Abstract
Mesenchymal stem cells are highly regarded for their potential in tissue repair and regenerative medicine due to their multipotency and self-renewal abilities. Recently, mesenchymal stem cells have been redefined as “medical signaling cells,” with their primary biological effects mediated through exosome secretion. These exosomes, which contain lipids, proteins, RNA, and metabolites, are crucial in regulating various biological processes and enhancing regenerative therapies. Exosomes replicate the effects of their parent cells while offering benefits such as reduced side effects, low immunogenicity, excellent biocompatibility, and high drug-loading capacity. Dental stem cells, including those from apical papilla, gingiva, dental pulp, and other sources, are key contributors to exosome-mediated regenerative effects, such as tumor cell apoptosis, neuroprotection, angiogenesis, osteogenesis, and immune modulation. Despite their promise, clinical application of exosomes is limited by challenges in isolation techniques. Current methods face issues of complexity, inefficiency, and insufficient purity, hindering detailed analysis. Recent advancements, such as micro-electromechanical systems, alternating current electroosmosis, and serum-free three-dimensional cell cultures, have improved exosome isolation efficacy. This review synthesizes nearly 200 studies on dental stem cell-derived exosomes, highlighting their potential in treating a wide range of conditions, including periodontal diseases, cancer, neurodegenerative disorders, diabetes, and more. Optimized isolation methods offer a path forward for overcoming current limitations and advancing the clinical use of exosome-based therapies.
Similar content being viewed by others
Introduction
Extracellular vesicles (EVs) are nanoscale particles encased by a lipid bilayer that are released by nearly all cell types.1,2 EVs play a crucial role in intercellular communication by mediating the transfer of bioactive molecules, thereby influencing various biological processes such as immune modulation, tissue regeneration, and oncogenesis. These vesicles are classified into various subtypes based on their origin, dimensions, and molecular composition, with exosomes being one of the most extensively researched categories.3 Exosomes, generally measuring between 30 and 150 nm in diameter, arise from the endosomal pathway and are discharged into the extracellular space following the merger of multivesicular bodies with the plasma membrane.4 Their molecular contents – consisting of proteins, lipids, and nucleic acids such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) – empower them to modulate cellular activities under both physiological and pathological conditions.5 The increasing focus on EVs, particularly exosomes, has highlighted their potential as valuable biomarkers and therapeutic agents with significant implications for regenerative medicine and tissue engineering.
The significance of exosomes in the field of regenerative medicine has gained increased interest due to their capacity to facilitate tissue regeneration and modulate immune reactions.6 Among the diverse origins of exosomes, mesenchymal stem cells (MSCs) have been thoroughly examined for their regenerative capabilities.7 Exosomes derived from MSCs (MSCs-Exos) have been demonstrated to enhance angiogenesis, regulate inflammatory responses, and promote tissue remodeling, rendering them promising candidates for therapeutic interventions.8 In contrast to direct MSCs transplantation, which is associated with challenges such as limited cell engraftment, potential immune rejection, and the risk of tumorigenicity, MSCs-Exos provide a cell-free therapeutic strategy characterized by lower immunogenicity and greater stability. The regenerative potential of MSCs-Exos is primarily attributed to their bioactive cargo, comprising cytokines, growth factors, microRNAs (miRNAs), and other signaling molecules that modulate cellular repair and tissue regeneration.
Dental stem cells (DSCs), a distinct subpopulation of MSCs, have gained recognition as a promising source of exosomes for regenerative applications.9,10 Numerous subpopulations of DSCs have been explored to date, including stem cells from apical papilla (SCAPs),11 stem cells derived from human exfoliated deciduous teeth (SHEDs),12 dental follicle stem cells (DFSCs),13 gingival mesenchymal stem cells (GMSCs),14 periodontal ligament stem cells (PDLSCs),15 and dental pulp stem cells (DPSCs)16 (Fig. 1). Compared to MSCs derived from bone marrow or adipose tissue, DSCs can be obtained through minimally invasive procedures, thereby mitigating ethical concerns and minimizing donor-site morbidity.17,18 Notably, DSCs possess unique regenerative attributes, including a high proliferative potential, multipotency, and enhanced immunomodulatory properties.19 Beyond their ability to regenerate dental tissues, DSCs have also demonstrated the capacity to contribute to the repair and regeneration of various somatic tissues,20,21,22,23,24 highlighting their potential for broader therapeutic applications beyond the field of dentistry.
The surface markers and tissue origins of various dental stem cells, confirming their mesenchymal lineage and regenerative potential. While all share core mesenchymal stem cell markers (CD44, CD73, CD90, CD105, STRO-1), unique markers including CD24, CD140a/β, and CD31/CD63 define specialized roles in tissue repair, regeneration, and immunomodulation
Although DSCs-Exos hold significant therapeutic promise, their clinical translation remains in its early stages, with several critical challenges that must be addressed to enable widespread adoption. Standardization of DSCs-Exos isolation and characterization protocols is essential to ensure consistency in yield, purity, and bioactivity.8 Additionally, scalable and reproducible bioprocessing techniques must be developed to facilitate cost-effective large-scale production for clinical applications.25 Optimizing delivery strategies, including the integration of biomaterial-based carriers, hydrogels, and scaffolds, is crucial for enhancing targeted exosome retention and therapeutic efficacy.26 Furthermore, rigorous evaluation of safety and regulatory considerations, including potential immunogenicity and off-target effects,27 is necessary through comprehensive preclinical and clinical studies to establish the reliability and safety of DSCs-Exos-based therapies.
Comparative analysis of dscs-exos and mscs-exos
DSCs-Exos and MSCs-Exos share a common regenerative potential; however, distinct biological characteristics and therapeutic advantages set them apart. While both exosome types exhibit the ability to modulate immune responses, promote angiogenesis, and enhance tissue regeneration,28,29 DSCs-Exos have demonstrated unique advantages that warrant further investigation for their clinical applications. One of the key distinctions between DSCs-Exos and MSCs-Exos lies in their origin. Unlike MSCs-Exos derived from bone marrow or adipose tissue, DSCs-Exos are sourced from dental tissues through minimally invasive procedures, reducing ethical concerns and donor-site morbidity.30,31 Moreover, DSCs possess a higher proliferation rate and exhibit enhanced neurogenic and odontogenic differentiation capabilities,32,33,34 making their exosomes particularly suitable for applications in craniofacial and neural tissue regeneration.
Molecular profiling studies have revealed variations in the bioactive cargo of DSCs-Exos compared to MSCs-Exos. DSCs-Exos have been shown to contain a distinct repertoire of miRNAs, growth factors, and extracellular matrix proteins that contribute to their specialized regenerative effects.28,35 For example, DSCs-Exos are enriched in miRNAs associated with osteogenesis, neuroprotection, and angiogenesis, which may explain their superior potential in bone and nerve regeneration.36 In contrast, MSCs-Exos have been widely recognized for their immunomodulatory properties, making them valuable in autoimmune and inflammatory disease treatments.37 Furthermore, DSCs-Exos exhibit a favorable immunological profile, with studies suggesting a lower risk of eliciting immune rejection compared to MSCs-Exos. This immunoprivileged status enhances their feasibility for allogeneic transplantation without the need for extensive immunosuppression.38 Additionally, the extracellular matrix components and adhesion molecules present in DSCs-Exos may enhance their retention at injury sites,30 potentially improving therapeutic efficacy.
Applications of exosomes in personalized medicine
For numerous reasons, exosomes have attracted considerable interest in scientific research as innovative vehicles for drug delivery. In contrast to synthetic nanoparticles, exosomes have demonstrated diminished toxicity and immunogenicity in preliminary investigations. These observations imply that exosomes may pose a lower risk of eliciting adverse reactions or immune responses when utilized as vehicles for drug delivery. Exosomes originate from cells and are constituted by lipid bilayers that closely resemble cellular membranes.39 This particular composition enhances the biocompatibility and stability of exosomes, enabling them to safeguard cargo molecules against degradation and unfavorable conditions within the body, such as enzymatic breakdown and acidic environments.26 Studies have indicated that exosomes can successfully reach their destination organs following circulation within the bloodstream. Furthermore, exosomes possess the capacity to deliver therapeutics through penetration of the blood-brain barrier.40 Consequently, extensive efforts have been undertaken to load a variety of therapeutic agents, thereby augmenting the therapeutic efficacy of these agents.41 A significant number of ongoing investigations are concentrated on optimizing the cargo loading of exosomes to fully exploit their potential as drug delivery systems. Diverse methodologies have been devised to incorporate therapeutic agents, including small-molecule pharmaceuticals and genetic materials, into exosomes.39
Two primary techniques employed to load drugs into exosomes are passive loading and active loading.42 The selection of technique may hinge upon the specific drug and its characteristics, alongside the intended release mechanism. Passive loading transpires when cells are cultured with the drug of interest, facilitating the natural incorporation of the drug into the exosomes during their formation. The cells internalize the drug, encapsulating it within exosomes, and subsequently release the drug-laden exosomes into the surrounding environment. This method is straightforward and facile to implement; however, it suffers from low loading efficiency and presents challenges in regulating the quantity of drug encapsulated within the exosomes. Passive loading is typically most effective for lipophilic drugs that can readily traverse cellular and vesicular membranes. Nevertheless, this approach may not be suitable for all drug categories. Alternatively, active loading entails the direct incorporation of the drug into isolated exosomes. This process is generally executed through various techniques that enhance the permeability of the exosomal membrane, such as exposure to detergents, electroporation, thermal shock, sonication, or the application of saponins. Active loading achieves superior loading efficiency relative to passive loading and permits more precise regulation of the drug loading quantity. However, this method can be intricate, and improper execution may compromise the process.43 In both approaches, the elimination of any unencapsulated drug following loading, utilizing diverse purification techniques such as ultrafiltration, size-exclusion chromatography (SEC), or ultracentrifugation, is essential.
Genetic engineering methodologies may also be employed to load genetic materials into exosomes. Cells can be genetically modified to express a protein or RNA of interest, which can subsequently be integrated into exosomes, during their formation.42 This approach can be employed for loading particular RNA or protein molecules into exosomes; however, necessitates intricate molecular biology strategies and might not be suitable for all kinds of therapeutic agents.44 Each of these approaches possesses benefits and limitations, and the best approach can rely on factors including the type of drug, the source of the exosome, and the intended drug delivery target.45
Exosome isolation techniques
To enable the comprehensive investigation and utilization of these distinctive EVs, exosomes must be meticulously extracted from an array of interfering constituents and cellular debris.46 The methodologies utilized in the exosome isolation process should demonstrate exceptional efficacy and possess the capability to extract exosomes from various sample matrices. With the rapid progress in scientific research and technological advancements, numerous approaches have been developed for the isolation of exosomes in significant quantities and with high purity.47 Each method capitalizes on specific characteristics of exosomes, such as their size, morphology, density, and surface proteins to facilitate their isolation.48 Variants within each category also introduce distinct sets of benefits and limitations concerning exosome isolation (Table 1)3,49,50,51,52,53,54
The selection and modification of isolation approaches ought to be tailored according to the specific biological fluid from which exosomes are extracted. An effective isolation approach must concentrate the exosomal signal for analysis while avoiding contamination from other molecules, such as EVs, lipoproteins, and protein aggregates. For example, blood plasma encompasses high-density lipoprotein (7–15 nm), LDL (20 to 40 nm), very-low-density lipoprotein (30–90 nm), and chylomicrons (200–600 nm), all of which exhibit similarities to EVs regarding density and size. This complicates the exosomal isolation process, as conventional methods struggle to exclude lipoprotein contamination.55 In this regard, it has been observed that LDL may co-purify with exosomes isolated from both platelet concentrations and blood plasma.56 Hence, it is recommended to utilize SEC in conjunction with a density gradient to effectively segregate EVs from lipoproteins.57 Additionally, the presence of fetal bovine serum (FBS) EVs and contaminants in cell culture media poses challenges to exosomal isolation in vitro. The exosomes found in FBS have been documented to possess biological activity. Hence, their co-isolation could disrupt subsequent in vivo or in vitro investigations. Numerous methods have been implemented to address this issue, such as culturing cells in serum-free media, which might lead to significant cellular starvation, altering cellular behavior, composition, and the number of exosomes secreted. An alternative strategy involves utilizing commercially available exosome-depleted or home-generated FBS. Another possibility is the application of bile salts, synthetic agents, pituitary extracts, or platelet lysates.58
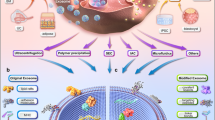
Currently, there is no one-size-fits-all protocol for the isolation of exosomes;59 hence, research on exosomes is significantly hindered by the application of variable extraction/isolation techniques, thereby constraining the understanding of studies focused on exosomes.60 Moreover, the heterogeneous protein composition of exosomes complicates their extraction through traditional immune-marking techniques, since not all exosomes exhibit the same classical protein markers, nor are all the recognized markers exclusive to exosomes, because they have also been identified in other categories of EVs. In fact, the ISEV has advocated for a new standardized nomenclature, contingent upon researchers being able to reliably ascertain the endosomal origin of their exosomal preparations. The 2018 MISEV (Minimal Information for Studies of Extracellular Vesicles) recommendations advise the characterization of EVs based on size (i.e., small EVs [sEVs] less than 200 nm; medium/large EVs more than 200 nm), density (i.e., high, medium, low), biochemical composition (i.e., CD63+/CD81+ EVs), or cellular origin (i.e., hypoxic EVs, oncosomes, and so forth).61 Regardless of nomenclature, exosomes and other EVs have been extracted using various methodologies, including microfluidics, immunoaffinity capture (IAC), precipitation, ultrafiltration, SEC, and ultracentrifugation (Fig. 2).62,63 The purpose of this review was to examine various techniques employed for the isolation of DSCs-derived exosomes for their applications in regenerative medicine and dentistry. Moreover, we also identified recently optimized methods for efficient isolation of the exosomes, demonstrating improved efficacy over conventional approaches.
Most utilized exosome isolation approaches. Traditional techniques for exosome isolation comprise differential ultracentrifugation (DUC) and size-exclusion chromatography (SEC). DUC focuses on the separation of exosomes through incrementally increasing centripetal acceleration. SEC employs biofluids as a mobile phase in conjunction with a porous stationary phase, allowing for the differential elution of molecules based on an inverse relationship with their size – specifically, larger particles elute prior to smaller ones, which, upon entering the pores, traverse a longer path resulting in extended elution times. Precipitation utilizes a solution to promote the formation of a polymer-encapsulated vesicle aggregate in substantial quantities. Immunoaffinity capture employs antibodies that target exosomal surface proteins, facilitating the isolation of specific vesicle subpopulations. The microfluidics approach utilizes chips designed for specific antibody-mediated interactions to efficiently capture exosomes. Ultrafiltration operates on the principle of using a filter with a defined pore size, resulting in a vesicle-rich filtrate tailored to the desired dimensions
Literature search strategy
An initial search using terms associated with exosomes (i.e., exosomes and extracellular vesicles) and dental stem cells without any limitation of publication year and field of study (medicine, dentistry, etc.) was performed on September 30th, 2024. The inclusion criteria comprised original research articles including clinical studies as well as studies conducted in animal or cellular models. Moreover, the included studies examined the therapeutic potential of DSCs-Exos for the treatment of medical and dental diseases. A total of 1 842 publications were retrieved including Elsevier’s Scopus (n = 939), Clarivate Analytics’ Web of Science (n = 419), and PubMed/MEDLINE (n = 484) (Table S1). Of these, 541 were selected and further assessed. Following the full-text screening, a total of 197 research articles were included.
Literature outcomes of dental stem cells-derived exosome isolation methods
Table S2 lists all 197 studies included in the present review including study references, field or topics covered, and exosomal isolation technique used.36,45,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255
Overall outcomes of DSCs-Exos isolation methods
Overall, in studies investigating exosomes derived from dental cells, a variety of isolation methods have been used, with DUC standing out as the most utilized approach. It was utilized in 132 studies (67%), confirming its position as the gold standard for exosome isolation due to its ability to achieve high-purity exosomes. PBP was the second most frequently used method, reported in 32 studies (16%), offering a cost-effective and accessible alternative. Several studies explored combining DUC with other methods to enhance yield and purity. For instance, DUC combined with PBP was applied in eight studies, with ultrafiltration in six studies, and with SEC in three studies. More complex multi-step approaches, such as the combination of DUC, ultrafiltration, and SEC, were utilized in two studies, indicating a focus on refining the isolation process for greater exosome quality and specificity. Other notable methods included the use of ultrafiltration combined with PBP (n = 5), SEC alone (n = 2), and microfluidics either as a standalone technique or in combination with ultrafiltration (one study each). Advanced approaches, such as combining ultrafiltration, SEC, and IAC, were explored in one study, reflecting efforts to increase precision in exosome isolation (Fig. 3). Figure 4 summarizes the subpopulations and applications of DSCs-Exos utilizing different isolation methods.
Recent advancements in dscs-exos isolation
Various isolation methods profoundly influence the yield, purity, and operational efficacy of exosomes.256,257,258 In this regard, Han et al.259 investigated the effect of DUC and SEC on the yield of salivary exosomes. They found that salivary exosomes isolated using the SEC approach had a higher yield of particles and particles/protein ratios compared to those isolated utilizing DUC.259 Kim et al.260 found that the tangential flow filtration (TFF) method showed a remarkable 92.5-fold superiority over ultracentrifugation approach to extract exosomes from umbilical cord mesenchymal stem cells (UCMSCs-Exos), yielding 1.85 × 1010 exosomes compared to ultracentrifugation’s 0.02 × 1010 for an equivalent cell count.260 This substantial scalability renders TFF particularly advantageous for extensive clinical applications. Moreover, employing TFF with optimized serum exosome depletion markedly diminished the presence of impurities (such as low-density lipoprotein [LDL] cholesterol) and augmented MSCs-Exos purity by a factor of 15.6, as verified through CD73 surface marker evaluation. The utilization of a 500 kDa filter enhanced purity relative to a 300 kDa filter by effectively eliminating non-exosomal contaminants.260 Additionally, both isolation methods successfully preserved the structural integrity of exosomes (characterized by double-layered spheres via transmission electron microscopy) and maintained tetraspanin markers (CD9, CD63, and CD81).260
Hadady and colleagues71 in 2022 evaluated two isolation approaches (ultrafiltration and micro-electromechanical systems [MEMS]) for the isolation of SCAPs-Exos. They found that the MEMS approach is capable of isolating a smaller and more effective EVs’ population that safeguards the visual acuity, highlighting the concept that exosomal isolation approaches determine the therapeutic efficacy and the compositions of the isolates. The suggested MEMS apparatus provides adaptable fabrication alternatives, accompanied by the significant benefit of comparatively modest expense. These initiatives are essential for an extensive array of clinical and biomedical applications as an exceptionally practical and essential contender, given that a MEMS device can be utilized to produce therapeutics tailored to individual patients.71
The application of alternating current (AC) electrokinetic forces for the manipulation of EVs within a microfluidic device has emerged as a robust and sensitive methodology for diagnostic and trapping applications. Since various AC electrokinetic forces might influence the overall dynamics of particles and fluids within such as device, the electrokinetic confinement of EVs necessitates a comprehensive investigation of all predominant forces at play, contingent upon the specific experimental parameters employed. Hence, Hadady et al.72 assessed the isolation of SCAPs-Exos on interdigitated electrode arrays by incorporating two significant AC electrokinetic phenomena, i.e., dielectrophoresis and AC electroosmosis (ACEO). The study revealed that the motion of ACEO fluid significantly influences the localization of SCAPs-Exos in experimental settings. The application of this optimization protocol facilitates a direct correlation with empirical findings, demonstrating a favorable qualitative alignment in behavior. Understanding the underlying physics governing the collection of EVs would aid researchers in manipulating their dynamics within microfluidic systems using AC electrokinetics, thereby advancing the development of innovative research instruments such as biochips and biosensors for exosomal isolation.72,261
The culture of three-dimensional (3D) dental pulp pluripotent-like stem cells (DPPSCs) for purposes except for exosome isolation has been documented. Given the benefits of 3D culture conditions for stem cells compared to traditional 2D monolayer systems—particularly regarding in-vivo mimicry, signaling pathways, and improved therapeutic outcomes—it was put forward that exosomes obtained from the 3D culture of these stem cells would similarly demonstrate heightened therapeutic potential for applications in regenerative medicine. Hence, a study by Faruqu et al.262 aimed to develop a practicable serum-free 3D culture environment for DPPSCs, facilitating the isolation of exosomes from the stem cells for prospective use in regenerative therapies, while ensuring that the phenotype of the parent cells remains unaltered. They found that a 12-day incubation of DPPSCs in a serum-free 3D culture permitted the isolation of DPPSCs-Exos with minimal contamination from non-exosomal vesicles.262 Future studies should aim to investigate the therapeutic potential of DPPSCs-Exos in vitro and within pre-clinical animal models. Additionally, examining the applicability of DPPSCs-Exos in drug delivery systems would be of substantial interest.
In 2024, Duan et al.45 presented an innovative approach utilizing ultracentrifugation for obtaining high-purity artificial cell-derived vesicles (ACDV) from DPSCs lysate. A comparative examination was conducted between ACDVs and those obtained via ultracentrifugation. The ACDVs isolated from the cellular lysate conform to the established criteria for EVs and exhibit a comparable protein secretion profile. Furthermore, the novel ACDVs significantly enhanced wound healing, modulated collagen regeneration, and diminished the synthesis of inflammatory mediators akin to EVs. Notably, the isolation efficacy was found to be augmented by 16-fold in comparison to the EVs isolated through the ultracentrifugation method.45
According to the International Extracellular Vesicle guidelines,61 EV purity is an essential parameter for subsequent analyses and therapeutic applications; nevertheless, current approaches for EV isolation fail to achieve particles of sufficient purity. Hence, Han et al.161 found that the purity of sEVs was quantified at 108 sEV particles/μg of protein, which is below the recommended threshold for highly pure EVs (i.e., 3 × 1010 EV particles/μg of protein).257 The presence of contaminants such as cellular debris, lipids, or proteins in impure EVs can significantly influence their biological functionality, thereby obstructing the precise interpretation of their impacts. Such contaminants might disrupt downstream processes, ultimately undermining effectiveness and leading to erroneous outcomes.61 This indicates that further refinement of the sEVs isolation approach is necessary, or that supplementary measures, such as RNase/proteinase/DNase treatments, should be implemented to improve EV purity.61 Han et al.161 successfully isolated exosomes, microvesicles, and apoptotic bodies from human gingival fibroblasts, human osteoblasts, and human PDLSCs utilizing their in-house SEC for sEV enrichment. Their isolation approach yielded approximately 6 × 107 sEV particles/mL of conditioned media,161 which is substantially greater than the previously documented sEV isolation from human PDLSCs via ultracentrifugation, which reported 1.08 × 107 sEV particles/mL of conditioned media146 and corroborates the earlier observations that the SEC technique can produce a roughly 5-fold increase in sEV enrichment compared to the ultracentrifugation method.259
Moving beyond traditional methods to isolate dsc-exos
Limitations of traditional exosome isolation methods
Since DUC was found to be the most widely used method to isolate DSCs-Exos, its inherent limitations, warrant detailed discussion. These limitations include suboptimal yield, variable outcomes, prolonged processing times, the requirement for substantial sample volumes, costly laboratory apparatus, and the necessity for highly skilled personnel.263,264 Furthermore, the exceedingly high centrifugal forces can compromise the integrity of exosomes, thereby diminishing the viability of their molecular constituents for subsequent analytical procedures. Crucially, this method is unsuitable for the processing of clinical samples, as it demands sample volumes that significantly exceed those typical of liquid biopsies, is labor intensive ( > 4 h per sample), entails multiple manual operations, and results in diminished exosome yield.265,266 Additional constraints pertain to the co-purification of exosomes alongside contaminants including protein aggregates and other particulate matter (i.e., lipoproteins) that exhibit comparable dimensions to exosomes, consequently undermining the precision of downstream analyses, which is essential for clinical applications.267
Regarding polymer-based precipitation, precipitation reagents (polyethylene glycol) might contaminate the exosome preparation and interfere with downstream applications such as proteomics or RNA analysis. Moreover, difficulties in the complete removal of the polymer residues can affect exosome characterization and functional studies.268 The columns of SEC typically handle small volumes of sample, making the approach unsuitable for large-scale isolation.269 Concerning ultrafiltration, filters can become clogged with other EVs, debris, or proteins reducing efficiency and recovery rates. Additionally, exosomes can adhere to the filtration membrane, leading to sample loss and inconsistent yield.268 Fractionation, similar to DUC, requires extensive preparation, and its execution is labor-intensive and time-consuming.270
Limitations of label-based exosome isolation approaches
In label-based isolation approaches (i.e., IAC, magnetic bead-based isolation, fluorescence labeling, biotinylated antibody-based isolation, and immunoglobulin-G antibody-based capture), capture molecules (such as aptamers and antibodies) that possess the capacity to physically or chemically bind to specific lipid composition or proteins on the surface of exosomes function as labels.271,272,273 Although physical entanglements and non-covalent interactions, such as hydrophobic interactions, van der Waal forces, ionic bonds, and hydrogen bonds, play a role in the attachment of capture molecules to target exosomes, the predominant labeling strategies rely on covalent interactions (for instance, fluorescent dyes covalently linked to exosomes, or peptides covalently conjugated to exosomes).274 Research has demonstrated that immunoaffinity-based microfluidic platforms, such as microplate-based enzyme-linked immunosorbent assays (ELISA), can effectively isolate specific populations of EVs, while minimizing the co-purification of extraneous particles.275
The literature suggests that label-based isolation approaches enhance the efficiency of recovering pure exosomes from biological fluids including urine, serum, saliva, and plasma.276,277,278,279 Despite ensuring the separation purity of labeled exosomes, these strategies require further refinement to achieve higher throughput and to target multiple EV populations utilizing diverse types of labels.280 Importantly, these methods incur significant cost due to the expensive labeling protocols. Moreover, they are generally intricate and time-consuming, necessitating complex preparation stages for labeling and additional washing steps to detach the labels from the exosomes. Crucially, capture molecules may inadvertently contaminate the sample and modify exosome characteristics potentially leading to erroneous results during subsequent characterization and undermining the reliability of downstream analyses in the clinical context.267
Potential of label-free approaches for isolation of dsc-exos
Sieving separation approaches
The sieving separation approach, especially when incorporated into sophisticated microfluidic systems, may present significant possibilities for the extraction of DSCs-Exos. This technique can effectively isolate DSCs-Exos from gingival crevicular fluid (GCF), saliva, or supernatants of various tissues. DSCs-Exos derived from these biological fluids contain molecular indicators (i.e., proteins, miRNAs) that are instrumental for the early detection of periodontal diseases, oral cancer, and other oral and dental diseases. Cutting-edge sieving systems, such as those using TFF, considerably reduce contamination from proteins and debris, hence enhancing the precision of biomarker-focused diagnostics.281
Sieving separation can be used to isolate DPSCs-Exos or PDLSCs-Exos for application in regenerative therapies. These exosomes can facilitate osteogenesis, angiogenesis, and tissue repair in periodontal defects and dental implants. Exosomes purified through sieving separation can be incorporated into biomaterial scaffolds to augment their osteoinductive and biocompatibility characteristics.7 Moreover, compact sieving devices allow for real-time exosome isolation at the point of care.282 This capability is particularly advantageous for monitoring therapeutic responses, such as during periodontal treatment or the integration of implants.
Sieving separation, particularly with ultrafiltration or TFF, is notably more expedient than traditional DUC, significantly decreasing isolation durations while maintaining exosome integrity.54 In contrast to DUC, sieving systems apply lower mechanical stress on exosomes, thereby preserving their structural and functional integrity, which is essential for therapeutic applications.283 Membrane-based systems are capable of processing diverse sample volumes, rendering them versatile for both research and clinical applications.284 Microfluidic sieving systems equipped with adjustable pore sizes facilitate precise exosome separation based on size.285 These systems can particularly be effective for isolating specific subpopulations of DSCs-Exos pertinent to dental diseases. TFF inhibits membrane fouling, potentially supporting continuous operation and enhancing recovery rates of DSCs-Exos.281 The exosomes isolated using sieving separation can serve as natural carriers for drugs, proteins, or miRNAs.286 These DSCs-Exos-based delivery systems could be used to treat dental diseases such as peri-apical infections or periodontitis.
Electrical separation approaches
The use of electrical separation methods, such as electrophoresis and dielectrophoresis (DEP), presents significant opportunities for isolating EVs,287 including DSCs-Exos. These techniques, which exploit the unique physical and electrical characteristics of exosomes,288 offer a range of advantages for dental and regenerative applications. Electrical separation methods have the potential to efficiently isolate DSCs-Exos from various biological fluids such as culture media,289 saliva, and GCF. Compared to conventional methods such as DUC, this method is particularly critical for dental applications, where intact and functional DSCs-Exos are required.
Microfluidic platforms that incorporate electrical separation mechanisms provide scalable and automated solutions for exosome isolation.290 These systems, which integrate microelectrodes or nanoporous membranes, are highly efficient for processing small sample volumes,287 such as those obtained from dental tissue fluids or culture media. Automated systems could facilitate high-throughput exosome isolation,263 making them practical for regenerative dentistry procedures, such as the preparation of bioengineered scaffolds or injectable therapeutic formulations. Achieving high purity is critical for clinical-grade exosome preparations.291 Electrical separation techniques are well-suited for this purpose (1) DEP-based isolation exploits differences in dielectric properties to improve the separation of DSCs-Exos from contaminants including protein aggregates or other nanoparticles;292 and (2) combined approaches, such as electrophoresis with membrane filtration,293 can further enhance the purity of DSCs-Exos obtained from dental culture supernatants.
Electrical separation approaches can facilitate the isolation of exosomes for incorporation into dental biomaterials. Isolated DSCs-Exos can be incorporated into hydrogels, scaffolds, or bone graft materials to enhance periodontal and alveolar bone regeneration. The gentle isolation process preserves exosome bioactivity,287 ensuring their therapeutic potential when integrated with biomaterials. DSCs-Exos can also be isolated from readily available fluids such as saliva or GCF, which are rich in biological information. Electrical separation methods are particularly suited for these applications (1) electrophoresis can exploit the negative charge of exosomes for efficient separation;294 and (2) DEP offers the capability to isolate exosomes rapidly, even in complex biological fluids, through precise control of electric fields.295
Inertial separation approaches
Inertial microfluidics may represent a cutting-edge platform for the isolation of DSCs-Exos. Leveraging size-dependent hydrodynamic forces, including inertial lift, viscous drag, and Dean vortices, these systems offer a precise and scalable approach to separate nanoscale EVs from complex biological fluids.296,297 Inertial separation systems provide a label-free method for isolating EVs,298 including DSC-Exos, from biofluids such as dental pulp culture media, saliva, and GCF. Spiral microchannels generate Dean vortices, directing smaller particles such as exosomes toward the inner channel wall while larger particles such as debris and cells are displaced outward.299 This enables high-throughput, single-step separation. The ability to continuously process biofluids makes these systems well-suited for both research and clinical applications,300 where rapid DSCs-Exos isolation is critical. The minimal mechanical stress in these systems ensures the preservation of DSCs-Exos integrity, including their bioactive components such as proteins, miRNAs, and cytokines.
The flexibility of inertial microfluidics to handle biofluids with varying viscosities can ensure compatibility with small-volume samples301 collected during dental procedures. These designs generate stronger Dean vortices compared to rectangular geometries,302 improving the lateral displacement of DSCs-Exos and enhancing separation efficiency. Optimized channel dimensions allow for higher sample processing rates, supporting clinical workflows and large-scale research studies.303
Viscoelastic separation approaches
Viscoelastic separation is a label-free method that exploits the elastic lift forces acting on particles of varying sizes within a viscoelastic fluid medium.304 Unlike affinity-based isolation techniques that rely on capture molecules such as antibodies or aptamers,305 this method preserves the native properties of exosomes, which is essential for ensuring their functional integrity in clinical applications. The viscoelastic separation process allows for the continuous and efficient fractionation of exosomes from larger EVs such as microvesicles and apoptotic bodies.306 This size-dependent separation is achieved by exploiting the differential lateral migration of particles in viscoelastic flow.305,307 Isolating purified exosome subpopulations from DSCs-Exos enhances the reproducibility and efficacy of their downstream therapeutic applications, particularly in dental tissue repair.
Viscoelastic microfluidic systems are capable of processing sample volumes at flow rates exceeding 1 mL/min,308 offering a significant advantage over conventional isolation methods such as DUC or polymer-based precipitation. This high-throughput capability is particularly relevant for clinical-scale applications, where large quantities of EVs are required. Stem cell cultures or tissue samples, such as dental pulp or periodontal ligament tissue, typically yield substantial EV quantities, and viscoelastic separation ensures efficient processing to achieve sufficient exosome yields. The performance of viscoelastic separation systems can be tuned by adjusting parameters such as the viscoelastic fluid composition, channel geometry, and flow rate.309 This flexibility allows for the optimization of isolation conditions specific to DSCs-Exos, ensuring effective separation from complex biological matrices such as stem cell culture supernatants or dental tissue extracts. Moreover, advancements in microchannel design, including wavy or curved geometries, can further enhance particle focusing and separation efficiency.310
Deterministic lateral displacement sorting
The application of deterministic lateral displacement (DLD) technology for isolating DSCs-Exos holds significant promise due to its ability to separate nanoparticles with high precision. DLD technology separates particles based on size,311 making it particularly suited for isolating exosomes from complex biological fluids such as dental tissue lysates. By tailoring the gap size, displacement angle, and total array length of the DLD device,312 it is possible to selectively isolate exosomes while removing larger particles such as apoptotic bodies, microvesicles, and debris. Dental tissue samples can be collected through minimally invasive procedures. The incorporation of DLD systems allows for streamlined processing of small volumes of fluid which is advantageous for dental applications where sample availability may be limited. DLD platforms are scalable, with parallelized nano-DLD arrays capable of processing larger sample volumes efficiently.313 For clinical applications, this scalability is critical for rapid and effective exosome isolation, addressing the time-sensitive nature of dental treatments.
Field flow fractionation
Field flow fractionation (FFF) is a sophisticated, flow-based separation technique314 with significant promise for isolating DSCs-Exos. FFF is well-suited for separating nanoparticles based on their biophysical properties such as size, density, and surface charge.315 By fine-tuning operational parameters—such as channel height, flow velocity, and applied external fields—FFF can effectively isolate exosome populations from complex biological fluids.314 This allows for the separation of pure exosome populations, free from other EVs and contaminants. In contrast to conventional DUC, FFF employs gentle hydrodynamic forces during separation.316 This approach preserves exosome morphology and bioactivity, ensuring their suitability for therapeutic applications in regenerative dentistry.
Advanced FFF modalities, such as asymmetrical flow FFF (AF4) and electrical FFF, enable the fractionation of exosome subpopulations based on subtle differences in size, surface charge, and density.317 For example, FFF can separate subpopulations of small (~60–80 nm) and large (~90–120 nm) exosomes, as well as exomeres (~35 nm), which may exhibit unique biological properties.318,319 This capability facilitates targeted investigations into the functional roles of different exosome subsets and their specific applications in dental tissue regeneration. FFF systems can be integrated with analytical detection methods, such as ultraviolet-visible spectroscopy, multiangle light scattering, and nanoparticle tracking analysis.320,321,322 These combined platforms provide comprehensive data on exosome size, concentration, and surface properties, ensuring high-quality exosome characterization.322 This integration is crucial for standardizing exosome preparations for therapeutic applications in dentistry.
Pinched flow fractionation
Pinched flow fractionation (PFF) is a microfluidic-based size-sorting technology that has demonstrated significant promise for the isolation of exosomes.323 Given its precise separation capabilities, PFF offers a valuable tool for isolating DSCs-Exos. PFF facilitates the efficient separation of particles based on size by leveraging laminar flow dynamics in a microchannel with a pinched and broadened segment.324 This technology is particularly effective for isolating exosomes from larger EVs, including apoptotic bodies and microvesicles.267 By aligning particles to the sidewall in the pinched segment and directing them along distinct streamlines based on size, PFF ensures high-resolution separation of pure exosome populations.325 This capability is critical for isolating DSCs-Exos for dental tissue regeneration.
PFF systems are capable of processing large sample volumes with high throughput. For example, recent studies demonstrated PFF devices capable of achieving flow rates up to 200 μL/min while maintaining separation efficiency.326 This scalability makes PFF suitable for processing clinical samples, enabling routine isolation of DSCs-Exos in dental research and practice. The size-specific separation capability of PFF allows for the fractionation of distinct exosome subpopulations. This feature is particularly advantageous for isolating exosome subsets with unique therapeutic potential.327 Small exosomes ( ~ 30 to 80 nm) may exhibit enhanced pro-angiogenic and neuroprotective properties beneficial for dental pulp and nerve regeneration. Large exosomes ( ~ 90 to 150 nm) may be enriched with osteogenic factors relevant to alveolar bone repair and periodontal ligament regeneration.
The microfluidic nature of PFF enables seamless integration with real-time analytical tools such as nanoparticle tracking analysis, fluorescence microscopy, and dynamic light scattering.328 This integration allows for immediate characterization of exosome size, concentration, and purity,329 enabling the development of standardized workflows for exosome isolation and quality control in dental research and therapeutic applications. The design of PFF devices can be optimized for specific dental applications. Adjustments in microchannel width, pinched segment dimensions, and outlet configurations allow for the fine-tuning of particle separation resolution.330 For instance, narrower pinched segments improve the isolation of smaller exosomes, while asymmetrically arranged outlets enhance separation precision and recovery of distinct exosome subpopulations.331 Such customization enables efficient isolation of exosomes tailored to therapeutic needs in dentistry.
Acoustic separation approaches
Acoustic separation is an innovative, label-free method for isolating exosomes with high precision and minimal damage to their structural and functional properties.332 By leveraging acoustic wave technology within microfluidic platforms, this approach offers significant advantages for isolating DSCs-Exos. Acoustic separation enables the efficient isolation of exosomes by exploiting differences in physical properties such as size, density, and compressibility.333 Larger EVs (e.g., microvesicles) are directed toward acoustic pressure nodes under the influence of acoustic radiation forces, whereas smaller exosomes remain confined in the central flow.334
Acoustic microfluidic devices are capable of continuous-flow processing,285 allowing for rapid and efficient isolation of DSCs-Exos from cell culture supernatants or biological fluids. The high throughput and minimal sample loss make acoustic separation highly suitable for clinical-scale exosome production, addressing the growing demand for exosome-based therapeutics in dentistry. Acoustic separation technology can be fine-tuned to selectively isolate exosome subpopulations based on unique biophysical properties335 (1) variations in membrane cholesterol content or lipid composition influence the acoustic contrast factor of exosomes,336 enabling their selective isolation for specific regenerative applications; and (2) By optimizing parameters such as acoustic wave frequency, amplitude, and channel dimensions,337 specific exosome subpopulations can be isolated to enhance angiogenesis, osteogenesis, or immunomodulation as required for dental applications.
Acoustic separation enables efficient discrimination between exosomes and impurities such as lipoproteins and protein aggregates, which often have overlapping physical properties. By optimizing the acoustic field parameters, high-purity exosome fractions can be obtained, reducing the risk of contamination and enhancing the safety of clinical applications in dentistry. The microfluidic devices used in acoustic separation can be tailored to meet the specific requirements of dental research and practice. Adjustments in transducer geometry, channel dimensions, and flow patterns allow for optimized isolation of DSCs-Exos, enabling precision-based therapeutic applications. Table S3 summarizes the abovementioned label-free isolation approaches with a set of their outcomes. DUC has also been included in Table S3 to enable a comparison of this frequently utilized method to report label-free exosome isolation techniques.48,55,305,314,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361
While various label-free exosome isolation techniques have been extensively studied in the broader field of EVs’ research, their specific application to DSCs-Exos remains largely unexplored and unverified. The authors believe that these isolation approaches successfully applied to non-DSCs-Exos hold significant potential for adaptation to DSCs-Exos. This highlights an important area for future investigations to optimize and standardize DSCs-Exos isolation methods.
Discussion
This benchmark study aimed to provide a comprehensive review of 197 studies regarding the methodologies utilized to isolate exosomes derived from DSCs as well as their potential applications in medical and dental fields. A clear trend emerged in the literature,47,62,296,362,363,364,365,366,367,368 highlighting differential adoption of isolation methods based on study focus and cell type. This study confirmed that DUC remains the dominant method across various research areas, maintaining its role as the conventional approach. For exosomes derived from SCAPs, DFSCs, GMSCs, SHEDs, PDLSCs, DPSCs, and ABMSCs, DUC was used in 52% – 82% of the studies, with popularity varying slightly across cell types. Notably, DUC’s dominance was observed particularly in studies emphasizing high-purity exosome isolation for regenerative applications.
Other approaches such as precipitation, ultrafiltration, and SEC were frequently applied alongside DUC to enhance exosome yield, purity, and scalability. Specifically, Moreover, precipitation method showed cost-effectiveness and scalability, making it a practical alternative, especially for GMSCs, PDLSCs, and DPSCs. Microfluidic and hybrid approaches, though less common, indicated a shift towards more precise and scalable isolation techniques, with studies increasingly testing their efficacy across multiple DSCs-Exos applications. Overall, an evolving trend emerged toward optimizing DSCs-Exos isolation for specific clinical applications, balancing purity, yield, and feasibility.
The widespread utilization of DUC was evident across various medical and dental fields, particularly in studies related to periodontitis, dental pulp regeneration, CBDs, diabetes, and CNS disorders. Periodontitis consistently appeared as a major focus, underscoring the demand for high-purity exosomes to investigate inflammatory and regenerative pathways. DUC, which relies on repeated high-speed centrifugation to separate exosomes by density,46 was particularly suited for applications in cell-derived biomaterials and regenerative medicine due to its ability to preserve exosome bioactivity without introducing chemical reagents.59,369
Precipitation emerged as a preferred method for studies requiring large exosome quantities, particularly in CBDs, ischemic heart disease, periodontitis, dental pulp regeneration, and immunomodulation applications. This method, which relies on polymer-based precipitation,370,371,372 was advantageous in studies requiring substantial exosome volumes to achieve therapeutic impact. It was frequently paired with DUC, especially in cancer and tissue regeneration studies, where a two-step process was used to ensure both high yield and enhanced purity. In cancer research, where exosome content is analyzed for biomarkers or therapeutic targeting, this hybrid approach ensured that exosomes were abundant yet free from contaminants. Similarly, in tissue regeneration, purified exosomes with retained bioactivity were crucial for promoting cellular repair and differentiation.
Ultrafiltration and precipitation methods were applied mainly in periodontal regeneration studies, often in combination with DUC to enhance specificity in exosome isolation. Ultrafiltration, which uses membrane-based size-selective filtering, was particularly effective in maintaining exosome integrity and functionality – critical for modulating inflammation and promoting periodontal tissue healing.
A more complex methodological landscape was observed in studies focusing on tissue regeneration, CNS diseases, and myocardial infarction, where DUC, ultrafiltration, and SEC were used in combination to maximize isolation efficiency. SEC, although less commonly used alone, was notable in diabetes, hyperthermia, and cancer studies, where high specificity in molecular differentiation is required.
Studies on DSCs-Exos demonstrated a broad range of applications across medicine and dentistry, reflecting a growing interest in their regenerative and therapeutic potential. Among the most frequently researched areas, periodontal regeneration (20%)64,81,88,109,146,181 emerged as a dominant focus, reinforcing the strong emphasis on using DCSs-Exos to promote periodontal tissue healing and regeneration. CBDs (13%)36,74,108,120,153,155,180,185,254 and dental pulp regeneration (13%)65,79,136,143,182,186 also received significant attention, highlighting the role of DSCs-Exos in hard and soft tissue repair within the oral and maxillofacial regions. Similarly, CNS disorders (6%)78,115,124,127,138,145,198,208 represented a key area of investigation, underscoring the expanding interest in DSCs-Exos-mediated neuroprotection and neural tissue repair.
Beyond regenerative applications, the research landscape reflected an increasing focus on refining DSCs-Exos isolation and functional enhancement. Studies on optimization techniques (4%)71,72,86,87,97,161,193 and tissue regeneration (4%)70,103,204 pointed to ongoing efforts to improve the exosome efficacy for therapeutic applications. In parallel, cancer research (3%)151,184,191,225 explored DSCs-Exos’ role in tumor progression, immune modulation, and potential biomarker discovery. Furthermore, studies on arthritis (3%),98,101,102,125,219,224 coronary artery disease (3%),215 diabetes (3%),68,174 and immunomodulation (3%)199,206,216,222 demonstrated the broadening of DSCs-Exos research into systemic and inflammatory diseases.
Research on OTM147,149,159 and omics profiling96,110,111,229 indicated a growing interest in DSXs-Exos-based precision medicine, leveraging exosomal content for diagnostic and therapeutic advancements. Investigations into Sjögren’s syndrome,122,131,192,239 wound healing,92,95,116,134,220 retinal degeneration,71,87 sciatic nerve injuries,104,211,236 and tooth avulsion66,144,189 (each < 2% suggested early-stage exploration into specialized applications, with potential for future translation into clinical practice. Additionally, studies on multiple sclerosis (1%),170,171 osteoporosis (1%),140,255 Parkinson’s disease (1%),129,141 salivary gland function (1%),178,179 and TMJ disorders (1%)83,139 reflected the expanding interdisciplinary interest in DSCs-Exos-mediated therapeutics. Finally, individual studies examined DSCs-Exos applications in acute renal injury,73 apical periodontitis,77 dental caries,168 dental implants,231 systemic lupus erythematosus,130 and trigeminal neuralgia,135 reinforcing the versatility of DSCs-Exos in diverse clinical contexts.
The paucity of comparative investigations evaluating DSCs-Exos and MSCs-Exos has been observed in the literature. While exosome-based regenerative strategies have been widely explored, direct comparisons between these two sources of exosomes remain insufficiently characterized. This lack of data hinders a comprehensive understanding of their distinct molecular compositions, bioactive cargo, and functional properties, limiting the ability to discern their relative advantages and therapeutic potential. Future studies should focus on systematically comparing the proteomics, transcriptomics, lipidomics, and metabolomics profiles of these exosomes, as well as their respective roles in key biological processes such as osteogenesis, angiogenesis, and immunomodulation. Addressing this knowledge gap through well-designed comparative analyses will provide valuable insights into the translational applications of DSCs-Exos and their potential superiority or complementarity to MSCs-Exos in regenerative medicine.
While this review has provided an overview of recent advancements in exosome isolation, the studies primarily focused on exosomes derived from SCAPs, PDLSCs, and DPSCs. This narrow focus may limit the comprehensiveness of this review in capturing the full potential of DSCs-Exos in regenerative applications. Despite the increasing interest in exosome-based therapies, there remains a paucity of research investigating newer isolation methods for exosomes from other DSCs, such as GMSCs, ABMSCs, SHEDs, and DFSCs. The absence of studies evaluating their novel isolation methods restricts our ability to provide a more inclusive and critical discussion. Future research should aim to explore novel exosome isolation strategies across a broader range of DSCs, enabling a more complete comprehension of their yield, purity, and functionalities.
Roadmap for dsc-exos isolation
The increased focus on the significance of DSCs-Exos in biomedical research has underscored the urgent requirement for innovative approaches aimed at the swift and precise isolation of DSCs-Exos while minimizing the need for extensive sample preparation. In this discourse, the authors outline the following critical domains for prospective advancements:
Characterization and biological validation
Presently, the approaches employed for quantifying the precise or optimal yield of DSCs-Exos remain predominantly semi-quantitative. There exists a significant demand for both qualitative and quantitative evaluations of DSCs-Exos, alongside the establishment of unequivocal biological controls.373 It is crucial to acknowledge that there is no universally applicable solution for the characterization of DSCs-Exos. The characterization will be contingent upon the specific clinical application. Incorporating labeled DSCs-Exos into experiments provides a dependable approach for determining yield, whether through quantitative reverse transcription polymerase chain reaction (qRT-PCR) or fluorescence measurement. Additionally, it is advisable to evaluate not only the yield (i.e., presence of selected markers) but also the specificity (i.e., absence of contaminants).373
Specificity and reproducibility
Devices equipped with on-chip technologies, such as protein-specific markers for sorting exosomes, may enable clinicians to access a pertinent subpopulation of exosomes relevant to their site of interest. The prevailing methodologies for isolation have not effectively ensured reproducibility in DSCs-Exos recovery, which can be attributed to inconsistencies in sample handling. Ideally, the emerging approaches such as label-free isolation, should undergo testing across various laboratories to evaluate their reproducibility and consistency, thereby demonstrating standardized performance.
Overcoming potential limitations of emerging label-free isolation approaches
In this review, most of the included studies have employed a singular isolation approach and are not devoid of challenges. Future label-free approaches might therefore benefit from hybrid methodologies that integrate two or more isolation techniques; for instance, an initial separation based on size could eliminate larger cells from dental tissue samples, followed by an immunological separation to access a specific subpopulation of exosome.
The primary challenge, as with all label-free approaches, is to deliver a solution to a real clinical question. Two distinct pathways are likely to develop:(1) to investigate exosome biology within the research domain, necessitating refined isolation techniques. For this, the benefit of label-free approaches will lie in providing a more economical alternative to DUC with minimal capital investment; and (2) concentrating on creating tools to enhance liquid biopsy preparation for next-generation sequencing. This difficulty will be in the swift extraction from complex biofluids. To integrate seamlessly into clinical settings, the approaches will need to be more robust and cost-effective compared to their existing counterparts. The developmental challenge will encompass (a) lowering approach costs, a factor that must be considered during the design phase, potentially involving the removal of the need for structures smaller than 80 μm, at this point, photolithography could be supplanted by rapid throughput and high-volume manufacturing processes; and (b) furthermore, achieving the appropriate throughput will be imperative, which might necessitate multiple units within a single device.
From laboratory to clinical settings
DUC is presently employed in research environments, yet it has not been widely adopted in clinical settings. Should a DSCs-Exos-based approach be implemented in real-world clinical settings, one might envision that in non-emergent scenarios, such as oral cancer diagnosis, the utilization of a centralized laboratory equipped with DUC technology could be viable, contingent upon the reproducibility of the isolation process. However, if standardized efficacy is established using label-free approaches, it would facilitate the development of more rapid, dependable, and economically viable DSCs-Exos-based interventions. Moreover, this advancement could permit enhanced analysis of DSCs-Exos, given that research has demonstrated a significant reduction in DSCs-Exos quantity from biofluids even when preserved at – 80 °C374 and within two hours.375 For DSCs-Exos application in clinical settings, the approach should ideally enable the clinician to swiftly and reliably isolate DSCs-Exos from biofluids with a 30-minute interval post-sample collection.
Conclusion
This benchmark analysis of exosomes highlights DUC as the primary method for isolating exosomes from diverse DSC sources, demonstrating its effectiveness in producing high yields while preserving exosomal structure and bioactivity. To further improve exosome purity, DUC was often used alongside precipitation and ultrafiltration techniques, especially in research areas requiring high exosome volume and specificity. The emergence of advanced methods, such as MEMS, signifies progress toward scalable and cost-efficient isolation strategies, highlighting a trend toward achieving the high-purity exosome preparations essential for therapeutic efficacy. This study underscores the critical need for continued refinement in exosome isolation techniques to support the purity and functionality of exosomal preparations, which is essential to advancing their application across regenerative, diagnostic, and therapeutic platforms in both dental and broader medical contexts.
Data availability
Data available on request from the corresponding author.
References
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Beer, K. B. & Wehman, A. M. Mechanisms and functions of extracellular vesicle release in vivo—what we can learn from flies and worms. Cell Adhes. Migr. 11, 135–150 (2017).
Miron, R. J. & Zhang, Y. Understanding exosomes: Part 1—Characterization, quantification and isolation techniques. Periodontology 2000 94, 231–256 (2024).
Miron, R. J., Estrin, N. E., Sculean, A. & Zhang, Y. Understanding exosomes: Part 2—Emerging leaders in regenerative medicine. Periodontology 2000 94, 257–414 (2024).
Miron, R. J., Estrin, N. E., Sculean, A. & Zhang, Y. Understanding exosomes: Part 3—therapeutic+ diagnostic potential in dentistry. Periodontology 2000 94, 415–482 (2024).
Hade, M. D., Suire, C. N. & Suo, Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10, 1959 (2021).
Hu, W., Wang, W., Chen, Z., Chen, Y. & Wang, Z. Engineered exosomes and composite biomaterials for tissue regeneration. Theranostics 14, 2099 (2024).
Tan, F. et al. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 9, 17 (2024).
Bojic, S., Volarevic, V., Ljujic, B. & Stojkovic, M. Dental stem cells-characteristics and potential. World Journal of Stem Cells 13, 1610–1624 (2014).
Nakajima, K. et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 497, 876–882 (2018).
Sonoyama, W. et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE1, e79 (2006).
Miura, M. et al. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl Acad. Sci. 100, 5807–5812 (2003).
Handa, K. et al. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect. Tissue Res. 43, 406–408 (2002).
Zhang, Q. et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 183, 7787–7798 (2009).
Seo, B.-M. et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155 (2004).
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G. & Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl Acad. Sci. 97, 13625–13630 (2000).
Aly, L. A. A. Stem cells: Sources, and regenerative therapies in dental research and practice. World J. Stem Cells 7, 1047 (2015).
Chalisserry, E. P., Nam, S. Y., Park, S. H. & Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 8, 2041731417702531 (2017).
Nuti, N., Corallo, C., Chan, B., Ferrari, M. & Gerami-Naini, B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev. Rep. 12, 511–523 (2016).
Sharpe, P. T. Dental mesenchymal stem cells. Development 143, 2273–2280 (2016).
Campanella, V. Dental stem cells: current research and future applications. Eur. J. Paediatr. Dent. 19, 257- (2018).
Sui, B. et al. Dental pulp stem cells: from discovery to clinical application. J. Endod. 46, S46–S55 (2020).
Yamada, Y., Nakamura-Yamada, S., Kusano, K. & Baba, S. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: a concise review. Int. J. Mol. Sci. 20, 1132 (2019).
Li, Y., Duan, X., Chen, Y., Liu, B. & Chen, G. Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases. Int. J. Oral. Sci. 14, 2 (2022).
Syromiatnikova, V., Prokopeva, A. & Gomzikova, M. Methods of the large-scale production of extracellular vesicles. Int. J. Mol. Sci. 23, 10522 (2022).
Chen, H. et al. Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 9, 751079 (2021).
He, J. et al. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 12, 2385–2402 (2022).
Mai, Z. et al. Translational and clinical applications of dental stem cell-derived exosomes. Front. Genet. 12, 750990 (2021).
Schepici, G., Silvestro, S. & Mazzon, E. Regenerative effects of exosomes-derived MSCs: an overview on spinal cord injury experimental studies. Biomedicines 11, 201 (2023).
Ning, X. et al. Dental stem cell-derived exosomes: a review of their isolation, classification, functions, and mechanisms. Stem Cells Int. 2024, 2187392 (2024).
Ren, S., Lin, Y., Liu, W., Yang, L. & Zhao, M. MSC-Exos: important active factor of bone regeneration. Front. Bioeng. Biotechnol. 11, 1136453 (2023).
Abdullah, M. F. et al. Proliferation rate of stem cells derived from human dental pulp and identification of differentially expressed genes. Cell Biol. Int. 38, 582–590 (2014).
Al-Maswary, A. A. et al. Exploring the neurogenic differentiation of human dental pulp stem cells. PLoS ONE17, e0277134 (2022).
Zhou, L., Zhao, S. & Xing, X. Effects of different signaling pathways on odontogenic differentiation of dental pulp stem cells: a review. Front. Physiol. 14, 1272764 (2023).
Zou, J. et al. Exosomes derived from odontogenic stem cells: its role in the dentin-pulp complex. Regenerative Ther. 24, 135–146 (2023).
Brunello, G. et al. Exosomes derived from dental pulp stem cells show different angiogenic and osteogenic properties in relation to the age of the donor. Pharmaceutics 14, 908 (2022).
Shen, Z. et al. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front. Immunol. 12, 749192 (2021).
Li, P., Ou, Q., Shi, S. & Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 20, 558–569 (2023).
Kim, H. I. et al. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 56, 836–849 (2024).
Liang, Y. et al. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Mol. Ther. 31, 1207–1224 (2023).
Kibria, G., Ramos, E. K., Wan, Y., Gius, D. R. & Liu, H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol. Pharmaceutics 15, 3625–3633 (2018).
Luan, X. et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sin. 38, 754–763 (2017).
Han, Y. et al. Overview and update on methods for cargo loading into extracellular vesicles. Processes 9, 356 (2021).
Koh, H. B., Kim, H. J., Kang, S.-W. & Yoo, T.-H. Exosome-based drug delivery: translation from bench to clinic. Pharmaceutics 15, 2042 (2023).
Duan, X. et al. A new subtype of artificial cell-derived vesicles from dental pulp stem cells with the bioequivalence and higher acquisition efficiency compared to extracellular vesicles. J. Extracell. Vesicles 13, e12473 (2024).
Kurian, T. K., Banik, S., Gopal, D., Chakrabarti, S. & Mazumder, N. Elucidating methods for isolation and quantification of exosomes: a review. Mol. Biotechnol. 63, 249–266 (2021).
Alzhrani, G. N. et al. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 45, 1807–1831 (2021).
Li, P., Kaslan, M., Lee, S. H., Yao, J. & Gao, Z. Progress in exosome isolation techniques. Theranostics 7, 789 (2017).
Lin, S. et al. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16, 1903916 (2020).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Ding, L. et al. A holistic review of the state-of-the-art microfluidics for exosome separation: an overview of the current status, existing obstacles, and future outlook. Small 17, 2007174 (2021).
Mohammadi, M. et al. Emerging technologies and commercial products in exosome-based cancer diagnosis and prognosis. Biosens. Bioelectron. 183, 113176 (2021).
Coumans, F. A. et al. Methodological guidelines to study extracellular vesicles. Circulation Res. 120, 1632–1648 (2017).
Chen, J. et al. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 9, 811971 (2022).
Wu, M. et al. Separating extracellular vesicles and lipoproteins via acoustofluidics. Lab Chip 19, 1174–1182 (2019).
Sódar, B. W. et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 6, 24316 (2016).
Karimi, N. et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell. Mol. Life Sci. 75, 2873–2886 (2018).
Ludwig, N., Whiteside, T. L. & Reichert, T. E. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 20, 4684 (2019).
Hua, S. et al. Periodontal and dental pulp cell-derived small extracellular vesicles: a review of the current status. Nanomaterials 11, 1858 (2021).
Liu, C. & Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 9, 1015 (2019).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V. & Laktionov, P. P. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed. Res. Int. 2018, 8545347 (2018).
Safdar, A., Saleem, A. & Tarnopolsky, M. A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol.12, 504–517 (2016).
Zhang, T. et al. Extracellular vesicles derived from human dental mesenchymal stem cells stimulated with low-intensity pulsed ultrasound alleviate inflammation-induced bone loss in a mouse model of periodontitis. Genes Dis. 10, 1613–1625 (2023).
Liu, D., Shi, B., Zhou, W. & Tao, G. Exosomes from hypoxia-conditioned apical papilla stem cells accelerate angiogenesis in vitro through Notch/JAG1/VEGF signaling. Tissue Cell 84, 102197 (2023).
Lin, X., Wang, H., Wu, T., Zhu, Y. & Jiang, L. Exosomes derived from stem cells from apical papilla promote angiogenesis via miR-126 under hypoxia. Oral. Dis. 29, 3408–3419 (2023).
Nie, Y.-F. et al. Apical papilla stem cell-derived exosomes regulate lipid metabolism and alleviate inflammation in the MCD-induced mouse NASH model. Biochem. Pharmacol. 222, 116073 (2024).
Jing, X. et al. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl. Mater. Interfaces 14, 16082–16099 (2022).
Yu, S. et al. Exosomes derived from stem cells from the apical papilla alleviate inflammation in rat pulpitis by upregulating regulatory T cells. Int. Endod. J. 55, 517–530 (2022).
Wang, A. et al. Identification and comparison of piRNA expression profiles of exosomes derived from human stem cells from the apical papilla and bone marrow mesenchymal stem cells. Stem Cells Dev. 29, 511–520 (2020).
Hadady, H., Karamali, F., Ejeian, F., Soroushzadeh, S. & Nasr-Esfahani, M. H. Potential neuroprotective effect of stem cells from apical papilla derived extracellular vesicles enriched by lab-on-chip approach during retinal degeneration. Cell. Mol. Life Sci. 79, 350 (2022).
Hadady, H. et al. AC electrokinetic isolation and detection of extracellular vesicles from dental pulp stem cells: Theoretical simulation incorporating fluid mechanics. Electrophoresis 42, 2018–2026 (2021).
Huang, T.-Y., Chien, M.-S. & Su, W.-T. Therapeutic potential of pretreatment with exosomes derived from stem cells from the apical papilla against cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 23, 5721 (2022).
Liu, Y. et al. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration through enhancing Cdc42-mediated vascularization. Stem Cell Research and Therapy 12, 76 (2020).
Zhuang, X. et al. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int. 2020, 5816723 (2020).
Wang, H. et al. Odontoblastic exosomes attenuate apoptosis in neighboring cells. J. Dent. Res. 98, 1271–1278 (2019).
Yang, S. et al. Extracellular vesicles delivering nuclear factor I/C for hard tissue engineering: treatment of apical periodontitis and dentin regeneration. J. Tissue Eng. 13, 20417314221084095 (2022).
Gratpain, V. et al. Influence of a pro-inflammatory stimulus on the miRNA and lipid content of human dental stem cell-derived extracellular vesicles and their impact on microglial activation. Heliyon 10 (2024).
Li, M. et al. A ROS-responsive hydrogel incorporated with dental follicle stem cell-derived small extracellular vesicles promotes dental pulp repair by ameliorating oxidative stress. Bioact. Mater. 36, 524–540 (2024).
Fu, H. et al. Mesenchymal stem cells-derived extracellular vesicles protect against oxidative stress-induced xenogeneic biological root injury via adaptive regulation of the PI3K/Akt/NRF2 pathway. J. Nanobiotechnol. 21, 466 (2023).
Liang, L. et al. High-yield nanovesicles extruded from dental follicle stem cells promote the regeneration of periodontal tissues as an alternative of exosomes. J. Clin. Periodontol. 51, 1395–1407 (2024).
Huang, Y. et al. Lipopolysaccharide-preconditioned dental follicle stem cells derived small extracellular vesicles treating periodontitis via reactive oxygen species/mitogen-activated protein kinase signaling-mediated antioxidant effect. Int. J. Nanomed. 17, 799–819 (2022).
Mao, E. et al. Human dental follicle cell-derived small extracellular vesicles attenuate temporomandibular joint cartilage damage through inhibiting HIF-2α. J. Tissue Eng. Regenerative Med. 2023, 6625123 (2023).
Ma, L. et al. Small extracellular vesicles from dental follicle stem cells provide biochemical cues for periodontal tissue regeneration. Stem Cell Res. Ther. 13, 92 (2022).
Yi, G. et al. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res. Ther. 13, 41 (2022).
Deng, Y., Liu, Z. & Lu, M. Extracellular vesicles deviced from hypoxia-3D-GMSCs rescue the mitochondrial dysfunction of aging-GMSCs. Biochem. Biophys. Res. Commun. 717, 150021 (2024).
Yu, Z. et al. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials 284, 121484 (2022).
Zarubova, J. et al. Engineered delivery of dental stem-cell-derived extracellular vesicles for periodontal tissue regeneration. Adv. Healthc. Mater. 11, 2102593 (2022).
Liang, X. et al. Nanoscale exosomes derived from gingiva mesenchymal stem cells for radiotherapy-induced apoptosis in non-small cell lung cancer cells. ACS Appl. Nano Mater. 6, 13533–13542 (2023).
Hu, Y. et al. Human gingival mesenchymal stem cell-derived exosomes cross-regulate the Wnt/β-catenin and NF-κB signalling pathways in the periodontal inflammation microenvironment. J. Clin. Periodontol. 50, 796–806 (2023).
Zeng, T. et al. Cartilaginous extracellular matrix enriched with human gingival mesenchymal stem cells derived “matrix bound extracellular vesicles” enabled functional reconstruction of tracheal defect. Adv. Sci. 9, 2102735 (2022).
Liu, Z. et al. Local transplantation of GMSC-derived exosomes to promote vascularized diabetic wound healing by regulating the Wnt/β-catenin pathways. Nanoscale Adv. 5, 916–926 (2023).
Della Rocca, Y. et al. Protective effect of oral stem cells extracellular vesicles on cardiomyocytes in hypoxia-reperfusion. Front. Cell Dev. Biol. 11, 1260019 (2024).
Sun, J. et al. Exosomes derived from human gingival mesenchymal stem cells attenuate the inflammatory response in periodontal ligament stem cells. Front. Chem. 10, 863364 (2022).
Shi, Q. et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 8, 904 (2017).
Silvestro, S. et al. Extracellular vesicles derived from human gingival mesenchymal stem cells: a transcriptomic analysis. Genes 11, 118 (2020).
Della Rocca, Y. et al. Autologous hGMSC-derived iPS: a new proposal for tissue regeneration. Int. J. Mol. Sci. 25, 9169 (2024).
Tian, X. et al. Gingival mesenchymal stem cell-derived exosomes are immunosuppressive in preventing collagen-induced arthritis. J. Cell. Mol. Med. 26, 693–708 (2022).
Wang, R. et al. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int. Immunopharmacol. 81, 106030 (2020).
Zhang, Y. et al. Effect of gingival mesenchymal stem cell-derived exosomes on inflammatory macrophages in a high-lipid microenvironment. Int. Immunopharmacol. 94, 107455 (2021).
Chen, J. et al. miRNA-148a–containing GMSC-derived EVs modulate Treg/Th17 balance via IKKB/NF-κB pathway and treat a rheumatoid arthritis model. JCI Insight 9 (2024).
Bruckner, S. et al. The therapeutic effects of gingival mesenchymal stem cells and their exosomes in a chimeric model of rheumatoid arthritis. Arthritis Res. Ther. 25, 211 (2023).
Diomede, F. et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res. Ther. 9, 1–21 (2018).
Rao, F. et al. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells Int. 2019, 2546367 (2019).
Zhang, Y. et al. SIS-ECM laden with GMSC-derived exosomes promote taste bud regeneration. J. Dent. Res. 98, 225–233 (2019).
Wang, S. et al. Extracellular vesicles secreted by human gingival mesenchymal stem cells promote bone regeneration in rat femoral bone defects. Front. Bioeng. Biotechnol. 11, 1098172 (2023).
Della Rocca, Y. et al. Role of extra-cellular vesicles derived by human gingival mesenchymal stem cells in cardiomyocytes acute hypoxia. Ital. J. Anat. Embryol. 126, 35–38 (2022).
Giuliani, A. et al. Could the enrichment of a biomaterial with conditioned medium or extracellular vesicles modify bone-remodeling kinetics during a defect healing? Evaluations on rat calvaria with synchrotron-based microtomography. Appl. Sci. 10, 2336 (2020).
Wang, M., Li, J., Ye, Y., Chen, D. & Song, J. SHED-derived exosomes improve the repair capacity and osteogenesis potential of hPDLCs. Oral. Dis. 29, 1692–1705 (2023).
Gao, Y. et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact. Mater. 14, 377–388 (2022).
Liu, F. et al. Preliminary study on the mechanism by which exosomes derived from human exfoliated deciduous teeth improve the proliferation and osteogenic inhibitory effect of glucocorticoid-induced BMSCs. Gene 923, 148575 (2024).
Zheng, Y. et al. Effects of sEV derived from SHED and DPSC on the proliferation, migration and osteogenesis of PDLSC. Regenerative Ther. 24, 489–498 (2023).
Fallah, A., Colagar, A. H., Khosravi, A. & Saeidi, M. Exosomes from SHED-MSC regulate polarization and stress oxidative indexes in THP-1 derived M1 macrophages. Arch. Biochem. Biophys.755, 109987 (2024).
Jin, S. et al. Young exosome bio-nanoparticles restore aging-impaired tendon stem/progenitor cell function and reparative capacity. Adv. Mater. 35, 2211602 (2023).
Li, Y. et al. Engineering antioxidant poly (citrate-gallic acid)-Exosome hybrid hydrogel with microglia immunoregulation for Traumatic Brain Injury-post neuro-restoration. Compos. B: Eng. 242, 110034 (2022).
Bastidas, J. G. et al. Secretome of stem cells from human exfoliated deciduous teeth (SHED) and its extracellular vesicles improves keratinocytes migration, viability, and attenuation of H2O2-induced cytotoxicity. Wound Repair Regeneration 31, 827–841 (2023).
Yu, T., Mi, N., Song, Y. & Xie, W. Exosomes miR-92a-3p from human exfoliated deciduous teeth inhibits periodontitis progression via the KLF4/PI3K/AKT pathway. J. Periodontal Res. 59, 771–782 (2024).
Wu, J. et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater. Sci. Eng. 5, 3561–3571 (2019).
Jing, Y. et al. Apoptotic vesicles modulate endothelial metabolism and ameliorate ischemic retinopathy via PD1/PDL1 Axis. Adv. Healthc. Mater. 13, e2303527 (2024).
Guo, J. et al. Exosome-shuttled mitochondrial transcription factor A mRNA promotes the osteogenesis of dental pulp stem cells through mitochondrial oxidative phosphorylation activation. Cell Prolif. 55, e13324 (2022).
Sunartvanichkul, T. et al. Stem cell-derived exosomes from human exfoliated deciduous teeth promote angiogenesis in hyperglycemic-induced human umbilical vein endothelial cells. J. Appl. Oral. Sci. 31, e20220427 (2023).
Du, Z. et al. SHED-derived exosomes ameliorate hyposalivation caused by Sjögren’s syndrome via Akt/GSK-3β/Slug-mediated ZO-1 expression. Chin. Med. J. 136, 2596–2608 (2023).
Katahira, Y. et al. Protective effects of conditioned media of immortalized stem cells from human exfoliated deciduous teeth on pressure ulcer formation. Front. Immunol. 13, 1010700 (2023).
Jonavičė, U. et al. Extracellular vesicles from human teeth stem cells trigger ATP release and promote migration of human microglia through P2X4 receptor/MFG-E8-dependent mechanisms. Int. J. Mol. Sci. 22, 10970 (2021).
Lin, C.-Y. et al. The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis. Int. J. Mol. Sci. 25, 8560 (2024).
Koga, S. & Horiguchi, Y. Efficacy of a cultured conditioned medium of exfoliated deciduous dental pulp stem cells in erectile dysfunction patients. J. Cell. Mol. Med. 26, 195–201 (2022).
Jonavičė, U., Tunaitis, V., Kriaučiūnaitė, K., Jarmalavičiūtė, A. & Pivoriūnas, A. Extracellular vesicles can act as a potent immunomodulators of human microglial cells. J. Tissue Eng. Regenerative Med. 13, 309–318 (2019).
Wang, M., Li, J., Ye, Y., He, S. & Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 111, 1–11 (2020).
Jarmalavičiūtė, A., Tunaitis, V., Pivoraitė, U., Venalis, A. & Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 17, 932–939 (2015).
Sonoda, S. et al. Targeting of deciduous tooth pulp stem cell–derived extracellular vesicles on telomerase-mediated stem cell niche and immune regulation in systemic lupus erythematosus. J. Immunol. 206, 3053–3063 (2021).
Chu, W. X. et al. SHED-exos promote saliva secretion by suppressing p-ERK1/2-mediated apoptosis in glandular cells. Oral. Dis. 30, 3066–3080 (2024).
Wei, J. et al. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J. Mol. Histol. 51, 455–466 (2020).
Pivoraitė, U. et al. Exosomes from human dental pulp stem cells suppress carrageenan-induced acute inflammation in mice. Inflammation 38, 1933–1941 (2015).
Xie, Y. et al. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J. Nanobiotechnol.20, 239 (2022).
Guo, R. et al. SHED-derived exosomes attenuate trigeminal neuralgia after CCI of the infraorbital nerve in mice via the miR-24-3p/IL-1R1/p-p38 MAPK pathway. J. Nanobiotechnol. 21, 458 (2023).
Lu, H. et al. Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration. J. Nanobiotechnol. 22, 265 (2024).
Peng, Y. et al. Young small extracellular vesicles rejuvenate replicative senescence by remodeling Drp1 translocation-mediated mitochondrial dynamics. J. Nanobiotechnol. 22, 543 (2024).
Li, Y. et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res. Ther. 8, 1–11 (2017).
Luo, P., Jiang, C., Ji, P., Wang, M. & Xu, J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 10, 1–12 (2019).
Sonoda, S. et al. Extracellular vesicles from deciduous pulp stem cells recover bone loss by regulating telomerase activity in an osteoporosis mouse model. Stem Cell Res. Ther. 11, 1–16 (2020).
Narbute, K. et al. Intranasal administration of extracellular vesicles derived from human teeth stem cells improves motor symptoms and normalizes tyrosine hydroxylase expression in the substantia nigra and striatum of the 6-hydroxydopamine-treated rats. Stem Cells Transl. Med. 8, 490–499 (2019).
Luo, L., Avery, S. J. & Waddington, R. J. Exploring a chemotactic role for EVs from progenitor cell populations of human exfoliated deciduous teeth for promoting migration of naïve BMSCs in bone repair process. Stem Cells Int. 2021, 6681771 (2021).
Wu, M. et al. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 54, e13074 (2021).
Huang, X. et al. Odontogenesis-empowered extracellular vesicles safeguard donor-recipient stem cell interplay to support tooth regeneration. Small 20, e2400260 (2024).
Li, Y. et al. Dental stem cell-derived extracellular vesicles transfer miR-330-5p to treat traumatic brain injury by regulating microglia polarization. Int. J. oral. Sci. 14, 44 (2022).
Lei, F. et al. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater.141, 333–343 (2022).
Huang, H.-M. et al. Mechanical force-promoted osteoclastic differentiation via periodontal ligament stem cell exosomal protein ANXA3. Stem Cell Rep. 17, 1842–1858 (2022).
Diomede, F. et al. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 13, 3805–3825 (2018).
Zheng, X. et al. Biological characteristics of microRNAs secreted by exosomes of periodontal ligament stem cells due to mechanical force. Eur. J. Orthod. 45, 408–417 (2023).
Lan, Q. et al. Curcumin-primed periodontal ligament stem cells-derived extracellular vesicles improve osteogenic ability through the Wnt/β-catenin pathway. Front. Cell Dev. Biol. 11, 1225449 (2023).
Chiricosta, L. et al. Extracellular vesicles of human periodontal ligament stem cells contain MicroRNAs associated to proto-oncogenes: Implications in cytokinesis. Front. Genet. 11, 582 (2020).
Niu, Q. et al. FoxO1-overexpressed small extracellular vesicles derived from hPDLSCs promote periodontal tissue regeneration by reducing mitochondrial dysfunction to regulate osteogenesis and inflammation. Int. J. Nanomed. 19, 8751–8768 (2024).
Pizzicannella, J. et al. Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front. Physiol. 10, 512 (2019).
Kang, H., Lee, M.-J., Park, S. J. & Lee, M.-S. Lipopolysaccharide-preconditioned periodontal ligament stem cells induce M1 polarization of macrophages through extracellular vesicles. Int. J. Mol. Sci. 19, 3843 (2018).
Wang, J. et al. Osteogenic differentiation effect of human periodontal ligament stem-cell initial cell density on autologous cells and human bone marrow stromal cells. Int. J. Mol. Sci. 24, 7133 (2023).
Zhang, Z. et al. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int. J. Med. Sci. 17, 558 (2020).
Zhao, B. et al. Periodontal ligament stem cell-derived small extracellular vesicles embedded in matrigel enhance bone repair through the adenosine receptor signaling pathway. Int. J. Nanomed. 17, 519-536.
Lu, J., Yu, N., Liu, Q., Xie, Y. & Zhen, L. Periodontal ligament stem cell exosomes key to regulate periodontal regeneration by miR-31-5p in mice model. Int. J. Nanomed. 18, 5327–5342 (2023).
Chang, M., Chen, Q., Wang, B., Zhang, Z. & Han, G. Exosomes from tension force-applied periodontal ligament cells promote mesenchymal stem cell recruitment by altering microRNA profiles. Int. J. Stem Cells 16, 202–214 (2023).
Kang, L., Miao, Y., Jin, Y., Shen, S. & Lin, X. Exosomal miR-205-5p derived from periodontal ligament stem cells attenuates the inflammation of chronic periodontitis via targeting XBP1. Immunity. Inflamm. Dis. 11, e743 (2023).
Han, P. et al. Effects of periodontal cells-derived extracellular vesicles on mesenchymal stromal cell function. J. Periodontal Res. 58, 1188–1200 (2023).
Zheng, Y. et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J. Cell. Physiol. 234, 20662–20674 (2019).
Wu, Y. et al. Exosomes from cyclic stretched periodontal ligament cells induced periodontal inflammation through miR-9-5p/SIRT1/NF-κB signaling pathway. J. Immunol. 210, 2001–2015 (2023).
Dai, Z. et al. Gallic acid ameliorates the inflammatory state of periodontal ligament stem cells and promotes pro-osteodifferentiation capabilities of inflammatory stem cell-derived exosomes. Life 12, 1392 (2022).
Novello, S., Tricot-Doleux, S., Novella, A., Pellen-Mussi, P. & Jeanne, S. Influence of periodontal ligament stem cell-derived conditioned medium on osteoblasts. Pharmaceutics 14, 729 (2022).
Zhao, Y. et al. The experimental study of periodontal ligament stem cells derived exosomes with hydrogel accelerating bone regeneration on alveolar bone defect. Pharmaceutics 14, 2189 (2022).
Cui, S. et al. Small extracellular vesicles from periodontal ligament stem cells primed by lipopolysaccharide regulate macrophage M1 polarization via miR-433-3p targeting TLR2/TLR4/NF-κB. Inflammation 46, 1849–1858 (2023).
Pourhajibagher, M. & Bahador, A. Periodontal ligament stem cell-derived exosome-loaded Emodin mediated antimicrobial photodynamic therapy against cariogenic bacteria. BMC Oral. Health 24, 311 (2024).
Lu, Y. et al. Rab27a-mediated extracellular vesicle secretion contributes to osteogenesis in periodontal ligament-bone niche communication. Sci. Rep. 13, 8479 (2023).
Soundara Rajan, T. et al. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 30, 238–252 (2017).
Rajan, T. S. et al. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci. Rep. 6, 38743 (2016).
Xu, X.-Y. et al. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl. Med. 9, 1414–1430 (2020).
Liu, T. et al. Human periodontal ligament stem cell-derived exosomes promote bone regeneration by altering MicroRNA profiles. Stem Cells Int. 2020, 8852307 (2020).
Liu, M. et al. Exosomal miR-141-3p from PDLSCs alleviates high glucose-induced senescence of PDLSCs by activating the KEAP1-NRF2 signaling pathway. Stem Cells Int. 2023, 7136819 (2023).
Han, P. et al. 3D bioprinted small extracellular vesicles from periodontal cells enhance mesenchymal stromal cell function. Biomater. Adv. 158, 213770 (2024).
Yu, J. et al. Effect of psoralen on the regulation of osteogenic differentiation induced by periodontal stem cell-derived exosomes. Hum. Cell 36, 1389–1402 (2023).
Xiang, M. et al. Metformin enhances the therapeutic effects of extracellular vesicles derived from human periodontal ligament stem cells on periodontitis. Sci. Rep. 14, 19940 (2024).
Dong, J. et al. Dental pulp stem cell-derived small extracellular vesicle in irradiation-induced senescence. Biochem. Biophys. Res. Commun. 575, 28–35 (2021).
Chansaenroj, A. et al. Magnetic bioassembly platforms towards the generation of extracellular vesicles from human salivary gland functional organoids for epithelial repair. Bioact. Mater. 18, 151–163 (2022).
Tian, J. et al. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact. Mater. 22, 326–342 (2023).
Shen, Z. et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 5, 1113–1126 (2020).
Zeng, J. et al. Exosomes from human umbilical cord mesenchymal stem cells and human dental pulp stem cells ameliorate lipopolysaccharide-induced inflammation in human dental pulp stem cells. Arch. Oral. Biol. 138, 105411 (2022).
Li, B. et al. Hypoxia preconditioned DPSC-derived exosomes regulate angiogenesis via transferring LOXL2. Exp. Cell Res. 425, 113543 (2023).
Vakhshiteh, F. et al. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: A new approach for drug delivery. Life Sci. 266, 118871 (2021).
Zhang, S. et al. Human dental pulp stem cell-derived exosomes decorated titanium scaffolds for promoting bone regeneration. Colloids Surf. B: Biointerfaces 235, 113775 (2024).
Chen, W.-J. et al. The role of small extracellular vesicles derived from lipopolysaccharide-preconditioned human dental pulp stem cells in dental pulp regeneration. J. Endod. 47, 961–969 (2021).
Han, S. et al. Programmed release of vascular endothelial growth factor and exosome from injectable chitosan nanofibrous microsphere-based PLGA-PEG-PLGA hydrogel for enhanced bone regeneration. Int. J. Biol. Macromol.253, 126721 (2023).
Huang, C.-C., Narayanan, R., Alapati, S. & Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials 111, 103–115 (2016).
Guo, H. et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials 279, 121223 (2021).
Swanson, W. B. et al. Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J. Control. Rel.324, 679–694 (2020).
Klimova, D. et al. Extracellular vesicles derived from dental mesenchymal stem/stromal cells with gemcitabine as a cargo have an inhibitory effect on the growth of pancreatic carcinoma cell lines in vitro. Mol. Cell. Probes 67, 101894 (2023).
Ogata, K. et al. Extracellular vesicles of iPS cells highly capable of producing HGF and TGF-β1 can attenuate Sjögren’s syndrome via innate immunity regulation. Cell. Signal. 113, 110980 (2024).
Winkel, A. et al. Cell culture media notably influence properties of human mesenchymal stroma/stem-like cells from different tissues. Cytotherapy 22, 653–668 (2020).
Li, Z. et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol. Ther. 30, 3193–3208 (2022).
Swanson, W. B. et al. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater.118, 215–232 (2020).
Lin, T.-Y. et al. 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside–stimulated dental pulp stem cells-derived exosomes for wound healing and bone regeneration. J. Dental Sci. 20, 154–163 (2024).
Mas-Bargues, C. et al. Small extracellular vesicles from senescent stem cells trigger adaptive mechanisms in young stem cells by increasing antioxidant enzyme expression. Redox Biol. 62, 102668 (2023).
Altanerova, U. et al. Dental pulp mesenchymal stem/stromal cells labeled with iron sucrose release exosomes and cells applied intra-nasally migrate to intracerebral glioblastoma. Neoplasma 63, 925–933 (2016).
Nakatsuka, R., Sasaki, Y., Masutani, M. & Nozaki, T. PARP1 regulates cellular processes mediated by exosomal miRNAs in dental pulp stem cells. J. Hard Tissue Biol. 30, 371–378 (2021).
Abdelgawad, L. M., Nghnughi, M. H. & Abdelgwad, M. Influence of photo biomodulation using 980 nm diode laser and exsosomes derived from dental pulp stem cells on pulp regeneration of dogs’ teeth. Reactions 9, 11 (2022).
Venugopal, C. et al. by human dental pulp mesenchymal stem cells: from billions to nano. Curr. Gene Ther. 18, 307–323 (2018).
Li, Y. et al. Deciphering the heterogeneity landscape of mesenchymal stem/stromal cell-derived extracellular vesicles for precise selection in translational medicine. Adv. Healthc. Mater. 12, 2202453 (2023).
Fei, Y., Ling, Z., Tong, Q.& Wang, J. Apoptotic extracellular vesicles from supernumerary tooth-derived pulp stem cells transfer COL1A1 to promote angiogenesis via PI3K/Akt/VEGF pathway. Int. J. Nanomed. 19, 6811–6828 (2024).
Diomede, F. et al. Extracellular vesicles (EVs): a promising therapeutic tool in the heart tissue regeneration. BioFactors 50, 509–522 (2024).
Xie, L. et al. Exosomal circLPAR1 promoted osteogenic differentiation of homotypic dental pulp stem cells by competitively binding to hsa-miR-31. Biomed. Res. Int. 2020, 6319395 (2020).
Mas-Bargues, C. et al. Extracellular vesicles from healthy cells improves cell function and stemness in premature senescent stem cells by miR-302b and HIF-1α activation. Biomolecules 10, 957 (2020).
Li, B. et al. Hypoxia alters the proteome profile and enhances the angiogenic potential of dental pulp stem cell-derived exosomes. Biomolecules 12, 575 (2022).
Li, S. et al. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 54, e13093 (2021).
Zhou, H. et al. Periodontitis-compromised dental pulp stem cells secrete extracellular vesicles carrying miRNA-378a promote local angiogenesis by targeting Sufu to activate the Hedgehog/Gli1 signalling. Cell Prolif. 54, e13026 (2021).
Merckx, G. et al. Angiogenic effects of human dental pulp and bone marrow-derived mesenchymal stromal cells and their extracellular vesicles. Cells 9, 312 (2020).
Chai, Y. et al. Dental pulp stem cell-derived exosomes promote sciatic nerve regeneration via optimizing schwann cell function. Cell. Reprogramming 26, 67–78 (2024).
Wang, S. et al. Fabrication of an exosome-loaded thermosensitive chitin-based hydrogel for dental pulp regeneration. J. Mater. Chem. B11, 1580–1590 (2023).
Li, J., Ju, Y., Liu, S., Fu, Y. & Zhao, S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect. Tissue Res. 62, 277–286 (2021).
Faruqu, F. N. et al. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB BioAdvances 2, 419 (2020).
Sánchez-Sánchez, R. et al. miR-4732-3p in extracellular vesicles from mesenchymal stromal cells is cardioprotective during myocardial ischemia. Front. Cell Dev. Biol. 9, 734143 (2021).
Umar, S. et al. Immunomodulatory properties of naïve and inflammation-informed dental pulp stem cell derived extracellular vesicles. Front. Immunol. 15, 1447536 (2024).
Guo, S. et al. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. 21, 2497–2504 (2021).
Altanerova, U. et al. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 12, 7923–7936 (2017).
Lin, T. et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR-140-5p-overexpressing human dental pulp stem cells. Int. J. Mol. Med. 47, 1- (2021).
Zhou, Z. et al. Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway. Int. J. Mol. Med. 50, 1–15 (2022).
Zhang, S. et al. Extracellular vesicles-loaded fibrin gel supports rapid neovascularization for dental pulp regeneration. Int. J. Mol. Sci. 21, 4226 (2020).
Gómez-Ferrer, M. et al. HIF-1α and pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int. J. Mol. Sci. 22, 3416 (2021).
Ganesh, V. et al. Exosome-based cell homing and angiogenic differentiation for dental pulp regeneration. Int. J. Mol. Sci. 24, 466 (2022).
Fu, Y., Cui, S., Zhou, Y. & Qiu, L. Dental pulp stem cell-derived exosomes alleviate mice knee osteoarthritis by inhibiting TRPV4-mediated osteoclast activation. Int. J. Mol. Sci. 24, 4926 (2023).
Teixeira, M. R., Alievi, A. L., da Costa, V. R., Kerkis, I. & Araldi, R. P. Exploring the therapeutic potential of extracellular vesicles derived from human immature dental pulp cells on papillary thyroid cancer. Int. J. Mol. Sci. 25, 8178 (2024).
Chen, Y. et al. The application of pulp tissue derived-exosomes in pulp regeneration: a novel cell-homing approach. Int. J. Nanomed. 17, 465-476 (2022).
Ivica, A., Ghayor, C., Zehnder, M., Valdec, S. & Weber, F. E. Pulp-derived exosomes in a fibrin-based regenerative root filling material. J. Clin. Med. 9, 491 (2020).
Jin, Q. et al. Extracellular vesicles derived from human dental pulp stem cells promote osteogenesis of adipose-derived stem cells via the MAPK pathway. J. Tissue Eng. 11, 2041731420975569 (2020).
Terunuma, A. et al. Extracellular vesicles from mesenchymal stem cells of dental pulp and adipose tissue display distinct transcriptomic characteristics suggestive of potential therapeutic targets. J. Stem Cells Regenerative Med. 17, 56 (2021).
Lee, A. et al. DPSC-derived extracellular vesicles promote rat jawbone regeneration. J. Dent. Res. 102, 313–321 (2023).
Eren Belgin, E., Genç, D., Tekin, L., Sezgin, S. & Aladağ, A. Anti-Inflammatory effect of dental pulpa mesenchymal stem cell exosomes loaded mucoadhesive hydrogel on mice with dental nickel hypersensitivity. Macromol. Biosci. 24, 2300352 (2024).
Li, L. & Ge, J. Exosome-derived lncRNA-Ankrd26 promotes dental pulp restoration by regulating miR-150-TLR4 signaling. Mol. Med. Rep. 25, 1–11 (2022).
Nasiri, K. Exosomes derived from human dental stem cell enhance the viability of odontoblasts. Nanomed. Res. J. 8, 365–372 (2023).
Kong, F. et al. Dental pulp stem cell-derived extracellular vesicles mitigate haematopoietic damage after radiation. Stem Cell Rev. Rep. 17, 318–331 (2021).
Ji, L. et al. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunologic Res. 67, 432–442 (2019).
Chai, Y. et al. Study on the role and mechanism of exosomes derived from dental pulp stem cells in promoting regeneration of myelin sheath in rats with sciatic nerve injury. Mol. Neurobiol. 61, 6175–6188 (2024).
Liu, C. et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J. Nanobiotechnol.20, 65 (2022).
Liang, X. et al. Dental pulp mesenchymal stem cell-derived exosomes inhibit neuroinflammation and microglial pyroptosis in subarachnoid hemorrhage via the miRNA-197-3p/FOXO3 axis. J. Nanobiotechnol. 22, 426 (2024).
Hu, S. et al. Dental pulp stem cell-derived exosomes revitalize salivary gland epithelial cell function in NOD mice via the GPER-mediated cAMP/PKA/CREB signaling pathway. J. Transl. Med. 21, 361 (2023).
Hu, X. et al. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 10, 1–14 (2019).
Zhou, H. et al. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem cell Res. Ther. 11, 1–18 (2020).
Zheng, J. et al. MicroRNA-enriched small extracellular vesicles possess odonto-immunomodulatory properties for modulating the immune response of macrophages and promoting odontogenesis. Stem Cell Res. Ther. 11, 1–14 (2020).
Luo, X. et al. Odontoblasts release exosomes to regulate the odontoblastic differentiation of dental pulp stem cells. Stem Cell Res. Ther. 14, 176 (2023).
Chi, Y., Liu, T., Jin, Q. & Liu, H. Extracellular vesicles carrying RUNX3 promote differentiation of dental pulp stem cells. Tissue Eng. Regenerative Med. 21, 111–122 (2024).
Imanishi, Y. et al. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm. Regeneration 41, 1–10 (2021).
Amaro-Prellezo, E. et al. Extracellular vesicles from dental pulp mesenchymal stem cells modulate macrophage phenotype during acute and chronic cardiac inflammation in athymic nude rats with myocardial infarction. Inflamm. Regeneration 44, 25 (2024).
Heitzer, M. et al. Evaluation of in vitro biocompatibility of human pulp stem cells with allogeneic, alloplastic, and xenogeneic grafts under the influence of extracellular vesicles. Sci. Rep. 13, 12475 (2023).
Shimizu, Y. et al. Exosomes from dental pulp cells attenuate bone loss in mouse experimental periodontitis. J. Periodontal Res. 57, 162–172 (2022).
Miao, Y. et al. Transfer of miR-877–3p via extracellular vesicles derived from dental pulp stem cells attenuates neuronal apoptosis and facilitates early neurological functional recovery after cerebral ischemia–reperfusion injury through the Bclaf1/P53 signaling pathway. Pharmacol. Res. 206, 107266 (2024).
Chen, T.-Y. et al. Exosomes derived from Polygonum multiflorum-treated human dental pulp stem cells (hDPSCs): New approach in regenerative medicine. J. Drug Deliv. Sci. Technol. 99, 105941 (2024).
Diomede, F. et al. Decellularized dental pulp, extracellular vesicles, and 5-azacytidine: a new tool for endodontic regeneration. Biomedicines 10, 403 (2022).
He, X. et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 7132708 (2019).
Li, X. et al. Exosomes derived from maxillary BMSCs enhanced the osteogenesis in iliac BMSCs. Oral. Dis. 26, 131–144 (2020).
Zhu, Q. et al. Exosomes derived from mesenchymal stromal cells promote bone regeneration by delivering miR-182–5p-inhibitor. Pharmacol. Res. 192, 106798 (2023).
Xu, S. & Wang, Z. Bone marrow mesenchymal stem cell-derived exosomes enhance osteoclastogenesis during alveolar bone deterioration in rats. RSC Adv. 7, 21153–21163 (2017).
Lamparski, H. G. et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 270, 211–226 (2002).
Webber, J. & Clayton, A. How pure are your vesicles?. J. Extracell. Vesicles 2, 19861 (2013).
Momen-Heravi, F. et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 394, 1253–1262 (2013).
Han, P., Lai, A., Salomon, C. & Ivanovski, S. Detection of salivary small extracellular vesicles associated inflammatory cytokines gene methylation in gingivitis. Int. J. Mol. Sci. 21, 5273 (2020).
Kim, J. Y. et al. Defined MSC exosome with high yield and purity to improve regenerative activity. J. Tissue Eng. 12, 20417314211008626 (2021).
Farshidfar, N. et al. The feasible application of microfluidic tissue/organ-on-a-chip as an impersonator of oral tissues and organs: a direction for future research. Bio-Des. Manuf. 6, 478–506 (2023).
Faruqu, F. N. et al. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB bioAdvances 2, 419–433 (2020).
Patel, G. K. et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 9, 5335 (2019).
He, M. & Zeng, Y. Microfluidic exosome analysis toward liquid biopsy for cancer. J. Lab. Autom. 21, 599–608 (2016).
Wang, J., Ma, P., Kim, D. H., Liu, B.-F. & Demirci, U. Towards microfluidic-based exosome isolation and detection for tumor therapy. Nano Today 37, 101066 (2021).
Zhao, Z., Yang, Y., Zeng, Y. & He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16, 489–496 (2016).
Hassanpour Tamrin, S., Sanati Nezhad, A. & Sen, A. Label-free isolation of exosomes using microfluidic technologies. ACS Nano 15, 17047–17079 (2021).
Yakubovich, E., Polischouk, A. & Evtushenko, V. Principles and problems of exosome isolation from biological fluids. Biochem. (Mosc.), Suppl. Ser. A: Membr. Cell Biol. 16, 115–126 (2022).
Liu, D. S. et al. Size-exclusion chromatography as a technique for the investigation of novel extracellular vesicles in cancer. Cancers 12, 3156 (2020).
Guo, J. et al. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J. Extracell. Vesicles 10, e12145 (2021).
Kang, Y. T. et al. Isolation and profiling of circulating tumor-associated exosomes using extracellular vesicular lipid–protein binding affinity based microfluidic device. Small 15, 1903600 (2019).
Farhana, F. Z. et al. Isolation and detection of exosomes using Fe2O3 nanoparticles. ACS Appl. Nano Mater. 4, 1175–1186 (2021).
Rayamajhi, S. & Aryal, S. Surface functionalization strategies of extracellular vesicles. J. Mater. Chem. B. 8, 4552–4569 (2020).
González, M. I. et al. Covalently labeled fluorescent exosomes for in vitro and in vivo applications. Biomedicines 9, 81 (2021).
Yamamoto, M. et al. Application of high-mannose-type glycan-specific lectin from Oscillatoria Agardhii for affinity isolation of tumor-derived extracellular vesicles. Anal. Biochem. 580, 21–29 (2019).
Kang, Y.-T. et al. High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale 9, 13495–13505 (2017).
Iliuk, A. et al. Plasma-derived extracellular vesicle phosphoproteomics through chemical affinity purification. J. Proteome Res. 19, 2563–2574 (2020).
Lou, D., Wang, Y., Yang, Q., Hu, L. & Zhu, Q. Ultrafiltration combing with phospholipid affinity-based isolation for metabolomic profiling of urinary extracellular vesicles. J. Chromatogr. A 1640, 461942 (2021).
Tayebi, M., Zhou, Y., Tripathi, P., Chandramohanadas, R. & Ai, Y. Exosome purification and analysis using a facile microfluidic hydrodynamic trapping device. Anal. Chem. 92, 10733–10742 (2020).
Kim, J., Lee, H., Park, K. & Shin, S. Rapid and efficient isolation of exosomes by clustering and scattering. J. Clin. Med. 9, 650 (2020).
Visan, K. S. et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 11, 12266 (2022).
Ramnauth, N. et al. Development of a microfluidic device for exosome isolation in point-of-care settings. Sensors 23, 8292 (2023).
Liga, A., Vliegenthart, A., Oosthuyzen, W., Dear, J. & Kersaudy-Kerhoas, M. Exosome isolation: a microfluidic road-map. Lab Chip 15, 2388–2394 (2015).
Sato, Y. T. et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 6, 21933 (2016).
Wu, Y. et al. Microfluidic technology for the isolation and analysis of exosomes. Micromachines 13, 1571 (2022).
Yong, T. et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 10, 3838 (2019).
Hu, T. et al. Flow-electricity coupling fields enhance microfluidic platforms for efficient exosome isolation. Anal. Methods 16, 5335–5344 (2024).
Sani, F. et al. Unveiling exosomes: Cutting-edge isolation techniques and their therapeutic potential. J. Cell. Mol. Med. 28, e70139 (2024).
Jia, Y. et al. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 12, 6548 (2022).
Kim, D. et al. Ultra-thin membrane filter with a uniformly arrayed nanopore structure for nanoscale separation of extracellular vesicles without cake formation. Nanoscale Adv. 5, 640–649 (2023).
Aliakbari, F. et al. A methodological primer of extracellular vesicles isolation and characterization via different techniques. Biol. Methods Protoc. 9, bpae009 (2024).
Shi, L. et al. Rapid and label-free isolation of small extracellular vesicles from biofluids utilizing a novel insulator based dielectrophoretic device. Lab Chip 19, 3726–3734 (2019).
Gao, J. et al. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol. 10, 1100892 (2023).
Brennan, K. et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 10, 1039 (2020).
Dou, Y., Ren, L., Kulabhusan, P. K., Zaripov, E. A. & Berezovski, M. V. Quantitative capillary electrophoresis for analysis of extracellular vesicles (EVqCE). Separations 8, 110 (2021).
Yang, D. et al. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics 10, 3684 (2020).
Bányai, A. et al. Dean-flow affected lateral focusing and separation of particles and cells in periodically inhomogeneous microfluidic channels. Sensors 23, 800 (2023).
Pouraria, H., Foudazi, R. & Houston, J. P. Exploitation of elasto-inertial fluid flow for the separation of nano-sized particles: Simulating the isolation of extracellular vesicles. Cytometry A 103, 786–795 (2023).
Shiri, F. et al. Separation of U87 glioblastoma cell-derived small and medium extracellular vesicles using elasto-inertial flow focusing (a spiral channel). Sci. Rep. 12, 6146 (2022).
Bhagat, A. A. S., Kuntaegowdanahalli, S. S. & Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 8, 1906–1914 (2008).
Patel, K. & Stark, H. Instability of a liquid sheet with viscosity contrast in inertial microfluidics. Eur. Phys. J. E 44, 144 (2021).
Zhang, J. et al. Fundamentals and applications of inertial microfluidics: a review. Lab a Chip 16, 10–34 (2016).
Shi, R. Numerical simulation of inertial microfluidics: a review. Eng. Appl. Comput. Fluid Mech. 17, 2177350 (2023).
Nam, J., Jang, W. S., Hong, D. H. & Lim, C. S. Viscoelastic separation and concentration of fungi from blood for highly sensitive molecular diagnostics. Sci. Rep. 9, 3067 (2019).
Liu, C. et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11, 6968–6976 (2017).
Hettiarachchi, S. et al. Viscoelastic microfluidics for enhanced separation resolution of submicron particles and extracellular vesicles. Nanoscale 16, 3560–3570 (2024).
Mukherjee, S. & Sarkar, K. Lateral migration of a viscoelastic drop in a Newtonian fluid in a shear flow near a wall. Phys. Fluids 26 (2014).
Sevenler, D. & Toner, M. High throughput intracellular delivery by viscoelastic mechanoporation. Nat. Commun. 15, 115 (2024).
Kumar, T. et al. High throughput viscoelastic particle focusing and separation in spiral microchannels. Sci. Rep. 11, 8467 (2021).
Jiang D., Ni C., Tang W., Huang D., Xiang N. Inertial microfluidics in contraction–expansion microchannels: a review. Biomicrofluidics 15 (2021).
Collins, D. J., Alan, T. & Neild, A. Particle separation using virtual deterministic lateral displacement (vDLD). Lab Chip 14, 1595–1603 (2014).
Tottori, N. & Nisisako, T. Tunable deterministic lateral displacement of particles flowing through thermo-responsive hydrogel micropillar arrays. Sci. Rep. 13, 4994 (2023).
Bowman T. J., Drazer G., Frechette J. Inertia and scaling in deterministic lateral displacement. Biomicrofluidics 7 (2013).
Zhang, H. & Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 14, 1027–1053 (2019).
Chiang, C., Yeh, J. & Yeh, C. Nanoparticles separation by different conditions at asymmetric flow field-flow fractionation. J. Mech. 39, 500–507 (2023).
Shendruk, T. N. et al. Field-flow fractionation and hydrodynamic chromatography on a microfluidic chip. Anal. Chem. 85, 5981–5988 (2013).
Petersen, K. E. et al. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal. Bioanal. Chem. 406, 7855–7866 (2014).
Kim, Y. B., Lee, G. B. & Moon, M. H. Size separation of exosomes and microvesicles using flow field-flow fractionation/multiangle light scattering and lipidomic comparison. Anal. Chem. 94, 8958–8965 (2022).
Priedols, M. et al. Bifurcated Asymmetric Field Flow Fractionation of Nanoparticles in PDMS-Free Microfluidic Devices for Applications in Label-Free Extracellular Vesicle Separation. Polymers 15, 789 (2023).
Chronakis, M. I., Von Der Au, M. & Meermann, B. Single cell-asymmetrical flow field-flow fractionation/ICP-time of flight-mass spectrometry (sc-AF4/ICP-ToF-MS): an efficient alternative for the cleaning and multielemental analysis of individual cells. J. Anal. Spectrom. 37, 2691–2700 (2022).
Drexel, R. et al. Fast and purification-free characterization of bio-nanoparticles in biological media by electrical asymmetrical flow field-flow fractionation hyphenated with multi-angle light scattering and nanoparticle tracking analysis detection. Molecules 25, 4703 (2020).
Mitrano, D., Neubauer, K., Ranville, J. & Thomas, R. Field-flow fractionation coupled with ICP-MS forthe analysis of engineered nanoparticles in environmental samples. Spectroscopy 25, 652–664 (2012).
Lu, X. & Xuan, X. Elasto-inertial pinched flow fractionation for continuous shape-based particle separation. Anal. Chem. 87, 11523–11530 (2015).
Chiriacò, M. S. et al. Lab-on-chip for exosomes and microvesicles detection and characterization. Sensors 18, 3175 (2018).
Shin, S. et al. Separation of extracellular nanovesicles and apoptotic bodies from cancer cell culture broth using tunable microfluidic systems. Sci. Rep. 7, 1–8 (2017).
Sunahiro, S., Senaha, M., Yamada, M. & Seki, M., editors. Pinched Flow Fractionization device for sizes and density dependent separation of particles utilizing centrifugal pumping. In: 12th International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Diego, California, USA (2008).
Liu, F. et al. IB-LBM study on cell sorting by pinched flow fractionation. Bio-Med. Mater. Eng. 24, 2547–2554 (2014).
Hickey, S. M. et al. Fluorescence microscopy—an outline of hardware, biological handling, and fluorophore considerations. Cells 11, 35 (2021).
Kim, A., Ng, W. B., Bernt, W. & Cho, N.-J. Validation of size estimation of nanoparticle tracking analysis on polydisperse macromolecule assembly. Sci. Rep. 9, 2639 (2019).
Jain, A. & Posner, J. D. Particle dispersion and separation resolution of pinched flow fractionation. Anal. Chem. 80, 1641–1648 (2008).
Risbud, S. R. & Drazer, G. Mechanism governing separation in microfluidic pinched flow fractionation devices. Microfluidics Nanofluidics 17, 1003–1009 (2014).
Wang, Z. et al. Acoustofluidic separation enables early diagnosis of traumatic brain injury based on circulating exosomes. Microsyst. Nanoeng. 7, 20 (2021).
Fan, Y., Wang, X., Ren, J., Lin, F. & Wu, J. Recent advances in acoustofluidic separation technology in biology. Microsyst. Nanoeng. 8, 94 (2022).
Naquin, T. D. et al. Acoustic separation and concentration of exosomes for nucleotide detection: ASCENDx. Sci. Adv. 10, eadm8597 (2024).
Wu, M. et al. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng.5, 32 (2019).
Tayebi, M., Yang, D., Collins, D. J. & Ai, Y. Deterministic sorting of submicrometer particles and extracellular vesicles using a combined electric and acoustic field. Nano Lett. 21, 6835–6842 (2021).
Zhang, J. et al. A solution to the biophysical fractionation of extracellular vesicles: acoustic nanoscale separation via wave-pillar excitation resonance (ANSWER). Sci. Adv. 8, eade0640 (2022).
Díaz-Reinoso, B. Concentration and Purification of Seaweed Extracts Using Membrane Technologies. Sustainable Seaweed Technologies 371–390 (Elsevier, 2020).
Liang, L.-G. et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 7, 46224 (2017).
Woo, H.-K. et al. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano 11, 1360–1370 (2017).
Liu, F. et al. The exosome total isolation chip. ACS Nano 11, 10712–10723 (2017).
Wang, Z. et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip 13, 2879–2882 (2013).
Yasui, T. et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci. Adv. 3, e1701133 (2017).
Han, Z. et al. Highly efficient exosome purification from human plasma by tangential flow filtration based microfluidic chip. Sens. Actuators B: Chem. 333, 129563 (2021).
Lewis, J. M. et al. Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano 12, 3311–3320 (2018).
Shi, L., Rana, A. & Esfandiari, L. A low voltage nanopipette dielectrophoretic device for rapid entrapment of nanoparticles and exosomes extracted from plasma of healthy donors. Sci. Rep. 8, 1–12 (2018).
Davies, R. T. et al. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip 12, 5202–5210 (2012).
Mogi, K., Hayashida, K. & Yamamoto, T. Damage-less handling of exosomes using an ion-depletion zone in a microchannel. Anal. Sci. 34, 875–880 (2018).
Marczak, S. et al. Simultaneous isolation and preconcentration of exosomes by ion concentration polarization. Electrophoresis 39, 2029–2038 (2018).
Cho, S. et al. Isolation of extracellular vesicle from blood plasma using electrophoretic migration through porous membrane. Sens. Actuators B: Chem. 233, 289–297 (2016).
Tay, H. M. et al. Rapid purification of sub-micrometer particles for enhanced drug release and microvesicles isolation. NPG Asia Mater. 9, e434-e (2017).
Zhou, Y., Ma, Z., Tayebi, M. & Ai, Y. Submicron particle focusing and exosome sorting by wavy microchannel structures within viscoelastic fluids. Anal. Chem. 91, 4577–4584 (2019).
Wunsch, B. H. et al. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 11, 936–940 (2016).
Santana, S. M., Antonyak, M. A., Cerione, R. A. & Kirby, B. J. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed. Microdevices 16, 869–877 (2014).
Smith, J. T. et al. Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip 18, 3913–3925 (2018).
Petersen, K. E. et al. Exosome isolation: cyclical electrical field flow fractionation in low-ionic-strength fluids. Anal. Chem. 90, 12783–12790 (2018).
Shin, S. et al. Separation of extracellular nanovesicles and apoptotic bodies from cancer cell culture broth using tunable microfluidic systems. Sci. Rep. 7, 9907 (2017).
Lee, K., Shao, H., Weissleder, R. & Lee, H. Acoustic purification of extracellular microvesicles. ACS nNano 9, 2321–2327 (2015).
Evander, M., Gidlöf, O., Olde, B., Erlinge, D. & Laurell, T. Non-contact acoustic capture of microparticles from small plasma volumes. Lab Chip 15, 2588–2596 (2015).
Wu, M. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. 114, 10584–10589 (2017).
Yeo, J. C. et al. Label-free extraction of extracellular vesicles using centrifugal microfluidics. Biomicrofluidics 12 (2018).
Sidhom, K., Obi, P. O. & Saleem, A. A review of exosomal isolation methods: is size exclusion chromatography the best option?. Int. J. Mol. Sci. 21, 6466 (2020).
Zhang, Y. et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 15, 6917-6934 (2020).
Yang, X.-X., Sun, C., Wang, L. & Guo, X.-L. New insight into isolation, identification techniques and medical applications of exosomes. J. Control. Rel.308, 119–129 (2019).
Théry, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 30, 3.22. 1–3. 9 (2006).
Liu, W.-Z., Ma, Z.-J. & Kang, X.-W. Current status and outlook of advances in exosome isolation. Anal. Bioanal. Chem. 414, 7123–7141 (2022).
He, L. et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 9, 8206 (2019).
Livshits, M. A. et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 5, 1–14 (2015).
Lin, H. et al. Advances of exosomes in periodontitis treatment. J. Transl. Med. 20, 279 (2022).
Weng, Y. et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst 141, 4640–4646 (2016).
Rider, M. A., Hurwitz, S. N. & Meckes, D. G. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 6, 1–14 (2016).
Ludwig, A.-K. et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 7, 1528109 (2018).
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013).
Zhou, H. et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 69, 1471–1476 (2006).
Oosthuyzen, W. et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J. Physiol. 591, 5833–5842 (2013).
Acknowledgements
N.F. is a recipient of the 2024 Research Scholarship from the Osteology Foundation.
Funding
This research was not supported by any funding.
Author information
Authors and Affiliations
Contributions
P.A., Writing—review and editing, Writing—original draft, Formal analysis, Data curation, Conceptualization. N.E., Writing—review and editing, Conceptualization. N.F., Writing—review and editing; Y.Z., Writing—review and editing; R.J.M., Writing—review and editing, Supervision. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
Richard J. Miron holds intellectual property on the production of platelet-rich fibrin and is the founder of Miron Research and Development in Dentistry LLC. All other authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmad, P., Estrin, N., Farshidfar, N. et al. Isolation methods of exosomes derived from dental stem cells. Int J Oral Sci 17, 50 (2025). https://doi.org/10.1038/s41368-025-00370-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41368-025-00370-y
This article is cited by
-
Extracellular vesicles derived from dental mesenchymal stem cells for regenerative medicine: a scoping review
Molecular Biology Reports (2026)
-
Efficient methods of isolation and purification of extracellular vesicles
Nano Convergence (2025)
-
Exosome-based therapeutics in bone regeneration: from fundamental biology to clinical translation
Stem Cell Research & Therapy (2025)
-
Mechanistic insights into periodontal ligament stem cell-derived exosomes in tissue regeneration
Clinical Oral Investigations (2025)