Abstract
Excessive alcohol use causes a great deal of harm and negative health outcomes. Corticotrophin releasing factor (CRF), a stress-related neuropeptide, has been implicated in binge ethanol intake and ethanol dependence in rodents. CRF containing neurons in the bed nucleus of the stria terminalis (BNSTCRF) can influence ethanol consumption. These BNSTCRF neurons also release GABA, raising the question, is it CRF release, GABA release, or both that is regulating alcohol consumption. Here, we used viral vectors to separate the effects of CRF and GABA release from BNSTCRF neurons on the escalation of ethanol intake in an operant self-administration procedure in male and female mice. We found that CRF deletion in BNST neurons reduces ethanol intake in both sexes, with a stronger effect in males. For sucrose self-administration there was no effect of CRF deletion. Suppression of GABA release, via knockdown of vGAT, from BNSTCRF produced a transient increase in ethanol operant self-administration in male mice, and reduced motivation to work for sucrose on a progressive ratio schedule of reinforcement in a sex-dependent manner. Together, these results highlight how different signaling molecules from the same populations of neurons can bidirectionally control behavior. Moreover, they suggest that BNST CRF release is important for high intensity ethanol drinking that precedes dependence, whereas GABA release from these neurons may play a role in regulating motivation.
Similar content being viewed by others
Introduction
In 2014 approximately 137.9 million people in the United States were current alcohol users, of whom 43.6% were binge alcohol drinkers and 6.4% met criteria for Alcohol Use Disorder (AUD) [1]. Binge drinking, which the NIAAA defines as a pattern of drinking over a short period of time that brings blood alcohol concentration levels to 0.08 g/dl or higher, may represent an important vulnerability to the development of AUD [2, 3]. Escalation of drinking is a key process in the transition from casual to excessive drinking in AUD; however, the discrete mechanisms that drive this process are not fully understood. Corticotrophin releasing factor (CRF) is a stress-responsive neuropeptide that is released from the paraventricular nucleus of the hypothalamus (PVN) and from additional regions like the bed nucleus of the stria terminalis (BNST). CRF has been proposed to be released during escalated alcohol intake, and is thought to promote a heightened negative behavioral state, enhancing negative reinforcement and promoting progression to alcohol use disorder [4,5,6,7,8].
While a clinical study exploring the impact of a corticotrophin releasing factor receptor type 1 (CRF-R1) antagonist on alcohol consumption was unsuccessful [9, 10], multiple independent genetic association studies have revealed a link between excessive alcohol consumption and this gene [11, 12]. However, the genetic association of CRHR1 does not unambiguously rule out other genes in the same region, such as MAPT, the gene that encodes for tau [13]. Basic mechanistic research into how corticotrophin releasing factor (CRF) signaling is engaged over the course of escalated alcohol consumption is thus warranted, and may elucidate up or downstream regulators of CRF that may serve as successful therapeutic targets. Repeated binge drinking is one form of escalated intake that activates CRF neurons in the BNST [14,15,16]. The majority of CRF-expressing neurons within the BNST are GABAergic [17,18,19,20]. Inhibition of CRF-BNST neurons reduces binge-like alcohol consumption [15].
Over the past 10 years, rates of AUD have increased in women by 84%, relative to a 35% increase in men [21]. More research is needed into sex differences in the neurobiological response to alcohol [22]. The BNST is a sexually dimorphic brain region, and differences in structure are due to organizational influences of sex hormones during early development [23, 24]. Additionally, sex-specific differences in CRF and GAD67 (one enzyme that synthesizes GABA) were observed within the BNST of mice [25], highlighting the importance of studying males and females. A key question has emerged in the field, as we attempt to understand how subpopulations of neurons are related to behavior: for neurons that release multiple signaling molecules, can we parse out the individual contribution of those different molecules. This is important, as different signals from the same neurons can drive temporally distinct effects on behavior [26]. Several elegant studies have begun to explore this in other components of the extended amygdala [27, 28], notably work from the Messing lab has examined how GABA and other peptides in the CRF neurons of the central amygdala can regulate anxiety and fear learning. However, to date there has not been an investigation of how different signals emanating from the same neurons in the BNST can regulate alcohol consumption. Here, we use viral vectors to selectively reduce expression of CRF or the vesicular GABA transporter (vGAT) in the BNST to determine the role of CRF release or GABA release on binge ethanol intake in an operant model of self-administration in male and female mice.
Methods and materials
Adult male and female mice were used in the current study. Transgenic mice (CRH-Cre [29] and CRH-flox [30]) were bred in-house. C57BL/6J mice (Stock #: 000664, Jackson Laboratories; Bar Harbor, ME, USA) arrived at 8 weeks, and allowed to acclimate to the vivarium for at least 7 days prior to the start of the experiment. Mice were group-housed with same-sex littermates in polycarbonate cages (GM500, Tecniplast; West Chester, PA, USA) on ventilated racks within a climate-controlled vivarium maintained in a 12 h light/dark cycle (lights off at 7 a.m.). All procedures were approved by the UNC Chapel Hill Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Operant behavior
Mice were maintained at 85–90% of free-feeding body weight for the duration of the experiment by feeding 2.5–3.0 grams of standard rodent chow (Prolab Isopro RMH 3000, LabDiet; St. Louis, MO, USA) per mouse per day. Water was available ad libitum in the vivarium but was removed prior to behavioral testing. Mice were fed for 15 min without water available to induce thirst just prior to behavioral sessions. Before beginning instrumental training, mice were exposed to their assigned reinforcing fluid ad libitum (9% ethanol v/v with 2% w/v sucrose or 2% w/v sucrose alone) in their home cage for 24 h in the vivarium.
Behavior was assessed during the dark cycle in 8 standard operant conditioning chambers housed within sound-attenuating cubicles (Med Associates; St. Albans, VT, USA). Each chamber was equipped with two retractable levers located on the back wall with a cue light centered above in between, with a magazine positioned in the center of the front wall. The magazine was equipped with a photobeam sensor to record entries, and a drinking trough connected to a syringe pump. Liquid reinforcers were delivered as 14 µL rewards and delivery was accompanied by a 2 s cue light. Responses during this time were counted but did not contribute to the response requirement. At the end of each session the drinking trough was checked for any residual fluid. Sessions were 45 min in duration, began with the levers extending, and ended with the levers retracting.
At the beginning of the experiment, one lever was assigned to deliver reward (referred to as “active”) and the other lever had no programmed consequence (referred to as “inactive”). Assignment of active lever was counterbalanced across mice and maintained throughout the duration of the experiment. Active lever responses were initially reinforced using a fixed ratio 1 (FR1) schedule, where each press resulted in delivery of a single reinforcer. Once individual mice earned a criterion of 10 reinforcers in a single FR1 session (1–5 days, mean 1.5 days), they were then trained on a fixed ratio 2 (FR2) schedule for 3 days. Mice that took longer than 2 days to achieve the performance criterion (n = 6 total in all experiments) underwent remedial FR1 sessions that were extended to a duration of 2 h. After 3 days of FR2 training, they were then trained on a fixed ratio 4 (FR4) schedule for 9 days. After 9 days of FR4 training, the mice underwent progressive ratio testing to examine the motivation to work for liquid reinforcers. These progressive ratio tests were modified for use with this ethanol and sucrose solution by changing the session timeout to 90 s from the last earned reward, which was three times the average C57BL/6J self-administration inter-reinforcer interval [31]). First mice were tested with an arithmetic progression, where the ratios progressed 1, 1, 2, 2, 3, 3, 4, 4, 5, 5, 7, 7, 9, 9, 11, 11, 13, 13, 15, 15, 18, 18, 21, 21, 24, 24,… [32]. Arithmetic progressive ratio tests are sensitive to modest shifts in reinforcement efficacy (motivation) because the ratio increases at a slower rate. This schedule is commonly used in ethanol self administration studies that focus on detecting subtle treatment effects [33]. Mice received FR4 self-administration days between progressive ratio sessions to re-establish stable responding. The second progressive ratio test followed an exponential progression where the ratios were determined using (5*e0.2*reward)‐5, rounded up to the nearest integer [34]. Exponential progressive ratio schedules rapidly accelerate response demands and have potential to detect a wider dynamic range in reinforcer efficacy. By employing both arithmetic and exponential schedules, this approach enhances methodological rigor and the external validity and translational potential of findings. Breakpoint is not a continuous measure, so we report rewards earned, which is proportional to the log transformed breakpoint [31, 34].
Blood ethanol concentration
In an experiment with male and female C57BL/6J mice, we measured blood ethanol concentration to compare this to our calculated grams per kilogram measure. Within 5 min of the end of the 9th FR4 session mice were deeply anesthetized with isoflurane, and trunk blood was taken. Blood was centrifuged and plasma was collected. Blood ethanol concentration was measured using an Analox-AM1 alcohol analyzer (Analox Technologies, Atlanta, GA, USA).
Stereotaxic surgery
Adult mice were anesthetized with isoflurane (1–3%) in oxygen (1–2 l/min), and aligned on a stereotaxic frame (Kopf Instruments; Tujunga, CA, USA) while on a heated pad (Homeothermic monitoring system, Harvard Apparatus; Holliston, MA, USA). All surgeries were conducted in a sterile environment using aseptic techniques. The scalp was sterilized with 70% ethanol and betadine, then a vertical incision was made before using a drill to burr small holes in the skull directly above the injection targets. Microinjections were performed with a 1 µL Neuros Hamilton syringe (Hamilton, Reno, NV, USA) and a micro-infusion pump (Nanoject III, Drummond Scientific; Broomall, PA, USA) that infused virus at 100 nL/min. Viruses were administered bilaterally with 200 nL per side (relative to bregma: ML ± 0.9 mm, AP, 0.23 mm, DV −4.75 mm), and the needle was left in place for 5 min to allow for diffusion of the virus before the needle was slowly withdrawn. Most mice were administered a single injection of buprenorphine (0.1 mg/kg, s.c.) for surgical analgesia, with supplemental pain management from Tylenol water. For a total of 9 mice in behavioral studies and 4 mice in the electrophysiology experiment, the approved surgical analgesia protocol changed to ketoprofen injections (5 mg/kg, s.c.) on the day of surgery and at least 2 days post-surgery. Mice were allowed to recover for at least 3 weeks prior to being used for behavioral studies, and 8 weeks prior to being used for slice electrophysiology.
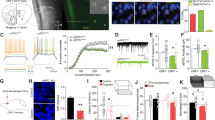
To selectively knockdown GABA release from CRF neurons, CRH-Cre mice were injected with either short hairpin RNAi directed at the vesicular GABA transporter, AAV8-hSyn-flex-GFP-shvGAT, or the control scrambled sequence, AAV8-hSyn-flex-GFP-shScramble (UNC Vector Core; Chapel Hill, NC, USA), as previously described [27, 35, 36]. For the functional validation of this construct, each of these viruses were mixed in a 4:1 ratio with AAV8-Ef1a-double floxed-hChR2(H134R)-mCherry-WPRE-HGHpA (Addgene; Watertown, MA, USA) and injected with 300 nL per side. To selectively knock down CRF expression in the BNST [30], CRH-flox mice were injected with either AAV8-hSyn-GFP or AAV8-hSyn-Cre-GFP (UNC Vector Core).
Viral placement validation for behavioral studies
Mice were anesthetized with an overdose of the anesthetic Tribromoethanol (Avertin, 1 mL, i.p.), and transcardially perfused with chilled 0.01 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Brains were extracted and post-fixed in 4% PFA for 24 h and then stored in PBS at 4 °C. Forty-five µm coronal sections were collected using a Leica VT1000S vibratome (Leica Biosystems; Deer Park, IL, USA) and then stored in 0.02% sodium azide (Sigma-Aldrich) in PBS. The tissue was then mounted on slides and allowed to dry before cover slipping with Vecta-Shield Hardset Mounting Medium with DAPI (Vector Laboratories; Newark, CA, USA). Viral placements were imaged at ×4 magnification using a Keyence BZ-X800 All-in-one Fluorescence microscope (Keyence; Itasca, IL, USA).
Slice electrophysiology
About 8 weeks post-surgery, mice were anesthetized with isoflurane and rapidly decapitated. Coronal sections through the BNST (300 μm) were prepared as previously described. Briefly, brains were quickly extracted, and slices were made using a Leica VT 1200 s vibratome (Leica Biosystems, IL, USA) in ice-cold, oxygenated sucrose solution containing in mM: 183 sucrose, 20 NaCl, 0.5 KCl, 2.5 MgCl2, 1.2 NaH2PO4, 10 glucose and 26 NaHCO3 saturated with 95% O2/5% CO2. Slices were incubated for at least 30 min in artificial cerebral spinal fluid (ACSF) maintained at 35 °C that contained in mM: 124 NaCl, 4.0 KCl, 1 NaH2PO4, 1.2 MgSO4, 10 D-glucose, 2 CaCl2, and 26 NaHCO3, saturated with 95% O2/5% CO2 before transferring to a submerged recording chamber (Warner Instruments, CT, USA) for experimental use.
Neurons were identified using infrared differential interference contrast on a Scientifica Slicescope II (Scientifica; East Sussex, UK). Fluorescent cells were visualized using a 470 nm LED. Whole-cell patch clamp recordings were performed on non-fluorescent neurons in the BNST. Identical optical stimulation parameters (5 ms of 470 nm with a power of 2.5 mW) were used to compare optically evoked currents in the BNST between the two groups. Optically evoked currents in the BNST were measured in voltage clamp using cesium methanesulfonate-based intracellular solution (Cs-Meth; in mM: 135 cesium methanesulfonate, 10 KCl, 1 MgCl2, 0.2 EGTA, 4 MgATP, 0.3 Na2GTP, 20 phosphocreatine, pH 7.3, 285–290 mOsm with 1 mg/mL QX-314) to detect light-evoked inhibitory postsynaptic currents (oIPSCs) at +10 mV. Data were sampled at 10 kHz and low pass filtered at 3 kHz. Access resistance was continuously monitored and changes greater than 20% from the initial value were excluded from data analyses.
Statistical analysis
Data were analyzed using Prism 9 (Graphpad, San Diego, CA, USA), and SPSS 26 (IBM, Armonk, NY, USA). Data are presented in figures as the mean ± standard error of the mean. Data are presented in sex-separated graphs for clarity, sex effects were analyzed and reported in the text. Response rates and rewards earned across virus, sex, and behavioral sessions were analyzed using repeated measures GLM with a Poisson distribution, because this distribution is the most appropriate for count data. Regression coefficients were tested with Wald χ2 to determine if they were significantly different from zero. Significant interaction effects were analyzed pairwise among relevant conditions (sex or virus) across days with a least significant difference adjustment for multiple comparisons. Significance level will be set at α = 0.05. For ethanol self-administration studies, grams per kilogram were calculated based on the volume consumed using the following formula ((0.014 L * number of rewards earned) – volume leftover in drinking trough after session) *0.09*0.789) / (body weight converted to kg). Grams per kilogram of ethanol consumed, and blood ethanol concentration were analyzed using the normal distribution since these are continuous measures. Blood ethanol concentration and calculated grams per kilogram were correlated with a simple linear regression. Percentage of responsive neurons in viral conditions was compared using Fisher’s exact test. Amplitude and latency of oIPSCs were analyzed with unpaired t-tests between viral conditions, with Welch’s correction applied for unequal variances when appropriate.
Results
Operant binge ethanol self-administration
High levels of ethanol consumption are achieved in our operant self-administration model for 9% (v/v) ethanol with 2% sucrose in food restricted, post-prandial mice during the dark cycle. This can potentially serve to model some of the critical interactions observed in humans regarding dieting, food security and sucrose consumption. The level of ethanol and sucrose included in the ethanol solution is about equivalent to a Moscato wine. Male and female C57BL/6J mice responded on the active lever to self-administer ethanol (Fig. 1A). Females tended to respond at higher rates than males did across days, with a significantly higher response rate on day 9 of FR4 (Main effect of sex: Wald χ2(1) = 3.4, p = 0.06; Main effect of day: Wald χ2(8) = 253.1, p < 0.0001; Sex-by-day interaction: Wald χ2(8) = 32.9, p < 0.0001; day 9 p < 0.05). Females have a smaller body mass than males do, which translated to significantly higher calculated grams per kilogram consumption of ethanol across days of FR4 (Fig. 1B. Two-way ANOVA g/kg: Main effect of Sex F(1,14) = 12.9, p = 0.003; Main effect of Day F(8) = 16.1, p < 0.0001; Sex-by-day interaction F(8,112) = 0.8, p = 0.6). Blood ethanol concentrations were measured immediately following the 9th FR4 self-administration session (Fig. 1C). All of the mice achieved above the binge intoxication threshold, and the ethanol concentrations in the blood correlated with the calculated ethanol consumed (R2 = 0.52, p = 0.0015). Subsequent experiments were performed in genetically modified mice as denoted.
A Male (n = 8) and female (n = 8) C57BL/6J mice learn to respond on the active lever for ethanol reinforcers. Females tended to respond on the active lever more than males did across days, with the effect being significant on day 9. B Female C57BL/6J mice consumed more ethanol on a grams per kilogram basis. Note that the standard error of the mean for Day 9 FR4 males is too small to be depicted (Mean 3.47 ± 0.16 SEM). C Rewards earned for male and female C57BL/6J mice. D On the ninth day of FR4, all mice had blood ethanol concentrations above 80 mg/dl, which is the threshold for binge intoxication. The blood ethanol concentration values significantly correlated with the calculated grams per kilogram consumed.
Ethanol self-administration BNST CRF knockdown
Knocking down expression of CRF peptide within the bed nucleus of the stria terminalis led to reductions in ethanol self-administration. Male CRF knockdown mice self-administered less ethanol than male GFP controls (Fig. 2A. Main effect of virus Wald χ2(1) = 18.9, p < 0.0001). There was a trend for a reduction in active lever responding in female CRF Knockdown mice (Fig. 2B. Main effect of virus: Wald χ2(1) = 3.3, p = 0.07). There were no differences in active lever responding in GFP-expressing control mice, indicating that knocking down CRF reduced active lever responding, and this effect is more pronounced in males than in females.
A Males CRF knockdown mice (n = 9) self-administered less ethanol than male GFP controls (n = 8). B Female CRF knockdown mice (n = 8) had a trend for reduced active lever responding on an FR4 schedule compared to GFP controls (n = 7). C Male CRF knockdown mice earned fewer rewards than male GFP controls. D Female CRF knockdown mice did not earn fewer rewards than female GFP controls. E Male CRF knockdown mice consumed less ethanol than male GFP controls. F Female CRF knockdown mice had a trend for reduced ethanol consumption compared to female GFP controls. G CRF knockdown reduced motivation to work for ethanol reinforcers on an arithmetic progressive ratio schedule. Female mice earned fewer rewards on this progressive ratio test than males did. H CRF knockdown reduced motivation to work for ethanol reinforcers on an exponential progressive ratio schedule. Female mice earned fewer rewards on this progressive ratio test than males did. I Representative images of viral placement in male mice expressing the control GFP virus (top) and the Cre expressing virus (bottom). J Representative images of viral placement in female mice expressing the control GFP virus (top) and the Cre expressing virus (bottom).
Knocking down expression of the CRF peptide also reduced the consumption of ethanol as measured by g/kg, with a similar pattern to the active lever responding. Male CRF knockdown mice consumed less ethanol than male GFP controls (Fig. 2C. Main effect of virus: Wald χ2(1) = 12.8, p < 0.0001, virus-by-day interaction: Wald χ2(8) = 72.5, p < 0.0001, all days’ p ≤ 0.01). There was a tendency for female CRF knockdown mice to consume less ethanol than female GFP controls, reflected in a trend for a day-by-virus interaction (Fig. 2D. Wald χ2(8) = 14.7, p = 0.066), and significantly lower g/kg values on day 6 (p = 0.001) and day 8 (p = 0.02).
Knocking down CRF peptide expression in the BNST reduced motivation to work for ethanol reinforcers in the progressive ratio tests. We used multiple progressive ratio approaches to provide more direct comparisons to other ethanol studies [31, 33, 37] and to measure behavior under the more demanding exponential progression. Rewards earned on the arithmetic progressive ratio test (Fig. 2E) were significantly lower for CRF knockdown animals (Main effect of virus: Wald χ2(1) = 6.4, p = 0.01) and also significantly lower for females (Main effect of sex: Wald χ2(1) = 3.8, p = 0.05). The same pattern of results was observed in the exponential progressive ratio test (Fig. 2F. Main effect of virus: Wald χ2(1) = 15.6, p < 0.0001, Main effect of sex: Wald χ2(1) = 4.7, p = 0.03).
Sucrose self-administration BNST CRF knockdown
Sucrose self-administration was not affected by knockdown of CRF peptide in the BNST. When examining active lever responses across days by sex and virus, there was no significant main effect of virus (Wald χ2(1) = 0.012, p = 0.9) or sex-by-virus interaction (Wald χ2(1) = 1.7, p = 0.2). There was a significant main effect of sex (Wald χ2(1) = 20.2, p < 0.0001), and sex-by-virus-by-day interaction (Wald χ2(8) = 15.7, p < 0.05), due to higher levels of active lever responses in females compared to males.
There was no significant effect of CRF knockdown among active lever responses in males self-administering sucrose (Fig. 3A. Main effect of virus: Wald χ2(1) = 0.64, p = 0.4). Also, there was no difference in active lever responding for sucrose between virus conditions in females (Fig. 3B. Main effect of virus: Wald χ2(1) = 1.7, p = 0.2).
A There was no significant effect of CRF knockdown among active lever responses in males self-administering sucrose (Male GFP n = 13, Cre n = 10). B There was no significant difference between virus conditions in females in active lever responding for sucrose (Female GFP n = 7, Cre n = 6). C There was no significant difference between virus conditions in males in rewards earned (Male GFP n = 13, Cre n = 10). D There was no significant difference between virus conditions in females in rewards earned (Female GFP n = 7, Cre n = 6). E CRF knockdown did not affect rewards earned in the arithmetic progressive ratio test. Females had higher motivation to work for sucrose reinforcers on this schedule. F CRF knockdown did not alter rewards earned in the exponential progressive ratio test. Females had higher motivation to work for sucrose reinforcers on this schedule.
Knocking down CRF peptide expression in the BNST did not alter motivation to work for sucrose reinforcers in the progressive ratio tests. In the arithmetic progressive ratio test, there was a significant main effect of sex (Fig. 3C. Main effect of sex: Wald χ2(1) = 12.9, p < 0.0001) reflecting the higher level of rewards earned in females. In the exponential progressive ratio test, there was a significant main effect of sex (Fig. 3D. Main effect of sex: Wald χ2(1) = 9.6, p = 0.002, sex-by-virus interaction: Wald χ2(1) = 4.5, p = 0.03). There were no significant effects of virus for males (p = 0.1) or for females (p = 0.1) on rewards earned in the exponential progressive ratio test.
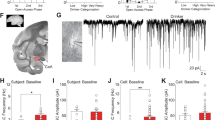
GABA knockdown functional validation
Using short hairpin RNA interference directed at the vesicular GABA transporter (referred to as shvGAT), we were able to selectively reduce GABA release from CRF neurons in the BNST. We injected a mixture of channelrhodopsin and either the shvGAT virus or the scrambled sequence control virus into the BNST of CRF-Cre mice to measure optically evoked inhibitory currents. Neurons in the BNST are densely inter-connected [38, 39], so whole-cell patch clamp recordings from non-fluorescent were able to detect reductions in GABA release from CRF neurons with GABA knockdown. There were no significant differences in the percentage of responsive neurons recorded from scramble controls (Supplementary Fig. 1) or shvGAT-expressing mice. There was a significant reduction in oIPSC amplitude in shvGAT-expressing mice compared with scramble controls (t(7.6) = 3.6, p = 0.007), indicating that GABA release was reduced by the viral manipulation. There was no difference in oIPSC latency between viral conditions (t(13) = 1.5, p = 0.15).
Ethanol self-administration following BNSTCRF neuron vGAT knockdown
Knocking down GABA release from BNSTCRF neurons led to a transient increase in ethanol self-administration in males. When analyzing active lever responding across days for effects of sex and virus, there was a significant main effect of virus (Wald χ2(1) = 22.0, p < 0.0001), a significant sex-by-virus interaction (Wald χ2(1) = 9.0, p = 0.003), and a significant sex-by-virus-by-day interaction (Wald χ2(8) = 15.3, p = 0.05). Male mice with reduced GABA release from BNSTCRF neurons self-administered significantly more ethanol compared with control males (Fig. 4A. Main effect of virus: Wald χ2(1) = 3.8, p = 0.05; virus-by-day interaction: Wald χ2(8) = 48.9, p < 0.0001), and with significantly more active lever responses on the first three days of FR4 (day 1 p = 0.016, Day 2 p = 0.018, day 3 p = 0.024, day 4 p = 0.06). Reducing GABA release from BNSTCRF neurons did not alter female ethanol active lever responding on an FR4 schedule of reinforcement (Fig. 4B. Main effect of virus: Wald χ2(1) = 0.63, p = 0.4, virus-by-day interaction: Wald χ2(8) = 11.6, p = 0.2).
A Male mice with reduced GABA release from BNST-CRF neurons (male shvGAT n = 7) self-administered significantly more ethanol compared with control males (male scramble n = 11), responses on the first 3 days of FR4 (day 1 p = 0.016, Day 2 p = 0.018, day 3 p = 0.024, day 4 p = 0.06). B Reducing GABA release from BNST-CRF neurons did not alter female ethanol active lever responding on an FR4 schedule of reinforcement (Females scramble n = 8, shvGAT n = 8). C Male mice with reduced GABA release from BNST-CRF neurons (male shvGAT n = 7) earned more rewards compared with controls on an FR4 schedule of reinforcement (male scramble n = 11). D Reducing GABA release from BNST-CRF neurons did not alter female rewards earned on a FR4 schedule of reinforcement (Females scramble n = 8, shvGAT n = 8). E Male mice with GABA knockdown in BNST CRF neurons tended to consume more ethanol, but this did not reach significance. F Female mice with GABA knockdown in BNST CRF neurons consumed similar amounts of ethanol as control females. G There were no significant effects of virus or sex for the arithmetic progressive ratio test. H There were no significant effects of virus or sex for the exponential progressive ratio test. I Representative images of viral placement in male mice expressing the control scramble virus and the shvGAT expressing virus (bottom). J Representative images of viral placement in female mice expressing the control scramble virus and the shvGAT expressing virus (bottom).
Knocking down GABA release from BNSTCRF neurons had less of an effect on g/kg consumed of ethanol. Male mice with GABA knockdown in BNST CRF neurons tended to consume more ethanol, but this did not reach significance (Fig. 4C. Wald χ2(1) = 3.4, p = 0.07). Female mice with GABA knockdown in BNST CRF neurons consumed similar amounts of ethanol as control females (Fig. 4D). In our progressive ratio tests there were no significant effects of virus or sex for either the arithmetic (Fig. 4E) or the exponential (Fig. 4F) versions.
Sucrose self-administration following BNSTCRF neuron vGAT knockdown
Reducing GABA release from BNSTCRF neurons had various impacts on sucrose self-administration. Within male mice (Fig. 5A), there was a significant virus-by-day interaction (Wald χ2(8) = 34.3, p < 0.0001), which is driven by the strong main effect of day (Wald χ2(8) = 146.0, p < 0.0001), as none of the individual days were significantly different between GABA knockdown males and controls. Within female mice (Fig. 5B), there was a similar pattern of results, with a significant main effect of day (Wald χ (8) = 171.1, p < 0.0001) and virus-by-day interaction (Wald χ (8) = 20.1, p = 0.01), yet none of the individual days were significantly different between GABA knockdown females and controls. Females responded more than males on the active lever regardless of viral expression.
A BNST-CRF GABA knockdown did not alter sucrose self-administration in male mice (male scramble n = 8, shvGAT n = 8). B BNST-CRF GABA knockdown did not alter sucrose self-administration in female mice (female scramble n = 7, shvGAT n = 7). C BNST-CRF GABA knockdown did not alter sucrose rewards obtained in male mice (male scramble n = 8, shvGAT n = 8). D BNST-CRF GABA knockdown did not alter sucrose rewards obtained in female mice (female scramble n = 7, shvGAT n = 7). E BNST-CRF GABA knockdown reduced motivation to work for sucrose reinforcement on an arithmetic progressive ratio schedule of reinforcement in male mice. F BNST-CRF GABA knockdown reduced motivation for both sexes to work for sucrose on an exponential progressive ratio schedule of reinforcement.
Knocking down GABA release from BNSTCRF neurons had a larger effect on males than females on motivation to work for sucrose reinforcement. In the arithmetic progressive ratio test (Fig. 5C) there was a significant main effect of sex (Wald χ (1) = 19.1, p < 0.0001), a trend for a main effect of virus (Wald χ (1) = 3.6, p = 0.06), and a significant sex-by-virus interaction (Wald χ (1) = 6.7, p = 0.009). GABA knockdown in BNST CRF neurons in males reduced motivation to work for sucrose on an arithmetic progressive ratio test (p = 0.015), but did not alter motivation to work for sucrose in females on this test (p = 0.3). In the exponential progressive ratio test, there was a significant main effect of virus (Wald χ (1) = 4.5, p = 0.04), indicating that GABA knockdown reduced motivation to work for sucrose for both males and females.
Discussion
In the present study, we found that deletion of CRF from the BNST led to a reduction of operant ethanol self-administration in male mice and reduced motivation to consume alcohol in both male and female mice. This appeared selective for ethanol, as we did not see similar effects of CRF knockdown when examining sucrose intake. Suppression of vGAT from BNSTCRF neurons transiently increased ethanol responses in male, but not female mice. Additionally, suppression of vGAT from BNSTCRF neurons reduced motivation to work for sucrose reinforcers, with females showing a reduction in motivation only on the exponential progressive ratio schedule. Together, these findings suggest that CRF release from BNST neurons supports the positive reinforcing effects of ethanol, which promote the development and maintenance of ethanol use prior to dependence [40]. This is especially interesting, as we recently found that deletion of 5HT2C-R, which is expressed in CRF neurons [41], does not robustly impact operant responding for alcohol [42], suggesting that another upstream signal beyond serotonin regulates CRF neurons. Suppressing GABA release from BNSTCRF neurons may produce reduced motivation or a state of anhedonia, which may underlie the transient increase in ethanol self-administration in males due to stronger negative reinforcement from the ethanol, as well as the reduction in motivation for sucrose reinforcers. Future studies should directly measure the role of GABA release from BNSTCRF neurons across additional behaviors in order to more deeply understand how this regulates behaviors. This study supports a model in which different compounds released from the same population of neurons can bidirectionally influence behavior. In support of this model, a recent report demonstrated opposing functions in anxiety-like behavior for CRF and GABA released from central amygdala CRF-expressing neurons in rats [27]. This is an important consideration to take into account when designing and interpreting experiments that drive activation and inhibition of neuronal populations.
Previous work in our lab found that CRF-containing neurons in the BNST appeared to be important for binge-like ethanol consumption. Specifically, we found that activating a Gi-coupled DREADD led to reduced alcohol consumption, in both male and female mice [14,15,16]. However it is notable that we did not explore if activation of a Gi-coupled DREADD in BNST CRF neurons reduced operant self-administration. These BNST CRF neurons are GABAergic as well, raising the question was it the CRF or the GABA, or both that was driving increased alcohol intake. In male mice, there was a clear model that emerged, with CRF driving alcohol responding and motivation, and GABA playing an opposing role on alcohol responding. In females, it was less clear. Deletion of CRF led to a reduction in motivation, but minimal impact on responding. This is interesting, as in a recent study we found that there were sex differences in the physiological properties of BNST CRF neurons, with the neurons from female mice being more excitable with greater synaptic excitation [16]. In addition, prior work shows that female mice exhibit enhanced performance on an arithmetic progressive ratio test as compared to males which suggests that the heightened demand of the exponential progressive ratio schedule was required to delineate differential reinforcer efficacy in females in this study [37]. Notably, while we focused our targeting on the ventral BNST, there was viral expression in both dorsal and ventral BNST, precluding a distinction from our work. To date, there is no clear evidence that BNST CRF neurons can release glutamate, however, there are glutamatergic neurons located in the ventral BNST [43], suggesting an interesting future direction to explore whether ventral BNST glutamatergic neurons express CRF and how that may contribute to behavior.
Reducing GABA release from BNST CRF neurons in male mice produced a transient increase in self-administration of ethanol, but did not alter motivation to work for ethanol on our progressive ratio tests. It is possible that if we had tested for motivation differences earlier in FR4 training, we may have seen an increase. It is also possible that there is some form of compensatory mechanism at play to adjust ethanol self-administration behavior following GABA release reductions, which seems more likely to happen with fast neurotransmitters like GABA compared to neuromodulators like CRF. Moreover, one intriguing possibility is that this may relate to a shift from motivation for natural rewards to alcohol as part of a transition to dependence, with a more dominant role for GABA. This also raises an important caveat: the relatively minor effects of the vGAT manipulation on ethanol consumption may be masked by the presence of sucrose. Taken together, these data sets support a model in which there may be different mechanisms and circuits engaged to support high level alcohol consumption. These sex-differences are important to consider translationally, as there have been negative results reported for CRFR1 antagonists in a clinical trial for AUD that has focused on female patient populations [44]. The potential reasons for this failure were discussed extensively by Pomerenze et al. [10]. Of note, much of the work that supported the premise for this trial was conducted in male animals, while the study was in females. This highlights the importance and utility of exploring across sexes.
Recent work in rats utilizing an operant two-choice task where both options gave reinforcement, but one option gave reinforcement paired with optogenetic laser stimulation demonstrated that pairing stimulation of BNST-CRF neurons with sucrose pellet reinforcement [45] led to avoidance of that operant response, whereas pairing stimulation of BNST-CRF neurons with cocaine reinforcement did not alter choice between operant responses [46]. Previous work using fiber photometry recordings has shown that food consumption increases [47], and cocaine administration decreases [48], bulk neural activity in the BNST. Combined with our results, this might indicate that GABA release from BNST-CRF neurons may lessen the motivational value of sucrose reinforcers. For cocaine reinforcers, the role for decreased BNST-CRF neural activity is not related to motivation, so optogenetic stimulation does not alter cocaine-motivated behavior.
Potential caveats of our model
Food restriction impacts the mouse behavior in this study, as does the inclusion of sweetener. Notably, in humans this is the case also. Food insecurity risk is associated with moderate and severe alcohol use disorder diagnosis in young adults [49]. According to the CDC, about half of American adults drink sugar-sweetened beverages on a given day [50], and about 1 in 5 American adults is dieting, which often involves some form of food restriction. Indeed, the term “drunkorexia” was coined 15 years ago to describe patterns of behavior involving food restriction and binge drinking of alcohol in humans that is associated with higher risk for alcohol-related problems [51]. The model used herein, rather than studying the impact of these factors in isolation to determine their individual effect, is using them as a model for common human behaviors and social determinants of health that represent important risk factors for adverse outcomes. However, it is important to note that these results may only apply to a subset of heavy drinkers who also diet and eat poorly. Other operant studies for ethanol [34, 52], food [53, 54], and other rewards including cocaine [55, 56] utilize food restriction as a means to enhance motivation. It is possible that enhanced motivation through food restriction may produce a ceiling effect for measuring increases in operant responding. Another issue is that this is not a model of dependence, which could potentially limit insight. Another key issue is that there may be compensation in neurons with reduced expression of CRF or vGAT, and these compensatory mechanisms may underlie the observed behavioral effects. This poses a very interesting question for future studies, measuring how the deletion of CRF may impact brain dynamics to reduce alcohol drinking. Finally, we only manipulated CRF neurons in this experiment; there are other neuronal subtypes in the BNST that may contribute to these behaviors, in particular elegant work from Nair et al. demonstrated that somatostatin containing neurons can regulate alcohol consumption [57].
Conclusion
These data demonstrate that the same genetically-defined neural population within a brain region can influence motivated behavior in opposing ways depending on which neurotransmitter is released. Additionally, neural circuits underlying motivated behavior are sexually dimorphic.
Data availability
The data are available on request.
References
Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health (NSDUH)|CBHSQ Data. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf.
Jennison KM. The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am J Drug Alcohol Abus. 2004;30:659–84.
Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094–101.
Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86.
de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, et al. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun. 2019;10:1238.
Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4.
Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–47.
Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–44.
Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–63.
Pomrenze MB, Fetterly TL, Winder DG, Messing RO. The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcohol Clin Exp Res 2017;41:1986–99.
Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602.
Gelernter J, Sun N, Polimanti R, Pietrzak RH, Levey DF, Lu Q, et al. Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol Psychiatry. 2019;86:365–76.
Sanchez-Roige S, Palmer AA, Clarke T-K. Recent efforts to dissect the genetic basis of alcohol use and abuse. Biol Psychiatry. 2020;87:609–18.
Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, et al. Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake. Biol Psychiatry. 2017;81:930–40.
Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci 2015;18:545–52.
Levine OB, Skelly MJ, Miller JD, Rivera-Irizarry JK, Rowson SA, DiBerto JF, et al. The paraventricular thalamus provides a polysynaptic brake on limbic CRF neurons to sex-dependently blunt binge alcohol drinking and avoidance behavior in mice. Nat Commun 2021;12:5080.
Nguyen AQ, Dela Cruz JAD, Sun Y, Holmes TC, Xu X. Genetic cell targeting uncovers specific neuronal types and distinct subregions in the bed nucleus of the stria terminalis. J Comp Neurol 2016;524:2379–99.
Partridge JG, Forcelli PA, Luo R, Cashdan JM, Schulkin J, Valentino RJ, et al. Stress increases GABAergic neurotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology. 2016;107:239–50.
Dedic N, Kühne C, Jakovcevski M, Hartmann J, Genewsky AJ, Gomes KS, et al. Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat Neurosci 2018;21:803–7.
Dabrowska J, Hazra R, Guo J-D, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 2013;7:156.
Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911–23.
Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, et al. Sex differences in stress-related alcohol use. Neurobiol Stress. 2019;10:100149.
Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706.
Hisasue S, Seney ML, Immerman E, Forger NG. Control of cell number in the bed nucleus of the stria terminalis of mice: role of testosterone metabolites and estrogen receptor subtypes. J Sex Med. 2010;7:1401–9.
Janitzky K, Peine A, Kröber A, Yanagawa Y, Schwegler H, Roskoden T, et al. Increased CRF mRNA expression in the sexually dimorphic BNST of male but not female GAD67 mice and TMT predator odor stress effects upon spatial memory retrieval. Behav Brain Res. 2014;272:141–9.
Tillage RP, Foster SL, Lustberg D, Liles LC, McCann KE, Weinshenker D, et al. Co-released norepinephrine and galanin act on different timescales to promote stress-induced anxiety-like behavior. Neuropsychopharmacology. 2021;46:1535–43.
Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO, et al. Dissecting the roles of GABA and neuropeptides from rat central amygdala CRF neurons in anxiety and fear learning. Cell Rep. 2019;29:13–21.e4.
Soden ME, Yee JX, Zweifel LS. Circuit coordination of opposing neuropeptide and neurotransmitter signals. Nature. 2023;619:332–7.
Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–42.
Yu W, Caira CM, del R, Rivera Sanchez N, Moseley GA, Kash TL. Corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis exhibit sex-specific pain encoding in mice. Sci Rep. 2021;11:12500.
Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11.
Walker BM, Koob GF. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18.
Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology. 2009;204:135–47. https://doi.org/10.1007/s00213-008-1444-9.
Gianessi CA, Groman SM, Thompson SL, Jiang M, van der Stelt M, Taylor JR, et al. Endocannabinoid contributions to alcohol habits and motivation: relevance to treatment. Addict Biol. 2020;25:e12768.
Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z, et al. Wakefulness is governed by GABA and histamine cotransmission. Neuron. 2015;87:164–78.
Gereau GB, Zhou D, Van Voorhies K, Tyler RE, Campbell J, Murray JG, et al. GABA release from central amygdala neurotensin neurons differentially modulates ethanol consumption in male and female mice. Neuropsychopharmacology. 2024;49:1151–61.
Faccidomo S, Eastman VR, Santanam TS, Swaim KS, Taylor SM, Hodge CW. Distinct sex differences in ethanol consumption and operant self-administration in C57BL/6J mice with uniform regulation by glutamate AMPAR activity. Front Behav Neurosci. 2025;18:1498201. https://doi.org/10.3389/fnbeh.2024.1498201.
Pati D, Marcinkiewcz CA, DiBerto JF, Cogan ES, McElligott ZA, Kash TL, et al. Chronic intermittent ethanol exposure dysregulates a GABAergic microcircuit in the bed nucleus of the stria terminalis. Neuropharmacology. 2020;168:107759.
Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101.
Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62. https://doi.org/10.1038/npp.2013.261.
Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, et al. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatry. 2018;23:143–53.
Flanigan ME, Gianessi C, Castle M, Dorlean W, Sides T, Kash TL, et al. Bed nucleus of the stria terminalis (BNST) neurons containing the serotonin 5HT2c receptor modulate operant alcohol self-administration behavior in mice. Neuropsychopharmacology. 2024;49:709–19.
Gungor NZ, Yamamoto R, Pare D. Glutamatergic and gabaergic ventral BNST neurons differ in their physiological properties and responsiveness to noradrenaline. Neuropsychopharmacology. 2018;43:2126–33.
Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41:2818–29.
Baumgartner HM, Schulkin J, Berridge KC. Activating corticotropin-releasing factor systems in the nucleus accumbens, amygdala, and bed nucleus of stria terminalis: incentive motivation or aversive motivation? Biol Psychiatry. 2021;89:1162–75.
Baumgartner HM, Granillo M, Schulkin J, Berridge KC. Corticotropin releasing factor (CRF) systems: promoting cocaine pursuit without distress via incentive motivation. PLoS ONE. 2022;17:e0267345.
Jaramillo AA, Williford KM, Marshall C, Winder DG, Centanni SW. BNST transient activity associates with approach behavior in a stressful environment and is modulated by the parabrachial nucleus. Neurobiol Stress. 2020;13:100247.
Melchior JR, Perez RE, Salimando GJ, Luchsinger JR, Basu A, Winder DG, et al. Cocaine augments dopamine-mediated inhibition of neuronal activity in the dorsal bed nucleus of the stria terminalis. J Neurosci. 2021;41:5876–93.
Nagata JM, Whittle HJ, Ganson KT, Tabler J, Hahn JA, Weiser SD, et al. Food insecurity risk and alcohol use disorder in US young adults: findings from the National Longitudinal Study of Adolescent to Adult Health. Am J Addict. 2021;30:601–8.
Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults, 2011-2014. NCHS Data Brief 2017;1–8.
Simons RM, Hansen JM, Simons JS, Hovrud L, Hahn AM. Drunkorexia: normative behavior or gateway to alcohol and eating pathology? Addict Behav. 2021;112:106577.
Serlin H, Torregrossa MM. Adolescent rats are resistant to forming ethanol seeking habits. Dev Cogn Neurosci. 2015;16:183–90.
Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–96.
Gianessi CA, Groman SM, Taylor JR. The effects of fatty acid amide hydrolase inhibition and monoacylglycerol lipase inhibition on habit formation in mice. Eur J Neurosci. 2022;55:922–38.
Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, et al. Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol Psychiatry. 2015;77:895–902.
Monsey MS, Ruiz SG, Taylor JR. Regulation of garcinol on histone acetylation in the amygdala and on the reconsolidation of a cocaine-associated memory. Front Behav Neurosci. 2019;13:281.
Suresh Nair M, Dao NC, Lopez Melean D, Griffith KR, Starnes WD, Moyer JB, et al. Somatostatin neurons in the bed nucleus of the stria terminalis play a sex-dependent role in binge Drinking. Brain Res Bull. 2022;186:38–46.
Acknowledgements
We would like to thank the reviewers for their helpful feedback on this manuscript.
Funding
This research was supported by grants from the National Institutes of Health’s (NIH) National Institute of Alcohol Abuse and Alcoholism (NIAAA): U01AA020911-06, U24AA025475-01, R01AA019454 (TLK) and T32AA007573-22 (CAG).
Author information
Authors and Affiliations
Contributions
CAG, TLK, and CWH designed experiments, CAG, TS, SLD, and GBG performed surgeries and conducted behavioral experiments. DP conducted slice physiology studies. CAG, HLH, RH, WPK, and KB performed IHC and prepared analysis. CAG and TLK wrote the paper with contributions from HLH, DP, and CWH.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gianessi, C.A., Haun, H.L., Pati, D. et al. Disentangling the effects of corticotrophin releasing factor and GABA release from the bed nucleus of the stria terminalis on ethanol self-administration in mice. Neuropsychopharmacol. 50, 2040–2050 (2025). https://doi.org/10.1038/s41386-025-02192-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02192-2