Abstract
The synthetic opioid fentanyl remains abundant in the illicit drug supply, contributing to tens of thousands of overdose deaths every year. Despite this, the neurobiological effects of fentanyl use remain largely understudied. The nucleus accumbens (NAc) is a central locus promoting persistent drug use and relapse, largely dependent on activity of dopamine D1 receptors. NAc D1 receptor-expressing medium spiny neurons (D1-MSNs) undergo molecular and physiological neuroadaptations in response to chronic fentanyl that may promote relapse. Here, we obtained Drd1-cre120Mxu mice to investigate D1-dependent mechanisms of fentanyl relapse. We serendipitously discovered this mouse line has reduced fentanyl seeking, despite similar intravenous fentanyl self-administration, similar sucrose self-administration and seeking, and greater fentanyl-induced locomotion compared to wildtype counterparts. We found drug-naïve Drd1-cre120Mxu mice have elevated D1 receptor expression in NAc and increased sensitivity to the D1 receptor agonist SKF-38393. After fentanyl self-administration, Drd1-cre120Mxu mice exhibit divergent expression of MSN markers, opioid receptors, glutamate receptor subunits, and TrkB which may underly their blunted fentanyl seeking. Finally, we show fentanyl-related behavior is unaltered by chemogenetic manipulation of NAc core D1-MSNs in Drd1-cre120Mxu mice. Conversely, chemogenetic stimulation of ventral mesencephalon-projecting NAc core MSNs (putative D1-MSNs) in wildtype mice recapitulated the blunted fentanyl seeking of Drd1-cre120Mxu mice, supporting a role for aberrant D1-MSN signaling in this behavior. Together, our data uncover alterations in NAc gene expression and function with implications for susceptibility and resistance to developing fentanyl use disorder.
Similar content being viewed by others
Introduction
Opioid use disorder (OUD) is a chronic, relapsing condition characterized by uncontrollable opioid use and craving despite negative consequences, and the development of tolerance and withdrawal [1]. In 2022, over 9 million adults in the U.S. required OUD treatment [2], and over 80,000 individuals died from fatal overdose involving opioids [3]. A massive contributor to this epidemic is the current abundance of fentanyl in the illicit drug supply [4]. Fentanyl is a potent synthetic opioid that possesses unique pharmacological properties compared to other opioids: it has high lipid solubility that allows for rapid passage through the blood brain barrier [5], unique binding interactions with the mu opioid receptor [6], and higher selectivity for mu over kappa and delta opioid receptors as compared to morphine [7]. Fentanyl exposure is associated with poorer treatment outcomes for OUD [8], necessitating novel treatment approaches to cases of OUD involving fentanyl.
The vicious cycle of OUD, in which an abstinence-induced negative affective state promotes persistent relapse, is thought to be driven by neural circuitry centered around the nucleus accumbens (NAc) [1, 9]. The NAc, located in the ventral striatum, is primarily comprised of GABAergic medium spiny neurons (MSNs) that are divided into two subtypes depending on whether they express Drd1 (encoding dopamine D1 receptor; D1-MSNs) or Drd2 (encoding dopamine D2 receptor; D2-MSNs). The MSN subtypes can be further identified by co-expression of additional markers: D1-MSNs co-express Chrm4 (muscarinic acetylcholine receptor M4), Pdyn (preprodynorphin), and Tac1 (preprotachkykinin-1); D2-MSNs co-express Adora2a (adenosine A2A receptor), Gpr6 (G protein-coupled receptor 6), and Penk (preproenkephalin) [10, 11]. D1- and D2-MSNs have distinct projection targets [11,12,13] that typically drive opponent processes in reward behavior, where D1-MSN activity is classically considered “pro-reward” while D2-MSN activity is “anti-reward” [14,15,16,17,18,19,20,21], although there are numerous exceptions [22,23,24,25,26,27,28,29].
NAc MSNs undergo extensive physiological [30,31,32,33,34,35,36,37,38,39,40,41,42] and transcriptional [43,44,45,46,47,48,49,50,51] adaptations following opioid abstinence that promote negative affect during withdrawal or increase relapse to drug seeking. However, neuroadaptations vary across opioid class and MSN subtype. Our prior work demonstrates homecage oral fentanyl produces adaptations specifically in D1- but not D2-MSNs that underly negative affect during abstinence [52]. In contrast, intravenous heroin self-administration produces similar adaptations to both D1- and D2-MSNs that promote drug seeking behavior [53, 54]. Whether intravenous fentanyl self-administration produces subtype-similar, or subtype-specific neuroadaptations remains unknown.
To further understand how NAc D1-MSNs influence intravenous fentanyl self-administration and relapse to fentanyl seeking, we obtained a Drd1-cre mouse line deposited to the Jackson Laboratory [55] that has been used by several other groups [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. First, we characterized baseline fentanyl self-administration and seeking behavior, and serendipitously discovered that the Drd1-cre120Mxu (MMRC #037156-JAX) line demonstrates reduced fentanyl seeking, despite similar acquisition and fentanyl intake as their wildtype littermates. Drd1-cre120Mxu mice also show reduced fentanyl conditioned place preference despite increased fentanyl-induced hyperlocomotion, as well as increased sensitivity to D1 receptor agonism, but normal sucrose self-administration and seeking. We further show these mice have differential mRNA expression of several NAc MSN markers both at baseline and after prolonged abstinence from fentanyl self-administration, including elevated Drd1 expression at both timepoints. Lastly, we show that chemogenetic stimulation of ventral mesencephalon-projecting NAc core MSNs (putative D1-MSNs) in wildtype mice recapitulates the blunted fentanyl seeking exhibited by Drd1-cre120Mxu mice, supporting aberrant signaling in NAc D1-MSNs as a mechanism promoting this behavior in the Drd1-cre120Mxu line.
Materials and methods
Experimental subjects
All procedures were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University College of Medicine (PSUCOM) and conducted in accordance with NIH guidelines for the use of laboratory animals. Drd1-cre120Mxu mice on a C57BL/6 J background were obtained from The Jackson Laboratory (B6;129-Tg(Drd1-cre)120Mxu/Mmjax; Jax strain #024860; RRID:MMRRC_037156-JAX) [55]. At PSUCOM, Drd1-cre120Mxu hemizygotes were bred with C57BL/6 J wildtype mice obtained from The Jackson Laboratory. Mice were given food and water ad libitum and housed in the PSUCOM vivarium on a 12:12 h light:dark cycle with lights on at 7:00. All mice were housed in corncob bedding and provided with nestlets. Mice were group housed until intravenous surgery, after which they were pair-housed across a perforated acrylic divider throughout self-administration to prevent isolation stress. All experiments used mice of both sexes aged 8–10 weeks at the start of behavior.
Procedures
All procedures are detailed in Supplemental.
Statistics
Data were analyzed with Graphpad Prism 10 (La Jolla, CA) and JASP [71]. In the absence of significant sex effects or interactions, we collapsed data by sex. Any repeated measures data (e.g. self-administration, locomotion) were analyzed with repeated-measures ANOVA (RM-ANOVA) employing a Greenhouse-Geisser sphericity correction, with sex and genotype as between-subjects factors. Seeking data were analyzed with ANOVA using sex and genotype as between-subjects factors, and nose-poke as within-subject factor. Data without repeated measures (e.g. conditioned place preference, stereotypy) were analyzed by unpaired t-test due to no sex effects, or ANOVA when sex was significant. Gene expression data were analyzed with 2-way ANOVA using sex and genotype. For chemogenetic experiments, seeking was analyzed with ANOVA using sex, genotype, and virus as between factors, and nose-poke as within-subject factor. c-fos expression was analyzed with Welch’s ANOVA due to unequal variance. All post-hoc tests employed Sidak’s correction, except for the chemogenetic conditioned place preference and c-fos experiments which employed Dunnett’s comparing to the mCherry control.
Results
Drd1-cre120Mxu mice exhibit attenuated fentanyl seeking behavior
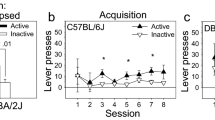
NAc dopamine D1 receptors (D1R) play key roles in mediating reward and addiction phenotypes [9]. To answer questions about the mechanisms of D1-neurons in fentanyl use and relapse, we obtained Drd1-cre120Mxu [55] mice from the Jackson Laboratory. While this Drd1-cre line has been used by other investigators in recent papers [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70], known issues in other lines [72,73,74,75] necessitate characterizing behavior of all transgenic mice. Thus, we first asked if Drd1-cre120Mxu mice would self-administer intravenous fentanyl. Similar to our previous work in wildtype C57/BL6J mice [76], Drd1-cre positive and negative mice underwent 10 d of fentanyl self-administration training (timeline in Fig. 1A). Both genotypes self-administered a similar number of fentanyl infusions (Fig. 1B, sexes combined due to no sex effects or interactions. RM-ANOVA, Day: F3.030,415.2 = 3.40, p = 0.018; Day x Genotype: F9,1233 = 1.06, p = 0.39). Both genotypes learned to discriminate between active and inactive nose-pokes as measured by discrimination index (Fig. 1C, D, RM-ANOVA, Day: F2.975, 407.6 = 6.27, p = 0.0004; Day x Genotype: F9,1233 = 0.67, p = 0.72). Across 10 d of self-administration, both genotypes had similar total fentanyl intake (wildtype female: 0.24 ± 0.02, male: 0.19 ± 0.02; Drd1-cre female: 0.21 ± 0.02, male: 0.22 ± 0.04 mg/kg).
A Experimental timeline for self-administration. Following recovery from jugular vein catheter surgery, mice underwent 5 days of fentanyl self-administration training (3 h sessions) under fixed ratio 1 (FR1), followed by 5 days under FR2. Half of the mice underwent a non-reinforced seeking test 24 h after the last self-administration session and the other half underwent seeking 14 d later. B Number of fentanyl infusions (1.5 µg/kg) earned during self-administration training in Drd1-cre120Mxu (magenta) and wildtype (black) mice during training (wildtype n = 38♀, 48♂; Drd1-cre120Mxu n = 27♀, 26♂). C Number of active and inactive responses during fentanyl self-administration training. D Discrimination index (active minus inactive responses) during fentanyl self-administration training. E Number of active and inactive responses during a 1h non-reinforced seeking test in wildtype and Drd1-cre120Mxu mice (**, p = 0.026. Sidak’s post-hoc after 2-way ANOVA. Triangles denote datapoints from males and circles from females). F Number of active and inactive responses during a seeking test in separate mice after 14 days abstinence (*, p = 0.03, Sidak’s post-hoc after 2-way ANOVA). G Experimental timeline for conditioned place preference. Mice freely explored the apparatus for 20 min during the pre-test day. For the following three days, mice received saline (10 mL/kg ip) in one compartment, followed 4 h later by fentanyl (0.2 mg/kg ip) in the other. On the fifth day, mice freely explored the apparatus. H Preference for the fentanyl-paired compartment expressed as time spent in fentanyl-paired chamber during the post-test minus time spent during pre-test (*, p = 0.03, unpaired t-test. Wildtype n = 33♀, 30♂; Drd1- cre120Mxu n = 33♀, 32♂). I Total number of movement counts during conditioning sessions (*, p = 0.0047, Sidak’s post-hoc after 2-way ANOVA). Data are presented as mean ± SEM with individual mice overlaid.
We next asked if Drd1-cre120Mxu mice exhibited fentanyl seeking under extinction conditions. As expected, after 24 h abstinence, wildtype mice exhibited fentanyl seeking behavior marked by increased responding at the active nose-poke. By contrast, Drd1-cre120Mxu mice exhibited reduced fentanyl seeking behavior (Fig. 1E, 2-way ANOVA, Genotype: F1,71 = 5.2, p = 0.02; Active nose-poke, wildtype vs. Drd1-cre, p = 0.006, Sidak’s). We tested a separate set of mice after 14 d abstinence. Like the 24 h timepoint, Drd1-cre120Mxu mice exhibited reduced fentanyl seeking behavior relative to wildtype mice (Fig. 1F, 2-way ANOVA, Genotype: F1,64 = 4.23, p = 0.04; Active nose-poke, wildtype vs. Drd1-cre120Mxu, p = 0.03, Sidak’s). To see if this generalized to other forms of fentanyl reward, we used conditioned place preference (CPP) in a separate set of mice (Timeline in Fig. 1G). We found slightly reduced CPP scores in Drd1-cre120Mxu mice relative to wildtype (Fig. 1H, t1,126 = 2.17, p = 0.03). When we examined fentanyl-induced hyperlocomotion, another proxy for drug reward, we found Drd1-Cre120Mxu mice exhibit greater movement counts during fentanyl conditioning (Fig. 1I, drug x genotype: F1,109 = 13.22 p = 0.0004; wildtype vs. Drd1-cre120Mxu fentanyl, p = 0.0047, Sidak’s). To determine if reduced drug reward was restricted to fentanyl, we asked if this generalized to other drugs with misuse potential, and compared cocaine self-administration and seeking in Drd1-cre120Mxu mice relative to wildtype mice in our published dataset [77] (Supplemental Fig. 1A). Unlike fentanyl, Drd1-cre120Mxu had reduced cocaine intake relative to wildtype mice (Supplemental Fig. 1B, C, Infusions: Genotype: F1,24 = 10.8, p = 0.003; Active responses: Genotype F1,24 = 17.3, p = 0.0003). Like fentanyl, Drd1-cre120Mxu had fewer active responses during the non-reinforced seeking test at 24 h (Supplemental Fig. 1D, Genotype: F1,48 = 22.6, p < 0.0001; Active responses, wildtype vs. Drd1-cre120Mxu, p = 0.0001, Sidak’s). Finally, to determine if Drd1-cre120Mxu mice had generally impaired reward seeking or if this behavior was specific to misused drugs, we compared sucrose self-administration and seeking in Drd1-cre120Mxu mice to wildtype mice (Supplemental Fig. 2A). We found that Drd1-cre120Mxu mice had no difference in number of sucrose pellets earned or consumed (Supplemental Fig. 2B, RMANOVA, Genotype F1,22 = 0.2; 2 C F1,22 = 0.8, p’s>0.3) and no differences in nose-poke discrimination (Supplemental Fig. 2D, E, Genotype F1,22 = 0.2, p = 0.7) relative to wildtype littermates. D1-cre120Mxu mice also exhibited similar sucrose seeking as wildtype littermates under both food-restriction and ad libitum conditions (Supplemental Fig. 2F, G, Genotype F’s< 0.21, p’s>0.6).
Drd1-cre120Mxu mice have altered baseline gene expression in nucleus accumbens
Previous work using Drd2-EGFP mice found elevated striatal Drd2 expression with effects on behavioral responses to cocaine [72]. Thus, we next asked if Drd1-cre120Mxu mice also have altered baseline expression of dopamine receptors that might drive the behavioral phenotypes. Given the importance of NAc in opioid-reward and opioid seeking [1], we looked at gene expression in total NAc. In experimentally naïve mice, we found elevated Drd1 expression in both sexes of Drd1-cre120Mxu mice compared to wildtype littermates (Fig. 2A, 2-way ANOVA, Genotype: F1,19 = 4.60, p = 0.045). Increased Drd1 mRNA is not due to an increase in the number of D1 relative to D2 MSNs, as viral labeling with a Cre-switch virus [78] revealed typical balance in D1 (Cre positive) vs. D2 (Cre negative) cells in NAc. (52.6 ± 4.4% D1, 47.4 ± 4.4% D2; Supplemental Fig. 3A–C). We also found elevated expression of D1-MSN marker Chrm4 in males (Fig. 2B, Sex x Genotype: F1,19 = 5.07, p = 0.036; wildtype vs. Drd1-cre male p = 0.01, Sidak’s). Not all D1-MSN markers were elevated, as there were no differences in Pdyn (Fig. 2C), and only baseline sex differences in Tac1 (Fig. 2D, Sex: F1,19 = 5.85, p = 0.026). We found no changes in D2-MSN markers Drd2, Adora2a, Gpr6, or Penk (Fig. 2E–H) in Drd1-cre120Mxu mice, with only a baseline sex difference in Adora2a (Fig. 2F, Sex: F1,19 = 5.67, p = 0.028). These differences in MSN marker genes did not generalize to all Drd1-cre mice, as we found no aberrant expression in a different Drd1-cre line (GensatFK150; Supplemental Fig. 3D). Given the differences in fentanyl seeking between genotypes, we also looked at expression of opioid receptors and related nociceptin receptor in NAc. In experimentally naïve mice, we found no differences in opioid receptor expression between genotypes (Fig. 2I–L).
Fold change mRNA in experimentally naïve wildtype (black) and Drd1-cre120Mxu (magenta) mice, relative to average of naïve male and female wildtype mice (wildtype n = 4♀, 6♂; Drd1-cre120Mxu n = 4♀, 9♂). A Dopamine D1 receptor, *, p = 0.045, main effect of genotype. B Muscarinic acetylcholine receptor M4, Sex x Genotype Interaction, p = 0.036; * p = 0.01, wildtype vs. Drd1-cre males, Sidak’s post-hoc. C Preprodynorphin. D Preprotachykinin-1, *, p = 0.026, main effect of sex. E Dopamine D2 receptor. F Adenosine A2a receptor, *, p = 0.028, main effect of sex. G G protein-coupled receptor 6. H Preproenkephalin. I Mu opioid receptor. J Delta opioid receptor. K Kappa opioid receptor. L Opioid related nociceptin receptor 1. Data are mean ± SEM with individual mice overlayed.
Drd1-cre120Mxu mice exhibit increased sensitivity to D1 receptor activation
Since Drd1-cre120Mxu mice had elevated Drd1 expression in NAc, we next asked if this would produce a baseline locomotor phenotype or increased sensitivity to D1R agonists. We administered D1R agonist SKF-38393 (SKF, 30 mg/kg sc), or saline, just prior to open field testing, which typically produces hyperlocomotion in unhabituated mice [79]. Both SKF-treated wildtype and Drd1-cre120Mxu mice exhibited increased locomotion relative to their saline-treated counterparts (Fig. 3A, time x drug: F4.9,186.4= 7.39, p < 0.001). Contrary to our prediction of increased distance traveled, both saline- and SKF-treated Drd1-cre120Mxu traveled less total distance across the 90 min relative to wildtype (Genotype: F1,38 = 4.1, p = 0.049), that was not mediated by differences in average velocity (Fig. 3B). Given the baseline differences in locomotion, we next looked at distance traveled as a percent of saline treatment. Drd1-cre120Mxu mice showed greater SKF-potentiated locomotion relative to wildtype mice, suggesting increased D1R sensitivity (Fig. 3C, t1,21 = 2.79, p = 0.011). Since D1R activation can elicit stereotyped movements [80], we also looked at jumps and stationary movement counts (stereotypy counts). Drd1-cre120Mxu mice exhibited more SKF-induced jumps (Fig. 3D, t1,21 = 3.33, p = 0.003) and stereotypy counts (Fig. 3E, t1,21 = 5.9 p < 0.0001). Together, these data suggest Drd1-cre120Mxu mice have reduced locomotion in a novel environment, but increased sensitivity to D1R activation.
Male and female wildtype (black) and Drd1-cre120Mxu (magenta) mice received saline or 30 mg/kg SKF-38393 s.c. prior to open field testing (wildtype saline n = 6♀, 6♂; wildtype SKF n = 6♀, 6♂; Drd1-cre saline n = 5♀, 6♂; Drd1-cre SKF n = 5♀, 6♂). A Distance traveled in the open field over 90 min in 5 min bins. ****, p < 0.0001, main effect of drug; *, p = 0.044, main effect of genotype. B Average velocity in the open field in saline and SKF treated mice. Triangles represent data points from male mice and circles from females. C Total distance traveled in SKF-treated wildtype and Drd1-cre relative to saline-treated mice. *, p = 0.011, unpaired t-test. D Jump counts in SKF-treated mice relative to saline-treated mice. **, p = 0.003, unpaired t-test. E Stereotypy counts in SKF-treated mice relative to saline-treated mice. ****, p < 0.0001, unpaired t-test. Data are presented as mean ± SEM with individual mice overlaid.
Drd1-cre120Mxu mice exhibit dysregulated nucleus accumbens gene expression after fentanyl experience
Given the increase in Drd1 expression, and increased response to a D1R agonist in experimentally naïve Drd1-cre120Mxu mice, we next asked if elevated Drd1 expression was maintained in mice with fentanyl self-administration experience, as this may underlie their behavioral differences (Timeline in Fig. 4A, behavior in Supplemental Fig. 4B). After 14 d abstinence, fentanyl-experienced Drd1-cre120Mxu mice maintained elevated Drd1 expression relative to naïve wildtype mice (Drd1-cre females: 1.25 ± 0.09, males: 1.7 ± 0.16; Fig. 4B, Sex x Genotype: F1,17 = 8.78, p = 0.009; females: p = 0.0005, males: p < 0.0001, Sidak’s). This is juxtaposed against a relative Drd1 downregulation in fentanyl-experienced wildtype mice (wildtype females: 0.69 ± 0.08, males: 0.62 ± 0.04; Fig. 4B). We also looked beyond Drd1, and additional genotype differences emerged that were not present in naïve mice. D1-MSN marker Tac1 was downregulated in Drd1-cre120Mxu mice with fentanyl experience (females: 0.47 ± 0.029, males: 0.6 ± 0.051; Fig. 4C, Genotype: F1,17 = 13.10, p = 0.002). D2-MSN marker Penk was also downregulated in fentanyl-experienced Drd1-cre120Mxu mice (females: 0.54 ± 0.02, males: 0.68 ± 0.12), juxtaposed against a relative upregulation in fentanyl-experienced wildtype mice (female: 3.15 ± 0.06; male: 2.46 ± 0.20; Fig. 4D, Genotype: F1,17 = 30.7, p < 0.0001). Additional D2-MSN marker genes Adora2a and Gpr6 were now upregulated in fentanyl-experienced Drd1-cre120Mxu mice, although Adora2a was only significantly upregulated in males (Fig. 4E, F, Adora2a, Sex x Genotype: F1,17 = 4.7, p = 0.044, male wildtype vs. Drd1-cre, p = 0.0018; Gpr6, Genotype: F1,17 = 15.8, p = 0.001). We also found upregulation of general MSN marker Ppp1r1b (DARPP-32) in fentanyl-experienced Drd1-cre120Mxu mice (Fig. 4G, Genotype F1,17 = 43.5, p < 0.0001), a difference that was absent in experimentally naïve mice (Supplemental Fig. 5A). When we looked at opioid receptor expression, we found differences that were absent in experimentally naïve mice. Compared to fentanyl-experienced wildtype mice, Drd1-cre120Mxu mice had less downregulation of Oprm1 (wildtype females: 0.82 ± 0.07, males: 0.76 ± 0.04; Drd1-cre females: 0.90 ± 0.09, males 0.98 ± 0.06; Fig. 4H, Genotype: F1,17 = 4.9, p = 0.04) and Oprl1 (wildtype females: 0.48 ± 0.04, males: 0.49 ± 0.04; Drd1-cre females: 0.88 ± 0.06, males: 0.71 ± 0.08; Fig. 4I, Genotype: F1,17 = 36.7, p < 0.0001). Drd1-cre120Mxu, but not wildtype mice, also upregulated Oprd1 (wildtype females: 0.95 ± 0.12, males: 1.26 ± 0.15; Drd1-cre females: 1.49 ± 0.15, males: 1.72 ± 0.22; Fig. 4J, Genotype F1,17 = 10.2, p = 0.0005). Because chronic opioid exposure is associated with reduction in NAc TrkB expression [17] and NAc NMDAR function [81], we also looked at expression of Ntrk2, and GluN2 subunits Grin2a and Grin2b. In fentanyl-experienced wildtype mice, we found expected downregulation of Ntrk2 that was completely absent in Drd1-cre120Mxu mice (wildtype female: 0.10 ± 0.044, male: 0.31 ± 0.013; Drd1-cre female: 1.17 ± 0.12, male: 1.23 ± 0.09; Fig. 4K, Genotype: F1,17 = 88.25, p < 0.0001). Similarly, downregulation of GluN2 subunits was more extensive in fentanyl-experienced wildtype mice compared with Drd1-cre120Mxu, especially for Grin2b (Grin2a: wildtype female 0.05 ± 0.01, male 0.12 ± 0.05; Drd1-cre female 0.43 ± 0.07, male 0.51 ± 0.03, Genotype F1,17 = 76.1, p < 0.0001; Grin2b: wildtype female 0.09 ± 0.02, male 0.29 ± 0.012; Drd1-cre female 1.15 ± 0.18, male 1.5 ± 0.11, Genotype F1,17 = 91.0, p < 0.0001; Fig. 4L, M) None of these differences were present in experimentally naïve mice (Supplemental Fig. 5B–D), nor did they arise due to different fentanyl exposure (qPCR subset, total intake Drd1-cre 0.21 ± 0.07, wildtype 0.25 ± 0.08 mg/kg).
A Experimental timeline. Following recovery from jugular vein catheter surgery, mice underwent 5 days of fentanyl self-administration under fixed ratio 1 (FR1), followed by 5 days under FR2. Following 14 days of homecage abstinence, mice underwent a non-reinforced seeking test, then nucleus accumbens tissue was collected ~4 h later. Fold change mRNA relative to experimentally naïve male and female wildtype mice (dashed line; wildtype n = 6♀, 6♂; Drd1-cre120Mxu n = 5♀, 4♂). B Dopamine D1 receptor, Sex x Drug Interaction, p = 0.0087; ***p = 0.0005, ****p < 0.0001, wildtype vs. Drd1-cre females, males, respectively, Sidak’s post-hoc. C Preprotachykinin-1, **p = 0.0021, main effect of genotype. D Preproenkephalin, ****p < 0.0001, main effect of genotype. E Adenosine A2a, Sex x Drug Interaction, p = 0.044; **p = 0.0018, wildtype vs. Drd1-cre males, Sidak’s post-hoc. F G protein-coupled receptor 6, ***p = 0.001, main effect of genotype. G Dopamine and cAMP-regulated phosphoprotein (DARPP-32), ****p < 0.0001, main effect of genotype. H Mu opioid receptor, *p = 0.041, main effect of genotype. I Opioid related nociceptin receptor 1, ****p < 0.0001, main effect of genotype. J Delta opioid receptor, **p = 0.005, main effect of genotype. K Tropomyosin receptor kinase B (TrkB) receptor, **** p < 0.0001, main effect of genotype. L NMDA receptor Subunit 2 A, ****p < 0.0001, main effect of genotype. M NMDAR Subunit 2B, ****p’s<0.0001, wildtype vs. Drd1-cre females, males, respectively, Sidak’s post-hoc. Data are presented as mean ± SEM with individual mice overlaid.
Chemogenetic activation of putative D1-MSNs in wildtype mice suppresses fentanyl seeking
Due to persistent upregulation of NAc Drd1 in the Drd1-cre120Mxu mice, we next asked if chemogenetically manipulating activity of NAc D1-MSNs using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) could restore fentanyl seeking to that of wildtype mice (Timeline in Fig. 5A). Immediately following fentanyl self-administration (Fig. 5B), we assigned Drd1-cre120Mxu mice to groups to ensure similar intake across sex and virus (future virus F2,33 = 0.4, future virus x sex F1,33 = 0.3). After two weeks of abstinence, we inhibited or activated NAc core D1-MSNs with the DREADDs ligand deschloroclozapine (DCZ) prior to a fentanyl seeking test. We found neither chemogenetic inhibition, nor activation, influenced fentanyl seeking behavior in Drd1-cre120Mxu mice (Fig. 5C, nose-poke x virus F2,35 = 2.15, p = 0.15). By contrast, chemogenetic activation of ventral mesencephalon-projecting NAc core MSNs (putative D1-MSNs, see Discussion) in wildtype mice suppressed fentanyl seeking (Self-administration in Fig. 5E, seeking in Fig. 5F; Nose-poke x sex x virus F2,34 = 3.44, p = 0.044; Active nose-pokes, hM3Dq vs. mCherry, male p = 0.024, female p < 0.0001, Sidak’s). This was not due to differences in self-administration behavior, as all mice, regardless of genotype or sex, had comparable fentanyl intake during acquisition (wildtype female 0.26 ± 0.02, male 0.21 ± 0.02, Drd1-cre female 0.21 ± 0.02, male 0.27 ± 0.04 mg/kg, Genotype F1,76 = 0.03). As with fentanyl seeking after self-administration, we found Drd1-cre120Mxu mice were also insensitive to chemogenetic manipulation during fentanyl conditioned-place preference. Using a within-subject design (Timeline in Fig. 5H), we found neither inhibiting, nor activating D1-MSNs significantly altered time spent in the fentanyl-paired chamber (Fig. 5I, drug x virus F2,54 = 2.8, p = 0.07). This was not due to DCZ inefficacy in Drd1-cre120Mxu mice, as stimulating NAc D1-neurons increased cfos in NAc (Fig. 5J, Welch’s ANOVA, F2,16.51 = 7.8 p = 0.0042, mCherry vs. hM3Dq p = 0.007, Dunnet’s T3), and decreased cfos in downstream ventral tegmental area (VTA) as expected (Fig. 5K, Welch’s ANOVA, F2,15.86 = 7.7, mCherry vs. hM3Dq = 0.049, Dunnet’s).
A Experimental timeline for IVSA experiments. Following jugular vein catheter surgery mice underwent 10 days of fentanyl self-administration training. Mice then underwent surgery to express DREADDs in NAc core D1-MSNs, using Cre-dependent DREADDs in NAc core of Drd1-cre120Mxu mice, or retrograde Cre in ventral mesencephalon and Cre-dependent DREADDs in NAc core of wildtype mice. Following 14 d abstinence and viral expression, mice were given 0.1 mg/kg DCZ i.p. 20 min prior to a 1h seeking test. B Infusions earned during fentanyl self-administration in male and female Drd1-cre120Mxu mice. C Active and inactive responses during the fentanyl seeking test in Drd1-cre120Mxu mice expressing mCherry control (n = 5♀, 7♂), inhibitory hM4Di (n = 7♀, 7♂), or stimulatory hM3Dq (n = 8♀, 6♂) in NAc core D1-MSNs. D Image demonstrating DREADDs expression in Drd1-cre120Mxu mice is restricted to the NAc core (ac, anterior commissure). E Fentanyl infusions earned during self-administration in male and female wildtype mice. F Active and inactive responses during the fentanyl seeking test in wildtype mice (mCherry, n = 7♀, 6♂; hM4Di, n = 7♀, 5♂; hM3Dq, n = 7♀, 8♂). Active responses: ****p < 0.0001, hM3Dq vs. mCherry females; *p = 0.024, hM3Dq vs. mCherry males, Sidak’s. Inactive responses: #p = 0.024, female hM3Dq vs. mCherry. G Image demonstrating DREADDs expression in wildtype mice is restricted to the NAc core H Experimental timeline for CPP experiments. Drd1-cre120Mxu mice underwent surgery for Cre-dependent DREADDs in NAc. Mice freely explored the apparatus during the pre-test day. For the following three days, mice received saline (10 mL/kg i.p.) in one compartment, followed 4 h later by fentanyl (0.2 mg/kg i.p.) in the other. On the fifth day, mice received saline (or DCZ) 20 min prior to the first post-test. Then, 4 h later, mice received 0.1 mg/kg DCZ (or saline) 20 min prior to the second post-test. Tissue was collected immediately following the second post-test to capture immediate early gene expression. I CPP score during the post-test under saline and DCZ conditions (mCherry, n = 12♀, 13♂; hM4Di, n = 12♀, 7♂; hM3Dq, n = 10♀, 6♂). J In mice receiving DCZ just prior to tissue collection, immediate early gene cfos is upregulated in NAc of Drd1-cre120Mxu mice expressing hM3Dq relative to mCherry, **p = 0.007, Dunnet’s T3 (mCherry, n = 5♀, 4♂; hM4Di, n = 7♀, 6♂; hM3Dq, n = 8♀, 4♂). K In downstream VTA, cfos is downregulated in hM3Dq relative to mCherry, *p = 0.049, Dunnet’s T3. Data are presented as mean ± SEM with individual mice overlaid.
Discussion
Here we report that the widely-used Drd1-cre120Mxu mouse line [55] exhibits several behavioral and transcriptional differences compared to wildtype mice. We serendipitously discovered that this line has reduced responding in a nonreinforced fentanyl seeking test following either 24 h or 14 days of abstinence, despite normal fentanyl acquisition. This resistance translates to other fentanyl administration paradigms, as Drd1-cre120Mxu mice also have reduced preference for a fentanyl-paired context, despite increased fentanyl-induced hyperlocomotion. This behavior is specific to misused drugs, since these mice also have reduced self-administration and seeking for cocaine, but not sucrose. These mice also exhibit baseline hypolocomotion in a novel environment, as well as relative increased behavioral response to D1R agonism. Although the mechanism underlying fentanyl resistance in these mice is not fully understood, key differences in gene expression in the Drd1-cre120Mxu mice, alongside evidence that stimulating putative D1-MSNs in wildtype mice produces similarly blunted fentanyl seeking, implicates that this phenotype is, at least in part, due to aberrant signaling in D1-MSNs.
Atypical drug-associated behavior in Drd1-cre120Mxu mice
We showed that Drd1-cre120Mxu mice have reduced self-administration of cocaine but not fentanyl. Our study is not the first to report on a transgenic line with abnormal drug self-administration behavior; for example, ChAT-Cre mice (B6.FVB(Cg)-Tg(Chat-cre)GM60Gsat/Mmucd) have reduced self-administration of intravenous nicotine [73]. However, our findings are unique in that despite normal acquisition of fentanyl self-administration, Drd1-cre120Mxu mice exhibited attenuated nonreinforced fentanyl seeking. This effect is strikingly robust: the blunted fentanyl seeking is observed at both 24 h and 14 days after self-administration, is also reflected in a reduced preference for a fentanyl-paired context, persists in both paradigms despite bidirectional chemogenetic manipulation of D1-MSNs, and it is specific to drugs as there is no impairment of sucrose seeking in these mice.
In wildtype mice, we found chemogenetic stimulation of putative NAc core D1-MSNs (ventral mesencephalon-projecting) attenuated fentanyl seeking. While reminiscent of D1R agonists decreasing cocaine seeking [82], this was also surprising, since research by O’Neal et al. shows activating VTA-projecting NAc neurons exacerbates heroin seeking in rats [83]. This discrepancy may reflect species differences in D1-MSN collaterals to the ventral pallidum (VP). In mice, over 90% of VTA-projecting MSNs send collaterals to VP [84]; in rats, O’Neal et al. report VTA-projecting MSNs do not send collaterals to VP [83]. MSNs make inhibitory synapses on mostly GABAergic neurons in VP [85]. Activating MSN collaterals in VP would increase inhibitory drive on GABAergic VP neurons, and inhibiting VP GABA neurons attenuates remifentanil seeking [86]. Thus, we suspect reduced fentanyl seeking with NAc core D1-MSN activation is explained by inhibition in VP, although this requires further investigation. It is worth noting these mechanisms may not extend to the NAc shell. For example, systemic D1R agonism attenuates cocaine seeking [82], but when delivered directly to the NAc shell, D1R agonists instead promotes cocaine seeking [87]. Since DREADDs expression in our study was limited to NAc core, future work is necessary to elucidate the specific contributions of NAc subregions to fentanyl seeking.
Unlike wildtype mice, we found that chemogenetic manipulation of D1-MSNs did not affect seeking in Drd1-cre120Mxu mice. It is likely D1-MSN stimulation had no effect in Drd1-cre120Mxu mice because their seeking responses are so low at baseline, they cannot be further attenuated (a “floor effect”); additionally, it is perhaps unsurprising that chemogenetic inhibition of D1-MSNs did not increase seeking in Drd1-cre120Mxu mice since this did not increase seeking in wildtype mice. Importantly, the lack of response in Drd1-cre120Mxu mice does not reflect an inability for DREADDs to stimulate or inhibit D1-MSNs, as we identified expected increases and decreases in cfos expression after DCZ treatment. However, the DREADDs manipulations in wildtype and Drd1-cre120Mxu are not identical. In Drd1-cre120Mxu mice, we expressed DREADDs in all NAc core D1-MSNs; in wildtype mice, we targeted only ventral mesencephalon-projecting MSNs in NAc core. While these neurons almost exclusively express D1 receptor, there may be minor differences in the populations targeted by both approaches, given the collateralization discussed above.
It is worth noting that neither Drd1-cre120Mxu nor wildtype mice demonstrated increased fentanyl seeking after a longer period of abstinence, a phenomenon termed “incubation of craving” that is well-documented for other misused drugs [88, 89]. A potential explanation is the time course of our seeking experiments. Indeed, incubation of heroin seeking follows an inverted U-shaped time course, with higher responding at 6 and 12 days compared to 1 and 25 [90]. The specific time course for incubation of fentanyl seeking in mice is unknown, although we suspect it arises later than the 14 day timepoint tested here, since rats demonstrate incubation of fentanyl seeking after 30 days [91], and mice exhibit high rates of fentanyl seeking after 4 weeks [92]. Additional work is necessary to fully understand the nuance of time-dependent changes to fentanyl seeking behavior, and whether this differs between genotypes.
Atypical locomotor responses in Drd1-cre120Mxu mice
We showed that Drd1-cre120Mxu mice exhibit several locomotor phenotypes, including increased locomotor response to fentanyl, reduced locomotion in a novel environment, and increased sensitivity to a D1R agonist. There are several reports on transgenic mouse lines with abnormal locomotor responses to misused drugs. For example, Drd2-EGFP mice [93] have increased striatal Drd2 expression and reduced locomotor response to cocaine [72]. Similarly, DAT-Ires-Cre mice (B6.SJL-Slc6a3tm1.1(cre)Bkmn/J, Jackson Laboratory No: 006660) have reduced dopamine transporter (DAT) expression [94] and function [95], and exhibit attenuated amphetamine-induced locomotion [74] that generalizes to a different DAT-Cre line (Slc6a3tm1(cre)Xz, Jackson Laboratory No: 020080) [75]. Here we show Drd1-cre120Mxu mice have elevated expression of NAc Drd1 and a greater locomotor response to fentanyl. D1-MSNs form inhibitory synapses on GABAergic interneurons in VTA [96, 97]. Increased D1R activation may potentiate dopamine neuron disinhibition, increasing the locomotor response to fentanyl. In support of increased D1R sensitivity, Drd1-cre120Mxu mice exhibited a relatively stronger locomotor stimulatory effect with D1R agonist SKF-38393. Interestingly, Drd1-cre120Mxu mice exhibit baseline hypolocomotion in a novel environment. Altered baseline locomotion is similar to observations in the Drd2-EGFP and the DAT-IRES-Cre line, which exhibit baseline hyperactivity in a novel environment [72, 74] and dysregulated dopamine regulation mechanisms [72, 95]. These baseline locomotor differences are to be expected given the importance of D1- and D2-MSN signaling for normal locomotion [98]. Importantly, the increase in Drd1 does not reflect an increase in the number of D1-MSNs relative to D2-MSNs in NAc, as our viral labeling indicates there are an equivalent number of D1 positive and negative neurons in Drd1-cre120Mxu mice.
Nucleus accumbens transcriptional adaptations implicate potential mechanisms of fentanyl resistance
Although the precise mechanisms underlying reduced fentanyl seeking in Drd1-cre120Mxu mice are unknown, looking at the transcriptional differences provides some insight. At baseline, we found Drd1-cre120Mxu mice had greater Drd1 expression relative to wildtype mice. Increased Drd1 was maintained following fentanyl self-administration in Drd1-cre120Mxu mice, resulting in an exaggerated genotype difference, since fentanyl-experienced wildtype mice downregulated Drd1 expression. Tac1, another D1-MSN marker, demonstrated baseline sex differences that are consistent with findings in rat striatum [99], but did not initially differ between genotypes. Following fentanyl, wildtype mice upregulated Tac1, similar to findings in morphine-exposed rats [51]. In stark contrast, Drd1-cre120Mxu mice downregulated Tac1 after fentanyl. Tac1 encodes the precursor for Substance P, a neuropeptide that is induced by morphine [100] and particularly relevant to opioid-dependent behaviors [101]. Substance P is released by local MSN collaterals [102] onto cholinergic interneurons expressing Substance P receptors [103, 104], where it participates in an excitatory microcircuit that promotes coordinated activity between D1- and D2-MSNs [105]. The downregulation of Tac1 in the NAc of Drd1-cre120Mxu mice likely impairs the function of this local circuit, and as such, may contribute to the blunted fentanyl seeking in these mice.
We also looked at NAc expression of D2-MSN markers, including Drd2, Gpr6, Adora2a, and Penk. While expression of these genes did not differ between Drd1-cre120Mxu and wildtype mice at baseline, fentanyl-experienced Drd1-cre120Mxu mice had increased Gpr6 expression in both sexes, and increased Adora2a expression in males. Gpr6, which encodes G protein-coupled receptor 6, is enriched in NAc indirect pathway MSNs, where it plays a role in regulating instrumental conditioning [10], but a link to drug misuse has not yet been identified. Adora2a encodes adenosine A2A receptors, which are colocalized on D2-MSNs [106, 107] and antagonize activity at the D2 receptor [108]. NAc A2A activity has been shown to regulate cocaine seeking [109], but its role in opioid use is less studied. Two of the most promising molecular targets are Penk (encoding the enkephalin precursor) and Oprd1 (encoding delta opioid receptor, which binds enkephalin). The enkephalinergic system has close interactions with reward pathways: enkephalins released from D2-MSNs modulate neurotransmission in the VP, and enkephalins released locally in NAc modulate NAc signaling at various sites [110]. Prior studies in rats implicated a direct role for NAc Penk in opioid seeking, where Penk overexpression in NAc potentiates heroin seeking [111], consistent with upregulated Penk in our fentanyl-seeking wildtype mice. Since Drd1-cre120Mxu mice downregulate Penk, and upregulate Oprd1 only after fentanyl, these adaptations could drive decreased fentanyl seeking and warrant further investigation.
In addition to the MSN markers, we also investigated other genes that, in the NAc, have known roles in opioid misuse. We found that Ntrk2, which encodes BDNF receptor TrkB, was downregulated following fentanyl abstinence in wildtype mice but was unchanged in Drd1-cre120Mxu mice. This finding is consistent with previous work showing morphine exposure downregulates TrkB in D1-MSNs, resulting in impaired BDNF signaling that promotes morphine reward [17]. It is tempting to speculate that, given unchanged Ntrk2 expression, Drd1-cre120Mxu mice preserve normal BDNF signaling in D1-MSNs after fentanyl, thereby reducing fentanyl seeking. We also found Drd1-cre120Mxu mice have blunted downregulation of Grin2a and Grin2b following fentanyl abstinence. These genes encode the GluN2 subunits of the glutamatergic NMDA receptor. NAc glutamatergic signaling is essential for opioid seeking [112], and previous work shows chronic opioid use alters NMDA-mediated signaling in NAc [81]. The observed downregulation of NMDA receptor components in wildtype mice aligns with findings of an increased NAc AMPA/NMDA ratio that results from chronic morphine use and persists throughout protracted withdrawal [32, 35]. This adaptation is believed to contribute to an increased excitatory drive onto D1-MSNs that promotes drug seeking [113]. A similar adaptation results from fentanyl abstinence [52], and this process is likely disrupted in Drd1-cre120Mxu mice, thereby impairing fentanyl seeking.
Conclusions
Although we did not identify the precise circuit and molecular mechanisms underlying reduced relapse to fentanyl seeking in Drd1-cre120Mxu mice, it is clear these mice have aberrant NAc D1-MSN adaptations with implications for circuit function. The fentanyl abstinence-induced changes in NAc gene expression we observed in Drd1-cre120Mxu mice suggest some of these adaptations include impaired Substance P-dependent and enkephalinergic signaling, conserved BDNF-dependent signaling, and altered NMDA plasticity, all of which could reduce fentanyl seeking, although additional experiments are necessary to confirm these possibilities, including fully characterizing cell-type and subregion differences in gene expression. A recent study proposed that imbalanced D1- to D2-MSN plasticity promotes negative affect during opioid abstinence, and restoring this plasticity through a D1R-dependent process attenuated negative affect and relapse [114]. Given that Drd1-cre120Mxu mice exhibit increased sensitivity to D1R activation, combined with the potential adaptations to NAc plasticity, it is tempting to speculate that the Drd1-cre120Mxu mice preserve D1- to D2-MSN balance after fentanyl experience. Future work should investigate whether Drd1-cre120Mxu mice exhibit abstinence-induced negative affective behavior comparable to wildtype mice, as the neuroadaptations described here may promote resilience during abstinence, thereby decreasing relapse. In conclusion, our findings provide potential new molecular mechanisms and neurobiological targets for understanding susceptibility and resistance to fentanyl relapse.
Data availability
All gene expression, and the majority of behavioral data are presented as individual data points in either main or supplemental figures. Any additional behavioral data are available from the corresponding author upon reasonable request.
References
Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44–53.
Dowell D, Brown S, Gyawali S, Hoenig J, Ko J, Mikosz C, et al. Treatment for Opioid Use Disorder: Population Estimates — United States, 2022. MMWR Morb Mortal Wkly Rep. 2024;73:567–74.
NIDA. Drug Overdose Deaths: Facts and Figures. 2024. 21 August 2024.
Palamar JJ, Fitzgerald N, Carr TH, Cottler LB, Ciccarone D. National and regional trends in fentanyl seizures in the United States, 2017–2023. Int J Drug Pol. 2024:104417.
Kelly E, Sutcliffe K, Cavallo D, Ramos‐Gonzalez N, Alhosan N, Henderson G. The anomalous pharmacology of fentanyl. Br J Pharmacol. 2023;180:797–812.
Sutcliffe KJ, Corey RA, Alhosan N, Cavallo D, Groom S, Santiago M, et al. Interaction with the lipid membrane influences fentanyl pharmacology. Adv Drug Alcohol Res. 2022;2:10280.
Eshleman AJ, Nagarajan S, Wolfrum KM, Reed JF, Nilsen A, Torralva R, et al. Affinity, potency, efficacy, selectivity, and molecular modeling of substituted fentanyls at opioid receptors. Biochem Pharmacol. 2020;182:114293.
Socias ME, Wood E, Le Foll B, Lim R, Choi JC, Mok WY, et al. Impact of fentanyl use on initiation and discontinuation of methadone and buprenorphine/naloxone among people with prescription‐type opioid use disorder: secondary analysis of a Canadian treatment trial. Addiction. 2022;117:2662–72.
Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–25.
Lobo MK, Cui Y, Ostlund SB, Balleine BW, William Yang X. Genetic control of instrumental conditioning by striatopallidal neuron–specific S1P receptor Gpr6. Nat Neurosci. 2007;10:1395–7.
Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol. 2013;23:546–52.
Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66.
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2.
Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA. 2016;113:2726–31.
Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90.
Hauser SR, Deehan GA, Dhaher R, Knight CP, Wilden JA, McBride WJ, et al. D1 receptors in the nucleus accumbens-shell, but not the core, are involved in mediating ethanol-seeking behavior of alcohol-preferring (P) rats. Neuroscience. 2015;295:243–51.
Koo JW, Lobo MK, Chaudhury D, Labonté B, Friedman A, Heller E, et al. Loss of BDNF Signaling in D1R-Expressing NAc Neurons Enhances Morphine Reward by Reducing GABA Inhibition. Neuropsychopharmacol. 2014;39:2646–53.
Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8.
James AS, Chen JY, Cepeda C, Mittal N, Jentsch JD, Levine MS, et al. Opioid self-administration results in cell-type specific adaptations of striatal medium spiny neurons. Behav Brain Res. 2013;256:279–83.
Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907.
Tai L-H, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–9.
Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun. 2016;7:11829.
Soares-Cunha C, De Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2020;25:3241–55.
Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun. 2018;9:1086.
Gibson GD, Prasad AA, Jean-Richard-dit-Bressel P, Yau JOY, Millan EZ, Liu Y, et al. Distinct accumbens shell output pathways promote versus prevent relapse to alcohol seeking. Neuron. 2018;98:512–20.e6.
Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M, et al. Ventrolateral striatal medium spiny neurons positively regulate food-incentive, goal-directed behavior independently of D1 and D2 selectivity. J Neurosci. 2017;37:2723–33.
Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–42.
Soares-Cunha C, Coimbra B, Domingues AV, Vasconcelos N, Sousa N, Rodrigues AJ. Nucleus accumbens microcircuit underlying D2-MSN-driven increase in motivation. eNeuro. 2018;5:ENEURO.0386-18.2018.
Vicente AM, Galvão-Ferreira P, Tecuapetla F, Costa RM. Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr Biol. 2016;26:R267–9.
McDevitt DS, Jonik B, Graziane NM. Morphine differentially alters the synaptic and intrinsic properties of D1R- and D2R-expressing medium spiny neurons in the nucleus accumbens. Front Synaptic Neurosci. 2019;11:35.
Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19:915–25.
Wu X, Shi M, Ling H, Wei C, Liu Y, Liu Z, et al. Effects of morphine withdrawal on the membrane properties of medium spiny neurons in the nucleus accumbens shell. Brain Res Bull. 2013;90:92–9.
Madayag AC, Gomez D, Anderson EM, Ingebretson AE, Thomas MJ, Hearing MC. Cell-type and region-specific nucleus accumbens AMPAR plasticity associated with morphine reward, reinstatement, and spontaneous withdrawal. Brain Struct Funct. 2019;224:2311–24.
Wu X, Shi M, Wei C, Yang M, Liu Y, Liu Z, et al. Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. J Neurosci Res. 2012;90:1270–83.
Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type–specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62.
Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal‐induced morphological changes in the nucleus accumbens. Eur J Neurosci. 2005;22:2332–40.
Diana M, Spiga S, Acquas E. Persistent and reversible morphine withdrawal‐induced morphological changes in the nucleus accumbens. Ann N Y Acad Sci. 2006;1074:446–57.
Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46.
Heng L, Yang J, Liu Y, Wang W, Hu S, Gao G. Repeated morphine exposure decreased the nucleus accumbens excitability during short‐term withdrawal. Synapse. 2008;62:775–82.
Thompson BL, Oscar-Berman M, Kaplan GB. Opioid-induced structural and functional plasticity of medium-spiny neurons in the nucleus accumbens. Neurosci Biobehav Rev. 2021;120:417–30.
Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–2.
Pal A, Das S. Chronic morphine exposure and its abstinence alters dendritic spine morphology and upregulates Shank1. Neurochem Int. 2013;62:956–64.
Cahill ME, Browne CJ, Wang J, Hamilton PJ, Dong Y, Nestler EJ. Withdrawal from repeated morphine administration augments expression of the RhoA network in the nucleus accumbens to control synaptic structure. J Neurochem. 2018;147:84–98.
Sun H, Martin JA, Werner CT, Wang Z-J, Damez-Werno DM, Scobie KN, et al. BAZ1B in Nucleus accumbens regulates reward-related behaviors in response to distinct emotional stimuli. J Neurosci. 2016;36:3954–61.
Mayberry HL, Bavley CC, Karbalaei R, Peterson DR, Bongiovanni AR, Ellis AS, et al. Transcriptomics in the nucleus accumbens shell reveal sex- and reinforcer-specific signatures associated with morphine and sucrose craving. Neuropsychopharmacol. 2022;47:1764–75.
Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, et al. Essential Role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci. 2013;33:16088–98.
Spijker S, Houtzager SWJ, Gunst MCM, Boer WPH, Schoffelmeer ANM, Smit AB. Morphine exposure and abstinence define specific stages of gene expression in the rat nucleus accumbens. FASEB J. 2004;18:848–50.
Martin JA, Werner CT, Mitra S, Zhong P, Wang Z-J, Gobira PH, et al. A novel role for the actin-binding protein drebrin in regulating opiate addiction. Nat Commun. 2019;10:4140.
Townsend EA, Kim RK, Robinson HL, Marsh SA, Banks ML, Hamilton PJ. Opioid withdrawal produces sex-specific effects on fentanyl-versus-food choice and mesolimbic transcription. Biol Psychiatry Glob Open Sci. 2021;1:112–22.
Hofford RS, Mervosh NL, Euston TJ, Meckel KR, Orr AT, Kiraly DD. Alterations in microbiome composition and metabolic byproducts drive behavioral and transcriptional responses to morphine. Neuropsychopharmacol. 2021;46:2062–72.
Reiner BC, Zhang Y, Stein LM, Perea ED, Arauco-Shapiro G, Ben Nathan J, et al. Single nucleus transcriptomic analysis of rat nucleus accumbens reveals cell type-specific patterns of gene expression associated with volitional morphine intake. Transl Psychiatry. 2022;12:374.
Fox ME, Wulff AB, Franco D, Choi EY, Calarco CA, Engeln M, et al. Adaptations in nucleus accumbens neuron subtypes mediate negative affective behaviors in fentanyl abstinence. Biol Psychiatry. 2023;93:489–501.
Anderson EM, Tsvetkov E, Galante A, DeVries D, McCue LM, Wood D, et al. Epigenetic function during heroin self-administration controls future relapse-associated behavior in a cell type-specific manner. Proc Natl Acad Sci USA. 2023;120:e2210953120.
Anderson EM, Tsvetkov E, Wood D, Akiki RM, Al Hasanieh K, McCue LM, et al. Heroin regulates the voltage-gated sodium channel auxiliary subunit, SCN1b, to modulate nucleus accumbens medium spiny neuron intrinsic excitability and cue-induced heroin seeking. eNeuro. 2025;12:ENEURO.0017-25.2025.
Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, et al. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–96.
Han Y, Xia G, He Y, He Y, Farias M, Xu Y, et al. A hindbrain dopaminergic neural circuit prevents weight gain by reinforcing food satiation. Sci Adv. 2021;7:eabf8719.
Chen APF, Malgady JM, Chen L, Shi KW, Cheng E, Plotkin JL, et al. Nigrostriatal dopamine pathway regulates auditory discrimination behavior. Nat Commun. 2022;13:5942.
Tzanoulinou S, Musardo S, Contestabile A, Bariselli S, Casarotto G, Magrinelli E, et al. Inhibition of Trpv4 rescues circuit and social deficits unmasked by acute inflammatory response in a Shank3 mouse model of Autism. Mol Psychiatry. 2022;27:2080–94.
Tan B, Browne CJ, Nöbauer T, Vaziri A, Friedman JM, Nestler EJ. Drugs of abuse hijack a mesolimbic pathway that processes homeostatic need. Science. 2024;384:eadk6742.
Liu Y, Wang Y, Zhao Z, Xie G, Zhang C, Chen R, et al. A subset of dopamine receptor-expressing neurons in the nucleus accumbens controls feeding and energy homeostasis. Nat Metab. 2024;6:1616–31.
Zhang Y-F, Wu J, Wang Y, Johnson NL, Bhattarai JP, Li G, et al. Ventral striatal islands of Calleja neurons bidirectionally mediate depression-like behaviors in mice. Nat Commun. 2023;14:6887.
Boxer EE, Kim J, Dunn B, Aoto J. Ventral subiculum inputs to nucleus accumbens medial shell preferentially innervate D2R medium spiny neurons and contain calcium permeable AMPARs. J Neurosci. 2023;43:1166–77.
Petter EA, Fallon IP, Hughes RN, Watson GD, Meck WH, Ulloa Severino FP, et al. Elucidating a locus coeruleus-dentate gyrus dopamine pathway for operant reinforcement. eLife. 2023;12:e83600.
Assali DR, Sidikpramana M, Villa AP, Falkenstein J, Steele AD. Type 1 dopamine receptor (D1R)-independent circadian food anticipatory activity in mice. PLoS ONE. 2021;16:e0242897.
Hagihara KM, Bukalo O, Zeller M, Aksoy-Aksel A, Karalis N, Limoges A, et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature. 2021;594:403–7.
Vachez YM, Tooley JR, Abiraman K, Matikainen-Ankney B, Casey E, Earnest T, et al. Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption. Nat Neurosci. 2021;24:379–90.
Sneddon EA, Schuh KM, Frankel JW, Radke AK. The contribution of medium spiny neuron subtypes in the nucleus accumbens core to compulsive-like ethanol drinking. Neuropharmacology. 2021;187:108497.
Smith ACW, Jonkman S, Difeliceantonio AG, O’Connor RM, Ghoshal S, Romano MF, et al. Opposing roles for striatonigral and striatopallidal neurons in dorsolateral striatum in consolidating new instrumental actions. Nat Commun. 2021;12:5121.
Lafferty CK, Yang AK, Mendoza JA, Britt JP. Nucleus accumbens cell type- and input-specific suppression of unproductive reward seeking. Cell Rep. 2020;30:3729–42.e3.
Thoeni S, Loureiro M, O’Connor EC, Lüscher C. Depression of accumbal to lateral hypothalamic synapses gates overeating. Neuron. 2020;107:158–72.e4.
Love J, Selker R, Marsman M, Jamil T, Dropmann D, Verhagen J, et al. JASP: Graphical statistical software for common statistical designs. Journal of Statistical Software. 2019;88:1–17.
Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, et al. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP Mice. J Neurosci. 2011;31:126–32.
Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler J, et al. Altered baseline and nicotine-mediated behavioral and cholinergic profiles in ChAT-Cre mouse lines. J Neurosci. 2018;38:2177–88.
Chohan MO, Esses S, Haft J, Ahmari SE, Veenstra-VanderWeele J. Altered baseline and amphetamine-mediated behavioral profiles in dopamine transporter Cre (DAT-Ires-Cre) mice compared to tyrosine hydroxylase Cre (TH-Cre) mice. Psychopharmacology. 2020;237:3553–68.
Costa KM, Schenkel D, Roeper J. Sex-dependent alterations in behavior, drug responses and dopamine transporter expression in heterozygous DAT-Cre mice. Sci Rep. 2021;11:3334.
Fox ME, Montemarano A, Ostman AE, Basu M, Herb B, Ament SA, et al. Transcriptional signatures of fentanyl use in the mouse ventral tegmental area. Addict Biol. 2024;29:e13403.
Engeln M, Fox ME, Lobo MK. Housing conditions during self-administration determine motivation for cocaine in mice following chronic social defeat stress. Psychopharmacology. 2021;238:41–54.
Saunders A, Johnson CA, Sabatini BL. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front Neural Circuits. 2012;6:47.
Tirelli E, Terry P. Biphasic locomotor effects of the dopamine D1 agonist SKF 38393 and their attenuation in non-habituated mice. Psychopharmacology. 1993;110:69–75.
White FJ, Bednarz LM, Wachtel SR, Hjorth S, Brooderson RJ. Is stimulation of both D1 and D2 receptors necessary for the expression of dopamine-mediated behaviors? Pharmacol Biochem Behav. 1988;30:189–93.
Martin G, Ahmed SH, Blank T, Spiess J, Koob GF, Siggins GR. Chronic morphine treatment alters NMDA receptor-mediated synaptic transmission in the nucleus accumbens. J Neurosci. 1999;19:9081–9.
Self DW, Barnhart WJ, Leham DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–9.
O’Neal TJ, Nooney MN, Thien K, Ferguson SM. Chemogenetic modulation of accumbens direct or indirect pathways bidirectionally alters reinstatement of heroin-seeking in high- but not low-risk rats. Neuropsychopharmacol. 2020;45:1251–62.
Pardo-Garcia TR, Garcia-Keller C, Penaloza T, Richie CT, Pickel J, Hope BT, et al. Ventral pallidum is the primary target for accumbens D1 projections driving cocaine seeking. J Neurosci. 2019;39:2041–51.
Morais-Silva G, Lobo MK. Refining the circuits of drug addiction: The ventral pallidum. Curr Opin Neurobiol. 2024;86:102883.
Farrell MR, Ye Q, Xie Y, Esteban JSD, Mahler SV. Ventral pallidum GABA neurons bidirectionally control opioid relapse across rat behavioral models. Addict Neurosci. 2022;3:100026.
Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1‐like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine‐seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–28.
Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2.
Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini‐review. Addict Biol. 2015;20:872–6.
Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107.
Gyawali U, Martin DA, Sulima A, Rice KC, Calu DJ. Role of BNST CRFR1 receptors in incubation of fentanyl seeking. Front Behav Neurosci. 2020;14:153.
Chen Y, Xiao T, Kimbrough A. Escalation of intravenous fentanyl self-administration and assessment of withdrawal behavior in male and female mice. Psychopharmacology. 2024. https://doi.org/10.1007/s00213-024-06739-x. Online Ahead of Print.
Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25.
Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–90.
O’Neill B, Patel JC, Rice ME. Characterization of optically and electrically evoked dopamine release in striatal slices from digenic Knock-in Mice with DAT-driven expression of channelrhodopsin. ACS Chem Neurosci. 2017;8:310–9.
Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–6.
Liu Z, Le Q, Lv Y, Chen X, Cui J, Zhou Y, et al. A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state. Cell Res. 2022;32:139–56.
Bariselli S, Fobbs WC, Creed MC, Kravitz AV. A competitive model for striatal action selection. Brain Res. 2019;1713:70–79.
Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci. 2009;29:768–76.
Rosén A, Zhang Y-X, Lund I, Lundeberg T, Yu L-C. Substance P microinjected into the periaqueductal gray matter induces antinociception and is released following morphine administration. Brain Res. 2004;1001:87–94.
Commons KG. Neuronal pathways linking substance P to drug addiction and stress. Brain Res. 2010;1314:175–82.
Lee T, Kaneko T, Shigemoto R, Nomura S, Mizuno N. Collateral projections from striatonigral neurons to substance P receptor- expressing intrinsic neurons in the striatum of the rat. J Comp Neurol. 1997;388:250–64.
Martone ME, Armstrong DM, Young SJ, Groves PM. Ultrastructural examination of enkephalin and substance P input to cholinergic neurons within the rat neostriatum. Brain Res. 1992;594:253–62.
Pickel VM, Douglas J, Chan J, Gamp PD, Bunnett NW. Neurokinin 1 receptor distribution in cholinergic neurons and targets of substance P terminals in the rat nucleus accumbens. J Comp Neurol. 2000;423:500–11.
Francis TC, Yano H, Demarest TG, Shen H, Bonci A. High-frequency activation of nucleus accumbens D1-MSNs Drives Excitatory Potentiation on D2-MSNs. Neuron. 2019;103:432–44.e3.
Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Mol Brain Res. 1992;14:186–95.
Svenningsson P. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–96.
Ferre S, Von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–41.
O’Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens Bi-directionally alter cocaine seeking in rats. Neuropsychopharmacol. 2012;37:1245–56.
Rysztak LG, Jutkiewicz EM. The role of enkephalinergic systems in substance use disorders. Front Syst Neurosci. 2022;16:932546.
Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry. 2012;72:803–10.
LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7.
Hearing M, Graziane N, Dong Y, Thomas MJ. Opioid and psychostimulant plasticity: targeting overlap in nucleus accumbens glutamate signaling. Trends Pharmacol Sci. 2018;39:276–94.
Zhu Y, Wang K, Ma T, Ji Y, Lou Y, Fu X, et al. Nucleus accumbens D1/D2 circuits control opioid withdrawal symptoms in mice. J Clin Investig. 2023;133:e163266.
Acknowledgements
The authors thank Daniela Franco and Mary Kay Lobo at the University of Maryland School of Medicine for providing tissue from the FK150 Drd1-cre mouse line. The authors thank Aubri Noll for assistance with cell counts, and the Penn State College of Medicine Advanced Light Microscopy Core (RRID:SCR_022526).
Funding
This work was supported by NIH grants DA050575 and DA058661, and National Alliance for Research on Schizophrenia and Depression Young Investigator Award (Award No. 31325) to MEF.
Author information
Authors and Affiliations
Contributions
MEF designed the experiments. AM, LDF, AEO, HS, SP, LBM, and MEF performed behavioral experiments and analyzed data. AM, FAA and MEF extracted RNA and/or performed qRT-PCR experiments and analyzed data. AM and MEF wrote the manuscript and prepared the figures with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montemarano, A., Fox, L.D., Alkhaleel, F.A. et al. A Drd1-cre mouse line with nucleus accumbens gene dysregulation exhibits blunted fentanyl seeking. Neuropsychopharmacol. 50, 1993–2005 (2025). https://doi.org/10.1038/s41386-025-02116-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02116-0