Abstract
Although airway fibrosis and epithelial–mesenchymal transition (EMT) contribute to airway remodeling in chronic obstructive pulmonary disease (COPD), the mechanisms underlying their development have not been fully elucidated. In the present study, we aimed to assess heparin-binding epidermal growth factor (HB-EGF) expression in the airways of patients with COPD and to elucidate the possible role of HB-EGF in the pathology of COPD. Sputum and lung tissue HB-EGF expression was evaluated in control subjects and patients with COPD. The relationships between HB-EGF expression, disease severity, collagen deposition (fibrosis), and EMT were investigated. In vitro, human bronchial epithelial (HBE) cells and lung fibroblast cells exposed to the recombinant HB-EGF, collagen deposition and EMT were assessed. We found that sputum HB-EGF expression was significantly increased in patients with COPD compared with non-smokers and smokers without COPD. There was a significant positive correlation between sputum HB-EGF and COPD assessment test (CAT) score. HB-EGF expression was significantly increased in the lung tissue samples of patients with COPD and associated with collagen deposition and N- and E-cadherin, and vimentin expression. In vitro, HB-EGF promoted collagen production in lung fibroblasts. Moreover, HB-EGF induced the EMT process through induction of N-and E-cadherin, and vimentin expression in HBE cells. Collectively, HB-EGF induces airway remodeling by modulating airway fibrosis and pulmonary EMT, and contributes to the COPD severity. The current data may provide insight into the underlying pathogenesis of COPD, in which HB-EGF has an important pathogenic role.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory respiratory condition, characterized by progressive airflow limitation that is not fully reversible [1]. Airway fibrosis and epithelial–mesenchymal transition (EMT) contribute to airflow limitation and resistant to current therapies, including corticosteroids [2, 3]. Despite advances in understanding the inflammatory features of COPD, the mechanisms underlying the airway fibrosis and EMT remain poorly understood.
Heparin-binding epidermal growth factor (HB-EGF) is a member of the epidermal growth factor (EGF) superfamily [4]. HB-EGF is a potent chemotactic factor for epithelial, smooth muscle, and mesenchymal stem cells [4, 5]. Wang et al. [6] reported the level of HB-EGF expression correlated to airway smooth muscle (ASM) mass thickening, and that it induced ASM migration and proliferation in vitro. Hassan et al. [7] showed that elevated HB-EGF expression within the asthmatic ASM mass occurred. However, information is still lacking regarding whether HB-EGF occurs at an early stage of disease or accelerates COPD disease progression.
The airway epithelium assures frontline innate defense mechanisms via a physical barrier and secretion of protective factors. Epithelial–mesenchymal transition (EMT) is a process through which epithelial cells that normally interact with basement membranes via their basal surface undergo multiple biochemical changes [8, 9]. EMT is involved in bronchial remodeling and loss of lung function [10]. Peribronchial and subepithelial fibrosis in peripheral airways are major causes for airway remodeling in COPD and are responsible for the airflow limitation. It has been previously shown that increased extracellular matrix (ECM) deposition is a key structural change of the remodeled airway wall and drives the cellular processes of proliferation, migration, repair, and inflammation [2, 3, 11]. Among lung resident cells, fibroblasts play a key role in tissue remodeling and fibrosis. Fibroblast-mediated airway fibrosis and ECM protein deposition play an important role in peripheral airway narrowing in COPD [3, 11]. However, whether HB-EGF participates in EMT’s functional consequences and fibrosis in the COPD patients’ conducting airways remains unclear.
Based on this evidence, we designed the present study to determine three points: (1) whether the levels of sputum HB-EGF are elevated in COPD patients’ airways and to identify its correlations to clinical parameters (such as lung function and disease severity); (2) whether HB-EGF expression is upregulated in COPD patients’ lung tissues and to identify its correlations to airway fibrosis and EMT; and (3) human lung fibroblasts and bronchial epithelial (HBE) cells were stimulated by HB-EGF in order to analyze collagen deposition and EMT.
Materials and methods
Patient selection and clinical data
To investigate the levels of HB-EGF in the airways, a total of 35 smokers with stable COPD as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [12], who had a postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio <0.7 were recruited for the induced sputum study after receiving written informed consent. Patients didn’t receive oral or intravenous corticosteroids or any other anti-inflammatory drugs during the preceding 4 weeks. Stable COPD was defined as no change in patients’ treatment course for four or more weeks with no evident acute COPD exacerbation during that same time period [12]. We also recruited 20 age-matched non-smokers with normal spirometry and 26 smokers with normal spirometry (as controls) from the communities surrounding our hospital. They were free of respiratory tract infection during. the 4 weeks prior to the study. Patients’ and controls’ characteristics are shown in Table 1.
To investigate the expression of HB-EGF in the lung tissue and identify its correlation to airway epithelial–mesenchymal transition (EMT) and fibrosis, lung tissue specimens were obtained from the subpleural parenchyma of the lobe that was resected at surgery. Specimens were dissected at a distance of ≥5 cm away from the tumor. Patients who had respiratory infections or received oral or intravenous corticosteroids during the preceding 4 weeks, a previous history of other cancer, and had undergone radiotherapy or chemotherapy were excluded. A total of nine non-smokers with normal spirometry, 10 smokers without COPD, and 30 smokers with COPD were enrolled. Twelve GOLD I/II patients and 18 GOLD III/IV patients had undergone lung cancer surgery. Patients’ characteristics are shown in Table 2. Primary human lung fibroblasts were isolated from lung parenchyma from a subgroup (n = 4) of these subjects.
The corresponding protocol was approved by the Ethics of Research Committee of the Medical University of Guangdong. Informed and written consents were obtained from all participating subjects, which was in accordance with the Declaration of Helsinki.
Questionnaires
The COPD assessment test (CAT) consists of eight items with scores ranging from 0 to 5 (0 = no impairment, 5 = greatest impairment). An overall score is calculated by adding the score from each item with total scores ranging from 0 to 40. Higher scores indicated more severe health status impairment or a poorer COPD control [12].
Collection and processing of induced sputum
Sputum was induced as previously described [13]. Briefly, induced sputum from each subject was collected by means of inhalation of a nebulized 3% saline solution over a 2-min period. Three volumes of dithiothreitol were added to sputum, and the sample was mixed vigorously on a shaker plate for 15 to 20 min in order to solubilize mucus. Cell counting was performed by an observer blind to the clinical characteristics of the subjects. A sample was considered adequate when the patient was able to expectorate at least 2 ml of sputum, and slides contained <10% squamous cells on differential cell counting. The percentage of sputum inflammatory cells was used for analysis. Sputum supernatants were kept at −80 °C for further measurement of cytokines.
Laboratory measurements
Sputum HB-EGF levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Uscn Life Science, Inc., Wuhan, China). The minimum detection limit of the HB-EGF assay was 1.5 pg/ml.
Immunohistochemistry
HB-EGF expression (Abcam, Cambridge, UK), N-cadherin and E-cadherin (Proteinthech, Chicago, USA), and vimentin (D21H3) (CST, Beverly, MA, USA) in lung tissues were investigated by immunocytochemical examination. Quantitative measurements of positive cells in the lung tissue were performed according to previously described methods [14]. Positive cells were expressed as a percentage of total cells. Peribronchial collagen deposition was detected by Masson trichrome staining. The area of peribronchial Masson trichrome staining (blue color) was visualized and was quantified by the software as a percentage of the total band area as previously described [15]. Sections were examined using a light microscope (BX51; Olympus, Japan) and quantified by computer algorithm using the Image Pro 6.1 software (Media Cybernetics).
Immunofluorescence
Immunofluorescent staining was performed on paraffin-embedded lung tissue sections. The antibodies that were used in appropriate combinations were rabbit anti-human collagen I antibody (Proteinthech, Chicago, USA), rabbit anti-human collagen III antibody (Proteinthech, Chicago, USA), and mouse anti-human α-SMA antibody (Abcam, Cambridge, UK).
Western blotting assay
Western blot analysis was used to detect changes in collagen types I and III, α-smooth muscle actin (SMA), mitogen-activated protein kinase (ERK/MAPK), phosphorylated ERK (p-ERK), p38, phosphorylated (p-p38) (Cell Signaling Technology, USA), N-cadherin and E-cadherin (Proteinthech, Chicago, USA), and vimentin (D21H3) (CST, Beverly, MA, USA) as previously described [11].
Cell viability assays, migration and proliferation
To investigate HB-EGF’s effects on tissue fibrosis, human lung fibroblasts were treated with different doses of recombinant human HB-EGF protein (R&D Systems, Minneapolis, USA) for 48 h. Cells were seeded into a 96-well plate at a density of 1 × 104 cells/well. The cells were assayed with a cell counting kit (CCK)-8 (Liankebio, Hangzhou, China), which was used in order to determine cell proliferation according to the manufacturer’s instructions. A ‘scratch-wound’ assay was used to assess fibroblast migration fibroblast as described previously [16].
Statistical analysis
Data were presented as the mean ± SEM or median (IQR) unless otherwise stated. Statistical analyses were performed using the parametric two-tailed Student’s t-test or non-parametric Mann–Whitney U-test for the comparison of two groups and ANOVA with post-hoc Tukey’s corrections for the comparison of more than two groups. Spearman’s rank correlation was used to test for dependence between two numeric variables. Statistical analysis was performed using GraphPad Prism 7.0 software (San Diego, CA, USA). p < 0.05 was considered statistically significant.
Results
Associations between sputum HB-EGF levels and clinical parameters
HB-EGF levels were significantly higher in patients with COPD than those smokers without COPD and non-smokers (34.2 ± 1.5 pg/ml vs. 20.4 ± 1.3 pg/ml, p < 0.01 and 34.2 ± 1.5 pg/ml vs. 12.4 ± 1.0 pg/ml, p < 0.001, respectively). HB-EGF expression in the sputum of current smokers with COPD was significantly higher than ex-smokers with COPD (38.2 ± 2.0 pg/ml vs. 28.4 ± 1.7 pg/ml, p = 0.036; Fig. 1a). Moreover, patients with GOLD III/IV had significantly higher HB-EGF levels than patients with GOLD I/II (39.4 ± 1.9 pg/ml vs. 29.3 ± 1.6 pg/ml, p = 0.0004; Fig. 1b). Although sputum HB-EGF levels were not associated with CAT scores or lung function (FEV1 %Pred) in patients with GOLD I/II (Fig. 1c, e), HB-EGF levels presented a significant positive association with CAT scores (r = 0.587, p = 0.015), but negative association with FEV1 %Pred in patients with GOLD III/IV (r = −0.602, p = 0.008; Fig. 1d, f). When investigating all patients with COPD, those with HB-EGF levels above the mean (34.2 pg/ml) had higher sputum neutrophils and CAT scores, but lower lung function (FEV1 %Pred, FEV1/FVC) compared to those with HB-EGF levels below the mean (Table 3). Collectively, these results suggest that sputum HB-EGF was increased in patients with COPD and may be associated with disease severity.
HB-EGF expression in the sputum and correlations between sputum HB-EGF expression and disease severity. Sputum HB-EGF levels were quantified using ELISA (a, b). The correlations between the levels of HB-EGF in the sputum and COPD assess test (CAT) scores, and FEV1 %Pred were investigated. The r is the correlation coefficient using Spearman’s test (c–f). Data are presented as mean ± SEM
HB-EGF expression is correlated with collagen deposition
We next investigated HB-EGF expression and collagen deposition in lung tissues of subjects who had undergone surgery for lung tumors. We found that very few immunopositive cells were observed in the lung tissues from the control subjects, whereas there was a significant increase in HB-EGF staining density in COPD patients’ airways when compared with smokers and non-smokers. HB-EGF expression in the lungs of current smokers with COPD was significantly higher than ex-smokers with COPD (40.6 ± 1.3% vs. 33.1 ± 2.7%, p = 0.026; Fig. 2a, b). Moreover, patients with GOLD III/IV had significantly higher HB-EGF levels than patients with GOLD I/II (41.3 ± 1.4% vs. 34.0 ± 2.1%, p = 0.005; Fig. 2c). Patients with COPD showed a tendency for an increase in collagen deposition in small airways when compared with smokers and non-smokers (Fig. 2d). Patients with GOLD III/IV had significantly higher collagen deposition than patients with GOLD I/II (43.3 ± 2.0% vs. 33.7 ± 1.9%, p = 0.003; Fig. 2e). Moreover, HB-EGF immunoreactivity positively correlated with collagen deposition in patients with GOLD III/IV (r = 0.570; p = 0.014; Fig. 2g). However, similar results were not observed in patients with GOLD I/II (r = 0.228, p = 0.474; Fig. 2f).
HB-EGF expression and collagen deposition in the lung tissues of patients with COPD. The localization of HB-EGF in the lung tissues was investigated by immunostaining. Photomicrographs of blue staining showing collagen expression around the small airway walls. Representative photographs are shown (a). (all images are ×400 magnification). HB-EGF expression and collagen deposition in the lung tissues were quantified using the Image Pro 6.1 software (b–e). The relationship between HB-EGF expression and collagen deposition was assessed by Spearman’s test (f, g). Data were presented as the mean ± SEM
HB-EGF induced lung fibroblast activation
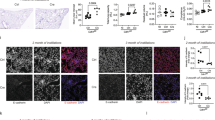
The lung matrix is predominantly deposited by fibroblasts [17, 18]. We next attempted to investigate whether HB-EGF exerted direct effects on lung fibroblasts in vitro. We found that exposure to recombinant human HB-EGF (rhHB-EGF) significantly upregulated lung fibroblast migration capacity and proliferation (Supplementary Fig. 1a, b). Treatment with rhHB-EGF also increased collagen I and collagen III production in fibroblasts (Fig. 3a‒d).
The effect of HB-EGF treatment on human lung fibroblast cells. Collagen I, collagen III production and, α-SMA expression upon stimulation by HB-EGF as determined by immunofluorescence staining (a, c, e) and western blot analysis (b, d, f). Results were expressed as mean ± SEM of three independent experiments
The upregulation of α-SMA expression is a characteristic marker for the fibroblast differentiation into activated fibroblasts (so-called myofibroblasts). Myofibroblasts have a role in the pathogenesis of airway remodeling in COPD [19]. Thus, we further detected α-SMA expression in lung fibroblasts after treatment with rhHB-EGF using immunofluorescence and western blotting. We found that rhHB-EGF significantly caused an increase in fibroblast α-SMA protein expression (Fig. 3e, f). Together, these findings demonstrated that HB-EGF induced fibroblast activation and contributed to the pathogenesis of airway collagen deposition in COPD.
Increased phosphorylation of p-38 and ERK in HB-EGF-induced collagen production
The mitogen-activated protein kinase (ERK/MAPK) family is well known to play a key role in mediating inflammatory responses [11]. To assess whether ERK/MAPK pathways are involved in HB-EGF-induced collagen production, the effect of HB-EGF on activation of p38 and ERK was evaluated by western blotting. As shown in Fig. 4, HB-EGF activated p38 and ERK phosphorylation in a time-dependent and dose-dependent manner.
HB-EGF induced phosphorylation of ERK and p38 in lung fibroblast cells. HB-EGF induced phosphorylation of extracellular signal related kinase (ERK), and p38 in lung fibroblast cells. Stimulation of lung fibroblast cells with 100 ng/ml of recombinant human HB-EGF protein also induced phosphorylation of ERK and p38 in a time-dependent manner (0, 10, 30, 60, 90, and 120 min) (a); Recombinant human HB-EGF protein activated ERK and p38 phosphorylaton in a dose-dependent manner (0, 25, 50, 75, 100, and 150 ng/ml for 2 h) (b)
HB-EGF expression in lung tissues and associated with airway EMT
The percentages of N-cadherin and vimentin positive cells had significantly increased in patients with COPD over that seen in smokers and non-smokers. (Fig. 5a, b; Fig. 6a, c). In contrast, E-cadherin expression had significantly decreased in patients with COPD over that in non-smokers (Fig. 5c; Fig. 6e). N-cadherin expression in GOLD IV was significantly higher than those in GOLD I (Fig. 6b). Although there was a trend to higher E-cadherin and vimentin expression in GOLD IV, there was no significant difference among these different groups (Fig. 6d, f). Moreover, these mesenchymal markers significantly correlated with HB-EGF expression in patients with GOLD III/IV (Fig. 6h, j, l), but similar results were not observed in patients with GOLD I/II (Fig. 6g, i, k).
Correlations between the expression of EMT-related markers in lung tissues and sputum HB-EGF levels. The expression of EMT-related markers (N-cadherin, vimentin, E-cadherin) in lung tissues was quantified using the Image Pro 6.1 software (a–f). The relationships between EMT-related markers and HB-EGF expression were assessed using Spearman’s test. Data were presented as the mean ± SEM
HB-EGF induces EMT-related gene expression in human airway epithelial cells
Using immunofluorescence and western blotting, we detected N-cadherin and vimentin expression in HBE cells after HB-EGF stimulation and found that it significantly increased N-cadherin and vimentin expression in a concentration-dependent manner when compared with untreated control cells (Fig. 7a‒d). In contrast, E-cadherin expression significantly decreased in HBE cells (Fig. 7e, f). Collectively, these data indicate that HB-EGF might induce EMT in HBE cells.
HB-EGF promoted EMT production in HBE cells. HBE cells were treated with HB-EGF for 48 h. Measurements of N-cadherin, Vimentin, and E-cadherin expression upon stimulation by HB-EGF as determined by immunofluorescence staining (a, c, e), and western blot analysis (b, d, f). Results were expressed of three independent experiments. Data were presented as the mean ± SEM of three independent experiments
Discussion
In this study, we demonstrated that HB-EGF expression in sputum and lung tissues had significantly increased in patients with COPD compared to control subjects. HB-EGF expression was associated with disease severity, collagen deposition, EMT-related markers (N-and E-cadherin and vimentin). In vitro, stimulation with HB-EGF promoted collagen production in lung fibroblasts and induced the EMT process in HBE cells. These results suggest that EGF blockade may be a treatment option for airway remodeling in COPD.
COPD is accompanied by systemic inflammation and airflow limitations that occurs as a result of many mechanisms, particularly airway inflammation and smoking. HB-EGF, a member of the EGF superfamily, is expressed mainly in the airway epithelium [4]. In the present study, we found that HB-EGF expression in the sputum and lungs of current smokers with COPD was significantly higher than in the ex-smokers with COPD. Moreover, we observed that lung vimentin expression in current smokers with COPD was significantly higher than ex-smokers with COPD. These results indicated that smoking cessation may improve HB-EGF and vimentin expression in smokers with COPD. However, a large prospective study is needed to assess whether smoking-induced airway inflammation and structural changes are reversible after smoking cessation. Patients with GOLD III/IV had significantly higher HB-EGF levels than patients with GOLD I/II. Moreover, patients with high levels of sputum HB-EGF had higher cytokine levels, CAT scores, and lower lung function compared to those with HB-EGF levels below the mean, thus revealing a cluster of clinical and pathophysiological variables associated with high HB-EGF levels. Together, these results strengthen the view that HB-EGF is implicated in COPD pathogenesis.
The remodeling process involves tissue repair and collagen deposition that contributes to lung function decline and airflow obstruction in COPD [12]. Collagens are synthetized primarily by fibroblasts as precursor molecules with the propeptides being cleaved during the process of secretion of the newly formed collagens [20]. However, it is unknown whether the increased HB-EGF levels participate in fibrosis onset in the small airways and are involved in airway remodeling pathogenesis in COPD. We examined collagen deposition in airways from patients with COPD and controls. There was a significant increase in collagen deposition in airways of COPD patients and correlated closely with HB-EGF expression. A previous study indicated that HB-EGF-promoted airway smooth muscle cell and their progenitor’s migration contribute to airway smooth muscle remodeling in asthmatic mice [5]. These data suggest that HB-EGF is involved in the airway remodeling process, a finding that could help elucidate the mechanism of airway remodeling in COPD.
Peribronchiolar fibrosis is an important feature of COPD and is mainly caused by the increased deposition of extracellular matrix proteins. Collagens are the classical components of the extracellular matrix. Lung fibroblasts have been shown to contribute to airway remodeling in airway diseases via synthesis and secretion of the main ECM components (such as collagens and proteoglycans) [21, 22]. We found that HB-EGF promoted lung fibroblast proliferation, migration, collagen secretion, and α-SMA expression. Given the role of HB-EGF in lung fibroblasts, we sought to determine the key signaling mechanisms by which this occurs. The present study showed MAPK signaling was required for HB-EGF-induced collagen production in lung fibroblasts. Collectively, we presume that HB-EGF could activate lung fibroblasts and its downstream MAPK pathway and promote collagen production, which may enhance the airway remodeling process in COPD.
Airway epithelium represents the first line of defense against microorganisms and acts as a physical barrier between the host and the environment. Airway epithelium is composed of apical tight junctions and underlying adherent junctions [19]. It is now recognized that airway epithelium is involved in a variety of immunological mechanisms by releasing cytokines and interacting with immune cells [19]. EMT is a process essential in wound healing and tissue remodeling after injury [23]. Prevention of EMT is considered an effective measure for the inhibition of tissue remodeling. EMT was observed in several chronic inflammatory airway diseases such as asthma and COPD [19, 20, 23, 24]. Small airway fibrosis is the main contributor to airflow obstruction in COPD. EMT has been implicated in this process, and in large airways, it is associated with angiogenesis. Mahmood et al. showed that there was increased expression of EMT-related markers (EGFR, vimentin, S100A4) in small airways of COPD compared to controls, but this was less than that in large airways [25]. Furthermore, they demonstrated that inhaled corticosteroids (ICS) has an anti-EMT effect in large airway samples in COPD [26]. Our study showed that the expression of N- and E-cadherin as a marker of EMT was associated with HB-EGF expression in COPD patients. In vitro, stimulation with HB-EGF promoted the EMT process in HBE cells. Collectively, these results suggest that EGF blockade may be a treatment option for airway remodeling in COPD.
There are some limitations to this study that need to be considered. First, a previous study showed that HB-EGF silencing with RNA interference inhibited cell cycle progression in lung cancer cells. HB-EGF was significantly and positively associated with primary lung tumors, and HB-EGF expression predicted a poor survival outcome in patients [27]. Although resected normal tissues >5 cm away from the tumor in our study, it should be noted that HB-EGF influenced tumor growth and metastasis. Therefore, HB-EGF expression in lung tissues should be interpreted cautiously due to this limitation. Second, a previous study showed that EMT-related protein expression was significantly high in the peripheral leading edge of non-small-cell lung cancer and related to tumor characteristics associated with poor prognosis [28]. EMT-related markers were also associated with clinicopathological parameters in adenocarcinomas and squamous cell lung carcinoma [29]. It should be noted that patients are not from a pure COPD population so lung cancer might have affected the results. Therefore, the expression of EMT-related markers in lung tissues should be interpreted cautiously due to this limitation.
In summary, we demonstrated that elevated HB-EGF expression is associated with disease severity in COPD patients. In addition, in vitro data showed that HB-EGF may promote airway remodeling in COPD by modulating airway fibrosis and pulmonary EMT. These findings suggest that HB-EGF may be a therapeutic target for intervention against airway remodeling in COPD.
References
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:347–365.
Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653.
Lai T, Tian B, Cao C, et al. HDAC2 Suppresses IL17A-Mediated Airway Remodeling in Human and Experimental Modeling of COPD. Chest 2017. pii: S001–3692(17)33044-1.
Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–F199.
Wang Q, Li H, Yao Y, et al. HB-EGF-Promoted Airway Smooth Muscle Cells and Their Progenitor Migration Contribute to Airway Smooth Muscle Remodeling in Asthmatic Mouse. J Immunol. 2016;196:2361–7.
Wang Q, Li H, Yao Y, et al. The overexpression of heparin-binding epidermal growth factor is responsible for Th17-induced airway remodeling in an experimental asthma model. J Immunol. 2010;185:834–41.
Hassan M, Jo T, Risse PA, et al. Airway smooth muscle remodeling is a dynamic process in severe long-standing asthma. J Allergy Clin Immunol. 2010;125:1037–.e3.
Gohy ST, Hupin C, Fregimilicka C, et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur Respir J. 2015;45:1258–72.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Bartis D, Mise N, Mahida RY, et al. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–5.
Lai T, Wu D, Chen M, et al. YKL-40 expression in chronic obstructive pulmonary disease: relation to acute exacerbations and airway remodeling. Respir Res. 2016;17:31. 24
GOLD Committee. Global strategy for the diagnosis, management, and prevention of COPD. http://www.goldcopd.org/guidelines-pocket-guide-to-copd -diagnosis.html. Accessed 11 Jan 2016.
Kips JC, Fahy JV, Hargreave FE, et al. Methods for sputum induction and analysis of induced sputum: a method for assessing airway inflammation in asthma. Eur Respir J Suppl. 1998;26:9e12S.
Xanthou G, Alissafi T, Semitekolou M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–8.
Fattouh R, Midence NG, Arias K, et al. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med. 2008;177:593–603.
Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33.
Krimmer DI, Burgess JK, Wooi TK, et al. Matrix proteins from smoke-exposed fibroblasts are pro-proliferative. Am J Respir Cell Mol Biol. 2012;46:34–39.
Kranenburg AR, Willems-Widyastuti A, Moori WJ, et al. Enhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;126:725–35.
Park IH, Kang JH, Shin JM, et al. Trichostatin A Inhibits Epithelial Mesenchymal Transition Induced by TGF-β1 in Airway Epithelium. PLoS ONE. 2016;11:e0162058.
Weber CE, Li NY, Wai PY, et al. Epithelial-mesenchymal transition, TGF-beta, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res. 2012;33:311–8.
Harju T, Kinnula VL, Pääkkö P, et al. Variability in the precursor proteins of collagen I and III in different stages of COPD. Respir Res. 2010;30:165.
Sun C, Zhu M, Yang Z, et al. LL-37 secreted by epithelium promotes fibroblast collagen production: a potential mechanism of small airway remodeling in chronic obstructive pulmonary disease. Lab Invest. 2014;94:991–1002.
Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–20.
Milara J, Peiro T, Serrano A, et al. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–20.
Mahmood MQ, Sohal SS, Shukla SD, et al. Epithelial mesenchymal transition in smokers: large versus small airways and relation to airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2015;10:1515–24.
Sohal SS, Soltani A, Reid D, et al. A randomized controlled trial of inhaled corticosteroids (ICS) on markers of epithelial-mesenchymal transition (EMT) in large airway samples in COPD: an exploratory proof of concept study. Int J Chron Obstruct Pulmon Dis. 2014;9:533–42.
Hsieh CH, Chou YT, Kuo MH, et al. A targetable HB-EGF-CITED4 axis controls oncogenesis in lung cancer. Oncogene. 2017;36:2946–56.
Mahmood MQ, Ward C, Muller HK, et al. Epithelial mesenchymal transition (EMT) and non-small cell lung cancer (NSCLC): a mutual association with airway disease. Med Oncol. 2017;34:45.
Kim SH, Kim JM, Shin MH, et al. Correlation of epithelial-mesenchymal transition markers with clinicopathologic parameters in adenocarcinomas and squamous cell carcinoma of the lung. Histol Histopathol. 2012;27:581–91.
Acknowledgements
Grant support: This work was supported by the Doctoral Startup Foundation of Affiliated Hospital of Guangdong Medical University (Tianwen Lai), the National Natural Science Foundation of China (81420108001), the National Natural Science Foundation of China (81700025), Medical and Health Science and Technology Project of Zhejiang (2018245859), and Beijing Medical Health Foundation (YWJKJJHKYJJ-HX32).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lai, T., Li, Y., Chen, M. et al. Heparin-binding epidermal growth factor contributes to COPD disease severity by modulating airway fibrosis and pulmonary epithelial–mesenchymal transition. Lab Invest 98, 1159–1169 (2018). https://doi.org/10.1038/s41374-018-0049-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41374-018-0049-0
This article is cited by
-
Fibroblast EGFR signaling mediates ricin toxin-induced acute lung injury via EGR1/CXCL1 axis
Archives of Toxicology (2025)
-
Keratin 15 protects against cigarette smoke-induced epithelial mesenchymal transformation by MMP-9
Respiratory Research (2023)
-
Identification of the shared genes and immune signatures between systemic lupus erythematosus and idiopathic pulmonary fibrosis
Hereditas (2023)
-
A blood and bronchoalveolar lavage protein signature of rapid FEV1 decline in smoking-associated COPD
Scientific Reports (2023)
-
HB-EGF-induced IL-8 secretion from airway epithelium leads to lung fibroblast proliferation and migration
BMC Pulmonary Medicine (2021)