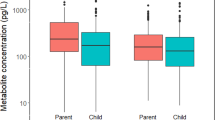
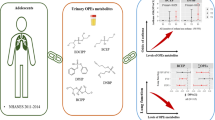
Abstract
Background
The biochemical dysregulation underlying the adverse health effects of exposure to air pollution (AP) remains unclear.
Objective
The objective of this study was to explore associations between long-term exposure to AP and the urinary metabolome.
Methods
In the Swedish birth cohort BAMSE (n = 4089), urine samples were collected from a subset of participants attending clinical examination at the 4-year follow-up and from all participants attending clinical examination at the 24-year follow-up. Among paired samples and children with diagnosis of asthma and/or low lung function, non-targeted screening using liquid chromatography high-resolution mass spectrometry was applied to 4-year samples (n = 612) and 24-year samples (n = 846) and metabolites were annotated based on standard matching to in-house compound libraries (n = 260 metabolites). Time-weighted average exposure to air pollutants (i.e., particulate matter with diameter ≤10μm (PM10), ≤2.5 μm (PM2.5), and nitrogen oxides (NOx)) during the first year of life and the year prior to urine collection was estimated using validated dispersion models. The association between AP exposure and urine metabolites was estimated cross-sectionally using exponential regression.
Results
AP exposure was overall positively associated with metabolite abundance (p < 0.002). However, metabolite-specific associations exhibited variability. At the 4-year follow-up, the first-year-of-life and prior-year AP exposures were positively associated with 8 purine/pyrimidine derivative metabolites (e.g., an increase of 2.8 μg/m3 (interquartile range) in PM10 during the first year of life was associated with a 1.21-fold increase in 1,7-dimethylxanthine, p = 3.87E−05). We also observed interactions between AP exposures and metabolism-related genetic variants on metabolite levels. At the 24-year follow-up, prior year AP was negatively associated with levels of six long-chain fatty acids.
Impact
-
Long-term exposure to air pollution alters urinary metabolites in children and young adults, revealing environmental impacts on systemic metabolism even at low levels of air pollution.
Similar content being viewed by others
Introduction
Air pollution is a significant public health issue worldwide and an established risk factor for morbidity and mortality [1]. Accumulating research has identified inflammation and oxidative stress to have important roles in the health effects of air pollution [2,3,4]. However, significant gaps remain in our understanding of the etiological mechanisms and the extent to which underlying molecular changes induced by air pollution can be detected during early childhood, particularly in the metabolome [5].
Metabolomic epidemiology has emerged as a powerful methodology to explore how air pollution exposure is affecting biological responses [6]. Metabolites are low molecular weight compounds that reflect systemic biochemistry [7, 8]. The metabolome can serve as a proxy for the internal exposome [9], which consists of the exogenous compounds entering the internal environment (e.g., dietary, medication-related, and ambient environmental molecules), endogenous compounds that are the products of genetic and endogenous processes stimulated by the external environment (e.g., oxidative stress, inflammation), and the interactions between exogenous and endogenous compounds. While metabolomics has been performed in many biofluids, urine offers some distinct advantages including: (1) non-invasive collection, (2) higher concentration of conjugated metabolites, (3) reflects end-state metabolism, (4) represents a wide range of biochemical processes (although it captures a narrower spectrum of biochemical processes compared to blood), and (5) provides an integrative snapshot of systemic biochemical imbalances [8, 10].
Experimental studies have found that short-term exposure to particulate air pollution can lead to metabolic dysregulation in the urine and blood metabolomes in animal models [11, 12]. Although the identified metabolic signatures vary across studies, metabolome-wide epidemiological studies in humans consistently support the role of air pollution in the perturbation of pathways related to inflammation, oxidative stress, and mitochondrial function [5, 13, 14]. The majority of studies have focused on investigating the effects of short-term air pollution exposure in adults. Data are scarce regarding the effects of air pollution on the metabolome in children [15] as well as the consequences of long-term air pollution exposure [16,17,18,19,20]. Furthermore, in younger populations, where genetic influences on omics profiles are generally more pronounced, the metabolism and systemic metabolome are largely shaped by both internal genetic processes and external exposures such as air pollution [21,22,23].
The aim of the current study was to investigate the association of long-term air pollution exposure at various life stages (i.e., during the first year of life and during the prior year to biosampling) with the internal exposome by measuring urine metabolites in children at 4 years of age and in young adults at 24 years of age, using a well-defined cohort with genetic data available. Given the potentially stronger genetic influence on metabolism in early life, we additionally explored the interaction between air pollution exposure and genetic variants in relation to the metabolism of some of the identified metabolites.
Methods
Study design and population
This is a cross-sectional analysis of urine metabolites at two time-points in a birth cohort with prospectively collected data. The study included participants from the population-based birth cohort BAMSE (Swedish acronym for Children, Allergy, Milieu, Stockholm, Epidemiology), consisting of 4089 infants born in four predefined areas of Stockholm, Sweden, between 1994 and 1996 [24]. Parents were invited to complete a baseline questionnaire that assessed environmental exposures, parental smoking habits, residential characteristics, lifestyle, and parental allergies. Questionnaires were answered repeatedly by the parents (up to age 16 years) and by the participants themselves (from age 12 years). For the current study, potential confounders were extracted from questionnaires from 3 months, 1 year, 4 years, and 24 years of age. Urine samples were collected at ages 4 and 24 years from 933 (23%) children and 2235 (55%) young adults, respectively. At age 4, children were assigned to urine sampling in a subgroup based on the prevalence of symptoms of allergic diseases, as described in detail previously [25]. At 24 years of age, all participants were asked to leave a urine sample at the clinical examination.
In total, 1460 urine samples were selected for urine metabolomics analyses at 4 and 24 years of age (Fig. 1), including all participants having urine samples at both time points (580 paired samples), and additional samples from participants with a diagnosis of asthma and/or low lung function (34 additional 4-year samples plus 266 additional 24-year samples). The study population was therefore a mild asthma- and allergic symptoms-enriched population compared to the general population. Low lung function was defined as the ratio of pre-bronchodilator forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) ratio below the lower limit of normal (LLN) based on the Global Lung Function Initiative (GLI) criteria (i.e., FEV1/FVC < LLN (GLI) at 24 years of age) [26].
Air pollution exposure assessment
The methodology for calculating individual long-term exposure to locally emitted air pollutants has been described in detail previously [27]. In order to explore the impact of air pollution exposure during the first year of life and the year prior to biosampling, time-weighted average exposures were calculated at the individual address level for these periods. The exposures included particulate matter with a diameter ≤10 μm (PM10), particulate matter with a diameter ≤2.5 μm (PM2.5), and nitrogen oxides (NOx), calculated using a validated Gaussian air quality dispersion model and a wind model, both part of the Airviro Air Quality Management System (http://airviro.smhi.se). The calculations were performed on a 35-m resolution grid for addresses in the more densely populated areas of Stockholm County, such as urban areas, and a 100-m or 500-m grid in less densely populated areas. In addition, a street canyon contribution was added for addresses in the most polluted street segments in the inner city of Stockholm with multistorey houses on both sides, using the Airviro street canyon model (until 2012; www.airviro.com/airviro/ modules) and the OSPM operational street pollution model (from 2013 onwards; www.au.dk/OSPM). Emission databases were available for the years 1990, 1995, 2000, 2002, 2003, 2004, 2006, 2010, 2015, and 2020. To obtain concentrations for all years during the period of interest, the model calculations were interpolated. Annual average long-range contributions were added to the locally modeled concentrations based on continuous measurements at regional background stations.
Outcome assessment
Urine samples were collected at clinical examination visits at the 4- and 24-year follow-ups, and stored at -80° C. A detailed description of the urine metabolomic profiling methods has been previously published [28]. Before analysis, samples were thawed and normalized based on specific gravity measurements [29]. Non-targeted screening based on liquid chromatography high-resolution mass spectrometry (LC-HRMS) was applied to acquire the metabolomic profile [28, 29]. Two independent injections were run: one in positive and one in negative acquisition mode. Quality control samples were injected after every five biological samples. ProteoWizard [30] was used for data quality check using MZmine2.53 [31]. Targeted peak detection was based on 3 technical standards (CHES, HEPES and PIPES) and 25 pre-defined metabolites [29]. The data quality was assessed based on the stability of binary pump pressures, overlaid total ion chromatograms (TICs) for QCs, extracted ion chromatograms (EICs) of lock masses for all samples and QCs only, 3D views (x = RT, y = intensity, z = m/z) of one QC sample and the reference blank sample, repeatability of the EICs of each technical standard extracted with 20 ppm tolerance and calculations of Coefficient of Variation (CVs) in the QCs (<10%) and the samples (<20%) using R scripts. Batch correction was performed using Quality Control-Robust Spline Correction [32]. The untargeted metabolomics analyses resulted in 9663 metabolite features, including 4733 features in negative ionization mode and 4930 features in positive ionization mode. The untargeted data were annotated based on an in-house library of 622 chemical standards resulting in 260 unique chemical standard-confirmed metabolites from both positive and negative ionization modes (Appendix A). Peak areas, as measures of relative abundance, were used in the statistical analyses. Extreme outliers were identified and subsequently excluded from the analysis based on the results of principal component analysis (PCA) using the 95% limit of Hotelling’s T2.
Statistical analyses
The association analyses were conducted with the Stata (version 16.1; StatCorp) and R software (version 4.2.2). All analyses were performed cross-sectionally for 4- and 24-year samples. The main analyses were applied to annotated metabolites (level 1). Firstly, we explored the overall effect of air pollution on the urine metabolome. This metabolome-level analysis was motivated by our hypothesis that air pollution exposure may induce a systematic metabolic shift rather than isolated effects on individual metabolites in a few pathways. To investigate the association between air pollution and the urine metabolome, we initially aggregated annotated metabolites into one outcome variable (referred to as “metabolome”; details described in Appendix A). After that, a parametric model using exponential regression with “metabolite id” as a gamma-distributed random effect (to model the heterogeneity of metabolites) and an exponential distribution was performed. This analysis has the benefit of dimensionality reduction and more statistical power, but the estimate could be diluted by opposing directions of different metabolites. Missing values of metabolites were considered to imply a very low level or a biological zero. Missing values were imputed using half the minimum observed value for each metabolite, given the low levels of missingness in annotated metabolites [33].
Next, to explore specific air pollution-associated metabolites, a left-censored parametric model using exponential regression was applied for the annotated metabolites. Instead of half-minimum imputation, measurements below the minimum observed value were treated as left-censored observations [34]. The left censoring was suitable because the missing observations were not missing at random, but were rather below the detectable values available in the dataset. The impact of influential observations was assessed by winsorization, where the modeling was checked by replacing values above the 99th percentile with the 99th percentile value [35]. For statistically significant associations (p < 0.05), we applied a visual bivariate assessment of the effect of influential observations via a density plot of the distribution of each metabolite over quartiles of air pollution exposure. As a complement to the main analysis, the same metabolite-specific model was applied to all-feature datasets, including non-annotated features, to perform supplementary pathway enrichment analysis.
All results are presented as fold change, comparing the predicted marginal medians when air pollution exposure was increased by one unit from the median. To enhance the comparability of the effect estimates between different exposure periods, one unit was set as one interquartile range (IQR) of air pollution levels averaged during the first year of life, corresponding to 2.8, 1.1, and 22.9 μg/m3 for PM10, PM2.5, and NOx, respectively. Multiple testing was accounted for in the metabolite-specific analyses by controlling the false discovery rate (FDR) at 5%, implementing the Benjamini-Hochberg adjustment [36]. FDR-corrected p < 0.05 was considered statistically significant unless otherwise specified.
Potential confounders were selected based on a literature review and data availability. We applied a directed acyclic graph to illustrate the hypothesis of the relationship between exposure, outcomes, and covariates (Figure C.1). Sex and potential confounders including municipality at birth, household socio-economic status (or parents’ occupation), study subjects’ occupation at 24 years of age, any parent born outside of Sweden, body mass index (BMI), maternal smoking during pregnancy, parental smoking, individual smoking, time of the day for the urine collection, family history of asthma and birth season were examined but not included into the final model as they did not significantly (>10%) change the estimates, based on the metabolome model. In the analyses of the impact of air pollution during the first year of life and one year prior to the 4-year sampling, the final models were adjusted for parents’ education at baseline, while the model for air pollution during the prior year before the 24-year sampling included study subjects’ education level at 24 years of age as a covariate.
Sex and overweight status were each further examined as effect modifiers by additionally including an interaction term in the metabolite-specific model. For metabolites (excluding xenobiotics) that were significantly associated with at least one air pollutant in the main model (p < 0.05) and in the interaction model (p of the interaction term <0.05), we applied a stratified analysis.
Sensitivity analysis
To explore to what extent the metabolite-specific associations may be influenced by lifestyle factors, we conducted sensitivity analyses additionally adjusting for smoking (maternal smoking during pregnancy and/or parental smoking for early life exposure, individual smoking for prior year exposure in 24-year samples), caffeine (biomarker of caffeine intake [37]) and ethyl-glucuronide (biomarker of alcohol consumption in 24-year samples [38]). Additionally, we conducted sensitivity analysis adjusted for occupation (parents’ occupation status at 3 months for early life exposure, individual occupation for prior year exposure in 24-year samples) instead of education. Pearson’s correlation test was used to compare the estimates in sensitivity analyses with the main model, and the Kolmogorov-Smirnov test was used to compare the distribution of p values in sensitivity analyses with the main model. Potential influence of a more allergic symptoms- asthma- and low lung function- enriched population was explored 1) in sensitivity analysis adjusting for asthma and allergic symptoms in 4-year follow-up and adjusting for asthma and diagnosis of low lung function in 24-year follow-up, as well as 2) in a weighted analysis [39] based on inverse probability of selection weighting (i.e., assigning weights based on prevalence ratios of allergic symptoms, allergy, and low lung function in the population-based BAMSE birth cohort vs. our study population).
Metabolic pathway enrichment analysis
To explore biological functions and molecular mechanisms associated with significant metabolic features, a pathway enrichment analysis was carried out using Metaboanalyst 6.0 [40]. We selected metabolic features at the top 10% based on the rank of the absolute value of the percentage change in predicted median and p-value for pathway analysis. For the annotated metabolites, compound names and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database were used [41]. For untargeted features, pathway analysis was conducted using Mummichog (v.2.0.1), a bioinformatics platform that infers and categorizes functional biological activity directly from mass spectrometry output, without prior metabolite validation [42, 43]. The retention time, m/z value, and the results of association analysis were input into the Mummichog. To further minimize the possibility of false positive discovery, candidate pathways were also inspected using the most common forms out of the 16 standard adduct forms in Mummichog [43]. For untargeted analyses, the pathway analyses were based on a Mummichog curated database originated from KEGG, Biochemical Genetic and Genomic knowledgebase of large-scale metabolic reconstructions (BiGG), Edinburgh Human Metabolic Network (Edinburgh Model), and a community-driven consensus reconstruction of human metabolism (Recon2) [43].
To assess the robustness of our untargeted approach, we performed sensitivity analyses using alternate parameters (5% significance cutoff; 2 adduct forms: [M + H]+ and [M–H]–). While these conditions yielded metabolite networks more closely aligned with our annotated results, it is not largely different from our current analyses, and therefore we retained the 10% cutoff and 16 adduct forms for untargeted analyses to maximize feature coverage in hypothesis generation, consistent with mummichog’s design for high-quality untargeted discovery [42].
Genetic variants and air pollution interaction analyses on urine metabolites
Guided by the metabolomics findings, we conducted a post hoc analysis of the interaction between genetic variants and air pollution on the identified metabolites to facilitate the interpretation of potential mechanisms. The OpenTargets Genetics database was used to search for single-nucleotide polymorphisms (SNPs) previously associated with selected urine metabolites that showed significant associations with air pollution (p < 0.05) [44, 45]. Genotyping has been conducted in two subsets of BAMSE participants, referred to as “Waves” (further details described in Appendix A) [46]. The effect of the interaction of the selected genetic variants with prior-year and first-year-of-life air pollutants on the levels of each of those metabolites at 4 and 24 years of age was assessed through exponential regression models. Moreover, the winsorization of metabolite levels was carried out following the approach described above. For each metabolite, the regression included the allele dosages of each SNP, air pollution levels, and a term of interaction between the SNP and air pollution as independent variables. In this study, the term “sub-additive” refers to an interaction where the joint association is less than the sum of independent associations of air pollution exposure and the allele (effect or reference) showing a direction of association consistent with the effect of the air pollution. Two principal components of genetic ancestry, sex, parental education level at birth of the participant, and genotyping Wave were also added as covariates. The regressions evaluating the effect of the genetic interaction with prior-year air pollutants on metabolites measured at 24 years of age were adjusted by the participant’s education level at the time of the data collection visit instead. The evidence of significant interaction was considered after a FDR adjustment of 5% (FDR p < 0.05). The Benjamini-Hochberg method was applied across all genetic variants per metabolite and air pollutant.
Sensitivity analyses were carried out accounting for the effect of tobacco smoke exposure. In the case of first-year-of-life air pollutants, the association testing with metabolites at 4 and 24 years of age was further adjusted by maternal smoking during pregnancy and parental smoking during the first year of life. A variable related to individual active smoking habits was included in the evaluation of the effect of the interaction with exposure to prior-year air pollution on metabolites measured at 24 years.
Ethics approval and consent to participate
This study was conducted in accordance with relevant guidelines and regulations. Ethical approvals for the BAMSE cohort and the analyses performed in this study were obtained from the Regional Ethics Review Board, Karolinska Institutet, Stockholm, Sweden DNR: 98-175, 2016/1380-31/2. All caregivers and adult study subjects provided written informed consent.
Results
Descriptive statistics of the study population
The study population consisted of 880 subjects: 614 subjects at the 4-year follow-up and 846 subjects at the 24-year follow-up. Two 4-year samples were excluded based on the PCA analyses as potential measurement errors, resulting in 612 4-year samples and 578 paired samples (samples from the same individual at 4 and 24 years). The distribution of air pollution exposures (PM10, PM2.5, and NOx), and relevant covariates at 4-year and 24-year follow-ups are described in Table 1. The background characteristics were generally comparable between the two follow-ups. A comparison between the study population and the original cohort is given in Table B.1, which shows that the study population did not differ from the complete cohort regarding sex and socioeconomic factors. As expected given the study design, the present study population had higher prevalence of symptoms of allergic disease at the 4-year follow-up (53% vs. 36%), higher prevalence of asthma at the 4-year (13% vs. 7%) and 24-year follow-ups (31% vs. 11%), and higher prevalence of low lung function at the 24-year follow-up (19% vs. 7%) compared to the BAMSE cohort at respective time point (Table B.1). The air pollution levels during the last year prior to biosampling were higher at the 4-year follow-up than the 24-year follow-up, particularly for NOx (median (q25, q75): 21.6 (13.9, 30.0) vs. 9.5 (6.5, 13.4)) (Table 1). Correlations were strong among air pollutants during the same time window (Spearman’s correlation mostly >0.9) (Table B.2). The distributions of 260 annotated metabolites are described in Table B.3. The annotated metabolites had a low percentage of missing (<9% for each metabolite at each follow-up) (Table B.3).
Associations between air pollution and urine metabolome
At the level of all 260 annotated metabolites, higher air pollution exposure was overall associated with higher levels of urinary metabolites. The estimated median fold change at 4 years of age for 1 IQR (2.8 μg/m3) increase in PM10 during the first year of life was 1.017 (p = 2.06e−09), and during the prior year 1.017 (p = 1.42e−07), also consistent for PM2.5 and NOx (Table 2). To interpret it, every 2.8 μg/m3 increment in first-year PM10 is associated with 1.7% increase in the abundance of urinary metabolites (an average trend for these 260 metabolites). The estimated variance of the random effect was 2.6 (95% Confidence Interval (CI): 2.3, 3.0), which suggests that the specific metabolites are heterogeneous (Intraclass Correlation Coefficient (ICC) = 56.6%). Similarly, at the level of all 260 annotated metabolites, both early life and prior year air pollution exposure were associated with higher levels in urinary metabolites at 24 years (Table 2). In each model based on the 24-year follow-up data, the estimated variance of the random effect also indicated heterogeneity (ICC = 51.2%). Parametric models using other common distributions showed similar model fit (Table B.4). Note that on an individual metabolite basis, some metabolites may increase, others may decrease or show no significant association with air pollutants, but overall exposure to higher air pollution levels were associated with higher levels of urinary metabolites at both 4 and 24 years, driven by an imbalance in upregulated versus downregulated associations, suggesting systematic metabolic perturbation.
Associations between air pollution and specific metabolites at 4 years of age
At 4 years of age, air pollution exposure both during the first year of life and the year prior to biosampling was associated with an increase in eight nucleotide-related compounds (fold change: 1.12 to 1.36; seven of eight FDR significant with at least one of the three exposures: PM10, PM2.5 or NOx; Fig. 2, Fig. D.1, D.2). These include six purines (1,7-dimethylxanthine, 1,3,7-trimethyluric acid, 1-methylxanthine, (1,7-)dimethyluric acid, 1,3-dimethyluric acid, and methyluric acid) and two pyrimidine derivatives (6-amino-5-formamido-1,3-dimethyluracil and 5-acetylamino-6-amino-3-methyluracil) (Table 3, Tables B.5, B.6). For example, compared with children exposed to median levels of PM10, PM2.5, or NOx during their first year of life, one IQR increase in air pollution exposure was associated with a 1.20 to 1.22-fold increase in estimated median of 1,7-dimethylxanthine (FDR p < 0.05).
The blue line indicates FDR p = 0.05. The red line indicates p = 0.05. Dimethylxanthine indicates 1,7-dimethylxanthine. The results are extracted from exponential regression models, adjusted for parents’ education at 3 months. For each metabolite, observations above the 99th percentile value were replaced with the 99th percentile value.
Further, first-year or prior-year air pollution exposure was mostly nominally associated with seven amino acid derivatives (fold change 0.83 to 1.47, e.g., anserine, succinylacetone, furosine, N-methyl-proline, tryptophan betaine, cis-urocanic acid, and methyl-histidine), three fatty acids (fold change 0.86 to 1.19, e.g., azelaic acid, suberic acid, and stearic acid), one vitamin panthenol (fold change: 1.05 to 1.14), and eight other metabolites (fold change: 0.86 to 1.35, e.g., umbelliferone, 4-hydroxycoumarin, quinic acid, ferulic acid 4-sulfate, 2-benzoxazolol, adipic acid, trimethylamine N-Oxide, 2-piperidone), as well as four xenobiotics (fold change: 0.68 to 1.09, e.g., cyclamic acid, saccharin, acesulfame, and acetaminophen) (Table 3).
Some xenobiotics showed a higher effect size (fold change: 0.68 to 1.09) than most other types of metabolites. However, when conducting a bivariate visual assessment of the associations between metabolites and air pollution exposures, xenobiotics were found to have a distribution with most values closer to zero and less clear distinction across quartiles of air pollution compared with other types of metabolites (Fig. E.1-E.32), implying that xenobiotics were more prone to the effects of extreme values.
In the enrichment analyses, the top 10% enriched metabolic pathways based on fold change were very similar to those based on p-value. Figure 3 shows the top pathways associated with air pollution at age 4, using PM10 as an example. Both first-year and prior-year exposures to PM10 were significantly associated with eight caffeine pathway metabolites (1,7-dimethylxanthine, 6-amino-5-formamido-1,3-dimethyluracil, 1,3,7-trimethyluric acid, 1-methylxanthine, 1,7-dimethyluric acid, 5-acetylamino-6-amino-3-methyluracil, 1,3-dimethyluric acid, and methyluric acid). Evidence was not strong enough to support the association between air pollution and caffeine itself (fold change: 1.07-1.15, p values ranging from 0.06 to 0.13, with five out of six p values between 0.09 and 0.13, for any first-year or prior year air pollution exposure). Prior-year exposure to PM10 was further associated with two histidine pathway metabolites (anserine and methyl-histidine), as well as weakly associated with some fatty acid-related pathways. Caffeine metabolism and fatty acid-related pathways were also found by supplementary enrichment pathway analyses based on the untargeted metabolomics feature datasets (Table B.7). A mechanistic plot of the hypothesized relationship between air pollution and caffeine metabolism can be found in Fig. C.2.
The Y-axis represents the negative logarithm of the p-value (base 10 logarithm) derived from the enrichment analysis. The X-axis indicates the structural impact of PM10-associated metabolites within the enriched pathways, calculated based on the cumulative importance of all significant metabolites in the pathway. The bubble size reflects the impact value, while the bubble color indicates the significance of the enrichment.
In the sensitivity analysis, the eight metabolites related to caffeine metabolism remained similar in magnitude and significance when adjusting for caffeine (Table B.8). Additional adjustment for maternal smoking during pregnancy and parental smoking during the first year of life, or adjustment for occupation instead of education, did not significantly change the associations (Tables B.9, B.10). Sensitivity analyses adjusting for asthma and allergic symptoms did not change the estimates, although in a weighted analysis the effect estimates were somewhat decreased (Fig. C.3).
Associations between air pollution and specific metabolites at 24 years of age
At age 24, prior year air pollution exposure was associated with a decrease in levels of six lipid derivatives (three were FDR significant with at least one of the exposures PM10, PM2.5 or NOx, Fig. 4, Figures D.3, D.4), particularly long-chain fatty acids (Table 4, Tables B.11, B.12). Compared with young adults exposed to median level of PM10, PM2.5 or NOx, during the prior year of life, one IQR increase in air pollution exposure was associated with 0.82 to 0.76-fold decrease in estimated median of stearic acid (for associations with NOx and PM10, nominal p values < 0.05; for PM2.5, FDR p < 0.05). Furthermore, one IQR increase in prior year air pollution exposure was associated with a decrease in palmitic acid (estimated median fold change: 0.76 to 0.83), oleic acid (fold change: 0.72 to 0.84), linoleic acid (fold change: 0.76 to 0.85), hexadecenoic acid (0.84 to 0.89), and erucamide (fold change: 0.62 to 0.77), a derivative of the long-chain fatty acid erucic acid.
The blue line indicates FDR p = 0.05, and the red line indicates nominal p = 0.05. All results are adjusted for parents’ education at 3 months (for first-year exposure) or individual education (for prior-year exposure). For each metabolite, observations above the 99th percentile value were replaced with the 99th percentile value.
Prior year air pollution was also associated with an increase in nine amino acid derivatives. In addition, first-year or prior-year air pollution exposure was associated with eight xenobiotics, one carbohydrate, four cofactors and vitamins, and seven other metabolites (Table 4). The associations with the eight caffeine-pathway metabolites found in 4-year samples were not observed in the analyses with 24-year samples. Similar to the results at age 4, when conducting a bivariate visual assessment of the associations between metabolites and air pollution exposures, the distribution of xenobiotics generally indicated effects of a few extreme values (Figures F.1–F.38).
In the enrichment analyses for 24-year samples, the strongest enriched pathways were also very similar based on fold change and p-value. Figure 5 shows the top most significantly enriched metabolic pathways associated with air pollution exposure at age 24, using PM10 as an example. Driven by four fatty acids (stearic acid, palmitic acid, oleic acid, and linoleic acid), prior-year exposure to PM10 at age 24 was associated with the biosynthesis of unsaturated fatty acids. Fatty acid-related pathways were also supported by enrichment pathway analyses based on the untargeted features (Table B.13).
The Y-axis represents the negative logarithm of the p-value (base 10 logarithm) derived from the enrichment analysis. The X-axis indicates the structural impact of PM10-associated metabolites within the enriched pathways, calculated based on the cumulative importance of all significant metabolites in the pathway. The bubble size reflects the impact value, while the bubble color indicates the significance of the enrichment.
In the sensitivity analyses, the direction and magnitude of the associations were not significantly changed after additionally adjusting for smoking, caffeine, and ethyl-glucuronide, or adjusting for occupation, respectively (Tables B.14–B.17). In both the 4-year and the 24-year samples, pathway enrichment analyses based on associations between metabolites and PM2.5 or NOx showed similar findings compared with PM10 (Figures G.1–G.4). Sensitivity analyses adjusting for asthma and low lung function or using a weighted approach did not alter the results (Figure C.3).
Results of stratified analysis by BMI or sex are presented in Tables B.18–B.25. Both BMI and sex showed some effect modification. In particular, in the 24-year samples, individuals with overweight showed an indication of upregulated long-chain fatty acids, albeit not statistically significant, while normal-weight or underweight individuals showed downregulated long-chain fatty acids (mostly FDR significant) in association with prior year air pollution (Table B.21).
Genetic variant - air pollution interaction on caffeine or coumarin pathway metabolites
To better understand if the identified caffeine pathway metabolites were more related to alterations in pathways instead of behavioral factors, we conducted post hoc analysis by including metabolism-related genetic variants. The interaction of genetic variants and air pollutants on the levels of metabolites potentially derived from caffeine or coumarin was explored. Metabolites from both caffeine and coumarin were included since they could be derived through the action of the same cytochrome P450 enzyme (CYP1A2) and have shared metabolic pathways [47,48,49,50]. Ten metabolites nominally associated with at least one air pollutant were included (1,7-dimethylxanthine, 1,3,7-trimethyluric acid, 1-methylxanthine, (1,7-)dimethyluric acid, 1,3-dimethyluric acid, methyluric acid, 6-amino-5-formamido-1,3-dimethyluracil, 5-acetylamino-6-amino-3-methyluracil, umbelliferone, and 4-hydroxycoumarin).
Ten SNPs previously associated with caffeine-derived metabolites were available in BAMSE and selected for the analyses (Table B.26). Among these, seven SNPs were found to be associated (p < 0.05) with the 4-year levels of at least one of the ten metabolites assessed (Table B.27). Four of these SNPs also showed significant associations at 24 years of age (Table B.28, Figs. H.1–H.10).
Further analyses showed significant interactions between caffeine metabolism-associated genetic variants and air pollutants on caffeine and coumarin-derived metabolites in urine at 4 years of age. Taking PM10 as an example, six of the SNPs showed significant interactions with exposure during the first year of life on several of the evaluated metabolites at age 4 years (Table B.29). The highest number of significant interactions was observed on umbelliferone levels. Sub-additive interaction effects were consistently observed between caffeine metabolism-associated SNPs and both first-year-of-life and prior-year PM10 exposure on umbelliferone levels (Fig. 6, Figure H.11, Table B.29, and Table B.30). A similar pattern was observed in metabolites measured in urine samples collected at the 24-year follow-up (Tables B.31–B.32). When compared to the results with PM10, similar results were observed for the interaction of genetic variants with exposure to PM2.5 or NOx on urine metabolites (Tables B.33–B.40).
Comparing the distribution of p-values of the interaction between PM10 and SNPs on caffeine and coumarin-derived metabolites at 4 and 24 years of age suggests that the results were more robust in children (Figure H.12). In the sensitivity analysis, the identified interactions remained similar in effect magnitude and significance when adjusting for smoking (Tables B.41–B.44).
Discussion
We assessed the associations between air pollution exposure (during the first year of life as well as during the year prior to biosampling) and the metabolite levels measured in urine samples at 4 and 24 years of age. We found that preceding PM10, PM2.5, and NOx exposures were strongly associated with higher levels of the 260 annotated urinary metabolites, although there was a heterogeneity of metabolite-specific associations in terms of both direction and magnitude. The top enriched pathways primarily included caffeine metabolism and histidine metabolism at 4 years, and fatty acid metabolism at 24 years. Further, we observed interactions between air pollution exposures and caffeine-metabolism-related genetic variants on identified caffeine and coumarin metabolites, especially at the 4-year follow-up.
Despite comparatively low air pollution levels, a multitude of associations between air pollution exposure and adverse health effects have been observed in Stockholm. Air pollution exposure during infancy has been linked to lung function decrement in 6-month-old infants [51], as well as an elevated risk of allergy, asthma, and lung function impairment in children up to school age [52,53,54,55], adolescence [56, 57], and even early adulthood [58, 59]. It is therefore reasonable to hypothesize that molecular alterations occur from childhood to young adulthood in response to air pollution exposure.
At 4 years, we found that air pollution was associated with upregulated metabolites in the caffeine pathway, including higher levels of six purine derivatives and two pyrimidine derivatives. However, no significant association with caffeine itself was observed. Among previous studies in children, the most relevant study on medium-term prenatal exposure to air pollution also reported upregulated levels of hypoxanthine in serum samples of newborns [15]. This is a purine-derivative that is related to caffeine metabolism via the purine metabolism pathway and inhibition of xanthine oxidase [60]. In our study, the 4-year samples were collected during 1998-2001 (4-year follow-up). The samples had much lower levels of caffeine (fold change in median caffeine from 4-year samples to 24-year samples: 31.7) and 1,7-dimethylxanthine (fold change in median 1,7-dimethylxanthine from 4-year samples to 24-year samples: 9.5), compared with the 24-year samples. Therefore, if the 4-year-olds consumed caffeine, it would only be in small amounts. A United States population-based study on young children aged 2-5 years conducted during 1999–2002 estimated that 62.7% of the total population at this age consumed caffeine, though the intake was low (17.4 mg/day during 1999–2000 and 20.6 mg/day during 2001), compared with adults (142.9 mg/day during 1999–2000) [61]. In more recent population-based studies, the caffeine intake was 13.07 mg/day in a Korean population aged 3–5 years, whereas 36% of Canadian children aged 1-5 years drank caffeinated beverages and consumed 7 mg/day caffeine on average [62, 63]. The most common sources of caffeine for this age group are soda, tea, flavored dairy, and cocoa products [61, 62].
One potential explanation behind the association of caffeine metabolism with air pollution could have been that parents who live in areas with higher air pollution were more likely to give products that contain caffeine-related metabolites (e.g., chocolate, caffeine-containing soft drinks) due to the disproportionate distribution of socioeconomic factors [64, 65]. Nonetheless, after adjusting for socio-economic status-related variables (i.e., education and occupation), caffeine metabolism-related metabolites at age 4 were consistently associated with air pollution. Furthermore, metabolites of caffeine were associated with preceding air pollution exposure, but caffeine itself was not associated with air pollution. This leads us to believe that the observed associations are not related to caffeine intake, but rather to a difference in metabolism between children with varying levels of air pollution exposure.
Another plausible explanation of the association of caffeine metabolism with air pollution would be that air pollution affects how the human body metabolizes caffeine, particularly in young children who are more vulnerable to exposure. Air pollution was associated with increased metabolites from not only the caffeine pathway but also the coumarin pathway, potentially linked to CYP1A2 enzyme activity [47,48,49,50]. Significant interactions between air pollution and caffeine-metabolism-associated SNPs on caffeine and coumarin-derived metabolites were also observed, especially at the 4-year follow-up. For example, significant interaction with SNPs located at genes encoding the Aryl Hydrocarbon Receptor (AHR), which has been shown to regulate CYP1A2 expression and to be activated by exposure to environmental pollutants [66,67,68,69].
While direct evidence of air pollution’s effect on CYP1A2 activity is limited, research on related environmental pollutants provides some insights. Nicotine or tobacco smoke is known to accelerate the metabolism of caffeine in the human body, potentially via increased CYP1A2 enzyme activity [70]. In addition, urinary caffeine metabolites (i.e., 1,7-dimethylxanthine, theobromine, theophylline, 1-methyluric acid, and 5-acetylamino-6-amino-3-methyluracil) have previously been used as a proxy for CYP1A2 activity [71, 72]. For example, one study reported that smokers had 1.55-fold higher CYP1A2 activity (measured as the ratio of 1,7-dimethylxanthine/caffeine) compared to smoking abstainers [73]. Moreover, polycyclic aromatic hydrocarbons (PAHs), primarily generated by incomplete combustion of coal or biomass and tobacco smoking [74], not only can induce CYP1A2 activity [75], but also have been found to interact with genetic variants at genes encoding enzymes involved in the metabolic activation or detoxification of PAHs (e.g., CYP1A1, CYP1B1, GSTM1, GSTT2) [76, 77]. Therefore, carrying the effect allele of these variants might modify the effect of air pollutants and the subsequent disease risk. Our findings not only illuminate caffeine metabolism but also suggest that there are SNPs that may indicate genetic susceptibility to air pollution effects more broadly, which merits further investigation in future health outcomes studies.
The gene-environment interactions we demonstrate in this study suggest that the metabolic changes might be due to alterations in pathways rather than caused by behavioral or socio-economic factors. The sub-additive nature of this interaction compared to the individual effects of the air pollutant and genetic variant suggests that both air pollution and SNPs influence the same pathway, potentially including CYP1A2 activity, with their combined effect less than the sum of individual effects, consistent with enzyme saturation. It has also been shown that exposure to air pollution can influence DNA methylation and gene expression, with genetic variants influencing enzyme activity [4, 78].
The same association with caffeine metabolism was not found in the 24-year samples, which is consistent with previous epidemiological studies in adults. In a review of 32 studies on air pollution and metabolomics, only three studies reported significant associations with caffeine metabolism [14]. The association with caffeine metabolism in the 4-year samples rather than 24-year samples can be explained by potential biological differences. It is important to note that human metabolism undergoes substantial changes during growth and development. Further, young adults are likely exposed to more diverse lifestyle risk factors than children [79]. Therefore, the association between long-term air pollution and caffeine pathway metabolites at 24 years could be small in magnitude and easier blurred by unmeasured factors.
At 24 years, we found that prior-year air pollution was associated with downregulated fatty acid metabolism. Among the few long-term air pollution studies that have been conducted, one focused on young adults, while others focused on later adulthood and elderhood. They have consistently reported associations between long-term exposure to PM or NO2 and long-chain fatty acids, or with compounds containing long-chain fatty acid component, or with fatty-acid-related pathways [16,17,18, 20]. However, these previous studies observed inconsistent results on the direction of the associations between long-term air pollution exposure and long-chain fatty acids. A study in southern California suggested that prior-year air pollution exposure is related to an upregulated trend in fatty acids in young adults with a history of overweight or obesity [20]. However, among three other studies on older populations, two reported downregulation in lipids containing long-chain fatty acids [16, 17] and one reported both up-and down-regulation in some long-chain fatty acids [18]. This inconsistency may be due to the variation in BMI across the study populations. Consistent with the study on a population with high BMI (mean BMI = 29.6) [20], in our stratified analysis, an overweight subgroup showed a trend of upregulation (which did not reach significance, likely due to a combination of small effect size and low statistical power). In the normal or underweight stratum we demonstrated downregulation of these fatty acids.
An implication of this finding is that downregulated long-chain fatty acids may explain some adverse health outcomes of air pollution exposure. In their untargeted metabolomics study using a meet-in-the-middle approach, Jeong et al. found linoleic acid metabolism to be a mediator of the association between air pollution and both asthma and cardio-cerebrovascular disease [18]. Furthermore, previous studies on mice indicate that diesel exhaust reduces the levels of linoleic acid and oleic acid, affecting inflammation resolution and mitochondrial β-oxidation [80, 81].
For the downregulated long-chain fatty acids with prior-year air pollution in the 24-year samples, many of the associations were of small magnitude and nominal significance in the main analysis, which is expected. First, since urine is not the main route of fatty acid elimination, the use of non-targeted measurement is not optimal for hydrophobic compound separation and detection. Importantly, the small magnitude of detected associations is expected, because the BAMSE birth cohort is a generally healthy population living with lower air pollution exposure compared to most previous studies. Therefore, it is interesting that we still replicated athe ssociation between air pollution and fatty acids metabolism in urine in this cohort.
In our 24-year samples, we also found some weakly enriched pathways such as β-alanine metabolism (underlying metabolite: carnosine), as well as taurine and hypotaurine metabolism (underlying metabolite: taurine), that has been suggested by a previous long-term air pollution study [17].
According to recent reviews [5, 13, 14], air pollution has been consistently associated with perturbations in metabolites such as hypoxanthine, histidine, serine, aspartate, glutamate, taurine and creatine, and metabolic pathways involving glycerophospholipid-, pyrimidine-, methionine/cysteine-, tyrosine-, and tryptophan metabolism. However, most of the previous studies have analyzed metabolites in plasma and serum, exploring associations with short-term air pollution in smaller samples (mostly <200). When we compared the significant metabolites identified in our study, we observed some results consistent with previous findings, for example, downregulated levels of taurine and creatine. We also replicated association with histidine metabolism in the 4-year samples, which has been consistently associated with short-term air pollution. Other previously suggested metabolites—such as anserine, tyrosine, and uracil—were not associated with air pollution in our study, but we did observe association with their derivatives or chemically related compounds.
In the 24-year follow-up, the FDR significant metabolite-specific associations were largely represented by exogenous compounds, typically dietary or medication-related compounds or exogenous compounds that could be associated with lower socio-economy such as ethyl-glucuronide and nicotine, many of which were affected by a small group of individuals with high levels.
Our study fills a research gap in the potential effects of long-term air pollution on metabolites in children and young adults. Further, using urinary metabolomics data, we were able to replicate some endogenous compounds and metabolic pathways identified in studies using blood-based metabolomics, such as long-chain fatty acids. The well-defined cohort, which provides extensive information from birth to adult age for the participants, enables sensitivity analyses, enhancing the robustness of our findings. We were also able to perform a post hoc analysis exploring interaction with genetic variants. Another strength of the current study is the large sample size.
The main limitations of this study are that the association between air pollution and exogenous compounds may have been influenced by dietary factors, which we could not explore further. Indoor air pollution and air pollution in occupational settings were not assessed, which might have introduced some misclassification bias, though likely non-differential. The primary results are based on only 260 annotated metabolites, which is significantly less than the 9663 detected features and may result in false negatives for associations of interest. However, the 260 annotated metabolites are high-quality annotations (level 1) and our main results were supported by our supplementary analyses based on the untargeted metabolomics data. Another limitation is that the strong correlations between the air pollutants under study made it difficult to disentangle pollutant-specific associations. The supplementary analyses based on untargeted feature-based datasets have a large amount of missing data; however, our statistical method using a left-censored exponential regression model, suitable for skewed distribution and for a large percentage of missing data, gives robust estimates that are easier to interpret compared with approaches based on transformed values. In addition, the generalizability of the findings is limited by the study population, including a higher proportion of people with allergic symptoms, asthma, and low lung function compared with the general population.
Conclusions
Both early-life and prior year-long-term air pollution exposures are associated with urine metabolites in childhood and young adulthood. Particularly, our study suggests that children exposed to higher levels of air pollutants during the first year of life or the prior year to biosampling had upregulated caffeine pathway metabolites likely related to perturbations in pathways, indicating possible changes in enzyme activity. Young adults with higher prior-year air pollution exposure had downregulated fatty acid-related metabolic pathways, and our results suggest that this effect was modified by BMI. Given that our study population is generally healthy and living in Stockholm with comparatively clean ambient air, it is noteworthy that metabolic alterations related to air pollution exposure could still be shown.
Data availability
Additional data are available from the corresponding author on reasonable request.
References
Fuller R, Landrigan PJ, Balakrishnan K, Bathan G, Bose-O’Reilly S, Brauer M, et al. Pollution and health: a progress update. Lancet Planet health. 2022;6:e535–e47.
Eckhardt CM, Wu H. Environmental Exposures and Lung Aging: Molecular Mechanisms and Implications for Improving Respiratory Health. Curr Environ health Rep. 2021;8:281–93.
Peters A, Nawrot TS, Baccarelli AA. Hallmarks of environmental insults. Cell. 2021;184:1455–68.
Merid SK, Bustamante M, Standl M, Sunyer J, Heinrich J, Lemonnier N, et al. Integration of gene expression and DNA methylation identifies epigenetically controlled modules related to PM(2.5) exposure. Environ Int. 2021;146:106248.
Gruzieva O, Jeong A, He S, Yu Z, de Bont J, Pinho MGM, et al. Air pollution, metabolites and respiratory health across the life-course. European respiratory review : an official journal of the European Respiratory Society. 2022;31.
Lasky-Su J, Kelly RS, Wheelock CE, Broadhurst D. A strategy for advancing for population-based scientific discovery using the metabolome: the establishment of the Metabolomics Society Metabolomic Epidemiology Task Group. Metabolomics. 2021;17:45.
Hicks KG, Cluntun AA, Schubert HL, Hackett SR, Berg JA, Leonard PG, et al. Protein-metabolite interactomics of carbohydrate metabolism reveal regulation of lactate dehydrogenase. Science. 2023;379:996–1003.
Khamis MM, Adamko DJ, El-Aneed A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom Rev. 2017;36:115–34.
Zhang P, Arora M, Chaleckis R, Isobe T, Jain M, Meister I, et al. Tackling the Complexity of the Exposome: Considerations from the Gunma University Initiative for Advanced Research (GIAR) Exposome Symposium. Metabolites. 2019;9:106.
Zhang A, Sun H, Wu X, Wang X. Urine metabolomics. Clin Chim Acta; Int J Clin Chem. 2012;414:65–9.
Zhang YN, Hu HJ, Shi YF, Yang XZ, Cao LG, Wu J, et al. H-1 NMR-based metabolomics study on repeat dose toxicity of fine particulate matter in rats after intratracheal instillation. Sci Total Environ. 2017;589:212–21.
Xu Y, Wang W, Zhou J, Chen M, Huang X, Zhu Y, et al. Metabolomics analysis of a mouse model for chronic exposure to ambient PM2.5. Environ Pollut. 2019;247:953–63.
Jin L, Godri Pollitt KJ, Liew Z, Rosen Vollmar AK, Vasiliou V, Johnson CH, et al. Use of Untargeted Metabolomics to Explore the Air Pollution-Related Disease Continuum. Curr Environ health Rep. 2021;8:7–22.
Liang D, Li Z, Vlaanderen J, Tang Z, Jones DP, Vermeulen R, et al. A State-of-the-Science Review on High-Resolution Metabolomics Application in Air Pollution Health Research: Current Progress, Analytical Challenges, and Recommendations for Future Direction. Environ Health Perspect. 2023;131:56002.
Ritz B, Yan Q, He D, Wu J, Walker DI, Uppal K, et al. Child serum metabolome and traffic-related air pollution exposure in pregnancy. Environ Res. 2022;203:111907.
Yao Y, Schneider A, Wolf K, Zhang S, Wang-Sattler R, Peters A, et al. Longitudinal associations between metabolites and long-term exposure to ambient air pollution: Results from the KORA cohort study. Environ Int. 2022;170:107632.
Nassan FL, Kelly RS, Kosheleva A, Koutrakis P, Vokonas PS, Lasky-Su JA, et al. Metabolomic signatures of the long-term exposure to air pollution and temperature. Environ Health. 2021;20:3.
Jeong A, Fiorito G, Keski-Rahkonen P, Imboden M, Kiss A, Robinot N, et al. Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environ Int. 2018;119:334–45.
Walker DI, Lane KJ, Liu K, Uppal K, Patton AP, Durant JL, et al. Metabolomic assessment of exposure to near-highway ultrafine particles. J Expos Sci Environ Epidemiol. 2019;29:469–83.
Liao J, Goodrich J, Walker DI, Lin Y, Lurmann F, Qiu C, et al. Metabolic pathways altered by air pollutant exposure in association with lipid profiles in young adults. Environ Pollut. 2023;327:121522.
Chen L, Zhernakova DV, Kurilshikov A, Andreu-Sánchez S, Wang D, Augustijn HE, et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat Med. 2022;28:2333–43.
van Dongen J, Nivard MG, Willemsen G, Hottenga JJ, Helmer Q, Dolan CV, et al. Genetic and environmental influences interact with age and sex in shaping the human methylome. Nat Commun. 2016;7:11115.
Lowe WL Jr, Karban J. Genetics, genomics and metabolomics: new insights into maternal metabolism during pregnancy. Diabet Med. 2014;31:254–62.
Mitselou N, Andersson N, Bergström A, Kull I, Georgelis A, van Hage M, et al. Preterm birth reduces the risk of IgE sensitization up to early adulthood: A population-based birth cohort study. Allergy. 2022;77:1570–82.
Zettergren A, Andersson N, Larsson K, Kull I, Melén E, Georgelis A, et al. Exposure to environmental phthalates during preschool age and obesity from childhood to young adulthood. Environ Res. 2021;192:110249.
Wang G, Hallberg J, Faner R, Koefoed HJ, Kebede Merid S, Klevebro S, et al. Plasticity of Individual Lung Function States from Childhood to Adulthood. Am J Respir Crit Care Med. 2023;207:406–15.
Yu Z, Merid SK, Bellander T, Bergström A, Eneroth K, Georgelis A, et al. Associations of improved air quality with lung function growth from childhood to adulthood: the BAMSE study. Eur Respir J. 2023;61.
Wang G, Hallberg J, Merid SK, Kumar A, Klevebro S, Habchi B, et al. Body mass index trajectories from birth to early adulthood and lung function development. Eur Respir J. 2025;65.
Meister I, Zhang P, Sinha A, Sköld CM, Wheelock ÅM, Izumi T, et al. High-Precision Automated Workflow for Urinary Untargeted Metabolomic Epidemiology. Anal Chem. 2021;93:5248–58.
Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30:918–20.
Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinforma. 2010;11:395.
Broadhurst D, Goodacre R, Reinke SN, Kuligowski J, Wilson ID, Lewis MR, et al. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics. 2018;14:72.
Wilson MD, Ponzini MD, Taylor SL, Kim K. Imputation of Missing Values for Multi-Biospecimen Metabolomics Studies: Bias and Effects on Statistical Validity. Metabolites. 2022;12.
Lindsey JK. A study of interval censoring in parametric regression models. Lifetime Data Anal. 1998;4:329–54.
Zhou Z, Li X, Zhang Z. The Peer Effect in Promoting Physical Activity among Adolescents: Evidence from the China Education Panel Survey. International journal of environmental research and public health. 2023;20.
Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–2.
Petrovic D, Estoppey Younes S, Pruijm M, Ponte B, Ackermann D, Ehret G, et al. Relation of 24-hour urinary caffeine and caffeine metabolite excretions with self-reported consumption of coffee and other caffeinated beverages in the general population. Nutr Metab. 2016;13:81.
van de Luitgaarden IAT, Schrieks IC, Kieneker LM, Touw DJ, van Ballegooijen AJ, van Oort S, et al. Urinary Ethyl Glucuronide as Measure of Alcohol Consumption and Risk of Cardiovascular Disease: A Population-Based Cohort Study. J Am Heart Assoc. 2020;9:e014324.
Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25.
Pang Z, Lu Y, Zhou G, Hui F, Xu L, Viau C, et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucl Acids Res. 2024.
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42:D199–205.
Karnovsky A, Li S. Pathway Analysis for Targeted and Untargeted Metabolomics. Methods Mol Biol. 2020;2104:387–400.
Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, et al. Predicting network activity from high throughput metabolomics. PLoS Comput Biol. 2013;9:e1003123.
Mountjoy E, Schmidt EM, Carmona M, Schwartzentruber J, Peat G, Miranda A, et al. An open approach to systematically prioritize causal variants and genes at all published human GWAS trait-associated loci. Nat Genet. 2021;53:1527–33.
Ghoussaini M, Mountjoy E, Carmona M, Peat G, Schmidt EM, Hercules A, et al. Open Targets Genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 2021;49:D1311–d20.
Hernandez-Pacheco N, Kilanowski A, Kumar A, Curtin JA, Olvera N, Kress S, et al. Exploring the genetics of airflow limitation in lung function across the lifespan – a polygenic risk score study. eClinicalMedicine. 2024.
Raunio H, Pentikäinen O, Juvonen RO. Coumarin-Based Profluorescent and Fluorescent Substrates for Determining Xenobiotic-Metabolizing Enzyme Activities In Vitro. Int J Mol Sci. 2020;21.
Juvonen RO, Ahinko M, Jokinen EM, Huuskonen J, Raunio H, Pentikäinen OT. Substrate Selectivity of Coumarin Derivatives by Human CYP1 Enzymes: In Vitro Enzyme Kinetics and In Silico Modeling. ACS omega. 2021;6:11286–96.
Thorn CF, Aklillu E, McDonagh EM, Klein TE, Altman RB. PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012;22:389–95.
Banks NF, Tomko PM, Colquhoun RJ, Muddle TWD, Emerson SR, Jenkins NDM. Genetic Polymorphisms in ADORA2A and CYP1A2 Influence Caffeine’s Effect on Postprandial Glycaemia. Sci Rep. 2019;9:10532.
Lundberg B, Gruzieva O, Eneroth K, Melén E, Persson Å, Hallberg J, et al. Air pollution exposure impairs lung function in infants. Acta paediatrica. 2022.
Gruzieva O, Bellander T, Eneroth K, Kull I, Melen E, Nordling E, et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol. 2012;129:240–6.
Gruzieva O, Bergstrom A, Hulchiy O, Kull I, Lind T, Melen E, et al. Exposure to Air Pollution from Traffic and Childhood Asthma Until 12 Years of Age. Epidemiology. 2013;24:54–61.
Schultz ES, Gruzieva O, Bellander T, Bottai M, Hallberg J, Kull I, et al. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Resp Crit Care Med. 2012;186:1286–91.
Nordling E, Berglind N, Melén E, Emenius G, Hallberg J, Nyberg F, et al. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology. 2008;19:401–8.
Schultz ES, Hallberg J, Bellander T, Bergström A, Bottai M, Chiesa F, et al. Early-Life Exposure to Traffic-related Air Pollution and Lung Function in Adolescence. Am J Respir Crit Care Med. 2016;193:171–7.
Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Bruske I, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Resp Med. 2015;3:933–42.
Wang G, Kull I, Bergström A, Hallberg J, Bergström PU, Guerra S, et al. Early-life risk factors for reversible and irreversible airflow limitation in young adults: findings from the BAMSE birth cohort. Thorax. 2021;76:503–7.
Wang G, Hallberg J, Um Bergström P, Janson C, Pershagen G, Gruzieva O, et al. Assessment of chronic bronchitis and risk factors in young adults: results from BAMSE. Eur Respir J. 2021;57:2002120.
Gibbs BF, Gonçalves Silva I, Prokhorov A, Abooali M, Yasinska IM, Casely-Hayford MA, et al. Caffeine affects the biological responses of human hematopoietic cells of myeloid lineage via downregulation of the mTOR pathway and xanthine oxidase activity. Oncotarget. 2015;6:28678–92.
Branum AM, Rossen LM, Schoendorf KC. Trends in caffeine intake among U.S. children and adolescents. Pediatrics. 2014;133:386–93.
Lim HS, Hwang JY, Choi JC, Kim M. Assessment of caffeine intake in the Korean population. Food Addit Contam A Chem Anal control Expos Risk Assess. 2015;32:1786–98.
Knight CA, Knight I, Mitchell DC. Beverage caffeine intakes in young children in Canada and the US. Canadian journal of dietetic practice and research : a publication of Dietitians of Canada. Rev Can. 2006;67:96–9.
Skinner JD, Carruth BR, Houck KS, Morris M, Moran J 3rd, Coletta F. Caffeine intake in young children differs by family socioeconomic status. J Am Diet Assoc. 2000;100:229–31.
Stucki L, Betnér S, Selander J, Lõhmus M, Åkesson A, Eriksson C. Sociodemographic inequalities in long-term exposure to air pollution, road traffic noise, and greenness: A population-based cohort study of women. 2023;7:e279.
Vogel CFA, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530.
Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11:481–94.
Liguori MJ, Lee CH, Liu H, Ciurlionis R, Ditewig AC, Doktor S, et al. AhR activation underlies the CYP1A autoinduction by A-998679 in rats. Front Genet. 2012;3:213.
Josse AR, Da Costa LA, Campos H, El-Sohemy A. Associations between polymorphisms in the AHR and CYP1A1-CYP1A2 gene regions and habitual caffeine consumption. Am J Clin Nutr. 2012;96:665–71.
Gunes A, Ozbey G, Vural EH, Uluoglu C, Scordo MG, Zengil H, et al. Influence of genetic polymorphisms, smoking, gender and age on CYP1A2 activity in a Turkish population. Pharmacogenomics. 2009;10:769–78.
Garduno A, Wu T. Tobacco Smoke and CYP1A2 Activity in a US Population with Normal Liver Enzyme Levels. Int J Environ Res Public Health. 2021;18.
Aranda JV, Beharry KD. Pharmacokinetics, pharmacodynamics and metabolism of caffeine in newborns. Semin Fetal Neonatal Med. 2020;25:101183.
Dobrinas M, Cornuz J, Oneda B, Kohler Serra M, Puhl M, Eap CB. Impact of smoking, smoking cessation, and genetic polymorphisms on CYP1A2 activity and inducibility. Clin Pharm Ther. 2011;90:117–25.
Marinković N, Pasalić D, Potocki S. Polymorphisms of genes involved in polycyclic aromatic hydrocarbons’ biotransformation and atherosclerosis. Biochem Med. 2013;23:255–65.
Skupinska K, Misiewicz-Krzeminska I, Stypulkowski R, Lubelska K, Kasprzycka-Guttman T. Sulforaphane and its analogues inhibit CYP1A1 and CYP1A2 activity induced by benzo[a]pyrene. J Biochem Mol Toxicol. 2009;23:18–28.
Wang L, Li Z, Jin L, Li K, Yuan Y, Fu Y, et al. Indoor air pollution and neural tube defects: effect modification by maternal genes. Epidemiology. 2014;25:658–65.
Wang S, Chanock S, Tang D, Li Z, Jedrychowski W, Perera FP. Assessment of interactions between PAH exposure and genetic polymorphisms on PAH-DNA adducts in African American, Dominican, and Caucasian mothers and newborns. Cancer Epidemiol Biomark Prev. 2008;17:405–13.
Huff RD, Carlsten C, Hirota JA. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J Allergy Clin Immunol. 2019;143:1989–2001.
Agustí A, Melén E, DeMeo DL, Breyer-Kohansal R, Faner R. Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir Med. 2022;10:512–24.
Yin F, Gupta R, Vergnes L, Driscoll WS, Ricks J, Ramanathan G, et al. Diesel Exhaust Induces Mitochondrial Dysfunction, Hyperlipidemia, and Liver Steatosis. Arterioscler Thromb Vasc Biol. 2019;39:1776–86.
Liang N, Emami S, Patten KT, Valenzuela AE, Wallis CD, Wexler AS, et al. Chronic exposure to traffic-related air pollution reduces lipid mediators of linoleic acid and soluble epoxide hydrolase in serum of female rats. Environ Toxicol Pharm. 2022;93:103875.
Acknowledgements
We thank all children and parents participating in the BAMSE cohort, as well as the research and clinical staff involved in the study.
Funding
The study was supported by grants from the Swedish Research Council (reference number 2020-01886, 2022-00796), the Swedish Research Council for Health, Working Life and Welfare (FORTE 2017-01146), the Swedish Heart-Lung Foundation (2021-2407, 2021-0519, 2023-0463), the European Research Council (TRIBAL, grant agreement 757919), the Swedish Asthma and Allergy Association and Region Stockholm (ALF project, cohort and database maintenance, and clinical appointment for SK). Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Shizhen He: Methodology, Formal analysis, Software, Validation, Visualization, Writing – original draft, Writing – review & editing; Baninia Habchi: Investigation, Methodology, Validation, Writing – review & editing; Romanas Chaleckis: Investigation, Methodology, Validation, Writing – review & editing; Natalia Hernandez-Pacheco: Methodology, Formal analysis, Software, Visualization, Writing – review & editing; Anna Bergström: Investigation, Project administration, Writing – review & editing; Anne-Sophie Merrit: Investigation, Project administration, Writing – review & editing; Inger Kull: Investigation, Project administration, Writing – review & editing; Kristina Eneroth: Methodology, Writing – review & editing; Matteo Bottai: Methodology, Software, Supervision, Writing – review & editing; Göran Pershagen: Investigation, Funding acquisition, Writing – review & editing; Simon Kebede Merid: Investigation, Software, Writing – review & editing; Sophia Björkander: Investigation, Data Curation, Writing – review & editing; Zhebin Yu: Investigation, Writing – review & editing; Erik Melén: Conceptualization, Funding acquisition, Methodology, Supervision, Project administration, Resources, Writing – review & editing; Olena Gruzieva: Conceptualization, Investigation, Writing – review & editing, Supervision, Funding acquisition; Craig E. Wheelock: Conceptualization, Investigation, Resources, Methodology, Supervision, Writing – review & editing; Susanna Klevebro: Conceptualization, Investigation, Methodology, Data Curation, Writing – original draft, Writing – review & editing, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, S., Habchi, B., Chaleckis, R. et al. Long-term exposure to air pollution and metabolites in children and young adults in a Swedish birth cohort. J Expo Sci Environ Epidemiol (2025). https://doi.org/10.1038/s41370-025-00810-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-025-00810-1