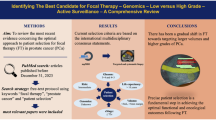
Abstract
Introduction
Prostate cancer (PCa) management poses challenges due to treatment-related morbidities associated with conventional therapies. Focal therapy (FT) is emerging as a promising alternative for intermediate-risk PCa, aiming to selectively target localized cancerous lesions while preserving healthy tissue. This review explores emerging FT modalities for PCa treatment, focusing on transrectal MRI-guided focused ultrasound surgery (MRgFUS), transurethral ultrasound ablation (TULSA), focal laser ablation (FLA), and histotripsy.
Methods
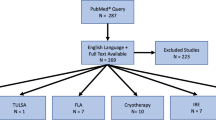
A comprehensive literature search was conducted to identify studies and clinical trials related to FT. Relevant articles were selected and data were synthesized to provide insights into the efficacy and feasibility of MRgFUS, TULSA, FLA, and histotripsy for FT.
Results
MRgFUS utilizes transrectal high-intensity focused ultrasound under MRI guidance to selectively ablate cancerous tissue, demonstrating positive outcomes in oncologic control and preservation of urinary and sexual function. TULSA employs transurethral delivery of high-intensity ultrasound energy under MRI guidance, showing promising results for whole gland treatment. FLA benefits from precise ablation, indicating effectiveness in tumor destruction while preserving quality-of-life. Histotripsy, a mechanical ablation method, exhibits promise by inducing tissue fractionation through bubble activity, offering advantages such as tissue selectivity and real-time treatment monitoring.
Conclusion
Emerging FT modalities present promising alternatives for the management of localized PCa, offering personalized treatment. Further research and clinical trials are warranted to establish the long-term efficacy of these techniques in PCa management.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is one of the most prevalent malignancies affecting men globally, with its management dependent on precise disease grading, staging, and risk assessment utilizing TNM staging, Gleason score, and prostate-specific antigen (PSA) levels [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
Conventional treatments for localized PCa, including radical prostatectomy (RP) and pelvic radiation therapy, offer disease control but are often associated with enduring urinary and sexual dysfunction, affecting over half of the treated patients [1, 2]. In contrast, while active surveillance is recommended for low-risk PCa and spares patients of treatment-related morbidities, its applicability in patients with intermediate-risk PCa remains contentious due to concerns regarding disease progression, particularly in men with magnetic resonance imaging (MRI) visible disease [3,4,5].
Advancements in disease localization techniques, particularly MRI, have facilitated the emergence of focal therapy (FT) as a promising treatment modality for PCa [6,7,8]. Targeted ablation of the clinically significant (cs) index lesion, often predictive of disease progression, while sparing adjacent healthy tissue, aims to minimize treatment-related morbidities [9]. By selectively treating only the cancerous areas that are likely to cause harm, there is an attempt to reduce the risk of side effects commonly associated with more aggressive treatments, such as urinary incontinence and sexual dysfunction. In 2022, the American Urological Association/American Society for Radiation Oncology guidelines were updated to state that minimally invasive FT lacks high quality data compared to existing conventional treatments for PCa but may be considered for intermediate-risk PCa in the appropriately counseled patient with clinical trial or prospective registry enrolment prioritized [10].
Guided by various imaging modalities, FT adopts a personalized approach tailored to individual patient characteristics. While ultrasound (US)-guided FT has undergone extensive investigation, more recently, there has been a growing interest in exploring and studying innovative MRI-guided techniques [6, 7, 11]. Since multiparametric MRI (mpMRI) is considered the standard of care for the detection of csPCa [6], recent studies have focused on MRI-guided FT. MRI-guidance allows better delineation of tumor in all 3 planes, enabling better targeting and planning. In addition, real-time thermal monitoring (MR-thermometry) allows instant real-time intra-procedure thermal feedback and provides an opportunity for optimization of ablation temperatures. However, since MRI may underestimate the histological tumor volume, targeted FT requires treatment beyond the visible tumor, ideally with a margin of 9-10 mm [12, 13]. MRI-guidance for FT, utilizing energy sources such as high-frequency focal ultrasound (HIFU), and interstitial laser thermal therapy (FLA) have been studied over the last few years.
In addition to developments in imaging, innovative energy sources such as histotripsy, a non-invasive non-thermal high-intensity ultrasound technique, have also been studied for FT. Histotripsy delivers short (microsecond to milliseconds duration) very-intense HIFU bursts (10-100 fold more intense than thermal exposures) to illicit bubble activity at the focus. Interactions between bubbles and ultrasound waves produce precise non-thermal mechanical ablation of targeted tissue [14]. Histotripsy is under development as a future FT of PCa [15, 16]. This review delves specifically into examining these newer techniques for FT for PCa and provides a comprehensive analysis of these emerging techniques in PCa management.
Transrectal MRI-guided focused ultrasound surgery (MRgFUS)
Transrectal MRI-guided focused ultrasound surgery (MRgFUS) has emerged as a promising modality for treating localized PCa in recent years. The ExAblate 2100 prostate device (Insightec Ltd, Haifa, Israel) is a non-invasive transrectal MRgFUS system [17]. Like other thermal HIFU devices, it utilizes the conversion of mechanical energy of sonication to thermal energy, raising the temperature to achieve tissue coagulation and allows for sharp ablation margins between the target zone and surrounding normal tissue [17,18,19] The phased-array transducer is made of approximately 1000 elements and operates within the range 0.8–3.5 MHz. The ultrasound beam can be electronically steered to the desired location in the gland planned for treatment.
The procedure is performed under general anesthesia or sedation, with the patient positioned in lithotomy on a modified MRI table. Following placement of a Foley catheter for continuous bladder drainage, the ExAblate endorectal probe is positioned in the rectum [17,18,19,20]. The endorectal balloon is then filled and circulated with degassed water at 14 °C for rectal and device cooling for protection. Initial imaging is obtained to ensure no air bubbles are present in the endorectal balloon, as air can disrupt ultrasound wave transmission. T2-weighted (T2WI) and diffusion-weighted (DWI) images are acquired for tumor localization and rectum, prostate and tumor contouring, including 10 mm margins when possible [17, 19]. The software then generates the treatment plan, specifying energy level and number of sonications. Subtherapeutic sonications are initially delivered for verification, followed by treatment sonications. Macrosonications are delivered on each axial slice covering the tumor and margins. Nominal sonication spots are delivered where required based on the MR-thermography feedback during treatment. Updated anatomical MRI between sonications allows intraoperative treatment plan modification. Post-ablation, dynamic contrast-enhanced (DCE) MRI confirms coverage and assesses the de-vascularized non-perfused volume (NPV) to confirm ablation [19] (Fig. 1).
A Pre-treatment axial T2-weighted fast spin‒echo MRI (repetition time (TR)/echo time (TE), 3820/97) and B apparent diffusion coefficient (ADC) map image, acquired on a 3T Siemens Skyra Fit scanner, showing the tumor in the left mid gland transition zone (arrows). C Intraoperative MRI obtained on a 1.5T GE Excite Twinspeed scanner showing the contoured rectal wall (red line), prostate margin (blue outline) and region of interest (orange outline). D Intraoperative MRI showing a focused ultrasound beam path (blue) overlaid on the treatment plan. The rectangular boxes within the region of interest illustrate each sonication spot. E MRI thermography image during treatment showing heat deposition color coded in red overlaid on the sonication spot. F Accumulated thermal dose map image at end of treatment depicting the predicted area of thermal damage color coded in blue. G Axial gadovist-enhanced MRI obtained immediately post-treatment showing the de-vascularized ablated volume (arrows). H T2-weighted fast spin‒echo MRI (TR/TE, 3820/97) obtained 24 months following the ablation on the same scanner, showing involution and volume loss at the treated area (arrow). Findings from a targeted biopsy of the treatment zone and the rest of the gland at 24 months were negative.
Over the past decade, various phase I and II clinical trials have demonstrated the safety and efficacy of MRgFUS FT as a treatment option in men with low and intermediate risk PCa yielding positive quality-of-life outcomes and oncologic responses. The primary findings of these trials are outlined in Table 1.
Initially, Napoli et al. conducted a proof-of-principle study, enrolling five patients with localized PCa, who underwent MRgFUS ablation followed by open radical prostatectomy [17]. No adverse surgical complications related to the MRgFUS procedure were noted. Histopathological analysis revealed extensive coagulative necrosis within the treatment zone, indicating the feasibility of MRgFUS ablation for localized PCa.
Subsequently, Tay et al. investigated MRgFUS focal ablation in 14 patients with low-risk PCa [21]. Follow-up assessments included monitoring of PSA levels, Expanded Prostate Cancer Index Composite (EPIC) questionnaire, mpMRI imaging, and biopsy. The procedure demonstrated good tolerance, with self-limiting hematuria being the most common early adverse effect. Functional outcomes related to sexual activity and urinary symptoms normalized in 3 months and thereafter. Although PSA levels decreased significantly by 38.8% at 3 months, some patients experienced an increase at 6 months, with biopsy revealing cancer outside the treatment area in 6 participants, one with Grade Group (GG) 2 PCa. At 2 years, template transperineal biopsy revealed two men with ≥GG2 disease.
In 2018, Ghai et al. reported a phase I study involving eight patients (10 lesions) with PCa [<GG3 PCa] [18]. Results indicated the feasibility and safety of MRgFUS therapy with favorable short-term oncologic outcomes. No adverse events were reported during the perioperative period, and functional outcomes, as assessed by International Prostate Symptom Score (IPSS) and International Index of Erectile Function (IIEF)-15 scores, remained largely unchanged post-treatment. At 6 months, one participant had persistent ≥ GG2 disease at the treatment site.
More recently, Ehdaie et al. conducted a multicenter phase II study involving 101 patients with unilateral intermediate-risk PCa (79 with GG2 and 22 with GG3 PCa) [22]. At the 6-month assessment, a significant decrease in PSA levels was observed, with most patients (96/101) showing no evidence of csPCa on biopsy. At 24 months, 88% (78/89) of participants did not harbor csPCa (≥GG2) at the treatment site on biopsy. There was a slight decline in IIEF-15 scores (difference of -3.5) at 2 years.
Additionally, a single-center phase II trial by Ghai et al. evaluated 2-year oncological and functional outcomes in 44 patients with unifocal csPCa (36 with GG2 and 8 GG3 PCa) [23, 24]. Median procedure and sonication times were 256 and 125 min respectively. The authors reported that men with larger ablation volumes (>15cc) had a greater decline in IIEF-15 scores during the early period following treatment. The majority of patients exhibited favorable outcomes, with 39/43 men (91%) exhibiting no residual csPCa at the treatment site over the 2-year period on biopsy. There were 3 additional men with de novo csPCa outside the treatment area. No significant change was noted in the median IIEF-15 and IPSS scores between baseline and 24 months, and no participant reported pad use. Overall, these studies collectively suggest that MRgFUS FT is a promising treatment option for PCa, offering favorable safety profiles and very encouraging oncological responses.
Advantages of MRgFUS FT for PCa include its minimally invasive approach, precise targeting enabled by MRI guidance, and real-time temperature feedback during treatment to ensure ablative temperatures (>65 °C) are reached [17, 25]. Additionally, post-treatment contrast-enhanced images enable immediate assessment of the NPV and coverage of the ablated area. While MRgFUS procedure times are longer than for US-guided HIFU, the overall safety profile and oncologic outcomes suggest MRgFUS as a favorable modality for focal PCa treatment [23, 26, 27]. It offers personalized treatment with the potential to enhance patient outcomes and quality-of-life, compared to existing FT literature.
Despite its advantages, MRgFUS has some limitations, similar to other transrectal HIFU devices. The transrectal MRgFUS approach may limit access to lesions in the anterior gland [28]. The Phase II studies described above did not include tumors >4–6 cm from the rectal wall. Additionally, the procedure requires access to MRI and expertise, leading to additional costs.
Transurethral ultrasound ablation (TULSA)
TULSA, or transurethral ultrasound ablation (TULSA-PRO, Profound Medical Inc, Toronto, Canada), is a minimally invasive procedure developed in 2012 for the treatment of PCa [29]. Using a transurethral approach, TULSA delivers ablative therapy directly to the prostate under MRI guidance for precise localization and MR thermometry for real-time monitoring [30]. The TULSA-PRO device, featuring rectal and urethral cooling, uses a linear array of 10 US transducers emitting high-intensity energy directionally into the prostate, intending consistent ablation while concurrently protecting the urethra and anterior rectal wall [30]. During the procedure, the US applicator is positioned within the prostatic urethra with the first US element placed 3 mm from the apical margin to provide safety for the apical sphincter [29]. Targeting temperatures of ≥55 °C, TULSA achieves acute thermal coagulation [29]. Immediately post-treatment, contrast-enhanced MRI assesses the NPV to confirm ablation (Fig. 2).
Treatment was circumferential and primarily using elements E2-E8 for this small (35cc) prostate. The Thermal Dose images show good distribution of ablation energy throughout the gland, while urethral sparing is most evident on the Maximum Temperature images. Red indicates the hottest temperatures, and yellow through red indicates that ablative temperatures have been reached. A 1-year post-treatment biopsy was negative and the patient’s PSA has fluctuated between 0.2 ng/ml and 0.6 ng/ml over 3 years post-treatment, with most recent PSA at 0.4 ng/ml.
The literature on TULSA showcases encouraging results in safety and efficacy for treating PCa in select patients (Table 2). In a proof-of-principle treat-and-resect investigation, Chopra et al. assessed its feasibility in 8 individuals with ≤GG3. They demonstrated a mean spatial targeting accuracy of −1.0 mm ± 2.6 mm, with no evidence of thermal effects on surrounding structures [30].
Subsequently, Chin et al. led a multi-center phase I investigation assessing whole-gland TULSA in 30 patients with low- to intermediate-risk PCa (24 GG1, 6 GG2) [29]. There were no rectal injuries or fistulae. At 12 months follow-up, there were no significant differences in mean functional outcomes, as assessed by the IPSS and IIEF-15 scores and erections sufficient for penetration were maintained in 85%. Median PSA decreased from 5.8 ng/ml at baseline to 0.8 ng/ml at 12 months. Of the 29 patients who completed a follow-up biopsy, 9 were positive for csPCa (≥GG2), of whom 2 underwent salvage prostatectomy at 12 months. In a 3-year follow-up of the initial phase I population, Nair et al. in 2021 reported that 32% (7 of 22) had recurrent PCa [31]. Of these 7 men, 4 underwent salvage prostatectomies. Pathology confirmed the location of the residual disease was congruent with the untreated peripheral zone safety region [31].
A pivotal multi-center evaluation of TULSA by Klotz et al. was reported on 115 men with low- to intermediate-risk PCa [43 GG1, 69 GG2, 3 GG3], undergoing whole gland evaluation [32]. Median procedure and ablation times were 243 and 51 minutes respectively, similar to those reported for the MRgFUS procedures. Twelve Grade 3 adverse events (UTI, urethral stricture, urinary retention, urethral calculus and urinoma) were recorded in 9 participants, all resolved by 12-months. No rectal injury was reported. Erections were maintained or regained at 12 months in 75% (69 of 92) of potent men at baseline. Three participants had moderate urinary incontinence at 12 months and <1% of men were incontinent to a >1 pad/day level. Primary efficacy endpoint of PSA reduction of ≥75% was achieved in 96% (110 of 115) of patients with decrease in median PSA from 6.3 ng/ml to 0.34 ng/ml. There was a concurrent 91% median prostate volume reduction from 37 cc at baseline to 2.8 cc at 12 months [32]. At 12 months follow-up, 65% (72 of 111) of patients had no evidence of cancer and 79% (54 of 68) with ≥GG2 disease at baseline were free of csPCa. Amongst the men with residual disease, 8 sought salvage treatment at 12 months (4 prostatectomy and 4 radiation therapy). The multivariate predictors of persistent csPCa at 12 months were intraprostatic calcifications at screening, suboptimal MRI thermal coverage of target volume, and PI-RADS ≥ 3 lesions on MRI (p < 0.05) [32].
While these multi-center studies assessed whole gland / sub-total ablation with the TULSA-PRO device, there have been a few recent treat-and-resect studies assessing sectoral FT. Ramsay et al. assessed safety and feasibility of sectoral ablation extending to the capsule in 5 patients with ≤GG2 PCa. Whole mount histology demonstrated an average target accuracy −1.5 mm ±2.8 mm [33]. In another trial by Anttinen et al., 6 patients (≤GG4) underwent lesion-targeted FT with the TULSA device, followed by robot-assisted-laparoscopic-prostatectomy at 3 weeks [34]. On histopathology, no viable tumor was noted in the ablation zones, but 4 of 6 patients had residual cancer outside of the planned ablation volume, within the pre-planned 3 mm safety margin, near the neurovascular bundle [34].
Anttinen et al. further conducted a phase I investigation of salvage TULSA in the treatment of radiorecurrent PCa in 11 patients [35]. At 12 months, there were no urethral strictures, rectal injuries, or fistulas. The median EPIC-26 irritative/obstructive domain decreased by 20% and 91% (10 of 11) men were free of any PCa in the targeted ablation zone [35].
Overall, the current literature suggests TULSA holds promise as a safe and effective treatment option for low to intermediate risk PCa, with encouraging early oncologic and quality-of-life outcomes.
The transurethral approach of TULSA is amenable to treating anterior lesions, which may lie beyond the reach of transrectal approaches [30]. Similar to other MRI-guided FT, MR thermometry for real-time monitoring of ablation temperatures further enhances treatment precision and safety [6]. One aspect unique to TULSA is that the measured temperatures are also used as a quantitative input to a feedback control algorithm that dynamically modulates the US intensity and frequency of each treatment element and the device rotation rate to ensure the target volume reaches therapeutic temperatures [29].
While TULSA offers a non-invasive transurethral approach, inserting a rigid US applicator into the prostatic urethra may lead to discomfort and potential complications, such as urethral injury or stricture [32]. Similar to other intra-procedural MRI guidance techniques, it adds complexity and time to the procedure, potentially increasing overall treatment costs and resource utilization. Notably, challenges related to intraprostatic calcifications may affect treatment efficacy and increase the risk of residual and recurrent disease [32]. It is recommended that patients with coarse calcifications in the beam path be excluded from TULSA treatment [32].
Focal laser ablation (FLA)
Focal Laser Ablation (FLA) is an interstitial procedure to treat localized PCa by inserting laser fibers into the prostate gland under image guidance, typically using transperineal or transrectal approaches [36, 37]. Once positioned, the laser emits beams of electromagnetic radiation, typically in the infrared spectrum (700–1064 nm), directly into the targeted tissue. The laser’s energy rapidly elevates the prostate tissue’s temperature, inducing protein denaturation and coagulative necrosis, leading to tissue destruction [38]. The diode laser fibers ablate a cylindrical zone with ablation diameters of <15 mm, and thereby multiple applicators are required to cover the planned ablation volume including margins beyond the MRI visible tumor (Fig. 3).
A Pre-treatment axial T2-weighted fast spin‒echo MRI (repetition time (TR)/echo time (TE), 7990/97) and B Corresponding diffusion weighted Image (DWI) image, b 1600 s/mm2, acquired on a 3T Siemens Skyra Fit scanner, showing the tumor in the right mid gland peripheral zone (arrows). C Intraoperative MRI obtained on a 1.5T GE Excite Twinspeed scanner showing the contoured rectal wall (orange line), prostate margin (blue outline) and region of interest (red outline) with 5 mm and 10 mm margins (maroon outline). D MRI thermography image during treatment showing heat deposition color coded in green overlaid on the contoured region of interest. E Axial gadovist-enhanced MRI obtained immediately post-treatment showing the de-vascularized ablated volume (arrows). F T2-weighted fast spin‒echo MRI (TR/TE, 7990/97) obtained 6 months following the ablation on the same scanner, showing involution and volume loss at the treated area (arrow). Findings from a targeted biopsy of the treatment zone at 6 months were negative.
FLA has garnered increasing attention as a FT option for localized PCa, with numerous recent studies shedding light on its efficacy, safety profile, and potential as an alternative to radical treatments in selected patients (Table 3).
Although, FLA has been predominantly evaluated under MRI guidance, some studies have assessed its feasibility under US / MRI- Transrectal (TRUS) fusion guidance, and with the new high-resolution micro-US. MRI guidance allows definition of the tumor in all 3 planes in addition to MRI thermometry. On the other hand, ultrasound guidance enables shorter treatment durations and requires fewer resources compared to MRI guidance, resulting in reduced costs.
An initial Phase 1 feasibility and proof-of-principle study by Lindner et al. from the University of Toronto, validated laser energy as an ablative modality using imaging and histopathology correlation [39, 40]. Twelve men with low-risk PCa underwent FLA with water-cooled 980 nm diode laser fibers (Visualase Inc., Houston, Texas) using a prototype MRI-TRUS fusion device, and four of these men underwent radical prostatectomy 1 week following FLA. The ablation volume measured on MR images was, on average, 1.1 times larger than the ablation volumes calculated using vital stain histopathology images (range 0.96–1.29). The same group also published the first study establishing the feasibility of FT under MRI guidance. Two patients with low-risk PCa were treated with outpatient MRI-guided FLA and MRI thermometry monitoring. Accumulated thermal damage was calculated in real time, and immediate post-contrast images confirmed the devascularization of the target [41].
Subsequently, Phase 1 and Phase II studies assessing in-bore transperineal FLA utilizing Visualase were reported by Oto et al. and Eggener et al. from University of Chicago [37, 42]. Nine men with low-risk MRI visible PCa (≤GG2) were successfully treated in the Phase 1 study. 7/9 (78%) men had no residual PCa at the treatment site on follow-up biopsy, and there was no significant change from baseline in the average Sexual Health Inventory for Men (SHIM) and IPSS scores. In their Phase II study, 27 men [23 GG1, 3 GG2 and 1 GG3 PCa] with MRI-visible stage T1c-T2a disease and PSA < 15 ng/ml were treated by transperineal MRI-guided FLA and followed for 12 months. Persistent disease at the treatment site was detected in 3 (11%) men on biopsy. No significant change in IPSS and SHIM scores were noted over the 12-month period.
In 2015, Lepor et al. reported 3-month early results from 25 consecutive men with MRI-visible localized disease (11 GG1, 13 GG2, 1 GG3 PCa) who underwent in-bore MRI-guided FLA for PCa with Visualase using MRI-thermometry temperature monitoring [43]. The study found no significant differences in SHIM scores between baseline and the 3-month mark, and none of the participants required pads. Mean PSA levels decreased by 2.3 ng/ml (44.2%) between baseline and 3-months. 26/28 sites subjected to target biopsy (96%) showed no evidence of PCa. While early results of this study were very promising, longer-term follow-up was lacking to establish the durability of oncologic control.
Al-Hakeem et al. evaluated oncological and functional outcomes in 49 patients (53 lesions) treated with FLA for low- and intermediate-risk PCa (13 GG1, 29 GG2, 7GG3) [44]. The procedure was performed in-bore either transperineally or transrectally based on the location of the tumor. All men underwent per protocol biopsy at 6 months and then as indicated based on PSA or mpMRI. At 18 months post-treatment, 39 patients (79.6%) experienced treatment success per the Donaldson criteria, with no cancer or only insignificant cancer in the ablated area [44]. No significant complications were noted. IPSS scores remained unchanged. SHIM scores dropped in the first year after treatment but were not different from baseline at 18 months. PSA levels remained significantly lower at 18 months vs. baseline.
In another study of 120 patients with low- to intermediate-risk PCa (37 GG1, 56 GG2, 27 GG3) treated by transrectal MRI-guided FLA using Visualase, 17% of patients required additional oncological treatment after 1 year, with no significant change in their quality-of-life or urologic function [45]. However, only men with suspicious post-treatment MRI or PSA elevation underwent a biopsy. With further refined techniques, including hemi-ablations with larger margins, this clinically significant residual tumor rate at 1-year follow-up post-FLA decreased to 6.8% of patients [45]. Moreover, it was identified that the tumor size was the only predictor for a positive post-ablation MRI risk, suggesting its significance in aiding future patient selection.
Chao and Lepor’s prospective investigation assessed 5-year failure-free survival (FFS) following transrectal FLA with Visualase and found that 83% patients (25 of 30) exhibited 5-year FFS [46]. Notably, 10 patients (40%) developed in-field recurrence, with 9 patients undergoing further salvage therapy with partial ablation with FLA, Cryoablation, or HIFU [46]. Additionally, Mehralivand et al. reported oncological and functional outcomes in 15 patients treated with transperineal FLA using Visualase over a 3-year follow-up [47]. No severe or persistent post-procedural complications were reported [47]. 47% (7 of 15 patients had PCa recurrence at follow-up. More recently, Magee et al. demonstrated the feasibility of salvage MRI-guided FLA for HIFU recurrences utilizing non-water cooled 1063 nm laser fibers [Clinical Laserthermia System (CLS) Inc, Lund, Sweden] [48].
Following the initial Phase I FLA study for localized PCa by Lindner et al. utilizing a prototype MRI-TRUS guidance for guidance in 2010, Natarajan et al. confirmed the feasibility of performing FLA under MRI-TRUS guidance using Visualase in 11 men [40, 49]. More recently, van Riel et al. demonstrated safety and feasibility of transperineal FLA using the Echolaser system (Calenzano, Italy) in 12 men prior to radical prostatectomy [50]. Ablation zone volumes on MRI and contrast enhanced US (CEUS) showed good correlation with histology (Pearson r = 0.94 [95% CI]). On-going studies are also exploring FLA under micro-US guidance [51]. With studies reporting real-time visualization of MRI lesions with micro-US, it may obviate the need for MRI-TRUS fusion [52, 53].
FLA offers several advantages over traditional treatments for localized PCa. Its real-time MRI imaging guidance ensures precise real-time targeting of lesions, minimizing damage to surrounding healthy tissue [38]. Moreover, due to the absorption rate and low vascularity, prostate tissue is well-suited for FLA and allows for finely controlled ablation [36, 54]. Similar to other focal therapies for PCa, studies have highlighted the preservation of urinary and sexual function in patients post-FLA, highlighting its potential to maintain quality-of-life compared to more invasive radical therapies [38]. Numerous studies have previously found that FLA or secondary whole-gland therapies remain viable options for post-FLA treatment.
One limitation of FLA is that within the current literature, most investigations for FLA for PCa treatment are small, non-randomized studies with relatively short-term follow-up periods [55]. Notably, there are currently numerous active phase II clinical trials assessing in-bore FLA for treating PCa including at the Princess Margaret Hospital, Toronto; Radboud University Medical Center, Nijmegan; and Mayo clinic, Rochester [56,57,58].
Histotripsy
Histotripsy is a non-invasive pulsed HIFU technique that produces non-thermal mechanical ablation of tissue through delivery of short bursts (microseconds to milliseconds-long) of high-amplitude HIFU waves containing shock fronts [14, 59]. These bursts induce bubble activity at the focal point, and interactions between bubbles and ultrasound waves breaks tissue into subcellular components. Compared to thermal HIFU ablation, histotripsy employs pulses with higher intensity (10-100 fold greater) delivered at a lower duty factor (≤1%) [14, 60, 61].
There are two primary methods of initiating bubble activity in histotripsy: ‘cavitation cloud’ histotripsy and ‘boiling’ histotripsy. Cavitation cloud histotripsy can be performed using shock scattering or intrinsic cavitation techniques using pulses ≤20 microseconds in duration at pulse repetition frequencies up to several hundred pulses per second. Conversely, boiling histotripsy uses pulses of 1–10 milliseconds duration with pulse repetition frequencies up to 10 pulses per second [14]. Both techniques rely on nonlinear propagation of sound waves, leading to the formation of shock waves at the focal point to create bubbles. With cavitation-based techniques the high peak negative pressure of the shocks result in dense cavitation bubble clouds that produce tissue fractionation [14, 62]. In boiling histotripsy, extremely rapid shock induced heating generates a vapor bubble (i.e., boiling) at the focus. As long as pulse duration is just longer than time to boil, and duty factor is low (≤1%), boiling histotripsy produces identical tissue fractionation without thermal effects [61, 63].
Histotripsy techniques offer advantages over thermal ablation, such as tissue selectivity, where cells are more sensitive to damage than extracellular structures (e.g., blood vessels and ducts) [64]. Additionally, histotripsy is not affected by heat sink effects or increased perfusion, addressing challenges faced by thermal ablation methods [65, 66]. Further, owing to the appearance of hyperechoic bubbles at the focus, and subsequent production of a hypoechoic cavity with treatment (from mechanical loss of tissue scatters), histotripsy enables ultrasound imaging based real-time treatment monitoring and feedback that is not possible with thermal techniques [59,60,61].
Histotripsy technology for prostate ablation has been tested in several preclinical animal studies [67,68,69,70,71,72]. The feasibility of prostate histotripsy was demonstrated in canine subjects using a 750 kHz transducer with a pulse repetition frequency (PRF) of 100–500 Hz (duty cycle <0.4%) [68]. It was noted that the dense peri-urethral tissue required a greater number of pulses for tissue fractionation [68, 71]. A pilot ex vivo study using boiling histotripsy showed subcellular tissue fractionation in both human PCa and benign prostate parenchyma, thereby establishing that human PCa tissue can be mechanically ablated using the boiling histotripsy method [15] [Fig. 4].
B-mode ultrasound appearance of tissue (A) pre-treatment, (B) during treatment demonstrating hyperechoic bubbles at the focus (within red oval), and (C) after treatment demonstrating a hypoechoic cavity consistent with histotripsy induced mechanical fractionation. Red arrow indicates the direction of BH sonications. Histologic appearance of H&E stained human prostate tissue from a rapid autopsy with Gleason 5 + 5 = 10 prostate cancer treated with boiling hisotripsy at low power (D), medium power (E), and high power (F). Histotripsy induced cellular fractionation is seen on lower power within the dashed boundary. At higher power sharp demarcation between treated (top half of panels E and D) and untreated tissue (bottom half of panels E and D) is seen with a boundary of ~100 μm. Untreated prostate cancer cells (black arrowhead) are present outside the treatment zone. Figure adapted from Rosnitskiy PB, et al. Ultrasonics 2023; 133: 107029 with permission.
Existing pre-clinical data for prostate histotripsy is encouraging and suggests that with further refinement it could be assessed in clinical trials as a novel PCa FT technique.
Conclusion
In conclusion, emerging FT techniques, including MRgFUS, TULSA, and FLA, represent innovative approaches for treating localized PCa while minimizing treatment-related side effects (Table 4). Clinical trials have demonstrated the safety and efficacy of these modalities, offering promising outcomes in oncological control and preservation of quality-of-life. Ablation of smaller volumes is associated with reduced functional decline and the precision of MRI allows for better targeting in all planes and thereby minimizes unnecessary treatment of surrounding healthy tissue [23, 26, 27]. While still in development, histotripsy offers advantages such as tissue selectivity, lack of heat sink effects and improved real-time treatment monitoring.
Different FT technologies have proven efficacy in managing localized prostate cancer. The collective body of evidence underscores the evolving landscape of FT in PCa management.
Beyond the clinical evidence, discussed for newer technologies in this review, factors such as tumor location and characteristics play a key role. Local infrastructure and expertise are also crucial in selecting a platform for focal treatment. We advocate for a tailored ‘a la carte’ approach, choosing the most appropriate technology based on the tumor’s specific features (size, location, distance from rectum, presence or calcification etc), local expertise and technology availability.
Despite the advantages, challenges such as limited long-term data and logistical complexities remain. Future research endeavors, including randomized controlled trials such as FARP (Focal Prostate Ablation versus Radical Prostatectomy) and ENFORCE (Effectiveness of Focal therapy in Men with PCa) with long-term follow-up and advancements in technology, are crucial to facilitate the integration of these novel therapies into clinical practice [73, 74]. As the field continues to advance, personalized approaches tailored to individual patient characteristics and preferences will likely play a pivotal role in optimizing treatment strategies for localized PCa, ultimately enhancing patient care and prognosis.
References
Hopstaken JS, Bomers JGR, Sedelaar MJP, Valerio M, Fütterer JJ, Rovers MM. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur Urol. 2022;81:5–33.
Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules. 2022;27:5730.
Baboudjian M, Breda A, Rajwa P, Gallioli A, Gondran-Tellier B, Sanguedolce F, et al. Active Surveillance for Intermediate-risk Prostate Cancer: A Systematic Review, Meta-analysis, and Metaregression. Eur Urol Oncol. 2022;5:617–27.
Li P, You S, Nguyen C, Wang Y, Kim J, Sirohi D, et al. Genes involved in prostate cancer progression determine MRI visibility. Theranostics. 2018;8:1752–65.
Wibmer AG, Lefkowitz RA, Lakhman Y, Chaim J, Nikolovski I, Sala E, et al. MRI-Detectability of Clinically Significant Prostate Cancer Relates to Oncologic Outcomes After Prostatectomy. Clin Genitourin Cancer. 2022;20:319–25.
Alabousi M, Ghai S, Haider MA. MRI-guided Minimally Invasive Focal Therapies for Prostate Cancer. Radiology. 2023;309:e230431.
Ghai S, Perlis N. Beyond the AJR: MRI-Guided Focused Ultrasound Focal Therapy for Intermediate-Risk Prostate Cancer. Am J Roentgenol. 2023;220:610–610.
Tay KJ, Scheltema MJ, Ahmed HU, Barret E, Coleman JA, Dominguez-Escrig J, et al. Patient selection for prostate focal therapy in the era of active surveillance: an International Delphi Consensus Project. Prostate Cancer Prostatic Dis. 2017;20:294–9.
Jaipuria J, Ahmed HU. Clinical and pathologic characteristics to select patients for focal therapy or partial gland ablation of nonmetastatic prostate cancer. Curr Opin Urol. 2022;32:224–30.
Eastham JA, Boorjian SA, Kirkby E. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. J Urol. 2022;208:505–7.
Bomers JGR, Sedelaar JPM, Barentsz JO, Fütterer JJ. MRI-Guided Interventions for the Treatment of Prostate Cancer. Am J Roentgenol. 2012;199:714–20.
Le Nobin J, Rosenkrantz AB, Villers A, Orczyk C, Deng F-M, Melamed J, et al. Image Guided Focal Therapy for Magnetic Resonance Imaging Visible Prostate Cancer: Defining a 3-Dimensional Treatment Margin Based on Magnetic Resonance Imaging Histology Co-Registration Analysis. J Urol. 2015;194:364–70.
Pooli A, Johnson DC, Shirk J, Markovic D, Sadun TY, Sisk AE, et al. Predicting Pathological Tumor Size in Prostate Cancer Based on Multiparametric Prostate Magnetic Resonance Imaging and Preoperative Findings. J Urol. 2021;205:444–51.
Khokhlova VA, Fowlkes JB, Roberts WW, Schade GR, Xu Z, Khokhlova TD, et al. Histotripsy methods in mechanical disintegration of tissue: towards clinical applications. Int J Hyperthermia. 2015;31:145–62.
Rosnitskiy PB, Tsysar SA, Karzova MM, Buravkov SV, Malkov PG, Danilova NV, et al. Pilot ex vivo study on non-thermal ablation of human prostate adenocarcinoma tissue using boiling histotripsy. Ultrasonics. 2023;133:107029.
Schade GR, Keller J, Ives K, Cheng X, Rosol TJ, Keller E, et al. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J Urol. 2012;188:1957–64.
Napoli A, Anzidei M, Ciolina F, Marotta E, Cavallo Marincola B, Brachetti G, et al. MR-guided high-intensity focused ultrasound: current status of an emerging technology. Cardiovasc Intervent Radiol. 2013;36:1190–203.
Ghai S, Perlis N, Lindner U, Hlasny E, Haider MA, Finelli A, et al. Magnetic resonance guided focused high frequency ultrasound ablation for focal therapy in prostate cancer - phase 1 trial. Eur Radiol. 2018;28:4281–7.
Ghai S, Louis AS, Van Vliet M, Lindner U, Haider MA, Hlasny E, et al. Real-Time MRI-Guided Focused Ultrasound for Focal Therapy of Locally Confined Low-Risk Prostate Cancer: Feasibility and Preliminary Outcomes. AJR Am J Roentgenol. 2015;205:W177–184.
Orihuela E, Pow-Sang M, Motamedi M, Cowan DF, Warren MM. Mechanism of healing of the human prostatic urethra following thermal injury. Urology. 1996;48:600–8.
Tay KJ, Cheng CWS, Lau WKO, Khoo J, Thng CH, Kwek JW. Focal Therapy for Prostate Cancer with In-Bore MR-guided Focused Ultrasound: Two-Year Follow-up of a Phase I Trial-Complications and Functional Outcomes. Radiology. 2017;285:620–8.
Ehdaie B, Tempany CM, Holland F, Sjoberg DD, Kibel AS, Trinh Q-D, et al. MRI-Guided Focused Ultrasound (MRgFUS) Focal Therapy for Intermediate-Risk Prostate Cancer: A Phase 2b Multicenter Study. Lancet Oncol. 2022;23:910–8.
Ghai S, Finelli A, Corr K, Chan R, Jokhu S, Li X, et al. MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial. Radiology. 2021;298:695–703.
Ghai S, Finelli A, Corr K, Lajkosz K, McCluskey S, Chan R, et al. MRI-guided Focused Ultrasound Focal Therapy for Intermediate-Risk Prostate Cancer: Final Results from a 2-year Phase II Clinical Trial. Radiology. 2024;310:e231473.
Napoli A, Alfieri G, Scipione R, Leonardi A, Fierro D, Panebianco V, et al. High-intensity focused ultrasound for prostate cancer. Expert Rev Med Devices. 2020;17:427–33.
Ahmed HU, Dickinson L, Charman S, Weir S, McCartan N, Hindley RG, et al. Focal Ablation Targeted to the Index Lesion in Multifocal Localised Prostate Cancer: a Prospective Development Study. Eur Urol. 2015;68:927–36.
Feijoo ERC, Sivaraman A, Barret E, Sanchez-Salas R, Galiano M, Rozet F, et al. Focal High-intensity Focused Ultrasound Targeted Hemiablation for Unilateral Prostate Cancer: A Prospective Evaluation of Oncologic and Functional Outcomes. Eur Urol. 2016;69:214–20.
Huber PM, Afzal N, Arya M, Boxler S, Dudderidge T, Emberton M, et al. Focal HIFU therapy for anterior compared to posterior prostate cancer lesions. World J Urol. 2021;39:1115–9.
Chin JL, Billia M, Relle J, Roethke MC, Popeneciu IV, Kuru TH, et al. Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Tissue in Patients with Localized Prostate Cancer: A Prospective Phase 1 Clinical Trial. Eur Urol. 2016;70:447–55.
Chopra R, Colquhoun A, Burtnyk M, N’djin WA, Kobelevskiy I, Boyes A, et al. MR imaging-controlled transurethral ultrasound therapy for conformal treatment of prostate tissue: initial feasibility in humans. Radiology. 2012;265:303–13.
Nair SM, Hatiboglu G, Relle J, Hetou K, Hafron J, Harle C, et al. Magnetic resonance imaging-guided transurethral ultrasound ablation in patients with localised prostate cancer: 3-year outcomes of a prospective Phase I study. BJU Int. 2021;127:544–52.
Klotz L, Pavlovich CP, Chin J, Hatiboglu G, Koch M, Penson D, et al. Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Cancer. J Urol. 2021;205:769–79.
Ramsay E, Mougenot C, Staruch R, Boyes A, Kazem M, Bronskill M, et al. Evaluation of focal ablation of MRI-defined prostate cancer using MRI-controlled transurethral ultrasound therapy with prostatectomy as the reference standard. J Urol. 2017;197:255–61.
Anttinen M, Mäkelä P, Suomi V, Kiviniemi A, Saunavaara J, Sainio T, et al. Feasibility of MRI-guided transurethral ultrasound for lesion-targeted ablation of prostate cancer. Scand J Urol. 2019;53:295–302.
Anttinen M, Mäkelä P, Viitala A, Nurminen P, Suomi V, Sainio T, et al. Salvage Magnetic Resonance Imaging–guided Transurethral Ultrasound Ablation for Localized Radiorecurrent Prostate Cancer: 12-Month Functional and Oncological Results. Eur Urol Open Sci. 2020;22:79–87.
Lee T, Mendhiratta N, Sperling D, Lepor H. Focal Laser Ablation for Localized Prostate Cancer: Principles, Clinical Trials, and Our Initial Experience. Rev Urol. 2014;16:55–66.
Oto A, Sethi I, Karczmar G, McNichols R, Ivancevic MK, Stadler WM, et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267:932–40.
Lindner U, Lawrentschuk N, Trachtenberg J. Focal laser ablation for localized prostate cancer. J Endourol. 2010;24:791–7.
Lindner U, Lawrentschuk N, Weersink RA, Davidson SRH, Raz O, Hlasny E, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol. 2010;57:1111–4.
Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SRH, Atri M, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–7.
Raz O, Haider MA, Davidson SRH, Lindner U, Hlasny E, Weersink R, et al. Real-time magnetic resonance imaging-guided focal laser therapy in patients with low-risk prostate cancer. Eur Urol. 2010;58:173–7.
Eggener SE, Yousuf A, Watson S, Wang S, Oto A. Phase II Evaluation of Magnetic Resonance Imaging Guided Focal Laser Ablation of Prostate Cancer. J Urol. 2016;196:1670–5.
Lepor H, Llukani E, Sperling D, Fütterer JJ. Complications, Recovery, and Early Functional Outcomes and Oncologic Control Following In-bore Focal Laser Ablation of Prostate Cancer. Eur Urol. 2015;68:924–6.
Al-Hakeem Y, Raz O, Gacs Z, Maclean F, Varol C. Magnetic resonance image-guided focal laser ablation in clinically localized prostate cancer: safety and efficacy. ANZ J Surg. 2019;89:1610–4.
Walser E, Nance A, Ynalvez L, Yong S, Aoughsten JS, Eyzaguirre EJ, et al. Focal Laser Ablation of Prostate Cancer: Results in 120 Patients with Low to Intermediate Risk Disease. J Vasc Interv Radiol. 2019;30:401–9.e2.
Chao B, Lepor H. 5-Year Outcomes Following Focal Laser Ablation of Prostate Cancer. Urology. 2021;155:124–9.
Mehralivand S, George AK, Hoang AN, Rais-Bahrami S, Rastinehad AR, Lebastchi AH, et al. MRI-guided focal laser ablation of prostate cancer: a prospective single-arm, single-center trial with 3 years of follow-up. Diagn Interv Radiol. 2021;27:394–400.
Magee D, Perlis N, Corr K, Chan R, Gertner M, Zisman A, et al. Salvage interstitial laser thermal therapy under MRI guidance (MRgFLA) for high-intensity focal ultrasound (HIFU) recurrences: feasibility study. Clin Imaging. 2021;76:217–21.
Natarajan S, Jones TA, Priester AM, Geoghegan R, Lieu P, Delfin M, et al. Focal Laser Ablation of Prostate Cancer: Feasibility of Magnetic Resonance Imaging-Ultrasound Fusion for Guidance. J Urol. 2017;198:839–47.
van Riel LAMJG, van Kollenburg RAA, Vis AN, van Leeuwen PJ, de Reijke TM, de Bruin DM, et al. Safety and Feasibility of Soractelite Transperineal Focal Laser Ablation for Prostate Cancer and Short-term Quality of Life Analysis from a Multicenter Pilot Study. Eur Urol Open Sci. 2022;39:48–54.
Genesis Research LLC. Investigator Initiated Trial to Further Evaluate the Safety and Efficacy of Trans-perineal Focal Laser Ablation of Localized Prostate Cancer Using High Frequency Micro-ultrasound Imaging. 2023. https://clinicaltrials.gov/study/NCT05826470. Accessed 31 Dec 2023.
Ghai S, Perlis N, Atallah C, Jokhu S, Corr K, Lajkosz K, et al. Comparison of Micro-US and Multiparametric MRI for Prostate Cancer Detection in Biopsy-Naive Men. Radiology. 2022;305:390–8.
Cornud F, Lefevre A, Flam T, Dumonceau O, Galiano M, Soyer P, et al. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: initial results of a single-center study. Eur Radiol. 2020;30:4838.
Wenger H, Yousuf A, Oto A, Eggener S. Laser Ablation as Focal Therapy for Prostate Cancer. Curr Opin Urol. 2014;24:236–40.
van Luijtelaar A, Greenwood BM, Ahmed HU, Barqawi AB, Barret E, Bomers JGR, et al. Focal laser ablation as clinical treatment of prostate cancer: report from a Delphi consensus project. World J Urol. 2019;37:2147–53.
University Health Network, Toronto. MRI Guided Focal Laser Ablation of Prostate Cancer. 2020. https://clinicaltrials.gov/study/NCT03650595. Accessed 31 Dec 2023.
Radboud University Medical Center. Magnetic Resonance Imaging-guided Focal Laser Ablation of Prostate Cancer. 2022. https://clinicaltrials.gov/study/NCT05370482. Accessed 31 Dec 2023.
Woodrum DA. A Pilot Study to Evaluate Magnetic Resonance Thermal Image-Guided Focal Laser Ablation of Prostate Cancer Tumors. 2023. https://clinicaltrials.gov/study/NCT02600156. Accessed 31 Dec 2023.
Dubinsky TJ, Khokhlova TD, Khokhlova V, Schade GR. Histotripsy: The Next Generation of High-Intensity Focused Ultrasound for Focal Prostate Cancer Therapy. J Ultrasound Med. 2020;39:1057–67.
Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32:115–29.
Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, Bailey MR. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am. 2011;130:3498–510.
Bader KB, Vlaisavljevich E, Maxwell AD. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med Biol. 2019;45:1056–80.
Simon JC, Sapozhnikov OA, Khokhlova VA, Wang Y-N, Crum LA, Bailey MR. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol. 2012;57:8061–78.
Luo J, Ren X, Yu T. Efficacy of extracorporeal ultrasound-guided high intensity focused ultrasound: An evaluation based on controlled trials in China. Int J Radiat Biol. 2015;91:480–5.
Khokhlova TD, Wang Y-N, Simon JC, Cunitz BW, Starr F, Paun M, et al. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc Natl Acad Sci USA. 2014;111:8161–6.
Lafond M, Asquier N, Mestas J-LA, Carpentier A, Umemura S-I, Lafon C. Evaluation of a Three-Hydrophone Method for 2-D Cavitation Localization. IEEE Trans Ultrason Ferroelectr Freq Control. 2018;65:1093–101.
Hall TL, Hempel CR, Wojno K, Xu Z, Cain CA, Roberts WW. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74:932–7.
Lake AM, Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72:682–6.
Wheat JC, Hall TL, Hempel CR, Cain CA, Xu Z, Roberts WW. Prostate histotripsy in an anticoagulated model. Urology. 2010;75:207–11.
Hempel CR, Hall TL, Cain CA, Fowlkes JB, Xu Z, Roberts WW. Histotripsy fractionation of prostate tissue: local effects and systemic response in a canine model. J Urol. 2011;185:1484–9.
Schade GR, Styn NR, Hall TL, Roberts WW. Endoscopic assessment and prediction of prostate urethral disintegration after histotripsy treatment in a canine model. J Endourol. 2012;26:183–9.
Schade GR, Hall TL, Roberts WW. Urethral-sparing histotripsy of the prostate in a canine model. Urology. 2012;80:730–5.
Radboud University Medical Center. Effectiveness of Focal Therapy in Men With Prostate Cancer. 2024. https://clinicaltrials.gov/study/NCT06223295. Accessed 31 Dec 2023.
Baco E. A Randomized Control Trial of Focal Prostate Ablation Versus Radical Prostatectomy. 2021. https://clinicaltrials.gov/study/NCT03668652. Accessed 31 Dec 2023.
Author information
Authors and Affiliations
Contributions
SG conceived the study, and TN and SG wrote the initial manuscript draft and prepared the manuscript revisions. CP, JF, GS, RS, FC, SE, JF, AG, AV, and JR provided expert feedback and revisions on the manuscript content.
Corresponding author
Ethics declarations
Competing interests
SG served as Principal Investigator for Phase I and Phase II studies assessing MRgFUS sponsored by Insightec Ltd. GS currently serves as a consultant to EDAP Technomed Inc., an advisor to Immunity Bio, owns intellectual property licensed to Petal Surgical, and his work has been funded by the NIH (NIH R01DK119310 and R01CA258581) and American Cancer Society (RSG2117101). SE previously served as a member of the medical advisory board for Profound Medical approximately from the years 2016-2020. All other co-authors declare no potential competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghai, S., Ni, T.T., Pavlovich, C.P. et al. New kids on the block: MRI guided transrectal focused US, TULSA, focal laser ablation, histotripsy – a comprehensive review. Prostate Cancer Prostatic Dis 29, 12–27 (2026). https://doi.org/10.1038/s41391-025-00956-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-025-00956-x