PI4P levels are reduced in HCC fibroblasts. Immunofluorescence analysis of the plasma membrane pool of PI4P, using an anti-PI4P antibody, in control and HCC patient fibroblasts. Shown are representative average intensity projection images of a confocal z-stack. Quantification and statistical information is provided in Fig. 5c. (e) FAM126A KO results in a more severe impact on PI4KIIIα complex levels in oligodendrocytes compared to neuronal cells. Left, Immunoblot analysis of PI4KIIIα complex components in cells of the oligodendrocyte (Oligo) and neuronal (Neu) lineage that were immunoisolated from wild-type and FAM126A KO mice at postnatal day 8. Right, Quantification of these immunoblots, showing the relative amount of each PI4KIIIα complex component in the corresponding cell type in FAM126A KO compared to in wild-type. Black bars, oligodendrocytes; gray bars, neuronal cells. Two-tailed Student’s t-test, unequal variance, ∗, PI4KIIIα:p = 0.0068. FAM126B: p = 0.000050. TTC7A: p = 0.0457. TTC7B: p = 0.0411. EFR3A: p = 0.0162. EFR3B: p = 0.0611; n = 3 biological replicates (3 total technical replicates for all, except 4 for PI4KIIIα). Error bars represent standard deviation.
Supplementary Figure 5 Morphological analysis of myelination in FAM126A KO and control mice.
Light and transmission electron micrographs of myelin in transverse sections of spinal cord (ventral funiculus cervical region), optic nerve, and sagittal sections of two regions of the central area of corpus callosum from WT and FAM126A KO male mice at age P15. No substantial differences in extent of myelination between the two genotypes can be observed. (a) Representative light microscopy images of semithin (1 μm) sections, stained with toluidine blue. (b) Representative transmission electron microscopy images of ultrathin sections (60 nm) contrasted with osmium tetroxide and uranyl acetate. Shown are representative images (n = 3 KO mice; n = 2 WT littermate control mice). Scale bars: 20 μm (a); 2 μm (b).
Supplementary information
Supplementary Information
Supplementary Information (PDF 1372 kb)
Supplementary Table 1
Supplementary Information (XLSX 698 kb)
Supplementary Table 2
Supplementary Information (XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Baskin, J., Wu, X., Christiano, R. et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat Cell Biol 18, 132–138 (2016). https://doi.org/10.1038/ncb3271
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ncb3271
This article is cited by
-
Adaptor proteins mediate CXCR4 and PI4KA crosstalk in prostate cancer cells and the significance of PI4KA in bone tumor growth
Scientific Reports (2023)
-
Fatty acids homeostasis during fasting predicts protection from chemotherapy toxicity
Nature Communications (2022)
-
Hypomyelinating leukodystrophies — unravelling myelin biology
Nature Reviews Neurology (2021)
-
Palmitoylation targets the calcineurin phosphatase to the phosphatidylinositol 4-kinase complex at the plasma membrane
Nature Communications (2021)
-
Oncogenic KRAS is dependent upon an EFR3A-PI4KA signaling axis for potent tumorigenic activity
Nature Communications (2021)