Abstract
Background
There is a paucity of data on neurodevelopmental outcomes in preterm infants who undergo transcatheter patent ductus arteriosus (PDA) closure (TCPC).
Objective
To evaluate neurodevelopmental impairment (NDI) or death at 2 years among preterm infants treated with TCPC compared to surgical ligation.
Methods
Retrospective cohort study of infants born at <27 weeks’ gestation at NICHD NRN sites. Comparisons were made between infants who underwent TCPC and PDA ligation.
Results
TCPC and surgical ligation were performed on 99 and 279 infants, respectively. Death or severe NDI occurred in 49% of infants with TCPC and 40% with surgical ligation. There was no difference in odds of death or severe NDI between the two groups [aOR 1.12 95% CI: 0.55–2.26)].
Conclusion
TCPC had similar odds of death or severe NDI compared to surgical ligation. These findings need to be evaluated in large prospective studies as the management practice around the TCPC evolves.
Clinical trial registration
ClinicalTrials.gov ID: Generic Database: NCT00063063.
Similar content being viewed by others
Introduction
Transcatheter patent ductus arteriosus (PDA) closure (TCPC) has become an increasingly common treatment for preterm infants [1,2,3]. Between 2016 and 2021, there has been a 4-fold increase in the number of patients undergoing TCPC [1]. In 2021, there were more than twice as many episodes of TCPC as surgical ligation [1]. This trend has continued, despite calls for caution by experts in the field due to limited evidence [4, 5]. Data from several observational studies showed superior short-term outcomes following TCPC compared to surgical ligation [6,7,8,9]. In contrast, multicenter data from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) showed similar respiratory outcomes in infants treated with TCPC and surgical ligation [10].
Previous data have shown an association between surgical ligation and neurodevelopmental impairment (NDI); however, some of these studies did not consider pre-ligation morbidities, the procedure itself, or post ligation cardiac syndrome [11,12,13,14]. A study conducted by Weisz and colleagues, reported no difference in the odds of death or NDI between surgical ligation or medical treatment after adjusting for differences in perinatal characteristics and pre-ligation morbidities [15].
There is a paucity of data on neurodevelopmental outcomes in extremely preterm infants who undergo TCPC. Data from two small observational studies that included a total of 25 subjects undergoing TCPC found no difference in neurodevelopmental outcomes in patients undergoing TCPC compared to surgical ligation [16, 17]. No large multicenter studies has evaluated neurodevelopmental outcomes in preterm infants undergoing TCPC.
The objective of the current study was to evaluate death or neurodevelopmental outcomes of extremely preterm infants undergoing TCPC compared to surgical ligation.
Methods
We performed a retrospective cohort study of preterm infants born at 22 0/7 through 26 6/7 weeks’ gestation at centers participating in the NICHD NRN between 1/1/2016 and 12/31/2019. Infants who died before 12 postnatal hours, were born outside of NRN hospitals or had major congenital anomalies were excluded.
We used prospectively collected data from the NRN Generic Database and linked Follow-up Database. Information on PDA diagnosis and treatment was prospectively entered by trained data abstractors. PDA diagnosis (“yes or no”) was defined as documentation of clinical or echocardiographic evidence of left-to-right PDA physiology determined by the clinical team in routine care. Medical PDA treatment was defined as any medication used specifically to induce PDA closure (indomethacin, ibuprofen, or acetaminophen) regardless of timing, duration, or dose. Procedural PDA closure included transcatheter and transthoracic surgical closure. Neonatal characteristics (e.g., gestational age at birth, birth weight, sex) were also collected.
The follow-up visit at 22–26 months’ corrected age included standardized physical and neurologic examination and the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) [18], both of which were administered by certified examiners who completed annual training to ensure interrater reliability [19]. Bayley-III cognitive, language, and motor composite scores are normalized to a mean of 100 (standard deviation 15). Severity of motor impairment was determined by the Gross Motor Function Classification system (GMFCS) of Palisano et al. [20]. Mild cerebral palsy (CP) as defined as GMFCS level 1, moderate as GMFCS level 2 or 3, and severe as GMFCS level 4 or 5.
Outcomes
The primary outcome was severe NDI or death before developmental assessment. Severe NDI was defined as any of the following: motor and/or cognitive composite score <70 on Bayley-III, gross motor impairment with GMFCS level 4 or 5, bilateral blindness or deafness. Secondary outcomes were death, severe NDI, moderate to severe NDI (any of Bayley-III cognitive or motor composite score < 85, GMFCS > 2, bilateral blindness or deafness), any CP, severity of CP, Bayley-III cognitive, language and motor composite scores, Bayley-III cognitive, language, and motor composite score <70 and head growth (Z score at follow-up).
Statistical analyses
Descriptive statistics included median (IQR) for continuous variables and frequencies and proportions for categorical variables. Unadjusted comparisons of baseline characteristics and neonatal morbidities among infants grouped by treatment received (TCPC vs surgery) were performed using chi-squared or Fisher’s exact tests for categorical variables and analysis of variance or Kruskal-Wallis tests for continuous variables.
To assess the relationship between PDA procedural treatment strategy and outcomes, we performed a multivariable logistic regression analysis with the outcome of severe NDI or death adjusting for the center, birth year, gestational age (in weeks), birth weight (in grams) and postnatal age (in days) at PDA intervention.
Statistical significance was defined as a two-sided p-value < 0.05 with no adjustment for multiple testing. All analyses were done using SAS (v. 9.4).
Ethics approval and consent to participate
Participating centers received local institutional review board (IRB) approval for data collection. Per individual IRB requirements, data were collected under a waiver of consent or after informed consent was obtained from parents or legal guardians. All study procedures were performed in accordance with the relevant guidelines and regulations.
Results
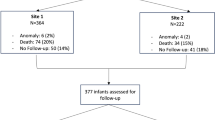
The study cohort included 378 infants who received procedural closure of their PDA. TCPC and surgical ligation were performed on 99 and 279 infants, respectively (Fig. 1). Neurodevelopmental follow up data were available for 78 and 197 infants respectively.
Characteristics of infants in these groups are presented in Table 1. Median gestational age (25.0 vs 24.7 weeks) and birth weight (690 vs 680 g) were not significantly different between the TCPC and surgical ligation groups. The median age at TCPC was almost twofold greater than the age at ligation (62 vs. 32 days, p < 0.01). There were more females in the TCPC group compared to the surgical ligation group. Median postmenstrual age was 33.9 weeks for TCPC and 29.1 for surgical ligation. The incidence of intraventricular hemorrhage was similar between the groups, but there was more periventricular leukomalacia in the TCPC group (16% vs 8%, p = 0.03). Data on PDA treatment and inpatient morbidities are presented in Table 2.
The primary outcome of death or severe NDI occurred in 49% of infants in TCPC group and 40% in surgical ligation group. Death before neurodevelopmental follow-up occurred in 3% of TCPC group compared to 6% in surgical ligation group but this difference was not statistically significant. Severe NDI among survivors was higher in the TCPC group compared to surgical ligation (47% vs 34%, p = 0.04) (Table 3).
In the adjusted analysis, there was no difference in odds of death or severe NDI between the transcatheter closure and surgical ligation groups (aOR 1.12 [95% CI: 0.55–2.26]) (Table 4). Similarly, there was no difference in odds of death before follow up (aOR 1.41 [95% CI: 0.67–2.96]) or severe NDI (aOR 0.19 [95% CI: 0.03–1.11]) between groups.
Discussion
This multicenter retrospective cohort study showed that the odds of severe NDI or death were similar among extremely preterm infants undergoing TCPC and surgical ligation. This is the first large multicenter study to evaluate 2-year neurodevelopmental outcomes in preterm infants undergoing TCPC.
Our results are consistent with the findings from two studies comparing neurodevelopmental outcomes of infants undergoing TCPC compared to surgical ligation [16, 17]. It should be noted that these comparison studies were single-center studies with small sample sizes and grossly underpowered. The study by Fernandez and colleagues [16] reported similar timing of PDA procedural closure to the current study, where surgical ligation was performed at an earlier age compared to TCPC.
Patients undergoing surgical PDA ligation compared to patients undergoing TCPC have been shown to be more likely to require longer duration of general anesthesia, have increased need for pain and sedation medications, have longer duration of mechanical ventilation and have increased risk for post-ligation cardiac syndrome and recurrent laryngeal nerve palsy [21]. Theoretically, the increased likelihood of adverse effects after surgical ligation may place this population at increased risk of developing neurodevelopmental impairment. Evidence suggests that suboptimal cerebral oxygenation due to prolonged PDA shunting may affect brain growth leading to adverse neurodevelopmental outcomes [22, 23]. Of note, infants in the TCPC group had higher incidence of PVL (16% vs 8%) and higher incidence of severe NDI (47% vs 34%) compared to the surgical ligation group. However, the TCPC group had much later median age of intervention and so presumably had a greater duration of PDA shunt exposure. Whether earlier TCPC resulting in shorter duration of altered cerebral oxygenation could have led to improved neurodevelopmental outcomes is not known.
Since the FDA approval of the Amplatzer Piccolo Occluder in 2019, a device for TCPC in infants as small as 700 g, TCPC has been increasingly utilized as a strategy to achieve definitive closure of the PDA [1,2,3]. In more recent years, TCPC has surpassed surgical ligation as the most common method of procedural closure [1,2,3, 9, 10]. The safety profile of TCPC has improved over time with increasing case volume and expertise [24]. Along with the rapid adoption of TCPC, the age and size of infants undergoing TCPC have declined over time [2, 3]. It should be noted that this study includes subjects born only until 12/31/2019 indicating that likely a small percentage of infants could have gotten the Amplatzer Piccolo Occluder as centers were adapting the use of this device into clinical practice.
Beyond how TCPC compares to surgical ligation, it is important for neonatologists and interventional cardiologists to understand how TCPC compares to conservative management of hemodynamically significant PDA. This question is currently being addressed by the ongoing clinical trial “Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Low Weight Infants (PIVOTAL).” [25] PIVOTAL is assessing neurodevelopmental outcomes at 36 weeks’ postmenstrual age and 3-4 months’ corrected age as secondary outcomes.
Strengths of this study include its large sample size compared to previously published studies. Data were prospectively collected by trained research staff which is another important strength of the study. However, our results have several important limitations. First, there was no standardized echocardiography protocol across NRN sites nor a standardized definition of a hemodynamically significant PDA. Second, neither the timing of diagnosis of PDA nor the burden (severity or duration) of shunt exposure was available. Third, the study cohort included infants born through the end of 2019 and so describe several years prior to widespread adoption of the Amplatzer Piccolo Occluder into clinical practice. Fourth, confounding by indication and residual confounding by factors not accounted for in our models (eg., PDA-targeted medication exposure, severity of illness contributing to patency of the PDA) could have biased our results. Fifth, occurrence of post-ligation cardiac syndrome (or post-transcatheter cardiorespiratory syndrome) and development of chronic pulmonary hypertension, both of which could be associated with adverse neurodevelopmental outcomes [21, 26], were not recorded in our dataset.
Conclusion
These data provide the first large multisite comparison of TCPC and surgical ligation on 2-year follow-up outcomes including neurodevelopmental impairment. As the age at TCPC declines, our findings should be re-evaluated in prospective studies that include infants receiving non-procedural treatment of the PDA as the comparison group and with better assessment of the magnitude and duration of PDA shunt physiology and related hemodynamic factors.
Data Sharing
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
Data availability
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
References
Kaluarachchi DC, Rysavy MA, Carper BA, Chock VY, Laughon MM, Backes CH, et al. Secular Trends in Patent Ductus Arteriosus Management in Infants Born Preterm in the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 2024;266:113877.
Lai KC, Richardson T, Berman D, DeMauro SB, King BC, Lagatta J, et al. Current Trends in Invasive Closure of Patent Ductus Arteriosus in Very Low Birth Weight Infants in United States Children’s Hospitals, 2016-2021. J Pediatr. 2023;263:113712.
Shah ZS, Clark RH, Patt HA, Backes CH Jr, Tolia VN. Trends in Procedural Closure of the Patent Ductus Arteriosus among Infants Born at 22 to 30 Weeks’ Gestation. J Pediatr. 2023;263:113716.
Clyman RI, Benitz WE. Trans catheter patent ductus arteriosus closure— will history repeat itself? J Perinatol. 2019;39:1435–6.
Backes CH, Giesinger RE, Rivera BK, Berman DP, Smith CV, Cua CL, et al. Percutaneous closure of the patent ductus arteriosus in very low weight infants: considerations following US Food and Drug Administration approval of a novel device. J Pediatr. 2019;213:218–21.
Ogando AR, Asensio IP, Rodriguez Sanchez de la Blanca A, Tejerizo FB, Luna MS, Jaurena JMG, et al. Surgical ligation versus percutaneous closure of patent ductus arteriosus in very low-weight preterm infants: which are the real benefits of the percutaneous approach? Pediatr Cardiol. 2018;39:398–410.
Sathanandam S, Balduf K, Chilakala S, Washington K, Allen K, Knott- CraigC, et al. Role of transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv. 2019;93:89–96.
Regan W, Benbrik N, Sharma SR, Auriau J, Bouvaist H, Bautista Rodriguez C, et al. Improved ventilation in premature babies after transcatheter versus surgical closure of patent ductus arteriosus. Int J Cardiol. 2020;311:22–27.
Kuntz MT, Staffa SJ, Graham D, Faraoni D, Levy P, DiNardo J, et al. Trend and Outcomes for Surgical Versus Transcatheter Patent Ductus Arteriosus Closure in Neonates and Infants at US Children’s Hospitals. Journal Am Heart Assoc. 2022;11:e022776.
Chock VY, Bhombal S, Davis AS, Sankar MN, Do BT, Laughon MM, et al. Respiratory Outcomes After Transcatheter vs Surgical Patent Ductus Arteriosus Closure in Preterm Infants. JAMA Netw Open. 2025;8:e2513366.
Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Trial of Indomethacin Prophylaxis in Preterms Investigators. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150:229–234, 234.e1.
Wickremasinghe AC, Rogers EE, Piecuch RE, Johnson BC, Golden S, Moon-Grady AJ, et al. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr. 2012;161:1065–72.
Madan JC, Kendrick D, Hagadorn JI, Frantz ID. Patent Ductus Arteriosus Therapy: Impact on Neonatal and 18-Month Outcome. Pediatrics. 2009;123:674–81.
Bravo MC, Ybarra M, Madero R, Pellicer A. Childhood Neurodevelopmental Outcome in Low Birth Weight Infants With Post-ligation Cardiac Syndrome After Ductus Arteriosus Closure. Front Physiol. 2019;10:718.
Weisz DE, Mirea L, Rosenberg E, Jang M, Ly L, Church PT, et al. Association of Patent Ductus Arteriosus Ligation with Death or Neurodevelopmental Impairment Among Extremely Preterm Infants. JAMA Pediatr. 2017;171:443–9.
Fernandez MC, Kase JS, Giamelli J, Reichlin A. Morbidity and neurodevelopmental outcomes at 2 years in preterm infants undergoing percutaneous transcatheter closure vs. surgical ligation of the PDA. J Perinatol 2024; https://doi.org/10.1038/s41372-024-02019-w.
Koenigsaecker T, Callas C, McElroy S, Ing F, Rottkamp CA. Neurodevelopmental Outcomes Following Transcatheter Occlusion of PD A versus Surgical Ligation. Pediatric Academic Societies Meeting. 2022.
Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition. Harcourt Assessment; 2006.
Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD. Follow-up Study Group of Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Improving the Neonatal Research Network annual certification for neurologic examination of the 18-22 month child. J Pediatr. 2012;161:1041–6.
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23.
Weisz DE, More K, McNamara PJ, Shah PS. PDA ligation and health outcomes: a meta-analysis. Pediatrics. 2014;133:e1024–46.
Lemmers PMA, Benders MJNL, D’Ascenzo R, Zethof J, Alderliesten T, Kersbergen K, et al. Patent ductus arteriosus and brain volume. Pediatrics. 2016;137. e20153090.
Lemmers P, Vijlbrief D, Benders M, Alderliesten T, Veldhuis M, Baerts W, et al. Delayed Surgical Closure of the Patent Ductus Arteriosus: Does the Brain Pay the Price? J Pediatr. 2023;254:25–32.
Bischoff AR, Kennedy KF, Backes CH, Sathanandam S, McNamara PJ. Percutaneous closure of the patent ductus arteriosus in infants ≤2 kg: IMPACT Registry insights. Pediatrics. 2023;152:e2023061460.
Nakanishi H, Uchiyama A, Kasuda S. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: A cohort study. J Perinatol. 2016;36:890–6.
Acknowledgements
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network for the Generic Database and the Follow-up Study. NICHD staff provided input into the study design, conduct, analysis, and manuscript drafting; NCATS cooperative agreements provided infrastructure support to the NRN. While NICHD staff had input into the study design,conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data included in this study. On behalf of the NRN, RTI International had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chair: Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-2023). Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904) – Abbot R. Laptook, MD; Martin Keszler, MD; Betty R. Vohr, MD; Angelita M. Hensman, PhD RNC-NIC; Elisa Vieira, BSN RN; Lucille St. Pierre, BS; Barbara Alksninis, RNC PNP; Andrea Knoll; Mary L. Keszler, MD; Teresa M. Leach, MEd CAES; Elisabeth C. McGowan, MD; Victoria E. Watson, MS CAS. Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364) – Anna Maria Hibbs, MD MSCE; Michele C. Walsh, MD MS; Nancy S. Newman, RN; Deanne E. Wilson-Costello, MD; Bonnie S. Siner, RN; Elizabeth Roth, PhD. Children’s Mercy Hospital (UG1 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Allison Scott, BSN RNC-NIC; Prabhu S. Parimi, MD; Lisa Gaetano, RN MSN. Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (UG1 HD27853, UL1 TR77) – Stephanie L. Merhar, MD MS; Brenda B. Poindexter, MD MS; Kurt Schibler, MD; Tanya E. Cahill, MD; Cathy Grisby, BSN CCRC; Kristin Kirker, CRC; Sandra Wuertz, RN BSN CLC; Juanita Dudley, RN BSN; Julia Thompson, RN BSN; Lisa Henkes, RN BSN; Sara Stacey, BA; Devan Hayes, BS. Duke University School of Medicine, University Hospital, University of North Carolina, Duke Regional Hospital, WakeMed Health & Hospitals, and Maynard Children’s Hospital at ECU Health (UG1 HD40492, UL1 TR1117) – C. Michael Cotten, MD MHS; Ronald N. Goldberg, MD; William F. Malcolm, MD; Patricia L. Ashley, MD; Deesha Mago-Shah, MD; Mollie Warren, MD; Joanne Propst, RN JD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Kathryn E. Gustafson, PhD; Caitlin Stone, MA; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Cindy Clark, RN; Diane Warner, MD MPH; Janice Wereszczak, CPNP-AC/PC; Andrea Trembath, MD MPH; Jennifer Talbert, MS RN; Michael O’Shea, MD MPH; Stephen D. Kicklighter, MD; Alexandra Bentley, MD; Laura Edwards, MD; Ginger Rhodes-Ryan, ARNP MSN, NNP-BC; Donna White, RN-BC BSN; Ryan Moore, MD; Kelly Bear, MD; Uduak Akpan, MD; Vickie Bergstedt, RN; Sherry Moseley, RN. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851, UL1 TR454) – Ravi M. Patel, MD MSc; David P. Carlton, MD; Ira Adams-Chapman, MD (deceased); Yvonne Loggins, RN; Diane Bottcher, RN; Sheena L. Carter, PhD; Salathiel Kendrick-Allwood, MD; Maureen Mulligan LaRossa, RN; Judith Laursen, RN; Colleen Mackie, RRT; Amy Sanders, PsyD; Gloria Smikle, PNP; Lynn Wineski, NNP. Eunice Kennedy Shriver National Institute of Child Health and Human Development – Michele C. Walsh, MD MS; Andrew A. Bremer, MD PhD; Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA. Indiana University, Methodist Hospital, and Riley Hospital for Children (UG1 HD27856, UL1 TR6) – Gregory M. Sokol, MD; Abbey C. Hines, PsyD; Dianne E. Herron, RN CCRC; Donna Watkins, MSN NNP-BC; Susan Gunn, NNP-BC CCRC; Jeff Joyce, CCRC (deceased). McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, and Memorial Hermann Southwest Hospital (U10 HD21373, UG1 HD87229) – Jon E. Tyson, MD MPH; Amir M. Khan, MD; Kathleen A. Kennedy, MD MPH; Barbara J. Stoll, MD; Ricardo A. Mosquera, MD MS; Andrea F. Duncan, MD MS; Elizabeth Eason, MD; Nora I. Alaniz, BS; Fatima Boricha, MD; Allison G. Dempsey, PhD; Donna J. Hall, RN; Janice John, CPNP; Karen Martin, RN; Georgia E. McDavid, RN, Kimberly Rennie, PhD; Tina Reddy, MD; Debasree Sana Boral, BS IMG; Daniel K. Sperry, RN; Emily Stephens, BSN RNC-NIC; Michelle White, BSN RNC-NIC; Sharon L. Wright, MT (ASCP); Dinorah Zanger, PhD. Nationwide Children’s Hospital, The Abigail Wexner Research Institute at Nationwide Children’s Hospital, Center for Perinatal Research, The Ohio State University College of Medicine, The Ohio State University Wexner Medical Center, and Riverside Methodist Hospital (UG1 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Jonathan L. Slaughter, MD MPH; Sudarshan R. Jadcherla, MD; Nathalie L. Maitre, MD PhD; Christopher Timan, MD; Keith O. Yeates, MD PhD; Patricia Luzader, RN; Julie Gutentag, RN BSN; Jennifer L. Grothause, BA RN BSN; Melanie Stein, RRT BBS; Rox Ann Sullivan, RN BSN; Cole D. Hague, BA MS; Helen Carey, PT DHSc PCS; Michelle Chao, BS; Stephanie Burkhardt, BS MPH; Margaret Sullivan, BS; Lina Yossef-Salameh, MD; Mary Ann Nelin, MD; Erna Clark, BA; Julie C. Shadd, BSN RD; Courtney Park, RN BSN; Courtney Cira, BS; Erin Fearns; Kristi Small, BS; Sarah A. Keim, PhD MA MS; Christine A. Fortney, RN PhD; Aubrey Fowler, BS, Jacqueline McCool; Lindsay Pietruszewski, PT DPT; Jessica Purnell, BS CCRC; Kyrstin Warnimont, BS; Laura Marzec, MD; Bethany Miller, RN BSN; Demi R. Beckford, MHS; Hallie Baugher, BS MSN; Julia Newton, MPH; Katelyn Levengood, PT DPT; Nancy Batterson, OT/L; Brittany DeSantis, BS. RTI International (UG1 HD36790) – Abhik Das, PhD; Carla M. Bann, PhD; Dennis Wallace, PhD; Marie G. Gantz, PhD; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS; Jenna Gabrio, BS MPH; Jamie E. Newman, PhD MPH; Lindsay Parlberg, BS; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN; Amanda Lewis. Stanford University, El Camino Hospital, and Lucile Packard Children’s Hospital (UG1 HD27880, UL1 TR93) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC;; Dharshi Sivakumar, MD; Dona Bahmani, CRC; Barbara P. Recine, MA; Jennifer E. Chuck, MS; Lilia Rutkowska, MA; Barbara Bentley, PsychD MSEd; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP PhD; Beth Earhart, PhD; Lynne C. Huffman, MD; Casey E. Krueger, PhD; Ryan E. Lucash, PhD; Melinda S. Proud, RCP; Elizabeth N. Reichert, MA CCRC; Heather Taylor, PhD; Hali E. Weiss, MD; R. Jordan Williams, MD. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Fred J. Biasini, PhD; Shirley S. Cosby, RN BSN; Kristy A. Domnanovich, PhD; Chantel J. Jno-Finn, PT DPT; Morissa Ladinsky, MD; Tara E. McNair, RN BSN; Kimberlly Stringer, MD MPH; Sally Whitley, MA OTR-L FAOTA; Sheree York Chapman, PT DPT PCS; Cindie L. Buie, RN BSN; Kelli P. Hagood, RN BSN. University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Neha Kumbhat MD MS Epi; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN. University of Iowa, Mercy Medical Center, and Sanford Health (UG1 HD53109, UL1 TR442) –Jane E. Brumbaugh, MD; Heidi M. Harmon, MD; Karen J. Johnson, RN BSN; Mendi L. Schmelzel, RN MSN; Jacky R. Walker, RN; Claire A. Goeke, RN; Diane L. Eastman, RN CPNP MA; Michelle L. Baack, MD; Laurie A. Hogden, MD; Megan M. Henning, RN; Chelsey Elenkiwich, BSN RN; Megan Broadbent, RN BSN; Sarah Van Muyden, RN BSN; Dan L. Ellsbury, MD; Tracy L. Tud, RN. University of New Mexico Health Sciences Center (UG1 HD53089, UL1 TR41) – Janell Fuller, MD; Kristi L. Watterberg, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Carol Hartenberger, BSN MPH; Sandra Sundquist Beauman, MSN RNC; Mary Ruffner Hanson, RN BSN; Jean R. Lowe, PhD; Elizabeth Kuan, RN BSN. University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, Children’s Hospital of Philadelphia, and Virtua Voorhees Hospital (UG1 HD68244) – Sara B. DeMauro, MD MSCE; Eric C. Eichenwald, MD; Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Soraya Abbasi, MD; Aasma S. Chaudhary, BS RRT; Toni Mancini, RN BSN CCRC; Sarvin Ghavam, MD; Megan A. Dhawan, MSN CRNP; Christine Catts, CRNP; Jonathan Snyder, RN BSN, Kristina Ziolkowski, CMA(AAMA) CCRP. University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, UL1 TR42) – Carl T. D’Angio, MD; Ronnie Guillet, MD PhD; Gary J. Myers, MD; Anne Marie Reynolds, MD; Holly I.M. Wadkins; Michael G. Sacilowski, BS; Rosemary L. Jensen; Joan Merzbach, LMSW; William Zorn, PhD; Osman Farooq, MD; Stephanie Guilford, BS; Kelley Yost, PhD; Mary Rowan, RN; Diane Prinzing; Ann Marie Scorsone, MS CCRC; Michelle Hartley-McAndrew, MD; Kyle Binion, BS; Constance Orme; Premini Sabaratnam, MPH; Alison Kent, BMBS FRACP MD; Rachel Jones; Elizabeth Boylin, BA; Daisy Rochez, BS MHA; Emily Li, BA; Jennifer Kachelmeyer, BS; Kimberly G. McKee, BS; Kelly R. Coleman, PsyD; Deanna Maffett, RN; Satyan Lakshminrusimha, MD. University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689) – Myra H. Wyckoff, MD; Luc P. Brion, MD; Roy J. Heyne, MD; Diana M. Vasil, MSN BSN RNC-NIC; Maria M. De Leon, RN BSN; Frances Eubanks, RN BSN; E. Rebecca McDougald, MSN APRN CPNP-PC/AC; Pollieanna Sepulveda, RN; Alicia Guzman; Elizabeth Heyne, PsyD PA-C; Lizette E. Lee, RN; Anna Puentez, MSN RN CPNP-PC; Azucena Vera, AS; Jillian Waterbury, DNP RN CPNP-PC; Cathy Twell Boatman, MS CIMI; Kristine Tolentino-Plata, MS. University of Utah Medical Center, Intermountain Medical Center, McKay-Dee Hospital, Utah Valley Hospital, and Primary Children’s Medical Center (UG1 HD87226, UL1 TR105) – Bradley A. Yoder, MD; Mariana Baserga, MD MSCI; Sarah Winter, MD; Stephen D. Minton, MD; Mark J. Sheffield, MD; Carrie A. Rau, RN BSN CCRC; Shawna Baker, RN; Jill Burnett, RNC BSN; Susan Christensen, RN; Sean D. Cunningham, PhD; Brandy Davis, RN BSN; Jennifer O. Elmont, RN BSN; Becky Hall, APRN; Erika R. Jensen, APRN; Jamie Jordan, RN BSN; Manndi C. Loertscher, BS CCRP; Trisha Marchant, RNC BSN; Earl Maxson, RN CCRN; Kandace M. McGrath, BS; Hena G. Mickelsen, BA; Galina Morshedzadeh, BSN APRN; D. Melody Parry, RN BSN; Susan T. Schaefer, RN BSN RRT; Kelly Stout, PhD; Ashley L. Stuart, PhD; Katherine Tice, RN BSN; Kimberlee Weaver-Lewis, RN MS; Kathryn D. Woodbury, RN BSN; Erick B. Gerday, MD; Laura Cole Bledsoe, RN; Kathleen Coleman, RN; Barbara L. Francom, BSN RN; Brixen A. Reich, RN, MSN. Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (UG1 HD21385) – Seetha Shankaran, MD; Beena G. Sood, MD MS; Athina Pappas, MD; Monika Bajaj, MD; Melissa February, MD; Prashant Agarwal, MD; Sanjay Chawla, MD; Rebecca Bara, RN BSN; Kirsten Childs, RN BSN; Eunice Hinz Woldt, RN MSN; Laura Goldston, MA, John Barks, MD; Stephanie A. Wiggins, MS; Mary K. Christensen, BA RRT; Martha Carlson, MD; Diane F. White, RRT CCRP.
Funding
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880,UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68270, UG1 HD68278, UG1 HD68263, UG1 HD68284; UG1 HD87226, UG1 HD87229) and the National Center for Advancing Translational Sciences (NCATS) (UL1 TR6, UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR105, UL1 TR442, UL1 TR454, UL1 TR1117, provided grant support for the Neonatal Research Network, including for the Follow-up Study.
Author information
Authors and Affiliations
Contributions
Dinushan C. Kaluarachchi: Methodology, Conceptualization, Writing original draft. Valerie Y. Chock: Methodology, Conceptualization, Reviewing & editing manuscript. Barbara T. Do: Writing –Methodology, Formal analysis, Data curation, Conceptualization, Reviewing & editing manuscript. Matthew A. Rysavy: Methodology, Conceptualization, Reviewing & editing manuscript. Meera N. Sankar: Methodology, Conceptualization, Reviewing & editing manuscript, Matthew M. Laughon: Methodology, Conceptualization, Reviewing & editing manuscript. Carl H. Backes: Methodology, Conceptualization, Reviewing & editing manuscript. Tarah T. Colaizy: Methodology, Conceptualization, Reviewing & editing manuscript, Edward F. Bell: Methodology, Conceptualization, Reviewing & editing manuscript. Patrick J. McNamara: Methodology, Conceptualization, Supervision, Reviewing & editing manuscript. Susan R Hintz: Methodology, Conceptualization, Supervision, Reviewing & editing manuscript. Girija Natarajan: Methodology, Conceptualization, Supervision, Reviewing & editing manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Kaluarachchi serves as a consultant for ONY Biotech Inc. Dr. Backes has following disclosures—Abbott Laboratories: funding of ongoing multicenter trial, in partnership with National Institutes of Health, entitled PIVOTAL (NCT05547165). Abbott Laboratories has no part in the design or execution of PIVOTAL. Dr. McNamara serves as a consultant for Aspect Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaluarachchi, D.C., Chock, V.Y., Do, B.T. et al. Comparison of neurodevelopmental outcomes of extremely preterm infants undergoing trans-catheter closure of the patent ductus arteriosus compared to surgical ligation. J Perinatol (2025). https://doi.org/10.1038/s41372-025-02417-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-025-02417-8