Abstract
Background
Understanding the complex cardiac adaptations following patent ductus arteriosus (PDA) ligation in preterm infants is essential for optimizing postoperative care. This study tested the hypothesis that left ventricular (LV), left atrial (LA) and right ventricular (RV) longitudinal strain are acutely impaired and then recover after PDA surgery in preterm infants.
Methods
Thirty-two preterm infants who underwent PDA ligation (median gestational age: 25 weeks) were evaluated using speckle-tracking echocardiography to quantify LV, RV, and LA longitudinal strain before and at 4–8 and 24–48 h post-surgery. These data were compared with 36 preterm infants without PDA (non-PDA).
Results
LV global longitudinal strain (LVGLS) was higher and RV free wall longitudinal strain (RVFWSL) was lower in the PDA group than in the non-PDA group preoperatively (both p < 0.01). In the non-PDA group, LVGLS and RVFWSL were −16% and −28%, respectively. In the PDA group, LVGLS at the three time points was −20%, −13%, and −15%, and RVFWSL was −21%, −21%, and −25%, respectively (all p < 0.05). LA reservoir strain (LASr) initially decreased and then increased.
Conclusions
After PDA ligation, LVGLS and LASr transiently decrease then increase within 24–48 h, while RVFWSL normalizes without a decrease.
Impact
-
Preoperatively, preterm infants with PDA had higher left ventricular global longitudinal strain (LVGLS), and lower right ventricular free wall strain (RVFWSL) than those without PDA.
-
After PDA ligation, LVGLS and left atrial reservoir strain (LASr) initially decreased and then recovered within 24–48 h, while RVFWSL rapidly improved.
-
Our findings indicate dynamic and different post-surgical cardiac adaptation in the right ventricle and left ventricle after PDA ligation.
Similar content being viewed by others
Introduction
Patent ductus arteriosus (PDA) is one of the major complications of preterm infants1. With a decrease in pulmonary resistance after birth, PDA may cause a larger left-to-right shunt throughout the cardiac cycle, causing high pulmonary flow and ischemia of systemic organs. Therefore, instability induced by PDA is associated with intraventricular hemorrhage or necrotizing enterocolitis. Moreover, regardless of whether there is surgical or percutaneous closure, closure of PDA causes an abrupt change in left ventricular (LV) loading conditions. In this situation, preterm infants are at a high risk of hemodynamic compromise, leading to hypotension, reduced cardiac output, and compromised oxygenation and ventilation2,3,4 after the closure of PDA5. Approximately 10% to 45% of preterm infants undergoing surgical PDA ligation encounter post-ligation hemodynamic instability, which may affect their long-term outcomes6.
However, how hemodynamic instability occurs following the surgical closure of PDA remains unclear. Several studies that used conventional echocardiography reported the changes in LV function associated with an increase in LV afterload induced by PDA closure3,4,7,8,9,10,11. Such instability in the LV after PDA closure and preoperative excessive preload in the LV and left atrium (LA) suggest that PDA is a disease of the left heart system. Our recent study using three-dimensional (3D) echocardiography showed that the right ventricular (RV) volume was significantly larger and the RV ejection fraction (EF) was significantly smaller in preterm infants before PDA surgery12,13,14. These findings suggest that the function of not only the LV and LA, but also the RV, is crucial in preterm infants with PDA.
Assessment of LV, LA, and RV function using speckle-tracking echocardiography has recently become a standard clinical tool for the evaluation of cardiac function in adults. Longitudinal strain analysis using speckle-tracking echocardiography equipped with artificial intelligence provides a simple, reproducible, and promising echocardiographic evaluation for the early detection of cardiac dysfunction and heart failure. LV global longitudinal strain (LVGLS) measured by speckle-tracking echocardiography is increasingly being recognized as a more effective technique than the conventional LV ejection fraction (LVEF) in detecting subtle changes in ventricular function and in predicting outcomes15,16. Longitudinal strain of the LA and RV measurements have also been standardized and are being used to assess cardiac function17,18,19,20,21. The longitudinal strain assessment of LV, LA, and RV using speckle-tracking echocardiography has also been sporadically reported in preterm infants22,23,24,25,26,27,28,29,30. However, in preterm infants with PDA, previous studies using speckle-tracking echocardiography have assessed the LV27,28 and LA23,25, but not the RV. Our previous study briefly described the values of GLS and global circumferential strain measured by 3D echocardiography for the LV and LA as a ref. 14. Our institution initiated the use of AutoStrain (AutoStrain LV/LA/RV; TomTec Imaging Systems, Unterschleissheim, Germany), which is a machine learning-based speckle-tracking software, during the middle of the observation period of our previous study14, using 3D echocardiography.
Although we measured LVGLS using 3D echocardiography in our previous study, its lower frame rate and different computational principles compared with 2D speckle-tracking echocardiography make direct comparisons challenging. The use of AutoStrain may provide further insight in myocardial longitudinal strain and essential reference values for LV, RV or LA longitudinal strain variables in preterm infants. This software may allow standardized and reproducible assessment of myocardial strain in preterm infants, and may help identify functional differences between those with severe PDA requiring surgical ligation and those without PDA.
This study aimed to test the hypothesis that not only LVGLS, but also LA longitudinal strain (reservoir, conduit, and contraction) and RV free wall longitudinal strain (RVFWSL), may be acutely impaired and then recover after PDA surgery in preterm infants as shown by speckle-tracking echocardiography using artificial intelligence.
Methods
Study design and population
This retrospective study, which was conducted at a single center, enrolled preterm infants with a gestational age ranging from 23 to 33 weeks who underwent PDA surgery and echocardiographic evaluation including AutoStrain between November 2019 and December 2022. We excluded preterm infants with (1) cardiac anomalies other than a patent foramen ovale and a persistent left superior vena cava, (2) chromosomal abnormalities, and (3) apparent clinical syndrome or multiple abnormalities. These patients who underwent PDA surgery were also included in our previous study14.
Referrals for PDA ligation were triaged on the basis of clinical and echocardiographic findings. The indications for surgical PDA closure were as follows: (1) difficulty in weaning from mechanical ventilation; (2) worsening congestive heart failure despite medical management or contraindication to cyclooxygenase inhibitors; (3) a transductal diameter > 1.5 mm, predominantly left-to-right flow; (4) LA enlargement as indicated by an LA/aortic (LA/Ao) diameter ratio > 1.3 or LA volume index > 1.0 ml/kg; and (5) left pulmonary artery end-diastolic velocity (LPAedv) > 15 cm/s on echocardiography. Speckle-tracking echocardiography has been part of our standard protocol since November 2019. The indication for PDA surgery was determined by neonatologists who were blinded to the speckle-tracking echocardiographic data. LV, LA, and RV function in patients who underwent PDA ligation (PDA group) were evaluated by transthoracic echocardiography within 12 h before PDA ligation, within 4–8 h after PDA ligation, and between 24 and 48 h postoperatively.
Preoperative LVGLS, RVFWSL, RV four-chamber longitudinal strain including the ventricular septum (RV4cSL) and phase-specific LA longitudinal strain (LA contraction [LASct], conduit [LAScd], and reservoir [LASr]) in preterm infants in the PDA group were compared with those in preterm infants without PDA (non-PDA group). The inclusion criteria of the non-PDA group were as follows: (1) 14-day-old neonates with a gestational age between 23 and 28 weeks and those with a gestational age between 29 and 31 weeks who required mechanical ventilation who were admitted to our neonatal intensive care unit between October 2020 and December 2022; (2) preterm infants who did not have PDA ligation; (3) PDA closure before day 14; and (4) preterm infants who did not have any congenital heart diseases or pulmonary hypertension as indicated by a non-circular LV shape at the peak of systole and/or tricuspid regurgitation pressure gradient > 32 mmHg31. An echocardiography dataset including speckle-tracking echocardiography was acquired in all preterm infants with these criteria as part of the institutional protocol.
This study was conducted in accordance with the principles contained in the Declaration of Helsinki and was approved by the institutional review board of Kanagawa Children’s Medical Center (No. 1806-07).
Clinical characteristics
Data of the gestational age in weeks, birth weight, sex, Apgar scores, age (days) at PDA surgery, corrected gestational age in weeks, and body weight at the surgery day were collected from medical records. In the PDA group, details of the treatment received, survival or death at discharge, and additional characteristics were obtained.
All data, comprising respiratory characteristics, baseline hemodynamics, and echocardiograms, were collected at the three specified time points. N-terminal pro-brain natriuretic peptide concentrations were quantified at the pre-ligation stage and at 24–48 h following surgical intervention as part of our standard protocol.
The collected clinical data included heart rate, oxygen saturation, the fraction of inspired oxygen, mean airway pressure, and blood pressure. Blood pressure was measured immediately before an echocardiogram. Postoperative blood pressure at all time points in the PDA group was measured using an arterial line. Blood pressure was measured using the oscillometer technique in all preterm infants in the non-PDA group and at certain preoperative points in those in the PDA group who did not have an arterial line.
When the echocardiogram was performed, the respiratory severity score was calculated as the mean airway pressure (mmHg) × the fraction of inspired oxygen32. The vasoactive–inotropic score at the initial echocardiographic examination was calculated as the dopamine dose (μg/kg/min) + the dobutamine dose (μg/kg/min) + 100 × the epinephrine dose (μg/kg/min) + 100 × the norepinephrine dose (μg/kg/min) + 10,000 × the vasopressin dose (U/kg/min) + 10 × the olprinone dose (μg/kg/min)33.
Speckle-tracking echocardiography
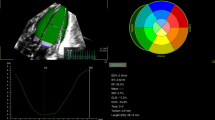
An experienced echocardiographer (K. Toyoshima) performed the echocardiography with an ultrasound device (EPIC 7 G or EPIQ CVx with an S9-2 probe; Philips Healthcare, Andover, MA). As part of the protocol, speckle-tracking echocardiography was used to assess LVGLS, RVFWSL, RV4cSL, LASct, LAScd, and LASr. Speckle-tracking analysis was performed by the same observer (K. Toyoshima) using AutoStrain LV/LA/RV. Two-dimensional images were acquired from apical four-chamber (A4C), apical two-chamber (A2C), and apical three-chamber (A3C) views for LV longitudinal distortion analysis. Frame rates of 80–100 frames/s were used for storage and analysis. AutoStrain is an automated strain measurement application equipped with artificial intelligence integrated into the Philips ultrasound system. This software automatically detects and labels selected apical views and applies a contour specific to each view (Fig. 1). Automatic endocardial contour recognition consisted of three steps. First, a complete R-R cycle was selected from the beginning of the end-diastolic (ED) cycle to the end of the ED cycle. Second, the LV was automatically detected in the first R-wave frame of the selected cycle. Finally, view-specific deformable endocardial contour models were aligned to the individual image content. Once the endocardial boundaries were set in ED, the software automatically tracked the heart motion throughout the cardiac cycle using speckle-tracking and displayed the results of the GLS analysis (Fig. 1). The entire process, from automatic view detection to the display of analysis results, took less than 1 s. If boundary editing was required, editing in ED triggered new speckle tracking of the LV cavity boundary throughout the cardiac cycle.
Longitudinal strain (LS) was measured at the endocardial border (Fig. 1), and instantaneous strain values near the border were color-coded for visual display. In addition, changes in the strain waveform during the cardiac cycle were displayed. Segmental strain values were displayed as end-systolic strain or peak-systolic strain, and an 18-segment bull’s-eye plot showed end-systolic strain or peak-systolic strain with the time to peak (ms)15,34.
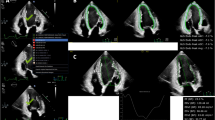
The same measurement of LS with automatic cross-section detection and automatic boundary detection as in the LV was also applied to the RV and LA (Fig. 2)17. Once the endocardial boundary was automatically placed in ED (RV) or ES (LA), it followed the heart motion using speckle tracking throughout the cardiac cycle. If the boundaries needed to be edited, they were edited in ED (LV, RV) or ES (LA). Editing the boundaries in ED (RV) or ES (LA) triggered new speckle tracking of the boundaries throughout the cardiac cycle.
a Right ventricular free wall strain and four-chamber strain obtained from apical four-chamber view (RV A4C). b Left atrial reservoir, conduit, and contraction strain measured from LA apical view using end-diastole as reference. RV right ventricular, LA left atrial, A4C apical four-chamber view, RVFWSL right ventricular free wall longitudinal strain, RV4cSL right ventricular four-chamber longitudinal strain, LASr_ED left atrial reservoir strain with a reference time point at end-diastole, LAScd_ED left atrial conduit strain with a reference time point at end-diastole, LASct_ED left atrial contraction strain with a reference time point at end-diastole.
Images were optimized to visualize each myocardial wall. RV systolic function was evaluated in the apical four-chamber view, which focused on the RV with all segments of the free wall and septal wall. RVFWSL and RV4cSL were evaluated using the mean of the longitudinal systolic strain peaks (Fig. 2). The software automatically generated LA myocardial surface tracings. Manual adjustments were required to optimize tracking around the LA appendage, pulmonary vein confluence, and atrial septum when necessary. To ensure accurate tracking of the atrial myocardium, the tracking performance was visually checked and the tracking geometry was further adjusted if necessary before reapplying the algorithm. Longitudinal strain of the LA was analyzed by triggering the onset of the P wave, and LASct, LAScd, and LASr were obtained from the strain curve (Fig. 2).
Conventional transthoracic echocardiography
The following conventional echocardiographic variables were measured. We measured LV diastolic and LV systolic dimensions (mm) using the M-mode in the long-axis view. The LA and Ao diameters (mm) were measured in the long-axis view using the leading-edge method35. The LA area (cm2) and LA long-axis length (LA length, cm) were measured in the four-chamber view. The narrowest internal diameter of the PDA (mm) using 2D echocardiography was measured in the ductal long-axis view, and the PDA flow pattern was measured using the pulse wave mode (left to right, right to left, bidirectional, and none). LPAedv was measured using the pulse wave mode13,36,37. RV function was evaluated using the fractional area change and corrected tricuspid annular plane systolic excursion (tricuspid annular plane systolic excursion/RV long-axis diameter)38. LA volume was calculated using the single-plane area–length method in the four-chamber view by applying the following equation: LA volume = 0.85 × (LA area)2 / (LA length) (cm3)13. The LV end-systolic wall stress was calculated using mean blood pressure measurements39,40. Superior vena cava flow was assessed using pulse-wave Doppler as described by Kluckow and Evans41. The average of the maximum and minimum diameters from a still 2D image was calculated over five heart cycles. The velocity time integral was derived from Doppler velocity tracings and averaged across five consecutive cardiac cycles. Heart rate was determined from the peak-to-peak intervals of the Doppler velocity signals.
Reproducibility analyses
Fifteen studies were randomly selected from the PDA group to investigate how measurements change over time. One observer (K. Toyoshima) measured LVGLS, RVFWSL, and LASr at 3-month intervals. The second observer (H. Aoki) who was unaware of the first measurement analyzed these data to investigate interobserver variability. We used the intraclass correlation coefficient (ICC) and Bland–Altman analysis to examine intraobserver and interobserver variabilities.
Statistical analyses
Descriptive statistics (e.g., mean ± standard deviation, median [interquartile range]) were used to summarize the demographic or clinical data of preterm infants in the PDA and non-PDA groups. The two groups were compared using the t-test, Mann–Whitney U-test, or Fisher’s exact test. We compared hemodynamic, respiratory, and echocardiographic parameters across three time points using one-way analysis of variance with repeated measures.
Statistical analyses were conducted using EZR (version 1.54; Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria), along with MedCalc (version 20; MedCalc Software Ltd., Ostend, Belgium). A p value < 0.05 was regarded as statistically significant.
Results
Clinical data of the PDA group
Thirty-two preterm infants who underwent PDA surgery were enrolled in this study between November 2019 and December 2022 (Fig. 3). The median gestational age at birth was 25 weeks (interquartile range [IQR]: 24–27), and the median birth weight was 695 g (IQR: 584–860). Pharmacological closure with cyclooxygenase inhibitors had failed in all 32 patients. PDA surgery was performed at a median age of 20 days (IQR: 15–27), with a median corrected gestational age of 28 weeks (IQR: 27–31) and a median weight of 760 g (IQR: 600–960). The median duration of surgery was 42 mi (IQR: 37–47), and the median fluid volume administered during the surgery was 27 ml/kg (IQR: 23–45). No major complications occurred in any patient during the procedure. Speckle-tracking and conventional echocardiographic measurements were successfully acquired for all preterm infants, with no missing scans. All preterm infants in this group survived to discharge.
In the PDA group, 32 preterm infants underwent PDA surgery at a gestational age of 23–33 weeks (November 2019 to December 2022) in our hospital. Two patients were excluded owing to the presence of multiple anomalies. Thirty-two patients were included in the final analysis, of whom 32 survived (100%). In the non-PDA group, between October 2020 and 2022, 82 inborn preterm infants were admitted to our hospital. Their gestational ages ranged from 23 to 28 weeks, and those aged between 29 and 31 weeks who required mechanical ventilation were also included. Of these, 46 patients were excluded for various reasons. As a result, 36 patients were ultimately included in the non-PDA group and 35 (97.2%) of them survived. PDA patent ductus arteriosus.
Reproducibility of speckle-tracking echocardiographic measurements
The analysis of intra- and interobserver variability in preterm infants with PDA, including the percentage bias, 95% limits of agreements, and ICCs for LVGLS, RVFWSL, and LASr, are shown in Table 1. The intrarater reproducibility (ICCs: LVGLS, 0.91; RVFWSL, 0.97; and LASr, 0.88) and interrater reproducibility (ICCs: LVGLS, 0.83; RVFWSL, 0.80; and LASr, 0.82) demonstrated very good. Figure 4 shows Bland–Altman plots, which indicated minimal bias values with acceptable limits of agreement.
a Intraobserver variability plots for LVGLS, RVFWSL, and LASr strain parameters. b Interobserver variability plots for the same parameters across two different observers. The three dashed lines show biases (means of differences) and limits of agreement. Bias is expressed as the mean of the difference (95% confidence interval). The limit of agreement is shown as the bias ± 2 SDs. LVGLS left ventricular global longitudinal strain (average), RVFWSL right ventricular free wall longitudinal strain, LASrED left atrial reservoir strain with a reference time point at end-diastole, LASctED left atrial contraction strain with a reference time point at end-diastole, SD standard deviation.
Comparison of the PDA and non-PDA groups preoperatively
Figure 3 and Tables 2 and 3 show the comparison of demographic and echocardiographic data between preterm infants in the PDA group (n = 32) and those in the non-PDA group (n = 36). All patients with PDA had a left-to-right ductal shunt. All patients had a left-to-right atrial shunt in both groups. There was no significant difference in gestational age, sex, 1- and 5-min Apgar scores, or the prevalence of small for gestational age between the groups. No significant differences were observed in corrected gestational age, body weight, or heart rate on echocardiography between the groups. However, the proportion of in-hospital birth was significantly lower in the PDA group than in the non-PDA group (p < 0.001). N-terminal prohormone brain natriuretic peptide concentrations were significantly higher in the PDA group than in the non-PDA group (p < 0.001).
Pre-ligation blood pressure was measured using an arterial line in 21 of 32 (66%) preterm infants. In the remaining 11 of 32 (34%) preterm infants in the PDA group and in all of those in the non-PDA group, blood pressure was assessed using the oscillometric technique. Systolic and diastolic blood pressure were significantly lower in the PDA group than in the non-PDA group (both p < 0.001).
The PDA group had a significantly larger LVEF and LVGLS than the non-PDA group (both p < 0.001) (Table 3).
In contrast, the PDA group had less RV wall motion indicated by a significantly smaller RVFWSL, and lower corrected tricuspid annular plane systolic excursion and RV fractional area exchange than the non-PDA group (Table 3). However, the difference in RV4cSL between the groups was not significant (p = 0.081).
The indices of LA enlargement (LA/Ao, LA volume) in the PDA group were higher than those in the non-PDA group (both p < 0.001). The LAEF and LASr were not significantly different between the groups, but LASct was lower in the PDA group than in the non-PDA group (p = 0.04) (Table 3).
Comparison of pre-ligation, 4–8 h post-ligation, and 24–48 h post-ligation in the PDA group
Heart rate at 4–8 and 24–48 h after surgery was significantly lower than that preoperatively (both p < 0.05). LVDD was also significantly decreased at 4–8 and 24–48 h postoperatively compared with that preoperatively (both P < 0.001). Additionally, the LPAedv, LVEF, LA/Ao ratio, and LA volume index were significantly reduced postoperatively compared with preoperatively (all p < 0.001) (Table 3).
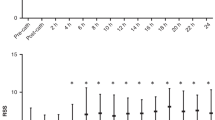
LVGLS was significantly decreased at 4–8 h (p < 0.001) and slightly increased at 24–48 h (p < 0.01) postoperatively compared with preoperatively (Fig. 5 and Table 3). RVFWSL and RV4cSL were increased at 24–48 h postoperatively compared with preoperatively (both p < 0.001) (Fig. 5 and Table 3), without a reduction at 4–8 h postoperatively. LASr was significantly decreased at 4–8 h and slightly increased at 24–48 h postoperatively compared with preoperatively (both p < 0.01) (Fig. 5 and Table 3).
Changes in LVGLS, RVFWSL, and LASr across four time points: non-PDA group, pre-ligation, 4–8 h post-ligation, and 24–48 h post-ligation. Error bars represent mean ± SD. Statistical annotations: *p < 0.05 vs. non-PDA; †p < 0.05 vs. pre-ligation; ‡p < 0.05 vs. 4–8 h post-ligation. |LVGLS| absolute value of left ventricular global longitudinal strain (average of A4C, A2C, and A3C), |RVFWSL| absolute value of right ventricular free wall longitudinal strain, LASr left atrial reservoir strain, PDA(-) non-PDA, PDA patent ductus arteriosus.
Discussion
The present study provides a detailed strain analysis, including phase-specific LA and RV in addition to LV, which were calculated using the highly adaptable AutoStrain software powered by artificial intelligence. To the best of our knowledge, this is the first study to show RVFWSL in this population. This strain approach allows a more comprehensive understanding of the loading and functional status, which dramatically changes after PDA surgical closure, and may provide insight into optimizing individual treatment in a tailored fashion in these preterm infants40.
Comparison of the PDA and non-PDA groups preoperatively
In our study, LV, RV, and LA volumes were successfully quantified by speckle-tracking echocardiography in preterm infants with and without PDA. Significant differences in LVGLS and RVFWSL values were observed between the PDA and non-PDA groups. The absolute values of LVGLS, RVFWSL, and LASct in the PDA group were 125%, 77%, and 87% of those in the non-PDA group, respectively. In the PDA group, LVGLS was increased, reflecting excessive preload and reduced afterload. In contrast, preoperative RVFWSL was reduced. This observation may be attributed to several factors, such as increased RV preload due to a left-to-right shunt via a stretched foramen ovale caused by an enlarged LA by PDA shunt, increased RV afterload due to elevated LA and pulmonary arterial pressure, and decreased coronary flow induced by an increased aorta-to-pulmonary artery shunt via PDA.
Regarding the LA, there was no significant difference in LASr or LAScd between the PDA and non-PDA groups preoperatively. Only LASct was lower in the PDA group than in the non-PDA group. Although the LA enlarges as PDA becomes more severe, LA strain does not necessarily decrease, possibly due to compensatory mechanisms.
Comparison of pre-ligation, 4–8 h post-ligation, and 24–48 h post-ligation
In this study, LVGLS and LASr were significantly decreased 4–8 h postoperatively. This decrease is likely due to the increased LV afterload after PDA ligation and the sudden reduction in LV and LA volumes14. By 24–48 h after surgery, LVGLS, LASr, and LAScd had returned to the levels similar to those of preterm infants without PDA. The recovery of LVGLS and LASr between 4–8 and 24–48 h after surgery may reflect compensatory mechanisms for increased afterload after surgery, which include recovery of the volume status and that from the effects of anesthesia.
However, LASct remained low in the PDA group, suggesting that recovery of LA contractile function may take longer even after PDA-induced LA enlargement. In contrast to the LVGLS, RVFWSL did not decrease 4–8 h after surgery and actually increased 24–48 h after surgery to the level of that in the non-PDA group. This finding suggests that RV dysfunction associated with PDA may rapidly improve owing to a decrease in LA pressure and loss of the PDA shunt to the pulmonary vasculature. However, severe RV dysfunction in premature infants with PDA may be another sign of severity of PDA that has not been focused on to date.
These postoperative changes in LVGLS, LASr, and RVFWSL strain may serve as useful indices for postoperative management of the circulation. They may help determine and predict the pathogenesis of post-ligature syndrome.
Demonstrating the reliability of speckle-tracking echocardiography in serial assessments before and after PDA ligation
We showed the reliability of speckle-tracking echocardiography in serial assessments before and after PDA ligation. Assessment of LV, LA, and RV function using the speckle-tracking technique has recently become a standard clinical tool for evaluating cardiac function in adults. LVGLS decreases before the LVEF, which reflects potential LV systolic dysfunction in heart failure with a preserved LVEF15. LVGLS is an important predictive marker of early cardiac dysfunction, and LVGLS is also an independent prognostic factor in heart failure with a reduced LVEF42. LASr can be used to evaluate reservoir function, which stores blood during systole by relaxation and extension of the LA, and the booster function, which ejects stored blood to the LV during late diastole20,21.
The assessment of LV, LA, and RV longitudinal strain using speckle-tracking echocardiography has only been sporadically reported in preterm infants16,22,23,24,25,26,27,28,29,30. RVFWSL is considered more important in assessing RV function because it excludes the intraventricular septum, which is strongly affected by LV function17,19. RVFWSL is used for diagnosing and predicting the prognosis of right heart failure and pulmonary hypertension18,19.
The study confirmed the robustness of speckle-tracking echocardiography in detecting changes in cardiac function, and showed high intra- and interobserver reliability. The AutoStrain application uses the automation technologies Auto View Recognition and Auto Contour Placement. The implementation of these automation tools provides a simple, fast workflow for robust and reproducible longitudinal strain measurements. However, editing and overriding this automation could make AutoStrain a convenient, non-invasive and useful echocardiographic indicator in clinical practice. We have reported that echocardiography-based tailored management of PDA in preterm infants may save lives with fewer complications40,43. Speckle-tracking echocardiography using artificial intelligence (machine learning) techniques may be useful in understanding the hemodynamics of PDA in preterm infants.
Finally, this study provides novel insights into cardiac adaptation after PDA ligation using machine learning-based speckle-tracking echocardiography. While our previous study evaluated biventricular volume and function using 3D echocardiography14, this study extends this analysis to myocardial deformation, offering a more comprehensive assessment of ventricular and atrial mechanics. Notably, to the best of our knowledge, this is the first study to comprehensively assess LA longitudinal strain in each phase (LASct, LAScd, and LASr), as well as RVFWSL and RV4cSL. These parameters provide crucial insights into how preterm infants with severe PDA and those without PDA differ in myocardial strain, and they were not measured in prior volume-based assessments. Although our previous study reported LVGLS using 3D echocardiography, the lower frame rate and different computational principles limit direct comparisons with 2D speckle-tracking echocardiography. In contrast, AutoStrain enables a high frame rate and standardized strain analysis, making it a clinically relevant feasible tool for assessing myocardial deformation in preterm infants.
Study limitations
This study has several limitations. First, because of the small sample size and the absence of patients who developed post-ligation instability syndrome, we were unable to assess the effect of speckle-tracking methods on clinical outcomes compared with traditional echocardiographic assessment. Nevertheless, we successfully obtained continuous hemodynamic data after PDA ligation in preterm infants, and captured the acute (4–8 h) and subacute (24–48 h) phases using semi-automated measurements with machine learning algorithms.
Second, this study was conducted at a single center, and there is substantial variability in the evaluation and management of preterm infants with PDA across different centers and countries. Postoperative outcomes may widely vary depending on preoperative conditions, the management approach, and anesthesia during surgery. Therefore, the generalizability of these findings should be considered with caution.
The echocardiographer had access to the patients’ clinical information and background data during the data extraction process, which could have introduced potential bias. Nevertheless, the extraction procedures were semi-automated and the inter- and intraobserver variability was within acceptable limits. Notably, only one vendor’s algorithm was used.
Conclusions
Speckle-tracking echocardiography shows the different functional abnormalities and post-surgical adaptation processes in LVGLS, LASr, and RVFWSL in preterm infants requiring a PDA ligation. Notably, RVFWSL normalized within 24–48 h after surgery, while LVGLS and LASr showed partial recovery. Future prospective studies are required to determine whether assessment of LVGLS, RVFWSL, and LASr using speckle-tracking echocardiography could help to optimize tailored management and improve outcomes in this patient population.
Data availability
The data underlying the findings of this study can be accessed upon reasonable request by contacting the corresponding author (K. Toyoshima).
Change history
01 August 2025
Reference 14 was missing journal name, volume, and pages range. This has been amended.
References
Hamrick, S. E. G. et al. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 146 (2020).
Giesinger, R. E., Bischoff, A. R. & McNamara, P. J. Anticipatory Perioperative Management for Patent Ductus Arteriosus Surgery: Understanding Postligation Cardiac Syndrome. Congenit Heart Dis 14, 311–316 (2019).
Jain, A. et al. Use of Targeted Neonatal Echocardiography to Prevent Postoperative Cardiorespiratory Instability after Patent Ductus Arteriosus Ligation. J Pediatr 160, 584–589.e581 (2012).
Teixeira, L. S., Shivananda, S. P., Stephens, D., Van Arsdell, G. & McNamara, P. J. Postoperative Cardiorespiratory Instability Following Ligation of the Preterm Ductus Arteriosus Is Related to Early Need for Intervention. J Perinatol 28, 803–810 (2008).
Weisz, D. E. et al. Association of Patent Ductus Arteriosus Ligation with Death or Neurodevelopmental Impairment among Extremely Preterm Infants. JAMA Pediatr 171, 443–449 (2017).
Noori, S. & Kumar, S. R. Pre-Dicting Post-Ligation Syndrome. The Journal of thoracic and cardiovascular surgery 154, 2060–2061 (2017).
El-Khuffash, A. F., Jain, A., Dragulescu, A., McNamara, P. J. & Mertens, L. Acute Changes in Myocardial Systolic Function in Preterm Infants Undergoing Patent Ductus Arteriosus Ligation: A Tissue Doppler and Myocardial Deformation Study. J Am Soc Echocardiogr 25, 1058–1067 (2012).
McNamara, P. J., Stewart, L., Shivananda, S. P., Stephens, D. & Sehgal, A. Patent Ductus Arteriosus Ligation Is Associated with Impaired Left Ventricular Systolic Performance in Premature Infants Weighing Less Than 1000 G. The Journal of thoracic and cardiovascular surgery 140, 150–157 (2010).
Nagata, H. et al. Left Ventricular Efficiency after Ligation of Patent Ductus Arteriosus for Premature Infants. The Journal of thoracic and cardiovascular surgery 146, 1353–1358 (2013).
Noori, S., Friedlich, P., Seri, I. & Wong, P. Changes in Myocardial Function and Hemodynamics after Ligation of the Ductus Arteriosus in Preterm Infants. The Journal of pediatrics 150, 597–602 (2007).
Ting, J. Y. et al. Predictors of Respiratory Instability in Neonates Undergoing Patient Ductus Arteriosus Ligation after the Introduction of Targeted Milrinone Treatment. J Thorac Cardiovasc Surg 152, 498–504 (2016).
Toyoshima, K., Masutani, S., Senzaki, H., Kawataki, M. & Itani, Y. Left Atrial Volume Is Superior to the Ratio of the Left Atrium to Aorta Diameter for Assessment of the Severity of Patent Ductus Arteriosus in Extremely Low Birth Weight Infants. Circ J 78, 1701–1709 (2014).
Toyoshima, K. et al. What Echocardiographic Indices Are Predictive of Patent Ductus Arteriosus Surgical Closure in Early Preterm Infants? A Prospective Multicenter Cohort Study. Journal of cardiology 74, 512–518 (2019).
Toyoshima, K. et al. Biventricular Function in Preterm Infants with Patent Ductus Arteriosus Ligation: A Three-Dimensional Echocardiographic Study. Pediatr Res 96, 773–784 (2024).
Voigt, J. U. et al. Definitions for a Common Standard for 2d Speckle Tracking Echocardiography: Consensus Document of the Eacvi/Ase/Industry Task Force to Standardize Deformation Imaging. J Am Soc Echocardiogr 28, 183–193 (2015).
Biering-Sørensen, T. et al. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging 10 (2017).
Badano, L. P. et al. Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: A Consensus Document of the Eacvi/Ase/Industry Task Force to Standardize Deformation Imaging. Eur Heart J Cardiovasc Imaging 19, 591–600 (2018).
Hamada-Harimura, Y. et al. Incremental Prognostic Value of Right Ventricular Strain in Patients with Acute Decompensated Heart Failure. Circ Cardiovasc Imaging 11, e007249 (2018).
Kemal, H. S. et al. Right Ventricular Free-Wall Longitudinal Speckle Tracking Strain in Patients with Pulmonary Arterial Hypertension under Specific Treatment. Echocardiography 34, 530–536 (2017).
Rimbaş, R. C., Mihăilă, S. & Vinereanu, D. Sources of Variation in Assessing Left Atrial Functions by 2d Speckle-Tracking Echocardiography. Heart Vessels 31, 370–381 (2016).
Pathan, F., D’Elia, N., Nolan, M. T., Marwick, T. H. & Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr 30, 59–70.e58 (2017).
Toma, D. et al. Comparative Assessment of Myocardial Function between Late Premature Newborns and Term Neonates Using the 2d Speckle Tracking Method. Front Pediatr 12, 1302383 (2024).
de Waal, K., Phad, N. & Crendal, E. Echocardiography Algorithms to Assess High Left Atrial Pressure and Grade Diastolic Function in Preterm Infants. Echocardiography 40, 1099–1106 (2023).
Bussmann, N., Franklin, O., McCallion, N., McNamara, P. J. & El-Khuffash, A. The Impact Preload on Left Ventricular Three-Plane Deformation Measurements in Extremely Premature Infants. Early Hum Dev 153, 105291 (2021).
de Waal, K., Phad, N. & Boyle, A. Left Atrium Function and Deformation in Very Preterm Infants with and without Volume Load. Echocardiography 35, 1818–1826 (2018).
De Waal, K., Phad, N., Lakkundi, A. & Tan, P. Post-Transitional Adaptation of the Left Heart in Uncomplicated, Very Preterm Infants. Cardiol Young 27, 1167–1173 (2017).
de Waal, K., Phad, N., Collins, N. & Boyle, A. Cardiac Remodeling in Preterm Infants with Prolonged Exposure to a Patent Ductus Arteriosus. Congenit Heart Dis 12, 364–372 (2017).
de Waal, K., Phad, N., Lakkundi, A. & Tan, P. Cardiac Function after the Immediate Transitional Period in Very Preterm Infants Using Speckle Tracking Analysis. Pediatr Cardiol 37, 295–303 (2016).
de Waal, K., Lakkundi, A. & Othman, F. Speckle Tracking Echocardiography in Very Preterm Infants: Feasibility and Reference Values. Early Hum Dev 90, 275–279 (2014).
Castaldi, B. et al. Early Modifications of Cardiac Function in Preterm Neonates Using Speckle Tracking Echocardiography. Echocardiography 35, 849–854 (2018).
Vyas-Read, S. et al. Early Characteristics of Infants with Pulmonary Hypertension in a Referral Neonatal Intensive Care Unit. BMC Pediatr 17, 163 (2017).
Iyer, N. P. & Mhanna, M. J. Non-Invasively Derived Respiratory Severity Score and Oxygenation Index in Ventilated Newborn Infants. Pediatr Pulmonol 48, 364–369 (2013).
Belletti, A., Lerose, C. C., Zangrillo, A. & Landoni, G. Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J Cardiothorac Vasc Anesth 35, 3067–3077 (2021).
Otani, K., Higa, Y., Kitano, T., Nabeshima, Y. & Takeuchi, M. Prediction of Cardiac Events Using Fully Automated Gls and Bnp Titers in Patients with Known or Suspected Heart Failure. PLoS One 15, e0234294 (2020).
Lang, R. M. et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28, 1–39.e14 (2015).
Desandes, R. et al. Echocardiography as a Guide for Patent Ductus Arteriosus Ibuprofen Treatment and Efficacy Prediction. Pediatr Crit Care Med 13, 324–327 (2012).
El Hajjar, M., Vaksmann, G., Rakza, T., Kongolo, G. & Storme, L. Severity of the Ductal Shunt: A Comparison of Different Markers. Arch Dis Child Fetal Neonatal Ed 90, F419–F422 (2005).
Szabo, D. et al. Influencing Factors of Cardiac Adaptation in Adolescent Athletes. Int J Sports Med 42, 1209–1221 (2021).
Rowland, D. G. & Gutgesell, H. P. Use of Mean Arterial Pressure for Noninvasive Determination of Left Ventricular End-Systolic Wall Stress in Infants and Children. Am J Cardiol 74, 98–99 (1994).
Toyoshima, K. et al. Tailor-Made Circulatory Management Based on the Stress-Velocity Relationship in Preterm Infants. J Formos Med Assoc 112, 510–517 (2013).
Kluckow, M. & Evans, N. Superior Vena Cava Flow in Newborn Infants: A Novel Marker of Systemic Blood Flow. Arch Dis Child Fetal Neonatal Ed 82, F182–F187 (2000).
Sengeløv, M. et al. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure with Reduced Ejection Fraction. JACC Cardiovasc Imaging 8, 1351–1359 (2015).
Tomotaki, S. et al. Proactive Diagnosis and Tailor-Made Treatment of Patent Ductus Arteriosus in Very Preterm Infants with Routine Echocardiography in Japan: A Post Hoc Analysis of the Plase Study. Neonatology, 1-9 (2024).
Acknowledgements
We thank all preterm infants and their families, and the staff at Kanagawa Children’s Medical Center, Yokohama. We thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This work was supported by JSPS KAKENHI (Grant Number: JP 21K07879).
Author information
Authors and Affiliations
Contributions
K Toyoshima and S Masutani designed the study. K Toyoshima, H Aoki, and N Saito performed the statistical analyses. K Toyoshima and S Masutani drafted the manuscript. All authors contributed to the interpretation of the findings and participated in the collection of data. All authors critically reviewed and revised the manuscript and provided final approval of the published version. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
This retrospective study was approved by the local ethicscommittee of Kanagawa Children’s Medical Center (No. 1806-07, December 2018), and written informed consent was obtained from all parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toyoshima, K., Aoki, H., Noguchi, T. et al. Cardiac adaptations to patent ductus arteriosus ligation in preterm infants: a speckle-tracking study. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04182-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04182-y
This article is cited by
-
Cardiac function in 6-year-old children born extremely preterm and associations to prolonged patent ductus arteriosus shunting
Scientific Reports (2026)
-
Biventricular adaptation after patent ductus arteriosus ligation
Pediatric Research (2025)
-
Early Echocardiographic Indicators of Pulmonary Vascular Disease in Preterm Infants at Risk for Bronchopulmonary Dysplasia
Pediatric Cardiology (2025)