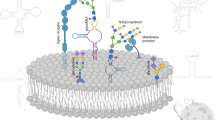
Abstract
Recent studies have identified glycosylated RNAs (glycoRNAs) as a novel class of biomolecules potentially involved in cancers and immunological diseases. However, their presence and functional roles in glioma remain unexplored. GlycoRNAs were extracted from glioma cells and detected using Ac4ManNAz labeling and Northern blot. Small RNA deep sequencing and qRT-PCR were employed to determine RNA types and content. A sequence-specific RNA-capture magnetic bead system was developed to enrich specific glycoRNAs, such as U2 and U4. Glycan components were analyzed using liquid chromatography-mass spectrometry. CCK-8, adhesion, ki67, TUNEL staining assays were used to evaluate cell viability, adhesion, proliferation and apoptosis. Glioma cells were found to be rich in glycoRNAs, predominantly small RNAs, with U2 and U4 being particularly abundant. These glycoRNAs primarily contained fucosylated and sialylated complex glycans. The depletion of cell-surface glycoRNAs at the observed time point significantly inhibited glioma cell viability and proliferation, without altering cell adhesion or apoptosis levels. This study underscored the significant role of glycoRNAs in glioma proliferation and provided a foundation for further research into their potential as novel biomarkers and therapeutic targets for glioma.

Glioma cells U87 and LN229 showed significant enrichment in glycoRNAs, predominantly small RNAs, with U2 and U4 being particularly abundant and specific. Furthermore, these glycoRNAs were found to be modified by multiple glycans, primarily complex, fucosylated, and sialylated structures.
Similar content being viewed by others
Introduction
Glycoconjugates, which include glycoprotein, proteoglycan, glycopeptides, glycosides, and glycolipids, play a pivotal role in a multitude of biological processes, such as cell proliferation/migration and immune response. Additionally, they are involved in various pathological processes, such as tumor formation and metastasis. Despite the prior discovery of numerous glycoconjugates, it was not until the identification of glycoRNAs that RNA was regarded as a potential substrate for glycan modification. In a study published in 2021, Flynn et al. employed a series of distinctive purification techniques and demonstrated the existence of glycosylated RNAs (glycoRNAs) in a range of cell types and in vivo [1]. Furthermore, glycoRNAs have been demonstrated to interact with members of the Siglec receptor family [1]. Another study by Zhang et al. revealed that glycoRNAs located on the cell surface of neutrophils were involved in the interaction with endothelial cells through P-selectin (Selp) [2]. These findings support the hypothesis that glycoRNAs play a critical role in both physiological and pathological processes.
Glioma represents the most prevalent primary intracranial tumor in adults, accounting for 40–60% of all primary tumors in the central nervous system (CNS) [3]. The standard treatment, comprising surgical resection in conjunction with concurrent chemoradiotherapy, has been demonstrated to have minimal impact on prolonging the survival of patients [4]. The aforementioned circumstances can be attributed to the high proliferation and invasiveness of glioma, in addition to the glioma microenvironment, which exhibits tumor-promoting and immunosuppressive functions. Glycoconjugates are of great importance in the development of gliomas. For example, glycosylated proteins, such as MUC1 and MUC4, are overexpressed in glioblastoma and associated with increased proliferation and invasion, and poor prognosis [5, 6]. Sialylation, defined as the process of covalent addition of sialic acid to the end of glycoproteins, is postulated to mediate β1 integrin signaling and to facilitate the growth and invasion of glioma cells [7]. Furthermore, the plasma membrane glycocalyx of glioma cells plays a pivotal role in the formation and maintenance of the tumor microenvironment. Its principal functions, including cell recognition, adhesion and signal transduction, are intimately associated with glioma invasion, migration, angiogenesis and immune regulation [8]. Conventional anti-cancer strategies that target glycans primarily concentrate on the creation of glycosylation inhibitors of tumor-related proteins and antibodies that target tumor glycoproteins and glycolipids [9, 10]. Despite some advances, the therapeutic effect remains limited. Consequently, there is an urgent need to develop new and more effective tumor markers and therapeutic targets. The study on glycoRNAs, a novel class of glycosylated RNAs located in the cell membrane, and the underlying mechanisms, may offer significant insights into the pathogenesis and potential therapeutic targets of tumors, including gliomas. However, it is currently unclear whether glycoRNAs are present in gliomas and what their precise composition may be.
In this study, glycoRNAs were enriched and purified from glioma cell lines LN229 and U87 using the N-Azidoacetylmannosamine-tetraacylated (Ac4ManNAz) labeling method, with their presence confirmed by Northern blot analysis. Notably, we developed a sequence-specific RNA-capture magnetic bead system to enrich specific glycoRNAs, including U2, U4, and Y5. The components of glycoRNAs were analyzed and identified through RNA sequencing and chromatography-mass spectrometry. The results revealed that glioma cells U87 and LN229 were significantly enriched in glycoRNAs, predominantly small RNAs, with U2 and U4 being particularly abundant. These glycoRNAs were found to be modified by multiple glycans, primarily complex, fucosylated, and sialylated structures. Additionally, CCK-8, adhesion, ki67 and TUNEL assays demonstrated that glycoRNA depletion at the observed time point significantly inhibited glioma cell viability and proliferation, without altering cell adhesion or apoptosis levels. Our study suggested that glycoRNAs may play a critical role in glioma development, providing new insights into their potential as biomarkers and therapeutic targets for glioma.
Results
U87 and LN229 glioma cells are enriched in glycoRNAs, which were predominantly small RNAs
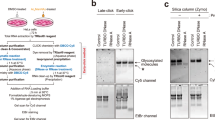
The flow diagram for this study was shown in Fig. 1. Ac4ManNAz is an azide group-containing mannose derivative that modifies cell surface proteins with N-linked glycosylation. It is commonly used to study glycoproteins through in vivo metabolic labeling and chemoselective ligation. In this study, to find out whether glycoRNAs are present in glioma cells, Ac4ManNAz was used to label the glycoRNAs in glioma cells (U87 and LN229) for 24 h. Subsequent to extraction, purification, and DBCO-biotin treatment, the RNA samples underwent separation by gel electrophoresis, followed by visualization through blotting with anti-biotin streptavitin-HRP (strep, Fig. 2A). Prior to RNA transfer to the membrane, total RNA in the gel was visualized and used as a contrast to strep-binding RNA in the membrane. As demonstrated in Fig. 2B, the biotin signals were observed in RNA samples from the U87, LN229, and Hela cells treated with Ac4ManNAz for 24 h (the 24 h groups). Conversely, no such biotin signal was detected in RNA samples from the U87, LN229, and Hela cells without Ac4ManNAz treatment (the 0 h groups). RNase and DNase were used to determine whether the biotin signals were from RNA or DNA. Upon digesting the RNA samples with RNase Cocktail prior to electrophoresis, the signals disappeared on blotting. Conversely, digestion with DNase or RNase preincubated with RNase inhibitor (RNasin) did not result in loss of signals (Fig. 2C). It was also evident that there was no signal in the gel for RNA samples treated with RNase (Fig. 2C). These results suggested that the purified samples were primarily composed of RNA with little or no DNA contamination. To determine the approximate range of molecular weights of glycoRNAs, small (<200 bp) and large (>200 bp) RNAs were isolated using the RNAzol Isolation Kit and then analyzed by gel electrophoresis and blotting, respectively. The results showed significant biotin signals in the small RNA samples derived from both U87 and LN229 cells, while there was minimal signal in the large RNA samples (Fig. 2D). It suggested that the glycoRNAs from glioma U87 and LN229 cells might be predominantly composed of small RNAs. The slow migration of glycoRNAs on the gels could be attributed to the glycans affecting the mobility of the RNAs. Furthermore, the biotin signals for glycoRNAs significantly decreased in the samples treated with sialidase, PNGase F, endo F2, and endo F3, respectively (Fig. 2E, F), suggesting that the glycoRNAs were sensitive to these enzymes. The efficiency of column cleanup was validated through blotting and the results were shown in Supplementary Fig. S1.
A Visualization of glycoRNAs from glioma cells through RNA blotting; B Sequencing of glycoRNAs from glioma cells and validation of the sequencing results; C Extraction and analysis of the glycans released from glycoRNAs; D Detection of glioma cell viability, adhesion, proliferation and apoptosis after treatment with either RNaseA alone or RNaseA plus RNasin.
A Schematic of the protocols for the detection of glycoRNAs by Northern blot. B RNA blotting of RNA from LN229, U87, and hela cells treated with Ac4ManNAz for 0 h and 24 h, respectively. strep: RNA-transferred membrane was treated with anti-biotin streptavitin-HRP and imaged. sybr: before RNA transfer to the membrane, the RNA in the gel was stained with SYBR Gold and imaged. C RNA blotting of Ac4ManNAz-labeled LN229, U87, and Hela cell RNA treated with DNase I or RNase A+/− RNasin (RNase inhibitor). D RNA blotting of total RNA, and large and small RNA fractions from Ac4ManNAz-labeled U87 and LN229 cells. The “total RNA” lane contained 25 µg of RNA, while the lanes for the “<200 bp” and the “>200 bp” represented the samples collected after the separation and purification of small and large RNAs, respectively, from the original 25 µg of the total RNA. For U87(E) and LN229 (F) derived samples, the RNA blotting of Ac4ManNAz-labeled RNAs treated with different enzymes, respectively.
Analysis of the abundant glycoRNAs in glioma cells
Subsequently, we sought to analyze the composition of glycoRNAs enriched in glioma cells. Through a series of processes including Ac4ManNAz labeling, small RNA extraction, DBCO-biotin treatment, purification by streptavidin magnetic beads and Zymo column, glycoRNAs from U87, LN229 and Hela cells were purified and used for small RNA deep sequencing analysis (Fig. 3A). The efficacy of the streptavidin magnetic beads in enriching glycoRNAs was verified (Supplementary Fig. S2). RNA samples from U87, LN229 and Hela cells treated with Ac4ManNAz were designated U87-24h, LN229-24h and Hela-24h, respectively. RNA samples from U87, LN229 and Hela cells without Ac4ManNAz treatment served as inputs and were designated U87-0h, LN229-0h and Hela-0h, respectively. The A260/A280 ratios of all glycoRNA samples ranged from 1.8 to 2.0, indicating high RNA purity. To assess potential protein contamination, we calculated the protein-to-RNA concentration ratio in the experimental groups (U87-24h, LN229-24h, and HeLa-24h). The results revealed average ratios of 5.38e–05, 3.96e–05, and 6.76e–05, respectively (Supplementary Fig. S3), indicating negligible protein contamination in the purified glycoRNA samples. The analysis of small RNA expression revealed significant differences in the expression profiles among the three cell types (Fig. 3B). Of particular interest were the RNAs exhibiting high expression in U87-24h and LN229-24h and low expression in U87-0h, LN229-0h, Hela-24h, and Hela-0h. Several small nuclear RNAs (snRNAs), including U2, U4, U1 and Y5, caught our attention: U2 and U4 were enriched in U87-24h and LN229-24h, whereas they were barely detectable in Hela-24h, Hela-0h, U87-0h, and LN229-0h. U1 and Y5 (a member of Y RNA family) were enriched in Hela-24h, U87-24h and LN229-24h, but were lower in Hela-0h, U87-0h and LN229-0h (Fig. 3B). These RNAs may be candidate RNAs for subsequent studies. Then the upregulated RNAs in the 24 h groups of the three cell lines were screened by using the 0 h groups as the input groups, with the following principles employed in the screening process: (1). P < 0.05, log2FC > 1, and UMI (0 h) < 100; or (2). P < 0.05, UMI (24 h) > 0, and UMI (0 h) = 0. The screened RNAs were considered to be glycoRNAs detected in three cell types, excluding non-specific RNAs. The number of glycoRNAs detected in U87, LN229, and Hela was 40, 24, and 39, respectively. An intersection of the RNAs detected in the three cell types was plotted on an upset plot (Fig. 3C). The number of intersecting RNAs between U87 and LN229, between U87 and Hela, and between LN229 and Hela was 11, 13, and 9, respectively (Fig. 3C). It was evident that the types of glycoRNAs exhibited considerable variation between the three cell types, including the two glioma cell lines U87 and LN229. Through the classification and enumeration of the detected RNAs across the three cell types, a diverse array of small RNA species was identified, including small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), ribosomal RNAs (rRNAs), Y RNAs, and small Cajal body-specific RNAs (scaRNAs), etc (Fig. 3D). Among these RNAs, some were enriched in both U87 and LN229, including U7, U1, U2, U3, U4, U6, Y5, 7SK, snoU13 and Metazoa_SRP (Fig. 4A). Of these ten types of RNA, several, including Y5, 7SK, U1, U7, U3, U6 and Metazoa_SRP were also enriched in Hela (Supplementary Fig. S4). We selected U2 and U4 as candidate RNAs because of their enrichment in U87 and LN229, but lack of enrichment in Hela. Conversely, Y5 and U1 were selected as positive control RNAs due to their enrichment in all three cell types.
A Flow plot showing the process of glycoRNA isolation and purification before sequencing. B Heatmap showing the small RNA expression profiles of six groups, including Hela-0h, Hela-24h, U87-0h, U87-24h, LN229-0h, and LN229-24h. Δ: The RNAs enriched in Hela-24h, U87-24h and LN229-24h, but lower in Hela-0h, U87-0h and LN229-0h. *: The RNAs enriched in U87-24h and LN229-24h, but barely detectable in Hela-24h, Hela-0h, U87-0h, and LN229-0h. C Upset plot showing the number of detected small RNAs in Hela, U87 and LN229 cells, and the number of intersecting RNAs. D Sector graphs showing the proportions of the different RNA species detected in the three cell types. Other: small RNAs that do not fall into the major categories of snoRNAs, snRNAs, rRNAs, etc.
A Scatterplot showing the enriched glycoRNAs in the U87 and LN229 groups. The vertical axis: U87, log2FC. The horizontal axis: LN229, log2FC. The size and color of the scatters were used to represent the -log10(Pvalue) of each RNA in the LN229 and U87 groups, respectively. B qRT-PCR analysis showing the expression levels of glycoRNA U1, U2, U4, and Y5 in glioma cells including U87 and LN229, and non-glioma cells, such as Hela, MCF7 and HEK-293T. 0 h represents the control group that did not undergo Ac4ManNAz labeling but went through a series of extraction and purification processes of glycoRNAs. While the 24 h group represents the products of cells after the entire process of Ac4ManNAz labeling and extraction and purification of glycoRNAs, i.e. glycoRNAs. *p < 0.05; **p < 0.01; ***p < 0.001; ns p > 0.05.
Next, the glycoRNAs were separated and the levels of glycoRNA U1, U2, U4 and Y5 were validated by qRT-PCR in several cell lines including U87, LN229, Hela, MCF7 and HEK-293T. The qRT-PCR results demonstrated that glycoRNA U2 was enriched in U87 and LN229 cells, but not in Hela, MCF7 and HEK-293T cells (p < 0.05 for U87, p < 0.01 for LN229, p > 0.05 for Hela, MCF7 and HEK-293T; Fig. 4B). GlycoRNA U4 was found to be enriched in U87, LN229 and Hela cells, but not in MCF7 and HEK-293T cells (p < 0.01 for U87, p < 0.05 for LN229, p < 0.01 for Hela, p > 0.05 for MCF7 and HEK-293T; Fig. 4B). GlycoRNA Y5 was found to be enriched in all five cell types (p < 0.001 for U87, p < 0.01 for LN229, p < 0.05 for Hela, p < 0.05 for MCF7 and p < 0.01 for HEK-293T; Fig. 4B). GlycoRNA U1 was found to be enriched in U87, LN229 and Hela cells, but not in MCF7 and HEK-293T cells (p < 0.05 for U87, p < 0.001 for LN229, p < 0.05 for Hela, p > 0.05 for MCF7 and HEK-293T; Fig. 4B). A combination of the RNA sequencing results led to the identification of U2 and U4 as potential glioma-related glycoRNAs for further investigation, with Y5 designated as a positive control glycoRNA. Both U2 and U4 are members of the snRNA family. snRNAs are classified into seven categories, including U1-U7, which are the primary components of the RNA spliceosome during the post-transcriptional processing in eukaryotes and are involved in the processing of mRNA precursors.
Identification of the glycans present on the U2 and U4 glycoRNAs
In order to gain insight into the composition of glycoRNAs U2 and U4, it was necessary to further identify the glycans present on them. In this study, a sequence-specific RNA-capture magnetic bead system was developed, comprising carboxyl-derivatized magnetic beads and amino-oligos. This system was designed to enrich specific RNA species, including U2 and U4. The amino-oligos were amino-linked oligonucleotide sequences that were specifically targeting a specific RNA segment. The specific probe sequences for U2 and U4 were shown in Table 1. The efficiency of the sequence-specific RNA capture magnetic beads was validated and the results were shown in Supplementary Fig. S5.
The enriched glycoRNA U2 or U4 was subjected to a series of processes such as PNGaseF treatment and purification on PGC-SPE columns to cleave, release and purify the contained glycans (Fig. 5A). The glycan samples were detected and analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). There are 6 groups of glycan samples, including glycans cleaved from U2, U4 and total RNA of U87 cells (U87-U2, U87-U4, U87-Total), and glycans cleaved from U2, U4 and total RNA of LN229 cells (LN229-U2, LN229-U4, LN229-Total). The total groups (U87-Total and LN229-Total) represented the glycans from the total glycoRNAs that were not enriched by the sequence-specific RNA capture magnetic bead system. The unsupervised clustering analysis of glycans revealed notable differences in the clustering of glycans from U87-U2 and LN229-U2, as well as in the clustering of glycans from U87-U4 and LN229-U4 (Fig. 5B). Next, the glycans derived from LN229 and U87 cells were classified and the percentages were calculated. The glycans detected included tri-antennary, tetra-antennary, and bi-antennary types, with no statistically significant differences observed in the proportions of these glycan types between the two cell lines (Fig. 5C). However, a higher percentage of tri-fucosylated glycans was detected in U87 cells compared to LN229 cells (Fig. 5D). It was noteworthy that there were significant discrepancies in proportions of glycans exhibiting varying degrees of sialylation, as observed in the U87 and LN229 samples (Fig. 5E). Furthermore, a greater proportion of hybrid glycans and a lesser proportion of complex glycans were identified in U87 samples in comparison to LN229 samples (Fig. 5F). The findings indicated that the two glioma cell lines, U87 and LN229, exhibited distinct glycan compositions. The intersections of the glycans from the total, U2, and U4 samples were then analyzed. There was a notable difference in the glycan types detected in the U2, U4 and total samples, whether derived from U87 or LN229 (Fig. 5G, H). Among the glycans detected in the samples from U87, the most common glycoforms or modifications were complex, sialylation and fucosylation (Fig. 5I–K). For the glycans detected in the samples from LN229, the most common glycoforms or modifications were fucosylation, sialylation, and complex (Fig. 5L–N). In the glycans of the U87-Total, U87-U2 and U87-U4 groups, complex glycans accounted for 93.1%, 88.7% and 92.0%, respectively (Fig. 5O–Q). While in the glycans of the LN229-Total, LN229-U2 and LN229-U4 groups, complex glycans accounted for 57.7%, 77.8% and 82.7%, respectively (Fig. 5R–T). Complex glycans have more structural changes than hybrid glycans, thus the glycan samples derived from U87 appeared to have more structural variation than those derived from LN229. In addition, the majority of the detected glycans in each sample were sialylated. The Sankey diagram analysis categorized the detected glycans into six distinct categories, namely core fucose, branch & terminal fucose, non-fucose, core & branch fucose, branch fucose, and core & branch & terminal fucose (Fig. 6A, B). The majority of these glycans were non-bisecting and sialylated (Fig. 6A, B). Subsequently, the glycans detected in each sample were ranked based on their relative abundance. The glycan with the highest relative abundance in each group was N5H6F0S3 (U87-Total), N4H5F1S0 (U87-U2), N5H6F1S2 (U87-U4), N5H6F2S2 (LN229-Total), N4H5F1S2 (LN229-U2), and N4H5F1S2 (LN229-U4), respectively (Fig. 6C–H). It is evident that multiple glycan types were present on U2 or U4.
A Flow plot showing the isolation, purification, and analysis process of the glycans on the glycoRNAs. B Unsupervised clustering analysis of glycans released from glycoRNAs of U87-U2, U87-U4, U87-Total, LN229-U2, LN229-U4, and LN229-Total groups. C Relative abundance of glycans with different numbers of antennae in LN229 and U87. D Relative abundance of glycans with different degrees of fucosylation in LN229 and U87. E Relative abundance of glycans with different degrees of sialylation in LN229 and U87. F Relative abundance of complex and hybrid glycans in LN229 and U87. G Venn diagram showing the number of glycans detected in the U87-Total, U87-U2, and U87-U4 groups, and the number of intersecting glycans. H Venn diagram showing the number of glycans detected in the LN229-Total, LN229-U2, and LN229-U4 groups, and the number of intersecting glycans. Bar charts showing the number of different glycan types in the U87-Total (I), U87-U2 (J), U87-U4 (K), LN229-Total (L), LN229-U2 (M), and LN229-U4 (N) groups. Sector graphs showing the percentages of complex and hybrid glycans in the U87-Total (O), U87-U2 (P), U87-U4 (Q), LN229-Total (R), LN229-U2 (S), and LN229-U4 (T) groups.
A Sankey plot showing the specific classification of the glycans detected in the U87-Total, U87-U2, and U87-U4 groups. B Sankey plot showing the specific classification of the glycans detected in the LN229-Total, LN229-U2, and LN229-U4 groups. C–H The glycans detected in each group were ranked based on their relative abundance. Bar charts showed the top 20 detected glycans in the U87-Total (C), U87-U2 (D), U87-U4 (E), LN229-Total (F), LN229-U2 (G), and LN229-U4 (H) groups. The top-ranking glycan in each group was N5H6F0S3 (U87-Total), N4H5F1S0 (U87-U2), N5H6F1S2 (U87-U4), N5H6F2S2 (LN229-Total), N4H5F1S2 (LN229-U2), and N4H5F1S2 (LN229-U4), respectively. N N-acetylhexosamines, H hexoses, F fucose residues, S sialic acids. e.g., N5H6F0S3 represents a glycan with 5 N-acetylhexosamines, 6 hexoses, 0 fucose residues, and 3 sialic acids.
Removal of total glycoRNAs inhibited the proliferation of U87 and LN229 cells
We then investigated the role of the glycoRNAs in glioma cell viability, adhesion, and proliferation. The basis for studying their functions is that they can be knocked down or knocked out. However, as there are currently no specific inhibitors for glycoRNAs, we removed the total glycoRNAs from the cell surface through RNase A treatment. The experimental design and procedures were shown in Fig. 7A. The RNaseA-FITC imaging showed that the FITC fluorescence was localized outside the CellMask fluorescence at the treatment endpoint (30 min), demonstrating that the FITC-labeled RNaseA showed minimal cellular internalization (Fig. 7B). GlycoRNA signals were nearly undetectable in RNaseA-treated (30 min) U87 and LN229 cell-derived RNA samples compared to control groups (Fig. 7C). However, glycoRNA signals were better preserved in the RNaseA+RNasin-treated group than in the RNaseA-treated group (Fig. 7C). Furthermore, glycoRNA signals remained undetectable in RNA samples from treated U87 and LN229 cells, which were treated with RNaseA for 30 min and cultured in complete medium for 12 h (Fig. 7D).
A Flow plot showing the procedures of glycoRNA depletion experiments. B Fluorescence imaging showing the distribution of FITC-labeled RNaseA following 30 min of incubation. C, D RNA blotting showing the biotin signals in RNA samples isolated from treated U87 and LN229 cells. Treatment conditions were: C 30-min exposure to RNaseA alone or RNaseA plus RNasin; D treatment as in C followed by 12-h culture in complete medium. strep: RNA-transferred membrane was treated with anti-biotin streptavitin-HRP and imaged. sybr: before RNA transfer to the membrane, the RNA in the gel was stained with SYBR Gold and imaged. CCK-8 analysis showing the cell viability of U87 (E) and LN229 (F) cells treated with either RNaseA alone or RNaseA plus RNasin for 30 min, followed by 12-h culture in complete medium. Cell adhesion analysis on U87 (G) and LN229 (H) cells treated with either RNaseA alone or RNaseA plus RNasin for 30 min, followed by 12-h culture in complete medium. ****p < 0.0001; ns: p > 0.05.
The Cell Counting Kit-8 (CCK-8) assay demonstrated that RNaseA treatment significantly impaired viability of U87 cells (p < 0.0001, Fig. 7E). However, the cytotoxic effect of RNaseA was significantly attenuated by RNasin co-treatment (p < 0.0001, Fig. 7E). Similarly, the cell viability of RNaseA-treated LN229 cells was significantly lower than that of the control or RNaseA+RNasin-treated LN229 cells (p < 0.0001; p < 0.0001, Fig. 7F).
Cell adhesion assays showed comparable adhesion rates across control, RNaseA-treated, and RNaseA+RNasin-treated groups (p > 0.05, Fig. 7G, H), indicating that RNaseA treatment did not significantly alter glioma cell adhesion.
Ki67 is a nuclear antigen that serves to identify the state of cell proliferation. It is predominantly expressed in cells that are in the G1, S, and G2 phases of the cell cycle, as well as in cells undergoing interphase mitosis. However, it is not expressed in cells that are in the G0 phase, which refers to resting cells. Given its function is closely related to cell proliferation, ki67 is frequently employed for the detection of tumor growth indices. The ki67 immunofluorescence assay revealed that RNaseA treatment significantly reduced the percentage of ki67-positive cells in both U87 (p < 0.0001) and LN229 (p < 0.0001) cell lines compared to controls (Fig. 8A–C). In contrast, RNaseA+RNasin co-treatment showed no significant effect on ki67 positivity relative to control cells (p > 0.05, Fig. 8A–C).
A Immunofluorescence images showing the ki67 and dapi expression in U87 and LN229 cells treated with either RNaseA alone or RNaseA plus RNasin for 30 min, followed by 12-h culture in complete medium. Box diagrams showing the percentage of ki67-positive U87 (B) and LN229 (C) cells with RNaseA or RNaseA+RNasin treatment. D Immunofluorescence images showing the TUNEL and dapi expression in U87 and LN229 cells treated with either RNaseA alone or RNaseA plus RNasin for 30 min, followed by 12-h culture in complete medium. Box diagrams showing the percentage of TUNEL-positive U87 (E) and LN229 (F) cells with RNaseA or RNaseA+RNasin treatment. ****p < 0.0001; ns: p > 0.05.
The Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay is a widely used method for detecting DNA fragmentation, a hallmark of programmed cell death (apoptosis). This technique labels the free 3’-hydroxyl termini of fragmented DNA with fluorescent or chromogenic dUTP nucleotides, allowing visualization and quantification of apoptotic cells in tissues or cell cultures. TUNEL assays revealed no significant differences in the percentages of TUNEL-positive cells among control, RNaseA-treated, and RNaseA+RNasin-treated groups in either U87 or LN229 cells (p > 0.05, Fig. 8D–F).
Collectively, our findings demonstrated that glycoRNA depletion at the observed time point significantly inhibited glioma cell viability and proliferation, without altering cell adhesion or apoptosis levels. However, the specific molecular mechanisms by which glycoRNAs affect glioma proliferation remain unclear.
Material and method
Labeling of glycoRNAs
Ac4ManNAz (MedChemExpress, HY-W728531) was dissolved into 500 mM and then diluted to 100 mM with DMSO. GalNAc and Gal were dissolved in sterile water to 500 mM and 50 mM, respectively, and diluted in sterile water to 100 mM and 10 mM. U87, LN229, or Hela cells (Pricella, authenticated by STR profiling) were treated with Ac4ManNAz at a final concentration of 100 μM for 0 and 24 h, and then were harvested for RNA extraction. Gal (Sigma, G0750, 10 μM, 1:1000 dilution) and GalNAc (Sigma, A2795, 100 μM, 1:1000 dilution) were used as medium additive and added simultaneously with Ac4ManNAz.
Extraction of total RNAs
Total RNA was extracted by Trizol method. The cells were collected, lyzed and mixed in TRIzol, and incubated at 37 °C for 10 min. Then 0.2 times the volume of chloroform was added to extract the RNA. After vortex mixing and centrifugation at 4 °C, the supernatant was transferred to a new tube and mixed with anhydrous ethanol. Above solution containing total RNA was added to the Zymo RNA purification and concentration columns (Zymo Research, 1017) and centrifuged at 10,000 × g for 20 s. The precipitation was washed with preparation buffer and washing buffer, and then centrifuged at 10,000 × g for 20 s. The purified total RNA was eluted with pure water.
DBCO-biotin treatment of RNAs
Protease K was added into the total RNA. After incubation at 37 °C for 45 min, RNA was purified with Zymo columns. The purified RNA was mixed with 1x volume of gel-loaded buffer II (df GLBII, formulated with 95% formamide, 18 mM EDTA, and 0.025%SDS) and 500 μM DBCO-biotin. DBCO-biotin was used to bind to Ac4ManNAz-labeled glycoRNAs. After incubation at 55 °C for 10 min, 80 μL water, 2× volume of RNA binding buffer and 3x volume of 100% ethanol was added to stop the reaction. Finally, Zymo columns were used to purified the RNAs.
Zymo column purification
Samples were mixed with two volumes of RNA Binding Buffer. An equal volume of ethanol2 (95–100%) was added to the samples, followed by vortex mixing and a brief incubation on ice. Samples were transferred to the Zymo-Spin™ IICR Column3 in collection tubes and were centrifuged. Four separate steps were adopted for cleaning: First, columns were cleaned with 400 μL of RNA Prep Buffer and centrifuged at 12,000 ×g for 30 s. Secondly, columns were washed with 80% ethanol (700 μL) and centrifuged at 12,000 × g for 30 s. Thirdly, columns were washed with 80% ethanol (400 μL) and centrifuged at 12,000 × g for 1 min. Finally, samples were eluted twice with 50 μL of ultrapure water.
Enzymatic treatment of RNAs
To digest RNA the following was used: 1 μL of RNase cocktail (0.5 U/μL RNaseA and 20 U/μL RNaseT1, Thermo Fisher Scientific) with 20 mM Tris-HCl (pH 8.0), 100 mM KCl and 0.1 mM MgCl2. To block the RNase activity of the RNase Cocktail, 1 μL of RNase Cocktail was pre-mixed with 8 μL of SUPERase•In™ RNase Inhibitor (20 U/μL, Thermo Fisher Scientific, AM2696) for 15 min at 25 °C before adding to the RNA solution. To digest DNA, 2 μL of TURBO DNase (2 U/μL, Thermo Fisher Scientific) with 1x TURBO DNase buffer (composition not provided by manufacture) was used. Following enzymatic treatment of the RNA, Zymo columns were used to purified the RNAs as described previously.
Identification of glycoRNAs
Visulation of glycoRNAs
3× volume of Formaldehyde Load Dye and 0.1x volume of 10x SYBR Gold nucleic acid gel dye was added to the solution containing biotinylated glycoRNAs. The glycoRNAs were denatured by incubation at 65 °C for 15 min and then left on ice. In order to remove RNA enzymes, all instruments required for electrophoresis and membrane transfer were sprayed with RNaseZap and washed with RNase free water to remove foam. 1% agarose form-aldehyde denatured gel was prepared. When it cooled to about 55 °C, 18 mL formaldehyde and 10 μL 10000X SYBR Gold nucleic acid gel dye was added into it. 1X MOPS gel flow buffer was used to cover the gel. The samples were added to 1% agarose formaldehyde denaturing gel and subjected to electrophoresis at 110 V for 45 min. Before RNA transfer to the membrane, total RNA in the gel was imaged. Membrane transfer was performed according to the NorthernMax™ kit operating instructions. Following irradiation by ultraviolet, the NC membrane was soaked in Odyssey Blocking Buffer added with streptavitin-HRP at 25 °C for 30 min. After washing the membrane with TBST for 3 times, imaging analysis was performed using Tanon 5200 series automatic chemi-luminescence image analysis system.
Small and large RNAs from Ac4ManNAz-treated U87 and LN229 cells were isolated using the RNAzol Isolation Kit, according to the kit instruction. The small and large RNAs were then subjected to denaturing gel electrophoresis and Nothern blot according to the methods similar to visualization of total glycoRNAs.
Enrichment of glycoRNAs for sequencing
Small RNAs were extracted from Ac4ManNAz-labeled U87, LN229, and hela cells by using RNAzol RT kit (Molecular Research Center, RN-190). The extracted small RNAs were treated with DBCO-biotin and then purified through Zymo columns. Initially, 10 μL of MyOne C1 streptavidin-coated beads (Thermo Fisher Scientific, REF: 65001) were added to 500 μL of Biotin Wash Buffer and incubated at 25 °C for 1 h to facilitate blocking of the beads. Following this step, the mixture was centrifuged at 3000 rpm for 2 min at 4 °C. The beads were then resuspended in 500 μL of Biotin Wash Buffer, and small RNAs were added to enrich for glycoRNAs, with the mixture rotated for 2 h at 4 °C. Subsequently, the beads bound with glycosylated RNA were resuspended in a solution containing 0.75 M NaCl, 10 mM EDTA, and 10 mM Tris-Cl (pH 7.4) and incubated at 22 °C for 5 min. To remove unbound RNA, the beads underwent a series of washes: twice with 1 mL of ChIRP wash buffer (2× SSC, 0.5% SDS), twice with 1 mL of Biotin Wash Buffer, and twice with NT2 Buffer (50 mM Tris HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.005% NP-40), all conducted at 25 °C for 3 min each. The glycoRNAs were eluted by mixing the beads with an equal volume of 5% β-mercaptoethanol (BME) solution and incubating at 22 °C for 5 min before centrifugation at 3000 rpm for 2 min. The resulting supernatant, enriched for glycoRNA, was collected and purified using a Zymo column for subsequent RNA sequencing analysis.
Protein detection on glycoRNA samples
To determine whether the glycoRNA samples were contaminated with proteins, we performed trace protein detection assay using the NanoOrange™ Protein Quantitation Kit (ThermoFisher, N6666). The NanoOrange™ assay is capable of detecting protein concentrations ranging from 10 ng/mL to 10 μg/mL. The specific procedure was performed following the manufacturer’s protocol. Specifically, the experimental procedure involved first preparing a 1X working solution by diluting the 500X NanoOrange™ reagent 1:500 with a 1X diluent (itself prepared by diluting the 10X stock 1:10 with water). A BSA standard curve was then generated by serially diluting the 2 mg/mL BSA stock to concentrations ranging from 0.01 to 10 µg/mL in the working solution. Samples and standards were incubated at 90–96 °C for 10 min, cooled to room temperature for 20 min, and protected from light. Fluorescence was measured at 485/590 nm (excitation/emission) using a fluorescence spectrophotometer (HITACHI, F-7000), with the reagent blank value subtracted for correction. The standard curve (fluorescence vs. concentration) was used to quantify protein in experimental samples. The RNA concentration of samples was determined using Qubit RNA XR Assay Kit (ThermoFisher, Q33223) and Qubit 3.0 Fluorometer (ThermoFisher, 033216). The detection was performed following the manufacturer’s protocol. Finally, the concentration ratio of protein to RNA was calculated.
Library construction and illumina sequencing of glycoRNAs
The library construction and illumina sequencing of glycoRNAs was conducted at Shanghai Outdo Biotech Co., Ltd., Shanghai, China. RNA quality was checked using Agilent 4200 TapeStation (Agilent technologies, Santa Clara, CA, US) according to standard procedures. Sequencing libraries were constructed by QIAseq miRNA Library Kit for Illumina (Cat #331505, Qiagen) according to manufacturer’s instructions. The sequencing was performed on an Illumina NovaSeq platform (Illumina, San Diego, CA, USA).
Sequencing data analysis
Raw data were processed with Cutadapt. In this step, the raw data were cleaned by removing adapters, short reads, and low-quality reads. Additionally, the Q30, and GC content were calculated to estimate the quality of clean reads. Next, the clean reads were aligned to the reference Database of miRBase22.1, piRNABank and RfamV14.1 using bowtie-1.3.0. The UMI counts of small RNA were calculated by perl. And the expression of small RNA was normalized to CPM (Descriptive indicators of gene expression levels). Data from three replicates were condensed by calculating the mean expression values for each RNA species. CPM refers to the number of miRNA, piRNA or other small RNA counts-per-million.
qRT-PCR analysis
Poly-A tailed qRT-PCR was applied to verify the results of RNA sequencing analysis. Tailed reverse transcription of small RNAs was performed using miRNA First-Strand cDNA Synthesis SuperMix kit (TransScript®, REF: AT351-01). After the construction of reverse transcription system according to the kit instructions, the reaction system was gently mixed and put into the reverse transcription apparatus, incubated at 37 °C for 1 h, heated at 85 °C for 5 s, and preserved at 4 °C. Then the qRT-PCR was performed as follows: ① 95 °C 30 s, 1 cycle; ② 95 °C 5 s, 60 °C 30 s, 45 cycles; ③ 65–95 °C, 1 cycle. RNA primer sequences used in this study were listed in Supplementary Table S1.
Enrichment of glycoRNAs U2, U4, and Y5
Through the binding of carboxyl derivatized beads and amino derivatives linked with specific nucleotide sequences (amino-oligos), glycoRNAs U2, U4, and Y5 were enriched. First, the MagnaBind™ carboxyl derivatized beads (Thermo Fisher Scientific, REF: 21353) were washed with reaction buffer (100 mM MES, PH = 4.5) for 3 times and separated by magnetic absorption. Amino-oligos dissolved in reaction buffer were mixed with washed carboxyl derivatized beads, vortexed at room temperature for 30 min, followed by addition of 1-(3-Dimethyl-aminopropyl)-3-ethylcarbodiimide solution (EDC, 100 mg/mL) into above mixture. Then the mixture was vortexed at room temperature for 12 h, and the beads binding to amino-oligos were separated through magnetic absorption and washed with washing buffer (1 × PBS, PH = 7.4). Second, the glycoRNAs U2, U4, and Y5 were enriched by the beads binding to amino-oligos. Specifically, the purified glycoRNA samples were denatured by incubating at 65 °C for 10 min in a vertical mixer. Then the beads binding to amino-oligos were added to above denatured samples, and the samples were hybridized at 37 °C for 30 min, incubated at 50 °C for 5 min, and hybridized at 37 °C for 180 min. The beads were separated, washed by shaking at 37 °C four times with washing buffer, and separated again by magnetic absorption. Third, the glycoRNAs U2, U4, and Y5 were eluted from beads. The samples were added with 50 μL RNA Elution Buffer and heated at 95 °C for 2 min. The supernatant and 400 μL binding buffer were added into RNA purification columns and centrifugated at 10000 g for 20 s. The precipitation was washed once with 400 μL prep buffer, twice with 400 μL washing buffer, and collected by EP tubes after centrifugation. The precipitation was enriched individual glycoRNA U2, U4, and Y5, respectively.
Identification and analysis of glycans in glycoRNAs
Firstly, the glycans were cut and released from glycoRNAs. glycoRNAs were diluted with RNA free water and added with 10 μL PNGaseF (New England Biolabs, P0704S) and 10 μL 10x GlycoBuffer 2 to a final volume of 100 μL. The mixture was incubated at 37 °C for 18 h. Secondly, the released glycans were purified. Each PGC-SPE purification column was pretreated with reagents in the following order: 15 mL 1 M NaOH, 15 mL HPLC-grade water, 15 mL 30% acetic acid, and 15 mL HPLC-grade water. Next, the purification columns were washed with 15 mL 50% acetonitrile/0.1% trifluoroacetic acid (TFA), and then washed with 15 mL 5% acetonitrile/0.1% trifluoroacetic acid. Then samples were suspended in water and purified using the pretreated PGC-SPE purification columns for two times. Then the samples were immediately freezed on dry ice until freeze-dried (16–24 h). After lyophilization, the sample was dissolved in 1 mL water, dried again in 1.5 mL EP tube, and stored at −20 °C. Thirdly, glycans in glycoRNAs were identified and analyzed using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). The glycan solution was dried in a vacuum concentrator and redissolved in pure water. CH3I and DMSO were added to the solution, and the NaOH micro spiral column was used for full methylation. The methylated glycans were extracted with chloroform and washed with water several times. Then the chloroform layer was collected and vacuum concentrated until dry. The separation parameters for LC were set as follows: analysis column: 360 μod × 75 μid, 75 cm; Filler: Phenomenex Jupiter C18, 5 μm, 300 Å; Isolation column: 360 μod × 200 μid, 5 cm; Filler: Phenomenex Jupiter C18, 5 μm, 300 Å; Mobile phase: Buffer A is a mixture of 99.9% H2O and 0.1% FA, and Buffer B is a mixture of 99.9% ACN and 0.1% FA. The parameters for Tandem Mass Spectrometry were as follows: the quality resolution: 22.5 k, the Data-Dependent-Acquisition: top 20, the control target value of automatic gain: 1 × 105, the maximum ion implantation time: 200 ms, the isolation window of parent ion: 3 m/z, the dynamic exclusion: 50.0 s, and the stepped NCEs: 10%. Based on the LC-MS/MS data, Glyseeker software was used for library identification (FDR ≤ 1%).
Enzymatic treatment of extracellular RNA
To digest the extracellular RNA on the cell surface, the wall-adherent U87 and LN229 cells were treated with RNase A (Thermo Fisher Scientific, EN0531) in serum-free medium at a concentration of 0.1 mg/mL at 37 °C for 30 min. To block the RNase A activity, 1 μg of RNase A was pre-incubated with 10 μL of RNase Inhibitor (40 U/μL, NEB, M0314S) at 25 °C for 30 min before adding to the serum-free culture medium. The cells were treated with RNaseA+RNasin at 37 °C for 30 min. The cells of control group were treated with serum-free medium at 37 °C for 30 min. The culture supernatant was first aspirated, and the cells were washed five times with PBS. Following this, they were cultured in complete medium for 12 h prior to performing cell viability, adhesion, proliferation, and apoptosis assays. Denaturing PAGE was performed to evaluate the level of glycoRNAs.
RNase A localization imaging
Cells were treated with FITC-labeled RNase A (RNaseA-FITC) and incubated at 37 °C for 30 min. After removal of supernatant and 5 washes with PBS, the cells were incubated in the cell membrane staining solution (CellMask™ Deep Red Plasma Membrane Stain, Thermo Fisher Scientific, C10046) at 37 °C for 10 min. Subsequently, the cells were washed again with PBS and fixed with 4% paraformaldehyde. The cells were washed three times with PBS. Finally, the cells were observed with a fluorescence microscope (Olympus, Tokyo, Japan) to determine the localization of RNaseA-FITC.
Cell viability, proliferation and apoptosis assays
CCK-8, ki67 and TUNEL assays were performed to detect the viability, proliferation and apoptosis of U87 and LN229. For the CCK-8 assay, 10 µl of CCK-8 reagent was added and the cells were incubated at 37 °C for 2 h. Finally, the optical density at 450 nm (OD450) was measured using a microplate reader. For the ki67 staining assay, cells were fixed with 4% paraformaldehyde at 25 °C for 30 min, penetrated with 0.5% Triton X-100, and blocked with 5% BSA. The cells were then incubated with anti-ki-67 monoclonal antibody (ORIGENE, AM01167RP-N) for 12 h at 4 °C and AF488-conjugated goat anti-mouse secondary antibody (Thermo Fisher, A-11001) at 25 °C for 1 h in sequence. The cell nuclei were stained with DAPI. The cells were then observed and photographed using a fluorescence microscope (Olympus, Tokyo, Japan). For the TUNEL assay, the cells were fixed with 4% paraformaldehyde at 25 °C for 30 min, penetrated with 0.5% Triton X-100. The cells were then incubated with TdT Equilibration Buffer (Elabscience® One-step TUNEL In Situ Apoptosis Kit, Elabscience, E-CK-A322) for 15 min at 37 °C and Labeling Solution at 37 °C for 1 h in sequence. The cells were washed three times with PBS, and the cell nuclei were subsequently stained with DAPI. Fluorescence microscopy was performed to visualize TUNEL-positive (apoptotic) cells.
Cell adhesion assay
An artificial substrate membrane gelatin solution (BeyoEmbryo™ 0.1% Gelatin Solution, Beyotime) was added to a 96-well plate and incubated at 37 °C for 2 h. The coating solution was aspirated, followed by the addition of 200 μL of 1% BSA, and the plate was incubated at 37 °C for 1 h. The well plate was then washed three times with serum-free DMEM medium to remove excess artificial basement membrane gelatin. Cells treated with RNase A were subsequently seeded into the 96-well plates and cultured at 37 °C for 1 h. Then 10 µl of CCK-8 reagent was added and the cells were incubated at 37 °C for 2 h. Finally, the optical density at 450 nm (OD450) was measured using a microplate reader. The cell adhesion rate was calculated as follows: [(Experimental group OD - Blank OD) / (Control group OD - Blank OD)] × 100%.
Discussion
The present study revealed the enrichment of glycoRNAs in glioma cell lines, which were predominantly small RNAs. Among the identified glycoRNAs, U2 and U4 were particularly abundant. Both U2 and U4 were covalently bound to a range of glycans, the majority of which were complex, fucosylated and sialylated. Furthermore, the removal of total glycoRNAs was observed to inhibit glioma cell proliferation. Overall, the findings indicated that glycoRNAs may play a significant role in glioma progression, and thus provided a foundation for the development of novel biomarkers and therapeutic targets for gliomas.
Glioma is the most prevalent primary intracranial tumor in adults, yet the precise aetiology remains uncertain. Given that early detection and treatment are key factors in the success of treatment, the discovery of early and effective diagnostic and therapeutic markers for glioma is of great clinical significance. There is increasing evidence that glycosylation alterations are common in glioma and are involved in a number of pathological physiological processes and signaling pathways in glioma [8, 11, 12]. N-glycosylation at Asn50 is essential for glioma cell proliferation and metastasis by affecting the folding and trafficking of SND1 [13]. Glycosylation-related genes have been linked to glioma cell growth and viability and have been associated with glioma prognosis [14]. Deficiency of a glycosylation enzyme (GnT-IX) inhibits glioma growth in vitro and in xenograft models by reducing PTPRZ protein levels [15]. A drug called penfluridol, which targets the MAN1A1 mannosidase involved in N-glycan maturation, has been shown to alter the glycosylation of PD-L1 and activate T-cell tumor immunity in glioma [16]. Despite the important role of glycosylation in glioma, the expression and function of glycoRNAs in glioma have not been studied.
Most glycoconjugates, including glycoproteins and glycolipids, are located on plasma membrane and play a key role in signaling transduction, cell–matrix interactions, and immunomodulation [17, 18]. Although glycoRNAs are recently discovered glycoconjugates, there is already evidence that glycoRNAs are located on the external surface of cells and bind to Siglec receptors such as Siglec-11 and Siglec-14 [1, 19]. Siglecs belong to the Ig superfamily (type I lectin) and are predominantly expressed on tumor cells and immune cells. Siglecs are involved in immune responses to tumors and microbial infections by recognizing glycans containing sialic acid. Many studies have shown that interfering with siglecs-sialic acid binding in cancer can prevent tumor cells, including glioma, from evading the immune system [20,21,22,23]. Therefore, we speculate that glycoRNAs may play an important role in establishing and maintaining the immune microenvironment of glioma.
Fucosylation is a type of glycosylation, which can affect a number of crucial biological processes, including protein stability, folding, localization, recognition and signal transduction. In the nervous system, fucosylation modification has been demonstrated to influence the development of neurons and synaptic plasticity [24]. In the context of tumorigenesis and tumor development, fucosylation-related enzymes and fucose-containing tumor antigens have been associated with the proliferation, invasion and metastasis of tumor cells [25]. It has been reported that the biantennary N-glycans with a core-fucosylated structure increased in glioblastoma tissue, and may represent a potential therapeutic target for glioblastoma [26]. In general, sialylation refers to the increase in sialic acid modification at the ends of glycoproteins on the surface of cell membranes mediated by sialic acid transferase. Aberrant sialylation has been identified as a cancer hallmark across many cancer types including lung, brain, and other cancers [12, 27]. The upregulation of sialylation has been demonstrated to play a role in the promotion of tumor growth, metastasis, immune evasion and drug resistance [28]. The concentration of serum sialic acid was confirmed as a reliable indicator for differentiating between benign and malignant intracranial tumors [29]. Increased sialylation on α2,3-linked glycoprotein has been observed in malignant gliomas [30]. Interestingly, one study has linked sialylated and fucosylated complex N-glycans with the malignant behavior of high-grade gliomas [31]. In this study, the glioma-related glycoRNAs U2 and U4 contained glycans that were predominantly complex, fucosylated and sialylated, suggesting that they may exert a pivotal influence on the malignant phenotype and progression of gliomas.
RNase A, a member of the ribonuclease superfamily, has garnered significant attention in cancer therapy research due to its ability to degrade RNA and induce cytotoxic effects in tumor cells [32, 33]. Its mechanism involves cleaving tRNA and rRNA, disrupting protein synthesis, and triggering cell death pathways. To enhance therapeutic efficacy, researchers have explored modifications such as PEGylation, hyaluronic acid, or conjugation with targeting ligands (e.g., antibodies or peptides) to improve stability, reduce immunogenicity, and increase tumor specificity [34,35,36]. Prior to the identification of glycoRNAs, RNA was generally not believed to be present on the extracellular surface of cell membranes. Consequently, earlier investigations into RNase A’s cellular effects primarily centered on its intracellular activity [37, 38]. In this study, to restrict RNase A activity to extracellular RNAs and prevent its internalization by glioma cells, we optimized the incubation time for RNase A treatment and validated its extracellular localization through fluorescent labeling. The extracellular localization of RNase A may be attributed to multiple biological and physicochemical factors: As a relatively large (13.7 kDa) and highly cationic protein, RNase A lacks inherent cell-penetrating capabilities and cannot passively diffuse across intact plasma membranes without specific transport mechanisms. Glioma cells, despite their malignant nature, may maintain sufficient membrane integrity to prevent nonspecific protein uptake during this relatively short incubation period. Additionally, the experimental conditions such as suboptimal concentration, absence of membrane permeabilizing agents, or unsuitable pH for endocytic uptake could collectively contribute to this extracellular restriction.
This study has several advantages and highlights. First, a sequence-specific RNA-capture magnetic bead system was developed for the enrichment of a specific glycoRNA. This system consists of carboxyl derivative magnetic beads and amino oligonucleotides, and was designed to enrich for specific RNA sequences such as U2, U4, and Y5. Amino oligonucleotides are amino-linked oligonucleotide sequences that specifically target specific RNA fragments, and the enrichment and precipitation of target RNAs can be achieved by the interaction of carboxyl and amino groups and the action of magnetic beads. Secondly, the current knowledge of glycoRNAs remains limited, with studies on the expression and function of glycoRNAs in gliomas being severely lacking. This study revealed the presence of multiple glycoRNAs in glioma cells, suggesting a potential association with cell proliferation. The findings provided a foundation for further research, particularly in exploring the potential significance of glycoRNAs in gliomas. Thirdly, the integration of RNA sequencing analysis, glycomics analysis and functional studies offered a reliable support for advancing in-depth research on glycoRNAs.
It should be noted that this study is not without limitations. First, although we demonstrated that total glycoRNAs play a vital role in glioma cell proliferation, the specific glycoRNA species that exert this effect remains to be identified. It is anticipated that this issue will be addressed in future research through the development of specific inhibitors against glycoRNAs. Secondly, it lacks verification in an orthotopic intracranial glioma mouse model, which will be improved in the next research. Thirdly, the underlying molecular mechanism of the effects of glycoRNAs on glioma cell viability and proliferation requires further investigation.
In conclusion, we found that glioma cells contained a large number and variety of glycoRNAs, which were mainly small RNAs. Each glycoRNA can bind various glycans, mainly through covalent and hydrogen bonds. The glycoRNAs U2 and U4, which were abundant in glioma cells U87 and LN229, contained mainly fucosylated, sialylated, and complex glycans. Furthermore, the removal of total glycoRNAs inhibited glioma cell viability and proliferation, without altering cell adhesion or apoptosis levels. This study highlighted an important role of glycoRNAs in glioma and laid the groundwork for further research into them as novel markers and therapeutic targets for glioma.
Data availability
The data presented in this study are openly available in Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/, reference number [GSE284296].
References
Flynn RA, Pedram K, Malaker SA, Batista PJ, Smith BAH, Johnson AG, et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. 2021;184:3109–24. e3122.
Zhang N, Tang W, Torres L, Wang X, Ajaj Y, Zhu L, et al. Cell surface RNAs control neutrophil recruitment. Cell. 2024;187:846–60. e817.
Huang R, Qian D, Hu M, Zhang X, Song J, Li L, et al. Association between human cytomegalovirus infection and histone acetylation level in various histological types of glioma. Oncol Lett. 2015;10:2812–20.
Louvel G, Metellus P, Noel G, Peeters S, Guyotat J, Duntze J, et al. Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother Oncol. 2016;118:9–15.
Kim S, Seo Y, Chowdhury T, Yu HJ, Lee CE, Kim KM, et al. Inhibition of MUC1 exerts cell-cycle arrest and telomerase suppression in glioblastoma cells. Sci Rep. 2020;10:18238.
Li W, Wu C, Yao Y, Dong B, Wei Z, Lv X, et al. MUC4 modulates human glioblastoma cell proliferation and invasion by upregulating EGFR expression. Neurosci Lett. 2014;566:82–87.
Zhou F, Cui C, Ge Y, Chen H, Li Q, Yang Z, et al. Alpha2,3-Sialylation regulates the stability of stem cell marker CD133. J Biochem. 2010;148:273–80.
Tondepu C, Karumbaiah L. Glycomaterials to investigate the functional role of aberrant glycosylation in glioblastoma. Adv Health Mater. 2022;11:e2101956.
Singh A, Mhlongo N, Soliman ME. Anti-cancer glycosidase inhibitors from natural products: a computational and molecular modelling perspective. Anticancer Agents Med Chem. 2015;15:933–46.
Zhang Y, Fan C, Zhang L, Ma X. Glycosylation-dependent antitumor therapeutic monoclonal antibodies. Prog Mol Biol Transl Sci. 2019;163:471–85.
Pace A, Scirocchi F, Napoletano C, Zizzari IG, D’Angelo L, Santoro A, et al. Glycan-lectin interactions as novel immunosuppression drivers in glioblastoma. Int J Mol Sci. 2022;23:6312.
Veillon L, Fakih C, Abou-El-Hassan H, Kobeissy F, Mechref Y. Glycosylation changes in brain cancer. ACS Chem Neurosci. 2018;9:51–72.
Zhou Y, Li Q, Zheng J, Lin N. N-Glycosylation on Asn50 of SND1 is required for glioma U87 cell proliferation and metastasis. J Immunol Res. 2022;2022:5239006.
Sun YF, Zhang LC, Niu RZ, Chen L, Xia QJ, Xiong LL, et al. Predictive potentials of glycosylation-related genes in glioma prognosis and their correlation with immune infiltration. Sci Rep. 2024;14:4478.
Nagai K, Muto Y, Miura S, Takahashi K, Naruse Y, Hiruta R, et al. Brain-specific glycosylation enzyme GnT-IX maintains levels of protein tyrosine phosphatase receptor PTPRZ, thereby mediating glioma growth. J Biol Chem. 2023;299:105128.
Xu W, Wang Y, Zhang N, Lin X, Zhu D, Shen C, et al. The antipsychotic drug penfluridol inhibits N-linked glycoprotein processing and enhances T-cell-mediated tumor immunity. Mol Cancer Ther. 2024;23:648–61.
Johannssen T, Lepenies B. Glycan-based cell targeting to modulate immune responses. Trends Biotechnol. 2017;35:334–46.
Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49.
Ma Y, Guo W, Mou Q, Shao X, Lyu M, Garcia V, et al. Spatial imaging of glycoRNA in single cells with ARPLA. Nat Biotechnol. 2024;42:608–16.
Hodgson K, Orozco-Moreno M, Goode EA, Fisher M, Garnham R, Beatson R, et al. Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone. EBioMedicine. 2024;104:105163.
Rodriguez E, Boelaars K, Brown K, Eveline Li RJ, Kruijssen L, Bruijns SCM, et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat Commun. 2021;12:1270.
Schmassmann P, Roux J, Buck A, Tatari N, Hogan S, Wang J, et al. Targeting the Siglec-sialic acid axis promotes antitumor immune responses in preclinical models of glioblastoma. Sci Transl Med. 2023;15:eadf5302.
Wang Y, Xu Z, Wu KL, Yu L, Wang C, Ding H, et al. Siglec-15/sialic acid axis as a central glyco-immune checkpoint in breast cancer bone metastasis. Proc Natl Acad Sci USA. 2024;121:e2312929121.
Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, et al. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci USA. 2006;103:21–26.
Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–46.
Tsuchiya N, Yamanaka R, Yajima N, Homma J, Sano M, Komata T, et al. Isolation and characterization of an N-linked oligosaccharide that is increased in glioblastoma tissue and cell lines. Int J Oncol. 2005;27:1231–9.
Pietrobono S, Stecca B. Aberrant sialylation in cancer: biomarker and potential target for therapeutic intervention?. Cancers. 2021;13:2014.
Pearce OM, Laubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111–28.
Marth E, Flaschka G, Stiegler S, Mose JR. Sialic acid as a marker for differentiation between benign and malignant intracranial tumors. Clin Chim Acta. 1988;176:251–7.
Yamamoto H, Saito T, Kaneko Y, Kersey D, Yong VW, Bremer EG, et al. alpha2,3-sialyltransferase mRNA and alpha2,3-linked glycoprotein sialylation are increased in malignant gliomas. Brain Res. 1997;755:175–9.
Cuello HA, Ferreira GM, Gulino CA, Toledo AG, Segatori VI, Gabri MR. Terminally sialylated and fucosylated complex N-glycans are involved in the malignant behavior of high-grade glioma. Oncotarget. 2020;11:4822–35.
Forouharmehr A, Nassiri M, Ghovvati Roudsari S, Javadmanesh A. Production and introduction of a novel immunotoxin based on engineered RNase A for inducing death to Her1-positive cell lines. J Cell Physiol. 2020;235:4679–87.
Patutina OA, Mironova NL, Ryabchikova EI, Popova NA, Nikolin VP, Kaledin VI, et al. Tumoricidal activity of RNase A and DNase I. Acta Nat. 2010;2:88–94.
Jafary B, Akbarzadeh-Khiavi M, Farzi-Khajeh H, Safary A, Adibkia K. EGFR-targeting RNase A-cetuximab antibody-drug conjugate induces ROS-mediated apoptosis to overcome drug resistance in KRAS mutant cancer cells. Sci Rep. 2025;15:1483.
Rutkoski TJ, Kink JA, Strong LE, Raines RT. Site-specific PEGylation endows a mammalian ribonuclease with antitumor activity. Cancer Biol Ther. 2011;12:208–14.
Wang X, Li Y, Li Q, Neufeld CI, Pouli D, Sun S, et al. Hyaluronic acid modification of RNase A and its intracellular delivery using lipid-like nanoparticles. J Control Rel. 2017;263:39–45.
Choi JH, Jang JY, Joung YK, Kwon MH, Park KD. Intracellular delivery and anti-cancer effect of self-assembled heparin-Pluronic nanogels with RNase A. J Control Rel. 2010;147:420–7.
Jia J, Zhang S, Wen K, Li Q. Nano-scaled zeolitic imidazole framework-8 as an efficient carrier for the intracellular delivery of RNase A in cancer treatment. Int J Nanomed. 2019;14:9971–81.
Acknowledgements
We would like to express our sincerest gratitude to Dr Ryan A. Flynn and other scholars for their invaluable contributions to the field of glycoRNAs, which have provided us with immense inspiration.
Funding
This study was supported by the National Natural Science Foundation of China [82203647], and Natural Science Foundation of Jilin Province [YDZJ202501ZYTS728].
Author information
Authors and Affiliations
Contributions
Benkai Xin and Jiajun Chen: Writing–original draft, Data curation, Methodology, Project administration, Software. Xin Hu, Jingtong Yang, Xiaoyu Wang, Ziqian Wang: Data curation, Investigation, Formal analysis. Youzhong: Supervision, Validation, Writing–review & editing. Lin Wang: Conceptualization, Supervision, Validation, Writing–review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xin, B., Chen, J., Hu, X. et al. GlycoRNAs are abundant in glioma and involved in glioma cell proliferation. Oncogenesis 14, 29 (2025). https://doi.org/10.1038/s41389-025-00570-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41389-025-00570-5