Abstract
Background
Approximately 5% of very premature infants delivered at less than 30 weeks’ gestation have systemic hypertension. In adult human and animal models, intermittent hypoxemia events are associated with systemic hypertension. In neonates, intermittent hypoxemia events are associated with adverse outcomes, but it is unknown if they are a risk factor for hypertension. We hypothesize that early intermittent hypoxemia events in very preterm neonates are associated with systemic hypertension at 34–36 weeks’ postmenstrual age.
Methods
Secondary analysis of a single-center cohort study of 164 infants, <31 weeks’ gestational age. Intermittent hypoxemia events were continuously recorded during the first 21 days of age.
Results
There was a significant association between the number of intermittent hypoxemia events (per 100) and systemic hypertension (OR (95% CI) = 1.08 (1.01–1.15)), and both the number of intermittent hypoxemia events (per 100 β (95% CI) = 0.22 (0.10–0.34)) and percent of time with hypoxemia (β (95% CI) = 0.10 (0.01–0.19)) and systolic blood pressure at 34–36 weeks’ postmenstrual age.
Conclusion
This study demonstrated a higher incidence of early intermittent hypoxemia events in preterm infants with hypertension. Decreasing intermittent hypoxemia during this critical period may reduce incidence of later vascular stress in this population.
Impact
-
Intermittent hypoxemia events are very common in premature infants and increased frequency of intermittent hypoxemia events is associated with morbidity.
-
Intermittent hypoxemia events in adult human as well as adult and neonatal animal models are associated with systemic hypertension.
-
This study demonstrated an association between early intermittent hypoxemia events and systemic hypertension in very preterm neonates, adding to the body of literature of possible morbidities caused by intermittent hypoxemia events.
-
This study addresses the common, though under-recognized, issue of neonatal hypertension, and suggests increased intermittent hypoxemia events may be contributory.
Similar content being viewed by others
Introduction
Systemic hypertension (HTN) of the neonate affects approximately 5% of premature infants delivered at less than 30 weeks’ gestational age (GA) at some point prior to their initial hospital discharge.1 Literature suggests a variety of risk factors for HTN in this population, such as the history of placement of an umbilical arterial catheter,2,3,4,5,6 acute kidney injury1,2,4,5,6 (AKI), and bronchopulmonary dysplasia2,3,4,5,6,7,8 (BPD). However, half of premature neonates with HTN have “unexplained HTN” with unclear or possibly multifactorial cause.6,8
In the premature neonatal population, there is a high frequency of intermittent hypoxemia (IH) events with an increase in events during the second to fourth week of age, followed by stabilization and gradual decrease.9,10 Proposed etiology includes immature respiratory control and movement, concomitant with decreased oxygen stores in the setting of decreased functional residual capacity.11 Most IH events peak in the first month of age and decline between 36 and 44 weeks’ postmenstrual age (PMA).10 Studies have demonstrated an association between IH events and a range of poor outcomes, including retinopathy of prematurity,12,13 BPD,14,15 unfavorable respiratory outcome at 40 weeks’ PMA,16 prolonged hospitalization,17 and cognitive or language impairment at 18 months of age.13
The association between IH and HTN has been well-documented in adult humans,18 which has been corroborated by numerous murine models implementing chronic IH to elicit HTN.19,20,21 Similarly, a direct link between chronic IH and elevated systemic blood pressure has also been demonstrated in neonatal animal models.22,23,24,25,26 It is unknown whether there is an association between IH and neonatal HTN in the preterm human population. In this cohort study, we aimed to determine whether IH events during the first month of age were associated with systemic HTN in preterm infants at 34–36 weeks’ PMA.
Methods
Subjects
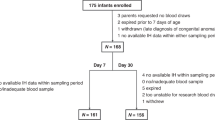
We conducted a secondary analysis of the Pre-Vent Case Western Reserve University (CWRU) single site study.27 Preterm infants <31 weeks’ GA and less than 7 days of age admitted to the UH Rainbow Babies and Children’s Hospital were eligible. Exclusion criteria for the Pre-Vent study included congenital or chromosomal anomalies and those who were unlikely to survive; 175 infants were enrolled at the CWRU site. For this study, infants were included if were enrolled in the Pre-Vent multicenter trial, were living at 36 weeks’ PMA, and had IH data available during the first 3 weeks of age. Parental consent was obtained for the initial prospective Pre-Vent study. For this secondary analysis and additional chart review, the University Hospitals Institutional Review Board approved a waiver of consent. Oversight was provided by the University of Virginia (UVA) Institutional Review Board and an observational and safety monitoring board, appointed by the National Heart, Lung, and Blood Institute (NHLBI).
Measurements
IH data and key demographic and Neonatal Intensive Care Unit (NICU) morbidities were captured as part of the main Pre-Vent multi-center trial. Per Pre-Vent standardized guidelines,10 based on current American Academy of Pediatrics recommended oxygen saturation target,28 the oxygen saturation target in the NICU during this study period was 90–95%. Oxygen saturation was continuously recorded (sample rate = 1 Hz, averaging time 8 s) from the clinical bedside pulse oximeter (Masimo, Radical-7, Irvine, CA) using a custom data acquisition system (National Instrument, Hungary and LabVIEW, Austin, TX). IH events were defined as oxygen saturation <90% for 10 s to 5 min using previously validated software (Matlab, Natick, MA)12 with IH frequency and total duration <90% assessed during the first 8–21 days of age. Analysis focused on days 8–21 when there is known to be a linear increase in the frequency of IH events.9,10
Retrospective chart reviews were performed to capture blood pressure-related data and additional risk factors for HTN. Blood pressures were obtained via unit protocol on one of the four limbs, using an automatic oscillometric blood pressure cuff (Welch Allyn, Skaneateles Falls, NY) or transduced via intra-arterial line, if present. One blood pressure per day was abstracted from a chart review when the infant was between 34 0/7 and 36 0/7 weeks’ PMA. If more than one blood pressure was obtained on a particular day, the blood pressure obtained closest to noon was abstracted. This number of blood pressures was chosen due to the unit norms of obtaining one blood pressure per day in lower acuity patients, which many of these patients were at this PMA. In the event of a mean arterial blood pressure (MAP) not being recorded, the formula [2 × diastolic blood pressure (DBP) + systolic blood pressure (SBP)]/3 was used to calculate the MAP. The median of these recorded blood pressures was calculated and compared to normative values as defined by Dionne et al.3 Patients were determined to have “hypertension by norms” if their median SBP or MAP during this period was greater than or equal to the 95th percentile value; that is, if at least 8 of the 15 daily blood pressures were greater than or equal to the 95th percentile.3 An infant was deemed to have clinically apparent elevated blood pressures if they met “hypertension by norms” criteria and if clinical progress notes between 34 0/7 to 36 0/7 weeks’ PMA contained one or more of any keywords in relation to blood pressures: “blood”, “elev”, “bp”, “htn”, and “hyperten” to account for misspellings and abbreviations. Data regarding diuretic, anti-hypertensive medication, sedating medication with anti-hypertensive effects, or beta blockers were documented as well.
Information was collected regarding renal ultrasounds obtained prior to 34 0/7 weeks’ PMA. Maximum serum creatinine (SCr) between 14 days of age and 34 0/7 weeks’ PMA was abstracted. Based on previously published studies on surviving premature infants, a normalized SCr cutoff of 0.5 was chosen to define acute kidney injury (AKI).29 Patients were identified who had an umbilical arterial catheter (UAC) placed. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at University Hospitals, supported by the Clinical and Translational Science Collaborative of Northern Ohio, funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Science Award Grant, UM1TR004528.30,31
Data analysis
Detectable difference calculation was conducted a priori based on an estimated 160 infants eligible for the study. Setting two-sided significance level at 0.05 and power 80%, we calculated a detectable difference of 0.51 standard deviation in the number of IH events between infants with and without HTN. The detectable difference was estimating 25% prevalence of HTN.
The data characteristics were described by presenting the median (interquartile range) for continuous variables and the frequency (%) for categorical variables, stratified by HTN groups. To account for the non-independence of twins or triplets, we applied generalized estimating equation models (GEE), which can handle correlated data.32 We applied GEE logistic regressions for the binary hypertension outcome and GEE linear regressions for the SBP continuous outcome. The univariate differences between HTN groups were assessed using GEE models or Chi-squared tests if GEE model failed to converge. Time series missing value imputation (R package imputeTS) was used in 18 infants to account for missing IH data for the chosen time windows. After imputation, subjects were excluded from the analysis of a particular week if IH variables were missing for more than two days in that week We conducted sensitivity analyses to assess the impact of missing data by analyzing the imputed data and raw data which found similar findings, and therefore imputed data was used. For the patients for whom imputed data was used, it was limited to one to two days only. The percentage difference in effect size for significant results ranged from 1.56% to 17.40%. For both GEE logistic regression model and GEE linear regression, the key predictors were total number of IH events summed over specific time windows (days 8–14 and days 15–21), median of daily percent of time with hypoxemia (SpO2 < 90%), and median duration of IH events over specified time windows. Although univariate analysis found GA to have p-value 0.21, it was included as a covariate a priori in the model because previous studies have found increased incidence of HTN in lower GA.1,3 Other potential confounders were considered for inclusion as covariates in multivariate GEE models: sex, race, small for gestational age (SGA), history of umbilical arterial catheter (UAC), patent ductus arteriosus (PDA) present prior to 34 0/7 weeks’ PMA, and SCr ≥0.5 between 14 days of age and 34 0/7 weeks’ PMA. A covariate was included in the multivariate GEE model if univariate analysis p values were <0.10. No covariates were found to meet the criteria of p < 0.10, so they were not included in the adjusted models. Analyses were performed using R Language software (Version 4.4.3).
Results
164 infants met inclusion criteria (Fig. 1). 23.2% (38/164) of enrolled children were found to have HTN by normative ref.3 Of these, only 13.2% (5/38) were documented to have clinically apparent elevated blood pressure. There was no difference in antenatal and early hospital course characteristics between those who were and were not found to have HTN by norms at 34–36 weeks’ PMA, including the use of diuretic therapy or other medications with a secondary side effect of lowering blood pressure (such as a beta blocker) (Table 1).When HTN was evaluated against IH parameters stratified by days of age 8–14 and 15–21, there was a significant (p = 0.02) association between the number of IH events 8–14 days of age and later HTN, but no association at days 15–21 (p = 0.17) (Table 2, Figs. 2 and 4). There was no association between the percentage of time with hypoxemia or the median duration of IH events and HTN (Table 2, Figs. 3 and 4).
The number of IH events on days 8–14, but not on days 15–21, showed a significant association with the continuous variable of SBP between 34- and 36-weeks’ PMA (days 8–14, p = 0.0004; days 15–21, p = 0.08). There was a significant association between the percentage of time with hypoxemia on days 8–14 and SBP (p = 0.03), but not on days 15–21 (p = 0.36). There was no significant association between the median duration of IH events and the continuous variable of SBP (Table 2).
Discussion
This study found a 23% rate of HTN, higher than previously documented incidence of HTN in neonates.1,2,3,4,5,6,7 Early IH events between 8 and 14 days of age were associated with HTN and SBP. The relationship between IH events at 15–21 days of age and HTN or SBP did not reach statistical significance. These findings showing a relationship between IH and elevated blood pressure are supported by both animal24,33 and adult human18 studies. In addition, our findings of statistical significance only with IH events on days 8–14 raise the question as to whether there is a critical window in which IH instigates potential pathways leading to vascular and/or sympathetic changes causing HTN. Missing data could have affected these findings, however sensitivity analysis was performed and had similar results.
Multiple possible mechanisms linking IH and HTN have been proposed, including inflammation, hypoxia-inducible factors (HIFs), reactive oxygen species (ROS), and sympathetic activation.19,21,25,26 IH has been shown to cause oxidative stress and generate ROS in both adult animal models of IH,34,35,36 and human infants.37,38 IH may also lead to HIF-induced inflammatory vascular remodeling39 and endothelial dysfunction.33,40,41 A systematic review and meta-analysis of studies of rodent data found exposure to IH increased blood pressures with signs of vascular remodeling including increased intima-media thickness and altered arterial reactivity.42 Adult animals exposed to IH have been noted to have increased sympathetic nerve activity,36,43,44 and remodeling of end organs innervated by the sympathetic nervous system.20 Adult mice exposed to neonatal IH were found to have persistent changes to peripheral baroreceptor22,23,24 and chemoreceptor function mediated by ROS-induced long-term sensory facilitation.26,45 An augmented peripheral chemoreflex contributes to sustained activation of the sympathetic nervous system. These data may be significant in the context of the results of the present study since preterm infants with a greater number of apneic episodes exhibited an augmented hypoxic ventilatory response, which is suggestive of enhanced peripheral chemoreceptor activity.46 These findings suggest potential multifactorial mechanisms by which IH may potentiate HTN.
The literature suggests multiple risk factors for HTN in premature neonates, including SGA status,2,6 PDA,3,5,7 UAC,2,3,4,5,6 and AKI.1,2,4,5,6 However, none of these were significantly associated with HTN at 34–36 weeks’ PMA in our population. This may be because our study is underpowered to see such a difference. We simplified our definition of AKI to include maximum serum creatinine ≥0.5 based on previously published data related to survivorship from the NICU.29 It is possible that a different definition of AKI, such as that used by Kidney Disease: Improving Global Outcome (KDIGO) may have identified alternate infants.47,48 Nevertheless, our 50% rate of AKI was similar to that of the Assessment of Worldwide Kidney Injury Epidemiology in Neonates (AWAKEN) study, which used the KDIGO definition.1 It has been noted that up to 50% of neonatal HTN are from unknown and multifactorial etiology6,8; perhaps IH plays a more impactful role in developing HTN in the preterm neonate than previously recognized.
This study found a higher rate of HTN (23%) compared to the AWAKEN study, which also evaluated raw clinical blood pressure data to define the cohort with HTN (5%).1 This large discrepancy could be due to our more frequent sampling (daily vs once per week), though shorter duration (15 days vs entirety of NICU hospitalization), of clinical blood pressure data. Our study used normative values based on Dionne et al.,3 while the AWAKEN study used different norms, which may affect the identification of infants with HTN. Of interest, the recently published Recombinant Erythropoietin for Protection of Infant Renal Disease (REPAIReD) study,49 which evaluated two-year blood pressure and renal outcomes of infants delivered at 28 weeks’ gestational age, found that 22% of their cohort had elevated systolic blood pressure for age and height. While our study does not follow infants after discharge, the elevated incidence in the study published by Hingorami et al. suggests there could be possible underestimation of hypertension rates in other published literature.
Our study is limited by retrospective chart abstraction of clinically obtained blood pressures, obtained on any of the four limbs, in a variety of infant behavioral states. Interestingly, both the dichotomous variable hypertension/no-hypertension and the continuous variable exploring the association between SBP and HTN were significant, suggesting that even if the 23% identification of HTN in our cohort was over-estimated, there is an association between IH and elevated blood pressures. Furthermore, misclassification (overestimation) of blood pressures due to an agitated state, for example, could bias the estimate towards the null. Nevertheless, our data demonstrated statistically significant association between IH and HTN, despite our data demonstrating a higher-than-expected rate of HTN.
In the current study, only 13% of the values meeting criteria for HTN were documented in daily progress notes (Table 1). This is consistent with the AWAKEN study, where true HTN (based on numeric BP data) was often clinically unrecognized (68% of cases overall, and 84% of cases 30–35 weeks’ GA).1 Interestingly, in our cohort, the incidence of infants whose HTN was both clinically recognized and met the criteria of blood pressures ≥95th percentile by normative values at 34–36 weeks’ PMA was 3% (5/164)—similar to other previously published incidence rates of neonatal HTN which did not evaluate raw blood pressure data.2,3,4,5,6,7
Future directions for this research include performing an inclusive prospective study with standardized blood pressure and renal function monitoring. Future studies could also address blood pressures in this population for the duration of and after hospital discharge, even into adulthood. A meta-analysis evaluating prematurity and later outcomes found that low birth weight and prematurity might predispose an individual to metabolic syndrome.50 A similar meta-analysis, while not finding increased risk of metabolic syndrome, did find elevated blood pressure in those individuals born preterm.51 It would be interesting to further stratify this population by IH events in early life. On a local level, there are opportunities for quality improvement work to recognize and treat hypertensive infants in the NICU.
In summary, this cohort study demonstrated that the number of IH and percentage of time with hypoxemia occurring during the second week of age was associated with systemic HTN at 34–36 weeks’ PMA. The median duration of IH events was not associated with HTN, nor were later IH events (15–21 days of age). These findings suggest there may be a critical window in which IH initiates potential pathologic pathways promoting vascular and/or sympathetic changes leading to HTN. Further studies could be helpful to determine the mechanism behind this association.
Data availability
All data generated or analyzed during this study are included in this article. Data is available upon reasonable request with IRB approval. Further inquiries can be directed to the corresponding author.
References
Kraut, E. J., Boohaker, L. J., Askenazi, D. J., Fletcher, J. & Kent, A. L. Incidence of neonatal hypertension from a large multicenter study [Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates—AWAKEN. Pediatr. Res. 84, 279–289 (2018).
Starr, M. C. & Flynn, J. T. Neonatal Hypertension: Cases, Causes and Clinical Approach. Pediatr. Nephrol. Berl. Ger. 34, 787–799 (2019).
Dionne, J. M., Abitbol, C. L. & Flynn, J. T. Hypertension in infancy: diagnosis, management and outcome. Pediatr. Nephrol. 27, 17–32 (2012).
Flynn, J. T. The hypertensive neonate. Semin. Fetal. Neonatal. Med. 25, 101138 (2020).
Seliem, W. A., Falk, M. C., Shadbolt, B. & Kent, A. L. Antenatal and postnatal risk factors for neonatal hypertension and infant follow-up. Pediatr. Nephrol. 22, 2081–2087 (2007).
Altemose, K. & Dionne, J. M. Neonatal hypertension: concerns within and beyond the neonatal intensive care unit. Clin. Exp. Pediatr. 65, 367–376 (2022).
Sahu, R. et al. Systemic Hypertension Requiring Treatment in the Neonatal Intensive Care Unit. J. Pediatr. 163, 84–88 (2013).
Jenkins, R. D. et al. Characteristics of hypertension in premature infants with and without chronic lung disease: a long-term multi-center study. Pediatr. Nephrol. 32, 2115–2124 (2017).
DiFiore, J., MMacFarlane, P., MMartinR. J. Intermittent Hypoxemia in Preterm Infants. Clin. Perinatol. 46, 553–565 (2019).
Weese-Mayer, D. E. et al. Maturation of cardioventilatory physiological trajectories in extremely preterm infants. Pediatr. Res. 95, 1060–1069 (2024).
Dormishian, A. et al. Etiology and Mechanism of Intermittent Hypoxemia Episodes in Spontaneously Breathing Extremely Premature Infants. J Pediatr. 262, 113623 (2023).
DiFiore, J. M. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157, 69–73 (2010).
Poets, C. F. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 314, 595–603 (2015).
Raffay, T. M. et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr. Res. 85, 318–323 (2019).
Fairchild, K. D., Nagraj, V. P., Sullivan, B. A., Moorman, J. R. & Lake, D. E. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr. Res. 85, 987–993 (2019).
Ambalavanan, N. et al. Cardiorespiratory Monitoring Data to Predict Respiratory Outcomes in Extremely Preterm Infants. Am. J. Respir. Crit. Care Med. 208, 79–97 (2023).
Hibbs, A. M. et al. Association between Intermittent Hypoxemia and NICU Length of Stay in Preterm Infants. Neonatology. 121, 327–335 (2024).
Tamisier, R. et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur. Respir. J. 37, 119–128 (2011).
Arnaud, C. Bochaton, T., Pépin, J. L. & Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 113, 350–358 (2020).
Fletcher, E. C., Lesske, J., Qian, W., Miller, C. C. & Unger, T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertens. Dallas Tex. 19, 555–561 (1992).
Bosc, L. V. G., Resta, T., Walker, B. & Kanagy, N. L. Mechanisms of intermittent hypoxia induced hypertension. J. Cell Mol. Med. 14, 3–17 (2010).
Julien, C. A., Niane, L., Kinkead, R., Bairam, A. & Joseph, V. Carotid sinus nerve stimulation, but not intermittent hypoxia, induces respiratory LTF in adult rats exposed to neonatal intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R192–R205 (2010).
Julien, C. A., Kinkead, R., Joseph, V. & Bairam, A. Neonatal Intermittent Hypoxia Induces Persistent Alteration of Baroreflex in Adult Male Rats. In Arterial Chemoreception (eds Nurse, C. A., Gonzalez, C., Peers, C. & Prabhakar, N.) 179–183 (Springer, 2012).
Soukhova-O’Hare, G. K. et al. Postnatal Intermittent Hypoxia and Developmental Programming of Hypertension in Spontaneously Hypertensive Rats. Hypertension 52, 156–162 (2008).
Prabhakar, N. R., Peng, Y. J. & Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Invest. 130, 5042–5051 (2020).
Prabhakar, N. R., Kumar, G. K. & Peng Y. J. Sympatho-adrenal activation by chronic intermittent hypoxia. J. Appl. Physiol. 113, 1304–1310, (2012).
Dennery, P. A. et al. Pre-Vent: The Prematurity-Related Ventilatory Control study. Pediatr. Res. 85, 769–776 (2019).
Cummings, J. J. Oxygen Targeting in Extremely Low Birth Weight Infants. Pediatrics 138, e20161576 (2016).
Walker, M. W., Clark, R. H. & Spitzer, A. R. Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. J. Perinatol. 31, 199–205 (2011).
Harris, P. A. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Harris, P. A. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).
Hibbs, A. M. Accounting for Multiple Births in Neonatal and Perinatal Trials: Systematic Review and Case Study. J. Pediatr. 156, 202 (2009).
Chu, A., Gozal, D., Cortese, R. & Wang, Y. Cardiovascular dysfunction in adult mice following postnatal intermittent hypoxia. Pediatr. Res. 77, 425–433 (2015).
MacFarlane, P. M., Wilkerson, J. E., RLovett-Barr, M. R. & Mitchell, G. S. Reactive Oxygen Species and Respiratory Plasticity Following Intermittent Hypoxia. Respir. Physiol. Neurobiol. 164, 263 (2008).
Nanduri, J. et al. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc. Natl. Acad. Sci. USA 106, 1199–1204 (2009).
Yuan, G. et al. H2S Production by Reactive Oxygen Species in the Carotid Body Triggers Hypertension in a Rodent Model of Sleep Apnea. Sci. Signal 9, ra80 (2016).
Shah, V. P. et al. The Relationship between Oxidative Stress, Intermittent Hypoxemia, and Hospital Duration in Moderate Preterm Infants. Neonatology 117, 577–583 (2020).
Raffay, T. M. et al. Hypoxemia events in preterm neonates are associated with urine oxidative biomarkers. Pediatr. Res. 94, 1444–1450 (2023).
Gras, E. et al. Endothelin-1 mediates intermittent hypoxia-induced inflammatory vascular remodeling through HIF-1 activation. J. Appl Physiol. 120, 437–443 (2016).
Makarenko, V. V. et al. Intermittent hypoxia-induced endothelial barrier dysfunction requires ROS-dependent MAP kinase activation. Am. J. Physiol. Cell Physiol. 306, C745–C752 (2014).
Arnaud, C. et al. Nonmuscle Myosin Light Chain Kinase: A Key Player in Intermittent Hypoxia-Induced Vascular Alterations. J. Am. Heart Assoc. Cardiovasc Cerebrovasc. Dis. 7, e007893 (2018).
Harki, O. et al. Intermittent hypoxia-related alterations in vascular structure and function: a systematic review and meta-analysis of rodent data. Eur. Respir. J. 59, 2100866 (2022).
Zoccal, D. B., Bonagamba, L. G. H., Oliveira, F. R. T., Antunes-Rodrigues, J. & Machado, B. H. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp. Physiol. 92, 79–85 (2007).
Silva, A. Q. & Schreihofer, A. M. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J. Physiol. 589, 1463–1476 (2011).
Julien, C. A., Joseph, V. & Bairam, A. Alteration of carotid body chemoreflexes after neonatal intermittent hypoxia and caffeine treatment in rat pups. Respir. Physiol. Neurobiol. 177, 301–312 (2011).
Nock, M. L., DiFiore, J. M., Arko, M. K. & Martin, R. J. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J. Pediatr. 144, 291–295 (2004).
Zappitelli, M. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr. Res. 82, 569–573, (2017).
Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pr. 120, c179–c184 (2012).
Hingorani, S. et al. Prevalence and Risk Factors for Kidney Disease and Elevated BP in 2-Year-Old Children Born Extremely Premature. Clin. J. Am. Soc. Nephrol. CJASN 17, 1129–1138 (2022).
Liao, L., Deng, Y., Zhao, D. Association of Low Birth Weight and Premature Birth With the Risk of Metabolic Syndrome: A Meta-Analysis. Front. Pediatr. 8, 405 (2020).
Parkinson, J. R. C., Hyde, M. J., Gale, C., Santhakumaran, S. & Modi, N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 131, e1240–e1263 (2013).
Acknowledgements
Dr. Thomas Raffay and Dr. Peter MacFarlane for reviewing portions of the manuscript. The Rainbow Babies and Children’s Fellowship Research Award Program (FRAP) provided funding allowing for presentations at local and national conferences and offset publication costs. NIH grants U01H133643, U01HL1333708, UM1TR004528, and K24HL143291. The views expressed in this article are those of the authors and do not necessarily represent those of the National Institutes of Health or the U.S. Department of Health and Human Services.
Author information
Authors and Affiliations
Contributions
S.M., Z.C., J.M.D., C.N., N.M.M., A.M.H. made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and contributed final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conset for publication
Parental consent was obtained for the initial prospective Pre-Vent study. For this secondary analysis and additional chart review, the University Hospitals Institutional Review Board approved a waiver of consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinez, S., Chen, Z., Di Fiore, J.M. et al. Neonatal intermittent hypoxemia events are associated with later systemic hypertension. Pediatr Res 98, 1075–1082 (2025). https://doi.org/10.1038/s41390-025-03881-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-03881-w