Abstract
Melanoma is the leading cause of death among cutaneous neoplasms. Best outcome relies on early detection and accurate pathologic diagnosis. For the great majority of melanocytic tumors, histopathologic examination can reliably distinguish nevi from melanomas. However, there is a subset of melanocytic tumors that cannot be definitively classified as benign or malignant using histopathological criteria alone. These tumors are usually diagnosed using terms that imply various degrees of uncertainty in regards to their malignant potential and create the possibility for over or undertreatment. For such tumors, additional ancillary tests would be beneficial in adjudicating a more definitive diagnosis. In recent years, DNA-based molecular ancillary tests, specifically comparative genomic hybridization and fluorescence in situ hybridization, have been developed to help guide the diagnosis of ambiguous melanocytic proliferations. This study will present an updated overview of these two major ancillary tests, which are currently being used in clinical practice to assist in the diagnosis of challenging melanocytic neoplasms.
Similar content being viewed by others
Introduction
From the time of the early pioneers of dermatopathology such as Simon and Unna, until the second half of the twentieth century, the pathologic diagnosis of melanocytic lesions was accomplished largely by traditional means: histological examination being the gold standard, ideally in conjunction with adequate clinical history and correlation. Although the microscope used by Unna in those early days would be considered primitive by current standards, the basic technology remains the same today. Light microscopic evaluation by a trained pathologist can provide an accurate diagnosis for the great majority of melanocytic lesions. There is, however, a small subset of melanocytic lesions that eludes appropriate classification by conventional light microscopy alone, preventing accurate prediction of clinical behavior and recommendations for appropriate treatment. Furthermore, inter-observer variability in the classification of melanocytic lesions has been well documented [1,2,3] and uncertainties can lead to both over and undertreatment. Histologically ambiguous melanocytic lesions are often treated more aggressively in order to avoid “missing” a bad actor, as the realm of melanocytic lesions has been one of the well-recognized legal peril [4]. In recent years, two major ancillary tests, comparative genomic hybridization (CGH) and fluorescence in situ hybridization (FISH), have been developed to help guide the diagnosis of ambiguous melanocytic proliferations.
In this overview, we discuss CGH and FISH, and their application to melanocytic lesions. We provide a brief survey of their technical aspects, appropriate use, and limitations.
Comparative genomic hybridzation
The idea that melanomas are characterized by multiple chromosomal copy number abnormalities (CNAs) was suggested a few decades ago by studies that reported chromosomal copy number differences between nevi and melanomas using conventional cytogenetics [5,6,7]. These initial discoveries formed the theoretical basis for further studies using CGH, and later FISH.
Background of the technique
The power of CGH lies in its ability to detect DNA copy number alterations across the entire genome. There are two major variations of this technique: classic and array-based.
Classic CGH
In classic CGH, tumor and reference DNA (from normal human tissue) are differentially labeled using fluorochromes, mixed in a 1:1 ratio, and hybridized onto a substrate of normal metaphase chromosomes. Following a washing step, the metaphase slides are scanned. The resulting wavelength is reflective of differences in relative proportion of tumor vs. normal DNA and can be used to detect genomic areas with gains or losses of DNA material [8,9,10]. The technique was subsequently improved to allow for analysis of archival paraffin-embedded samples [11]. CGH has a significant advantage over standard cytogenetics in that it does not require culturing the cells of interest and thus can be performed on archival formalin-fixed paraffin-embedded (FFPE) tissue. It also obviates any biological selection that may take place during the cell culturing used in traditional cytogenetics [12].
Array-based CGH and SNP arrays
The development of array-based CGH, where arrayed artificial genomic clones replace normal metaphase chromosomes as a substrate, led to increased resolution, reproducibility, and robustness of the assay, and has replaced classic CGH [13,14,15]. Each dot on the array contains genomic DNA from a specific locus and the resolution of the array is proportional with the number of dots. The assay can be performed on some platforms by co-hybridizing tumor and normal DNA onto the array, similar to classic CGH, or on other platforms by hybridizing only labeled tumor DNA. For the latter variation, after scanning, the copy number status at a certain locus is estimated by comparing the signal intensity of the tumor with that of a reference from a control series of non-tumoral tissue.
Recently, single-nucleotide polymorphism (SNP) arrays have been employed as alternatives to CGH. For these arrays, the probed loci are selected to flank known SNPs and each genomic locus is represented by two dots on the array corresponding to the two alleles. SNP arrays have the additional advantage of being able to detect allelic ratio and loss of heterozygosity (LOH), including copy-neutral LOH alterations that are missed by CGH arrays [16]. In addition, SNP arrays can also be used to identify selected point mutations. In recent years, protocols employing molecular inversion probes (MIPs) that use low amounts of tumor DNA from FFPE tissue have been developed. The probes have a footprint of only 40 bp, which allows evaluation of highly degraded DNA. In addition, as at the completion of the procedure the inverted MIPs are hybridized to the microarray, the signal-to-noise ratio is greatly improved compared with conventional SNP arrays in which the tumor DNA is directly hybridized [17, 18].
A typical SNP array plot consists of two tracks (Fig. 1). The upper track shows copy number status (log ratio on the vertical and chromosome locus on the horizontal). Gains and losses are reflected by deflections of the average line above or below 0, respectively (Fig. 1, red arrow). The lower track shows the B-allele frequency for each SNP on the array (B-allele frequency on the vertical and chromosome locus on the horizontal). In the normal state, this track consists of three lines, a middle heterozygous line and two outer lines that are homozygous for the A and B alleles. An LOH event is represented by a split in the middle heterozygous line. This is usually associated with corresponding losses or gains at that locus; however, occasionally, an LOH without numerical abnormalities can be encountered, representing a copy-neutral LOH, which is a form of acquired uniparental disomy (Fig. 1, black arrow).
The upper track shows copy number status (log ratio on the vertical and chromosome locus on the horizontal). Gains and losses are reflected by deflections of the average yellow line above or below 0, respectively. Red arrow indicates a one copy number gain of chromosome 11p. The lower track shows the B-allele frequency for each SNP on the array (B-allele frequency on the vertical and chromosome locus on the horizontal). In the normal state, this track consists of three lines: a middle heterozygous line and two outer lines that are homozygous for the A and B alleles. Black arrow indicated a copy-neutral LOH of chromosome 14q (note that there are no copy number changes detected in the upper track for 14q).
CGH in the evaluation of melanocytic lesions
In 1994, uveal melanomas were the first melanocytic tumors to be studied by CGH and were found to harbor frequent gains of chromosome arms 6p and 8q, and losses of chromosome 3, 6q, and 9p [12]. A much cited early study by Bastian et al. [19] indicated that loses of chromosomes 9 and 10 occurred early in melanoma progression, with gains of chromosome 7 occurring later. A later study found that among 16 primary and 12 metastatic melanomas, the mean number of genetic changes was 6.3 (range 1–14) in primary melanomas and 7.8 (range 1–16) in metastatic lesions. The number of genetic alterations was significantly higher in primary tumors that developed metastases within a year of surgery, in comparison with those that did not [20].
CGH as a diagnostic tool
The potential utility of CGH as a diagnostic tool was suggested most forthrightly by Bastian et al. [21] in a study from 2003, in which the authors found that 96.2% of melanomas displayed numerical aberrations involving segments of chromosomes, in comparison with 13.0% of benign nevi (out of a total of 132 melanomas and 54 benign nevi). This non-overlapping pattern of genomic abnormalities provided the impetus for developing diagnostic strategies based on tests evaluating CNAs such as CGH or SNP arrays (Fig. 2). A more recent study showed a specificity and sensitivity of 94.7% in separating unambiguous nevi from melanomas, using array-based CGH [22]. Array-based CGH was also used to compare copy number changes in melanomas from chronically sun-exposed vs. melanomas arising in sun-protected areas; it was found that melanomas on protected surfaces (acral and mucosal) had a significantly higher degree of chromosomal changes in comparison with melanomas on the skin with various degrees of exposure to the sun [23].
a An example of nodular melanoma. b SNP-array plot of melanoma from a showing numerous gains and losses of segments of chromosomes. c An example of conventional nevus. d SNP-array plot of nevus from c showing no CNAs. e Aggregate frequency of CNAs in a series of 41 melanomas and 18 nevi from University of Michigan Dermatopathology database (frequency of gains (blue) and losses (red) on the vertical and chromosome locus on the horizontal). Top panel shows numerous gains and losses in melanomas. Bottom panel shows a low frequency of isolated abnormalities in nevi with isolated losses of 3p (red) and gains of 11p and 15q (blue).
Several studies investigated the role of CGH as a potential ancillary tool for melanocytic tumors that traditionally pose diagnostic challenges using histology alone. These include the blue nevus-like group (cellular blue nevus, atypical cellular blue nevus, and melanoma arising in/resembling blue nevus) and spitzoid tumors (Spitz nevus, atypical Spitz tumor (AST), and spitzoid melanoma). Proliferative nodules in congenital nevi can also be problematic.
CGH in blue nevus-like melanocytic lesions
For the blue nevus-like group, an initial study of 10 histologically unequivocal cellular blue nevi and 1 deep penetrating nevus (by CGH) showed no chromosomal aberrations, in comparison with atypical cellular blue nevi, which showed abnormalities in 3 of 11 lesions, and overt melanomas arising in the blue nevus, which showed abnormalities in all 7 lesions studied [24]. Following this, other investigators have reported a non-overlapping pattern of chromosomal abnormalities between cellular blue nevi and melanoma [25,26,27,28,29]. The melanomas arising in cellular blue nevi tend to display multiple segmental copy number variations, similar to other melanomas. In contrast, cellular and atypical cellular blue nevi demonstrate no abnormalities or a small fraction may show one or two isolated CNAs [25]. An important finding was the association between deletion of 3p21 spanning the locus for BAP1 gene (associated with lack of BAP1 expression by immunohistochemistry) and poor prognosis in melanomas arising in blue nevi [25, 26].
CGH in spitzoid (and related) melanocytic neoplasms
Application of molecular techniques to spitzoid neoplasms is particularly relevant, as these lesions can be a source of diagnostic uncertainty among pathologists [30]. A study in the late 1990s showed clear molecular differences between Spitz nevi and melanomas, with the majority of Spitz nevi being of normal chromosome complement (with the exception of 11p gains in 4 of 17 cases) [31]. A later study of spitzoid neoplasms showed no chromosomal aberrations in seven of eight Spitz nevi; one Spitz nevus showed gains in both 7p and 11p (both of which are now commonly recognized to occur in benign Spitz nevi). In contrast, melanomas with spitzoid features showed multiple segmental chromosomal abnormalities [32].
Initial studies have shown that the majority of nevi have no CNAs by CGH. However, in recent years it has become apparent that certain types of benign nevi or indolent melanocytic proliferations are characterized by isolated genomic abnormalities, which in some cases can be detected by CGH/SNP array (Fig. 3). These findings are important if one is to use CGH to support a diagnosis of melanoma or nevus. Finding isolated CNAs in a melanocytic neoplasm does not imply necessarily that it is a melanoma; in fact, specific abnormalities, when present in isolation in a histologically atypical tumor, may support an indolent biologic behavior. Among spitzoid tumors, much progress has been made in recent years in identifying driver genetic alterations [33,34,35,36]. The first observation was made over 20 years ago when a subset of Spitz nevi was found to harbor HRAS gene mutations and to demonstrate gains of 11p by CGH [33]. On histology, these lesions are intradermal, associated with marked desmoplasia, have an infiltrative pattern, and may show some cytologic atypia and increased proliferation, features now recognized as characteristic for desmoplastic variant of Spitz nevus (Fig. 3a, b). In this context, finding an isolated 11p gain by CGH/SNP array in an atypical spitzoid neoplasm supports a diagnosis of Spitz nevus (Fig. 3c). A more recent study has found that almost half of spitzoid lesions including Spitz nevi, ASTs, and spitzoid melanomas harbor specific rearrangements resulting in kinase fusions of ROS1, NTRK1, NTRK3, ALK, BRAF, MET, and RET [34, 37]. The fusions can be detected by CGH/SNP array if they are the result of unbalanced translocations (Fig. 3j–l) [38].
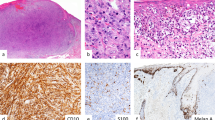
a–c Desmoplastic Spitz nevus. a Intradermal melanocytic proliferation with pronounced desmoplastic stromal reaction and infiltrative growth pattern. b Epithelioid cells with a large nuclei, prominent nucleoli, and abundant amphophilic cytoplasm. Two mitotic figures are noted in the center of the field (arrows). c SNP array showing a gain of 11p (arrow) with no additional abnormalities, suggesting a desmoplastic Spitz nevus. d–f BAP1-inactivated tumor. d Large, predominantly intradermal tumor with biphenotypic morphology and a lymphocytic host response. e Top, majority of the lesion is composed of epithelioid cells with large nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm with distinct cell membrane. Bottom, a component or ordinary nevus is identified. f SNP array showing a loss of 3p21 (arrow) with no additional abnormalities, suggesting an indolent BAP1-inactivated tumor/nevus. g–i Proliferative nodule arising in congenital nevus. g Hypercellular non-expansile dermal nodule composed of densely packed uniform melanocytes. h Melanocytes with round to oval hyperchromatic and slightly irregularly shaped nuclei with a moderate amount of eosinophilic cytoplasm, high nuclear to cytoplasmic ratio, and increased mitotic rate. i SNP array showing gains of whole chromosomes 2, 6, 13, 15, 16, and 22 suggestive of a proliferative nodule (arrow). j–l Low-risk atypical Spitz tumor with NTRK1 rearrangements. j Predominately intradermal melanocytic proliferation with a bulbous profile. k Epithelioid cells with intermediate size nuclei and moderate amount of eosinophilic cytoplasm arranged in back to back small nests with occasional mitotic figures (arrow). l SNP array results showing chromosome 1q. A small deletion is noted with breakpoints within LMNA and NTRK1 genes (arrows) resulting in an LMNA-NTRK1 fusion product.
Another important discovery was of a subset of melanocytic tumors with epithelioid morphology, atypical features, and inactivation of the BAP1 gene. These lesions in the past were frequently diagnosed as atypical Spitz tumors [39, 40]. Histology shows a dermal proliferation of epithelioid melanocytes with abundant eosinophilic cytoplasm, distinct cellular membranes, large nuclei with vesicular chromatin, and prominent nucleoli, imparting a spitzoid appearance. In contrast to most Spitz nevi, the epidermis is atrophic. An associated banal nevus component and a conspicuous lymphocytic infiltrate are often present. Worrisome histologic features include cytological atypia and lack of maturation (Fig. 3d, e). BAP1 stain shows loss of expression in the large epithelioid cells with preservation of staining in the banal nevus component and can be used as a diagnostic tool. These lesions also harbor BRAF gene mutations. Despite being atypical, these lesions are generally characterized by an indolent biologic behavior. By CGH, BAP1-inactivated nevi are characterized by an isolated deletion on chromosome 3 of various size but always spanning the 3p21 area where the BAP1 gene resides (Fig. 3f).
CGH in congenital melanocytic proliferations and proliferative nodules
Congenital melanocytic proliferations and, more specifically, proliferative nodules developing in congenital nevi can also present diagnostic challenges. These are cellular melanocytic nodules with varying degrees of cytologic atypia and proliferative activity (Fig. 3g, h). There is significant clinical importance in differentiating them from melanomas arising in congenital nevi [41, 42]. By CGH, these lesions demonstrate gains and/or losses of entire chromosomes in contrast to melanomas that harbor CNAs involving partial segments of chromosomes (Fig. 3i) [43, 44].
CGH and difficult-to-classify melanocytic lesions
One of the major limitations of our current knowledge in regards to CGH/SNP arrays is the relative lack of robust studies correlating the patterns of CNAs with outcomes data in the histologically ambiguous melanocytic lesions for which CGH is needed the most. There are studies documenting that in melanoma, the number of CNAs correlate with progression from in situ to invasive and to distant spread, and that high number of focal CNAs and the presence of chromothripsis (an event where numerous genomic rearrangements occur at once [45]) correlate with poor outcome [46, 47]. One group found that that melanocytic lesions that developed of metastases showed significantly more chromosomal aberrations on array CGH compared with those without metastases [48].
One can extrapolate these results to suggest that the same holds true for histologically borderline melanocytic tumors. A study by Ali et al. [32] on ten spitzoid neoplasms included some clinical follow-up data: a spitzoid neoplasm in this study from a 6-year-old male had one identifiable copy number change, gain in 19p, and clinically had lymph node involvement and local recurrence. A melanoma in that same study showed multiple chromosomal aberrations and a clinical course significant for distant metastases [32]. Future studies may allow for a better correlation with outcome. A second major limitation of CGH is the lack of clearly defined cutoff values or patterns that correlate with poor outcome in cases with small number of CNAs and more research is needed towards this end.
Fluorescence in situ hybridization
Background of the technique
FISH is a technique in which fluorescently labeled probes are directed against specific segments of genomic DNA and hybridized to tissue samples. Samples can be fresh, frozen, or paraffin-embedded tissue sections and a fluorescent microscope is used for detection. Advantages of this technique include limited tissue requirements and the ability to directly visualize cells of interest. The direct visualization allowed by this technique is particularly helpful for tissue specimens that are obscured by inflammation or other non-tumoral cells. Similar to CGH, FISH allows for the detection of chromosomal numerical abnormalities, based on losses or gains of fluorescent signals. A normal/non-neoplastic cell is expected to have two copies of any given chromosome or chromosomal fragment; therefore, identification of more or of less than two fluorescent signals is indicative of chromosomal gains or losses, respectively [49].
The original application of FISH to melanocytic lesions utilized a four-probe dataset, derived from earlier data on DNA copy number alterations. As the technique has evolved, refinements to this original four-probe dataset have been made in order to improve the assay, as discussed below.
FISH in the evaluation of melanocytic lesions
Investigation of melanocytic lesions using FISH began in earnest in the late 1990s. In 1994, Matsuta et al. [50] applied FISH using a centromeric probe specific for chromosome 17 and found that melanomas display more signals in comparison with benign nevi. In a follow-up study, some of the same authors reported their evaluation of 22 melanomas (14 primary and 8 metastatic) for chromosomal copy number changes and observed gains of chromosome 6 and 7, and/or losses of chromosome 9 and 10, which they suggested might play a role in melanoma development and progression [51].
Initial studies and proofs of principle
Similar to CGH, FISH has been used as an ancillary technique to assist in differentiating benign nevi from melanomas by identifying numerical abnormalities at probed loci. Selecting probes on the basis of existing CGH data [19], an initial study by Gerami et al. [52] aimed to develop a multiplex FISH assay by selecting the minimum number of probes capable of providing the highest discrimination between nevus and melanoma from a larger set of probes targeting loci found to be more frequently altered. The first probe set employed probes for 6p25 (RREB1), 6q23 (MYB), 11q13 (CCND1), and centromere 6 (CEP6), and had a sensitivity of 86.7% and a specificity of 95.4% in differentiating unequivocal melanomas from nevi [52].
Using this four-probe set, FISH analysis is performed by evaluating 30 nuclei and calculating the percentage of nuclei with more than 2 signals per nucleus for 6p25 and 11q13, and with less signals for 6q23 compared with CEP6. The test is considered positive if it demonstrates gains for 6p25 or 11q13, or losses of 6q23 (compared with CEP6) relative to validated cutoff values. In a follow-up study, the sample size was expanded and a sensitivity of 83.0% and specificity of 94.0% were reported [53]. Different authors, using the same probe set, studied 40 histologically unequivocal melanocytic tumors (10 metastatic melanoma, 10 primary melanomas, and 20 benign melanocytic nevi) and showed distinction of malignant from benign lesions with 90% sensitivity and 95% specificity [54]. Furthermore, in a study of 144 primary melanomas, it was found that a positive FISH result was an independent adverse prognostic marker, and that FISH-positive primary melanomas had a significantly increased risk of metastasis or melanoma-related death in comparison with FISH-negative cases [55].
Further studies examined the use of this four-probe set on various types of melanocytic lesions. A study on melanocytic deposits in sentinel lymph nodes supported the use of FISH in differentiating nodal nevi from metastatic melanoma [56]. The standard four-probe set was also found to assist in the distinction of nevoid melanoma from mitotically active nevi [57], conjunctival nevi from conjunctival melanoma [58], and epithelioid blue nevus from blue nevus-like melanoma [59]. Similarly, FISH has been suggested as a tool for microstaging of melanoma associated with nevi [60]. A modestly sized study of 12 unequivocal spitzoid melanomas and 6 Spitz nevi showed a sensitivity of 87.5% and a specificity of 100% in differentiating spitzoid melanomas from Spitz nevi [61]. FISH testing was found to have a lower sensitivity for spindle cell melanoma and spitzoid melanoma; a study of 22 pigmented spindle cell nevi and 24 spindle cell melanomas found that FISH testing had a 73% sensitivity and 93% specificity in separating malignant from benign [62]. Another study of 43 unequivocal spitzoid melanomas using the same standard probes against chromosomes 6 and 11 found a sensitivity of only 70%. This sensitivity was improved to 85% when the standard assay was combined with 9p21 [63].
FISH and difficult-to-classify melanocytic lesions
Studies investigating the utility of FISH in ambiguous or difficult-to-classify melanocytic lesions have, in general, found lower sensitivity and specificity than studies comparing only unequivocal lesions. The most robust data regarding ambiguous lesions comes from association with outcomes data. Robust data, however, is difficult to obtain, as many ambiguous melanocytic lesions are treated (altering the natural disease course) and adverse events can be experienced years (sometimes many years) later. What follows is a highlighting of some of the most pertinent available data.
The original study by Gerami et al. [52] included 27 histologically ambiguous lesions, 6 of which developed metastasis. All six cases that developed metastasis were positive by FISH [52]. In a study of 38 atypical spitzoid lesions, the only fatal outcome showed multiple chromosomal alterations by standard FISH [64]. In a separate study with nine histologically challenging lesions, a positive FISH result was obtained in one lesion only. This patient developed lymph node metastasis 14 years later [65]. A study of two spitzoid lesions with fatal outcomes showed a sensitivity of 50% (one of two lesions accurately detected) by FISH using standard probes. The authors of that study concluded that the current panel of FISH probes failed to detect the ASTs that had copy number variations by array CGH, including a metastatic and fatal AST. This group suggested that a comprehensive, genome-wide approach would offer great sensitivity than FISH probes alone [66]. In one of the more sizeable studies, Vergier et al. [67] showed a sensitivity of 85% and a specificity of 90% using a four-probe FISH assay on 43 non-equivocal (non-ambiguous) melanomas and nevi. However, when FISH was evaluated on 90 ambiguous lesions with outcomes data (the presence of metastases at minimum follow-up of 5 years), the sensitivity and specificity of FISH was more modest (43% and 80%, respectively). Sensitivity and specificity increased to 90% and 76%, respectively, when combined with histological review. These authors conclude that a positive FISH result reinforces a diagnosis of melanoma [67].
Gaiser et al. [48] found a somewhat modest sensitivity when FISH was applied to 12 histologically ambiguous lesions in comparison with clinical behavior (the presence or absence of metastases; mean follow-up of 65 months) with a sensitivity of 60% and a specificity of 50%. These authors reached the conclusion that FISH testing did not reach clinically useful sensitivity and specificity [48]. However, it has been suggested that their data may be less robust due to methodologies, specifically in the diagnostic criteria used [68].
Tetzlaff et al. [69] studied 34 histologically ambiguous melanocytic lesions, with follow-up data available for 25, and found that no metastases were experienced in the 17 lesions favored to be benign (with a mean follow-up of 16.8 months). Among lesions favored to be malignant, during a mean follow-up of 14.6 months, a single lymph node metastasis was identified in a 4-year-old girl, who had negative FISH testing. Based on integrated final diagnosis of all features together, the sensitivity of FISH in this study for the diagnosis of melanoma was 50% and specificity was 87.5%. In all cases, the initial diagnosis remained unchanged. The authors concluded that in their experience, in the setting of a lesion with predominately benign findings, a negative FISH test is a reassuring finding. In contrast, a positive FISH result should be carefully interpreted in the context [69].
Several other groups have published experiential data with FISH. In examining 804 ambiguous melanocytic neoplasms, a group from the University of California San Francisco found that FISH allowed for a more definitive diagnosis in 88% of cases, and was particularly helping if positive [70]. A separate group, who studied 140 histologically ambiguous lesions (with limited follow-up data), also found the use of FISH helpful, while warning about the risk of false-positive results from tetraploidy (see below). Ten lesions with abnormal FISH results were further classified as likely melanoma. No diagnoses were altered based upon negative FISH results [71]. A final group took a more tempered view, highlighting the challenges in studying melanocytic tumors of uncertain malignant potential in the context of a less-than-perfect gold standard [72].
Polyploidy/tetraploidy
One major source of false-positive results is the presence of polyploidy/tetraploidy or balanced chromosomal duplications involving all loci. Tetraploidy has been observed in benign melanocytic lesions, such as Spitz nevi [73]. Rejection of cells showing copy number increases of all probed targets is important in limiting false-positive results [71, 74]. Use of an assay with four separate loci and 9p21 also allows tetraploidy to be recognized with greater confidence, as balanced gains otherwise become statistically unlikely [74].
Beyond the four-probe assay
Incorporating probes for 9p21 (CDKN2A) and 8q24 (MYC) into the four-probe FISH assay was found to improve sensitivity for spitzoid melanomas [63, 74]. A multicenter study described the association of 9p21 homozygous deletion with aggressive clinical behavior in spitzoid melanocytic tumors and also found that 6p25 or 11q13 gains have an increased risk for aggressive clinical behavior compared with FISH-negative ASTs. Interestingly, the presence of isolated 6q23 deletion was not associated with adverse events in spitzoid neoplasms [75]. These studies formed the basis for the addition of 9p21/ Cep9 and 8q24 to the standard four-probe set in some assays. However, even with this improved probe set, not all investigators experienced robust results. One group found low correlation between FISH results and a final diagnosis of melanoma or metastasis [76].
Comparison of FISH and CGH
Concordance between the two methods has been observed to be reasonably high (90% using the original four-probe set) [77] and, from a practical standpoint, both of these techniques have advantages and disadvantages (Table 1). The major advantage of CGH/SNP array is that it allows for interrogation of the entire genome (in comparison with the limited number of genomic loci evaluated via FISH) and is therefore likely more sensitive. In the setting of tetraploidy, CGH/SNP array is also likely to be more specific due to the possible false-positive results seen with FISH [73]. A study by our group showed that for borderline melanocytic lesions FISH has a sensitivity and specificity of only 61% and 84%, respectively, when compared with an SNP array [78].
There are some circumstances, however, when FISH testing may be preferred. FISH can be performed on superficial specimens with limited material (only two unstained slides are needed) or on specimens in which the tumor cells are infiltrated by a significant component of other cell types, as the cells of interest are directly visualized during the test. In contrast, CGH/SNP array requires 20–30% tumor purity and, especially for small biopsies, 10 tissue sections. FISH is less labor intensive than CGH/SNP array in general, with typically lower turnaround times and cost.
Conclusions
Recent years have seen an increase in the use of ancillary molecular tests in the diagnosis of histologically ambiguous or challenging melanocytic tumors. Although there are certain limitations in our knowledge regarding the exact correlation between various patterns of chromosomal abnormalities and outcome, especially in borderline melanocytic tumors, the existing body of literature suggests that both CGH and FISH can be useful in selected clinical scenarios and are indeed being used (Figs. 4 and 5). A study that surveyed a group of dermatopathologists on the use of these tests showed that 54% reported routine use of molecular testing for ambiguous melanocytic lesions with an additional 37% reporting rare use and only 8% reporting to never have used the tests [79].
Histologic diagnosis: Suspicious for melanoma arising in blue nevus vs. atypical cellular blue nevus. a Large dermal and subcutaneous melanocytic lesion displaying two distinct morphologies. There is a background of spindle cells with melanophages consistent with cellular blue nevus and an expansile cellular nodule. b Top, the cellular nodule contains spindle to epithelioid cells with prominent nucleoli, mild hypechromasia, and rare mitoses (arrow). Bottom, spindle cells with intervening melanophages consistent with cellular blue nevus. c SNP array reveals numerous CNAs including gains of 6p supporting a diagnosis of melanoma. Final diagnosis: melanoma arising in blue nevus.
Histologic diagnosis: atypical Spitz tumor of uncertain malignant potential. a Dermal proliferation of melanocytes with an infiltrative pattern associated with a sclerotic stroma and lymphoid aggregates. b Cells show a spitzoid morphology with occasional nuclear hyperchromasia and pleomorphism. c p16 immunohistochemistry shows diffuse loss of expression. d FISH with 9p21 (CDKN2A) probe (red) and centromere 9 probe (green). Neoplastic cells show one green signal (white arrow), indicating one copy of centromere 9 but no red signals. On the left side there is a non-neoplastic cell showing two copies of 9p21 (red arrows). Rendered diagnosis: high-risk atypical Spitz tumor concerning for spitzoid melanoma.
The use of FISH and CGH will likely increase, as their roles are worked out and refined. The greatest utility for these tests is for lesions situated in the center of the biological spectrum (i.e., in lesions that are not obviously benign or malignant by histologic examination). Results of a study involving a panel of 17 experts that were asked to rate the appropriateness of using molecular testing in various clinical scenarios showed that CGH/FISH assays were considered usually appropriate in most clinical scenarios in which the diagnosis of melanoma is in question but histology is not definitively diagnostic (i.e., borderline melanocytic tumors). As expected, molecular testing was not considered appropriate when pathology is definitive for melanoma or nevus [80].
Unfortunately, borderline melanocytic tumors with ambiguous histology may also demonstrate equivocal molecular results such as a low number of chromosomal abnormalities with uncertain biological significance in relation to outcome [35]. In order to make sense of the molecular results, they need always to be interpreted in conjunction with histological findings on routine light microscopy and with the clinical presentation. In an ambiguous lesion, which is favored to be benign by histology, negative FISH and/or CGH testing is reassuring for an indolent biologic behavior and the lesion may be managed as an atypical nevus (i.e., conservative excision), with the caveat that FISH lacks sensitivity compared with CGH. In an ambiguous lesion, which is worrisome for melanoma, a positive FISH and/or CGH test is supportive of that diagnosis and may warrant more aggressive treatment. However, abnormal testing in a lesion that is ambiguous but favored to be benign and negative testing in a lesion favored to be melanoma should be interpreted with caution, as both false-positive and false-negative results can occur [69].
It should be noted that although this study is focused on CGH and FISH, there are other types of ancillary molecular tests designed to differentiate between melanoma and nevus or evaluate prognosis in melanoma. PCR analysis of RNA expression from genes found to be differentially expressed in melanoma (“gene expression signature analysis”) has also shown promising results. Both 23- and 28-gene expression profile tests have been studied and are available commercially [81, 82]. Analysis of telomerase reverse transcriptase promoter mutations with PCR may be helpful in some cases, particularly when positive [83]. The study of epigenetic alterations, such as DNA methylation, has also shown promise [84]. The advantages, disadvantages, and precise roles of each technique will be clarified, as more data and experience is accrued.
The past few decades have seen substantial advances in how we are able to examine melanocytic lesions, with CGH and FISH being prime examples. As more clinical outcome data are collected, the role of these techniques for ambiguous lesions will likely be further refined.
References
Elmore JG, Barnhill RL, Elder DE, Longton GM, Pepe MS, Reisch LM, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ 2017;357:j2813.
Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996;27:528–31.
Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751–6.
Troxel DB. Medicolegal aspects of error in pathology. Arch Pathol Lab Med. 2006;130:617–9.
Balaban G, Herlyn M, Guerry Dt, Bartolo R, Koprowski H, Clark WH, et al. Cytogenetics of human malignant melanoma and premalignant lesions. Cancer Genet Cytogenet. 1984;11:429–39.
Cowan JM, Halaban R, Francke U. Cytogenetic analysis of melanocytes from premalignant nevi and melanomas. J Natl Cancer Inst. 1988;80:1159–64.
Thompson FH, Emerson J, Olson S, Weinstein R, Leavitt SA, Leong SP, et al. Cytogenetics of 158 patients with regional or disseminated melanoma. Subset analysis of near-diploid and simple karyotypes. Cancer Genet Cytogenet. 1995;83:93–104.
Houldsworth J, Chaganti RS. Comparative genomic hybridization: an overview. Am J Pathol. 1994;145:1253–60.
North JP, Vemula SS, Bastian BC. Chromosomal copy number analysis in melanoma diagnostics. Methods Mol Biol. 2014;1102:199–226.
Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science 1992;258:818–21.
Isola J, DeVries S, Chu L, Ghazvini S, Waldman F. Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples. Am J Pathol. 1994;145:1301–8.
Speicher MR, Prescher G, du Manoir S, Jauch A, Horsthemke B, Bornfeld N, et al. Chromosomal gains and losses in uveal melanomas detected by comparative genomic hybridization. Cancer Res. 1994;54:3817–23.
Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet. 1998;20:207–11.
Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, et al. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41–6.
Solinas-Toldo S, Lampel S, Stilgenbauer S, Nickolenko J, Benner A, Dohner H, et al. Matrix-based comparative genomic hybridization: biochips to screen for genomic imbalances. Genes Chromosomes Cancer 1997;20:399–407.
Jacobs S, Thompson ER, Nannya Y, Yamamoto G, Pillai R, Ogawa S, et al. Genome-wide, high-resolution detection of copy number, loss of heterozygosity, and genotypes from formalin-fixed, paraffin-embedded tumor tissue using microarrays. Cancer Res. 2007;67:2544–51.
Chandler WM, Rowe LR, Florell SR, Jahromi MS, Schiffman JD, South ST. Differentiation of malignant melanoma from benign nevus using a novel genomic microarray with low specimen requirements. Arch Pathol Lab Med. 2012;136:947–55.
Wang Y, Carlton VE, Karlin-Neumann G, Sapolsky R, Zhang L, Moorhead M, et al. High quality copy number and genotype data from FFPE samples using Molecular Inversion Probe (MIP) microarrays. BMC Med Genomics 2009;2:8.
Bastian BC, LeBoit PE, Hamm H, Brocker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998;58:2170–5.
Balazs M, Adam Z, Treszl A, Begany A, Hunyadi J, Adany R. Chromosomal imbalances in primary and metastatic melanomas revealed by comparative genomic hybridization. Cytometry 2001;46:222–32.
Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003;163:1765–70.
Mesbah Ardakani N, Thomas C, Robinson C, Mina K, Harvey NT, Amanuel B, et al. Detection of copy number variations in melanocytic lesions utilising array based comparative genomic hybridisation. Pathology 2017;49:285–91.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47.
Maize JC Jr., McCalmont TH, Carlson JA, Busam KJ, Kutzner H, Bastian BC. Genomic analysis of blue nevi and related dermal melanocytic proliferations. Am J Surg Pathol. 2005;29:1214–20.
Chan MP, Andea AA, Harms PW, Durham AB, Patel RM, Wang M, et al. Genomic copy number analysis of a spectrum of blue nevi identifies recurrent aberrations of entire chromosomal arms in melanoma ex blue nevus. Mod Pathol 2016;29:227–39.
Costa S, Byrne M, Pissaloux D, Haddad V, Paindavoine S, Thomas L, et al. Melanomas associated with blue nevi or mimicking cellular blue nevi: clinical, pathologic, and molecular study of 11 cases displaying a high frequency of GNA11 mutations, BAP1 expression loss, and a predilection for the scalp. Am J Surg Pathol. 2016;40:368–77.
Dai J, Tetzlaff MT, Schuchter LM, Elder DE, Elenitsas R. Histopathologic and mutational analysis of a case of blue nevus-like melanoma. J Cutan Pathol. 2016;43:776–80.
North JP, Yeh I, McCalmont TH, LeBoit PE. Melanoma ex blue nevus: two cases resembling large plaque-type blue nevus with subcutaneous cellular nodules. J Cutan Pathol. 2012;39:1094–9.
Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258–63.
Barnhill RL, Argenyi ZB, From L, Glass LF, Maize JC, Mihm MC Jr., et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30:513–20.
Bastian BC, Wesselmann U, Pinkel D, Leboit PE. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J Invest Dermatol. 1999;113:1065–9.
Ali L, Helm T, Cheney R, Conroy J, Sait S, Guitart J, et al. Correlating array comparative genomic hybridization findings with histology and outcome in spitzoid melanocytic neoplasms. Int J Clin Exp Pathol. 2010;3:593–9.
Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000;157:967–72.
Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
Wiesner T, Kutzner H, Cerroni L, Mihm MC Jr., Busam KJ, Murali R. Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology 2016;48:113–31.
Yeh I, de la Fouchardiere A, Pissaloux D, Mully TW, Garrido MC, Vemula SS, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol. 2015;39:581–91.
Yeh I, Tee MK, Botton T, Shain AH, Sparatta AJ, Gagnon A, et al. NTRK3 kinase fusions in Spitz tumours. J Pathol. 2016;240:282–90.
Yeh I, Busam KJ, McCalmont TH, LeBoit PE, Pissaloux D, Alberti L, et al. Filigree-like rete ridges, lobulated nests, rosette-like structures, and exaggerated maturation characterize Spitz tumors with NTRK1 fusion. Am J Surg Pathol. 2019;43:737–46.
Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H, et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J surgical Pathol. 2012;36:818–30.
Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–21.
van Houten AH, van Dijk MC, Schuttelaar ML. Proliferative nodules in a giant congenital melanocytic nevus-case report and review of the literature. J Cutan Pathol. 2010;37:764–76.
Yelamos O, Arva NC, Obregon R, Yazdan P, Wagner A, Guitart J, et al. A comparative study of proliferative nodules and lethal melanomas in congenital nevi from children. Am J Surg Pathol. 2015;39:405–15.
Bastian BC, Xiong J, Frieden IJ, Williams ML, Chou P, Busam K, et al. Genetic changes in neoplasms arising in congenital melanocytic nevi: differences between nodular proliferations and melanomas. Am J Pathol. 2002;161:1163–9.
Nguyen TL, Theos A, Kelly DR, Busam K, Andea AA. Mitotically active proliferative nodule arising in a giant congenital melanocytic nevus: a diagnostic pitfall. Am J Dermatopathol. 2013;35:e16–21.
Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011;144:27–40.
Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med. 2015;373:1926–36.
Hirsch D, Kemmerling R, Davis S, Camps J, Meltzer PS, Ried T, et al. Chromothripsis and focal copy number alterations determine poor outcome in malignant melanoma. Cancer Res. 2013;73:1454–60.
Gaiser T, Kutzner H, Palmedo G, Siegelin MD, Wiesner T, Bruckner T, et al. Classifying ambiguous melanocytic lesions with FISH and correlation with clinical long-term follow up. Mod Pathol. 2010;23:413–9.
Waters JJ, Barlow AL, Gould CP. Demystified… FISH. Mol Pathol. 1998;51:62–70.
Matsuta M, Matsuta M, Kon S, Thompson C, LeBoit PE, Weier HU, et al. Interphase cytogenetics of melanocytic neoplasms: numerical aberrations of chromosomes can be detected in interphase nuclei using centromeric DNA probes. J Cutan Pathol. 1994;21:1–6.
Matsuta M, Imamura Y, Matsuta M, Sasaki K, Kon S. Detection of numerical chromosomal aberrations in malignant melanomas using fluorescence in situ hybridization. J Cutan Pathol. 1997;24:201–5.
Gerami P, Jewell SS, Morrison LE, Blondin B, Schulz J, Ruffalo T, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol. 2009;33:1146–56.
Gerami P, Mafee M, Lurtsbarapa T, Guitart J, Haghighat Z, Newman M. Sensitivity of fluorescence in situ hybridization for melanoma diagnosis using RREB1, MYB, Cep6, and 11q13 probes in melanoma subtypes. Arch Dermatol 2010;146:273–8.
Morey AL, Murali R, McCarthy SW, Mann GJ, Scolyer RA. Diagnosis of cutaneous melanocytic tumours by four-colour fluorescence in situ hybridisation. Pathology 2009;41:383–7.
North JP, Vetto JT, Murali R, White KP, White CR Jr., Bastian BC. Assessment of copy number status of chromosomes 6 and 11 by FISH provides independent prognostic information in primary melanoma. Am J Surg Pathol. 2011;35:1146–50.
Dalton SR, Gerami P, Kolaitis NA, Charzan S, Werling R, LeBoit PE, et al. Use of fluorescence in situ hybridization (FISH) to distinguish intranodal nevus from metastatic melanoma. Am J Surg Pathol. 2010;34:231–7.
Gerami P, Wass A, Mafee M, Fang Y, Pulitzer MP, Busam KJ. Fluorescence in situ hybridization for distinguishing nevoid melanomas from mitotically active nevi. Am J Surg Pathol. 2009;33:1783–8.
Busam KJ, Fang Y, Jhanwar SC, Pulitzer MP, Marr B, Abramson DH. Distinction of conjunctival melanocytic nevi from melanomas by fluorescence in situ hybridization. J Cutan Pathol. 2010;37:196–203.
Pouryazdanparast P, Newman M, Mafee M, Haghighat Z, Guitart J, Gerami P. Distinguishing epithelioid blue nevus from blue nevus-like cutaneous melanoma metastasis using fluorescence in situ hybridization. Am J Surg Pathol. 2009;33:1396–400.
Newman MD, Lertsburapa T, Mirzabeigi M, Mafee M, Guitart J, Gerami P. Fluorescence in situ hybridization as a tool for microstaging in malignant melanoma. Mod Pathol. 2009;22:989–95.
Requena C, Rubio L, Traves V, Sanmartin O, Nagore E, Llombart B, et al. Fluorescence in situ hybridization for the differential diagnosis between Spitz naevus and spitzoid melanoma. Histopathology 2012;61:899–909.
Diaz A, Valera A, Carrera C, Hakim S, Aguilera P, Garcia A, et al. Pigmented spindle cell nevus: clues for differentiating it from spindle cell malignant melanoma. A comprehensive survey including clinicopathologic, immunohistochemical, and FISH studies. Am J Surg Pathol. 2011;35:1733–42.
Gammon B, Beilfuss B, Guitart J, Gerami P. Enhanced detection of spitzoid melanomas using fluorescence in situ hybridization with 9p21 as an adjunctive probe. Am J Surg Pathol. 2012;36:81–8.
Massi D, Cesinaro AM, Tomasini C, Paglierani M, Bettelli S, Dal Maso L, et al. Atypical Spitzoid melanocytic tumors: a morphological, mutational, and FISH analysis. J Am Acad Dermatol. 2011;64:919–35.
Abasolo A, Vargas MT, Rios-Martin JJ, Trigo I, Arjona A, Gonzalez-Campora R. Application of fluorescence in situ hybridization as a diagnostic tool in melanocytic lesions, using paraffin wax-embedded tissues and imprint-cytology specimens. Clin Exp Dermatol. 2012;37:838–43.
Raskin L, Ludgate M, Iyer RK, Ackley TE, Bradford CR, Johnson TM, et al. Copy number variations and clinical outcome in atypical spitz tumors. Am J Surg Pathol. 2011;35:243–52.
Vergier B, Prochazkova-Carlotti M, de la Fouchardiere A, Cerroni L, Massi D, De Giorgi V, et al. Fluorescence in situ hybridization, a diagnostic aid in ambiguous melanocytic tumors: European study of 113 cases. Mod Pathol. 2011;24:613–23.
Gerami P, Zembowicz A. Update on fluorescence in situ hybridization in melanoma: state of the art. Arch Pathol Lab Med. 2011;135:830–7.
Tetzlaff MT, Wang WL, Milless TL, Curry JL, Torres-Cabala CA, McLemore MS, et al. Ambiguous melanocytic tumors in a tertiary referral center: the contribution of fluorescence in situ hybridization (FISH) to conventional histopathologic and immunophenotypic analyses. Am J Surg Pathol. 2013;37:1783–96.
North JP, Garrido MC, Kolaitis NA, LeBoit PE, McCalmont TH, Bastian BC. Fluorescence in situ hybridization as an ancillary tool in the diagnosis of ambiguous melanocytic neoplasms: a review of 804 cases. Am J Surg Pathol. 2014;38:824–31.
Zembowicz A, Yang SE, Kafanas A, Lyle SR. Correlation between histologic assessment and fluorescence in situ hybridization using MelanoSITE in evaluation of histologically ambiguous melanocytic lesions. Arch Pathol Lab Med. 2012;136:1571–9.
Muhlbauer A, Momtahen S, Mihm MC, Wang J, Magro CM. The correlation of the standard 5 probe FISH assay with melanocytic tumors of uncertain malignant potential. Ann Diagn Pathol. 2017;28:30–6.
Isaac AK, Lertsburapa T, Pathria Mundi J, Martini M, Guitart J, Gerami P. Polyploidy in spitz nevi: a not uncommon karyotypic abnormality identifiable by fluorescence in situ hybridization. Am J Dermatopathol. 2010;32:144–8.
Gerami P, Li G, Pouryazdanparast P, Blondin B, Beilfuss B, Slenk C, et al. A highly specific and discriminatory FISH assay for distinguishing between benign and malignant melanocytic neoplasms. Am J Surg Pathol. 2012;36:808–17.
Gerami P, Scolyer RA, Xu X, Elder DE, Abraham RM, Fullen D, et al. Risk assessment for atypical spitzoid melanocytic neoplasms using FISH to identify chromosomal copy number aberrations. Am J Surg Pathol. 2013;37:676–84.
Al-Rohil RN, Curry JL, Torres-Cabala CA, Nagarajan P, Ivan D, Aung PP, et al. Proliferation indices correlate with diagnosis and metastasis in diagnostically challenging melanocytic tumors. Hum Pathol. 2016;53:73–81.
Wang L, Rao M, Fang Y, Hameed M, Viale A, Busam K, et al. A genome-wide high-resolution array-CGH analysis of cutaneous melanoma and comparison of array-CGH to FISH in diagnostic evaluation. J Mol Diagn. 2013;15:581–91.
Carter MD, Durham AB, Miedema JR, Harms PW, Chan MP, Patel RM, et al. Molecular testing of borderline cutaneous melanocytic lesions: SNP array is more sensitive and specific than FISH. Hum Pathol. 2019;86:115–23.
Emanuel PO, Andea AA, Vidal CI, Missall TA, Novoa RA, Bohlke AK, et al. Evidence behind the use of molecular tests in melanocytic lesions and practice patterns of these tests by dermatopathologists. J Cutan Pathol. 2018;45:839–46.
Vidal CI, Armbrect EA, Andea AA, Bohlke AK, Comfere NI, Hughes SR, et al. Appropriate use criteria in dermatopathology: Initial recommendations from the American Society of Dermatopathology. J Cutan Pathol. 2018;45:563–80.
Clarke LE, Warf MB, Flake DD II, Hartman AR, Tahan S, Shea CR, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244–52.
Gerami P, Cook RW, Wilkinson J, Russell MC, Dhillon N, Amaria RN, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res. 2015;21:175–83.
Thomas NE, Edmiston SN, Tsai YS, Parker JS, Googe PB, Busam KJ, et al. Utility of TERT promoter mutations for cutaneous primary melanoma diagnosis. Am J Dermatopathol. 2019;41:264–72.
Conway K, Edmiston SN, Parker JS, Kuan PF, Tsai YH, Groben PA, et al. Identification of a robust methylation classifier for cutaneous melanoma diagnosis. J Invest Dermatol. 2019;139:1349–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miedema, J., Andea, A.A. Through the looking glass and what you find there: making sense of comparative genomic hybridization and fluorescence in situ hybridization for melanoma diagnosis. Mod Pathol 33, 1318–1330 (2020). https://doi.org/10.1038/s41379-020-0490-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41379-020-0490-7