Abstract
Sarcomas with MEIS1-NCOA2 fusions have been so far reported in 2 cases each of primitive renal sarcomas and intraosseous pelvic rhabdomyosarcomas. Their histologic spectrum, anatomic distribution, and clinical behavior remain poorly defined. In this study, we report 6 additional spindle cell sarcomas with MEIS1-NCOA2 or NCOA1 fusions that fall into the same disease spectrum with the previously reported renal sarcomas. The patients’ age range was wide (20–76 years, mean 46) and all except one were female. The tumors arose in the kidney (n = 2), and one each in the uterine corpus, vagina, scrotum, and para-rectal region. The consistent morphology was that of monomorphic spindle to ovoid cells in a storiform, whorling, or solid pattern. Alternating cellularity, myxoid stroma, and microcystic changes were seen in some cases. Mitotic activity varied greatly (<1–33/10 high power fields). The immunophenotype was nonspecific, with most cases expressing variable degrees of TLE1, WT1, cyclin D1, CD56, and CD10. Using various platforms of RNA-based targeted sequencing, MEIS1-NCOA2 fusions were recurrently identified in 5 cases, and a novel MEIS1-NCOA1 fusion was found in one renal tumor. The gene fusions were validated by fluorescence in situ hybridization using custom BAC probes. Of the 5 patients with available follow-up (5 months to 8 years), all experienced local recurrences, but no distant spread or death from disease. Our results expand the clinicopathologic spectrum of sarcomas with MEIS1-NCOA2/1 fusions, providing evidence of an undifferentiated spindle cell phenotype with nonspecific immunoprofile and low-grade clinical behavior.
Similar content being viewed by others
Introduction
MEIS1-NCOA2 fusions have been recently described in a handful of spindle cell sarcomas, including 2 cases each of primitive sarcomas of the kidney and intraosseous rhabdomyosarcomas (RMS) [1, 2]. The renal tumors displayed a monomorphic spindle cell cytomorphology with alternating cellularity, arranged in a fascicular or whorling growth pattern. Due to their primitive-appearing round to fusiform nuclei and scant cytoplasm, the tumors resembled synovial sarcomas, BCOR family sarcomas, malignant peripheral nerve sheath tumors, and NTRK fusion tumors. Unlike the RMS with MEIS1-NCOA2 fusions showing convincing myogenic markers, these primitive renal sarcomas have a nonspecific immunoprofile, with positive CD56, focal WT-1, focal ER, and variable TLE1 and cyclin D1 staining. Based on their primitive morphology and undetermined histogenesis, their diagnosis remains challenging without molecular analysis. Moreover, as only 2 cases have been so far reported, their clinical behavior remains unclear based on the limited follow-up available and it is unknown whether this family of tumors is restricted to the kidneys.
In this study, we expand our understanding of tumors characterized by MEIS1-NCOA fusions by investigating the clinicopathologic and molecular findings of 6 new cases of spindle cell sarcomas with extended clinical follow-up, arising from various organs, including the uterus, vagina, scrotum, para-rectal soft tissue, and kidney. Moreover, we also report an additional spindle cell RMS with MEIS1-NCOA2 fusion arising in the vulva, representing the first extraosseous RMS with this fusion.
Materials and methods
Case selection
The cases were retrieved from the consultation files and institutional archives of the authors (C.R.A. and J.C.L.). Six sarcomas with MEIS1-NCOA2 or MEIS1-NCOA1 fusions were collected. These cases were submitted for various RNA sequencing methods either at the time of diagnosis to provide information for further tumor classification and potential treatment guidance or during retrospective case review for translocation-associated sarcomas. The hematoxylin and eosin stained slides and the immunohistochemical stains were reviewed. Clinicopathologic parameters, including relevant clinical history, age, gender, tumor location, tumor size, and follow-up information, were collected from the pathologic reports and electronic medical record. This study was approved by the institutional review board.
RNA sequencing
The samples were investigated on different targeted RNA sequencing platforms, including University of Chicago Medicine OncoPlus next-generation sequencing (n = 1, case 1), TruSight RNA Fusion Panel (Illumina, San Diego, CA) (n = 3, cases 2–4), and Archer FusionPlex Custom Solid Panel (n = 2, cases 5–6), using formalin-fixed paraffin-embedded (FFPE) tissues. The above gene panels include 1213, 507, and 85 cancer-related genes, respectively, with NCOA2 and NCOA1 genes being included in all three panels. The detailed methods have been described previously [1,2,3]. In addition, case 1 was also submitted to FoundationOne CDx (Cambridge, MA) for mutation discovery and copy number assessment.
Fluorescence in situ hybridization (FISH)
FISH for NCOA2 and MEIS1 was performed using custom designed probes made by BACs flanking respective genes as previously described to validate the fusions [1, 2]. BAC clones were selected based on the information on UCSC genome browser (http://genome.ucsc.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (CHORI) (Oakland, CA) (http://bacpac.chori.org) and Life Technologies Corporation (Carlsbad, CA). After plasmid DNA extraction and nick translation, the probes were validated on metaphases. Four µm-thick FFPE tissue sections of our cases were pretreated and hybridized with the probes.
Immunohistochemistry
Most of the immunohistochemical stains were performed at the time of diagnosis and therefore were based on the differential diagnoses of each case. The immunostained slides were reviewed and results summarized in Supplementary Table 1. After the identification of the gene fusion, an additional NCOA2 immunohistochemical stain (1:500, LS‑B12201, Lifespan Biosciences, Seattle, WA) was performed on case 2. Retrospective MDM2 staining was also performed after the identification of MDM2 gene amplification in case 1.
Results
Spindle cell sarcomas with MEIS1-NCOA2/1 fusions have a predilection for the genitourinary tract and gynecologic organs
A total of 6 cases, including 5 with MEIS1-NCOA2 fusions and one with MEIS1-NCOA1, were included in the study. Interestingly, all tumors arose within the abdominal cavity or pelvis and involved various organs, including 2 in the kidneys and one each in the uterine corpus, vagina, scrotum (involving dermis to subcutis), and para-rectal soft tissue. There were 5 females and one male, with a wide age range at diagnosis of 20–76 years (mean: 46, median: 44). The 76-year-old patient presented with a recurrent scrotal tumor years after resection of the primary tumor, and the age at the primary resection was unknown. The primary tumors ranged from 11 to 21 cm (mean: 17). The clinicopathologic features of the study group, along with those of the 2 previously reported kidney cases [1], were summarized in Table 1.
Two patients had available preoperative history. Case 2, a 35-year-old woman, had been receiving ovulation induction medication, including clomiphene, r-hFSH, and chorionic gonadotropin, for 8 months in an infertility clinic before a hemorrhagic vaginal mass was identified. Another patient (case 4) was a 38-year-old woman with an intrapelvic para-rectal tumor that was present for 2 years and enlarged during pregnancy. She received surgical removal of the tumor one month after delivery.
MEIS1-NCOA2/1 fusion sarcomas show primitive round to spindle cells with whorling and storiform patterns and alternating cellularity
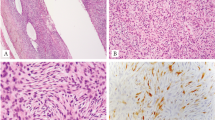
The tumor borders were unencapsulated and focally infiltrative (cases 1 and 3) or relatively well circumscribed (case 6). Histologically, most tumors showed primitive, monomorphic spindle to ovoid cells with scant cytoplasm, arranged in short fascicular, vague whorling, storiform and/or solid patterns (Figs. 1–2). Cases 1, 2 and 6 showed alternating cellularity, with myxoid to hyalinized stroma in the hypocellular area (Figs. 1A–D, and 2G). Necrosis was present in 2 renal cases. Mitotic activity ranged from <1–33 per 10 high power fields (HPFs), with 4 cases having ≤10/10HPFs and 2 mitotically active cases in the uterus (33/10HPFs) and kidney (25/10 HPFs).
The uterine tumor (case 1, A–C) shows short spindle to ovoid cells with alternating cellularity (A), vague short fascicles, cellular aggregates of primitive cells (B), and myxoid to hyalinized stroma in the hypocellular area (C). The vaginal tumor (case 2, D–F) shows monomorphic spindle cells with alternating cellularity and rich vasculature (D), in whorling (D) to storiform (E) patterns. NCOA2 immunostaining shows diffuse moderate staining (F).
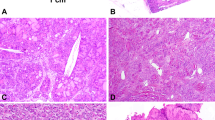
The superficial scrotal tumor (case 3) shows mildly infiltrative border (A) and is composed of compact spindle cells in storiform pattern (B), mimicking dermatofibrosarcoma protuberans. The para-rectal tumor (case 4) shows solid sheets of ovoid cells (C) with vesicular chromatin and indistinct cell border, accompanied by microcystic spaces (D). Case 5 shows vague fascicular pattern (E) and areas with increased pleomorphism and hyperchromasia (F). Case 6 displays sheets of ovoid cells with variation in cellularity (G). Epithelioid cells were observed in the second recurrent lesion (H).
In addition to this predominant histomorphology, the tumors showed a spectrum of appearances. Case 1 also had areas of cellular aggregates of primitive cells (Fig. 1B), reminiscent of the primitive cell clusters described in the previously reported MEIS1-NCOA2 renal sarcomas [1]. Case 2 was highly vascular displaying a rich capillary network (Fig. 1D) and scattered thick-walled vessels, and had prominent areas of hemorrhagic change. A few multinucleated tumor giant cells were noted. Case 4 was composed of sheets of ovoid cells with convoluted nuclei, vesicular chromatin and small nucleoli, and interspersed microcystic spaces (Fig. 2D). Case 5 showed mostly uniform cells, but also exhibited areas of increased pleomorphism and hyperchromasia (Fig. 2F). In highly cellular areas of case 6, the tumor cells appeared more rounded, and epithelioid cells were seen in the second recurrence (Fig. 2H).
Sarcomas with MEIS1-NCOA2/1 fusions are characterized by a nonspecific immunoprofile
Immunohistochemically, these tumors had a nonspecific phenotype, with variable degree of staining for TLE1, WT1, cyclin D1, CD56, and CD10, ranging from diffuse to focal (Supplementary Table 1). Variable ER (2 of 4) and CD99 (2 of 3) expressions were also seen in some cases. Myogenic markers were mostly negative, except for focal desmin staining in the 2 renal tumors, focal smooth muscle actin in the uterine tumor, and diffuse caldesmon expression in the scrotal tumor. All cases tested for myogenin were negative.
Other negative markers included BCOR (n = 3), AE1/AE3 (n = 5), EMA (n = 3), S100 (n = 5), SOX10 (n = 3), CD34 (n = 3), CD117 (n = 4), α-inhibin (n = 3), ALK (n = 3), HMB45 (n = 3), H3K27me3 (retained, n = 2), ERG (n = 2), and PAX8 (n = 2). NCOA2 immunostaining was performed in one case (case 2) and showed diffuse moderate staining (Fig. 1F). Pan-Trk staining (EPR17341) was performed in case 3 and was diffusely positive, but no gene rearrangement for NTRK1-3 was found by FISH and the subsequent targeted RNA sequencing. Pan-Trk staining was subsequently tested on 3 other cases and multifocal moderate expression was found in one (case 2).
The original diagnoses rendered in these tumors included undifferentiated uterine sarcoma, low-grade endometrial stromal sarcoma, low-grade sarcoma, not otherwise specified (NOS), undifferentiated spindle cell sarcoma, and sarcoma, NOS (Table 1).
MEIS1-NCOA2/1 fusions identified by RNA sequencing
RNA sequencing identified MEIS1-NCOA2 fusion in 5 cases, with exon 6 of MEIS1 (NM_002398.2) fused to exon 12 of NCOA2 (NM_001321703.1) (n = 3) or exon 7 of MEIS1 fused to exon 13 or 14 of NCOA2 (n = 2) (Table 1, Fig. 3A). The latter fusion junction was identical to the previously reported kidney tumor [1]. Another patient (case 5) had an alternative 3’ fusion partner gene, with a novel MEIS1 exon 7-NCOA1 (NM_003743) exon 13 fusion. All the fusion transcripts were predicted to be in-frame. FISH confirmed the translocations, showing break-apart of MEIS1 and NCOA2 genes (Fig. 3B, C).
A. Left panel, chromosomal locations and transcription directions of MEIS1 (2p14), NCOA1 (2p23.3), and NCOA2 (8q13.3) genes. Right panel, schematic diagram illustrating variants of MEIS1-NCOA2/1 fusions transcripts, including exon 6 of MEIS1 fused to exon 12 of NCOA2, exon 7 of MEIS1 fused to exon 13 or 14 of NCOA2, and exon 7 of MEIS1 fused to exon 13 of NCOA1. All the fusion variants are in-frame, as demonstrated by the fusion junction sequences and predicted amino acid beneath each fusion variant. Representative FISH of MEIS1 (B) and NCOA2 (C) of case 3 showing break-apart of orange and green signals (arrows).
In case 1 (uterine tumor), additional genetic alterations identified by FoundationOne CDx (Cambridge, MA) included CTNNB1 S33C, MDM2 amplification, ATRX C280fs*3, and MPL Y252H. Retrospective immunostains showed aberrant nuclear expression of beta-catenin and MDM2 (~70%) expression. Tumor mutational burden was 3 muts/Mb. Immunohistochemical stain for beta-catenin was performed on three other cases (cases 2–4) and were all negative for nuclear staining. MDM2 was also tested by immunohistochemistry in the remaining 5 cases and by FISH in 2 (cases 2 and 5), which all showed negative results for MDM2 overexpression or amplification.
MEIS1-NCOA2/1 fusion-positive sarcomas share a low-grade malignant potential
Five patients had available follow-up, ranging from 5 months to 8 years (Table 1). All 5 patients developed local recurrences, with 3 of them having multiple recurrences over the years. None developed distant metastases or died of disease at last follow-up. The follow-up information of each patient is as follows:
Patient 1 presented with a uterine tumor and underwent total hysterectomy and bilateral salpingo-oophorectomy. The primary tumor involved the uterine corpus without extending to the cervix or adnexae. During surgery, however, there were extensive adhesions, resulting in intraoperative rupture of the tumor. Follow-up computed tomography scan at 5 months after diagnosis showed multiple mesenteric masses. Biopsy of the mass confirmed recurrent disease.
Patient 2 was managed with an intralesional excision for a primary vaginal mass and subsequently developed multiple local recurrences 3 months, 1.5 years, and 7 years later. No metastasis was found at last gynecologic follow-up (7 years). The patient was still alive 10 years after the initial diagnosis, with unknown disease status.
Patient 3 was a male patient with a small recurrent scrotal lesion (6 mm), with the primary lesion had been excised years ago.
Patient 4 presented with a para-rectal tumor and subsequently developed multiple intrapelvic recurrences which were surgically removed multiple times (4, 6, and 8 years after initial diagnosis). The uterus and left ovary removed were free of tumor. The patient was still alive 12 years after the initial diagnosis, with unknown disease status.
Patient 6, a 50-year-old woman, had a large tumor replacing most of the right kidney. The tumor was confined to the renal capsule, without invading the perinephric fat, the renal sinus, or the renal vein. She underwent total nephrectomy and removal of a portion of 10th rib. One and a half years post surgery, she developed a local recurrence in the retroperitoneum, which was removed surgically and the additionally sampled margins were free from tumor. However, six years after initial diagnosis, she developed a second recurrence involving the right retrocaval region and right chest wall/diaphragm, which was also resected.
Vulvar spindle cell RMS with MEIS1-NCOA2 fusion
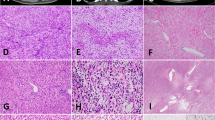
During our case selection, we also identified the first extra-osseous spindle cell RMS with MEIS1-NCOA2 fusion. The patient was a 33-year-old woman with an enlarging vulvar tumor for 2 years. Microscopically, the tumor showed spindle cells with increased mitotic activity arranged in fascicles (Fig. 4A), accompanied by focal larger tumor cells with eosinophilic cytoplasm (Fig. 4B), some multinucleated cells, and tumor-infiltrating mast cells. Unlike other MEIS1-NCOA2 tumors in this study group, this tumor expressed multiple myogenic markers including desmin, caldesmon, myogenin, MyoD1, focal calponin and smooth muscle myosin heavy chain. ER was also positive. Other stains performed retrospectively included MDM2, beta-catenin, pan-Trk, ALK, and p53 and were all negative. Targeted panel of RNA sequencing (Archer FusionPlex) identified a similar fusion of MEIS1 exon 7 to NCOA2 exon 13. The fusion was confirmed by break-apart FISH for MEIS1 and NCOA2 genes. The tumor involved the initial excisional margin, and the patient received a re-resection after neoadjuvant therapy. A small residual tumor (0.6 cm) with negative margins was noted. This was a recent case and no follow-up data was available.
Discussion
In this study, we report 6 undifferentiated spindle cell sarcomas harboring MEIS1-NCOA2/1 fusions, demonstrating that these tumors can occur not only in the kidneys, but also in other locations, such as uterus, vagina, scrotum, and para-rectal soft tissues. Similar to the YWHAE gene fusions in high-grade endometrial stromal sarcomas and clear cell sarcoma of kidney, sarcomas with MEIS1-NCOA fusions identified in this study also show a predilection for the gynecologic and genitourinary tracts. These neoplasms show a spectrum of histomorphology from monotonous spindle cells arranged in whorls or a storiform pattern with alternating cellularity to sheets of ovoid cells with microcystic change. Due to a nonspecific immunoprofile, many of the cases remained initially unclassified, being designated as undifferentiated sarcomas.
The differential diagnosis can be broad and depends on the location and histomorphology of individual tumors. For example, those in the kidneys can mimic synovial sarcoma, BCOR family of tumors, metanephric stromal tumor, or mixed epithelial stromal tumor of kidney. TLE1 expression in these tumors further simulates the immunoprofile of synovial sarcoma and BCOR family of tumors. Compared to sarcomas with MEIS1-NCOA fusions, synovial sarcomas typically are more uniformly hypercellular with elongated spindle cell fascicles. TLE1 staining is usually diffusely positive in synovial sarcoma, while it is more variable in sarcomas with MEIS1-NCOA fusions. BCOR immunoreactivity is observed in one of the two previously reported renal sarcomas with MEIS1-NCOA2, with moderate intensity in ~60% of the tumor cells [1]. In this study, three additional cases were tested for BCOR and all showed negative results (cases 1–3), suggesting that BCOR immunoreactivity is present in only a minority of sarcomas with MEIS1-NCOA fusions.
Sarcomas with MEIS1-NCOA fusions involving the gynecologic organs are most likely to be misdiagnosed as endometrial stromal sarcoma or undifferentiated uterine sarcoma. Case 1 showed a mitotically active uterine tumor with a primarily pushing border and only focally irregular interface with the myometrium and a CD10+/cyclin D1 focally+/BCOR−/ER−immunoprofile. The tumor was negative for BCOR, JAZF1, PHF1, and YWHAE rearrangements by FISH and was classified as an undifferentiated uterine sarcoma. Case 2, a vaginal tumor, showed a low-grade spindle cell sarcoma morphology with a whorling pattern accompanied by rich vasculature. The tumor was intra-lesionally excised, and therefore the tumor border could not be evaluated. The tumor was positive for ER and focally positive for CD10. FISH study was not available at the time of initial diagnosis. It was classified as a low-grade endometrial stromal sarcoma based on morphology and immunohistochemistry. In the gynecological tract, a small subset of sarcomas, be it ER-positive or -negative, do not fit into the common groups of low-grade and high-grade endometrial stromal sarcomas, both morphologically and genetically. Few examples of lesions associated with rare gene fusions include sarcomas harboring NTRK, PDGFB, and MEIS1-NCOA fusions, which can be encountered in the gynecologic tract as well as other locations.
For tumors involving superficial soft tissues, such as case 3 in the scrotum, the storiform pattern and monotonous short spindle cells raised the possibility of dermatofibrosarcoma protuberans. However, CD34 stain and FISH for PDGFB and PDGFD gene abnormalities were negative. The positive pan-Trk immunostaining triggered FISH for NTRK1-3 gene rearrangements, which were all negative. This tumor was therefore classified as a low-grade sarcoma, not otherwise specified.
These cases illustrate that in unclassifiable low-grade spindle cell sarcomas, especially those with alternating cellularity and whorling or storiform patterns, the nonspecific panel of TLE1, WT1, cyclinD1, CD56 and CD10 may point the need for further molecular testing such as targeted RNA sequencing or FISH for NCOA2.
NCOA2 gene encodes a nuclear hormone receptor transcriptional coactivator. Gene fusions involving NCOA2 with other partner genes have been identified in several distinct groups of mesenchymal tumors, including mesenchymal chondrosarcoma (HEY1-NCOA2) [4], soft tissue angiofibroma (AHRR-NCOA2) [5], congenital/infantile spindle cell RMS (VGLL2-NCOA2, SRF-NCOA2, TEAD1-NCOA2) [6], biphenotypic sinonasal sarcoma (PAX3-NCOA1/2) [7, 8], PRRX-NCOA1/2 rearranged fibroblastic neoplasm [9], and uterine tumor resembling ovarian sex cord tumor (ESR1-NCOA2/3, GREB1-NCOA1/2) [10]. Our uterine case did not show sex-cord-like differentiation as seen in uterine tumor resembling ovarian sex cord tumor. Each of these tumors has unique clinicopathologic features distinct from the low-grade spindle cell sarcomas with MEIS1-NCOA2/1 fusions reported in this study.
NCOA2 immunostain was positive in case 2, the only case tested. A previous study in soft tissue angiofibroma showed that NCOA2 (polyclonal; ITK Diagnostics BV, Uithoorn, The Netherlands) was positive not only in soft tissue angiofibromas but also in other spindle cell lesions without NCOA2 fusions, such as intramuscular myxoma, myxoid liposarcoma, myxofibrosarcoma, solitary fibrous tumor, schwannoma, gastrointestinal stromal tumor, etc., and therefore does not serve as a good screening marker [11].
In this study, a novel MEIS1-NCOA1 fusion was identified in a kidney sarcoma as an alternative fusion to MEIS1-NCOA2. The breakpoint of MEIS1 exon 7 fused to NCOA1 exon 13 is the same as one of the common fusion variants of MEIS1-NCOA2 fusions, and both preserve the C-terminal transcription activation domains of NCOA proteins in the deduced chimeric MEIS1-NCOA protein. Similar interchangeable role of NCOA1 and NCOA2 fusions has also been observed in other tumors, such as biphenotypic sinonasal sarcoma (PAX3-NCOA1/2) [7, 8], PRRX-NCOA1/2 rearranged fibroblastic neoplasm [9], and uterine tumor resembling ovarian sex cord tumor (GREB1-NCOA1/2) [10].
In addition to the MEIS1-NCOA2 fusion, case 1, a uterine sarcoma, was also identified to have co-existing CTNNB1 mutation and MDM2 amplification, among other genetic changes. As these changes were only identified in a single case of MEIS1-NCOA2 fusion sarcoma, their pathogenetic contributions are uncertain. In uterine sarcomas, CDK4 and/or MDM2 amplifications are recurrent genetic events in BCOR-rearranged uterine sarcoma (38% CDK4 and 45–100% MDM2 amplifications) and Müllerian adenosarcoma (22–37.5% CDK4 and 11–31% MDM2 amplifications) [12,13,14,15,16,17]. Whether the same is true for sarcomas with MEIS1-NCOA fusions awaits further research. The significance of CTNNB1 mutations in uterine sarcomas is also unclear although activating CTNNB1 mutations have been reported in rare uterine sarcomas and activation of Wnt signaling pathway is observed in low-grade endometrial stromal sarcomas [18, 19].
In this study, we provided an extended follow-up data demonstrating that these tumors have at least a low-grade malignant potential. The clinical courses of these patients were characterized by multiple local recurrences even years after the initial operation, suggesting that long-term follow-up is needed. Moreover, since only limited follow-up was available for our two mitotically active cases in this study (cases 1 and 5), it remains to be seen if there will be a high-grade subset of MEIS1-NCOA sarcomas with more aggressive clinical behavior that can be predicted by certain histologic parameters. Our case 1 had intraoperative rupture of the uterine tumor and later developed mesenteric locoregional recurrence/metastasis. Distant metastasis was not observed during the relatively short follow-up period (5 months). Case 5 was a renal tumor with areas of increased pleomorphism, hyperchromasia, and brisk mitotic activity. However, no follow-up information was available for this patient.
Lastly, we also describe the first extraosseous spindle cell RMS with MEIS1-NCOA2 fusion involving the vulva. Like the previous 2 intraosseous spindle cell RMS with MEIS1-NCOA2 fusions in the iliac bones of 22- and 39-year-old patients, this tumor also occurred in a young adult patient (33 years old). It also showed a similar histomorphology to the previously reported intraosseous cases, with hypercellular and monotonous spindle cells in intersecting short fascicles [2]. No rhabdomyoblastic type cells were noted on H&E. Immunohistochemically, it demonstrated multiple myogenic markers including desmin, caldesmon, myogenin, and MyoD1, indicating rhabdomyoblastic differentiation. In contrast, our study group of undifferentiated tumors with MEIS1-NCOA fusions showed very limited expressions of myogenic markers, with only focal desmin (cases 5 and 6), focal smooth muscle actin (case 1) or sole caldesmon staining (case 3). None of the 5 cases tested was positive for myogenin. The fusion junctions of MEIS1 and NCOA2 were similar in cases with or without rhabdomyoblastic differentiation, with in-frame fusions of MEIS1 exon 6 to NCOA2 exon 12 or MEIS1 exon 7 to NCOA1/2 exon 13 or 14. Currently, the clinical follow-up and treatment response of RMS with MEIS1-NCOA2 fusions are still unclear.
In conclusion, undifferentiated spindle cell sarcomas with MEIS1-NCOA fusions can occur in a variety of different anatomic locations in the genitourinary tract and gynecologic organs. In addition to the typical monomorphic spindle cell morphology with whorling or a storiform pattern and alternating cellularity, some cases may show a wider spectrum of morphology, including sheets of ovoid cells, microcystic change, and increased nuclear pleomorphism. MEIS1-NCOA2 fusions are the predominant fusion type, and a minority of cases may harbor the alternative MEIS1-NCOA1 fusion. Clinical follow-up suggests a low-grade malignant behavior with multiple local recurrences of these tumors.
References
Argani P, Reuter VE, Kapur P, Brown JE, Sung YS, Zhang L, et al. Novel MEIS1-NCOA2 gene fusions define a distinct primitive spindle cell sarcoma of the kidney. Am J Surg Pathol. 2018;42:1562–70.
Agaram NP, Zhang L, Sung YS, Cavalcanti MS, Torrence D, Wexler L, et al. Expanding the spectrum of intraosseous rhabdomyosarcoma: correlation between 2 distinct gene fusions and phenotype. Am J Surg Pathol. 2019;43:695–702.
Bennett JA, Ritterhouse LL, Furtado LV, Lastra RR, Pesci A, Newell JM, et al. Female adnexal tumors of probable Wolffian origin: morphological, immunohistochemical, and molecular analysis of 15 cases. Mod Pathol. 2020;33:734–47.
Wang L, Motoi T, Khanin R, Olshen A, Mertens F, Bridge J, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer. 2012;51:127–39.
Jin Y, Moller E, Nord KH, Mandahl N, Von Steyern FV, Domanski HA, et al. Fusion of the AHRR and NCOA2 genes through a recurrent translocation t(5;8)(p15;q13) in soft tissue angiofibroma results in upregulation of aryl hydrocarbon receptor target genes. Genes Chromosomes Cancer. 2012;51:510–20.
Alaggio R, Zhang L, Sung YS, Huang SC, Chen CL, Bisogno G, et al. A molecular study of pediatric spindle and sclerosing rhabdomyosarcoma: identification of novel and recurrent VGLL2-related fusions in infantile cases. Am J Surg Pathol. 2016;40:224–35.
Huang SC, Ghossein RA, Bishop JA, Zhang L, Chen TC, Huang HY, et al. Novel PAX3-NCOA1 fusions in biphenotypic sinonasal sarcoma with focal rhabdomyoblastic differentiation. Am J Surg Pathol. 2016;40:51–9.
Le Loarer F, Laffont S, Lesluyes T, Tirode F, Antonescu C, Baglin AC, et al. Clinicopathologic and molecular features of a series of 41 biphenotypic sinonasal sarcomas expanding their molecular spectrum. Am J Surg Pathol. 2019;43:747–54.
Lacambra MD, Weinreb I, Demicco EG, Chow C, Sung YS, Swanson D, et al. PRRX-NCOA1/2 rearrangement characterizes a distinctive fibroblastic neoplasm. Genes Chromosomes Cancer. 2019;58:705–12.
Goebel EA, Hernandez Bonilla S, Dong F, Dickson BC, Hoang LN, Hardisson D, et al. Uterine tumor resembling ovarian sex cord tumor (UTROSCT): a morphologic and molecular study of 26 cases confirms recurrent NCOA1-3 rearrangement. Am J Surg Pathol. 2020;44:30–42.
Bekers EM, Groenen P, Verdijk MAJ, Raaijmakers-van Geloof WL, Roepman P, Vink R, et al. Soft tissue angiofibroma: clinicopathologic, immunohistochemical and molecular analysis of 14 cases. Genes Chromosomes Cancer. 2017;56:750–57.
Lin DI, Hemmerich A, Edgerly C, Duncan D, Severson EA, Huang RSP, et al. Genomic profiling of BCOR-rearranged uterine sarcomas reveals novel gene fusion partners, frequent CDK4 amplification and CDKN2A loss. Gynecol Oncol. 2020;157:357–66.
Kommoss FK, Chang KT, Stichel D, Banito A, Jones DT, Heilig CE, et al. Endometrial stromal sarcomas with BCOR-rearrangement harbor MDM2 amplifications. J Pathol Clin Res. 2020;6:178–84.
Lee JC, Lu TP, Changou CA, Liang CW, Huang HN, Lauria A, et al. Genome wide copy number analysis of Mullerian adenosarcoma identified chromosomal instability in the aggressive subgroup. Mod Pathol. 2016;29:1070–82.
Hodgson A, Amemiya Y, Seth A, Djordjevic B, Parra-Herran C. High-grade Mullerian adenosarcoma: genomic and clinicopathologic characterization of a distinct neoplasm with prevalent TP53 pathway alterations and aggressive behavior. Am J Surg Pathol. 2017;41:1513–22.
Howitt BE, Sholl LM, Dal Cin P, Jia Y, Yuan L, MacConaill L, et al. Targeted genomic analysis of Mullerian adenosarcoma. J Pathol. 2015;235:37–49.
Piscuoglio S, Burke KA, Ng CK, Papanastasiou AD, Geyer FC, Macedo GS, et al. Uterine adenosarcomas are mesenchymal neoplasms. J Pathol. 2016;238:381–8.
Patel SB, McCormack C, Hodge JC. Non-fusion mutations in endometrial stromal sarcomas: what is the potential impact on tumourigenesis through cell cycle dysregulation? J Clin Pathol. 2020;73:830–5.
Przybyl J, Kidzinski L, Hastie T, Debiec-Rychter M, Nusse R, van de Rijn M. Gene expression profiling of low-grade endometrial stromal sarcoma indicates fusion protein-mediated activation of the Wnt signaling pathway. Gynecol Oncol. 2018;149:388–93.
Acknowledgements
The authors would like to thank Jeremy P. Segal (University of Chicago Medical Center) for assistance in analyzing the RNA sequencing results (case 1). This work is partly supported by P50 CA 140146-01, P30 CA008748, Cycle for Survival, St Baldrick Foundation, and Kristin Ann Carr Foundation granted to CRA, TMU108-AE1-B20 granted to YCK by Taipei Medical University, and 108-S4295 granted to JCL by National Taiwan University Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kao, YC., Bennett, J.A., Suurmeijer, A.J.H. et al. Recurrent MEIS1-NCOA2/1 fusions in a subset of low-grade spindle cell sarcomas frequently involving the genitourinary and gynecologic tracts. Mod Pathol 34, 1203–1212 (2021). https://doi.org/10.1038/s41379-021-00744-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41379-021-00744-7
This article is cited by
-
ESR1::NCOA2/3 fusions in uterine neoplasms with adenosarcoma-like morphology: clinicopathologic and molecular features of 12 cases and review of the literature
Virchows Archiv (2026)
-
A locally aggressive pelvic MEIS1::NCOA1 fusion sarcoma in a young adult female: a case report and review of the literature
Diagnostic Pathology (2025)
-
Pelvic spindle cell sarcomas harboring MEIS1::NCOA2 fusion and novel gene amplifications in 10q23-26 region: a potential predictor for tumor progression
Virchows Archiv (2025)
-
Low-grade undifferentiated sarcoma with MEIS1::NCOA2-rearrangement primary to the lung: a case report
Diagnostic Pathology (2024)
-
Current challenges and practical aspects of molecular pathology for bone and soft tissue tumors
Virchows Archiv (2024)