Abstract
The current classification systems for mental disorders provide a uniform symptom-based language to describe and diagnose mental disorders. Clinicians use these classifications to communicate with their patients and colleagues, to treat patients, and when applicable, to request reimbursement from payers. In clinical research and drug development, diagnostic categories are used as enrollment criteria for clinical trials and to inform prescribing information for the appropriate use of therapeutic interventions. However, like other neuropsychiatric diseases, mental disorders arise from the biology of the brain and its bidirectional interaction with the environment. Current classification systems do not reflect this knowledge. With scientific progress in neuroscience, the time has come for global stakeholders to align research efforts to work toward integrating symptomatic, biological, and behavioral information into the definition of mental disorders to advance the development of effective treatments. The European College of Neuropsychopharmacology (ECNP), following the 2024 New Frontiers Meeting, is coordinating a global initiative to design and implement a Precision Psychiatry Roadmap. By mobilizing resources and harmonizing translational methodologies and datasets, the aim is to discuss, design, and implement an iterative framework that incorporates biology-informed evidence into symptom-based syndromes, allowing for more discovery and implementation of mechanism-based effective treatments for mental disorders.
Similar content being viewed by others
Introduction
Classification systems currently used in psychiatry, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the International Classification of Diseases (ICD), have significant utility. They offer a common language for clinicians and researchers based on a framework, iteratively developed over more than 70 years in the case of DSM [1]. Also, they provide the basis for research, prescribing guidelines, organization of health services, and reimbursement from payers. However, there is substantial heterogeneity within current categorical diagnoses, and there is also overlap of symptoms across disorders, including co-morbidity [2,3,4,5,6]. This suggests that the biological validity of these classifications is poor.
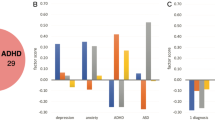
Typically, diagnoses are based on a qualitative clinical assessment of symptoms and do not routinely use biological and quantitative behavioral data. Indeed, in some cases, individuals with distinct biological alterations are grouped within a single diagnostic category (e.g., in autism spectrum disorder (ASD) or depression and anxiety [4,5,6]). We are now learning that, in many cases, symptoms may be driven by different and even opposing biological processes [5, 7]. In addition, most evaluations depend on subjective patient reporting, which is prone to recall or disease-related biases [8]. The lack of continuous, objective assessments of symptom profiles therefore increases heterogeneity of research findings and could contribute to limited effectiveness of treatments.
It is necessary to include multiple types of data, namely, biological and behavioral data as well as symptoms, to improve the biological validity of current classification systems. Dimensional aspects must also be considered, as symptomatic, biological, and behavioral data almost all display a continuum that includes normal-range values. Diagnosis based on the current classification systems can lead to overdiagnosis, as is the case for attention-deficit/hyperactivity disorder (ADHD) [9] and ASD [10], or underdiagnosis, as is the case for schizophrenia [11].
Despite the limited understanding of the complex biology of most psychiatric syndromes, there are at least two reasons why this is the right time to conceptualize a new framework for defining mental disorders. First, the burden of mental disorders is increasing worldwide [12] and available treatments are still suboptimal. The roadblock for developing more effective treatments can, in part, be attributed to the disconnect between symptoms (e.g., feeling sad) and quantitative measures from biology (e.g. reduced content of catecholamines in the synaptic cleft), behavior, and digital data. The consequence is that recruitment to clinical trials results in biologically heterogeneous patient populations with different responses to treatment. For instance, in the Bipolar and Schizophrenia Network for Intermediate Phenotypes (B-SNIP) consortium, this has now been shown in one biotype to be driven by hyper-glutamatergic function, in a second biotype to no strong link to glutamatergic function, and in a third biotype to hypo-glutamatergic function [7]. Therefore, hypothetically, a treatment aimed at attenuating glutamatergic function would only work in one biotype. Because of biological heterogeneity, in large-scale clinical trials, it is proving increasingly difficult to detect meaningful clinical benefit [13] and hence capture the data needed to support regulatory approval of innovative treatments. As in the precision psychiatry paradigm, clinical benefit, as well as improved clinical trial efficiency, is achieved by giving the right treatment to the right patient at the right time.
Second, modern technologies, such as computational tools, high throughput sequencing methods, and digital data, provide the ability to integrate a quantitative understanding of psychological symptoms, environmental exposure, biological activity, and behavioral functioning that can improve the biological validity of our definitions of mental disorders. For example, digital passive remote behavioral monitoring using smartphone or wearable technology can fill the gap in continuous behavioral monitoring by providing real-time, objective tracking of behavioral deviations [14]. Many initiatives using these technologies to develop quantitative measures of biological processes and behavioral data are ongoing – but often not aggregated, not harmonized, and not organized with similar tools and language. Digital measures could eventually, among others, be used as a biomarker (e.g., to measure the cyclicity in manic and depressive episodes in bipolar depression), or as efficacy endpoints (e.g., recently a digital efficacy endpoint for Duchenne muscle atrophy has been qualified by the EMA [15]).
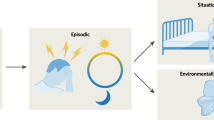
Importantly, a focus on the biological underpinnings of mental disorders does not indicate that environmental experiences are not relevant. Rather, the bidirectional interaction between biology and what has been termed the exposome [16] determines how an individual presents with mental disorders [17, 18]. The exposome consists of (a) specific external exposures, including lifestyle, stress, and social factors, such as trauma, abuse, and discrimination; (b) general external exposures, such as air pollution, socioeconomic status, and rural living; and (c) the internal exposome, which indicates how the human body reacts to, e.g., inflammation or changes in the microbiome. Examining and addressing the presentations that result from these common roots of the exposome can be considered biology-based.
The Precision Psychiatry Roadmap (PPR) is conceptualized as a dynamic process that incorporates new scientific evidence on an ongoing basis into a biologically-informed framework for mental disorders. To achieve this vision, the scientific community must collaborate using harmonized approaches and data-sharing initiatives. We acknowledge that this endeavor is ambitious and will take many years. However, with the appropriate short-, medium-, and long-term goals and milestones, this may be achievable.
The goal is to have evidence-based and quantitative biological and behavioral measurements complement the current symptom-based classification system for mental disorders. This would enable more precise diagnoses that are based on such quantitative measures. The consequence of this would be twofold: first, accurate stratification of heterogenous populations to biologically homogenous subpopulations, and, second, the development of mechanism-based treatments beyond the existing diagnostic boundaries. This means that the current static diagnostic framework focused on signs and symptoms would be transformed to a continuously flexible and iteratively evolving biology-informed framework. This flexibility will allow for innovative and validated biological findings to define the patients progressively better and rapidly allocate appropriate and effective treatment (Fig. 1).
Not everything can happen in parallel, nor does it all have to happen sequentially. Initially, broad changes can be made, e.g., based on our understanding of the relationship between symptoms and behavior via brain circuits and systems. These will be driven, at least in part, by the rapidly emerging digital technologies that are revolutionizing our ability to collect, collate, and interpret complex data sets. As these foundations are put in place, the full power of the omics revolution can be unleashed and the complexity of the interaction with the environment understood and integrated into the envisioned framework.
Implementation of the precision psychiatry roadmap (PPR)
Here we propose a PPR that comprises three main components, namely, (1) developing global alignment on principles and procedures, (2) building consensus on the predictive validity from emerging data, and (3) operationalizing new knowledge into an evolving biology-informed framework. Implementation of these main components will require a large variety of stakeholders to work together on specific tasks and according to a certain timeline. Each of those have been summarized in Table 1 and in the corresponding text sections below.
Global alignment of principles and procedures
Engagement and communication with all stakeholders, including funders and partner organisations
Implementation of the PPR, initially as a research effort, subsequently shaping a new conceptual organization of mental disorders, and eventually bridging into clinical practice, will require collaborations with a broad range of stakeholders, including scientists, scientific organizations, clinicians, industry partners, regulators, policy makers, funding bodies, payers, patients, and educators – the mental health ecosystem. Implementing the road map will be an iterative process and will mean different things to different stakeholders. Also, it will be important to make this a truly global alignment on principles and procedures by involving stakeholders across all continents. This is critical for a global adoption of the PPR as well as for inclusion of contextual factors like ethnicity, race, culture and socioeconomic disparities in the PPR.
The implementation of the PPR will not only require a change in mindset at different organizational levels but will also be highly dependent on coordinated actions from funding agencies to recognize the need to fund the initiatives. The burden from mental disorders is enormous. Recently, it was estimated that “418 million disability-adjusted life years (DALYs) could be attributable to mental disorders in 2019 (16% of global DALYs)—a more than three-fold increase compared to conventional estimates. The economic value associated with this burden is estimated at about USD 5 trillion [12].”
The various steps of the implementation of the PPR will follow different timelines. The key first steps are to discuss, disseminate, and align both the concept and proposed way forward. Some action has already taken place. For example, several meetings and interactions with all stakeholders have already occurred (ECNP New Frontiers Meeting, Global Summit on Precision Psychiatry 2024, session at the ECNP annual congress 2024, ECNP PPR meeting on biomarker validation 2025, ECNP New Frontiers meeting 2025 on targeting neurocircuitry in psychiatry). These meetings demonstrate that there is consensus for the need to discuss the approach’s basic concept.
Harmonisation and platform development
An important requirement for reaching consensus on relevant quantitative biological measures in precision psychiatry is to further harmonize and validate research approaches at a much larger level. Clinical research in psychiatry uses many different self-reporting, clinical, subjective, and objective tools to measure symptom dimensions and behaviors. In addition, biomarker research is sparse and lacks prioritization of methods and modalities. Research tools are often specific for a certain diagnostic area, complicating cross-disorder research with instruments that do not use similar assessments and definitions to measure cross-disorder phenomena. Harmonization of research assessments and approaches across different diagnostic populations is therefore a very much needed and key step. Stimulating examples for projects that use harmonized data and analytic approaches are those conducted by the Psychiatric Genomics Consortium (https://pgc.unc.edu) and the ENIGMA consortium (https://enigma.ini.usc.edu) where hundreds of members collaborate on cross-disorder genomics and neuroimaging research, respectively [20]. Such collaborative initiatives are needed as they stimulate harmonization of variables, analyses, and assessment tools and provide cross-disorder insights. Due to their large sample size and cross-disorder set-up, population-based studies such as the UK-Biobank [21] and the ABCD-study [22] will also further advance the discovery of relevant quantitative biological measures for precision psychiatry. However, these should be complemented by longitudinal patient-based cohort studies with richer mental-health relevant phenotypes. Current initiatives to collaborate across the world in phenotyping large patient groups, such as in the Accelerating Medicines Partnership® Program for Schizophrenia (AMP® SCZ) [23], are essential to reliably examine subdimensions and course prediction. Large-scale patient-based cohort studies that are linked together and where approaches have been harmonized will be instrumental for further precision psychiatry research. In addition, incorporating treatment data into these cohort studies will be an equally crucial aspect as there is a strong need for multi-modal analyses into treatment response prediction.
Also, in the field of interventional clinical research, we need to upscale our activities by conducting larger, multi-site clinical trials as our field is suffering from non-replicable findings from small, single-site studies. In addition, we need to provide strong methodological rigor to our research approaches. Transparent reporting of analyses and findings is essential, for which standard guidelines should be followed. An example is the TRIPOD guideline [24], which provides clear guidelines for building multivariable prediction models. Methodological rigor also includes a pre-specified statistical analysis plan. Unfortunately, there are various barriers to global data sharing across datasets. Issues like privacy regulations, complex legal agreements around data sharing, and proprietary data ownership hinder global collaboration. Constant awareness raising of such obstacles with governmental institutions remains important. Mitigation strategies need to be further developed and include standardization of data and material sharing regulations and legal collaborative agreements at the international level. In addition, as a contingency plan, federated data analyses could help circumvent some barriers as this privacy-preserving analytical framework would allow for the computation of data analytics over multiple remote parties or silo-ed institutional entities without sharing the data among the parties. Another barrier for harmonization approaches is the disparity in infrastructure and variables collected across different study sites. While retrospective harmonization has been successfully applied in large-scale, global genetics and imaging consortia on rather crude phenotypes [2, 20, 25], prospective harmonization with uniform and deeper phenotyped assessments across cross-disorder cohorts and experimental studies remains essential.
To validate or fine-tune quantitative biological measures for precision psychiatry, it is relevant to scale up and speed up testing of such measures across diverse preclinical and clinical research projects. New platform development could support such a process by implementing similar quantitative measures on a large scale across different studies and in different patient populations. To establish well-developed, large-scale platforms, public-private partnerships should be created and regulatory agencies should be consulted early in the process to support this development. New platforms should embrace open-science approaches so that generated data are quickly available for testing by the greater research community. Such platforms should encompass preclinical, clinical, observational, and experimental studies, so that translationally validated measures can be generated with biological understanding and proven clinical validation and utility. An example of a shared biomarker discovery platform is the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a longitudinal multi-site study designed to develop clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of AD. An important lesson from ADNI is the critical role of user-friendly repositories for data and biological samples and the use of harmonized procedures and protocols for tests (http://adni.loni.usc.edu/datasamples/access-data/).
There are also several relatively large scale, multi-site biomarker depression trials that have identified multi-modal predictors of treatment response, along with differential moderators of treatment response, based on imaging, genetic, physiological and behavior measures. These studies each meet several of the criteria, including published preregistered protocols. A number of the findings from these trials survive cross-validation and direct replication [26,27,28,29], illustrating that scaling up collaborative analyses of treatment studies is both possible and necessary to provide valid biology-based mechanisms of treatment response.
Identifying and reaching consensus on relevant quantitative biological measures
Integration of biomarkers into the framework
Despite advances in the understanding of biological processes, the field of psychiatry lacks validated biomarkers for defining populations and tracking disease progression. Biomarkes are defined as a characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or interventions (FDA, 2016). Biomarkers are essential tools for building a biology-informed framework for mental disorders that encompasses biological evidence in the current symptom-based diagnostic classification. Biomarkers are also instrumental in drug development for identifying targets and designing clinical trials. Biomarkers are defined as characteristics that are objectively measured as indicators of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions [30]. The goal of precision psychiatry cannot be achieved without biomarkers.
Categories of biomarkers defined for use in drug development range from diagnostic, monitoring, predictive, and prognostic to pharmacodynamic/response, safety, and susceptibility/risk (Fig. 2). Biomarker modalities are diverse and commonly include imaging, physiological, biofluid-based, genetics/omics, and digital measures. As technologies and use cases emerge, the list continues to expand.
Because of the complexity of psychiatric diseases and the likely contribution of multiple biological processes and comorbidities, multicomponent biomarkers, possibly obtained with multiple modalities, will be needed. These biomarkers may be discovered and validated independently and combined in the framework to describe different biological processes and multiple aspects of the disease and its course (e.g., diagnosis, staging, progression, stratification) or analyzed in the context of a predictive model [23]. Furthermore, it is important for biomarkers to distinguish between causation from correlation; in other words, are these markers indicative of underlying neurobiology or secondary to symptom presentations?
In Alzheimer’s disease (AD), increased biological understanding and the development of validated biomarkers has already shifted the diagnostic framework. Classically, AD was diagnosed post-mortem through findings of amyloid plaques and tau tangles; in vivo, diagnosis was made based on cognitive impairment and altered functioning. However, advances in biomarker development revealed that cognitive symptoms are the late stage of a decades-long pathological process that can be uncovered using positron emission tomography and cerebrospinal fluid (CSF) markers. This paradigm shift prompted revisions in AD diagnostic criteria and staging methodologies, transitioning from a syndrome-based approach to one incorporating biomarkers [31]. Findings have now led to the Aβ, tau, and neurodegeneration (ATN) framework, which assigned biomarkers to mechanistic groups based on the pathogenic entity [32]. The ATN framework proposed assigning individuals to a biomarker status independent of their cognitive status, fostering a more precise and biologically grounded understanding of AD. These findings contributed to the first approval by the US Food and Drug Administration (FDA) of a monoclonal antibody targeting amyloid plaques as a treatment for AD [33]. The 2018 research framework has now further evolved to the 2024 revised criteria for diagnosis and staging that are intended to replace both the 2018 research criteria and the 2011 clinical criteria [34, 35], emphasizing biomarker-based diagnosis and staging, while restricting clinical diagnosis to symptomatic individuals.
Importance of biological systems across biomarkers, molecules, circuits, behavior
Building biology-driven hypotheses
A critical component of the framework comprises tying mechanistic biology-driven hypotheses to precision diagnostics and targeted therapeutics. In psychiatry, syndromes with known causative biology represent only a small fraction of mental disorders, e.g., NMDA receptor autoantibodies in specific psychosis syndromes [36] or syndromes with known genetic etiology, such as autism spectrum symptoms in Fragile X syndrome [37]. Even in these rare cases, the biological processes that link the genetic etiology to the clinical manifestations are still unclear.
For common mental disorders, such as schizophrenia, depression, anxiety, and substance use disorders, there are currently no discrete causative hypotheses, but rather diverse biological pathways that contribute to these largely heterogeneous phenotypes. For this reason, in order to build biology-driven hypotheses, collecting multimodal data based on neuroimaging, neurophysiology, and/or digitally-derived measures of behavior, in addition to molecular biomarkers, is imperative. Large longitudinal datasets are needed for identifying more biomarker-based, mechanistic hypotheses on which to build future efforts.
Clearly addressing the whole of psychiatry to this level of detail is a massive undertaking. Therefore, an important, early component to the implementation of the Roadmap would be the identification of areas of initial focus. These initial focus areas could be based on emerging science, assessment of relative clinical unmet need, available technology etc. The intention being to use the process laid out in Table 1 to iteratively complete the framework as shown in Fig. 3. To support the merit of this approach there are already good examples of what can be achieved. For example, the IMI (the European Union’s innovative medicines initiative) funded first the PRISM project which explored the potential to find a previously undetected transdiagnostic biology of social dysfunction across dementia and schizophrenia [38, 39]. The success of this project led to a refunding by the IMI of PRISM2 to probe the robustness of the concept, further develop the novel endpoints towards qualification as biomarkers and explore if the findings could be extended to major depression. This success has now prompted a new industry consortium to launch a new call (April 2025) within the IHI (the innovative health initiative, the EU’s successor to IMI) framework to explore a second transdiagnostic concept.
Implementation and operational delivery of a continuously evolving biology-informed diagnostic framework requires global alignment on principles and procedures and building consensus on the predictive validity of the new biology-informed findings to improve patient stratification and treatment. This framework will evolve based on scientific innovations in research and development and clinical implementation and will involve acceptance by professional organizations. Indeed, implementation of this biology-informed framework will require stakeholder engagement at all stages to deliver precision diagnostic and treatments for mental disorders. Stakeholders include patients and patient-family organisations, representatives from broad research initiatives in psychiatric disorders (such as the FNIH led AMP® SCZ initiative [23]), professional organisations (such as the International College of Neuropsychopharmacology CINP, the American College of Neuropsychopharmacology ACNP, the European College of Neuropsychopharmacology ECNP, the European Psychiatric Association EPA, funders, representatives from industry (such as big pharma and small and medium-sized enterprises) and regulatory bodies, health care professionals and educators, among others.
Dysfunction across systems and circuits
An important component of the development of a biology-informed framework may require a ‘bottom-up’ rebuilding of syndromes and symptoms, starting from biological processes and behavioral functions. Progress has been made, e.g., in our understanding of specific, albeit transdiagnostic concepts, such as hedonic dysfunction, focusing on ventral striatum mechanisms of reward processing and its hypofunction in anhedonia [40], or hyperfunction (or lack of cortical regulation) in substance use disorders [41]. Similarly, progress has been made in our understanding of negative valence systems, which also vary from hyperfunction to hypofunction. For instance, several studies have showen hyperactive threat processing in PTSD [42] and panic disorder [43], driven in part by an over-reactive threat response by the amygdala and brainstem, leading to increased hypervigilance, hyperarousal, sympathetic activation, startle response, and cardiopulmonary activation [44,45,46,47]. However, multiple laboratories have observed blunted reactivity in PTSD and multiple anxiety disorders [48,49,50,51]. Reward-based disorders and negative valence disorders both have components of altered cognition, likely in part due to cortical and hippocampal dysfunction, leading to increased generalization of cues and lack of goal-directed behaviors over habitual behaviors that then allow emotional behavior to over-ride top-down control [41, 52]. While there is less understanding of circuit models of some disorders and syndromes (e.g., psychosis and delirium) that appear to involve more general brain dysfunction [53], compared to the more ‘circuit-specific’ syndromes above, this provides additional cues for biomarker development – in the form of circuit function – to contribute to an hypothesis-driven classification of behavior and symptoms.
One way of investigating and validating circuit dysfunction-based hypotheses requires using innovative clinical trial designs. Clinical trial populations could be defined based on the specific circuit or system dysfunction hypotheses encompassing multiple diagnostic categories [38]. This will advance our understanding of the relationship between symptoms and behavior via brain circuits and systems.
Mechanism-based treatments
Most neuropsychopharmacology to date is based on our understanding of neurotransmitter systems in the brain, receptor functions, and cellular and molecular pathways connected to them. Drugs targeting the monoaminergic systems – primarily the serotonergic and noradrenergic systems in depression, stress, and anxiety disorders, and the dopaminergic system in psychosis and reward-based disorders – have been well studied for many years. Targeting the fast neurotransmitter systems has broad efficacy, ranging from anesthesia and epilepsy to dampening anxiety and hyperarousal symptoms. While much progress has been made, most of these agents are used very broadly in psychiatry. As we reach a greater understanding of disorder subtypes, it is likely that we will have more targeted regional and circuit specificity at the level of neuropeptides, nuclear hormones, and other atypical transmitter systems. Additional ways of targeting brain biology from epigenetic studies, targeted RNA and noncoding RNA, and protein modification approaches are exciting. As more mechanism-based neuroscience is integrated into drug development, the nomenclature could potentially become mechanism-based, such as that proposed in the ‘Neuroscience Based Nomenclature’ initiative [54]. With this approach, drugs would not be referred to as ‘antidepressants’ or ‘antipsychotics’ but rather using language that directly refers to their mechanism of action.
In addition to pharmacological treatments, there remains tremendous importance in understanding the mechanisms of psychotherapy and/or behavioral therapy at a biological level. Psychotherapy will likely continue to be a critical process by which specific memory traces, patient history, and complex concepts such as cognitive beliefs can be both evaluated and treated. Our understanding of neural plasticity – either where it is diminished (e.g., in depression or psychosis) or enhanced (through medications or neural stimulation) – will likely come together with our understanding of how to use psychological therapy or non-invasive brain stimulation in biologically informed ways to target critical aspects of psychological functioning.
Building and expanding on other frameworks
Addressing the biology of mental disorders will require novel frameworks and concepts. As an example, the Research Domain Criteria (RDoC) project was initiated by NIMH in 2009 to develop new approaches to research on mental disorders [55]. RDoC was intended to address these issues by creating a research framework based on normal dimensions of functioning that may be implicated in psychopathology when disrupted, thus connecting specific symptoms with particular aspects of behavior and neural circuits. In contrast to the PPR, RDoC was not intended to develop global alignment on principles and procedures, building consensus on the predictive validity from emerging data, and operationalizing new knowledge into an evolving biology-informed framework.
Importance of translational predictive model systems
Preclinical studies contribute to our understanding of the underlying mechanisms of mental disorders and to treatment development. First, preclinical in vivo studies in animals and/or in vitro studies in cellular assays can be used to enhance our fundamental biological knowledge of the brain. Second, preclinical studies will be needed to evaluate correlative human observations. Human studies provide relationships between various biological measures, such as brain region activity and behavioral performance. To evaluate the causality of such a finding, backtranslation in animal studies can be instrumental, e.g., by activating the corresponding brain area and testing for effects on the specific behavior using a variety of methodologies (e.g., chemo- or opto-genetic activation of the neurocircuit identified). In this way, biology-informed diagnostics for mental disorders will not only provide more precise diagnoses, but they will also enhance the translational validity of predictive animal and cellular model systems to accelerate the biological understanding of mental disorders and the drug discovery process.
Importance of predisposing factors, age, and the environment
Because many neuropsychiatric syndromes are neurodevelopmental, when modeling mental disorders, it is important to consider at what age damage occurs, i.e., preconception, in utero, prenatal, infancy, adolescence, or adulthood. Importantly, puberty, pregnancy, and menopause, typically associated with hormonal changes, can also precipitate psychopathology in women [56]. In addition to maturational brain events, additional environmental stressors must be considered. Apart from biological life stages and sex, personality features, such as neuroticism, but also general external factors, such as poverty, discrimination, and childhood trauma, predispose to mental disorders. Three-quarters of mental disorders develop before the age of 25 years [57] and therefore resilience features also need to be studied. To better understand the interaction of risk and resilience factors on healthy individuals, population-based longitudinal cohorts are needed. For example, the US-based Adolescent Brain Cognitive Development Study (ABCD Study®)(currently with 12,000 participants) examines long-term brain development and child health, and in the German National Kohort (NAKO [58]), 60,000 people have been phenotypically assessed in detail, including a whole-body MRI-scan (at least twice to date). Such studies allow us to disentangle genetic and environmental risk factors over a lifetime, including birth and obstetric complications, socioeconomic status of the family, and early cannabis use. The goal must be to identify individual risk and resilience features over a lifetime, including the likelihood of developing mental and somatic disease [59] preventive measures to reduce the incidence of mental disorders [60].
Validation biomarkers and identification of new biotypes
Validation is critical for the development of biomarkers as reliable quantitative measures of biological phenomena. Biomarker validation is a multi-layer process. Analytical validation is necessary to demonstrate that the test, and the biomarker through its test, accurately measures what it is intended to measure. Clinical validation establishes how to interpret the biomarker value or change in the biomarker value at the individual level. Importantly, clinical validation begins with internal validation intended to demonstrate that a relevant measure reflects the underlying process of interest, independently from confounders in the population in which it is developed. Thereafter, it must be demonstrated that the measure has sufficient predictive validity in an independent sample (external validation) to prove its generalizability. Unfortunately, most biomarkers under development fail to demonstrate generalizability; a critical impediment to external validation is overfitting. Overfitting is an error that occurs when a model excessively reflects the idiosyncratic features of the dataset in which it is developed, so that it underperforms when applied to new data. This could happen when a biomarker is developed in a dataset that is too small or that contains substantial amounts of irrelevant information, called noisy data. To guide the use and implementation of quantitative measures, we must define minimum standards for internal and external validation. By establishing whether biomarkers (and the tests used to assess them) are fit-for-purpose, validation informs any potential use of a biomarker, and only properly validated biomarkers can be used for regulatory purposes. Implementing minimum standards for internal and external validation requires a variety of steps, such as identification of clinically relevant biological constructs, global alignment on the definitions and assessment methodologies and outcome measures for relevant biological constructs, and internal and external validation of biomarkers based on these biological constructs, thus providing consensus on the predictive validity of the validated biomarkers. These steps, a corresponding timeline and the stakeholders involved have been indicated in Table 1.
Consensus on the predictive validity from emerging new data
Perform clinical intervention trials in stratified patient populations
Industry is already moving forward with innovative programs that rely far more on the existing biological understanding of the maladapted circuitry than in the past. New clinical trials will have to be conducted that incorporate quantitative biological outcome measures and stratification biomarkers to study treatment efficacy based on biology-informed subtype analyses. The transition from one approach to another will not happen overnight – it will be iterative and evolutionary.
Obtaining regulatory approval for biomarkers, outcome measures and treatments
The implementation of emerging biological and behavioral findings into a continuously evolving diagnostic framework will need to be based on consensus about the predictive validity of these biological data. A mechanism by which this consensus will be reached has yet to be developed and implemented. Furthermore, scientific innovations will be an important driver for the development and validation of novel quantitative biomarkers and mechanism-based treatments to optimize treatment success for mental disorders. Interactions with regulators will be a key component throughout the process to ensure that hypotheses and measures can be translated into validated tools for use in drug development.
Implementation of an evolving biology-informed diagnostic framework
Incorporation of biological information in diagnostic classification and treatment guidelines
A procedure for incorporating novel science on a continuous basis into an adaptive diagnostic framework has yet to be established as part of the PPR. This step will have to overcome the complexity that symptoms at the level of diagnostic classes often do not relate convincingly to neural systems or functional performance. Two of the authors of this paper are members of the current future DSM strategic committee to incorporate the importance a biology-informed continuously evolving framework for mental disorders in these forward looking discussions.
In parallel, collaborative research initiatives will be implemented and aligned with ongoing initiatives to identify, curate, analyze, and interpret quantitative biological measures to refine and improve the current diagnostic framework.
Developing strategies for implementation of precision diagnostics and treatments into health care practice
Once in place, the ongoing refinement of the biology-informed diagnostic framework will also require education of clinicians and patients, which is another critical component of the PPR, as they will be the ones who need to recognize the clinical relevance of this biology-informed framework for mental disorders to implement more precise diagnosis and treatments. As part of the PPR, we will reach out to clinical organisations (e.g., EPA, APA, national societies) to disseminate knowledge, discuss and implement strategies necessary for health care implementation.
Summary and conclusions
This manuscript describes the concept, principles, and important aspects of the PPR intended as the process to build and implement a biology-informed framework for mental disorders that encompasses continuously evolving biological evidence into the current symptom-based classification of psychiatric syndromes. This PPR initiative calls for multi-stakeholder global alignment on principles and objectives, coordination of preclinical and clinical research methods, data sharing, and funding to identify and validate quantitative measures of psychiatric phenotypes and to implement hypothesis-driven treatment development (Fig. 3).
The implementation of the PPR will be a long-lasting effort that will involve many different stakeholders at all stages of the process, with the scope of progressively validating biological measures and hypotheses with the necessary degree of confidence and scientific consensus. As biological knowledge progresses and technological advancements are made, the time has come to support this global initiative and align data, principles, and objectives to eventually bring the right treatments to the right patients at the right time.
It is uncertain how long it will take to observe noticeable advances in precision psychiatry, and the consequent steady reduction in the burden of mental disorders. As a former Director of the National Institute of Mental Health noted regarding the complexity of mental disorders and their components, “The missing scientific pieces of these puzzles will take years to discover and integrate into a fully useful picture; however, intellectual support for both the clinical enterprise and much-needed research both demand alternatives to the categorical approach of the [current] DSM” [61]. Given this need for action, the milestones for the PPR demonstrate an aspiration to move with alacrity.
References
Diagnostic and statistical manual: mental disorders (DSM-I). Washington D.C.: American Psychiatric Association; 1952.
Lee PH, Anttila V, Won H, Feng Y-CA, Rosenthal J, Zhu Z, et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469–82.e11.
Kas MJH, Fernandes C, Schalkwyk LC, Collier DA. Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol Psychiatry. 2007;12:324–30.
Fountain C, Winter AS, Bearman PS. Six developmental trajectories characterize children with autism. Pediatrics. 2012;129:e1112–20.
Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84.
Tozzi L, Zhang X, Pines A, Olmsted AM, Zhai ES, Anene ET, et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat Med. 2024;30:2076–87. https://doi.org/10.1038/s41591-024-03057-9
Bolo N, Zeng V, Clementz B, Tamminga C, Pearlson G, Gershon E, et al. Hippocampal glutamate, verbal episodic memory and intrinsic neural activity in the psychosis spectrum. Biol Psychiatry. 2021;89:S345.
Jongs N, Penninx B, Arango C, Ayuso-Mateos JL, van der Wee N, Rossum IW, et al. Effect of disease related biases on the subjective assessment of social functioning in Alzheimer’s disease and schizophrenia patients. J Psychiatr Res. 2022;145:302–8.
Kazda L, Bell K, Thomas R, McGeechan K, Sims R, Barratt A. Overdiagnosis of attention-deficit/hyperactivity disorder in children and adolescents. JAMA Netw Open. 2021;4:e215335.
Rødgaard E-M, Jensen K, Vergnes J-N, Soulières I, Mottron L. Temporal changes in effect sizes of studies comparing individuals with and without autism. JAMA Psychiatry. 2019;76:1124.
Lopez-Castroman J, Leiva-Murillo JM, Cegla-Schvartzman F, Blasco-Fontecilla H, Garcia-Nieto R, Artes-Rodriguez A, et al. Onset of schizophrenia diagnoses in a large clinical cohort. Sci Rep. 2019;9:9865.
Arias D, Saxena S, Verguet S. Quantifying the global burden of mental disorders and their economic value. EClinicalMedicine. 2022;54:101675.
Leichsenring F, Steinert C, Rabung S, Ioannidis JPA. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta‐analytic evaluation of recent meta‐analyses. World Psychiatry. 2022;21:133–45.
Jongs N, Jagesar R, van Haren NEM, Penninx BWJH, Reus L, Visser PJ, et al. A framework for assessing neuropsychiatric phenotypes by using smartphone-based location data. Transl Psychiatry. 2020;10:211.
Servais L, Strijbos P, Poleur M, Mirea A, Butoianu N, Sansone VA, et al. Evidentiary basis of the first regulatory qualification of a digital primary efficacy endpoint. Sci Rep. 2024;14:29681.
Wild CP. Complementing the genome with an “Exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–50.
Barzilay R, Pries L-K, Moore TM, Gur RE, van Os J, Rutten BPF, et al. Exposome and trans-syndromal developmental trajectories toward psychosis. Biol Psychiatry Glob Open Sci. 2022;2:197–205.
Xu J, Liu N, Polemiti E, Garcia-Mondragon L, Tang J, Liu X, et al. Effects of urban living environments on mental health in adults. Nat Med. 2023;29:1456–67.
Kas MJH, Hyman S, Williams LM, Hidalgo-Mazzei D, Huys QJM, Hotopf M, et al. Towards a consensus roadmap for a new diagnostic framework for mental disorders. Eur Neuropsychopharmacol. 2025;90:16–27.
Georgiadis F, Larivière S, Glahn D, Hong LE, Kochunov P, Mowry B, et al. Connectome architecture shapes large-scale cortical alterations in schizophrenia: a worldwide ENIGMA study. Mol Psychiatry. 2024;29:1869–81. https://doi.org/10.1038/s41380-024-02442-7
Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, et al. Inflammation and brain structure in schizophrenia and other neuropsychiatric disorders. JAMA Psychiatry. 2022;79:498.
Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. 2022;604:525–33.
Wannan CMJ, Nelson B, Addington J, Allott K, Anticevic A, Arango C, et al. Accelerating medicines partnership® schizophrenia (AMP® SCZ): rationale and study design of the largest global prospective cohort study of clinical high risk for psychosis. Schizophr Bull. 2024;50:496–512.
Moons KGM, Altman DG, Reitsma JB, Collins GS. New guideline for the reporting of studies developing, validating, or updating a multivariable clinical prediction model. Adv Anat Pathol. 2015;22:303–5.
Xia Y, Xia C, Jiang Y, Chen Y, Zhou J, Dai R, et al. Transcriptomic sex differences in postmortem brain samples from patients with psychiatric disorders. Sci Transl Med. 2024;16:eadh9974.
Brannan SK, Mayberg HS, McGinnis S, Silva JA, Tekell J, Mahurin RK, et al. 355. Cingulate metabolism predicts treatment response: a replication. Biol Psychiatry. 2000;47:S107.
Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression. Neuroreport. 1997;8:1057–61.
Williams LM, Whitfield Gabrieli S. Neuroimaging for precision medicine in psychiatry. Neuropsychopharmacology. 2025;50:246–57.
Vreijling SR, Chin Fatt CR, Williams LM, Schatzberg AF, Usherwood T, Nemeroff CB, et al. Features of immunometabolic depression as predictors of antidepressant treatment outcomes: pooled analysis of four clinical trials. Br J Psychiatry. 2024;224:89–97.
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) resource. Silver Spring, MD: Food and Drug Administration (US); 2016.
Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47.
Harris E. Alzheimer drug lecanemab gains traditional FDA approval. JAMA. 2023;330:495.
Jack CR, Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024;20:5143–69. https://doi.org/10.1002/alz.13859
Dubois B, Villain N, Schneider L, Fox N, Campbell N, Galasko D, et al. Alzheimer disease as a clinical-biological construct—An international working group recommendation. JAMA Neurol. 2024;81:1304.
Pollak TA, Kempton MJ, Iyegbe C, Vincent A, Irani SR, Coutinho E, et al. Clinical, cognitive and neuroanatomical associations of serum NMDAR autoantibodies in people at clinical high risk for psychosis. Mol Psychiatry. 2021;26:2590–604.
Brown WT, Friedman E, Jenkins EC, Brooks J, Wisniewski K, Raguthu S, et al. Association of fragile X syndrome with autism. Lancet. 1982;319:100.
Kas MJ, Penninx B, Sommer B, Serretti A, Arango C, Marston H. A quantitative approach to neuropsychiatry: the why and the how. Neurosci Biobehav Rev. 2019;97:3–9.
Saris IMJ, Aghajani M, Reus LM, Visser P-J, Pijnenburg Y, van der Wee NJA, et al. Social dysfunction is transdiagnostically associated with default mode network dysconnectivity in schizophrenia and Alzheimer’s disease. World J Biol Psychiatry. 2022;23:264–77.
Pizzagalli DA. Toward a better understanding of the mechanisms and pathophysiology of anhedonia: are we ready for translation? Am J Psychiatry. 2022;179:458–69.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Ressler KJ, Berretta S, Bolshakov VY, Rosso IM, Meloni EG, Rauch SL, et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol. 2022;18:273–88.
Guan X, Cao P. Brain mechanisms underlying panic attack and panic disorder. Neurosci Bull. 2024;40:795–814.
Seligowski AV, Webber TK, Marvar PJ, Ressler KJ, Philip NS. Involvement of the brain–heart axis in the link between PTSD and cardiovascular disease. Depress Anxiety. 2022;39:663–74.
Lanius RA, Rabellino D, Boyd JE, Harricharan S, Frewen PA, McKinnon MC. The innate alarm system in PTSD: conscious and subconscious processing of threat. Curr Opin Psychol. 2017;14:109–15.
Alexandra Kredlow M, Fenster RJ, Laurent ES, Ressler KJ, Phelps EA. Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology. 2022;47:247–59.
Fitzgerald JM, DiGangi JA, Phan KL. Functional neuroanatomy of emotion and its regulation in PTSD. Harv Rev Psychiatry. 2018;26:116–28.
Frewen PA, Lanius RA. Neurobiology of dissociation: unity and disunity in mind–body–brain. Psychiatr Clin North Am. 2006;29:113–28.
D’Andrea W, Pole N, DePierro J, Freed S, Wallace DB. Heterogeneity of defensive responses after exposure to trauma: blunted autonomic reactivity in response to startling sounds. Int J Psychophysiol. 2013;90:80–89.
Sambuco N, Bradley M, Herring D, Hillbrandt K, Lang PJ. Transdiagnostic trauma severity in anxiety and mood disorders: functional brain activity during emotional scene processing. Psychophysiology. 2020;57:e13349.
McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–56.
Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N Engl J Med. 2017;376:2459–69.
Tandon R, Nasrallah H, Akbarian S, Carpenter WT, DeLisi LE, Gaebel W, et al. The schizophrenia syndrome, circa 2024: what we know and how that informs its nature. Schizophr Res. 2024;264:1–28.
Zohar J, Stahl S, Moller H-J, Blier P, Kupfer D, Yamawaki S, et al. A review of the current nomenclature for psychotropic agents and an introduction to the neuroscience-based nomenclature. Eur Neuropsychopharmacol. 2015;25:2318–25.
Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: The NIMH research domain criteria project. Schizophr Bull. 2010;36:1061–2.
Kundakovic M, Rocks D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: from clinical evidence to molecular mechanisms. Front Neuroendocrinol. 2022;66:101010.
Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–95.
Peters A, Peters A, Greiser KH, Göttlicher S, Ahrens W, Albrecht M, et al. Framework and baseline examination of the German National Cohort (NAKO). Eur J Epidemiol. 2022;37:1107–24.
Wen J, Varol E, Yang Z, Hwang G, Dwyer D, Kazerooni AF, et al. Subtyping brain diseases from imaging data. In: Machine learning for brain disorders. New York, NY: Humana; 2023. p. 491–510.
Wasserman D, Hoven CW, Wasserman C, Wall M, Eisenberg R, Hadlaczky G, et al. School-based suicide prevention programmes: the SEYLE cluster-randomised, controlled trial. Lancet. 2015;385:1536–44.
Hyman SE. Psychiatric disorders: grounded in human biology but not natural kinds. Perspect Biol Med. 2021;64:6–28.
Acknowledgements
The authors would like to thank GAMIAN Europe, and especially Shaun Johnson, for their careful review and valuable feedback on an earlier version of the manuscript.
Author information
Authors and Affiliations
Contributions
MJHK, BWJHP, GMK, BC, PF, GSS, KJR, ML, HM, JL and VM contributed to the conceptualization of the paper outline. MJHK generated the first draft of the manuscript. BWJHP, GMK, BC, PF, GSS, KJR, ML, HM, JL and VM reviewed and edited the paper. EBI and FBD reviewed and edited the near final version of the manuscript. MJHK led and coordinated the project, and incorporated the co-authors’ feedback.
Corresponding author
Ethics declarations
Competing interests
MJHK, BWJHP, BC declare no conflict of interest. GMK served as a speaker for Angelini, Abbvie, Cybin, and H Lundbeck, as an advisor for Sanos, Onsero, Pangea Botanica, Gilgamesh, and Pure Technologies and as a research site for Reunion and Delix Therapeutics. BC: The views in this article do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the United States Government. PF is on the advisory board of Gedeon Richter, JNJ and Boehringer-Ingelheim, and gave paid talks for Gedeon Richter, Ostuka, JNJ, Boehringer-Ingelheim and Lundbeck. GSS is a full-time employee of Signant Health and has consulting agreements with Rapport Therapeutics, Intracellular, Merck, Kintsugi, and Cognitiv. KJR has performed consultation for Bionomics, Acer and Jazz Pharma; Scientific Advisory Boards for Sage, Boehringer Ingelheim, Senseye, Brain & Behavior Research Foundation and Brain Research Foundation, and received sponsored research support from Alto Neuroscience. FBD was an employee of EMA during the conceptual design, drafting and review of the manuscript. The views expressed in this article are the personal views of the author and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency with which the author is/was affiliated. HM is a full-time employee of Boehringer Ingelheim GmbH. JL is a full-time employee of H Lundbeck A/S. VM has no conflict of interest to declare. VM views and opinions presented in this manuscript are her own and should not be construed to represent an official FDA position or policy.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kas, M.J.H., Penninx, B.W.J.H., Knudsen, G.M. et al. Precision psychiatry roadmap: towards a biology-informed framework for mental disorders. Mol Psychiatry 30, 3846–3855 (2025). https://doi.org/10.1038/s41380-025-03070-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03070-5