Abstract
Objective
To review the evolution of golden hour management and outcomes for infants with congenital diaphragmatic hernia (CDH).
Study design
Retrospective single center cohort study of infants with CDH born 2008–2023 at a quaternary children’s hospital. Infants were grouped into 3 epochs: 2008–2013, 2014–2018, and 2019–2023. Outcome measures included extracorporeal membrane oxygenation therapy and survival.
Result
There were 454 infants, including 106 (2008–2013), 156 (2014–2018), and 192 (2019–2023). Despite increased disease severity, survival improved over time, from 71% (2008–2013) to 82% (2014–2018) and 83% (2019–2023), p = 0.02 for trend, with no difference in ECMO utilization.
Conclusion
Management of infants with CDH continues to evolve with ongoing experience at our high-volume center. Despite increasing severity of illness, survival outcomes have improved over time. In the absence of clinical trial data, observational data should be evaluated rigorously to inform care in a data-driven manner.
Similar content being viewed by others
Introduction
Congenital diaphragmatic hernia (CDH) occurs when the fetal abdominal viscera herniate into the thoracic cavity through a diaphragmatic defect. Pulmonary hypoplasia and pulmonary hypertension may occur as a result of this defect and may complicate the typical postnatal cardiorespiratory transition. Effective delivery room (DR) resuscitation of infants with CDH is complex and requires critical interventions to occur simultaneously or in quick succession [1]. Rapid intubation and early gentle ventilation optimize gas exchange and are associated with improved survival [2,3,4,5]. Annibale et al. introduced the concept of adapting the Golden Hour of Trauma to a golden hour of DR management in preterm infants from birth through initial stabilization [6]. The goal of the golden hour is to rapidly stabilize an infant with critical interventions and systematic decision making. While initially described for the DR management of preterm infants, the concept of a golden hour of stabilization can be extrapolated to infants with complex congenital anomalies like CDH.
Existing CDH management guidelines are based predominantly on expert opinion, particularly regarding DR management [7,8,9,10,11]. Large trials are lacking in this population, and many changes in DR management strategies have occurred based solely on observational data and expert opinion.
The Garbose Family Special Delivery Unit (SDU) at the Children’s Hospital of Philadelphia is the world’s first birth facility located in a children’s hospital, allowing infants with congenital anomalies to be born with immediate access to quaternary level care [12, 13]. Each year, there are approximately 500 deliveries in the SDU, including about 50 infants with CDH [14].
The objective of this study was to leverage this large single center experience delivering and managing infants with CDH to review the evolution of golden hour care and outcomes.
Methods
Design
This was a retrospective single center cohort study of infants with CDH born 2008–2023. We have structured our CDH DR program using the framework of a Learning Health System [15] to inform and improve care. In this model, observations of clinical performance and outcomes prompted changes to clinical management, which were then assessed through ongoing review of patient data. Temporal changes in golden hour management included initiating a lower fraction of inspired oxygen, reducing use of empiric inhaled nitric oxide (iNO), developing separate DR algorithms based on anticipated severity, and utilizing high frequency oscillatory ventilation as the initial mode of ventilation immediately after birth. With these temporal changes in management and introduction of an electronic medical record, infants were grouped into 3 epochs: 2008–2013, 2014–2018, and 2019–2023. Epochs were divided into roughly equal increments and marked by changes in clinical management as outlined below. Using the Clinical Outcomes Data Archive (CODA) Registry [16], we included all infants who received active treatment and excluded any infants with a planned palliative delivery. Outcome measures included extracorporeal membrane oxygenation (ECMO) therapy and survival. The CHOP Institutional Review Board approved this study (IRB 21-018553) with a waiver of informed parental consent.
Hospital delivery room resuscitation protocols
Uniform interventions throughout all epochs: 2008–2023
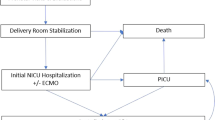
Throughout the entire study period, all infants with CDH were intubated immediately after birth, and received intermittent positive pressure ventilation (PPV) with a T-piece ventilator; settings included peak inspiratory pressures (PIPs) of 20–25 cm H2O, positive end expiratory pressure (PEEP) of 5 cm H2O, and a ventilation rate of 40–50 breaths/minute. A gastric decompression tube was placed immediately following intubation to promote adequate ventilation and stability by minimizing dilation of the intrathoracic bowel. Infants transitioned to a ventilator as soon as the endotracheal tube was secured. Vascular access was established and a blood gas, arterial if available, was used to guide ventilator adjustments (Fig. 1). Imaging and vascular access were obtained in the DR. It was our clinical practice to administer fentanyl with or without vecuronium to infants with significant hypoxia presumed to be secondary to pulmonary hypertension to improve pulmonary blood flow and reduce asynchrony with the ventilator. Throughout the study period, all new providers joining the SDU team participated in multidisciplinary delivery simulations focused on unique aspects of DR resuscitations of infants with surgical anomalies. The SDU DR team included a neonatologist, a neonatal fellow physician, an advanced practice provider, two to three neonatal intensive care unit (NICU) nurses, and a respiratory therapist. A pediatric surgeon frequently attended DR resuscitations.
Changes introduced to the resuscitation protocol throughout the 15-year study period are outlined with significant events highlighted in Fig. 1.
Epoch 1: 2008–2013
Interventions
Following the opening of the SDU in 2008, infants with CDH were born within the freestanding children’s hospital, thereby eliminating the need for postnatal transfer after delivery. Initial resuscitation protocols were grounded in historical perspectives and expert consensus. In addition to the interventions above, protocols included intubation with a conventional laryngoscope and conventional mechanical ventilation with an initial FiO2 of 100%. Empiric iNO was used frequently prior to echocardiogram in the setting of profound hypoxia on 100% FiO2 and based on provider discretion.
Monitoring
Monitoring was limited to pulse oximetry and electrocardiography.
Epoch 2: 2014–2018
Interventions
Beginning in 2014, initial DR FiO2 was decreased from 100% to 50%. FiO2 was then subsequently titrated to achieve pre-ductal oxygen saturation goals of ≥85% by 10 minutes of life and beyond. Riley et al. studied this practice change and demonstrated that an initial FiO2 of 50% was safe and effective [17]. Intubation in infants with CDH can be challenging due to significant airway deviation. Following review, our institutional experience demonstrated improved intubation outcomes with video laryngoscopy and in 2014 [18], video laryngoscopy was made standard of care.
In 2016, fetoscopic endoluminal tracheal occlusion (FETO) was introduced to our center for infants with severe left CDH. The Tracheal Occlusion to Accelerate Lung Growth trials found that FETO resulted in increased fetal lung volume and survival in infants with severe, isolated, left CDH with less clear benefit for infants with moderate CDH [19, 20]. In 2018, a pilot feasibility trial assessed physiologic based cord clamping, whereby the umbilical cord was clamped after intubation and lung aeration were established. Although physiologic based cord clamping was safe and feasible for infants with CDH, there was no clear benefit in clinical outcomes compared with historical controls [21]; therefore, this approach was not adopted for standard practice in our center.
Epoch 3: 2019–2023
Interventions
Beginning in 2019, our DR protocol was updated to reflect two cohorts of infants with CDH based on anticipated severity, with severe CDH defined as an intrathoracic liver or right CDH. For infants with mild to moderate left CDH, initial conventional mechanical ventilation in the DR remained standard. Initial FiO2 was decreased further from 50% to 30% following close monitoring of infants started on 50% FiO2 with no adverse events noted and an overall reduction in exposure to supplemental oxygen. Patient level outcomes including delivery room outcomes (bradycardia, hypotension, Apgar scores) and NICU outcomes (survival, duration of invasive ventilation, incidence and duration of ECMO, days to surgery) were compared pre and post policy change to assess patient safety, as described in Riley et al. [17]. In contrast, as a response to high rates of hypercarbia in infants with severe CDH, high frequency oscillatory ventilation (HFOV) was established as the initial mode of ventilation for infants with severe CDH immediately after the endotracheal tube was secured; initial FiO2 remained at 50% for this population. Recommended HFOV settings include Mean Airway Pressure (MAP) of 11–13 mm H2O, amplitude of 30–35 adjusted to achieve appropriate chest wall vibration, and frequency or Hertz (Hz) of 6 for term infants that was increased with decreasing gestational age. Within these ranges, clinicians were encouraged to use the lowest possible settings to minimize barotrauma. The clinical impact of initial HFOV was assessed four years after this practice change; this analysis demonstrated significantly improved early gas exchange with no adverse differences in hospital outcomes among infants with severe CDH [22].
iNO was considered as an adjunctive DR therapy in infants predicted to have severe CDH with severe hypoxia on 100% FiO2. However, with a growing appreciation for left ventricle (LV) hypoplasia and dysfunction as a contraindication to iNO due to increased risk for pulmonary edema [23,24,25], we evaluated our experience and found increased ECMO need and higher mortality in infants with LV dysfunction that were treated with iNO [26]. Thus, in 2021 we changed our guidelines to recommend against use of iNO in infants with evidence of LV dysfunction.
We socialized these new guidelines and recommended obtaining an echocardiogram to evaluate LV function prior to starting iNO. Instead of empiric iNO use, we now use iNO on a case-by-case basis in a subset of infants with RV dysfunction without LV dysfunction and do not use iNO in the delivery room.
In 2021, we briefly introduced Microcuff® endotracheal tubes in the DR to allow inflation of the cuff peri-operatively to improve ventilation during the dynamic respiratory challenges during CDH repair. However, the practice was discontinued after an interim review of 22 patients revealed that more intubation attempts were needed to successfully intubate infants with CDH in the DR when Microcuff® tubes were used. Finally, in 2022, we undertook a quality improvement project to reduce rates of admission hypothermia for infants with congenital anomalies, given the evidence demonstrating both the association between admission hypothermia and increased morbidity and mortality in preterm infants and the increased risk of hypothermia in infants with congenital anomalies [27]. At baseline, 27% of infants were hypothermic (<36.5 degrees Celsius) on NICU admission. Plan, Do, Study Act cycles included standardizing the temperature of the DR and resuscitation bed, recommendations for increased frequency of temperature monitoring, trialing polyethylene lined hats, and implementing a DR thermoregulation checklist; in conjunction, these interventions led to an improvement with hypothermia seen in only 9% of infants in a recent cohort [28].
Monitoring
In 2020, we introduced enhanced monitoring in the DR beginning with respiratory function monitoring (RFM) to characterize the transitional pulmonary physiology of infants with CDH in real time during DR resuscitation [29]. RFM allows for evaluation of exhaled tidal volumes and end-tidal carbon dioxide monitoring during invasive positive pressure ventilation immediately after birth to guide ongoing ventilatory support. DR resuscitation video recording was also introduced in 2021 as routine practice for quality assurance [1]. In 2022, we introduced near infrared spectroscopy (NIRS) to evaluate cerebral hypoxia during the perinatal transition with ongoing evaluation of these data. In 2023, we started a quality improvement study to improve time to lead placement and vital sign acquisition in the DR given the unique challenges and competing interests of immediate intubation and invasive ventilation for infants with CDH. Plan, Do, Study, Act cycles have included transitioning to different electrocardiography leads with faster time to signal acquisition as well as rotating infants on the warmer bed to give bedside nurses improved access to placing leads on the infant.
Planning/team communication
In 2019, we implemented biweekly multidisciplinary CDH clinical management meetings to review DR and neonatal care. In 2022, we added alternating biweekly SDU management meetings to review upcoming deliveries and management plans, recent deliveries, and areas for education or improvement. In 2022, we implemented monthly video review conferences to identify facilitators and challenges to optimal stabilization that inform education initiatives, quality improvement projects, and guideline changes.
Main outcome measures
Main hospital-based outcome measures included extracorporeal membrane oxygenation (ECMO) therapy and survival.
Data analysis
We evaluated the proportion of infants who experienced these outcomes across epochs. The Chi-squared test of trend was used to evaluate systematic increase or decrease in categorical variables across the time epochs. A linear regression model with the epochs coded as a continuous variable was used to evaluate trends in continuous variables. Additionally, a multivariable logistic regression was used to assess the confounder adjusted association between survival at NICU discharge and time. All analyses were conducted in R V.4.1.2.
Results
From 2008–2023, 454 infants with CDH were born in the SDU including 106 (2008–2013), 156 (2014–2018), and 192 (2019–2023) (Table 1). There was a trend toward increased severity across epochs. Differences in DR characteristics reflect temporal changes in DR management including lower initial fraction of inspired oxygen, two DR algorithms based on anticipated severity, and HFOV as the initial mode of ventilation after birth in infants with severe CDH (Table 2, Fig. 2). Tables 1 and 2 list the median and interquartile range for each variable, not the overall range. Despite increased severity, survival improved significantly over time, from 71% (2008–2013) to 82% (2014–2018) and 83% (2019–2023), p = 0.02 for trend. There was no difference in ECMO utilization over time, but there was a significant increase in CDH repair, p < 0.001 (Fig. 3). CDH repair details are shown in Supplementary Table 1.
Survival (A), ECMO utilization (B), CDH repair (C) and length of stay (D). Survival improved significantly over time (A), from 71% (2008–2013) to 82% (2014–2018) and 83% (2019–2023), p = 0.02 for trend. There was no difference in ECMO utilization over time (B) but there was a significant increase in CDH repair (C), p < 0.001.
Discussion
Management of infants with CDH continues to evolve. As large trials are lacking in this population, we reviewed the evolution of DR practice and outcomes in our large volume center. Notable changes to golden hour management for infants with CDH included lower initial fraction of inspired oxygen, severity-specific DR algorithms including HFOV as the initial mode of ventilation in severe CDH, and reduction of empiric inhaled nitric oxide. Despite increasing severity of illness among infants with CDH, survival to discharge improved over time with no difference in ECMO utilization. We believe this survival improvement is reflective of ongoing improvements we have implemented to ensure an experienced and collaborative team is available to respond to deliveries 24/7. While an ideal transition to postnatal life may help to optimize an infant’s overall course, it is the totality of CDH care (including the DR) that may explain improved survival outcomes.
There was a significant increase in CDH repair which likely reflects a more aggressive approach to infants with CDH in our center, despite increasing severity of illness. Over time, our approach to limitations of care changed to attempt stabilization, utilize ECMO if indicated, and attempt CDH repair prior to redirection of care if in line with a family’s goals of care. There has also been an increase in CDH repair of infants with concomitant complex congenital heart disease and other anomalies who would not have been ECMO candidates in the early years of this cohort. CDH repair is discussed on a case-by-case and was performed for infants with double outlet right ventricle, truncus arteriosus, and Tetralogy of Fallot in the later years of this cohort. Length of stay has increased over time, likely as a result of increased severity and an increase in CDH repair in the most severe infants.
Clinical trials, particularly randomized controlled trials, are rare in infants with CDH [19, 20, 30,31,32,33,34]. When attempted, these trials have been difficult to complete due to the rarity of CDH as well as high cost and low enrollment. Multicenter randomized controlled trials are even more challenging given the combination of a heterogeneous disease with a large spectrum of severity and significant practice variation across centers. Given these difficulties, CDH management is predominantly based on guidelines driven by expert opinion. As a result, large volume centers have a responsibility to evaluate and share observational data rigorously. While survival and CDH repair were higher than those reported by other groups, ECMO utilization was comparable [19, 20, 35]. As we have introduced changes to our golden hour management for this population, we have systematically studied these changes to ensure that expert-driven changes did not result in harm.
We have structured our CDH DR program using the framework of a Learning Health System [15] to inform and improve care. A Learning Health System begins with a learning community and is an iterative cycle to continuously improve care as we learn from each patient. We have multiple venues to review our data and revise our guidelines continuously including a team debrief after each delivery, weekly alternating CDH and SDU management meetings, and a monthly SDU video review meeting. Our interdisciplinary Clinical Outcomes Data Archive (CODA) Registry with data from both the electronic medical record and chart abstraction support this effort. To advance the care of infants with CDH without the benefit of clinical trial data, we employ a physiologic and data-driven approach to guide DR management. We have recently adopted advanced monitoring modalities such as NIRs and RFM to allow for real-time adaptation and individualization of DR resuscitations as needed. Finally, fetal interventions such as FETO have improved survival and decreased ECMO utilization for infants with severe CDH and have resulted in a different DR phenotype for infants on the most severe end of the CDH spectrum [36]. As FETO utilization and inclusion criteria increase, there may be an increased number of infants with this unique post-FETO phenotype that will need to be evaluated.
We acknowledge study limitations and unique strengths. This was an observational cohort study and therefore a non-randomized sample. We adjusted for known confounders, such as CDH side and severity in our analysis. As a single center study, outcomes may also reflect center-specific practice management differences. Therefore, we recommend that each center review their own key outcomes systematically as they make changes to CDH guidelines as our outcomes may not be generalizable.
Study strengths include one of the largest cohorts of infants with CDH born in a DR within a children’s hospital. DR practices and changes in management are not always represented in clinical trials; this study fills an important evidence gap specific to this high-risk population.
Conclusion
Management of infants with CDH continues to evolve with ongoing experience and continual review of individual outcomes at our high-volume center. Despite increasing severity of illness, survival outcomes have improved over time. In the absence of clinical trial data, observational data should be evaluated within an established framework such as a Learning Health System to inform care in a data-driven manner.
Data availability
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.
Change history
16 June 2025
The name of author K. Taylor Wild has been corrected.
References
Wild KT, Rintoul N, Hedrick HL, Heimall L, Soorikian L, Foglia EE, et al. Delivery room resuscitation of infants with congenital diaphragmatic hernia: lessons learned through video review. Fetal Diagn Ther. 2024:1–9. https://doi.org/10.1159/000538536.
Schultz CM, Digeronimo RJ, Yoder BA. Congenital diaphragmatic hernia: a simplified postnatal predictor of outcome. J Pediatr Surg. 2007;42:510–6. https://doi.org/10.1016/j.jpedsurg.2006.10.043.
Salas AA, Bhat R, Dabrowska K, Leadford A, Anderson S, Harmon CM, et al. The value of Pa(CO2) in relation to outcome in congenital diaphragmatic hernia. Am J Perinatol. 2014;31:939–46. https://doi.org/10.1055/s-0034-1368088.
Khmour AY, Konduri GG, Sato TT, Uhing MR, Basir MA. Role of admission gas exchange measurement in predicting congenital diaphragmatic hernia survival in the era of gentle ventilation. J Pediatr Surg. 2014;49:1197–201. https://doi.org/10.1016/j.jpedsurg.2014.03.011.
Abbas PI, Cass DL, Olutoye OO, Zamora IJ, Akinkuoto AC, Sheikh F, et al. Persistent hypercarbia after resuscitation is associated with increased mortality in congenital diaphragmatic hernia patients. J Pediatr Surg. 2015;50:739–43. https://doi.org/10.1016/j.jpedsurg.2015.02.028.
Annibale DJ, Bissinger RL. The golden hour. Adv Neonatal Care. 2010;10:221–223. https://doi.org/10.1097/ANC.0b013e3181e9e244.
Snoek KG, Reiss IKM, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus - 2015 Update. Neonatology. 2016;110:66–74. https://doi.org/10.1159/000444210.
Lazar DA, Cass DL, Rodriguez MA, Hassan SF, Cassady CI, Johnson YR, et al. Impact of prenatal evaluation and protocol-based perinatal management on congenital diaphragmatic hernia outcomes. J Pediatr Surg. 2011;46:808–813. https://doi.org/10.1016/j.jpedsurg.2011.02.009.
Jancelewicz T, Brindle ME, Guner YS, Lally PA, Lally KP, Harting MT. Toward standardized management of congenital diaphragmatic hernia: an analysis of practice guidelines. J Surg Res. 2019;243:229–235. https://doi.org/10.1016/j.jss.2019.05.007.
Puligandla PS, Skarsgard ED. The canadian pediatric surgery network congenital diaphragmatic hernia evidence review project: developing national guidelines for care. Paediatr Child Health. 2016;21:183–186. https://doi.org/10.1093/pch/21.4.183.
Wild KT, Hedrick HL, Ades AM, Fraga MV, Avitabile CM, Gebb JS, et al. Update on management and outcomes of congenital diaphragmatic hernia. J Intensive Care Med. 2023. https://doi.org/10.1177/08850666231212874.
Howell LJ, Adzick NS. Establishing a fetal therapy center: lessons learned. Semin Pediatr Surg. 2003;12:209–217. https://doi.org/10.1016/s1055-8586(03)00023-4.
Howell LJ. The Garbose Family Special Delivery Unit: A new paradigm for maternal–fetal and neonatal care. Semin Pediatr Surg. 2013;22:3–9. https://doi.org/10.1053/j.sempedsurg.2012.10.002.
Goldshore M, Land S, Flohr S, Mathew L, Reynolds T, Eppley E, et al. The impact of comprehensive fetal care on mortality of children with congenital diaphragmatic hernia when delivery is co-located in a pediatric hospital. J Pediatr Surg. 2024;59:445–450. https://doi.org/10.1016/j.jpedsurg.2023.09.039.
Institute of Medicine. 2013. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press. https://doi.org/10.17226/13444.
Reynolds T, Goldshore MA, Flohr S, Land L, Mathew L, Gebb JS, et al. A clinical outcomes data archive for a comprehensive fetal diagnosis and treatment center. Fetal Diagn Ther. 2024;1–9. https://doi.org/10.1159/000541877.
Riley JS, Antiel RM, Rintoul NE, Ades AM, Waqar LN, Lin N, et al. Reduced oxygen concentration for the resuscitation of infants with congenital diaphragmatic hernia. J Perinatol. 2018;38:834–843. https://doi.org/10.1038/s41372-017-0031-5.
Pouppirt NR, Nassar R, Napolitano N, Nawab U, Nishisaki A, Nadkarni V, et al. Association between video laryngoscopy and adverse tracheal intubation-associated events in the neonatal intensive care unit. J Pediatr. 2018;201:281–284.e1. https://doi.org/10.1016/j.jpeds.2018.05.046.
Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C, Persico N, et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med. 2021;385:119–129. https://doi.org/10.1056/NEJMoa2026983.
Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med. 2021;385:107–118. https://doi.org/10.1056/NEJMoa2027030.
Foglia EE, Ades A, Hedrick HL, Rintoul N, Munson D, Moldenhauer J, et al. Initiating resuscitation before umbilical cord clamping in infants with congenital diaphragmatic hernia: a pilot feasibility trial. Arch Dis Child Fetal Neonatal Ed. 2020;105:322–326. https://doi.org/10.1136/archdischild-2019-317477.
Wild KT, Mathew L, Ades AM, Rintoul NR, Soorikian L, Matthews K, et al. Association between initial ventilation mode and hospital outcomes for severe congenital diaphragmatic hernia. J Perinatol. 2024. https://doi.org/10.1038/s41372-024-02024-z.
Patel N, Lally PA, Kipfmueller F, Massolo AC, Luco M, Van Meurs KP, et al. Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. Am J Resp Crit Care Med. 2019;200:1522–1530. https://doi.org/10.1164/rccm.201904-0731OC.
Kinsella JP, Steinhorn RH, Mullen MP, Hopper RK, Keller RL, Ivy DD, et al. The left ventricle in congenital diaphragmatic hernia: implications for the management of pulmonary hypertension. J Pediatr. 2018;197:17–22. https://doi.org/10.1016/j.jpeds.2018.02.040.
Patel N, Massolo AC, Paria A, Stenhouse EJ, Hutner L, Finlay E, et al. Early postnatal ventricular dysfunction is associated with disease severity in patients with congenital diaphragmatic hernia. J Pediatr. 2018;203:400–407.e1. https://doi.org/10.1016/j.jpeds.2018.07.062.
Lawrence KM, Monos S, Adams S, Herkert L, Peranteau WH, Munson DA, et al. Inhaled nitric oxide is associated with improved oxygenation in a subpopulation of infants with congenital diaphragmatic hernia and pulmonary hypertension. J Pediatr. 2020;219:167–172. https://doi.org/10.1016/j.jpeds.2019.09.052.
Laptook AR, Salhab W, Bhaskar B. and the Neonatal Research Network. Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics. 2007;119:e643–e649. https://doi.org/10.1542/peds.2006-0943.
Heimall L, Barrila-Yetman M, McCray KR, Cestare D, Duran M, Wild KT, et al. Preventing hypothermia in newborns with congenital anomalies in the delivery room. Adv Neonatal Care. 2024. https://doi.org/10.1097/ANC.0000000000001184.
Wild KT, Mathew L, Hedrick HL, Rintoul NR, Ades A, Soorikian L, et al. Respiratory function after birth in infants with congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2023;108:535–539. https://doi.org/10.1136/archdischild-2022-324415.
Snoek KG, Capolupo I, van Rosmalen J, Hout LdeJ, Vijfhuize S, Greenough A, et al. Conventional mechanical ventilation versus high- frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomized clinical trial (The VICI-trial). Ann Surg. 2016;263:867–74. https://doi.org/10.1097/SLA.0000000000001533.
Cochius-den Otter S, Schaible T, Greenough A, van Heijst A, Patel N, Allegaert K, et al. The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia. BMJ Open. 2019;9:e032122. https://doi.org/10.1136/bmjopen-2019-032122.
Lakshminrusimha S, Keszler M, Kirpalani H, Van Meurs K, Chess P, Ambalavanan N, et al. Milrinone in congenital diaphragmatic hernia – a randomized pilot trial: study protocol, review of literature and survey of current practices. Matern Health Neonatol Perinatol. 2017;3:27. https://doi.org/10.1186/s40748-017-0066-9.
Le Duc K, Mur S, Rakza T, Boukhris MR, Rousset C, Vaast P, et al. Efficacy of intact cord resuscitation compared to immediate cord clamping on cardiorespiratory adaptation at birth in infants with isolated congenital diaphragmatic hernia (CHIC). Children. 2021;8:e339. https://doi.org/10.3390/children8050339.
Cochius - den Otter S, Deprest JA, Storme L, Greenough A, Tibboel D. Challenges and pitfalls: performing clinical trials in patients with congenital diaphragmatic hernia. Front Pediatr. 2022;10:852843. https://doi.org/10.3389/fped.2022.852843.
Gupta VS, Harting MT, Lally PA, Miller CC, Hirschl RB, Davis CF, et al. Mortality in congenital diaphragmatic hernia: a multicenter registry study of over 5000 patients over 25 years. Ann Surg. 2023;277:520–527. https://doi.org/10.1053/j.semperi.2019.07.006.
Wild KT, Rintoul NE, Ades AM, Gebb JS, Moldenhauer JS, Mathew L, et al. The delivery room resuscitation of infants with congenital diaphragmatic hernia treated with fetoscopic endoluminal tracheal occlusion: beyond the balloon. Fetal Diagn Ther. 2024. https://doi.org/10.1159/00053620924.
Funding
Children’s Hospital of Philadelphia Delivery Room of the Future Frontier Program.
Author information
Authors and Affiliations
Contributions
KTW and EEF conceptualized and designed the study, collected and analyzed the data, drafted the initial manuscript, and reviewed and revised the manuscript. HLH, NER, AMA, JSG, LM, TR, AB, EE, SF, and NSA assisted in designing the study, analyzing the data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
All authors have indicated they have no potential conflicts of interest relevant to this article to disclose.
Ethics approval
This study was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia (IRB 21-018553) and was performed in accordance with the Declaration of Helsinki.
Consent to participate
The CHOP Institutional Review Board approved this observational study with a waiver of informed parental consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wild, K.T., Hedrick, H.L., Rintoul, N.E. et al. Golden hour management of infants with congenital diaphragmatic hernia: 15 year experience at a high-volume center. J Perinatol 45, 1247–1254 (2025). https://doi.org/10.1038/s41372-025-02226-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02226-z