Abstract
Cancer has emerged as a critical global health concern due to its elevated heterogeneity and mortality rate. Despite the continuous efforts put into medical research, current clinical approaches still face limitations, including dependency on costly facilities, trained operators and suboptimal diagnostic precision. Therefore, innovative strategies for accurate, personalized and patient-friendly cancer theranostic solutions are urgently required. Among all the platforms, wearable bioelectronics stand out for advantages such as non-invasive detection, responsive therapy and long-term monitoring, owing to their functional bioelectronic interfaces. This review summarizes the latest advances in wearable bioelectronics, aiming to provide a new strategy for cancer theranostics. Initially, the clinical status and emerging wearable bioelectronics are briefly described to provide a general overview. The subsequent contents focus on the implementation of wearable bioelectronics across early diagnosis, treatment, prognostic monitoring and rehabilitation with multiple device configurations. Finally, we discuss the current challenges and prospective future development in advanced cancer theranostics.

Similar content being viewed by others
Introduction
Characterized by unlimited cell proliferation and its ability to form malignant tumors, cancer remains a worldwide leading threat to both public health and society1,2,3. Despite the efforts of people in various fields in recent years, advances have already been made annually to improve patient outcomes4,5. Although current cancer theranostic strategies, such as liquid biopsy, chemotherapy and radiotherapy, have gained popularity in the clinic, they still face inevitable restrictions. For example, the limited sensitivity and tumor correlation of liquid biopsy result in misdiagnosis in identifying early-stage cancers6,7,8. Highly toxic intravenous drugs may trigger severe side effects9,10, whereas radiation can cause damage to both cancer cells and surrounding normal cells11,12. Therefore, these limitations appeal the development of advanced technologies that are accurate, continuous, effective and patient-friendly for cancer theranostics.
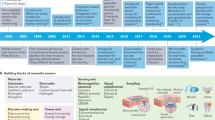
Wearable bioelectronics have emerged as transformative platforms that can bridge clinical approaches and engineered designs. They normally integrate flexible substrates, electronic components, wireless communication modules and sensors or actuators to interact with the environment13,14. As a method of interface communication, wearable bioelectronics work by connecting different functions through energy transformation and propagation, extracting or exporting information from the environment on the basis of passive or active controls. Owing to the inherently flexible, lightweight, biocompatible and financially affordable nature of wearable devices, they have the potential to provide better patient compliance as well as painless and low-burden experiences in whole cancer theranostics. Meanwhile, as wearable bioelectronics serve as high-quality unified frameworks for realizing multidisciplinary integration, advances in multiple subjects, such as molecular imaging15, machine learning16 and nanomedicine17, can also further facilitate the applications of wearable platforms in precision oncology. Moreover, tailored by unique pathophysiological characteristics or clinical requirements, device configurations can be customized to optimize medical resource utilization. Additional technological innovations in the system integration of wearable devices, including self-powered modules18,19, closed-loop systems20,21 and artificial intelligence-empowered evaluation22,23, continue to broaden their design capability, paving the way for autonomous and self-regulating cancer theranostics.
A wide range of wearable bioelectronics, including operational mechanisms: optical24,25, electrical26,27,28, mechanical29,30, thermal31 and ultrasonic32,33 triggered or responsive devices, have been developed for diverse applications. In oncological applications, they can be designed specifically for different types of cancer and different theranostic purposes. Many cancers like breast cancer34, nasopharyngeal carcinoma35, melanoma36, lipomas37, laryngeal cancer38 and tongue cancer39 have already been included in this platform. Meanwhile, diagnostics, therapeutics, prognosis and rehabilitation are also discussed accordingly. Among them, each type of cancer may correspond to different device options. The predictable tumor biomarkers and prognostic indicators required for monitoring of most cancers can be detected in biofluids with wearable sensors. However, limited by the penetration depth owing to certain energy and architecture design, some wearable devices for imaging and treatment focus more on superficial tumors and tumors between superficial and deep tumors, such as melanoma40 and breast cancer41. Furthermore, some cancers, like head and neck cancers, can cause significant damage to bodily functions. Wearable devices targeting these cancers are more focused on functional reconstruction to reduce the impact of cancer treatment on patients’ quality of life.
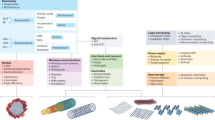
In this review, the potential of wearable bioelectronics in cancer theranostics for diverse malignancies is discussed across diagnostics, therapeutics, prognostic monitoring and rehabilitation (Fig. 1). Initiating detection methodologies, including biomarker sensing and tissue imaging, we then investigate the potential of wearable bioelectronics in cancer therapeutics from transdermal drug delivery and ROS-based therapy to thermal therapy. Consequently, tumor inhibition and drug concentration, as well as specific rehabilitation methods in selected cancer examples, have been explored for the application of wearable bioelectronics in personalized post-therapeutic care. Ultimately, we examine the limitations of the current solution and discuss possible future directions, aiming to bridge wearable breakthroughs with clinical requirements and offering a proposed framework for next-generation cancer theranostics.
Biological overview of wearable bioelectronics in cancer theranostics
The occurrence, development and intervention of cancer involve a series of complex biological mechanisms. According to physiological changes, corresponding wearable bioelectronics can be selected to offer tailored solutions for each stage. This section employs conventional cancer theranostics, spanning early diagnosis, therapeutic intervention, prognostic monitoring and recovery, as a framework to explain the biological principles of oncology and design considerations for wearable bioelectronics (Fig. 2).
The development of cancer is associated with diverse mutagenicity and carcinogenicity. Factors such as gene mutations, epigenetic changes, exposure to carcinogens, radiation, and viruses may all induce cancer. Under the influence of these factors, cancer development begins with the transformation of normal cells, proceeds through uncontrolled proliferation of malignant cells, and culminates in tumor microenvironment formation and metastasis into other tissues. During this process, tumor biomarkers emerge in tissues, biofluids (e.g. blood, interstitial fluid, urine, and saliva) and feces through passive liberation or active secretion from malignant cells or the tumor microenvironment, which indicate the occurrence and progression of cancer42. According to biochemical properties, they can be classified into proteins (e.g., alpha-fetoprotein (AFP) for liver cancer diagnosis43), nucleic acids (e.g., p53 gene sequencing for non-small cell lung cancer44) and small molecule metabolites (e.g., pyruvate kinase45). Corresponding technologies, including immune histochemistry (IHC)46, liquid biopsy47, point-of-care biosensors48, polymerase chain reaction (PCR)49 and next generation gene sequencing50, are developed to achieve rapid and highly specific cancer diagnosis. Recently, microRNA51, incRNA52, extracellular vesicles53, circulating tumor cells (CTCs)54 and circulating tumor DNA (ctDNA)55 have been discussed as novel alternatives to classic tumor biomarkers, as traditional biomarkers may hold the limitation of low sensitivity56. New biomarker detection technologies are also constantly being invented to adapt to new tumor markers. Besides, imaging methods, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), allow the visualization of tumor size and location. Although issues, such as exposure to electromagnetic radiation, contrast agent intake and limited spatial resolution, limit their applicability, their availability for molecular detection and dynamic observation still provides an alternative for cancer detection15.
The core targets of cancer treatment methods mainly focus on destroying the key survival structures or functional pathways of cancer cells. Therapies targeting cellular nucleic acids, cell replication and direct cell toxicity processes include radiotherapy and chemotherapy57. Chemotherapeutic agents predominantly target the cell cycle machinery, with antimetabolites disrupting DNA synthesis and taxanes stabilizing microtubules58. Radiotherapy induces DNA double-strand breaks through localized ionization and generates free radicals to kill cancer cells59. Immunotherapy modulates immune checkpoint pathways, and therapies that modify immune cells to target cancer cell antigens are also being widely studied (e.g., CAR-T cell therapy)60. Emerging modalities now target cancer-specific redox homeostasis and stress response pathways, which exploit distinct biological vulnerabilities of malignant cells. Thermal therapy exploits heat shock protein overexpression in tumors, where localized hyperthermia triggers protein denaturation while sparing normal tissues with functional thermotolerance pathways61. ROS-based strategies capitalize basal oxidative stress in malignant cells, via the use of catalytic nanomaterials along with external stimuli to disrupt redox balance, generating excess reactive oxygen species62. In short, cancer treatment uses chemical interventions (drugs) or physical interventions (radiation or heat) to block the progression of cancer in different ways.
Prognostic monitoring plays a pivotal role in evaluating therapeutic efficacy and tumor evolution by tracking dynamic biomarkers that reflect real-time biological changes in cancers. Unlike static diagnostic markers, these evolving indicators capture tumor heterogeneity information and adaptive resistance mechanisms, which place higher demands on the sensitivity of sensors. Circulating biomarkers, such as ctDNA63,64 and CTC65, provide a monitoring window into tumor dynamics, revealing shifts in driver mutation dominance or metastatic potential. Furthermore, since most drugs have a clear concentration-effect relationship, they can warn of acquired resistance and organ damage66. Moreover, immune markers may also indicate immune escape and immune activation, indicating the therapeutic efficacy of cancer treatment67. Therefore, drug concentration and immune responses are also valuable indicators of prognosis.
In cancer rehabilitation, precise monitoring of functional reconstruction markers and long-term biological follow-up constitute the core pillars of survivorship care. The specific mechanisms of functional impairment across different cancer types dictate the variability in recovery focus. For laryngeal cancer patients, the quality of recurrent laryngeal nerve regeneration and mucosal wave dynamics are emphasized68,69, whereas swallowing function rehabilitation relies on the movement trajectory of the hyoid-laryngeal complex70. These biomechanical parameters fundamentally reflect the molecular processes of synaptic remodeling at neuromuscular junctions and extracellular matrix remodeling. Other widely studied examples are taste restoration after tongue cancer therapy71 and epidermal wound healing72, which are also of particular interest. Meanwhile, rehabilitation is a long physiological process requiring long-term collaboration between patients and physicians. Therefore, the development of a digital health platform is essential, which reduces communication costs for patients and physicians while enabling longitudinal monitoring and analysis of various indicators.
In conclusion, understanding the biological pathways involved in cancer development is crucial for advancing cancer theranostic strategies. The integration of wearable technology into cancer care represents a significant opportunity for improving patients’ quality of life. As research continues to evolve, the synergy between biological insights and technological innovations will pave the way for more effective and personalized cancer management strategies. Here, the sensing/driving modes, application scenarios, and performance metrics of four biomedical platforms (electrical responsive, optical responsive, ultrasonic responsive, and thermal responsive devices) are summarized, demonstrating the key parameter differences between different technologies in cancer theranostics (Table 1)35,37,40,41,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94. In the following sections, more specific applications of wearable bioelectronics will be discussed, with the aim of constructing a more integrated and automated cancer theranostic process.
Early diagnosis
Early cancer diagnosis plays a crucial role, as early detection tends to be correlated with higher cure rates and quality of life in patients95,96. This raises the need to build highly accessible platforms for frequent testing to improve the chances of early diagnosis97,98. Thus, wearable bioelectronics have been proposed because of their high portability and low manufacturing costs. In this section, the latest advances in wearable bioelectronics, including optical sensing, electrical sensing, ultrasound imaging and thermal imaging, are discussed to provide innovative solutions for early cancer diagnostics.
Optical sensing
Wearable optical sensing is a technology that converts biomarker or tissue information into light differences discernible to the human eyes or detectable by machines. Among them, methods such as colorimetry99, fluorescence76,77, and surface-enhanced Raman spectroscopy (SERS)100 are widely recognized by researchers. Under external light excitation, substances interact with electromagnetic waves through absorption, emission and scattering. By detecting variations in the intensity or frequency of light, optical sensors allow noninvasive, spatially and temporally resolved diagnostics. Compared with the popular solutions, colorimetry offers the advantages of direct observation of color reactions, low cost and low power consumption, but it has the disadvantage of low sensitivity101. Fluorescence methods have a lower detection limit, but they face issues such as photobleaching and poor photostability102. Among them, surface-enhanced Raman spectroscopy, as a label-free method, offers an alternative for distinguishing structurally similar substances. It amplifies the Raman signals of adsorbed molecules by 1010-1011 times through the enhancement of localized electromagnetic fields generated by precious metal nanostructures and chemical adsorption103. To conduct the clinical application of wearable sensors, a sensing platform integrating dopamine-modified microneedles (MNs) with ternary metal Au@Ag-Pt nanoparticles was developed for dual-mode detection of tyrosinase (TYR) activity via SERS and colorimetry, enabling in situ melanoma monitoring (Fig. 3a)78. The experimental data revealed that the detection sensitivity of this system reached 0.01 U/mL (SERS mode) and 0.03 U/mL (colorimetric mode), significantly improving the performance indicators of traditional detection methods. Moreover, spectral imaging methods have also been developed for subcutaneous tumor mapping, which has bright prospects for application104.
a Wearable microneedle patch with SERS/colorimetric dual-mode sensing enables in situ tyrosinase monitoring for early melanoma screening and treatment prognosis. Reprinted from ref. 78. with permission. b An electrochemical microneedle patch for the diagnosis of transdermal melanoma. Reprinted from ref. 74. with permission. c A wearable triboelectric impedance tomography (TIT) system for noninvasive, dynamic pathological tissue detection. Reprinted from ref. 37. with permission. d A conformable breast honeycomb patch for the standardized and multi-directional ultrasound imaging. Reprinted from ref. 86. with permission
Electrical sensing
Wearable electrochemical sensors, which capture abnormalities before the tumor forms discernible masses or produces clinical symptoms, are of particular interest because of their minute-level responses and high sensitivity73,105. With working electrodes modified with biorecognition elements (such as antibodies106, aptamers107,108 and enzymes109), it can specifically capture target molecules and initiate oxidation-reduction reactions that generate electrical responses. Figure 3b showed a microneedle (MN) array-based electrochemical sensor patch for melanoma screening that targeted tyrosinase (Tyr), an important enzyme in melanin formation74. By conducting cyclic voltammetry on the captured electrodes, reversible reduction of the oxidized products could be executed for sensor reusability. To validate its performance under realistic conditions, the device was tested on a full-thickness human skin model obtained from plastic surgery, which demonstrated excellent prolonged measurement and effective regenerative performance in Tyr-injected skin samples. In addition to melanoma, electrochemical sensors have been developed for the detection of breast cancer75 and nasopharyngeal carcinoma35. These findings support the potential of wearable electrochemical sensors to provide real-time, long-term, noninvasive or minimally invasive cancer diagnosis through highly sensitive biomarker detection.
Electrical signals can also be leveraged to excite different skin responses in wearable imaging devices. Owing to the heterogeneous conductivity of tumor tissues, electrical impedance tomography (EIT) enables abnormal tissue identification by applying weak alternating currents on the skin, measuring boundary voltage changes and constructing an impedance distribution110. A wearable impedance tomography (TIT) system based on triboelectric nanogenerators (TENGs) was developed for high-precision, low-power dynamic imaging of biological tissues (Fig. 3c)37. By inputting a current density of only 79.58–99.47 mA/m², which is much lower than the safe current for humans, this system can achieve a spatial resolution of 1 mm/50 mm, enabling precise localization of lipomas. However, although EIT has high biosafety and dynamic imaging capabilities, its sensitivity can be limited by current penetration111. Motion artifacts can be caused by dynamic factors, such as electrode movement, human fluids and neural activities, which are not fully considered by algorithms111,112. Optimizing electrode array design and advanced algorithms and combining prior information would be an effective solution.
Ultrasound imaging
Medical imaging plays a pivotal role in early cancer detection, which enables estimation of tumor location, size and infiltration depth. However, conventional imaging methods are particularly dependent on bulky, rigid imaging devices, which increase patients’ transportation burdens. Therefore, lightweight portable alternatives are advocated, and wearable bioelectronics have been developed for continuous imaging. In addition to the ionizing radiation utilized or high energy consumption in other imaging devices113,114, electric-mediated, ultrasound-mediated and thermal-mediated imaging arrays are especially suitable for integration into wearable platforms because of their high biosafety and low power requirements.
Ultrasound imaging has emerged as a promising detection method via the active emission of mechanical waves. By emitting high-frequency sound waves through probes and calculating differences in time delay, frequency shift and echo intensity, the impedance distributions and structural properties can be constructed as possible indicators of tumor formation115. Recent advances in microelectromechanical system (MEMS) technology have allowed flexible piezoelectric transducer arrays to realize conformal skin contact and real-time health monitoring87. However, achieving an optimal balance between imaging quality, biocompatibility and adhesion remains a key challenge for wearable ultrasound imaging systems116,117. To this end, a conformable breast ultrasound patch was constructed, including a honeycomb design for standardized imaging, a monocrystalline probe for superior acoustic properties and an adjustable tracker to expand the field of view, as shown in Fig. 3d86. Through simple rotation, the tracker could rotate to change the detection angles, enabling multiangle image reconstruction, while the fixed honeycomb structure minimized image artifacts possibly from misalignment or poor contact. For a patient with breast cancer, the imaging system could detect small residual tumors and predict tumor morphology through multidirectional imaging.
Thermal imaging
Additionally, wearable temperature sensor arrays can monitor heat dissipation disorders on the skin, which can be attributed to metabolic activities and vascular anomalies associated with cancer90. One type of cancer that widely utilizes this technology is breast cancer. In healthy patients, both breasts exhibit symmetrical temperature distributions, whereas in breast cancer patients, the side with the tumor shows an increase in temperature. Among these methods, infrared thermal imaging (IRT) on the basis of ionizing radiation requires patients to be exposed to an infrared camera for 15 minutes, increasing patient discomfort118. Therefore, a contact-based wearable thermal imaging bra system based on the medical Internet of Things (IoMT) was developed, which used 128 high-precision biosensors (±0.1°C) to monitor breast surface temperature gradients in real time, enabling early screening for breast cancer (2.08°C temperature difference)91. Compared with traditional infrared thermal imaging, the system employed thermal asymmetry analysis and cloud-based algorithm processing to maintain noninvasiveness while enhancing spatial resolution, which demonstrated its potential for detecting small tumors (3 mm). However, despite its noninvasiveness and safety, thermal imaging has limited specificity and low detection depth, restricting its further clinical application in cancer detection.
In conclusion, wearable early diagnostic tools enable precise and rapid identification and localization of tumors via both biomarker detection and imaging methods. However, simple indicators may restrict the precise evaluation of cancer staging, which calls for the construction of multimodal sensing platforms to comprehensively consider more related signals119,120. Moreover, parameters within the devices should be considered comprehensively. For example, owing to the flexibility requirements, rigid damping layers are discarded at the expense of shorter pulse lengths in wearable ultrasound arrays121. Therefore, innovative technologies still need to be invented to balance the internal subcomponents while integrating as much information as possible to guarantee effective diagnosis.
Cancer treatment
Wearable bioelectronics promise more convenient and personalized cancer treatment. By integrating tailored medicines, engineered configurations and flexible materials, they control processes that are inherently difficult to manage via external interventions, which consequently improves drug delivery efficiency and treatment effects. Therefore, the potential of wearable bioelectronics in cancer treatment has received immense attention.
Transdermal drug delivery
Transdermal drug delivery has emerged as a key approach for localized and systemic cancer treatment, which enables drugs to bypass the biological barriers of the stratum corneum. This method allows for the targeted delivery of drugs to tumor sites and potentially expands the narrow therapeutic window of certain drugs while minimizing invasiveness. Recent advances have been proposed in fabricating methods (e.g., 3D printing microneedles122,123), drug types (e.g., responsive drugs124 and engineered cells36,125) and physical enhancement technologies (e.g., electrical126, acoustic energy127 and heat energy128) to enhance manufacturing processes and delivery efficiency.
For transdermal drug delivery, several methods of different action mechanisms are widely studied (Fig. 4a). Microneedles (MNs) enhance drug permeation by physically bypassing the stratum corneum via micro-sized punctures, enabling direct intradermal delivery of therapeutics129. Iontophoresis employs mild electrical currents to drive charged molecules across the skin through electrorepulsion and electroosmosis130. Thermal ablation temporarily disrupts skin barriers by localized heating131, increasing permeability, while ultrasound cavitation generates microbubbles that induce microflows for transdermal transportation132. Among them, microneedles are ideal for wearable integration due to their miniaturizability, pain-free application, and compatibility with active methods. Their modular design allows hybrid platforms, offering precise spatiotemporal control which is critical for personalized therapy. Therefore, in the following part, microneedle-based wearable platforms will be mainly discussed.
a Operational mechanisms of mechanical structure-mediated, electrical mediated, thermal mediated, ultrasonic mediated transdermal drug delivery. b Bioinspired microneedles based on anisotropic porous microstructures for Adoptive T cells therapy. Reprinted from ref. 133. with permission. c A self-powered microneedle patch integrated with triboelectric nanogenerators for iontophoresis-driven deep melanoma chemotherapy. Reprinted from ref. 140. with permission. d A silk-based soluble microneedle enabling active smart phone control and synergistic sequential hydrogen/ immunity therapy. Reprinted from ref. 141. with permission
MNs employ both passive and active release mechanisms. Inspired by the wood xylem vessel structure, a porous cryogen MN system loaded with γδ T cells was developed for cancer immunotherapy, as shown in Fig. 4b133. Its unidirectional pore structure enabled the rapid absorption of macromolecular drugs without external assistance and allowed for long-term preservation by freezing. Compared with conventional intravenous injections, γδ T-cell delivery via MNs resulted in improved survival rates in both skin and organ tumor models, highlighting the therapeutic advantages of MN-based systems. Additionally, active pressurization strategies can also be employed for deeper drug penetration. In this strategy, an oxygen pump MN was constructed by integrating chemical oxygen sources and sodium percarbonate in the MN matrix134. Upon contact with tissue fluid, the oxygen source could be converted into oxygen to propel the drug preload and relieve tumor hypoxia. In addition to preload diversification, structural design enhancement, such as self-locking135, has been explored to meet specific therapeutic needs. However, passive methods often lack precise control over release rates, leading to high heterogeneity among different tumor types and patient cohorts.
To address these limitations, external stimuli can be introduced for actively controlled, on-demand drug release with better performance. Electricity and heat are two widely studied energies that can be integrated into microneedle systems to enhance advanced features such as release stability, penetration depth, and control of droplets136,137,138,139. To achieve deep tumor treatment, a self-powered MN integrated with a flexible triboelectric nanogenerator was developed for combined chemotherapy and photodynamic therapy (Fig. 4c)140. By harvesting the biomechanical energy from body movement, the system generated an alternating current that activated pH-responsive nanoparticles, facilitating their delivery into a 750 µm-deep subcutaneous melanoma in mice. Once localized, acid-sensitive drugs decomposed into DOX and Ce6 to induce apoptosis. Moreover, to increase therapeutic sensitivity and actively control the amount of drug delivered, a heat-responsive silk-based MN device was constructed to target cancer stem cells for synergistic immunization and gas therapy (Fig. 4d)141. Combined with a heating film for heat generation, flexible electric components for wireless control and a wrist strap for localization, the MN patch allowed real-time temperature monitoring and controlled release via a smartphone. Simultaneously, a coated structure was designed for sequential release. Wrapped around the MNs, the first dissolved AB-MSNs produced hydrogen to reduce the amount of reactive oxygen species, followed by the central release of aPD-1 for targeted immunosuppression. With these advancements, a closed-loop drug delivery system can be further designed on this basis to realize automatic dynamic treatment adjustments without user intervention142, and multimodal therapeutic strategies are needed for complex cancer treatment scenarios.
ROS-based therapies
Reactive oxygen species (ROS) are defined as highly reactive oxygen-containing chemical molecules, generally including superoxide radical anions (O2–), singlet oxygen (1O2), hydroxyl free-radicals (OH−) and hydrogen peroxide (H2O2)143. Endogenous ROS are produced by mitochondria and peroxisomes as byproducts of cellular reactions, with stable concentrations in normal tissues144,145. Low-abundance ROS can act as signaling molecules to promote cancer cell proliferation, whereas excessive ROS may lead to apoptosis and cytotoxicity146. Therefore, catalyzing the formation of excessive ROS by exogenous sensitizers is invented as an effective cancer treatment strategy147. Recently, the integration of wearable technologies has expanded the application scope of ROS-generating therapies, especially noninvasive or semi-invasive treatments such as photodynamic therapy (PDT), sonodynamic therapy (SDT) and thermodynamic therapy (TDT), which have different performance merits and limitations compared to traditional therapies (Table 2)57,148,149,150,151,152,153,154,155,156,157,158,159.
The photosensitizers used in PDT are activated by red, blue or near-infrared light to catalyze the production of ROS from endogenous substances within the tumor. More specifically, after excitation, PS is transferred from the ground state to a singlet state and then spin-converts to a triplet state, where it exchanges electrons or energy with its surroundings to catalyze ROS generation160. Here, wearable bioelectronics optimize both the delivery of sensitizers and the excitation process. To integrate the procedure of drug delivery and PDT, a wireless detachable metronomic injector was proposed for battery-free and recyclable breast cancer therapy (Fig. 5a)41. By converting mechanical energy from body movement into electrical energy, the device heated a thermal expansion layer, driving drug release from a reservoir into the tumor site. In tumor-bearing mouse models, this device ultimately maintained the tumor volume at a manageable level for 12 days, illustrating its effective and long-term anticancer efficacy. Among the ROS, 1O2 is considered the main cause of phototoxicity via a type II photo process, where energy from the triplet state of photosensitizers is transferred into triplet dioxygen (3O2)161. However, oxygen scarcity in tumor regions limits the effectiveness of this process. Wearable devices can relieve this hypoxic environment via the simultaneous transport of oxygen or peroxisomes, which facilitate oxygen generation and glutathione (GSH) depletion79,80,82. In addition to chemical components, the excitation efficiency can also be optimized by wearable devices through the development of high-power OLEDs162. These innovations significantly improve treatment efficacy, allow long-term use, increase patient compliance, and reduce discomfort associated with conventional endoscopic PDT through removable and lightweight structures.
a A self-powered and light irradiated drug injector for the metronomic photodynamic tumor treatment. Reprinted from ref. 41. with permission. b A wearable ultrasound patch for tumor sonodynamic therapy via the reactive oxygen generation and immune memory formation. Reprinted from ref. 88. with permission. c A smartphone-controlled wearable systems for tumor thermodynamic treatment by the pyroelectric and thermal cycling effect. Reprinted from ref. 93. with permission
SDT also relies on ROS generation but uses ultrasound as an external stimulus to activate acoustic sensitizers. The principle of ROS generation by SDT can be interpreted as the exploitation of sonoluminescence and the pyrolysis effect caused by the cavitation effect163,164. Compared with light-based methods, ultrasound can penetrate deeper into tissue, induce cavitation effects and mediate certain apoptosis pathways, making SDT a highly valuable noninvasive cancer therapy165,166. Figure 5b showed a flexible ultrasound MN patch for painless sensitizer delivery, conformal skin adhesion and ultrasound emission88. After subcutaneous drug delivery via MNs, embedded piezoelectric nanoparticles accumulated in the tumor via enhanced permeability and retention effects. Upon ultrasound stimulation, the nanoparticles generated separate electrons and holes, which catalyzed the production of ROS from water and oxygen to induce apoptosis. In addition, this device enhanced immunotherapy, which was supported by immune cell-related alterations, demonstrating long-term immune memory and efficient suppression of tumor recurrence in the post-SDT stages.
TDT is an emerging method that utilizes the thermoelectric effect to induce ROS generation. The pyroelectric effect between two pyroelectric terminals results in electron‒hole mobility within the internal crystal167. Similar to previous therapies, different endogenous substances are catalyzed to generate ROS at the electron end and the hole segments. Here, the wearable device often acts as a controlled heat source to energize the nanomedicine and reduce the effects of hyperthermia on surrounding cells. For example, a joint ferroelectric bismuth ferrite nano effector and smartphone-controlled wearable platform were developed to routinize the cancer treatment process, as shown in Fig. 5c93. After treatment, this approach yielded a posttreatment survival rate of approximately 80%, which was twice that of the control groups. For breast cancer recovery, a wearable system is designed later capable of automated temperature cycling on the basis of remote smartphone commands, illustrating the feasibility of practical applications.
By utilizing wearable devices, ROS-based therapies are evolving from short-term, hospital-centered interventions to continuous, daily use and patient-friendly solutions. Enabled by smartphone control, wearable bioelectronics support real-time adjustments of treatment parameters for tailored dosing, improved precision and reduced medical waste. With the development of intelligent nanomedicine, more innovative and integrated platforms are expected to emerge to further advance personalized cancer therapeutics. Notably, ROS-based therapies require the assistance of sensitizers, indicating that the body needs to ingest additional drugs to achieve therapeutic effects. Therefore, biosafety assessments, including major organ testing and blood biochemical analysis, are necessary. Moreover, drug metabolism, excretion, and biotoxicity are also key considerations for such therapies and represent major in their transition to clinical application168.
Thermal therapy
Owing to the difference in heat resistance between cancer and normal cells, thermal therapy has emerged as a promising approach that selectively induces apoptosis in cancer cells without harming surrounding healthy tissues with external heat energy169. Among the various temperature-based therapies, hyperthermia is a widely recognized thermal therapy that can be achieved by maintaining a temperature ranging from 42°C to approximately 48 °C170. For example, a unidirectional silver nanofiber (AgNF) transparent patch was developed for localized superficial epidermal tumor thermotherapy (Fig. 6a)94. The unidirectional AgNF network offered both high transparency and low resistance, allowing safe operation at low voltages while permitting real-time monitoring of the skin condition to prevent burns. Moreover, hyperthermia also has effective synergistic effects with other modalities. It promotes intratumor penetration and drug uptake of chemotherapeutic agents by vasodilating tumor blood vessels and increasing metabolism171. It can also sensitize cells to radiotherapy and increase the catalytic activity of catalytic therapy172. In addition to excitation energy, photothermal therapy (PTT), which converts light energy into heat, represents another promising thermal approach for cancer therapeutics. As shown in Fig. 6b, ionic gels doped with MXene were developed with dual cell death mechanisms for joint electrical stimulation and PTT85. The MXene-doped ionic gels possessed highly transparent properties that allowed laser penetration and real-time visualization of the treatment area. It also featured flexibility and conductivity, which enabled uniform electrical stimulation across the entire tumor area and eliminated treatment blind spots. A decrease in tumor volume was observed in the groups that received combined PTT and electrical stimulation, indicating a synergistic therapeutic effect. Therefore, cancer thermotherapy provides a multi-mechanism treatment approach with enhanced efficacy and relatively low toxicity.
Recent advances highlight the transformative role of wearable bioelectronics in cancer theranostics, significantly improving both therapeutic outcomes and patient experience. For traditional therapies, transdermal drug delivery systems enable painless and precise medication administration, whereas for ROS-based and thermal therapy, wearable bioelectronics miniaturize and simplify the delivery of external stimuli. Furthermore, they demonstrate the potential of closed-loop drug delivery by combining sensing and treatment components, which enables real-time feedback control to maintain drug homeostasis. By combining different therapies under similar external stimuli, wearable devices are also feasible for synergistic cancer therapies to achieve multifunctional integration.
Prognostic monitoring
Cancer prognosis refers to the prediction of disease progression and outcomes following medical intervention on the basis of information from tumor morphology, blood drugs or cells, and clinical manifestations173,174,175. Traditional monitoring tools tend to provide only discrete, point-in-time data; in contrast, wearable bioelectronics enable continuous monitoring, offering valuable insights into disease dynamics. Tumor regression monitoring in animal models, circulating biomarker detection and drug concentration detection in biofluid sensing are discussed in this section to highlight the long-term operation ability of wearable devices.
Tumor regression
Prognostic monitoring via wearable bioelectronics plays a crucial role in animal models, where tumor volume is an important indicator to assess drug efficacy. Wearable sensors enable fast and convenient data collection, thereby reducing the labor burden. Compared with other detection methods, strain sensors ensure noninvasive detection with satisfactory diagnostic accuracy. As shown in Fig. 7a, a strain sensor based on microcrack extension of a gold film was developed for automated tracking of dynamic changes in tumor size176. As the tumor grew or shrunk, changes in the circumference stretched the surrounding sensor, modulating the resistance of the gold film. In both the subcutaneous and internal tumor-bearing mouse models, the device demonstrated stable and continuous monitoring over several days. This method detected differences in tumor regression between the treatment and control groups much earlier than conventional methods did. Integrating such tumor regression monitoring tools with therapy development efforts may enhance comprehensive cancer management40.
a A flexible electronic cracked gold strain sensor measuring real-time and long-time tumor regression information. Reprinted from ref. 176. with permission. b A self-healing field effect transistor based bioelectronic for the in situ monitoring of interstitial fluid methylated circulating DNAs. Reprinted from ref. 178. with permission. c A printable multiplexing wearable sensor with molecule-selective core-shell nanoparticles for anti-cancer immunosuppressive drug concentration sensing. Reprinted from ref. 181. with permission
Circulating biomarkers
Another effective prognostic indicator is the continuous monitoring of tumor-associated circulating biomarkers177. Unlike early cancer diagnosis, prognosis requires long-term tracking of biomarker levels. To support this, sensors should be both durable and resilient to the mechanical stresses of daily wear. Figure 7b showed a field-effect transistor platform with self-healing circuitry developed for real-time and transdermal monitoring of methylated circulating tumor DNA (ctDNA)178. The functionalized antibodies on the gate could specifically bind to methylated ctDNA, modulating the channel current. Notably, the system incorporated liquid metal components capable of forming an oxide layer for self-healing. Although this study highlighted a promising direction for long-term use, wearable prognostic monitoring, antibody degradation and liquid metal biosafety remained challenging. Future advancements should focus on biosensors that are robust under long-term monitoring and capable of maintaining stable performance in complex living environments, thus supporting treatment effectiveness analysis and adaptive cancer treatment.
Drug concentration
Apart from direct measurement of the tumor, evaluating drug concentration levels is essential for predicting cancer recurrence, assessing drug resistance and determining the optimal dosage179. Owing to the extremely narrow therapeutic window of cancer drugs, it is assumed that the effective and toxic doses of cancer drugs are very close to each other, resulting in a greater risk of ineffective treatment or severe toxic side effects180. Moreover, individual differences in drug metabolism make standardized dosing ineffective for many patients. To address these issues, a reproducible inkjet-printed core‒shell nanoparticle microfluidic sensing platform was developed for real-time monitoring of multiple drugs (Fig. 7c)181. Designed for bedridden patients, this platform enabled sweat-based drug monitoring by using iontophoresis to stimulate sweat induction and microfluidics to guide the fluid into the sensor reservoir. The system incorporated selective cavities that binded target drugs, reducing the exposure of the magnetic nanoparticle core, hindering electron transfer, and decreasing the redox current. Because of its excellent long-term operational stability in a mouse model, this device represented an innovative approach in wearable bioelectronics for pharmacokinetic monitoring.
Wearable bioelectronics offer promise for cancer prognosis through continuous, real-time monitoring of tumor status, circulating biomarkers, and therapeutic drug levels. Their utility in preclinical animal models can streamline drug development by reducing costs and manual labor. Furthermore, these systems address the limitations of traditional tools by reducing time delays in prognostic evaluation and supporting data-driven medicine in cancer management.
Rehabilitation
Cancer rehabilitation marks the final yet essential stage in comprehensive cancer theranostics, requiring not only medical support but also social and psychological care. To automate the evaluation of recovery states, diverse sensor inputs and machine learning data processing methods are widely utilized in wearable bioelectronics182. Furthermore, devices can be customized with cancer-specific assistive functionalities to improve patients’ quality of life during recovery. Here, laryngeal and tongue cancer recovery environments are specifically described below to demonstrate the significance of wearable bioelectronics in scenario-customized healthcare.
Throat behavior evaluation
Head and neck cancer is one of the most specific cancer sites, as therapeutic treatment may result in difficulty swallowing or even vocalization disorders, greatly reducing the well-being of the patient183,184,185. As postoperative rehabilitation of assisted patients requires equipment for long-term monitoring of vocal cord movement and swallowing, wearable bioelectronics offer flexible alternatives for daily sensing. As shown in Fig. 8a, a lightweight and stretchable hydrogel platform was proposed, which integrated a built-in triaxial accelerometer, a surface electromyography (sEMG) sensor, and a convolutional neural network data analysis module for laryngeal rehabilitation management186. This platform captured both gross body movements and subtle laryngeal physiological vibrations, along with muscle electrical activity, enabling precise evaluation of swallowing behavior. The trained CNN model achieved an accuracy of 98.2% in classifying key laryngeal motions and demonstrated adaptability with new subjects, making it an ideal and accurate solution for postsurgical laryngeal cancer patients with personalized rehabilitation management. Moreover, mechanoacoustic sensors187, strain sensors188, vibration sensors189 and multimodal systems190 diversify the sensor choices for the precise monitoring of throats.
a A fully integrated stretchable platform with machine learning-based throat behavior and body motion classification for laryngeal cancer rehabilitation monitoring. Reprinted from ref. 186. with permission. b An artificial throat enabling multiplexed speech recognition and voice reproduction for the post-laryngectomy aphonic patient. Reprinted from ref. 38. with permission. c A high-density electrode arrays for the taste restoration of tongue cancer patients. Reprinted from ref. 39. with permission
Artificial Throat
Although laryngeal monitoring is a good approach to support rehabilitation, some patients face permanent or prolonged vocal impairments after laryngeal cancer surgery. Moreover, the conventional electronic larynx can only produce mechanical and unnatural sounds that limit effective communication. To address this gap, a wearable artificial larynx system has been developed by integrating machine learning algorithms and vocalization modules according to the structure of laryngeal monitoring for electromyographic signals. For example, a graphene strain sensor-based artificial larynx was developed to cope with signal perception and speech output in noisy environments (Fig. 8b)38. Graphene sensors affixed to the neck synchronized residual muscle contractions with skin-conducted vocal fold vibrations. By converting the signals into spectrograms and using them for AlexNet model-based deep learning classification, the device showed 91% classification accuracy for laryngeal cancer resection patients, demonstrating the device’s excellent immunity to interference and adaptive learning capability. Simultaneously, machine learning-integrated artificial larynxes have emerged based on different skin signal detection principles. By responding to sensing signals in real time, the artificial larynx, as a wearable device specifically targeting laryngeal cancer, provides inspiration for broader functional recovery applications tailored to specific cancer types. In addition to strain sensors, electromyograms191, piezoelectric transducers192 and soft magnetoelastic sensors193 are also promising alternatives for input formation to achieve more precise corresponding device responses.
Taste decoding
In tongue cancer, postoperative complications often include partial sensory loss, particularly in taste perception, which impacts quality of life. Patients with sensory loss acquire specific sensations or generate alternative sensation signals, which are important and relevant. Therefore, a flexible tongue electrode for postoperative recovery monitoring of tongue cancer and reconstruction of taste function was developed to achieve high-resolution whole tongue electrical signal recording, as depicted in Fig. 8c39. The device monitored electrophysiological differences between the reconstructed and natural tongues under gustatory stimulation, allowing visualization of recovery. Additionally, the simultaneously recorded electroencephalography (EEG) signals were compared by using a bimodal fusion algorithm to predict taste information. Although there was limited research on tongue signal decoding, this study highlighted the potential of wearable bioelectronics in restoring sensory functions. Moreover, as a growing area of interest, salivary biosensing could be leveraged to enhance recovery monitoring and rehabilitation in tongue cancer patients.
In summary, the complexity and diversity of cancer-related impairments necessitate tailored wearable solutions. Current advances in wearable bioelectronics for laryngeal and tongue cancer rehabilitation demonstrate how multimodal sensing and machine learning can support partial restoration of lost functions. The development of cancer-specific wearable interventions could play a key role in personalized and high-quality postoperative care, bridging wearable healthcare closer to the ideal of individualized medicine.
Conclusion and perspective
Wearable bioelectronics facilitate development in the whole loop of cancer theranostics, including early diagnosis, on-patient treatment, and recovery support. They have demonstrated notable advantages in the combination of molecular, cellular and anatomical information for monitoring the cancer progression. However, several challenges should be addressed before their successful translation to the clinical market (Fig. 9).
Reprinted from ref. 27. with permission
Device sensitivity and continuity
The development of highly sensitive detection systems remains paramount for improving diagnostic reliability. Sensitive materials and novel recognition elements are promising signal enhancement techniques for improving the detection limit of the sensor. Continuous monitoring is also essential because of the high spatial and temporal heterogeneity of tumors during the treatment cycle. This requires the implementation of stable sensing platforms with extended operational lifetimes, achieved through optimized interfacial contact mechanics and biocompatible material to reduce inflammatory reactions in long-term wear. Power management represents another key consideration for long-term monitoring. The development of lower energy consumption devices, higher energy density batteries and active energy harvesting methods are recommended. Ultimately, multimodal sensors are crucial methods to enhance both sensitivity and continuity. By integrating sensors targeting multiple tumor biomarkers, they can improve diagnostic accuracy and precision, while they can perform timely calibration to reduce the impact of environmental factors by combining both physical and chemical sensors.
Standardized fabrication
Cost-effective manufacturing methods are key elements for making multifunctional wearable bioelectronics accessible for economically disadvantaged patients. For manufacturers, to reduce the process complexity and manufacturing difficulty, all-in-one fabrication solutions, including roll-to-roll processing194, printing methods122 and laser patterning195, are also advocated, which facilitates the commercialization of wearable bioelectronics.
Clinical validation
Successful clinical translation requires rigorous validation of device performance in human populations. Comprehensive biocompatibility assessments must address both material components and energy delivery parameters. For instance, while traditional metallic microneedle arrays may present toxicity concerns, polymeric alternatives offer improved safety profiles without compromising mechanical integrity196. Similarly, stimulation parameters must be carefully optimized to prevent tissue damage. The implementation of robust data governance frameworks is equally important to ensure patient privacy and ethical data handling throughout continuous monitoring processes. Furthermore, device functionality should be tailored to specific clinical contexts, such as incorporating respiratory and swallowing function assessment in laryngeal cancer rehabilitation. The development of disease types is important. Current research mainly focuses on superficial cancers, while the development of deep cancers places higher demands on the penetration capabilities of devices. Although advanced actuators have been developed, devices that combine penetration depth, targeting ability and biosafety are still scarce.
Overall, wearable bioelectronics show great promise for advancing cancer theranostics. By overcoming key challenges in sensor sensitivity and continuous monitoring as well as the limitation in scenario consideration, these devices can enable real-time, precise and personalized health care in diverse cancer types. With further development of multimodal sensing, biocompatible materials, and standardized manufacturing, wearable bioelectronics may ultimately transform cancer theranostics through decentralized, data-driven approaches that improve the intervention outcomes.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 74, 229–263 (2024).
Emery, J. et al. Management of common clinical problems experienced by survivors of cancer. Lancet 399, 1537–1550 (2022).
Chen, S. M. et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 9, 465–472 (2023).
Singal, A. G., Kanwal, F. & Llovet, J. M. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 20, 864–884 (2023).
Markham, M. J. et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. J. Clin. Oncol. 38, 1081 (2020).
Lone, S. N. et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 21, 79 (2022).
Wang, K., Wang, X., Pan, Q. & Zhao, B. Liquid biopsy techniques and pancreatic cancer: diagnosis, monitoring, and evaluation. Mol. Cancer 22, 167 (2023).
Nikanjam, M., Kato, S. & Kurzrock, R. Liquid biopsy: current technology and clinical applications. J. Hematol. Oncol. 15, 131 (2022).
Verstappen, C. C., Heimans, J. J., Hoekman, K. & Postma, T. J. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 63, 1549–1563 (2003).
Sioka, C. & Kyritsis, A. P. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother. Pharmacol. 63, 761–767 (2009).
De Ruysscher, D. et al. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 5, 13 (2019).
Stone, H. B., Coleman, C. N., Anscher, M. S. & McBride, W. H. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 4, 529–536 (2003).
Gong, S., Lu, Y., Yin, J., Levin, A. & Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 124, 455–553 (2024).
Lee, J. et al. Conductive fiber-based ultrasensitive textile pressure sensor for wearable electronics. Adv. Mater. 27, 2433–2439 (2015).
Hussain, T. & Nguyen, Q. T. Molecular imaging for cancer diagnosis and surgery. Adv. Drug Deliv. Rev. 66, 90–100 (2014).
Tran, K. A. et al. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 13, 152 (2021).
Chaturvedi, V. K., Singh, A., Singh, V. K. & Singh, M. P. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr. Drug Metab. 20, 416–429 (2019).
Miao, L. et al. 3D Temporary-magnetized soft robotic structures for enhanced energy harvesting. Adv. Mater. 33, 2102691 (2021).
Su, Z. et al. Digitalized self-powered strain gauge for static and dynamic measurement. Nano Energy 42, 129–137 (2017).
Ghanim, R., Kaushik, A., Park, J. & Abramson, A. Communication protocols integrating wearables, ingestibles, and implantables for closed-loop therapies. Device 1, 100092 (2023).
Li, J., Liang, J. Y., Laken, S. J., Langer, R. & Traverso, G. Clinical opportunities for continuous biosensing and closed-loop therapies. Trends Chem. 2, 319–340 (2020).
Zheng, Y. B. et al. Smart materials enabled with artificial intelligence for healthcare wearables. Adv. Funct. Mater. 31, 2105482 (2021).
Chen, M. R., Cui, D. X., Haick, H. & Tang, N. Artificial intelligence-based medical sensors for healthcare system. Adv. Sens. Res. 3, 2300009 (2024).
Yi, L., Hou, B. & Liu, X. Optical integration in wearable, implantable and swallowable healthcare devices. ACS Nano 17, 19491–19501 (2023).
Lee, G.-H. et al. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 5, 149–165 (2020).
Braendlein, M. et al. Lactate detection in tumor cell cultures using organic transistor circuits. Adv. Mater. 29, 1605744 (2017).
Huang, C. et al. A flexible aptameric graphene field-effect nanosensor capable of automatic liquid collection/filtering for cytokine storm biomarker monitoring in undiluted sweat. Adv. Funct. Mater. 34, 2309447 (2024).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6, 1214–1224 (2022).
He, Q. et al. Conductive hydrogel for flexible bioelectronic device: current progress and future perspective. Adv. Funct. Mater. 34, 2308974 (2024).
Wang, T. et al. Mechano-based transductive sensing for wearable healthcare. Small 14, 1702933 (2018).
Yang, C. et al. Stretchable and microneedle-integrated electronic patches with actively controlled chemothermal therapy for cancer treatment. Adv. Funct. Mater. n/a, 2505261 (2025).
Yang, Y. et al. An implantable ultrasound-powered device for the treatment of brain cancer using electromagnetic fields. Sci. Adv. 8, eabm5023 (2022).
Park, C. K. S. et al. Cost-effective, portable, patient-dedicated three-dimensional automated breast ultrasound for point-of-care breast cancer screening. Sci. Rep. 13, 14390 (2023).
Huang, H. et al. A microneedle patch for breast cancer screening via minimally invasive interstitial fluid sampling. Chem. Eng. J. 472, 145036 (2023).
Yang, B., Fang, X. & Kong, J. Engineered microneedles for interstitial fluid cell-free DNA capture and sensing using iontophoretic dual-extraction wearable patch. Adv. Funct. Mater. 30, 2000591 (2020).
Zhou, R. et al. Grooved microneedle patch augments adoptive T cell therapy against solid tumors via diverting regulatory T cells. Adv. Mater. 36, 2401667 (2024).
Yang, P. et al. A wearable triboelectric impedance tomography system for noninvasive and dynamic imaging of biological tissues. Sci. Adv. 10, eadr9139 (2024).
Yang, Q. et al. Mixed-modality speech recognition and interaction using a wearable artificial throat. Nat. Mach. Intell. 5, 169–180 (2023).
Wang, X. et al. Gustatory interface for operative assessment and taste decoding in patients with tongue cancer. Nat. Commun. 15, 8967 (2024).
Siboro, P. Y. et al. Harnessing HfO2 nanoparticles for wearable tumor monitoring and sonodynamic therapy in advancing cancer care. ACS Nano 18, 2485–2499 (2024).
Lin, T. et al. A self-powered wireless detachable drug/light injector for metronomic photodynamic therapy in cancer treatment. Nano Energy 116, 108826 (2023).
Zhou, Y. et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 9, 132 (2024).
Kew, M. Alpha-fetoprotein in primary liver cancer and other diseases. Gut 15, 814 (1974).
Chiba, I. et al. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung cancer study group. Oncogene 5, 1603–1610 (1990).
Wang, W. et al. Cancer metabolites: promising biomarkers for cancer liquid biopsy. Biomark. Res. 11, 66 (2023).
Yatabe, Y. et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J. Thorac. Oncol. 14, 377–407 (2019).
De Rubis, G., Krishnan, S. R. & Bebawy, M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol. Sci. 40, 172–186 (2019).
Soper, S. A. et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens. Bioelectron. 21, 1932–1942 (2006).
Bernard, P. S. & Wittwer, C. T. Real-time PCR technology for cancer diagnostics. Clin. Chem. 48, 1178–1185 (2002).
Meldrum, C., Doyle, M. A. & Tothill, R. W. Next-generation sequencing for cancer diagnostics: a practical perspective. Clin. Biochem. Rev. 32, 177 (2011).
Tricoli, J. V. & Jacobson, J. W. MicroRNA: Potential for cancer detection, diagnosis, and prognosis. Cancer Res 67, 4553–4555 (2007).
Badowski, C., He, B. & Garmire, L. X. Blood-derived lncRNAs as biomarkers for cancer diagnosis: the good, the bad and the beauty. npj Precis. Oncol 6, 40 (2022).
Lee, Y. et al. Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Mol. Cancer 22, 33 (2023).
Ankeny, J. S. et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br. J. Cancer 114, 1367–1375 (2016).
Fiala, C. & Diamandis, E. P. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med 16, 1–10 (2018).
Edoo, M. I. A. et al. Serum biomarkers AFP, CEA and CA19-9 combined detection for early diagnosis of hepatocellular carcinoma. Iran. J. Public Health 48, 314–322 (2019).
Sridhar, T. & Symonds, R. P. Principles of chemotherapy and radiotherapy. Obstet. Gynaecol. Reprod. Med. 19, 61–67 (2009).
McKnight, J. A. Principles of chemotherapy. Clin. Tech. Small Anim. Pract. 18, 67–72 (2003).
Nickoloff, J. A., Sharma, N. & Taylor, L. Clustered DNA double-strand breaks: biological effects and relevance to cancer radiotherapy. Genes 11, 99 (2020).
Hosseinkhani, N. et al. Immune checkpoints and CAR-T cells: the pioneers in future cancer therapies?. Int. J. Mol. Sci. 21, 8305 (2020).
Sreedhar, A. S. & Csermely, P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol. Ther. 101, 227–257 (2004).
Wu, X., Zhou, Z., Li, K. & Liu, S. Nanomaterials-induced redox imbalance: challenged and opportunities for nanomaterials in cancer therapy. Adv. Sci. 11, 2308632 (2024).
Sanz-Garcia, E., Zhao, E., Bratman, S. V. & Siu, L. L. Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: current research, opportunities, and challenges. Sci. Adv. 8, eabi8618 (2022).
Jacob, S. et al. The use of serial circulating tumor DNA to detect resistance alterations in progressive metastatic breast cancer. Clin. Cancer Res. 27, 1361–1370 (2021).
Allen, T. A. The role of circulating tumor cells as a liquid biopsy for cancer: advances, biology, technical challenges, and clinical relevance. Cancers 16, 1377 (2024).
Gonzalez, D., Schmidt, S. & Derendorf, H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin. Microbiol. Rev. 26, 274–288 (2013).
Seliger, B. & Massa, C. Immune therapy resistance and immune escape of tumors. Cancers 13, 551 (2021).
Berke, G. S. & Gerratt, B. R. Laryngeal biomechanics: an overview of mucosal wave mechanics. J. Voice 7, 123–128 (1993).
Tian, H., Pan, J., Chen, L. & Wu, Y. A narrative review of current therapies in unilateral recurrent laryngeal nerve injury caused by thyroid surgery. Gland Surg. 11, 270 (2022).
Steele, C. M. et al. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin. Otolaryngol. 36, 30–36 (2011).
Sandow, P., Hejrat-Yazdi, M. & Heft, M. Taste loss and recovery following radiation therapy. J. Dent. Res. 85, 608–611 (2006).
Payne, W. G. et al. Wound healing in patients with cancer. Eplasty 8, e9 (2008).
Yang, B., Kong, J. & Fang, X. Programmable CRISPR-Cas9 microneedle patch for long-term capture and real-time monitoring of universal cell-free DNA. Nat. Commun. 13, 3999 (2022).
Poursharifi, N., Hassanpouramiri, M., Zink, A., Ucuncu, M. & Parlak, O. Transdermal sensing of enzyme biomarker enabled by chemo-responsive probe-modified epidermal microneedle patch in human skin tissue. Adv. Mater. 36, 2403758 (2024).
Dervisevic, M., Alba, M., Adams, T. E., Prieto-Simon, B. & Voelcker, N. H. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosens. Bioelectron. 192, 113496 (2021).
Yang, Q. et al. Microneedle array encapsulated with programmed DNA hydrogels for rapidly sampling and sensitively sensing of specific microRNA in dermal interstitial fluid. ACS Nano 16, 18366–18375 (2022).
Huang, R., Wan, P., Hu, S., Zhang, C. & Miao, W. Silver nanoclusters-decorated porous microneedles coupling duplex-specific nuclease-assisted signal amplification for sampling and detection of microRNA in interstitial fluid. ACS Sens 9, 5604–5612 (2024).
Huang, X. et al. In situ tyrosinase monitoring by wearable microneedle patch toward clinical melanoma screening. ACS Nano 17, 20073–20086 (2023).
He, G. et al. Synthetic biology-instructed transdermal microneedle patch for traceable photodynamic therapy. Nat. Commun. 13, 6238 (2022).
Li, Y. et al. A microneedle patch with self-oxygenation and glutathione depletion for repeatable photodynamic therapy. ACS Nano 16, 17298–17312 (2022).
Li, Y. et al. A versatile cryomicroneedle patch for traceable photodynamic therapy. Adv. Mater. 36, 2400933 (2024).
Liu, P. et al. Microneedle patches with O2 propellant for deeply and fast delivering photosensitizers: towards improved photodynamic therapy. Adv. Sci. 9, 2202591 (2022).
Liu, Z. et al. A microneedle patch delivers mitochondria-and lysosomes-dual targeting prodrug-like photosensitizers with regulated photoactivity for precise photodynamic therapy. Adv. Healthc. Mater. 14, 2403954 (2025).
Li, Y. et al. A flexible wearable device coupled with injectable Fe3O4 nanoparticles for capturing circulating tumor cells and triggering their deaths. Biosens. Bioelectron. 235, 115367 (2023).
Ju, X. et al. A wearable electrostimulation-augmented ionic-gel photothermal patch doped with MXene for skin tumor treatment. Nat. Commun. 15, 762 (2024).
Du, W. et al. Conformable ultrasound breast patch for deep tissue scanning and imaging. Sci. Adv. 9, eadh5325 (2023).
Hu, H. et al. Stretchable ultrasonic arrays for the three-dimensional mapping of the modulus of deep tissue. Nat. Biomed. Eng. 7, 1321–1334 (2023).
Xue, H. et al. Wearable flexible ultrasound microneedle patch for cancer immunotherapy. Nat. Commun. 16, 2650 (2025).
Zou, F., Luo, Y., Zhuang, W. & Xu, T. A fully integrated conformal wearable ultrasound patch for continuous sonodynamic therapy. Adv. Mater. 36, 2409528 (2024).
Kang, M. et al. Wireless graphene-based thermal patch for obtaining temperature distribution and performing thermography. Sci. Adv. 8, eabm6693 (2022).
Elouerghi, A., Bellarbi, L., Errachid, A. & Yaakoubi, N. An ioMT-based wearable thermography system for early breast cancer detection. IEEE Trans. Instrum. Meas. 73, 1–17 (2024).
Elouerghi, A. et al. A flexible wearable thermography system based on bioheat microsensors network for early breast cancer detection: IoT technology. J. Electr. Comput. Eng. 2022, 5921691 (2022).
Li, R.-Q. et al. mHealth: A smartphone-controlled, wearable platform for tumour treatment. Mater. Today 40, 91–100 (2020).
Wang, Q. et al. A skin-mountable hyperthermia patch based on metal nanofiber network with high transparency and low resistivity toward subcutaneous tumor treatment. Adv. Funct. Mater. 32, 2111228 (2022).
Crosby, D. et al. Early detection of cancer. Science 375, eaay9040 (2022).
Fitzgerald, R. C., Antoniou, A. C., Fruk, L. & Rosenfeld, N. The future of early cancer detection. Nat. Med. 28, 666–677 (2022).
Pashayan, N. & Pharoah, P. D. P. The challenge of early detection in cancer. Science 368, 589–590 (2020).
Robby, A. I., Lee, G., Lee, K. D., Jang, Y. C. & Park, S. Y. GSH-responsive self-healable conductive hydrogel of highly sensitive strain-pressure sensor for cancer cell detection. Nano Today 39, 101178 (2021).
Mazzone, P. J. et al. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 62, 565–568 (2007).
Chen, Z. et al. Hydrogel based flexible wearable sweat sensor for SERS-AI monitoring treatment effect of lung cancer. Sens. Actuators B 427, 137155 (2025).
Liu, B., Zhuang, J. & Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci.:Nano 7, 2195–2213 (2020).
Mamontova, A., Grigoryev, A., Tsarkova, A., Lukyanov, K. & Bogdanov, A. Struggle for photostability: bleaching mechanisms of fluorescent proteins. Russ. J. Bioorg. Chem. 43, 625–633 (2017).
Nie, C., Shaw, I. & Chen, C. Application of microfluidic technology based on surface-enhanced Raman scattering in cancer biomarker detection: a review. J. Pharm. Anal. 13, 1429–1451 (2023).
Wang, Y. et al. Near-infrared spectroscopy–enabled electromechanical systems for fast mapping of biomechanics and subcutaneous diagnosis. Sci. Adv. 10, eadq9358 (2024).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Ilkhani, H., Sarparast, M., Noori, A., Bathaie, S. Z. & Mousavi, M. F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 74, 491–497 (2015).
Parihar, A., Choudhary, N. K., Sharma, P. & Khan, R. Carbon nanomaterials-based electrochemical aptasensor for point-of-care diagnostics of cancer biomarkers. Mater. Today Chem. 30, 101499 (2023).
Sekhon, S. S., Kaur, P., Kim, Y. H. & Sekhon, S. S. 2D graphene oxide-aptamer conjugate materials for cancer diagnosis. NJP 2D Mater. Appl. 5, 21 (2021).
Kim, J. et al. Wearable bioelectronics: enzyme-based body-worn electronic devices.Acc. Chem. Res. 51, 2820–2828 (2018).
Hong, S. et al. A 4.9 mΩ-sensitivity mobile electrical impedance tomography IC for early breast-cancer detection system. IEEE J. Solid-State Circuits 50, 245–257 (2014).
Adler, A., Grychtol, B. & Bayford, R. Why is EIT so hard, and what are we doing about it. Physiol. Meas. 36, 1067–1073 (2015).
Soleimani, M., Gómez-Laberge, C. & Adler, A. Imaging of conductivity changes and electrode movement in EIT. Physiol. Meas. 27, S103 (2006).
Chen, J. et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. J. Am. Coll. Cardiol. 56, 702–711 (2010).
Fazel, R. et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N. Engl. J. Med. 361, 849–857 (2009).
Aldrich, J. E. Basic physics of ultrasound imaging. Crit. Care Med. 35, S131–S137 (2007).
Hu, H. Y., Hu, C. H., Guo, W., Zhu, B. P. & Wang, S. Y. Wearable ultrasound devices: an emerging era for biomedicine and clinical translation. Ultrasonics 142, 107401 (2024).
Liu, T. et al. Emerging wearable acoustic sensing technologies. Adv. Sci. 12, e2408653 (2025).
Gonzalez-Hernandez, J.-L. et al. Technology, application and potential of dynamic breast thermography for the detection of breast cancer. Int. J. Heat. Mass Transf. 131, 558–573 (2019).
Gu, M., Guo, C. F. & Song, Y. Multimodal bioelectronics: a pathway to digital health management. Matter 8, 102048 (2025).
Mahato, K. et al. Hybrid multimodal wearable sensors for comprehensive health monitoring. Nat. Electron. 7, 735–750 (2024).
La, T. G. & Le, L. H. Flexible and wearable ultrasound device for medical applications: a review on materials, structural designs, and current challenges. Adv. Mater. Technol. 7, 2100798 (2022).
Li, Y. et al. Multifunctional microneedle patches via direct ink drawing of nanocomposite inks for personalized transdermal drug delivery. ACS Nano 17, 19925–19937 (2023).
Yi, H.-G. et al. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Controlled Release 238, 231–241 (2016).
Cheng, X., Hu, S. & Cheng, K. Microneedle patch delivery of PROTACs for anti-cancer therapy. ACS Nano 17, 11855–11868 (2023).
Li, L. et al. A spatially distributed microneedle system for bioorthogonal T cell-guided cancer therapy. Adv. Sci. 12, 2416841 (2025).
Wang, H. et al. A wearable transdermal device for on-demand drug delivery. Matter 8, 102040 (2025).
Jeong, J., Jang, D., Kim, D., Lee, D. & Chung, S. K. Acoustic bubble-based drug manipulation: carrying, releasing and penetrating for targeted drug delivery using an electromagnetically actuated microrobot. Sens. Actuators, A 306, 111973 (2020).
Lee, J. W., Gadiraju, P., Park, J.-H., Allen, M. G. & Prausnitz, M. R. Microsecond thermal ablation of skin for transdermal drug delivery. J. Controlled Release 154, 58–68 (2011).
Nazary Abrbekoh, F. et al. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 13, 20417314221085390 (2022).
Pikal, M. J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 46, 281–305 (2001).
Parhi, R. & Mandru, A. Enhancement of skin permeability with thermal ablation techniques: concept to commercial products. Drug Deliv. Transl. Res. 11, 817–841 (2021).
Joshi, A. & Raje, J. Sonicated transdermal drug transport. J. Controlled Release 83, 13–22 (2002).
Hu, T. et al. Microneedles with an anisotropic porous microstructure facilitate the transdermal delivery of small molecules, lipid nanoparticles, and T cells. Matter 8, 102038 (2025).
Tao, J. et al. Dual metal nanoflower oxygen pump microneedles based on cuproptosis and STING pathway activation for cancer immunotherapy. Small 21, 2409187 (2025).
Joo, S. H., Kim, J., Hong, J., Fakhraei Lahiji, S. & Kim, Y. H. Dissolvable self-locking microneedle patches integrated with immunomodulators for cancer immunotherapy. Adv. Mater. 35, 2209966 (2023).
Jin, T. et al. A wireless operated flexible bioelectronic microneedle patch for actively controlled transdermal drug delivery. Adv. Mater. 37, 2417136 (2025).
Kim, T. H. et al. Smartphone-based iontophoresis transdermal drug delivery system for cancer treatment. J. Controlled Release 364, 383–392 (2023).
Ma, X. et al. Stretchable and skin-attachable electronic device for remotely controlled wearable cancer therapy. Adv. Sci. 10, 2205343 (2023).
Shao, J., Li, X., Li, Y., Lin, J. & Huang, P. Self-heating multistage microneedle patch for topical therapy of skin cancer. Adv. Mater. 36, 2308217 (2024).
Wang, C. et al. Enhancing deep-seated melanoma therapy through wearable self-powered microneedle patch. Adv. Mater. 36, 2311246 (2024).
Yang, J. et al. A smart silk-based microneedle for cancer stem cell synergistic immunity/hydrogen therapy. Adv. Funct. Mater. 32, 2206406 (2022).
Parrilla, M. et al. Wearable microneedle-based array patches for continuous electrochemical monitoring and drug delivery: toward a closed-loop system for methotrexate treatment. ACS Sens. 8, 4161–4170 (2023).
Zhang, Q., Luo, Q., Liu, Z., Sun, M. & Dong, X. Nano-ROS-generating approaches to cancer dynamic therapy: lessons from nanoparticles. Chem. Eng. J. 457, 141225 (2023).
del Río, L. A. & López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 57, 1364–1376 (2016).
Zandalinas, S. I. & Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 122, 21–27 (2018).
Peltier, A., Seban, R. D., Buvat, I., Bidard, F. C. & Mechta-Grigoriou, F. Fibroblast heterogeneity in solid tumors: from single cell analysis to whole-body imaging. Semin. Cancer Biol. 86, 262–272 (2022).
Kwon, S., Ko, H., You, D. G., Kataoka, K. & Park, J. H. Nanomedicines for reactive oxygen species mediated approach: an emerging paradigm for cancer treatment. Acc. Chem. Res. 52, 1771–1782 (2019).
Zafar, A., Khatoon, S., Khan, M. J., Abu, J. & Naeem, A. Advancements and limitations in traditional anti-cancer therapies: a comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 16, 607 (2025).
Lane, R. J. et al. Challenges in chemotherapy delivery: comparison of standard chemotherapy delivery to locoregional vascular mass fluid transfer. Future Oncol. 14, 647–663 (2018).
Chandra, R. A., Keane, F. K., Voncken, F. E. & Thomas, C. R. Contemporary radiotherapy: present and future. Lancet 398, 171–184 (2021).
Taefehshokr, S. et al. Cancer immunotherapy: Challenges and limitations. Pathol., Res. Pract. 229, 153723 (2022).
Tokumaru, Y., Joyce, D. & Takabe, K. Current status and limitations of immunotherapy for breast cancer. Surgery 167, 628–630 (2020).
Lahiri, A. et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol. Cancer 22, 40 (2023).
Jiang, W., Liang, M., Lei, Q., Li, G. & Wu, S. The current status of photodynamic therapy in cancer treatment. Cancers 15, 585 (2023).
Niculescu, A.-G. & Grumezescu, A. M. Photodynamic therapy—an up-to-date review. Appl. Sci. 11, 3626 (2021).
Gong, Z. & Dai, Z. Design and challenges of sonodynamic therapy system for cancer theranostics: from equipment to sensitizers. Adv. Sci. 8, 2002178 (2021).
Rengeng, L., Qianyu, Z., Yuehong, L., Zhongzhong, P. & Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagn. Photodyn. Ther. 19, 159–166 (2017).
Zhou, W. et al. Organic nanomaterials for near-infrared light-triggered photothermal/thermodynamic combination therapy. Dyes Pigm. 205, 110499 (2022).
Hu, H. et al. Emerging nanomedicine-enabled/enhanced nanodynamic therapies beyond traditional photodynamics. Adv. Mater. 33, 2005062 (2021).
Correia, J. H., Rodrigues, J. A., Pimenta, S., Dong, T. & Yang, Z. C. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics 13, 1332 (2021).
Calzavara-Pinton, P., Venturini, M. & Sala, R. Photodynamic therapy: update 2006 Part 1: photochemistry and photobiology. J. Eur. Acad. Dermatol. Venereol. 21, 293–302 (2007).
Jeon, Y. et al. Parallel-stacked flexible organic light-emitting diodes for wearable photodynamic therapeutics and color-tunable optoelectronics. ACS Nano 14, 15688–15699 (2020).
Costley, D. et al. Treating cancer with sonodynamic therapy: a review. Int. J. Hyperth. 31, 107–117 (2015).
Song, X. R. et al. Nanomedicine-enabled sonomechanical, sonopiezoelectric, sonodynamic, and sonothermal therapy. Adv. Mater. 35, e2212259 (2023).
Gong, Z. R. & Dai, Z. F. Design and challenges of sonodynamic therapy system for cancer theranostics: from equipment to sensitizers. Adv. Sci. 8 (2021).
Fabiano, A. R. et al. Applying ultrasound to mechanically and noninvasively sensitize prostate tumors to TRAIL-mediated apoptosis. Adv. Sci. 12, e2412995 (2025).
Yuan, X. et al. Piezoelectricity, pyroelectricity, and ferroelectricity in biomaterials and biomedical applications. Adv. Mater. 36, 2308726 (2024).
Dong, L. et al. Energy-converting biomaterials for cancer therapy: Category, efficiency, and biosafety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 13, e1663 (2021).
Zhu, Y. J. et al. Rational design of biomaterials to potentiate cancer thermal therapy. Chem. Rev. 123, 7326–7378 (2023).
Danewalia, S. & Singh, K. Bioactive glasses and glass–ceramics for hyperthermia treatment of cancer: state-of-art, challenges, and future perspectives. Mater. Today Bio 10, 100100 (2021).
Ribeiro, P. T., Moreira, J. A., Monterio, J. F. & Laranjeira, S. M. Nanomaterials in cancer: reviewing the combination of hyperthermia and triggered chemotherapy. J. Controlled Release 347, 89–103 (2022).
Mortezaee, K. et al. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 269, 119020 (2021).
Shi, C. R., Zhou, Z. J., Lin, H. Y. & Gao, J. H. Imaging beyond seeing: early prognosis of cancer treatment. Small Methods 5, e2001025 (2021).
Pickles, M. D., Lowry, M. & Gibbs, P. Pretreatment prognostic value of dynamic contrast-enhanced magnetic resonance imaging vascular, texture, shape, and size parameters compared with traditional survival indicators obtained from locally advanced breast cancer patients. Invest. Radiol. 51, 177–185 (2016).
Maltoni, M. et al. Prognostic factors in advanced cancer patients:: evidence-based clinical recommendations -: a study by the steering committee of the European Association for palliative care. J. Clin. Oncol. 23, 6240–6248 (2005).
Abramson, A. et al. A flexible electronic strain sensor for the real-time monitoring of tumor regression. Sci. Adv. 8, eabn6550 (2022).
Kurtz, D. M. et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat. Biotechnol. 39, 1537–1547 (2021).
Fang, P. et al. Self-healing electronics for prognostic monitoring of methylated circulating tumor DNAs. Adv. Mater. 35, 2207282 (2023).
Widmer, N. et al. Review of therapeutic drug monitoring of anticancer drugs part two - targeted therapies. Eur. J. Cancer 50, 2020–2036 (2014).
Lin, S. et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci. Adv. 8, eabq4539 (2022).
Wang, M. et al. Printable molecule-selective core–shell nanoparticles for wearable and implantable sensing. Nat. Mater. 24, 1–10 (2025).
Shin, B. et al. Automatic clinical assessment of swallowing behavior and diagnosis of silent aspiration using wireless multimodal wearable electronics. Adv. Sci. 11, e2404211 (2024).
Nguyen, N. P., Sallah, S., Karlsson, U. & Antoine, J. E. Combined chemotherapy and radiation therapy for head and neck malignancies - quality of life issues. Cancer 94, 1131–1141 (2002).
Rafeedi, T. et al. Wearable, epidermal devices for assessment of swallowing function. npj Flex. Electron 7, 52 (2023).
Qaiser, N. et al. A robust wearable point-of-care CNT-based strain sensor for wirelessly monitoring throat-related illnesses. Adv. Funct. Mater. 31, 2103375 (2021).
Xu, H. et al. A fully integrated, standalone stretchable device platform with in-sensor adaptive machine learning for rehabilitation. Nat. Commun. 14, 7769 (2023).
Kang, Y. J. et al. Soft skin-interfaced mechano-acoustic sensors for real-time monitoring and patient feedback on respiratory and swallowing biomechanics. npj Digital Med. 5, 147 (2022).
Ramírez, J. et al. Metallic nanoislands on graphene for monitoring swallowing activity in head and neck cancer patients. ACS Nano 12, 5913–5922 (2018).
Song, Y., Yun, I., Giovanoli, S., Easthope, C. A. & Chung, Y. Multimodal deep ensemble classification system with wearable vibration sensor for detecting throat-related events. npj Digital Med. 8, 14 (2025).
Shin, B. et al. Automatic clinical assessment of swallowing behavior and diagnosis of silent aspiration using wireless multimodal wearable electronics. Adv. Sci. 11, 2404211 (2024).
Qiao, Y. et al. Electromyogram-strain synergetic intelligent artificial throat. Chem. Eng. J. 449, 137741 (2022).
Liu, T. et al. Machine learning-assisted wearable sensing systems for speech recognition and interaction. Nat. Commun. 16, 2363 (2025).
Che, Z. et al. Speaking without vocal folds using a machine-learning-assisted wearable sensing-actuation system. Nat. Commun. 15, 1873 (2024).
Cheng, X. et al. A flexible large-area triboelectric generator by low-cost roll-to-roll process for location-based monitoring. Sens. Actuators A 247, 206–214 (2016).
Wang, M., Yang, Y. & Gao, W. Laser-engraved graphene for flexible and wearable electronics. Trends Chem. 3, 969–981 (2021).
Zhang, X. P. et al. An update on biomaterials as microneedle matrixes for biomedical applications. J. Mater. Chem. B 10, 6059–6077 (2022).
Acknowledgements
The work was supported by the Natural Science Foundation of Guangdong Province, China (2025A1515010362), City University of Hong Kong (9382003, 7020123), the University Grants Committee’s Teaching Start-up Grant (6000908) and National High Level Hospital Clinical Research Funding (BJ-2023-194). The human hand and six icons in Fig. 9 are created from Figdraw (https://www.figdraw.com/). The conceptual diagrams in Fig. 1 to Fig. 4 are drawn in Biorender.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hua, Z., Dai, C., Yang, Y. et al. Wearable bioelectronics for cancer theranostics. Microsyst Nanoeng 11, 180 (2025). https://doi.org/10.1038/s41378-025-01048-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41378-025-01048-5
This article is cited by
-
Harnessing the power of ternary nanocomposites in comprehensive cancer management
Discover Sensors (2025)