Abstract
The B cell lymphoma 2 (BCL2) protein family critically controls apoptosis by regulating the release of cytochrome c from mitochondria. In this cutting-edge review, we summarize the basic biology regulating the BCL2 family including canonical and non-canonical functions, and highlight milestones from basic research to clinical applications in cancer and other pathophysiological conditions. We review laboratory and clinical development of BH3-mimetics as well as more recent approaches including proteolysis targeting chimeras (PROTACs), antibody-drug conjugates (ADCs) and tools targeting the BH4 domain of BCL2. The first BCL2-selective BH3-mimetic, venetoclax, showed remarkable efficacy with manageable toxicities and has transformed the treatment of several hematologic malignancies. Following its success, several chemically similar BCL2 inhibitors such as sonrotoclax and lisaftoclax are currently under clinical evaluation, alone and in combination. Genetic analysis highlights the importance of BCL-XL and MCL1 across different cancer types and the possible utility of BH3-mimetics targeting these proteins. However, the development of BH3-mimetics targeting BCL-XL or MCL1 has been more challenging, with on-target toxicities including thrombocytopenia for BCL-XL and cardiac toxicities for MCL1 inhibitors precluding clinical development. Tumor-specific BCL-XL or MCL1 inhibition may be achieved by novel targeting approaches using PROTACs or selective drug delivery strategies and would be transformational in many subtypes of malignancy. Taken together, we envision that the targeting of BCL2 proteins, while already a success story of translational research, may in the foreseeable future have broader clinical applicability and improve the treatment of multiple diseases.
Similar content being viewed by others
Introduction
The induction of cell death is one of the fundamental aspects regulating tissue homeostasis and counterbalancing proliferation. It is vital for developmental tissue sculpting such as digit formation, the elimination of auto-reactive immune cells and the removal of cells which are damaged beyond repair. Dysregulation of cell death can lead to pathological conditions associated with increased cell mass or tissue loss.1 The most prominent form of regulated cell death is apoptosis, which can be initiated via extracellular ligands (extrinsic apoptosis) or internal signals (intrinsic apoptosis). Several modes of cross-talk exist between the pathways and both require the activation of caspases to execute cell death. Intrinsic apoptosis is critically regulated by the BCL2 protein family.2 This protein family contains both pro- and anti-apoptotic family members that interact with each other to regulate the release of cytochrome c and other noxious proteins from the mitochondria into the cytosol. Once cytosolic, cytochrome c leads to formation of the apoptosome complex and activation of caspase-9 and the caspase cascade, ultimately leading to cell death.3 In humans, the BCL2 protein family contains about 20 proteins, that either act to facilitate or prevent apoptosis. Shared and defining characteristics of the BCL2 protein family are structural elements called BCL2 homology (BH) domains consisting of stretches of up to 15 amino acids.4 The BCL2 family proteins can be divided into three groups, based on function and number of domains: multi-domain anti-apoptotic proteins (BCL2, BCL-XL, BCL-w, MCL1, BCL2A1, BCLB), multi-domain pro-apoptotic proteins (BAK, BAX and BOK) and BH3-only pro-apoptotic proteins (BID, BIM, BAD, BIK, NOXA, PUMA, BMF and HRK) (Fig. 1a and b). Thus, this family can be regarded as a tripartite apoptotic switch. Cellular stress causes the upregulation or activation of pro-apoptotic BH3-only proteins which inhibit the anti-apoptotic proteins and activate the multi-domain pro-apoptotic proteins. The BH sequence motifs are evolutionary highly conserved and hence BCL2 proteins are not only found in mammals, but also in metazoans and several viruses.5 For the latter, expression of BCL2 genes may maintain survival of infected cells and counteract attack by the innate immune cells6 highlighting that a deregulated BCL2 family leads to disturbed apoptosis in the affected cells. In humans, dysregulation of the BCL2 protein family is associated with several diseases including cancer, neurodegenerative diseases and autoimmune diseases. In cancer, most malignancies retain dependence on one or more anti-apoptotic BCL2 proteins and these therefore are prime candidates for therapeutic targeting. Thus, the detailed mechanistic and structural understanding of the BCL2 protein family has paved the way for rationally designed targeting strategies, which are summarized and discussed in this review.
Milestones in the development of BH3-mimetics. a Overview of the anti-apoptotic BCL2 family proteins containing the BH domains and alpha helical structures α1-α9. b General interaction pattern within the BCL2 family, with the anti-apoptotic proteins inhibiting the pore-forming pro-apoptotic proteins BAX and BAK. The BH3-only proteins inhibit the anti-apoptotic BCL2 proteins and may also induce direct activation of BAX and BAK. c Timeline illustrating milestones and achievements in the discovery of BCL2 proteins and the development of BH3-mimetics. a and b Created in BioRender
Discovery of BCL2 proteins and their importance in apoptosis regulation
The founding and eponymous anti-apoptotic protein, BCL2, was discovered in 1984 as the gene involved in the t(14;18)(q32.3;q21.3) chromosomal translocation found in 85% of follicular lymphoma (FL).7,8,9 This translocation juxtaposes the BCL2 gene on chromosome 18 with the immunoglobulin heavy chain (IGH) enhancer region on chromosome 14, which results in an overexpression of BCL2. Apart from FL, this chromosomal translocation has also been observed in diffuse large B cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL).10
Following on its initial description, BCL2 was discovered to function as an inhibitor of apoptosis, representing the first example of an oncogene that contributes to cancer by blocking cell death rather than promoting proliferation11 (Fig. 1c). In 1993, BAX was discovered as BCL2 homolog, and based on the binding of BAX to BCL2, a first model of heterodimerization between anti- and pro-apoptotic BCL2 proteins was suggested.12 This was further supported by structural studies describing the hydrophobic groove as main protein-protein interaction site within the BCL2 family.13,14 The discovery of the first BH3-only proteins, BIK15 and BID,16 led to the identification of the BH3 domain as the main binding partner for the hydrophobic groove. More mechanistic insight into the regulation of apoptosis was gained in 1997 by the identification of cytochrome c release as the main apoptotic event blocked by BCL2.17,18 Over the last decades, the main principles of apoptosis regulation by the BCL2 protein family have been characterized, with several models attempting to explain the complex interactions within this protein family.19,20,21
Milestones on the way to the first clinical compounds
The discovery of the hydrophobic groove on the surface of the anti-apoptotic BCL2 proteins13 and its importance for the binding to pro-apoptotic BCL2 family proteins14 has paved the way for the development of compounds that selectively bind into the hydrophobic groove and hence functionally neutralize the targeted anti-apoptotic BCL2 protein. While many early putative BH3-mimetics turned out to be unspecific and mainly induced apoptosis via endoplasmatic reticulum (ER) stress,22,23 the discovery of ABT-737 provided the first specific and potent tool compound for lab-based research.24,25 It was developed in 2005 using nuclear magnetic resonance (NMR)-based screening, parallel synthesis and structure-based design to inhibit BCL-XL. This technology is based on the linkage of proximally binding fragments to achieve specific and high-affinity binding.26 The discovery of ABT-737 represents one of the first successful attempts at targeting a protein-protein interface using a small molecule.
Modifications of ABT-737 led to the development of ABT-263 (navitoclax) with improved oral availability, which proceeded to clinical testing as described in detail below.27,28 Both ABT-737 and ABT-263 bind with nanomolar affinity to BCL2, BCL-XL and BCL-w but not to MCL1 or BCL2A1, which display less homology in their hydrophobic groove.29,30,31 Similarity in the long hydrophobic groove of BCL2, BCL-XL and BCL-w made the development of selective inhibitors more challenging, but in 2013 the first selective BCL2 inhibitor, ABT-199 (venetoclax) was generated.32 Rapid clinical development of venetoclax with highly promising results obtained in clinical studies33,34,35 led to a first FDA and EMA approval of venetoclax in 2016. The approval of venetoclax as a first-in-class BH3-mimetic and its clinical success in the treatment of leukemia showcase the impact that fundamental mechanistic research may have on patients’ lifes.
The BCL2 protein network regulates apoptosis
The importance of the anti-apoptotic BCL2 proteins in maintaining mitochondrial integrity
The release of cytochrome c from mitochondria into the cytosol is frequently recognized as a point of no return for cell death, and hence its regulation is essential for tissue homeostasis. In this context, the BCL2 proteins are key in regulating the mitochondrial outer membrane permeabilization (MOMP) leading to cytochrome c release. The BCL2 protein family comprises both pro- and anti-apoptotic proteins which may prevent or facilitate MOMP. Essential for the inhibition of MOMP are the six anti-apoptotic BCL2 proteins including BCL2 itself, BCL-XL (BCL2L1),36 Bfl-1 (BCL2A1),37 BCL-w (BCL2L2),38 BCL-B (BCL2L10)39 and MCL140 (Fig. 1a). These globular α-helical proteins share extensive sequence and structural similarity and contain four BH domains.41,42 Typically, there is an eight-helix bundle (encoded within the BH1, 2 and 3 domains) which forms a hydrophobic surface groove for the binding of BH3 domains of other BCL2 family members. Interactions are modulated by four hydrophobic pockets (P1-4) within the binding groove.14
Another shared function of the anti-apoptotic BCL2 proteins is their ability to integrate into the outer mitochondrial membrane (OMM) via a C-terminal transmembrane (TM) domain and to interact with pro-apoptotic BCL2 family members residing both in the OMM and in the cytoplasm43 (Fig. 2). Localization at the OMM is key to the canonical function of anti-apoptotic BCL2 proteins, and even BCL2A1, which does not contain a well-defined transmembrane domain, is localized at the mitochondria of healthy cells.44,45
Overview of the BCL2 family and the regulation of cytochrome c release. a In unstressed healthy cells, several mechanisms, including canonical and non-canonical functions of BCL2 family members, prevent MOMP and release of cytochrome c from mitochondria, including the retrotranslocation of BAX/BAK and the limited availability of BH3-only proteins at mitochondrial membranes and preventing mitochondrial Ca2+ overload. b Upon cellular stress, BH3-only proteins become available at the mitochondria and facilitate BAX/BAK oligomerization, leading to MOMP. This is also facilitated by increased Ca2+ signaling including Ca2+ transfers from ER stores towards mitochondria. Created in BioRender
Regulation of intracellular Ca2+ signaling at the ER
In addition to their mitochondrial localization, all anti-apoptotic BCL2 family members also reside at other compartments including the ER, the main Ca2+ storage organelle (reviewed in ref. 46,47). The steady-state ER Ca2+ levels as well as the ER Ca2+-release properties critically contribute to a broad variety of cell death processes, including apoptosis.48 Furthermore, the ER and mitochondria are closely connected to each other via ER-mitochondrial tethers, thereby enabling the efficient exchange of lipids, reactive oxygen species (ROS) and Ca2+ between ER and mitochondria.49 These ER-mitochondrial contact sites also harbor Ca2+-transport systems, including inositol 1,4,5-trisphosphate receptors (IP3R) at the ER, and voltage-dependent anion channels (VDACs) at the OMM.50 The inter-organellar Ca2+ exchanges between ER and mitochondria regulate cell death and survival by promoting cellular fitness and mitochondrial metabolism.50,51,52 Overload of mitochondrial Ca2+ triggers cell death by opening of the mitochondrial permeability transition pore.48,53 Several anti-apoptotic BCL2 family members protect against apoptosis by operating at the ER and influencing ER Ca2+ homeostasis and dynamics. Strong evidence for a key role for BCL2 at the ER came from BCL2-cytochrome B5 fusion strategies, which target BCL2 to the ER. The expression of BCL2-cytochrome B5 conferred apoptosis protection against a broad spectrum of cell death inducers (reviewed in ref. 54). Nowadays, it has become clear that anti-apoptotic BCL2 profoundly impacts ER Ca2+ handling in multiple ways. BCL2 can lower steady state ER Ca2+ levels, thereby indirectly limiting ER Ca2+ efflux and thus preventing mitochondrial Ca2+ overload.55,56,57 Via its N-terminal BH4 domain, BCL2 also directly suppresses the IP3R-mediated release of Ca2+ from the ER.58,59 Disrupting the IP3R/BCL2 complex using IP3R-derived, BH4 domain-targeting peptide-based decoys for BCL2 antagonizes BCL2’s protective effect against Ca2+-driven apoptosis.60 Several other anti-apoptotic BCL2 family members have also been reported to exert their anti-apoptotic function by intersecting with ER Ca2+ handling, controlling IP3Rs, and/or ER-mitochondrial Ca2+ exchanges,61 including BCL-XL,62,63,64,65 MCL1,66 BCL-B67,68,69 and BCL-w.70,71 In addition to IP3Rs, ryanodine receptors, another class of intracellular Ca2+-release channels mainly present at the ER in excitable cells, are also targeted by anti-apoptotic BCL2 family members.72,73,74 In particular, both BCL2 and BCL-XL can bind to ryanodine receptors, thereby inhibiting their Ca2+-flux properties.
The importance of the individual anti-apoptotic BCL2 proteins is not only determined by their different molecular function, but also by their cell type- and tissue-specific expression. In this regard, some anti-apoptotic BCL2 proteins like BCL-w75 and BCL2A176 are only expressed in certain tissues, while others like MCL1 appear to be more ubiquitously expressed. Of note, the expression patterns also display species differences, with e.g. BCL2A1 expression being restricted to the hematopoietic system in mice but more widespread expression in humans.37,77
The BH3-only proteins as sentinels of cellular stress
The pro-apoptotic BCL2 family members comprise two main groups based on their structure and function (Fig. 1a, b): the multidomain pro-apoptotic BCL2 proteins BAX, BAK (BAK1) and BOK, and the BH3-only proteins, including BIM (BCL2L11), PUMA (BBC3), BID, NOXA (PMAIP1), BAD (BCL2L8), BMF, BIK, HRK (DP5) and some less well-established family members like proteins of the BNIP family.78,79 In contrast to the multidomain BCL2 proteins, the BH3-only proteins are intrinsically unstructured proteins.80,81,82,83 They only share the BH3 domain, which is unstructured in solution but folds into an alpha helix when interacting with a BCL2 binding partner.29,80 Structurally, BID differs from other BH3-only proteins since, similar to the multidomain BCL2 proteins, it contains a core structure and needs to be cleaved at an interhelical loop for exposure of the BH3 domain for interaction with its binding partners.84,85,86 Since cleavage of BID to tBID can be mediated by caspase-8 in the extrinsic apoptotic pathway, BID serves as an important crosstalk between extrinsic and intrinsic apoptotic pathways.87 In addition to their BH3 domain, some BH3-only proteins also contain a C-terminal anchor domain, allowing their insertion into membranes.88,89 Via their BH3 domain, BH3-only proteins may bind into the hydrophobic groove of the anti-apoptotic BCL2 proteins, thus forming heterodimers and preventing BAX and BAK sequestration.90 Based on the chemical nature of their BH3 domain, some BH3-only proteins like BIM, BID and PUMA can bind to all anti-apoptotic BCL2 proteins, while others interact only with selected binding partners offering high affinity binding to the hydrophobic groove.91,92 BH3-only proteins can also be functionally divided into direct activators or sensitizers. Thereby, the direct activator BH3-only proteins BIM, tBID and PUMA may directly bind to the multidomain pro-apoptotic proteins BAX and BAK to facilitate their pore-forming ability.93,94 In contrast, the sensitizer BH3-only proteins only interact with anti-apoptotic BCL2 proteins and counteract their sequestration of BAX and BAK95 (Fig. 1b).
Given their role as stress sensors in a cell, the activity of BH3-only proteins is tightly regulated by multiple different mechanisms, including transcriptional regulation, protein stability, phosphorylation and accessibility at the mitochondria. Some BH3-only proteins are more constitutively expressed and kept in check by sequestration at cytoskeletal proteins, like dynein and myosin complexes, as shown for BIM and BMF.96,97
Mitochondrial perturbations mediated by BAX and BAK
The multidomain pro-apoptotic proteins BAX and BAK share an overall α-helical structure with the anti-apoptotic BCL2 proteins containing a C-terminal TM domain and all four BH domains which comprise a hydrophobic pocket.98 In addition to their ability to integrate into the OMM, they can oligomerize within the OMM and form macromolecular pores.12,99 Thus, BAX, BAK and to a less well-characterized extent also BOK are responsible for MOMP and the release of cytochrome c into cytosol. Their oligomerization within the OMM may be inhibited by sequestration by the anti-apoptotic BCL2 proteins via their hydrophobic groove.31 Thus, by binding to BAX and BAK, the anti-apoptotic BCL2 proteins are essential in preventing MOMP. The function of BOK in apoptosis regulation is less clear, with some studies suggesting a more prominent role of BOK at the Golgi and ER membranes.100,101,102
The events leading to the activation and oligomerization of BAX and BAK are in parts shared between both proteins. Both BAX and BAK can shuttle between a cytosolic location and an association with the OMM. BAX is mainly cytosolic in unstressed cells and undergoes constant retrotranslocation to the cytosol, thus preventing toxic accumulation of BAX at the OMM.103 In contrast, BAK is mainly mitochondrial even in healthy cells and undergoes cytosolic retrotranslocation at much slower rates than BAX.104 For apoptosis initiation, both BAX and BAK must undergo a series of conformational changes, which are initiated by interaction with BH3-only proteins upon cellular stress and lead to the accumulation of BAX/BAK at the mitochondria.105,106 BH3-only proteins can either interact with BAX and BAK at the hydrophobic groove, or alternatively via a rear activation site presented by α1-α6 helices.93,107 Ultimately, the conformational changes of BAX and BAK result into the exposure of their BH3 domain which enables dimerization via a BH3-in-groove interaction.108,109 Of note, while heterodimers of BAX and BAK have been observed, their occurrence is less prevalent than homodimers of either BAX or BAK, which may be explained by lower binding affinity and less compatibility for the opposite BH3 domains.110,111 At the lipid interface presented by the OMM, BAX or BAK dimers can assemble into higher-order complexes.112
The precise nature of the pore formed by BAX and BAK in the OMM has been a key question for the last decades, and advances in biophysics and superresolution microscopy have shed some light on the pore structures.113,114 Recent evidence supports a toroidal pore model characterized by the fusion of the outer and inner leaflets of the OMM.115 In the toroidal pore, a continuous surface is formed that consists of lipids and proteins and allows membrane permeabilization to facilitate the release of intermembrane space proteins. To this end, BAX oligomers have been shown to assemble into large ring-like structures, as well as arcs and lines.116,117,118 Pore formation in the OMM may be triggered by shallow insertion of BAX or BAK into the outer leaflet and focal assembly of oligomers, resulting in increased membrane tension and curvature. This model also implies a potential function of BAX and BAK in mitochondrial membrane remodeling, as observed previously.119,120,121 In line with this, next to their role in mediating MOMP, active BAX, and BAK also play a key role in shaping the mitochondria and influencing the rate of fission and fusion.120,122 Therefore, BAX and BAK may be regarded as key regulators of mitochondrial dynamics.
In addition to their mitochondrial function, BAX and BAK also operate in the ER. Cells deficient in BAX/BAK display a decreased ER Ca2+-store content,123 which contributes to apoptosis resistance.57 Restoring ER Ca2+ levels by overexpressing the ER Ca2+-uptake pump SERCA increased cell death in BAX/BAK-deficient cells.123 How BAX and BAK impact ER Ca2+ stores remains not fully understood. One contributing mechanism is an increased ER Ca2+ leak via Protein Kinase A-mediated phosphorylation of IP3Rs, which renders the channels hypersensitive to their ligand IP3.124 In addition, in the absence of BAX/BAK, anti-apoptotic BCL2 proteins may become available for other targets such as the protein BAX Inhibitor-1/TMBIM6,125,126 which has been proposed as an ER Ca2+ leak channel that operates downstream of BCL2.127 BAX/BAK also function at ER-mitochondrial contact sites. During apoptosis, BAX appears to be recruited to these organellar contact sites, which become stabilized by sumoylation of DRP1.128 These ER-mitochondrial contact sites serve as platforms to recruit the cell death machinery and inter-organellar Ca2+ fluxes, which drive cytochrome c release.
Besides BAX and BAK, BOK also operates at the ER. In contrast to BAX/BAK, BOK, which appears to be constitutively active, has been proposed to be mainly controlled through protein turnover. BOK is continuously ubiquitylated with subsequent proteasomal degradation via ER-associated degradation.102 Furthermore, BOK is strongly associated to IP3R channels.129 BOK scaffolded to these channels is stabilized and protected from proteolytic degradation. The recruitment of BOK to IP3Rs also counteracts BOK’s pro-apoptotic activity. In addition to this, BOK stabilizes ER-mitochondrial contact sites and promotes ER-mitochondrial Ca2+ exchanges, thereby contributing to its pro-apoptotic effect.130 In BOK-deficient cells, expression of BOK mutants that fail to bind to IP3Rs support ER-mitochondrial contact site formation but fail to restore inter-organellar Ca2+ fluxes and apoptosis. Other reports however indicated that BOK mainly affects mitochondrial fusion rates, enhancing mitochondrial fragmentation, though without impacting IP3R-mediated Ca2+ release or mitochondrial Ca2+ transfers.131
Regulation of BCL2 proteins in physiological and pathological conditions
Control of embryonal development and organogenesis by BCL2 proteins
Due to their central function in apoptosis regulation, BCL2 proteins are key for balanced tissue generation during embryonal development. Early studies performed in knockout mice indicated that the deletion of BCL2 family genes affects multiple organs and tissues. BCL2 knockout animals displayed growth retardation and early postnatal mortality132 and BCL2L1 or MCL1 knockout mice were embryonically lethal.133,134 Even mice with combined deletion of single alleles of MCL1 and BCL2L1 were unable to survive and died at birth with multiple severe defects, suggesting overlapping functions of MCL1 and BCL-XL during embryonic development.135 Taken together, these data confirm that these anti-apoptotic genes are essential in embryonic development. Furthermore, lineage-selective deletion of different anti-apoptotic BCL2 proteins demonstrated that different types of non-transformed, differentiated cells rely on anti-apoptotic BCL2 proteins.136,137,138,139,140,141 For example, the BCL2 proteins play a crucial role in neuronal survival and apoptosis regulation within the central nervous system (CNS). The expression of BCL2 proteins varies between developing and mature neurons and between different brain regions, highlighting an important function of the BCL2 proteins in brain development.142 This is further supported by a study showing that high expression of BAX in neurons regulates microglia maturation and shapes the brain milieu during embryogenesis.143
Mutation of BCL-w/BCL2L2 in a mouse strain resulted in male sterility and testicular degeneration associated with loss of Sertoli cells, highlighting an important role of BCL-w in testicular development and spermatogenesis.144,145 In addition, a role for BCL-w in B cell survival was reported, with loss of BCL-w leading to increased apoptosis upon growth factor withdrawal in B cells.146 Knockout of BCL2A1 has been technically challenging due to quadruplication of the gene locus in mice, which complicated conventional knockout studies. While a constitutive knockdown of BCL2A1 by RNAi indicated an essential function in multiple hematopoietic cell types,147,148 the development of a mouse strain devoid of all BCL2A1 variants showed only a minor impact of BCL2A1 on cellular survival selectively in conventional dendritic cells.149,150 To the best of our knowledge, a knockout model for the remaining anti-apoptotic family member BCL-B has not yet been described, and its role in embryonal development is unclear.
The genetic deletion of the main pore-forming proteins BAX or BAK resulted in surprisingly minor phenotypes. Despite some morphological abnormalities, the mice were found to be developmentally normal and viable.151 However, combined deletion of BAX and BAK resulted in the perinatal death of most mice. This highlights the overlapping function of BAX and BAK and their ability to functionally compensate loss of only one pore-forming protein.152 Surviving double knockout mice showed multiple developmental defects, including the persistence of interdigital webs and accumulation of excess hematopoietic cells leading to autoimmune phenotypes. Additional deletion of BOK in a hematopoietic reconstitution model showed only a mild increase in lymphocyte counts, suggesting some functional redundancies also between BAX/BAK and BOK.153 Interestingly, in many tissues of surviving BAX/BAK double knockout mice, cell death still occurred at a normal rate. Although cells deficient of BAX and BAK are resistant to apoptosis, induction of autophagy may represent an alternative way to die for cells deficient in BAX and BAK, highlighting the interconnection of the core apoptotic machinery with other non-apoptotic cell death pathways.154,155
Given their partially overlapping functions, the genetic deletion of individual BH3-only proteins is generally well tolerated in mice and the observed phenotypes are relatively mild. An exception was observed upon homozygous deletion of BIM, which resulted in embryonic lethality in half of the animals and indicated a non-redundant function of BIM during embryogenesis.156 The function of most BH3-only proteins only became apparent upon induction of cell death, e.g. by withdrawal of cytokines or upon irradiation. Loss of BBC3/PUMA did not affect the survival of mice but thymocytes isolated from knockout mice underwent less apoptosis upon DNA damage.157 Similarly, PMAIP1/NOXA knockout mice showed no overt phenotype, but the response to stress was affected in a cell type- and stimuli-dependent manner.158,159 Of note, combined knockout of multiple BH3-only proteins had a much more severe phenotype, with loss of BIM and PUMA or BMF leading to autoimmunity in several organs.160,161,162 The combined deletion of BID, BIM and PUMA resulted in a phenotype similar to BAX/BAK double knockout mice.163 Taken together, these studies highlight the importance of BH3-only proteins in regulating BAX/BAK activation and subsequent apoptosis induction, particularly in immune cell homeostasis.
Epigenetic and transcriptional regulation of BCL2 proteins
The expression of the BCL2 proteins is also regulated transcriptionally and post-transcriptionally.164,165,166 In contrast to mutations, epigenetic modifications are reversible and allow for quick changes of the expression of whole sets of genes. The epigenetic landscape is influenced by several epigenetic enzymes, such as histone acetyl-transferases and their counterpart histone deacetylases (HDAC) or DNA methyltransferases (DNMT). Hypermethylation of tumor suppressor genes or hypomethylation of oncogenic loci represent attractive targets for therapeutic approaches via targeting DNMTs or histone-modifying enzymes.167,168 Azacitidine and 5-aza-2’-deoxycytidine (decitabine) are clinically approved DNMT inhibitors that improved the treatment of myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML) and AML.169,170 Several compounds have been applied in clinical studies as single agents, but also in combinations with small molecule inhibitors or chemotherapy. The epigenetic changes often prime the cells to be more susceptible for additional cytotoxic treatments and may reverse acquired resistance.166,171,172,173 Most DNMTs impact the BCL2 proteins by means of upregulation of the BH3-only protein NOXA, as shown for several cancer types.174,175,176 Pro-apoptotic BMF and anti-apoptotic BCL2A1 are also regulated through HDAC inhibitors.177 The expression of MCL1 is additionally regulated by methylation status, which correlates with cisplatin sensitivity in osteosarcoma.178
Readers of acetylation, such as BET proteins, influence the transcription of genes by enabling transcription elongation at promoters or enhancer regions, especially at super-enhancers. These often regulate important cellular processes, like proliferation, metabolic or cell death regulation179, and the BCL2 protein family is often differentially regulated in response to BET inhibitor treatment in several cancer types.180,181,182 As with “methylation-related” enzyme inhibitors, BETi are in ongoing clinical trials as single agents, but more often as combination therapy. To date, it is not been fully investigated which of the “epi-drugs” directly influence the expression of BCL2 family members or whether the impact on prominent regulatory pathways, such as NFκB, p53, or STAT, to name some are the main cause for changes in the expression of the BCL2 proteins.
Gene expression regulation of the anti-apoptotic BCL2 proteins
The chromatin status directly influences the transcription of BCL2 family genes through recruitment of transcription factors. Expression of BCL-XL was increased by histone 3 Lys27 acetylation at the BCL2L1 promoter which determined the occupancy by p300 and the transcription factor Ets-1 to induce transcription.183 Also, upstream survival signaling strongly influences the expression of BCL2 proteins. Several anti-apoptotic BCL2 proteins are well-described targets of STAT and Rel/NF-κB transcription factors.184,185,186,187,188 In addition, the Forkhead box O (FOXO) family of transcription factors induced by PI3K-AKT signaling influenced BCL-XL and BCL2 expression.189,190 As a short-lived protein, MCL1 is strongly influenced by transcriptional regulation and induced by several transcription factors, such as HIF-1, Elk4, ATF4 or c-Myc, among others.191,192,193 E2F1 on the other hand led to transcriptional repression of MCL1.194
In addition to the chromatin status and upstream survival signaling, gene expression can be influenced by micro RNAs (miRNAs) or long non-coding RNAs (lncRNAs). The role of miRNAs can be oncogenic as well as tumor-suppressive, and many miRNAs are differentially expressed between healthy controls and cancerous tissues (reviewed in ref. 195). Several miRNAs targeting the anti-apoptotic BCL2 proteins have been identified in different diseases.165 BCL2 itself can be dysregulated via the downregulation or deletion of miR-15/16, microRNAs that suppress translation of multiple proteins over and above BCL2. Downregulation of miR-15/16 has been observed in CLL, prostate cancer, pituitary adenomas, and mesothelioma, thus contributing to high BCL2 expression in these malignancies.196,197,198,199,200 In leukemic cells, miR-145 downregulates BCL2 and simultaneously induces BAX expression.201 Additionally, the miRNAs themselves are influenced by lncRNAs that act as molecular sponges and thereby hinder the repressing effect of miRNAs.202
Furthermore, protein levels are influenced by mRNA stability. The stability of the BCL2 mRNA was increased by RNA binding protein nucleolin.203 The mRNA binding protein HuR promoted protein stability of BCL2, MCL1, and BCL-XL.204 Another RNA binding protein and ribonuclease, Regnase-1 (also known as MCPIP-1), on the other hand, inhibited the expression of anti-apoptotic genes such as BCL2L1, MCL1 or BCL2A1 by cleavage of the corresponding mRNA.205,206
Epigenetic regulation of pro-apoptotic BCL2 proteins
The activity of the BH3-only proteins is tightly regulated and induced by cellular stress.207 Modes of regulation include transcriptional induction, post-translational modifications like ubiquitination and phosphorylation, or subcellular compartmentalization. PUMA and NOXA are transcriptionally induced by p53, and hence they can exert their pro-apoptotic functions in multiple stress situations that lead to the activation of p53.159,208 In a similar manner, several other transcription factors, including E2F-1,209,210 HIF-1a211 and FOXO1/3,212,213,214,215,216 induced the expression of multiple BH3-only proteins. Additionally, BIM was described to be regulated through the transcription factors IRF4,217 the non-classical AP1 family member BATF218 or the EMT-inducing factor ZEB1.219 On the other hand, several regulators associated with pro-survival signaling have been described to silence and inactivate the expression of BH3-only proteins, thus ensuring cellular survival.220,221
Besides the induction of transcription, the mRNA stability of BH3-only proteins is highly regulated and may be affected by miRNAs or molecular chaperones.222,223,224 Several miRNAs have been described to be involved in the regulation of BIM in various diseases, e.g. the miR-17-92 cluster that is overexpressed in aggressive hematological malignancies.225 MiR-130a also suppresses BIM, however, in osteoarthritis the lncRNA CIR is upregulated, leading to inhibition of miR-130a and increased BIM expression.226 In AML the lncRNA MORRBID is overexpressed and related to poor overall survival, as it directly controls transcription at the BIM promoter and keeps the gene poised.227,228 In the case of PUMA, the lncRNA NEAT1 induced EZH2-mediated histone methylation on a promoter region of miR-139. In turn, the miRNA was suppressed, resulting in increased PUMA levels.229 In colorectal cancer cells, hypermethylation prevented the transcription of the lncRNA TSLC8 through FOXO1, which prevented TSCL8 to act as a tumor suppressor by stabilizing pro-apoptotic PUMA.230 In contrast, miR-221 is known to reduce PUMA mRNA and protein expression.231 Results in hypoxia cell models indicate that the lncRNA GAS5 can sponge miR-221 and thereby influence PUMA expression.232 NOXA expression is also influenced by several miRNAs that can either act inhibitory such as miR-21,233 miR-200b234 or miR-197,235 or induce NOXA expression like miR-23a.236 Furthermore, NOXA is impacted through the regulation by lncRNAs. These studies highlight the interconnectivity between chromatin state, lncRNAs, and miRNAs in the regulation of BCL2 protein expression. In colorectal cancer the c-Myc/miR-1271-5/NOXA/MCL1 axis can be targeted through BET inhibitor treatment in combination with BH3-mimetics to induce apoptosis, highlighting the interplay of many epigenetic mechanisms in one treatment.237 Often, miRNAs also target transcription factors or proteins of signaling pathways, whose dysregulation in turn affects the expression of BCL2 family genes. The proto-oncogene Bmi1 for example is upregulated in mantle cell lymphoma and a target of miR-16-1. Bmi1 furthermore directly inhibits NOXA and BIM expression.238
Phosphorylation and control by kinase signaling pathway
There are four major mechanisms by which signal transduction is regulated on the protein level: expression, localization, phosphorylation, and degradation. The phosphorylation of BCL2 family proteins is an underexplored topic, with a decreasing number of articles published on it in recent years. These often focus on the effect of a direct phosphorylation by the survival cascade PI3K/AKT on BCL2-mediated apoptosis,239 but even here the matter is not straightforward. BAD240,241 and BAX242,243 are direct targets of AKT, which thereby inhibits their pro-apoptotic function at the mitochondria. However, this signaling cascade also affects the BCL2 family via protein transcription.244,245
The other pathway that plays a central role in BCL2 family phosphorylation is the mitogen-activated protein (MAP) kinase signaling cascade. Here, different members of the signaling cascade, such as MAPK and JNK, can have opposite effects on the BCL2 family.246 JNK phosphorylates BIM and BMF at their conserved Thr112 sites, as well as Ser65 of BIM.246 This induces their translocation from the dynein and myosin V motor complexes to the mitochondria, where they contribute to apoptosis induction.247 Of note, also here the kinase might affect BIM protein expression. ERK1/2 also mediates the phosphorylation of BIMEL at Ser65, as well as Ser55 and Ser73, leading to its disassociation from MCL1 and BCL-XL and degradation, allowing MCL1 and BCL-XL to exert their anti-apoptotic function, for example after serum withdrawal.248 Indeed, phosphorylation of the BCL2 family proteins often mediates protein disassociation, as it has also been described for BAD/BCL-XL, where MAP2K-mediated phosphorylation of BAD disrupts BAD/BCL-XL interactions.249 Interestingly, phosphorylation of the BCL2 family often occurs in unstructured regions and might not be sufficient to prevent binding of pro-apoptotic factors, but instead creates binding sites for other proteins.248 This has been demonstrated for BAD/BCL-XL complexes, where BAD can be phosphorylated at different sites by AKT, MAPK-activated kinase RSK, and cAMP-dependent protein kinase.240,250,251 Phosphorylation by the latter at Ser155 has been clearly demonstrated to lead to disassociation from BCL-XL and promote its interaction with 14-3-3 proteins, which sequester BAD away from BCL-XL.251 In contrast, phosphorylation can also lead directly to protein degradation, as shown for MCL1. When mitotic cell death is induced, cyclin-dependent kinase 1 and several other kinases can phosphorylate MCL1 leading to its degradation. Interestingly, the same events also lead to BCL2 and BCL-XL phosphorylation, which does not affect their protein levels but weakens the interaction with BAX and other pro-apoptotic proteins.252
Not surprisingly, the best-studied member of this family in terms of regulation by phosphorylation is the BCL2 protein itself. The first report showing that phosphorylation of BCL2 correlates with survival was published in 1994.253 Through a series of elegant experiments, it was determined that Ser70 in the loop region of the protein was the main target of growth factor agonist-initiated phosphorylation mediated by protein kinase C.254 Later, additional kinases that phosphorylate BCL2 at the mitochondria and facilitate survival were identified, such as the stress kinase SAPK, MAPKs and ERK1/2. Taken together, the phosphorylation of Ser70 is a highly dynamic process that can be mediated via several signaling cascades and is antagonized by a PP2A phosphatase.254 Phosphorylated BCL2 is not a good target for caspase-mediated cleavage and does not interact well with the pro-apoptotic family members, while BCL2 de-phosphorylated by PP2A is degraded by the proteasome, possibly at the mitochondria and ER.255,256 Additionally, a second phosphorylation pattern was identified, induced by prolonged exposure to antimitotic agents and possibly associated with increased susceptibility to cell death, indicating an opposite function as Ser70 phosphorylation.254 Here, additional residues found in the loop region are phosphorylated on top of Ser70, among them Thr69 and Ser87. This hyperphosphorylation also seems to occur at the ER, where it affects the Ca2+ dynamics.257
In summary, the regulation of BCL2 family proteins via phosphorylation seems to control their signaling via three independent routes: 1. Indirectly, via (in)activation of gene expression, thus affecting protein expression; 2. directly, via affecting the ability to dimerize with other family members and sequestration partners, and 3. directly, by facilitating proteasomal degradation of BCL2 family proteins and, thus again, affecting their protein expression.
Proteasomal regulation of BCL2 proteins
Continuing from the previous argument, BCL2 localized at the ER membrane interacts rather stably with PP2A and blockage of this phosphatase led to proteasome-mediated degradation of BCL2.256 Interestingly, only BCL2 phosphorylated at Ser87 by MAPK and not phosphorylated by AKT, protein kinase C or cyclic AMP-dependent protein kinase, affected its proteasomal degradation.258 In addition to phosphorylation after specific stimuli, there is thus another level of regulation by the proteasome. Some BH3-only proteins like NOXA display a very short half-life of less than 2 hours and are rapidly degraded by the proteasome. Therefore, proteasome inhibitors were found to induce NOXA protein expression independently of p53 activity.259,260,261 In addition, BIM, BIK, and BAX were also found to be regulated by proteasomal degradation.262,263,264 This implies that the stability of these BH3-only proteins is regulated by post-translational modifications like ubiquitination or NEDDylation. In line with this, the E3 ubiquitin ligase CHIP has been reported to control NOXA stability.265 Another interesting target of proteasomal degradation is MCL1, which is often found upregulated in a compensatory fashion when BCL2 is therapeutically inhibited.266,267 Upon cellular stress MCL1, in a stable complex with NOXA at the mitochondria, is phosphorylated by CDK2 at Ser64 and Thr70 and thus primed for degradation.268 At least four ubiquitinases and two deubiquitinases so far have been identified as regulating MCL1 degradation, suggesting that this is a tightly regulated process.269,270 This finding was initially thought to open up new potential targeting strategies in which MCL1 may be indirectly targeted by CDK inhibitors, like alvocidib and dinaciclib. However, initial clinical trials produced rather discouraging results.186 Taken together, it is becoming clear that phosphorylation of BCL2 proteins upon specific stimuli can lead to their proteasomal degradation and shift the balance between pro- and anti-apoptotic members of the family. However, additional mechanisms regulate the half-life of short-lived family members, leading to a complex regulation of BCL2 proteins in a cell type- and situation-dependent manner.
BCL2 proteins as drug targets in cancer
BCL2 family dependencies in cancer
The mechanistic understanding of the protein interactions within the BCL2 family and their functional consequences on the induction of apoptosis highlights the importance of the anti-apoptotic BCL2 proteins for cancer development. For cancer cells to survive their hostile environment, the anti-apoptotic BCL2 proteins must manage to keep the BH3-only proteins in check to prevent BAX/BAK pore formation and MOMP. Therefore, it is not surprising that many cancer cells display genetic alterations leading to increased expression of one or multiple anti-apoptotic BCL2 proteins.
Some striking differences are observed in the frequency and nature of genetic events involving the anti-apoptotic BCL2 genes (Fig. 3). In particular BCL2 is affected differently in comparison with BCL-XL (encoded by BCL2L1) or MCL1. Apart from its involvement in chromosomal translocations,7,9 BCL2 can either be mutated or amplified in B cell malignancies, pointing to an important contribution of BCL2 in B cells. In both FL and DLBCL, the t(14;18)(q32.3;q21.3) translocation is an initiating event in disease pathogenesis, allowing survival of cells otherwise destined to die in the germinal center. In other malignancies however, BCL2 is rarely amplified and in complete contrast, BCL2 is homozygous deleted in about 5% of colorectal cancer cases. This may be explained by its genetic proximity to the tumor suppressor gene Deleted in Colon Cancer (DCC) which lies 11 Mb centromeric of BCL2 on the long arm of chromosome 18.271,272 BCL2L1 and MCL1 on the other hand are commonly (about 5% of cases) amplified in solid tumors; BCL2L1 in colorectal cancer, while MCL1 is most commonly amplified in breast cancer. Overall, MCL1 is amplified at higher frequencies than BCL2L1, in line with a previous landmark paper describing copy number alterations of MCL1 in up to 10% of patients for some cancer types.273
Genetic alterations in cancer. Analysis of genetic modifications involving BCL2, BCL2L1 and MCL1 using cBioportal541,542,543 was performed. Four main pan-cancer studies using targeted deep sequencing and encompassing 71,060 samples were selected (MSK-IMPACT,544 Cancer Therapy and Clonal Hematopoesis,545 China Pan-Cancer546 and MSK MetTropism547) and analyzed for genetic alterations involving BCL2, BCL2L1 or MCL1 in different cancer types. Thresholds were set for 100 samples / cancer type and a minimal frequency of 0.5%
Analysis of cellular dependencies using large-scale CRISPR screens provided in Depmap (https://depmap.org/portal/)274 reveals some striking observations (Fig. 4). Only a few malignancies depend on BCL2 for survival (mean Chronos Gene Dependency Score −0.0462), most notably B cell malignancies, AML, and neuroblastoma. BCL2L1 and to a lesser extent also MCL1 are classified as common essential genes (mean Chronos Gene Dependency Scores −1.11 and −0.673, respectively), with only some cancer types being unaffected by deletion of either BCL2L1 or MCL1. In line with its frequent amplification in colorectal malignancies, deletion of BCL2L1 is highly lethal in colorectal cancer. Of note, many colorectal cell lines displaying BCL2L1 dependency derive from rectal tumors, e.g. SW1463, SNU254, and C99. The most pronounced effect of MCL1 deletion was observed in cutaneous squamous cell carcinoma, rhabdomyosarcoma, and mature B cell neoplasms. Taken together, these data highlight that the anti-apoptotic BCL2 proteins BCL2, BCL-XL, and MCL1 are all highly promising therapeutic targets in multiple cancer types. In contrast to these three main anti-apoptotic BCL2 proteins, the genes for the related anti-apoptotic BCL2 proteins Bfl1 (BCL2A1), BCL-w (BCL2L2) and BCL-B (BCL2L10) display little perturbation effects in cancer (mean Chronos Gene Dependency Scores −0.0454, −0.103 and 0.006, respectively).
BCL2 family dependencies in cancer. Boxplots depicting the Chronos dependency scores of BCL2, BCL2L1 and MCL1 of cancer cell lines according to the DepMap data. Cancer subtypes (primary disease) with data available for n ≥ 5 cell lines were included. If multiple subtypes belonged to one lineage, the lineage was color-coded, the rest are shown in black
Targeting of BCL2 proteins with BH3-mimetics
The molecular function of BCL2 proteins in regulating cell survival highlights their potential as therapeutic targets for multiple diseases. Although the importance of BCL2 proteins is clearly demonstrated also in physiological tissues, this has not stopped the development and clinical assessment of multiple BH3-mimetics since the discovery of ABT-737 in 2005 (Fig. 5). While BCL2 proteins may also represent promising targets in autoimmune diseases (discussed below), the clinical development of BH3-mimetics has been centered on cancer, in particular lymphoid malignancies. The developed BH3-mimetics include bispecific inhibitors able to inhibit both BCL2 and BCL-XL, but also selective inhibitors binding only BCL2, BCL-XL or MCL1 (Fig. 5a). Any clinical efficacy of BH3-mimetics will however depend on sufficiently large differences between the sensitivity of malignant and normal cells to BCL2 family inhibition, a situation comparable to regular chemotherapy.
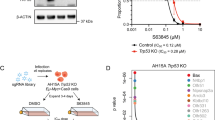
Clinically developed BH3-mimetics. a Overview of current clinically tested BH3-mimetics targeting BCL2, BCL-XL, MCL1 or multiple anti-apoptotic BCL2 proteins (created in Biorender). b Hydrophobic pockets P1, P2, P3 and P4 mapped onto the surface of the BCL2 structure (PDB-id: 1G5M31). c Zoom into SS55746, navitoclax, venetoclax or sonrotoclax bound to BCL2 (PDB-id: 6GL8,336 PDB-id: 4LVT,32 PDB-id: 6O0K,320 PDB-id: 8HOG). d Overlay of venetoclax bound to BCL2 (light grey) and BCL2-G101V (yellow). Mutation of glycine 101 (light grey spheres) to valine (yellow spheres) effects conformational change (indicated by black arrow) of the adjacent E152 (side chains as sticks in light grey and yellow, respectively), pushing it towards the chlorine (green) of venetoclax and slightly displacing it. (PDBids: 6O0K and 6O0L320). e 2D sketches of inhibitors shown in B-E, and pelcitoclax and lisaftoclax
Based on their mechanism of action, all true BH3-mimetics induce intrinsic apoptosis dependent on BAX and BAK, and therefore their effects should be inhibited in BAX/BAK-deficient cells.275 Selectivity of BH3-mimetics should also be confirmed by the high sensitivity of cell types that are known to be dependent on the targeted anti-apoptotic BCL2 protein for survival, e.g. CLL cells for BCL2 inhibitors276 and platelets for BCL-XL inhibitors.277,278 This sensitivity profile highlights that BH3-mimetics may have profound on-target effects on non-malignant cells as also indicated by the phenotype of gene deletions in embryonic development.
The small molecule BH3-mimetics navitoclax and venetoclax
ABT-737 and Navitoclax: Due to its dysregulated expression in some hematological malignancies, BCL2 is a promising therapeutic target despite its broad expression in normal cells. The first potent and specific BH3-mimetic, ABT-737, targeted not only BCL2 but also BCL-XL and BCL-w due to the structural similarities of their hydrophobic grooves. It binds to the hydrophobic pockets P2 and P4 of the anti-apoptotic proteins, displacing previously bound pro-apoptotic proteins like BIM or BAX (Fig. 5b and c). Navitoclax is an effective single-agent treatment for some B cell malignancies and non-small cell lung cancer cell and xenograft models with dependence on either BCL2 or BCL-XL and low MCL1 expression.27 Single-agent treatment and combinations with immuno/chemotherapy phase I and II trials in lymphoid malignancies, particularly CLL, showed promising results.279,280,281 However, neutropenia and thrombocytopenia were major dose-limiting toxicities, the latter due to on-target effects of BCL-XL inhibition.277,279,282 Consequently, many trials involving single-agent navitoclax have been completed/withdrawn. Current efforts are directed to combination regimes in Non-Hodgkin Lymphoma (NHL), myeloid neoplasms, ovarian cancer, triple-negative breast cancer, and non-squamous non-small cell lung carcinoma. Of particular note, two phase III trials (NCT04472598, NCT04468984) are currently investigating the combination of navitoclax with the JAK1/2 inhibitor, ruxolitinib, for the treatment of primary and relapsed/resistant (R/R) myelofibrosis, a form of myelodysplasia often characterized by increased platelet numbers and constitutive JAK/STAT activation.283 These trials followed a promising phase II trial.284,285 Early data from TRANSFORM-1 reported that the rate of spleen volume reduction was twice as high in patients who received navitoclax and ruxolitinib compared to placebo and ruxolitinib. Adverse effects of thrombocytopenia and neutropenia were commonly reported but manageable with navitoclax dose modification or interruption. Nonetheless, nearly one-third of patients discontinued treatment.286
Venetoclax: Navitoclax and related structures were reverse engineered through systematic removal or replacement of key binding elements to produce the first BCL2 specific inhibitor, venetoclax (ABT-199).32 The compound effectively kills various BCL2-dependent lymphoid cells and xenograft models in a BAK/BAX dependent manner, whilst sparing platelets.32,287 As of June 2024, venetoclax is investigated in 448 active clinical trials registered at clinicaltrials.gov, and among these are 40 phase III trials. In CLL, venetoclax is a transformative medicine. Clinically, nearly irrespective of their underlying genetic defects, all cases of CLL are exquisitely sensitive to BCL2 inhibition, usually with a low nanomolar EC50 in vitro. Consequently, venetoclax received several FDA breakthrough designations for the treatment of CLL following the success of several phase I and II studies.34,35,288,289 In May 2019, the FDA approved the use of venetoclax in combination with the CD20 antibody, obinutuzumab, as a chemotherapy free regimen for untreated CLL due the results of a phase III trial (CLL14) which found that venetoclax-obinutuzumab was associated with longer progression-free survival (PFS) than chlorambucil-obinutuzumab (88.2% vs 64.1% at 24 months).290 The 5-year follow up of this study reported patients treated with venetoclax-obinutuzumab continued to experience improved PFS (62.6% vs 27% in the chlorambucil-obinutuzumab arm) and higher rates of undetectable MRD.291 Early trials indicated a high risk of tumor lysis syndrome in the presence of bulk disease. Ramp-up dosage regimens and prophylaxis with hydration and rasburicase for high-risk patients were implemented which have greatly reduced the incidence and severity of this life-threatening complication34 and in most instances venetoclax can now be safely administered without hospital admission.
Additionally, full FDA approval was granted in 2020 for venetoclax in combination with hypomethylating agents for newly diagnosed AML patients older than 75 years who are not eligible for chemotherapy, based on the superior overall survival in the VIALE trial.292,293 Mechanistically, hypomethylating agents may reduce the expression of MCL1 while simultaneously increasing the expression of the BH3-only protein NOXA, which provides a rational molecular basis for the observed synergy with venetoclax.294,295 Additionally, increased T cell cytotoxicity has been reported following venetoclax treatment due to increased ROS formation and simultaneous activation of the cGAS/STING pathway activity. A more detailed understanding of the essential molecular mechanisms may pave the way for further refined combination treatments.296,297
In contrast, in FL and DLBCL, venetoclax monotherapy has been less successful, with reported response rates of only 38% and 18% respectively.298 Many FL and DLBCL cells express high levels of BCL2 due to either chromosomal translocation or amplification, and therefore such inherent resistance to BCL2 inhibition was unanticipated. Nonetheless, case reports of good responses after single-agent venetoclax in R/R DLBCL299 provide evidence for a subgroup of DLBCL with functional dependence on BCL2. Collectively, these data indicate that in DLBCL, unlike CLL, there is heterogenous dependence on different anti-apoptotic proteins, which is also supported by multiple preclinical studies.300,301 The disparity between anti-apoptotic protein expression and functional dependence is not exclusive to DLBCL and is therefore a poor biomarker to predict responses to BH3-mimetics.
Consequently, in DLBCL and other diseases, rational use of specific BH3-mimetics mandates robust identification of predictive biomarkers and this is now an area of intense research.300,301,302,303 Co-expression of alternative anti-apoptotic proteins, protein interactions and accessibility and phosphorylation status of BCL2 all likely contribute to venetoclax resistance in cases of high BCL2 expression. Additionally, in some instances, secondary genetic events in t(14;18) DLBCL may result in disruption of the BCL2 gene resulting in loss of BCL2 protein expression.304 However, high expression of BCL2 mRNA305 and BCL2 protein306 have been associated with poor outcomes in DLBCL; BCL2 “super-expressing” DLBCL may be associated with particularly poor outcomes.306 This has led to the empirical addition of venetoclax to various immuno-chemotherapy backbones in phase I/II clinical trials. Venetoclax in combination with immunochemotherapy (R-CHOP) in the CAVALLI phase II study indicated improved outcomes specifically in BCL2 highly expressing cases of DLBCL.307 More recently, the combination with the CD79B antibody drug conjugate (ADC), polatuzumab vedotin (NCT02611323) may be of interest since the ADC may cause downregulation of MCL1 via proteasomal degradation.308 Although the initial clinical results may look promising, in the absence of robust biomarkers associated with sensitivity to venetoclax, toxicities, both clinical and financial, have to be carefully weighed against perceived benefits.
In R/R multiple myeloma (MM), the chromosomal translocation t(11;14)(q13;q32.3) resulting in deregulated expression of Cyclin D1 has been suggested as a biomarker for venetoclax sensitivity.309 This subgroup of disease is associated with a B cell-specific transcriptional program resulting in upregulation of B cell genes such as MS4A1/CD20.310 Despite impressive results of single-agent treatment in R/R MM, adoption of venetoclax into combination regimens has been slowed by significant toxicities and deaths associated with increased infection rates in venetoclax-containing regimens.311 Although appearing in several treatment guidelines, venetoclax is not yet approved for use in MM.
Following the clinical success of venetoclax in the treatment of adult hematological malignancies, its use is currently also being evaluated in pediatric patients with leukemia, including AML and ALL. A phase I/II study of venetoclax in combination with chemotherapy shows promising response rates and manageable toxicities in pediatric AML patients.312,313 The combination of venetoclax with low-dose navitoclax and chemotherapy (NCT03181126) showed promising efficacy in ALL patients, and a safe dose of venetoclax could be identified for children.314 Of note, in pediatric T-ALL patients with a dismal prognosis, a beneficial effect of venetoclax was observed in R/R patients, highlighting the potential of venetoclax also in the treatment of childhood cancer (NCT03181126). However, as a note of caution it should be considered that developing neurons show increased expression of pro-apoptotic BCL2 proteins associated with higher apoptotic priming of neurons in children, indicating an important role of the BCL2 family proteins during brain development and neuronal differentiation.142,315 Therefore, the potential effects of venetoclax or similar drugs on the brain and neuronal development of children will need to be carefully assessed in long-term follow-up studies and may have to be compared to the serious long-term effects of intensive chemotherapy in children.
Resistance to venetoclax often emerges over prolonged treatment times and represents an important clinical challenge.316,317 Several acquired somatic BCL2 mutations have been identified at the point of progression, the most prevalent being either G101V or D103Y.318,319 G101 is adjacent to but not directly a part of P4, and the P4 pocket was unaffected by mutations of this residue. Instead, the G101V mutation causes a rotamer change of E152 within the P2 pocket. This ‘knock-on’ effect reduces the affinity of BCL2 for venetoclax 180-fold, whereas the affinity for BH3-only proteins is largely unaffected (Fig. 5d).320 However, BCL2 mutations are not identified in all CLL patients who relapse. Furthermore, in those that display mutated BCL2, the variant allele frequency is usually low, suggesting that there are multiple mechanisms that contribute to venetoclax resistance. Sub-clonal upregulation of alternative BCL2 family members, BCL-XL and MCL1, have also been identified.318,321 This has been associated with copy number gains or increased NF-κB signaling which drives protein expression.321,322 Additionally, acquired variants of the effector BAX or loss of BAX protein have been reported in the myeloid compartment of venetoclax-resistant AML and CLL patients.323,324
Despite their high selectivity and binding affinity for the targeted anti-apoptotic BCL2 proteins, off-target effects of venetoclax have been described.325 A recent study described venetoclax as immunometabolic regulator in a subset of Natural Killer cells, an effect that was observed mainly independently of BCL2 expression.326 This may also speak to the non-canonical functions of the BCL2 family, some of which may not be inhibited by BH3-mimetics. The autophagy promoter, beclin 1 (BECN1), contains a BH3 domain which can be inhibited via interactions with BCL2 and BCLXL. In line with this, BH3-mimetics such as ABT-737 have been proposed to induce autophagy.327 Additionally, the BH4 domain of BCL2 has been implicated in autophagy regulation. It has been reported to interact with GABA Receptor Associated Protein (GABARAP), a molecule involved in autophagosome biogenesis328 and overexpression of BCL2 lacking its BH4 domain commits melanoma cells to autophagy.329 Interactions of the BH4 domains of BCL2 and BCL-XL have also been linked to roles in proliferation, differentiation, DNA repair, cell migration and tumor progression.330 These interactions would not be hindered by BH3-mimetics. Other reported non-canonical functions of the anti-apoptotic BCL2 family members include regulation of senescence, inflammation, metabolism, mitochondrial morphology, and calcium homeostasis.331,332 With so many roles of these proteins above and beyond suppression of apoptosis, it is perhaps unsurprising that in a large screen across different cancer types there was a poor correlation between BCL2 family RNA expression and BH3-mimetic sensitivity (Fig. 6). Some of this disparity may be due to this correlation being based on RNA expression rather than protein expression. However, target protein expression has been defined as a poor biomarker of BH3-mimetic sensitivity in multiple cancer models.301,333,334,335 In a pan-cancer analysis, the gene knockout effects do not resemble the response to venetoclax or navitoclax, and only an association between navitoclax response and a BCL2L1 gene dependency is observed (Fig. 6). Taken together, these studies highlight that non-canonical functions of BCL2 proteins as well as unanticipated off-target effects of venetoclax or navitoclax need to be considered in the assessment of clinical responses.
Prediction of BH3-mimetic responses. Linear regression analyses between cancer cell line response to BH3-mimetics from the PRISM Drug Repurposing Library and BCL2 RNA interference (green), BCL2 dependence score (pink) and BCL2 gene expression (blue). a Linear regression analyses between response to ABT-199 (venetoclax) and BCL2 (myeloid and lymphoid malignancies were not included in PRISM repurposing screening for ABT-199). b Linear regression analyses between response to ABT-263 (navitoclax) and BCL2. c Linear regression analyses between response to ABT-263 (navitoclax) and BCL2L1/BCL-XL. d Linear regression analyses between response to S63845 and MCL1. The most significant correlation is a negative one between BCL2L1 expression and navitoclax response (c), however the R-squared value is only 0.06
Other BCL2 targeting BH3-mimetics
As frequently happens in the pharmaceutical industry, the success of venetoclax has spawned a plethora of structurally related molecules, some of which differ only slightly from venetoclax (Fig. 5e). In total, 11 selective BCL2 inhibitors have entered clinical trial; development of some has already been discontinued (Table 1).
ABBV-543 and ABBV-623: In addition to venetoclax, AbbVie (www.abbvie.com) developed two further BCL2 inhibitors. ABBV-453 is a BCL2 inhibitor in a phase I trial for patients with R/R t(11;14) positive and/or BCL2 high-status MM (NCT05308654). Another trial investigating the efficacy of ABBV-453 in R/R CLL/SLL patients who have had at least two prior therapies (NCT06291220) has been recruiting since August 2024. The other compound, ABBV-623 has been tested in a clinical trial (NCT04804254) but its development has now been discontinued. No chemical structures or biological data have been published for either compound.
S55746 and S65487: S55746 (BCL201) is a BCL2-specific inhibitor developed by Servier (https://servier.com) using structure-based drug design. The compound activated apoptosis in CLL and MCL patient samples and induced tumor regression in xenograft models at a comparable level to venetoclax.336 In marked contrast to venetoclax, S55746 binds via a different binding mode and mainly occupies pockets P1, P2 and P3 but not P4 of BCL2 (Fig. 5c). Despite alternative binding modalities, S55746 binds to G101V mutant BCL2 with 100-fold lower affinity compared to wildtype BCL2.320 Two clinical trials were completed; firstly, to assess the safety and tolerability of S55746 in CLL and NHL (NCT02920697), and secondly, to assess the combination of S55746 and the PI3K inhibitor, idelalisib, in FL and MCL (NCT02603445). Despite having been completed six years ago, no results from these trials are available. S65487 (VOB560), a prodrug of S55746, induced apoptosis in BCL2-dependent hematological cell lines and displayed synergy when combined with MCL1 inhibitors in AML patient-derived xenografts.337 Additionally, S65487 was potent in pre-clinical models resistant to venetoclax, including those that harbored BCL2 mutations.337 S65487 is being assessed clinically in combination with a MCL1 inhibitor for the treatment of R/R AML, MM or NHL (NCT04702425) or in combination with azacitidine in patients with untreated AML (NCT04742101), but both studies are currently not recruiting.
Sonrotoclax and BGB-21447: Beigene (www.beigene.com) produced a series of BCL2 inhibitors through scaffold screening and structure-based drug design leading to the development of sonrotoclax (BGB-11417), which binds to BCL2 with higher affinity than venetoclax due to a different binding mode within the P2 pocket338 (Fig. 5c). Stronger binding was attributed to van der Waals forces, additional hydrophobic interactions, and water-bridged hydrogen bonds not present in BCL2:venetoclax interaction. Moreover, it was more potent in BCL2-dependent hematological tumor cells and xenograft models compared to venetoclax. Interestingly, due to its unique binding mode, sonrotoclax can inhibit the G101V BCL2 variant and kill mutant cells.338,339 There are currently seven active phase I and II clinical trials involving sonrotoclax monotherapy for the treatment of mature B cell malignancies, R/R CLL and SLL, R/R MCL, myeloid malignancies and Waldenström Macroglobulinemia. There are an additional two studies (phase II and III) that combine sonrotoclax and the BTK inhibitor, zanubrutinib, in participants with untreated CLL. Early clinical trial data were promising, indicating that sonrotoclax was well tolerated and achieved good responses both as monotherapy340 and in combination with zanubrutinib in R/R and treatment naïve CLL/SLL.341,342 BGB-21447 is another BCL2 inhibitor, for which there are no published data, however there is a phase I clinical trial in mature B cell malignancies underway (NCT05828589).
Lisaftoclax: The development of lisaftoclax (APG2575) by Ascentage (www.ascentage.com) marked another selective BCL2 inhibitor that is orally active and potently inhibits BCL2.343 Its overall structure is based on venetoclax but displays key structural differences (Fig. 5e) leading to faster cellular uptake and slightly higher cell death induction in CLL cells. Data from the phase I clinical trial in CLL patients indicated promising results with rapid clinical responses and an acceptable safety profile.344 Similar to sonrotoclax, lisaftoclax may potentially maintain activity in AML cells with acquired resistance to venetoclax when applied in combination with agents that upregulate BAX or downregulate MCL1.345 Following promising early clinical data, lisaftoclax is currently being investigated in a phase III trial in China for the combination with azacitidine in AML patients (NCT06389292) and in a global phase III trial in combination with BTK inhibitors in CLL patients (NCT06104566).
FCN-338/LOXO-338: FCN-338 is a selective BCL2 inhibitor developed by Fochon Pharmaceutical (https://fochonpharma.com) which displayed nanomolar potency against a range of BCL2-dependent FL, DLBCL, AML and ALL cell lines in vitro.346,347 Dose-dependent tumor growth inhibition was observed in FL, AML, and ALL xenograft models without significant weight loss. In October 2020, LOXO Oncology at Lilly (https://www.loxooncology.com) obtained the rights to develop and commercialize the drug under the name LOXO-338. Initial results from the first-in-human phase I study with advanced hematological malignancies (NCT05024045) showed that LOXO-338 was well tolerated and efficacy was observed.348 A second part of this trial was to evaluate LOXO-338 plus the BTK inhibitor, pirtobrutinib (LOXO-305), which was effective in vitro.349 However, in November 2022, Lilly discontinued the development of this molecule. A phase I trial by Fochon Pharmaceuticals is still recruiting patients with R/R CLL/SLL in China to investigate FCN-338 (NCT04682808).
Zn-d5: Zn-d5 is a selective BCL2 inhibitor developed by Zentalis Pharmaceuticals (https://www.zentalis.com) which reportedly demonstrates significant single-agent and combination anti-tumor activity in xenograft models of NHL, AML, and other hematological and solid tumor types.344 It has been under clinical assessment for R/R NHL, AML and light chain amyloidosis. Additionally, the combination of Zn-d5 and the WEE1 inhibitor azenosertib was being evaluated in a phase I/II trial for AML (NCT05682170), which has been terminated.
LP-108: Newave Pharmaceuticals (http://www.newavepharma.com) is clinically developing a BCL2 inhibitor named LP-108 (lacutoclax). Initial data from a phase I clinical trial in R/R CLL and NHL (NCT04356846) indicate that the drug is well tolerated with an overall response rate (ORR) of 53.8%.350 For R/R CLL/SLL the ORR was 75%. Monotherapy of LP-108 and combination with azacitidine is also under assessment for the treatment of R/R AML, MDS, and chronic myelomonocytic leukemia. Early data shows the combination has an advantageous ORR and PFS compared to single-agent LP-108.351 The Newave pipeline also indicates an additional BCL2 inhibitor, NW-4-1191, still in the discovery stage.
TQB3909: In China, the Chia Tai Tianqing Pharmaceutical Group (www.cttq.com) are the sponsors of several phase I trials evaluating the efficacy of the BCL2 inhibitor, TQB3909, in patients with R/R NHL, AML, MCL, plasmacytoma, myelofibrosis, MDS or HR-positive and HER2 negative advanced or metastatic breast cancer. First results presented at the European Society for Medical Oncology in 2024 indicate clinical activity particularly in the CLL patient population with an acceptable safety profile.352
Taken together, several selective BCL2 targeting BH3-mimetics are emerging clinically that may compete with venetoclax for the treatment of hematological malignancies. Whether any will outperform venetoclax remains to be seen; in CLL, venetoclax, particularly in combination with BTK inhibitors and obinutuzumab has set a very high bar. Determination of clinically significant differences will mandate long-term, head-to-head phase III clinical trials with careful assessment of toxicities as well as efficacies in terms of minimal residual disease assessments. Additionally, associated treatment cost differentials may play a major role in determining which inhibitor will be used as standard of care. With sonrotoclax and lisaftoclax now in phase III clinical trials, further clinical results are eagerly awaited.
Other BCL-XL and BCL2/BCL-XL dual inhibitor BH3-mimetics
Since BCL-XL is widely overexpressed and an essential gene in multiple solid tumors (Figs. 3 and 4), compounds that selectively target BCL-XL may have the potential to translate promising results from hematological malignancies into the field of solid tumors dependent on BCL-XL for survival. AbbVie introduced the compound WEHI-539 in 2013 as the first highly selective inhibitor of BCL-XL.353 Although several preclinical studies showed effective antitumor activity either as a monotherapy or as a combination treatment for various entities, WEHI-539 was never clinically tested.354,355,356 AbbVie presented two further BCL-XL inhibitors with improved selectivity over the next few years, A1155463 and A1331852, which have been in preclinical research since 2014 and 2015 respectively.357,358
In preclinical research, A1155463 has been shown to be effective at low nanomolar concentrations against colorectal cancer cells with high expression of BCL-XL, as well as other solid malignancies.359,360 A1331852 similarly has been shown to induce apoptosis across a variety of entities, but as well as A1155463, was used mainly as a tool compound to investigate the importance of singular anti-apoptotic BCL2 family members. Of note, a third compound named A1293102 was presented in 2021 by AbbVie, however, no further preclinical studies have been conducted so far.361 None of these compounds have entered clinical assessment.
Following the dual BCL2/BCL-XL inhibitors ABT-737 and navitoclax, several other comparable approaches using dual inhibitors are being investigated with the goal of reducing dose-limiting side effects while maintaining the efficacy and wide-range applicability. APG-1252 (pelcitoclax) developed by Ascentage is a phosphate prodrug that is converted to APG-1252-M1 after reaching its target cells.362 It has higher affinity for BCL2 and BCL-XL compared to navitoclax and lower permeability in platelets. APG-1252 at submicromolar concentrations was shown to achieve strong in vitro effects in gastric cancer cells, as well as colorectal and non-small cell lung cancer cells.362,363,364 A phase I clinical trial investigated APG-1252 safety in a cohort of patients with locally advanced or metastatic solid tumors reporting good tolerance and antitumor effects.365 Four studies investigating APG-1252 for the treatment of NHL or solid tumors, alone and in combination with other precision medicines, are currently recruiting (NCT05186012, NCT04001777, NCT04893759, NCT05691504).
MCL1-selective BH3-mimetics
MCL1 amplification is associated with higher grade malignancy and poor prognosis in many malignancies.366,367,368 Structurally, in contrast to BCL2 and BCL-XL, MCL1 has only one central hydrophobic helix surrounded by six helices which are less densely packed giving rise to a wider hydrophobic binding pocket.29 This has made the development of inhibitors more challenging. Nonetheless, several MCL1-specific BH3-mimetics have been developed by different pharmaceutical companies or academic institutions. These compounds target one or more of the four pockets within the hydrophobic groove of MCL1, and several MCL1 inhibitors have entered clinical studies as single agent or in combination with other drugs. However, all but one clinical trials have been halted over safety concerns and none have yet received approval for clinical usage (Table 1).
S63845 and S64315: S63845 has been shown to be efficient in killing cells from various hematologic malignancies and displays potent anti-tumor activity as a single agent in numerous malignancies in vivo.369 Its derivative MIK665/S64315370 was tested in several phase I dose escalation studies for the treatment of R/R lymphoma and MM (NCT02992483), AML, and MDS (NCT02979366). In addition, a dose-escalation trial of S64315 in combination with venetoclax (NCT03672695) was conducted and a phase I/II trial (NCT04629443) evaluating the combination of S64315 and azacitidine in AML patients has been completed but formal results have not been presented. According to data posted on the clinical trials website (https://clinicaltrials.gov/study/NCT04629443?tab=results accessed 24JUN2024) 17 patients were entered, but none completed the study due either to progressive disease (8 patients) or adverse events (4 patients); there were four fatal adverse events. A major limitation of this molecule is the necessity for intravenous administration at three times per week. With this dosing schedule, efficacy may be limited given the short half-life of MCL1 protein.
ABBV-467: ABBV-467, a very potent and selective macrocyclic MCL1 inhibitor with a short half-life in humans, was tested for safety and tolerability in adults with R/R MM (NCT04178902). While treatment with ABBV-467 achieved some disease control, it also caused elevated cardiac troponin levels in the plasma in some patients, prompting the study’s termination, despite the absence of other cardiac findings.371 The effect of MCL1 inhibitors on cardiomyocytes was also predicted by genetic studies showing cardiomyopathy and heart failure in cardiomyocyte-specific MCL1 knockout mice,372 indicating on-target toxicity of the MCL1 inhibitors.
AMG176 and AMG397: AMG176 (tapotoclax) is a potent, specific, and orally available MCL1 inhibitor developed by Amgen (www.amgen.com).373 Following an initial safety study (NCT02675452), AMG176 was investigated in combination with venetoclax in patients with R/R hematologic malignancies (NCT03797261), which has been suspended, and in combination with azacitidine in patients with MDS/CML (NCT05209152), which has been completed. However, no results from either study have been posted, despite the former study completing in 2019. Improvement of AMG176 resulted in AMG397 (murizatoclax)374 which has high affinity for MCL1 and selectively competes with pro-apoptotic BCL2 family members for the binding to the BH3-binding groove of MCL1. AMG397 effectively disrupted the interaction between MCL1 and BIM, increased caspase-3/7 activity and reduced cell viability. Cell lines from hematologic malignancies were highly sensitive to AMG397, and in vivo oral treatment led to significant tumor regressions in xenograft mice.375 A phase I dose-escalation study of AMG397 in MM, NHL or AML was undertaken (NCT03465540) but terminated due to cardiac toxicity. As with S63415, preliminary data available on the clinical trials website indicate significant toxicities without major responses (https://clinicaltrials.gov/study/NCT03465540).
AZD5991: AZD5991 is another highly selective macrocyclic MCL1 inhibitor developed by Astrazeneca (www.astrazeneca.com). Clinical studies using it either alone or in combination with venetoclax in patients with R/R hematologic malignancies (NCT03218683) were conducted and stopped, most likely due to toxicities. A trial of biomarker-based therapy for AML initially incorporated AZD5991 (NCT03013998), but the arm containing AZD5991 has been closed.
PRT1419: PRT1419 is a selective inhibitor of MCL1 developed by Prelude Therapeutics (https://preludetx.com) that has demonstrated preclinical efficacy in hematologic malignancies and solid tumors. A phase I dose expansion study of PRT1419 in R/R hematologic malignancies (NCT04543305) was carried out to evaluate the dosing schedule, maximum tolerated dose, and/or the optimal biological dose. A separate study (NCT05107856) tested PRT1419 as a once weekly intravenous infusion in patients with hematologic malignancies, using it as monotherapy or in combination with azacitidine or venetoclax in R/R myeloid or B cell malignancies. Given that MCL1 overexpression is a modulator of venetoclax resistance317 and azacitidine downregulates MCL1,376 PRT1419 may provide additional or synergistic advantages to azacitidine or venetoclax in treatment-resistant myeloid or B cell malignancies. However, this study was terminated in January 2024; no results have thus far been posted. Furthermore, MCL1 inhibition with PRT1419 in patients with advanced solid tumors (R/R breast and lung cancer, sarcoma and melanoma) (NCT04837677) indicated satisfactory safety and tolerability, with no cardiac toxicity reported. BAX and caspase 3 activation were observed, suggesting potentially successful MCL1 inhibition, however no significant efficacy was observed.377 Whether PRT1419 is being further developed by the company is not clear; the molecule is not currently listed on the company website (accessed 24JUN2024).
GS9716: GS9716 (zamzetoclax) developed by Gilead (www.gilead.com) is a potent and specific small molecule inhibitor that binds directly to MCL1. In vivo and in vitro studies have demonstrated a strong single-agent effect as well as synergy with other anti-cancer therapies.378 GS9716 is currently undergoing a phase I clinical trial in patients with advanced solid tumors to assess its safety, tolerability, and pharmacokinetics as monotherapy and in combination with anticancer medicines (NCT05006794). This makes GS9716 the only MCL1 inhibitor currently being given to patients, but since its original presentation at AACR in 2022,378 no scientific update has been provided.
Additional putative inhibitors of MCL1 are currently emerging, which have not yet entered clinical testing. NA1-115-7 is a compound isolated from the Winteraceae plant Zygogynum pancheri native to eastern and south eastern New Caledonia.379 The physicochemical properties and structural complexity of natural products make them particularly interesting for use in the treatment of cancer. NA1-115-7 binds to MCL1 in the BH3 binding groove and forms a covalent bond, making it the first covalent BH3-mimetic that targets MCL1. In lymphoma cells, NA1-115-7 stabilized MCL1, disrupted the BAK/MCL1 interaction, and rapidly induced apoptosis in a BAK- and BAX-mediated process.380 Moreover, NA1-115-7 induced tumor cell death while demonstrating no toxicity to cardiomyocytes or normal blood cells.380 Like many other natural compounds, it has a lipophilic structure, and its susceptibility to acidic environments may impede its oral administration and, consequently, its clinical development. A NA1-115–7-loaded nanoemulsion was created to address these issues, which could potentially render it easier to evaluate in vivo.381
In summary, despite the successful development of potent and selective MCL1 inhibitors, their toxicities observed in patients have so far precluded clinical development. These toxicities highlight the need to develop novel strategies to safely deliver MCL1 inhibitors to the intended target cells while sparing healthy tissues.
Novel approaches to target BCL2 proteins
Cyclic peptides
As an alternative to small BH3-mimetics, also cyclic or stapled peptides may be employed to target BCL2 proteins. These peptides are usually based on the BH3 domain and modified to ensure high affinity binding to the targeted anti-apoptotic BCL2 protein as well as improved stability. Pioneering work by the Korsmeyer lab using hydrocarbon stapling resulted in the generation of the first stabilized alpha-helix of BCL2 domains (SAHBs) displaying high binding affinity to BCL2 or BAX and cell permeability.382,383 A stapled-modified BIM BH3 peptide has been shown to reactivate apoptosis and overcome resistance by simultaneously inhibiting multiple BCL2 proteins.384,385 Modification of the BH3 domain sequence and inclusion of non-natural amino acids and acrylamide moieties resulted in the development of SAHBs selectively targeting BCL2A1, highlighting the potential of this approach in targeting BCL2 proteins that were so far not amenable for the development of selective BH3-mimetics.386,387,388 Recent research has also demonstrated that SAHBs may be designed to inhibit specific functions of BCL2 proteins, e.g. the interactions with IP3Rs, to dissect the functions of BCL2 proteins at different subcellular compartments.389 However, so far these approaches have been limited to preclinical tool compounds and have not yet been tested clinically. To the best of our knowledge, the only stapled peptide to enter clinical trials (NCT02909972, NCT03654716)390 was the MDM2 inhibitor ALRN-6924 (sulanemadlin)391 and development of this compound has now been terminated, indicating that the translational value of stapled peptides beyond their use as tool compounds remains to be proven.
BH4 domain-targeting of BCL2
As anti-apoptotic BCL2 also operates as an inhibitor of IP3R channels, strategies to disrupt IP3R/BCL2 complexes in cancer cells have been investigated. Most strategies have focused on IP3R-derived peptides that comprise the BH4 domain-binding site of BCL2. As such, these tools serve as a decoy for BCL2 and strip IP3Rs from BCL2. A cell-permeable stabilized version of this peptide, BCL2/IP3R disrupter-2 (BIRD-2), has been applied in different BCL2-dependent cancer cell types. BIRD-2 application by itself, in the absence of other pro-apoptotic triggers, was sufficient to kill CLL and DLBCL cells by triggering excessive, pro-apoptotic Ca2+ release.392,393 Further mechanistic studies revealed that BIRD-2 sensitivity in B cell lymphoma cells was particularly due to the combination of two factors: first, high levels of IP3R2, the IP3R isoform with the highest sensitivity to IP3,393, and second, constitutive basal IP3 signaling downstream of the tonically activated B cell receptor.394 Moreover, a reciprocal sensitivity between BIRD-2 and venetoclax has been reported across a panel of DLBCL cell lines.395 However, BIRD-2 enhances the sensitivity of DLBCL cells to venetoclax by evoking Ca2+-dependent upregulation of BIM.395 The synergistic cell death effects between BIRD-2 and BH3-mimetics also have been exploited in several other cancer types including MM, FL and small cell lung cancer cells.396,397 In addition, BIRD-2 has been combined with chemotherapeutic agents, which enhanced the cell death response in ovarian cancer cells.398
Beyond peptides, also BH4 domain-targeting BCL2 inhibitors have emerged. BDA-366 was the first molecule reported to directly bind to the BH4 domain of BCL2 and thus to serve as a BH4 antagonist, capable of killing lung cancer and MM cells.399,400 The mechanism for this cell death effect was attributed to its ability to convert BCL2 into a pro-apoptotic protein by triggering the exposure of its BH3 domain, thereby resulting in BAX/BAK activation.401 As such, cancer cells with high levels of BCL2 were more susceptible to BDA-366. However, further work, at least in BCL2-dependent malignant B cells, indicated that BDA-366 evoked cell death independently of BCL2 protein levels, even potently killing cancer cells lacking substantial BCL2 protein levels.402 Furthermore, in vitro BAX pore-forming assays excluded that BDA-366 either directly or indirectly through BCL2 could activate BAX pore formation.402 Hence, the mechanism of action of BDA-366 in evoking cancer cell death requires further scrutiny.403 Furthermore, other BH4 domain-antagonizing small molecules have emerged such as CYD0281, which limited breast cancer growth404 and DC-B01, which kills lung cancer cells.405 DC-B01 has been reported to block the BCL2/cMYC complex, thereby suppressing the transcriptional activity of cMYC. Moreover, lung cancer cells in which BCL2 was knocked down became resistant to DC-B01, indicating an on-target effect of DC-B01. Yet, further work is needed to assess its effect in other BCL2-dependent cancer types. Finally, these peptides and small molecules have so far been used in pre-clinical settings, and thus further research is needed to determine whether a strategy targeting the BH4 domain may lead to novel therapeutics.
PROTACs
Therapeutic targeting of BCL-XL in men resulted in the dose-limiting on-target toxicity of thrombocytopenia. Therefore, a lot of effort has been devoted to developing other strategies to target BCL-XL without inducing platelet toxicity. One approach has been to develop BCL-XL or BCL-XL /BCL2 dual PROTACs (reviewed in406). PROTACs are advantageous in this setting as they promote proteasome-mediated degradation of the target protein through recruitment of E3 ubiquitinating ligases, allowing for tissue-specificity based on the expression of E3 ligases. Although there are around 600 E3 ligases in the human genome,407 most of the clinically advanced PROTACs are based on Von Hippel-Lindau (VHL) cullin-2, Cereblon (CRBN) cullin-4A RING, Mouse Doubleminute 2 homolog (MDM2) or Inhibitor of Apoptosis Proteins (IAP) as recruited E3 ligases.408 As VHL and CRBN ligases are only expressed at low levels in platelets,409 unwanted toxicity of BCL-XL inhibition may be strongly reduced. An additional advantage of PROTACs over small molecule inhibitors lies in their ability to promote target degradation catalytically, enabling more effective target neutralization at lower drug exposure.410
In addition to the E3 ligase ligand, PROTACs consist of a so-called warhead, which is often a small molecule inhibitor against the protein of interest. Those functional groups are connected through a linker of variable length and properties that are designed to ensure that the linker does not disturb the binding properties of the warhead. This general toolbox consisting of warhead, linker, and E3 ligase ligand offers various combinations and design strategies, which can be seen in the overview of PROTACs in Table 2.
The furthest advanced BCL-XL-targeting compound DT2216 developed by Dialectic Pharmaceuticals (www.dtsciences.com) is in clinical testing in a phase I trial in R/R malignancies, including solid and hematological cancers (NCT04886622). DT2216 is based on ABT-263 linked to a VHL recruiting ligand. Although ABT-263 has high binding affinity against BCL-XL and BCL2, it only forms ternary complexes with BCL-XL in living cells. In this ternary complex, VHL ligase can transfer ubiquitin to Lys87 of BCL-XL.411 The inability to degrade BCL2 might be caused by the absence of a stable ternary complex. The specific targeting of BCL-XL by DT2216 has proven effective against BCL-XL-dependent cancers412 and it has been shown that DT2216 is also able to overcome BCL-XL-mediated gemcitabine resistance, without causing organ toxicity.413 Furthermore, breast, prostate, liver and colon cancer cell lines with acquired BCL-XL-mediated resistance against commonly used chemotherapeutic agents could be sensitized by DT2216 treatment.411 In vitro studies combining DT2216 with small molecule MCL1 inhibitors in small-cell lung cancer showed promising results in addressing the co-dependency of the cancer cells without induction of toxicity in healthy cells.414 Initial preclinical studies comparing the effects of DT2216 and ABT-263 side-by-side in platelets and leukemia cells indicate a highly improved therapeutic window for DT2216.411 DT2216 has received FDA fast-track designation for adult patients with R/R peripheral and cutaneous T cell lymphomas (https://prn.to/3O2PiGm), but no recent updates have been provided.
Three other PROTACs targeting BCL-XL also through ABT-263 rely on pomalidomide as CRBN recruiting ligand. SIAIS361034, PZ15227 and XZ739 differ by their linker design, which can affect the ability to form stable ternary complexes and therefore the effectivity of the PROTAC to degrade the targeted protein. In PZ15227, ABT-263 is connected via a piperazine ring to the linker, similar to DT2216, whereas ABT-263 is tethered through a N-methylamino group to a PEG linker in XZ739. XZ739 showed improved potency compared to DT2216, induced apoptosis in T-ALL cell lines and displayed indications for senolytic activity,415 indicating on-target activity.416 While SIAIS361034 binds both BCL2 and BCL-XL, it only degrades BCL-XL and has proven selective in hepatocellular carcinoma.417,418 XZ424 is also a CRBN-based PROTAC, but instead of the dual inhibitor ABT-263 it uses the selective BCL-XL inhibitor A1155463. The PROTAC exhibited similar binding affinities to BCL-XL as the small molecule inhibitor, but proteasome-mediated BCL-XL degradation was not accompanied by platelet toxicity.419
To provide further options for cells with limited response to VHL or CRBN-based PROTACs, PROTAC-8a was developed using the IAP antagonist 1 bound to ABT-263 through a short alkane linker. BCL-XL degradation was observed across different entities of solid cancers, whereby the sensitivity seemed to correlate with XIAP E3 ligase expression.420
In addition to the linker properties, the orientation of the warhead also largely influences the interaction with the targeted protein and thereby its degradation specificity. This becomes obvious in the dual-targeting BCL-XL/BCL2 PROTACs PZ703B, 753B, and WH244 which are also based on ABT-263 as a warhead, but with altered orientations. This enables a dual targeting mechanism where PZ703B degrades BCL-XL and inhibits BCL2 function.421 753B differs in the linker domain. Furthermore, both compounds exerted enhanced degradation potency compared to their respective stereoisomers, highlighting that in addition to the tethering position of the warhead, also the steric orientation impacts the ternary complex formation. Although 753A and B displayed similar binding activity against both BCL-XL and BCL2, their binding was weaker than that of the parent inhibitor ABT-263. However, 753B showed single-agent potency in reducing both target proteins in AML models and senolytic activity through apoptosis induction.422 The capacity to also target BCL2 is given by the different ternary complex conformation compared to DT2216, allowing access to BCL2 surface lysines as ubiquitination sites.423 In small-cell lung cancer 753B was also more potent than DT2216 or ABT-263 in reducing cell viability and was well tolerated in mice.424 With PROTAC 753B as a starting point, the second-generation dual degrader WH244 was recently developed. By studying the ternary complex formation of VHL/753B and BCL2 or BCL-XL, it was possible to optimize the linker design and improve potency for both BCL-XL and BCL2 degradation, resulting in a highly promising compound that exhibited enhanced activity in leukemia cells.425
A slightly different approach was recently presented by NeoXBiotech (www.neoxbio.com) in the design of its lead compound NXD02.426 In comparison to the navitoclax-based PROTAC DT2216, NXD02 was designed to have less potent binding for BCL-XL, to prevent an inhibitory effect while maintaining the ability to degrade BCL-XL, and thus establishing a favorable therapeutic window and preventing platelet toxicity.
So far, only few reports describe specific and potent MCL1 targeting PROTACs. Compound C3 is described to selectively degrade MCL1 while compound C5 from the same synthesis trials targets BCL2.427 Both compounds work via CRBN recruitment and are based on the dual BCL2/MCL-1 inhibitor Nap-1,428 showcasing that the linker design influences the properties of the PROTAC molecule, not only concerning solubility and membrane permeability, but also regarding specificity and degrading potency.
Targeting of BCL2 may also be possible using molecular glue degraders, and very recently several thalidomide-related compounds have been described that selectively target BCL2. These thalidomide analogs bind close to the BH1 domain of BCL2 to recruit CRBN, thus holding the potential to also target mutant BCL2 proteins emerging as a mechanism of venetoclax resistance.429
Additionally, efforts are made to further reduce undesirable off-target effects of PROTACs by the introduction of PROTAC prodrugs that are recruited to cancer cells through biomarker recognition. Here, the PROTAC function is activated in a biomarker-dependent fashion, which ensures the degradation of the targeted BCL2 protein selectively in the cancer cells.430 This approach highlights an entirely new strategy, in which PROTACs may be more selectively delivered to the intended cell types.
Nanoparticles and antibody drug conjugates
The very first clinically tested attempts to target BCL2 relied on antisense technology used in G3139 (oblimersen),431,432 and hence the ongoing clinical trials with BP1002 (NCT05190471 and NCT04072458) read a bit like a trip down memory lane. This liposomal BCL2-targeting antisense DNA molecule has been developed by Bio-Path (https://www.biopathholdings.com) using neutral charge lipids as carriers to improve stability and overcome toxicity of previous approaches. An optimized siRNA-based approach to target MCL1 has recently been described that may also achieve better plasma stability due to increased albumin binding and that may be able to reduce MCL1 expression in vivo in tumors.433
Recent approaches to deliver BH3-mimetics more safely and more selectively to the targeted tumor cells relied on alternative drug formulations or packaging. Nanoparticles can be used to encapsulate drugs, thus ensuring a more controlled release of the drug.434 Thereby, the coating of the nanoparticles may achieve a more tumor-targeted drug delivery and thus increase their therapeutic efficacy. This may be particularly important for combination treatments, which are associated with increased toxicity and unwanted side effects. The first evidence that this approach may be feasible for BH3-mimetics originates from a study with nanoencapsulated ABT-737 in combination with camptothecin.435 More recently, preclinical studies have shown that the combined treatment with venetoclax and S63845 was more efficacious and less toxic if either drug was administered in nanoformulation.436 In a more targeted approach, hyaluronic acid-coated nanocrystals were used to deliver venetoclax to CD44-expressing breast cancer cells,437 highlighting that receptor-ligand interactions can be exploited to selectively deliver BH3-mimetic containing nanoparticles to the intended cell populations.
Due to its function in platelet survival,277 packaging into nanoparticles may be particularly important for BH3-mimetics targeting BCL-XL. To this end, the dual BCL2/BCL-XL inhibitor AZD4320 has been further developed as a PEGylated poly-lysine dendrimer conjugate.438 With a size of only 7–15 nm, the resulting dendrimer conjugate, AZD0466, is a small nanoparticle with good tumor penetration ability and a favorable therapeutic index.439 A first-in-human clinical trial indicated good tolerability of AZD0466 in patients with advanced solid tumors (NCT04214093). Further clinical development of AZD0466 was investigated in the phase I/II NIMBLE trial for AML and ALL patients (NCT04865419) as well as NHL patients (NCT05205161). However, both studies were terminated in 2023 due to an overall lack of significant clinical benefit.440
A different approach to delivering BH3-mimetics to the intended tumor cells may lie in the development of antibody-drug conjugates. To this end an antibody against B7H3 (mirzotamab) has been linked with the BCL-XL inhibitor clezutoclax resulting in ABBV-155. ABBV-155 has been in clinical trial (NCT03595059) since 2018 in patients with advanced lung cancer. Preliminary results presented in 2022 and 2023 indicate that thrombocytopenia due BCL-XL inhibition in platelets was not observed upon treatment with ABBV-155, suggesting that an antibody-targeted approach may indeed circumvent unwanted side effects of BCL-XL inhibition.441,442 However, in this heavily pre-treated patient cohort, ABBV-155 also did not show anti-tumor activity, and this molecule has now been discontinued by Abbvie. More promising results were obtained by ABBV-637, an EGFR-targeted antibody coupled with a BCL-XL inhibitor, which displayed some activity in a phase I study in R/R lung cancer patients (NCT04721015).443
Strategies targeting the pro-apoptotic BCL2 proteins to prevent apoptosis
Not only too little but also too much apoptosis can contribute to disease. In this regard, strategies that prevent cell death by interfering with the BCL2 family may also hold therapeutic promise. Hence, small molecules inhibiting pro-apoptotic BAX activity such as MSN-50 and MSN-125 have emerged.444 Moreover, these compounds have already been reported to exert neuroprotective actions against glutamate-induced toxicity,444 a process driven by pro-apoptotic BAX.445 However, MSN-50 and MSN-125 also inhibit BAK. More recently, BAI1 has been designed as a novel, selective BAX-inhibiting molecule.446 Hence, such BAX-selective inhibitors could be attractive compounds to preserve neuronal survival.
Targeting BCL2 proteins beyond cancer
Neurodegenerative diseases
BCL2 proteins in Alzheimer’s disease
The BCL2 family of proteins plays a crucial role in neuronal survival and apoptosis regulation within the central nervous system (CNS) and hence it is not surprising that BCL2 proteins are implicated in numerous neurological diseases including Alzheimer’s disease (AD).447,448 AD is characterized by the accumulation of amyloid plaques consisting of amyloid β (Aβ). BCL2 proteins are downregulated in AD, whereby Aβ plaques can evoke an imbalance in the protein levels of BCL2 family members.449,450 In vivo exposure of brains or ex vivo treatment of hippocampal slides with Aβ oligomers results in a decrease in BCL2 proteins and an increase in BIM, thereby triggering the activation of BAX and neuronal death. BAX inhibition either using peptide-based approaches or gene therapy could prevent the neuronal cell death induced by oligomeric Aβ.450 Thus, in the context of AD, selective BAX inhibitors could be promising to sustain the survival of neurons and thus potentially delay cognitive decline.451
Beyond the inhibition of BAX, an upregulation of BCL2 protein levels in neurons has been explored as a neuroprotective strategy. Overexpressing of anti-apoptotic BCL2 in the postmitotic neurons of an AD mouse models reduced the activation of caspase 9 and caspase 3, thereby limiting the caspase-mediated cleavage of tau and the formation of neurofibrillary tangles.452 Furthermore, the neurons of these mice showed increased intracellular accumulation of amyloid precursor protein, thus restricting the formation of Aβ plaques. Importantly, AD mice overexpressing BCL2 displayed improved memory retention, thereby delaying the decline in cognitive function. This neuroprotective function of BCL2 upregulation preceded neuronal cell death, suggesting additional mechanisms are in play beyond BCL2’s canonical anti-apoptotic function (see below). Moreover, a correlation between increased expression of BCL2 in brain regions rich in Aβ plaques and neuroprotection was observed in an amyloid precursor protein transgenic mouse model for AD.453 This study further supported the neuroprotective functions of BCL2, which reduced the caspase 3-mediated cleavage of amyloid precursor protein and subsequently prevented the formation of extracellular Aβ plaques.454
However, the neuroprotective properties of BCL2 in AD are not limited to BCL2s canonical function in apoptosis, but also extend to the non-canonical role in regulating Ca2+ homeostasis. Over the years, it has become clear that deranged neuronal Ca2+ signaling is a very early hallmark of AD pathology, thereby driving pathogenesis.455,456 Hence, strategies to normalize or preserve Ca2+ signaling hold therapeutic promise to delay the deleterious impacts of AD progression.457,458,459 At the mechanistic level, both excessive Ca2+ release through IP3Rs460,461,462 and ryanodine receptors,463,464,465,466,467,468 potentially related in part to BCL2 downregulation, have been implicated in AD. Genetic or pharmacological inhibition of these channels could provide neuroprotection. Hence, BCL2, which inhibits both IP3Rs and ryanodine receptors, is an attractive target to suppress deranged Ca2+ signaling in AD.72,469 Moreover, endogenous ryanodine receptors/BCL2 complexes have been identified in neuronal samples,72 though whether those are dysregulated in AD awaits further validation. In any case, strategies that elevate BCL2 protein levels may provide neuroprotective effects through the targeting of ryanodine receptors by BCL2.470 Adeno-associated viral vectors expressing BCL2 proteins stereotactically injected in the brain of AD mice exerted synaptoprotective and amyloid-protective effects. Furthermore, overexpression of BCL2K17D, a BCL2 mutant that fails to inhibit IP3Rs but still inhibits ryanodine receptors, retained neuroprotective properties. These results suggest that inhibition of deranged ryanodine receptor channels in AD likely contributes to the neuroprotective role of BCL2 thereby inhibiting excessive Ca2+ responses in AD.470 However, since the molecular determinants in BCL2 responsible for ryanodine receptor binding remain elusive, BCL2 mutants that are defective in ryanodine receptor binding are not yet available to firmly establish ryanodine receptors as the neuroprotective targets of BCL2.
Finally, as an alternative to direct overexpression models of BCL2, several therapeutic drugs can increase BCL2 protein levels and can thus be implemented to improve AD outcomes. in this regard, drugs currently used for the treatment of asthma, such as ibudilast, montelukast, and pranlukast, can prevent BCL2 downregulation and improve Aβ-induced memory impairment.471,472,473
BCL2 proteins in Parkinson’s disease
Increasing evidence supports a role of dysregulated lysosomal function as an early feature in Parkinson disease (PD), and more specifically impaired lysosomal membrane permeabilization (LMP).474 The role of BAX/BAK in LMP and autophagic cell death,475,476 and its functional link to PD-linked lysosomal dysregulation has become clearer in recent years. Interestingly, BAX inhibition is able to prevent LMP, restore lysosomal levels and prevent accumulation of undegraded autophagosomes both in vitro and in vivo.477 Besides lysosomal defects, PD is characterized by degeneration of dopaminergic neurons in substantia nigra pars compacta (SNpc) and the loss of nerve fibers in the striatum. In experimental settings, neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is used to replicate a PD-like syndrome by damaging SNpc dopaminergic neurons, mimicking the loss of these neurons.478 BAX is highly expressed in the SNpc and involved in degeneration of SNpc dopaminergic neurons in PD. Additionally, mice lacking BAX become resistant to MPTP-induced neurodegeneration.479 While MPTP administration increased BAX levels, a reduction in BCL2 protein levels was observed, thereby causing excessive BAX to exist free from neutralization by BCL2.479 Consistently, overexpression of BCL2 can also protect against MPTP-induced neurodegeneration.480,481
BCL2 in amyotrophic lateral sclerosis
The protein levels of different BCL2 proteins are altered in motor neuron disease-amyotrophic lateral sclerosis (ALS), which is characterized by excessive ER stress and disturbed protein homeostasis. Similarly to PD, BCL2 protein levels are reduced in ALS while BAK levels are increased.482 Familial forms of ALS are caused by mutations in superoxide dismutase-1 (SOD1). In SOD1G93A mice, neurodegeneration was prevented by overexpression of BCL2 and by delaying caspase activation, highlighting the importance of apoptosis in ALS.483 On the other hand, deletion of BAX improved the lifespan of SOD1G93A mice.484 Also, BIM targeting has been proposed as a potential strategy for ALS as upregulation of BIM in an ALS mouse model has been observed. By removal of BIM, the lifespan of the ALS mice was increased,485 highlighting the potential of targeting apoptosis as a potential therapeutic strategy for the treatment of ALS. However, so far this approach has only been tested in preclinical models, and no clinical trials are currently exploring whether this strategy may be translated to human disease.
Autoimmune disorders
Given their central role at regulating immune cell survival, BCL2 proteins may also be implicated in autoimmune diseases characterized by insufficient apoptosis of autoreactive immune cells. Initial studies in mice indicated that loss of BIM or overexpression of BCL2 leads to the production of antinuclear autoantibodies, resulting in autoimmune disease resembling Systemic Lupus Erythematosus (SLE).156,486 In humans, SLE is associated with high levels of T cell-dependent antinuclear autoantibodies and systemic inflammation. Evidence for the role of BCL2 in SLE was provided by a study showing high BCL2 expression in circulating lymphocytes in SLE patients.487,488,489 In addition, BCL2 polymorphisms were found to be associated with the pathogenesis of SLE.490 In B cells derived from SLE, reduced induction of BCL-XL and MCL1 levels were observed upon in vitro stimulation as compared to healthy control cells,491 which may indicate a higher functional importance of BCL2 also in B cells in SLE patients. These data suggest that targeting of BCL2 may represent a promising therapeutic strategy in SLE, and animal models of SLE treated with ABT-737 showed encouraging results.492 Based on these pre-clinical data, venetoclax has recently been tested in a phase I study in 73 women with SLE (NCT01686555). In these patients, venetoclax was well-tolerated and induced a concentration-dependent reduction in lymphocyte counts, especially in disease-relevant B cell subsets, highlighting the potential of venetoclax for the treatment of autoimmune diseases.493,494,495
Apart from SLE, a potential application of BH3-mimetics may be in the gastrointestinal autoimmune Crohn’s disease. Here, chronic inflammation is driven by hyperproliferating T cells with elevated BCL2 expression.496,497 BCL2 may also play a role in the associated intestinal fibrosis driven by activation of fibroblasts, which is linked to BCL2 and can be prevented by treatment with ABT-737 or ABT-263.498,499
More controversial is the role of BCL2 proteins in other autoimmune diseases including Type 1 diabetes mellitus (T1D).500 T1D is generally characterized by immune-mediated apoptosis of the β cells of the pancreas, resulting in insulin deficiency and chronic hyperglycemia. A recent study has indicated that in T1D, a subset of β cells acquires a senescence-associated secretory phenotype (SASP) associated with increased BCL2 expression.501 Treatment with senolytic BH3-mimetics (see below) may eliminate these senescent cells and prevent immune-mediated β cell destruction, indicating a new treatment strategy for T1D. In summary, although several studies indicate a potential for BH3-mimetics like venetoclax in different autoimmune diseases, there is currently no clinical development in this area.
Ageing and senescence
The BCL2 proteins have been shown to play a major role in the survival of senescent cells.502 Cellular senescence was discovered after observing that human fetal cells only engaged in 40-60 replicative cycles and then ceased to divide any further.503 This number of divisions marks the replicative capacity of a cell and was eventually known as Hayflick’s limit, after which replicative senescence is induced. The mechanism underlying this phenomenon was later discovered to be the progressive shortening of telomeres with each mitotic division, which happens in all cells except those that express the telomerase enzyme, capable of telomere extension.504 In order to continue to grow beyond their natural numbers of division, cancer cells must adapt strategies to overcome and evade cellular senescence.505,506 The accumulation of senescent cells has also been found to play an important role in ageing and age-related diseases.507 Thus, preventing the accumulation of senescent cells has become a strategy with a strong potential to foster healthy ageing and ameliorate the prognosis of diseases such as diabetes, AD or cancer itself.508
This is the goal of a new class of therapeutics called senolytics that target senescent cells by selectively killing them.508,509 Early senolytics were discovered utilizing a combined hypothesis-driven and bioinformatic approaches, focusing on natural compounds or repurposing drugs already in use for other applications.508 Dasatinib, a pan-tyrosine kinase inhibitor approved for the treatment of myeloid leukemia, and quercetin, a naturally occurring flavonoid that may directly or indirectly inhibit BCL-XL, were two of the fist senolytics to be discovered, and are often applied together.508 This combination has not only been shown to improve physical function and increase the lifespan in aged mice,510 but also to reduce the presence of senescent cells in a phase I clinical trial in patients with diabetic kidney disease.511
These and other observations led to the study of BH3-mimetics as potential senolytics. Similar to cancer cells, senescent cells are primed for death but protected from apoptosis by the overexpression of BCL2 proteins.512 Indeed, senescent cells share many features with cells undergoing apoptosis, such as expression of p53, elevation of ROS or changes in mitochondrial physiology, along with consistent overexpression of BCL-w, BCL-XL, and BCL2.513 In addition, it has recently been discovered that minority MOMP (meaning MOMP occurring in a subset of mitochondria) is associated with cellular senescence.514 Although the factors that determine whether a cell will undergo apoptosis or senescence after damage are not fully understood, the BCL2 family of proteins seems to play an important role in these cell fate decisions, with expression of pro-survival BCL2 proteins favoring the induction of senescence over cell death.
ABT-737 was one of the first BH3-mimetics reported to exhibit selective pro-apoptotic activity in different senescent cell types.513 As a result of this, navitoclax is now widely used as a senolytic compound in pre-clinical research.515 A1331852 has also been found to have senolytic properties, which suggests that BCL-XL may be the key protective protein in certain senescent cells.358 It has been shown to reduce senescence in cholangiocytes dependent on BCL-XL and activated stromal fibroblasts, reducing liver fibrosis in vivo in MRD2-/- mice.516 However, the senolytic activity of A1331852 and venetoclax is not as potent as that of navitoclax, suggesting that co-inhibition of BCL2, BCL-w and BCL-XL may be more effective than BCL2 inhibition alone. Although BH3-mimetics seem to be some of the strongest senolytics known, with many potential clinical applications,517 the mixed response of different senescent cells to them will likely prevent their widespread use. This is probably due to the large heterogeneity of the senescent phenotype, which is still poorly understood and may include dependence on other pro-survival signals. Also, their off-target effects and toxicity may also limit their benefits as senotherapies.515 Nevertheless, BH3-mimetics are currently being used as part of an effort to design more targeted second-generation senolytics, which may change the way these drugs are delivered to their targets and improve their effects.518,519
Future perspectives
BCL2 inhibitors: can venetoclax be bettered?
It is said that “imitation is the sincerest form of flattery”, which means that if someone is copying your work, you must be doing something right! The combination of the transformational clinical activity in some hematologic malignancies and the concurrent absence of major toxicities of venetoclax has spawned an increasing number of “imitators” (Table 1 and Fig. 5e).
Venetoclax not only clinically validated the choice of BCL2 family proteins as therapeutic targets for cancer but also demonstrated the potential efficacy of specifically inhibiting protein-protein interactions. Moreover, the careful modeling of venetoclax to the BH3 binding groove of BCL2 made it appear unlikely that BCL2 inhibitors of improved binding and perhaps efficacy could be obtained. However, the development of sonrotoclax and lisaftoclax in particular, indicated that this may be the case, reflecting structural flexibility within the BCL2 BH3 binding groove. How far such improvements of binding of BCL2 inhibitors to BCL2 can be driven with the aid of modern computational modeling (Alphafold3 etc) remains to be determined.520
Improved binding of BCL2 inhibitors to BCL2 may not be without problems. Like all BCL2 family proteins, BCL2 is broadly expressed in normal tissues, and the lack of severe on-target toxicities with venetoclax reflects the inherent resistance of most normal tissues to BCL2 inhibition. Whether improved BCL2 binding will alter the toxicity profiles remains to be determined, although initial clinical data appear promising. One unanticipated consequence of BCL2 inhibition in some patients with CLL treated with either venetoclax or sonrotoclax is the acquisition of biallelic BAX mutations in normal myeloid cells.323,521 Their possible clinical significance remains unknown and will require careful monitoring. Biologically, they are fascinating since they would be anticipated to result in a profound apoptotic block and yet have not been detected in lymphoid malignant cells. In contrast, 17% of patients with AML treated with venetoclax exhibited various BAX mutations, pointing to a special role of BAX in myeloid cells.
With the emergence of acquired resistance in CLL or AML patients treated with venetoclax, one potential advantage of other BCL2 inhibitors may lie in their slightly different binding affinities to the individual pockets within the hydrophobic groove. However, resistance to venetoclax is not only mediated by point mutations within the hydrophobic groove, but also by the induction of other anti-apoptotic BCL2 proteins like MCL1 or BCL2A1.276,522,523 In contrast to MCL1, there is still no high-affinity inhibitor for BCL2A1.77,524 Overall, the hydrophobic groove of BCL2A1 is very similar to that of MCL1, but displays a unique Cys55 residue that may enable covalent binding of thiol-containing inhibitors.525,526,527,528 Further development of small molecule inhibitors of BCL2A1 may give rise to compounds able to counteract venetoclax resistance in selected patients. Given that BCL2A1 knockout mice are viable with only a minor hematological phenotype,149 inhibition of BCL2A1 may be associated with a favorable toxicity profile compared to MCL1 inhibitors.
Novel applications of venetoclax
Since BH3-mimetics are designed to act downstream or independently of p53, a puzzling clinical observation was that despite initial responses, CLL or AML patients with mutated p53 are more likely to relapse early upon venetoclax treatment, indicating that functional p53 is required for durable responses to venetoclax.529,530,531 A molecular explanation for this observation was provided by the finding that BH3-mimetic induced MOMP activates p53 in a feedforward loop involving the release of mitochondrial DNA followed by cGAS/STING activation.532 Identification of this additional pathway also opens up the exciting possibility to intensify the responses to venetoclax in p53 mutated malignancies using STING agonists, and clinical trials investigating this combination are eagerly awaited.
Development of novel formulations and delivery methods
While a multitude of combination treatments are currently being investigated mainly in preclinical studies, it remains to be seen whether these can increase tumor cell killing without simultaneously increasing on-target toxicities. In regards to tumor cell-selective delivery methods, both the development of nanoformulations and antibody-drug conjugates holds the potential to selectively deliver BH3-mimetics to malignant cells. To further increase tumor selectivity, these approaches could be combined with the delivery of a PROTAC molecule instead of a BH3-mimetic, thus incorporating another selection criterion. These approaches are still in their infancy, but with the rapid advances in these fields and the high importance of MCL1 and BCL-XL as therapeutic targets, it is likely that progress will be rapid.
Tailoring BCL-XL or MCL1 inhibitors for selective tumor cell killing
A plethora of preclinical studies533 has established that for many tumor cells, the combined inhibition of BCL-XL and MCL1 may be highly synergistic and efficiently kill tumor cells, thus paving the way for a potential chemotherapy-free treatment of many cancer types. Based on their significance in solid tumors, selective inhibition of MCL1 or BCL-XL in cancer cells represents the “Holy Grail” in the field of BH3-mimetics. However, with the toxicities reported particularly for selective MCL1 inhibitors and the subsequent termination of multiple candidates, highly potent BH3-mimetics may not be the right way forward. Potential solutions could be provided either by combination treatments allowing for a subtoxic dosage regiments or by tumor cell-selective delivery of BH3-mimetics. An alternative approach for MCL1 is to target indirectly, either by selective protein degradation or suppression of mRNA synthesis. For example, the short half-lives of MCL1 mRNA and protein may permit selective suppression by CDK9 inhibition.534,535 MCL1 protein may also be selectively targeted for proteasomal degradation by the CD79B antibody-drug conjugate, polatuzamab vedotin.308
Therapeutic application beyond cancer
Mechanistic understanding of the BCL2 family and their role in regulating cellular survival also highlight potential applications beyond cancer. Currently developed treatment options may directly be applicable to some autoimmune diseases like SLE, where preclinical experiments and early clinical studies indicate some activity of BH3-mimetics. However, how BH3-mimetics may compare to other immune-dampening drugs currently applied for autoimmune diseases needs to be carefully assessed and toxicities need to be monitored. Besides autoimmune diseases, BH3-mimetics may serve as senolytic drugs, and thus improve some age-related diseases. Although studies on senolytics are still in their infancy, these may be of huge impact in an aging society. For the treatment of neurodegenerative diseases on the other hand, a potential therapeutic approach would be to modulate the BCL2 family in such a way that neurons are protected from pathological cell death. The advancements in technologies particularly for gene transfers may open up new possibilities to maintain neuronal survival and mitigate cell loss associated with neurodegenerative diseases like AD.
Conclusions
BCL2 represents a therapeutic target for some hematological malignancies, but not for most solid tumors. Venetoclax is highly successful in all CLL and some AML patients, but for other hematological malignancies most notably DLBCL, clinically actionable biomarkers are urgently required to define patient populations most likely to benefit from BCL2 inhibition. In their absence, the empirical addition of BCL2 inhibitors to therapeutic regimens may add to physical and financial toxicities for no additional clinical benefit.536 As we have argued previously, some form of functional assessment is necessary to match precision medicines to specific malignancies.537 In B cell malignancies, the field is moving rapidly towards “chemotherapy-free” combinations of precision medicines, as illustrated by the recently-reported ViPOR study, which utilized obinutuzumab, ibrutinib, venetoclax, lenalidomide, and prednisone in patients with R/R LBCL in a small single-center phase II study.536 This regimen was for the most part well tolerated and produced a complete remission rate of 38% in this difficult-to-treat group of patients. However, empirically, one can already consider potential improvements such as the addition of polatuzumab vedotin and replacing obinutuzumab with CD20xCD3 bispecific antibodies.538 It should be noted that none of these precision medicines are tumor-specific and in combination, may result in more severe long-term immunosuppression than regular chemotherapy, which, especially during viral pandemics, can have fatal consequences.
In solid tumors, both MCL1 and BCL-XL represent highly promising therapeutic targets, but current approaches to inhibit these proteins are limited by on-target toxicities and the clinical development of several highly promising compounds has been terminated. Novel approaches to deliver BH3-mimetics or similar compounds safely to the targeted cell types are currently being explored, and with the technological advances in nanotechnology and protein engineering, it appears to be just a matter of time before successful strategies will be translated into clinical applications. Therefore, targeting of the BCL2 protein family is entering an exciting new era, and multiple new applications for modulating the activity of BCL2 proteins selectively in diseased tissues are foreseeable even beyond the treatment of cancer.
References
Green, D. R. A Matter of Life and Death. Cold Spring Harb Perspect Biol. 14, a041004 (2022).
Singh, R., Letai, A. & Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 20, 175–193 (2019).
Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell 187, 235–256 (2024).
Sora, V. & Papaleo, E. Structural details of BH3 Motifs and BH3-mediated interactions: an updated perspective. Front Mol. Biosci. 9, 864874 (2022).
Banjara, S., Suraweera, C. D., Hinds, M. G. & Kvansakul, M. The Bcl-2 family: ancient origins, conserved structures, and divergent mechanisms. Biomolecules. 10, (2020).
Kvansakul, M., Caria, S. & Hinds, M. G. The Bcl-2 family in host-virus interactions. Viruses 9, 290 (2017).
Tsujimoto, Y. et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099 (1984).
Tsujimoto, Y. & Croce, C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc. Natl Acad. Sci. Usa. 83, 5214–5218 (1986).
Tsujimoto, Y. et al. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature 315, 340–343 (1985).
Barrans, S. L. et al. The t(14;18) is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome. Clin. Cancer Res. 9, 2133–2139 (2003).
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988).
Oltvai, Z. N., Milliman, C. L. & Korsmeyer, S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619 (1993).
Muchmore, S. W. et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381, 335–341 (1996).
Sattler, M. et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997).
Boyd, J. M. et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11, 1921–1928 (1995).
Wang, K. et al. BID: a novel BH3 domain-only death agonist. Genes Dev. 10, 2859–2869 (1996).
Yang, J. et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129–1132 (1997).
Kluck, R. M., Bossy-Wetzel, E., Green, D. R. & Newmeyer, D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136 (1997).
Leber, B., Lin, J. & Andrews, D. W. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis 12, 897–911 (2007).
Llambi, F. et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol. Cell. 44, 517–531 (2011).
Luo, X., O'Neill, K. L. & Huang, K. The third model of Bax/Bak activation: a Bcl-2 family feud finally resolved?. F1000Res. 9, F1000Res (2020).
Vogler, M. et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 16, 1030–1039 (2009).
Varadarajan, S. et al. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 20, 1475–1484 (2013).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005).
Vaux, D. L. ABT-737, proving to be a great tool even before it is proven in the clinic. Cell Death Differ. 15, 807–808 (2008).
Shuker, S. B., Hajduk, P. J., Meadows, R. P. & Fesik, S. W. Discovering high-affinity ligands for proteins: SAR by NMR. Science 274, 1531–1534 (1996).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Park, C. M. et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J. Med Chem. 51, 6902–6915 (2008).
Day, C. L. et al. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J. Biol. Chem. 280, 4738–4744 (2005).
Losonczi, J. A. et al. NMR studies of the anti-apoptotic protein Bcl-xL in micelles. Biochemistry 39, 11024–11033 (2000).
Petros, A. M. et al. Solution structure of the antiapoptotic protein bcl-2. Proc. Natl Acad. Sci. USA 98, 3012–3017 (2001).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Konopleva, M. et al. Efficacy and biological correlates of response in a Phase II Study of Venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 6, 1106–1117 (2016).
Roberts, A. W. et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016).
Stilgenbauer, S. et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 17, 768–778 (2016).
Boise, L. H. et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597–608 (1993).
Lin, E. Y., Orlofsky, A., Berger, M. S. & Prystowsky, M. B. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J. Immunol. 151, 1979–1988 (1993).
Gibson, L. et al. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene 13, 665–675 (1996).
Ke, N., Godzik, A. & Reed, J. C. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J. Biol. Chem. 276, 12481–12484 (2001).
Zhou, P., Qian, L., Kozopas, K. M. & Craig, R. W. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 89, 630–643 (1997).
Warren, C. F. A., Wong-Brown, M. W. & Bowden, N. A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 10, 177 (2019).
Petros, A. M., Olejniczak, E. T. & Fesik, S. W. Structural biology of the Bcl-2 family of proteins. Biochim Biophys. Acta 1644, 83–94 (2004).
Nguyen, D., Osterlund, E., Kale, J. & Andrews, D. W. The C-terminal sequences of Bcl-2 family proteins mediate interactions that regulate cell death. Biochem J. 481, 903–922 (2024).
Brien, G. et al. C-terminal residues regulate localization and function of the antiapoptotic protein Bfl-1. J. Biol. Chem. 284, 30257–30263 (2009).
D’Sa-Eipper, C. & Chinnadurai, G. Functional dissection of Bfl-1, a Bcl-2 homolog: anti-apoptosis, oncogene-cooperation and cell proliferation activities. Oncogene 16, 3105–3114 (1998).
Popgeorgiev, N., Jabbour, L. & Gillet, G. Subcellular localization and dynamics of the Bcl-2 family of proteins. Front Cell Dev. Biol. 6, 13 (2018).
Morris, J. L., Gillet, G., Prudent, J. & Popgeorgiev, N. Bcl-2 Family of Proteins In The Control Of Mitochondrial Calcium Signalling: An Old Chap With New Roles. Int. J. Mol. Sci. 22, 3730 (2021).
Dhaouadi, N. et al. Ca(2+) signaling and cell death. Cell Calcium 113, 102759 (2023).
Csordas, G., Weaver, D. & Hajnoczky, G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 28, 523–540 (2018).
Loncke, J. et al. Balancing ER-Mitochondrial Ca(2+) fluxes in health and disease. Trends Cell Biol. 31, 598–612 (2021).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 (2012).
de Ridder, I. et al. The ER-mitochondria interface, where Ca(2+) and cell death meet. Cell Calcium 112, 102743 (2023).
Giorgio, V. et al. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 70, 56–63 (2018).
Thomenius, M. J. & Distelhorst, C. W. Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J. Cell Sci. 116, 4493–4499 (2003).
Pinton, P. et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J. Cell Biol. 148, 857–862 (2000).
Foyouzi-Youssefi, R. et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 97, 5723–5728 (2000).
Pinton, P. et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701 (2001).
Chen, R. et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 166, 193–203 (2004).
Rong, Y. P. et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl Acad. Sci. USA 106, 14397–14402 (2009).
Rong, Y. P. et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol. Cell. 31, 255–265 (2008).
Ivanova, H. et al. Bcl-2-protein family as modulators of IP(3) receptors and other organellar Ca(2+) Channels. Cold Spring Harb. Perspect. Biol. 12, a035089 (2020).
White, C. et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat. Cell Biol. 7, 1021–1028 (2005).
Li, C. et al. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl Acad. Sci. Usa. 104, 12565–12570 (2007).
Rosa, N. et al. Bcl-xL acts as an inhibitor of IP(3)R channels, thereby antagonizing Ca(2+)-driven apoptosis. Cell Death Differ. 29, 788–805 (2022).
Gadet, R. et al. The endoplasmic reticulum pool of Bcl-xL prevents cell death through IP3R-dependent calcium release. Cell Death Discov. 10, 346 (2024).
Eckenrode, E. F. et al. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J. Biol. Chem. 285, 13678–13684 (2010).
Bonneau, B. et al. IRBIT controls apoptosis by interacting with the Bcl-2 homolog, Bcl2l10, and by promoting ER-mitochondria contact. Elife 5, e19896 (2016).
Nougarede, A. et al. Breast cancer targeting through inhibition of the endoplasmic reticulum-based apoptosis regulator Nrh/BCL2L10. Cancer Res. 78, 1404–1417 (2018).
Duong, M. Q. et al. Nrh L11R single nucleotide polymorphism, a new prediction biomarker in breast cancer, impacts endoplasmic reticulum-dependent Ca(2+) traffic and response to neoadjuvant chemotherapy. Cell Death Dis. 14, 392 (2023).
Pease-Raissi, S. E. et al. Paclitaxel reduces axonal Bclw to initiate IP(3)R1-dependent axon degeneration. Neuron 96, 373–386.e376 (2017).
Tang, S. X. et al. Dissecting the neuroprotective interaction between the BH4 domain of BCL-w and the IP3 receptor. Cell Chem. Biol. 31, 1815–1826.e1815 (2024).
Vervliet, T. et al. Bcl-2 binds to and inhibits ryanodine receptors. J. Cell Sci. 127, 2782–2792 (2014).
Vervliet, T. et al. Ryanodine receptors are targeted by anti-apoptotic Bcl-XL involving its BH4 domain and Lys87 from its BH3 domain. Sci. Rep. 5, 9641 (2015).
Vervliet, T. et al. Regulation of the ryanodine receptor by anti-apoptotic Bcl-2 is independent of its BH3-domain-binding properties. Biochem Biophys. Res Commun. 463, 174–179 (2015).
Hartman, M. L. & Czyz, M. BCL-w: apoptotic and non-apoptotic role in health and disease. Cell Death Dis. 11, 260 (2020).
Choi, S. S. et al. A novel Bcl-2 related gene, Bfl-1, is overexpressed in stomach cancer and preferentially expressed in bone marrow. Oncogene 11, 1693–1698 (1995).
Vogler, M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 19, 67–74 (2012).
Happo, L., Strasser, A. & Cory, S. BH3-only proteins in apoptosis at a glance. J. Cell Sci. 125, 1081–1087 (2012).
Zhang, J. & Ney, P. A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 16, 939–946 (2009).
Hinds, M. G. et al. BIM, BAD: and BMF: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 14, 128–136 (2007).
Rautureau, G. J., Day, C. L. & Hinds, M. G. Intrinsically disordered proteins in bcl-2 regulated apoptosis. Int J. Mol. Sci. 11, 1808–1824 (2010).
Barrera-Vilarmau, S., Obregon, P. & de Alba, E. Intrinsic order and disorder in the bcl-2 member harakiri: insights into its proapoptotic activity. PLoS One 6, e21413 (2011).
Peng, Z., Xue, B., Kurgan, L. & Uversky, V. N. Resilience of death: intrinsic disorder in proteins involved in the programmed cell death. Cell Death Differ. 20, 1257–1267 (2013).
Chou, J. J. et al. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96, 615–624 (1999).
McDonnell, J. M. et al. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96, 625–634 (1999).
Yao, Y., Bobkov, A. A., Plesniak, L. A. & Marassi, F. M. Mapping the interaction of pro-apoptotic tBID with pro-survival BCL-XL. Biochemistry 48, 8704–8711 (2009).
Billen, L. P., Shamas-Din, A. & Andrews, D. W. Bid: a Bax-like BH3 protein. Oncogene 27, S93–S104 (2008).
Andreu-Fernandez, V. et al. The C-terminal domains of apoptotic BH3-onLy proteins mediate their insertion into distinct biological membranes. J. Biol. Chem. 291, 25207–25216 (2016).
Wilfling, F. et al. BH3-only proteins are tail-anchored in the outer mitochondrial membrane and can initiate the activation of Bax. Cell Death Differ. 19, 1328–1336 (2012).
Shamas-Din, A., Brahmbhatt, H., Leber, B. & Andrews, D. W. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys. Acta 1813, 508–520 (2011).
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 9, 351–365 (2006).
Chen, L. et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 17, 393–403 (2005).
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 (2008).
Leshchiner, E. S., Braun, C. R., Bird, G. H. & Walensky, L. D. Direct activation of full-length proapoptotic BAK. Proc. Natl Acad. Sci. USA 110, E986–E995 (2013).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2, 183–192 (2002).
Puthalakath, H. et al. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 3, 287–296 (1999).
Puthalakath, H. et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832 (2001).
Suzuki, M., Youle, R. J. & Tjandra, N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645–654 (2000).
Vandenabeele, P., Bultynck, G. & Savvides, S. N. Pore-forming proteins as drivers of membrane permeabilization in cell death pathways. Nat. Rev. Mol. Cell Biol. 24, 312–333 (2023).
Bonzerato, C. G. & Wojcikiewicz, R. J. H. Bok: real killer or bystander with non-apoptotic roles? Front Cell Dev. Biol. 11, 1161910 (2023).
Echeverry, N. et al. Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ. 20, 785–799 (2013).
Llambi, F. et al. BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation. Cell 165, 421–433 (2016).
Edlich, F. et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116 (2011).
Todt, F. et al. Differential retrotranslocation of mitochondrial Bax and Bak. EMBO J. 34, 67–80 (2015).
Pena-Blanco, A. & Garcia-Saez, A. J. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 285, 416–431 (2018).
Sandow, J. J. et al. Dynamic reconfiguration of pro-apoptotic BAK on membranes. EMBO J. 40, e107237 (2021).
Li, M. X. et al. BAK alpha6 permits activation by BH3-only proteins and homooligomerization via the canonical hydrophobic groove. Proc. Natl Acad. Sci. Usa. 114, 7629–7634 (2017).
Zhang, Z. et al. BH3-in-groove dimerization initiates and helix 9 dimerization expands Bax pore assembly in membranes. EMBO J. 35, 208–236 (2016).
Czabotar, P. E. et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 (2013).
Mikhailov, V. et al. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278, 5367–5376 (2003).
Dewson, G. et al. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 19, 661–670 (2012).
Uren, R. T. et al. Disordered clusters of Bak dimers rupture mitochondria during apoptosis. Elife. 6, (2017).
Uren, R. T., Iyer, S. & Kluck, R. M.Pore formation by dimeric Bak and Bax: an unusual pore? Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160218 (2017).
Ugarte-Uribe, B. & Garcia-Saez, A. J. Apoptotic foci at mitochondria: in and around Bax pores. Philos Trans. R. Soc. Lond. B Biol. Sci 372, 20160217 (2017).
Bleicken, S. et al. Topology of active, membrane-embedded Bax in the context of a toroidal pore. Cell Death Differ. 25, 1717–1731 (2018).
Grosse, L. et al. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 35, 402–413 (2016).
Salvador-Gallego, R. et al. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 35, 389–401 (2016).
Schweighofer, S. V. et al. Endogenous BAX and BAK form mosaic rings of variable size and composition on apoptotic mitochondria. Cell Death Differ. 31, 469–478 (2024).
Wasiak, S., Zunino, R. & McBride, H. M. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol. 177, 439–450 (2007).
Karbowski, M. et al. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658–662 (2006).
Zhang, Y. et al. Altered mitochondrial morphology and defective protein import reveal novel roles for Bax and/or Bak in skeletal muscle. Am. J. Physiol. Cell Physiol. 305, C502–511 (2013).
Karbowski, M. et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 (2002).
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300, 135–139 (2003).
Oakes, S. A. et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 105–110 (2005).
Bultynck, G. et al. The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. J. Biol. Chem. 287, 2544–2557 (2012).
Bultynck, G., Kiviluoto, S. & Methner, A. Bax inhibitor-1 is likely a pH-sensitive calcium leak channel, not a H+/Ca2+ exchanger. Sci. Signal. 7, pe22 (2014).
Xu, C., Xu, W., Palmer, A. E. & Reed, J. C. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J. Biol. Chem. 283, 11477–11484 (2008).
Prudent, J. et al. MAPL SUMOylation of Drp1 stabilizes an ER/Mitochondrial platform required for cell death. Mol. Cell. 59, 941–955 (2015).
Schulman, J. J. et al. The stability and expression level of bok are governed by binding to Inositol 1,4,5-Trisphosphate receptors. J. Biol. Chem. 291, 11820–11828 (2016).
Carpio, M. A. et al. BOK controls apoptosis by Ca(2+) transfer through ER-mitochondrial contact sites. Cell Rep. 34, 108827 (2021).
Schulman, J. J. et al. Bok regulates mitochondrial fusion and morphology. Cell Death Differ. 26, 2682–2694 (2019).
Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267, 1506–1510 (1995).
Opferman, J. T. et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671–676 (2003).
Grabow, S. et al. Subtle changes in the levels of BCL-2 proteins cause severe craniofacial abnormalities. Cell Rep. 24, 3285–3295.e3284 (2018).
Staversky, R. J. et al. Epithelial ablation of Bcl-XL increases sensitivity to oxygen without disrupting lung development. Am. J. Respir. Cell Mol. Biol. 43, 376–385 (2010).
Kodama, T. et al. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ. 19, 1856–1869 (2012).
Sathe, P. et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat. Commun. 5, 4539 (2014).
Delbridge, A. R., Grabow, S., Strasser, A. & Vaux, D. L. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 16, 99–109 (2016).
Jain, R. et al. A critical epithelial survival axis regulated by MCL-1 maintains thymic function in mice. Blood 130, 2504–2515 (2017).
Healy, M. E. et al. MCL1 is required for maintenance of intestinal homeostasis and prevention of carcinogenesis in mice. Gastroenterology 159, 183–199 (2020).
Callens, M. et al. The role of Bcl-2 proteins in modulating neuronal Ca(2+) signaling in health and in Alzheimer’s disease. Biochim Biophys. Acta Mol. Cell Res. 1868, 118997 (2021).
Zhao, F. et al. Brain milieu induces early microglial maturation through the BAX-Notch axis. Nat. Commun. 13, 6117 (2022).
Ross, A. J. et al. Testicular degeneration in Bclw-deficient mice. Nat. Genet. 18, 251–256 (1998).
Russell, L. D. et al. Spermatogenesis in Bclw-deficient mice. Biol. Reprod. 65, 318–332 (2001).
Adams, C. M. et al. BCL-W has a fundamental role in B cell survival and lymphomagenesis. J. Clin. Invest. 127, 635–650 (2017).
Ottina, E. et al. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood 119, 6032–6042 (2012).
Ottina, E. et al. Knockdown of the antiapoptotic Bcl-2 family member A1/Bfl-1 protects mice from anaphylaxis. J. Immunol. 194, 1316–1322 (2015).
Schenk, R. L. et al. Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ. 24, 534–545 (2017).
Tuzlak, S. et al. The BCL-2 pro-survival protein A1 is dispensable for T cell homeostasis on viral infection. Cell Death Differ. 24, 523–533 (2017).
Knudson, C. M. et al. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270, 96–99 (1995).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 6, 1389–1399 (2000).
Ke, F. et al. Impact of the combined loss of BOK, BAX and BAK on the hematopoietic system is slightly more severe than compound loss of BAX and BAK. Cell Death Dis. 6, e1938 (2015).
Arakawa, S. et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 24, 1598–1608 (2017).
Shimizu, S. et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 6, 1221–1228 (2004).
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999).
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 4, 321–328 (2003).
Shibue, T. et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 17, 2233–2238 (2003).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003).
Gray, D. H. et al. The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity 37, 451–462 (2012).
Labi, V. et al. Deregulated cell death and lymphocyte homeostasis cause premature lethality in mice lacking the BH3-only proteins BIM and BMF. Blood 123, 2652–2662 (2014).
Erlacher, M. et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 203, 2939–2951 (2006).
Ren, D. et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 330, 1390–1393 (2010).
Aubrey, B. J. et al. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 25, 104–113 (2018).
Cui, J. & Placzek, W. J. Post-Transcriptional Regulation of Anti-Apoptotic BCL2 Family Members. Int. J. Mol. Sci. 19, 308 (2018).
Prado, G., Kaestner, C. L., Licht, J. D. & Bennett, R. L. Targeting epigenetic mechanisms to overcome venetoclax resistance. Biochim Biophys. Acta Mol. Cell Res. 1868, 119047 (2021).
Zhang, W. & Xu, J. DNA methyltransferases and their roles in tumorigenesis. Biomark. Res. 5, 1 (2017).
Schiffmann, I., Greve, G., Jung, M. & Lubbert, M. Epigenetic therapy approaches in non-small cell lung cancer: Update and perspectives. Epigenetics 11, 858–870 (2016).
El Fakih, R. et al. Azacitidine Use for Myeloid Neoplasms. Clin. Lymphoma Myeloma Leuk. 18, e147–e155 (2018).
Kantarjian, H. et al. Current status and research directions in acute myeloid leukemia. Blood Cancer J. 14, 163 (2024).
Kapoor, I. et al. Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis. 11, 941 (2020).
San-Miguel, J. F. et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 15, 1195–1206 (2014).
Thomalla, D. et al. Deregulation and epigenetic modification of BCL2-family genes cause resistance to venetoclax in hematologic malignancies. Blood 140, 2113–2126 (2022).
Fritsche, P. et al. HDAC2 mediates therapeutic resistance of pancreatic cancer cells via the BH3-only protein NOXA. Gut 58, 1399–1409 (2009).
Nakajima, W. et al. Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer. Cancer Biol. Ther. 17, 27–35 (2016).
Torres-Adorno, A. M. et al. Histone deacetylase inhibitor enhances the efficacy of MEK inhibitor through NOXA-mediated MCL1 degradation in triple-negative and inflammatory breast cancer. Clin. Cancer Res. 23, 4780–4792 (2017).
Bolden, J. E. et al. HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses. Cell Death Dis. 4, e519 (2013).
He, C. et al. Elevated H3K27me3 levels sensitize osteosarcoma to cisplatin. Clin. Epigenet. 11, 8 (2019).
Schwalm, M. P. & Knapp, S. BET bromodomain inhibitors. Curr. Opin. Chem. Biol. 68, 102148 (2022).
Cummin, T. E. C. et al. BET inhibitors synergize with venetoclax to induce apoptosis in MYC-driven lymphomas with high BCL-2 expression. Blood Adv. 4, 3316–3328 (2020).
Hogg, S. J. et al. BET inhibition induces apoptosis in aggressive B-cell lymphoma via epigenetic regulation of BCL-2 family members. Mol. Cancer Ther. 15, 2030–2041 (2016).
Tseng, H. Y. et al. Co-targeting bromodomain and extra-terminal proteins and MCL1 induces synergistic cell death in melanoma. Int J. Cancer 147, 2176–2189 (2020).
O’Hara, S. P. et al. The transcription factor ETS1 promotes apoptosis resistance of senescent cholangiocytes by epigenetically up-regulating the apoptosis suppressor BCL2L1. J. Biol. Chem. 294, 18698–18713 (2019).
Grad, J. M., Zeng, X. R. & Boise, L. H. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr. Opin. Oncol. 12, 543–549 (2000).
Pieper, N. M. et al. Inhibition of bromodomain and extra-terminal proteins targets constitutively active NFkappaB and STAT signaling in lymphoma and influences the expression of the antiapoptotic proteins BCL2A1 and c-MYC. Cell Commun. Signal. 22, 415 (2024).
Senichkin, V. V. et al. Saga of Mcl-1: regulation from transcription to degradation. Cell Death Differ. 27, 405–419 (2020).
Haselager, M. et al. Regulation of Bcl-XL by non-canonical NF-kappaB in the context of CD40-induced drug resistance in CLL. Cell Death Differ. 28, 1658–1668 (2021).
Lee, H. H. et al. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl Acad. Sci. USA 96, 9136–9141 (1999).
Fu, Z. & Tindall, D. J. FOXOs, cancer and regulation of apoptosis. Oncogene 27, 2312–2319 (2008).
Li, L. et al. Sevoflurane protects against intracerebral hemorrhage via microRNA-133b/FOXO4/BCL2 axis. Int. Immunopharmacol. 114, 109453 (2023).
Day, B. W. et al. ELK4 neutralization sensitizes glioblastoma to apoptosis through downregulation of the anti-apoptotic protein Mcl-1. Neuro Oncol. 13, 1202–1212 (2011).
Hu, J. et al. Activation of ATF4 mediates unwanted Mcl-1 accumulation by proteasome inhibition. Blood 119, 826–837 (2012).
Liu, X. H., Yu, E. Z., Li, Y. Y. & Kagan, E. HIF-1alpha has an anti-apoptotic effect in human airway epithelium that is mediated via Mcl-1 gene expression. J. Cell Biochem. 97, 755–765 (2006).
Croxton, R. et al. Direct repression of the Mcl-1 promoter by E2F1. Oncogene 21, 1359–1369 (2002).
Tagawa, H., Ikeda, S. & Sawada, K. Role of microRNA in the pathogenesis of malignant lymphoma. Cancer Sci. 104, 801–809 (2013).
Dong, J. T., Boyd, J. C. & Frierson, H. F. Jr Loss of heterozygosity at 13q14 and 13q21 in high grade, high stage prostate cancer. Prostate 49, 166–171 (2001).
Calin, G. A. et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA 99, 15524–15529 (2002).
Bottoni, A. et al. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J. Cell Physiol. 204, 280–285 (2005).
Cimmino, A. et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl Acad. Sci. USA 102, 13944–13949 (2005).
Bonci, D. et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 14, 1271–1277 (2008).
Li, Z. H., Zhang, Y. P., Xing, P. T. & Zhan, X. R. Effect of MiRNA-145 on APOPTOSIS OF LEUKemia HuT 78 CELLS AND ITS MEChanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 28, 104–109 (2020).
Paraskevopoulou, M. D. & Hatzigeorgiou, A. G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 1402, 271–286 (2016).
Willimott, S. & Wagner, S. D. Post-transcriptional and post-translational regulation of Bcl2. Biochem. Soc. Trans. 38, 1571–1575 (2010).
Filippova, N. et al. The RNA-binding protein HuR promotes glioma growth and treatment resistance. Mol. Cancer Res. 9, 648–659 (2011).
Dobosz, E. et al. MCPIP-1 restricts inflammation via promoting apoptosis of neutrophils. Front Immunol. 12, 627922 (2021).
Mao, R. et al. Regnase-1, a rapid response ribonuclease regulating inflammation and stress responses. Cell Mol. Immunol. 14, 412–422 (2017).
Puthalakath, H. & Strasser, A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 9, 505–512 (2002).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 7, 683–694 (2001).
Real, P. J. et al. Transcriptional activation of the proapoptotic bik gene by E2F proteins in cancer cells. FEBS Lett. 580, 5905–5909 (2006).
Subramanian, T. et al. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J. Virol. 81, 10486–10495 (2007).
Kim, J. Y. et al. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J. Exp. Med. 199, 113–124 (2004).
Dijkers, P. F. et al. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10, 1201–1204 (2000).
Sunters, A. et al. FoxO3a transcriptional regulation of BIM controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 278, 49795–49805 (2003).
You, H. et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J. Exp. Med. 203, 1657–1663 (2006).
Gogada, R. et al. Bim, a proapoptotic protein, up-regulated via transcription factor E2F1-dependent mechanism, functions as a prosurvival molecule in cancer. J. Biol. Chem. 288, 368–381 (2013).
Hu, Y. et al. Acetylation of FOXO1 activates BIM expression involved in CVB3 induced cardiomyocyte apoptosis. Apoptosis 29, 1271–1287 (2024).
Fedele, P. L. et al. The transcription factor IRF4 represses proapoptotic BMF and BIM to licence multiple myeloma survival. Leukemia 35, 2114–2118 (2021).
Titcombe, P. J., Silva Morales, M., Zhang, N. & Mueller, D. L. BATF represses BIM to sustain tolerant T cells in the periphery. J. Exp. Med. 220, e20230183 (2023).
Inoue-Yamauchi, A. & Oda, H. EMT-inducing transcription factor ZEB1-associated resistance to the BCL-2/BCL-X(L) inhibitor is overcome by BIM upregulation in ovarian clear cell carcinoma cells. Biochem. Biophys. Res. Commun. 526, 612–617 (2020).
Sanz, C. et al. Interleukin 3-dependent activation of DREAM is involved in transcriptional silencing of the apoptotic Hrk gene in hematopoietic progenitor cells. EMBO J. 20, 2286–2292 (2001).
Yamashita, M. et al. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J. Exp. Med. 205, 1109–1120 (2008).
Matsui, H., Asou, H. & Inaba, T. Cytokines direct the regulation of Bim mRNA stability by heat-shock cognate protein 70. Mol. Cell. 25, 99–112 (2007).
Xiao, C. et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 9, 405–414 (2008).
Li, Y. et al. MYC through miR-17-92 suppresses specific target genes to maintain survival, autonomous proliferation, and a neoplastic state. Cancer Cell. 26, 262–272 (2014).
Tagawa, H. & Seto, M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia 19, 2013–2016 (2005).
Lu, Z., Luo, M. & Huang, Y. lncRNA-CIR regulates cell apoptosis of chondrocytes in osteoarthritis. J. Cell Biochem. 120, 7229–7237 (2019).
Cai, Z. et al. Targeting BIM via a lncRNA Morrbid regulates the survival of preleukemic and leukemic cells. Cell Rep. 31, 107816 (2020).
Kotzin, J. J. et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243 (2016).
Wang, Q. et al. Silencing long noncoding RNA NEAT1 alleviates acute liver failure via the EZH2-mediated microRNA-139/PUMA axis. Aging. 13, 12537–12551 (2021).
Du, Z. et al. The long non-coding RNA TSLC8 inhibits colorectal cancer by stabilizing puma. Cell Cycle 19, 3317–3328 (2020).
Zhang, C. Z. et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 9, 229 (2010).
Zhou, X. B. et al. LncRNAGAS5 sponges miRNA-221 to promote neurons apoptosis by up-regulated PUMA under hypoxia condition. Neurol. Res. 42, 8–16 (2020).
Sun, H. et al. MicroRNA‑21 expression is associated with the clinical features of patients with gastric carcinoma and affects the proliferation, invasion and migration of gastric cancer cells by regulating Noxa. Mol. Med Rep. 13, 2701–2707 (2016).
Shi, J. et al. Noxa inhibits oncogenesis through ZNF519 in gastric cancer and is suppressed by hsa-miR-200b-3p. Sci. Rep. 14, 6568 (2024).
Fiori, M. E. et al. Antitumor effect of miR-197 targeting in p53 wild-type lung cancer. Cell Death Differ. 21, 774–782 (2014).
Morey, T. M. et al. Heat shock inhibition of CDK5 increases NOXA levels through miR-23a repression. J. Biol. Chem. 290, 11443–11454 (2015).
Wu, Z. et al. The BET-Bromodomain Inhibitor JQ1 synergized ABT-263 against colorectal cancer cells through suppressing c-Myc-induced miR-1271-5p expression. Biomed. Pharmacother. 95, 1574–1579 (2017).
Teshima, K. et al. Dysregulation of BMI1 and microRNA-16 collaborate to enhance an anti-apoptotic potential in the side population of refractory mantle cell lymphoma. Oncogene 33, 2191–2203 (2014).
Westhoff, M. A. et al. Killing me softly-future challenges in apoptosis research. Int J. Mol. Sci. 15, 3746–3767 (2014).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
del Peso, L. et al. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 (1997).
Gardai, S. J. et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J. Biol. Chem. 279, 21085–21095 (2004).
Kale, J. et al. Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep. 19, e45235 (2018).
Guo, H. et al. Modulation of the PI3K/Akt pathway and Bcl-2 family proteins involved in chicken's tubular apoptosis induced by Nickel Chloride (NiCl(2). Int. J. Mol. Sci. 16, 22989–23011 (2015).
Dai, Y., Jin, S., Li, X. & Wang, D. The involvement of Bcl-2 family proteins in AKT-regulated cell survival in cisplatin resistant epithelial ovarian cancer. Oncotarget 8, 1354–1368 (2017).
Pinon, J. D., Labi, V., Egle, A. & Villunger, A. BIM and BMF in tissue homeostasis and malignant disease. Oncogene 27, S41–S52 (2008).
Lei, K. & Davis, R. J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA 100, 2432–2437 (2003).
Ewings, K. E. et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 26, 2856–2867 (2007).
Scheid, M. P., Schubert, K. M. & Duronio, V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 274, 31108–31113 (1999).
Bonni, A. et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 (1999).
Lizcano, J. M., Morrice, N. & Cohen, P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J. 349, 547–557 (2000).
Eichhorn, J. M. et al. Critical role of anti-apoptotic Bcl-2 protein phosphorylation in mitotic death. Cell Death Dis. 4, e834 (2013).
May, W. S. et al. Interleukin-3 and bryostatin-1 mediate hyperphosphorylation of BCL2 alpha in association with suppression of apoptosis. J. Biol. Chem. 269, 26865–26870 (1994).
Ruvolo, P. P., Deng, X. & May, W. S. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 15, 515–522 (2001).
Tamura, Y., Simizu, S. & Osada, H. The phosphorylation status and anti-apoptotic activity of Bcl-2 are regulated by ERK and protein phosphatase 2A on the mitochondria. FEBS Lett. 569, 249–255 (2004).
Lin, S. S. et al. PP2A regulates BCL-2 phosphorylation and proteasome-mediated degradation at the endoplasmic reticulum. J. Biol. Chem. 281, 23003–23012 (2006).
Bassik, M. C. et al. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 23, 1207–1216 (2004).
Breitschopf, K. et al. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol. Cell Biol. 20, 1886–1896 (2000).
Fernandez, Y. et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 65, 6294–6304 (2005).
Perez-Galan, P. et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood 107, 257–264 (2006).
Qin, J. Z. et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 65, 6282–6293 (2005).
Nikrad, M. et al. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol. Cancer Ther. 4, 443–449 (2005).
Zhu, H. et al. Bik/NBK accumulation correlates with apoptosis-induction by bortezomib (PS-341, Velcade) and other proteasome inhibitors. Oncogene 24, 4993–4999 (2005).
Li, B. & Dou, Q. P. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc. Natl Acad. Sci. USA 97, 3850–3855 (2000).
Albert, M. C. et al. CHIP ubiquitylates NOXA and induces its lysosomal degradation in response to DNA damage. Cell Death Dis. 11, 740 (2020).
Carrington, E. M. et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 24, 878–888 (2017).
Michalski, M. et al. Simultaneous inhibition of Mcl-1 and Bcl-2 induces synergistic cell death in hepatocellular carcinoma. Biomedicines 11, 1666 (2023).
Nakajima, W. et al. DNA damaging agent-induced apoptosis is regulated by MCL-1 phosphorylation and degradation mediated by the Noxa/MCL-1/CDK2 complex. Oncotarget 7, 36353–36365 (2016).
Mojsa, B., Lassot, I. & Desagher, S. Mcl-1 ubiquitination: unique regulation of an essential survival protein. Cells 3, 418–437 (2014).
Tang, G. L. Q., Lai, J. X. H. & Pervaiz, S. Ubiquitin-proteasome pathway-mediated regulation of the Bcl-2 family: effects and therapeutic approaches. Haematologica 109, 33–43 (2024).
Turley, H. et al. The distribution of the deleted in colon cancer (DCC) protein in human tissues. Cancer Res. 55, 5628–5631 (1995).
Ayhan, A. et al. Loss of heterozygosity at the bcl-2 gene locus and expression of bcl-2 in human gastric and colorectal carcinomas. Jpn J. Cancer Res. 85, 584–591 (1994).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e516 (2017).
Lessene, G., Czabotar, P. E. & Colman, P. M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discov. 7, 989–1000 (2008).
Vogler, M. et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood 113, 4403–4413 (2009).
Mason, K. D. et al. Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 (2007).
Schoenwaelder, S. M. et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 118, 1663–1674 (2011).
Wilson, W. H. et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 11, 1149–1159 (2010).
Roberts, A. W. et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 30, 488–496 (2012).
Kipps, T. J. et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 56, 2826–2833 (2015).
Vogler, M. et al. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 117, 7145–7154 (2011).
Rampal, R. et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood 123, e123–133 (2014).
Harrison, C. N. et al. Addition of Navitoclax to ongoing Ruxolitinib therapy for patients with myelofibrosis with progression or suboptimal response: Phase II Safety and Efficacy. J. Clin. Oncol. 40, 1671–1680 (2022).
Pemmaraju, N. et al. Addition of navitoclax to ongoing ruxolitinib treatment in patients with myelofibrosis (REFINE): a post-hoc analysis of molecular biomarkers in a phase 2 study. Lancet Haematol. 9, e434–e444 (2022).
Pemmaraju, N. et al. Transform-1: A randomized, double-blind, placebo-controlled, multicenter, International Phase 3 study of Navitoclax in combination with Ruxolitinib Versus Ruxolitinib plus placebo in patients with untreated myelofibrosis. Blood 142, 620–620 (2023).
Vogler, M., Dinsdale, D., Dyer, M. J. & Cohen, G. M. ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. Br. J. Haematol. 163, 139–142 (2013).
Seymour, J. F. et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 18, 230–240 (2017).
Jones, J. A. et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 19, 65–75 (2018).
Fischer, K. et al. Venetoclax and Obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 380, 2225–2236 (2019).
Al-Sawaf, O. et al. Transcriptomic profiles and 5-year results from the randomized CLL14 study of venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab in chronic lymphocytic leukemia. Nat. Commun. 14, 2147 (2023).
DiNardo, C. D. et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 383, 617–629 (2020).
Pratz, K. W. et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 99, 615–624 (2024).
Jin, S. et al. 5-Azacitidine induces NOXA to Prime AML cells for Venetoclax-mediated apoptosis. Clin. Cancer Res. 26, 3371–3383 (2020).
Cojocari, D. et al. Pevonedistat and azacitidine upregulate NOXA (PMAIP1) to increase sensitivity to venetoclax in preclinical models of acute myeloid leukemia. Haematologica 107, 825–835 (2022).
Mishra, R., Zokaei Nikoo, M., Veeraballi, S. & Singh, A. Venetoclax and hypomethylating agent combination in myeloid malignancies: mechanisms of synergy and challenges of resistance. Int. J. Mol. Sci. 25, 484 (2023).
Lee, J. B. et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood 138, 234–245 (2021).
Davids, M. S. et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 35, 826–833 (2017).
Walter, H. S. et al. Successful treatment of primary cutaneous diffuse large B-cell lymphoma leg type with single-agent Venetoclax. JCO Precis Oncol. 3, 1–5 (2019).
Klanova, M. et al. Targeting of BCL2 family proteins with ABT-199 and Homoharringtonine reveals BCL2- and MCL1-dependent subgroups of diffuse large B-cell lymphoma. Clin. Cancer Res. 22, 1138–1149 (2016).
Smith, V. M. et al. Specific interactions of BCL-2 family proteins mediate sensitivity to BH3-mimetics in diffuse large B-cell lymphoma. Haematologica 105, 2150–2163 (2020).
Soderquist, R. S. et al. Systematic mapping of BCL-2 gene dependencies in cancer reveals molecular determinants of BH3 mimetic sensitivity. Nat. Commun. 9, 3513 (2018).
Bojarczuk, K. et al. Targeted inhibition of PI3Kalpha/delta is synergistic with BCL-2 blockade in genetically defined subtypes of DLBCL. Blood 133, 70–80 (2019).
Kiem, H. P. et al. Concurrent activation of c-myc and inactivation of bcl-2 by chromosomal translocation in a lymphoblastic lymphoma cell line. Oncogene 5, 1815–1819 (1990).
Ren, W. et al. Genetic and transcriptomic analyses of diffuse large B-cell lymphoma patients with poor outcomes within two years of diagnosis. Leukemia 38, 610–620 (2024).
Roh, J. et al. BCL2 super-expressor diffuse large B-cell lymphoma: a distinct subgroup associated with poor prognosis. Mod. Pathol. 35, 480–488 (2022).
Morschhauser, F. et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 137, 600–609 (2021).
Lasater, E. A. et al. Targeting MCL-1 and BCL-2 with polatuzumab vedotin and venetoclax overcomes treatment resistance in R/R non-Hodgkin lymphoma: Results from preclinical models and a Phase Ib study. Am. J. Hematol. 98, 449–463 (2023).
Kumar, S. et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130, 2401–2409 (2017).
Gupta, V. A. et al. Venetoclax sensitivity in multiple myeloma is associated with B-cell gene expression. Blood 137, 3604–3615 (2021).
Kumar, S. K. et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 21, 1630–1642 (2020).
Karol, S. E. et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. 21, 551–560 (2020).
Masetti, R. et al. Venetoclax-based therapies in pediatric advanced MDS and relapsed/refractory AML: a multicenter retrospective analysis. Blood Adv. 7, 4366–4370 (2023).
Pullarkat, V. A. et al. Venetoclax and Navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 11, 1440–1453 (2021).
Sarosiek, K. A. et al. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell. 31, 142–156 (2017).
DiNardo, C. D. et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 135, 791–803 (2020).
Pei, S. et al. Monocytic subclones confer resistance to Venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 10, 536–551 (2020).
Blombery, P. et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 9, 342–353 (2019).
Kotmayer, L. et al. Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax. Int. J. Mol. Sci. 24, 5802 (2023).
Birkinshaw, R. W. et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 10, 2385 (2019).
Thijssen, R. et al. Single-cell multiomics reveal the scale of multilayered adaptations enabling CLL relapse during venetoclax therapy. Blood 140, 2127–2141 (2022).
Guieze, R. et al. Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell. 36, 369–384.e313 (2019).
Blombery, P. et al. Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL. Blood 139, 1198–1207 (2022).
Moujalled, D. M. et al. Acquired mutations in BAX confer resistance to BH3-mimetic therapy in acute myeloid leukemia. Blood 141, 634–644 (2023).
Roca-Portoles, A. et al. Venetoclax causes metabolic reprogramming independent of BCL-2 inhibition. Cell Death Dis. 11, 616 (2020).
Wang, Y. et al. Venetoclax acts as an immunometabolic modulator to potentiate adoptive NK cell immunotherapy against leukemia. Cell Rep. Med. 5, 101580 (2024).
Pedro, J. M. et al. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy 11, 452–459 (2015).
Ma, P. et al. Interaction of Bcl-2 with the autophagy-related GABAA receptor-associated protein (GABARAP): biophysical characterization and functional implications. J. Biol. Chem. 288, 37204–37215 (2013).
Trisciuoglio, D. et al. Removal of the BH4 domain from Bcl-2 protein triggers an autophagic process that impairs tumor growth. Neoplasia 15, 315–327 (2013).
Gabellini, C., Trisciuoglio, D. & Del Bufalo, D. Non-canonical roles of Bcl-2 and Bcl-xL proteins: relevance of BH4 domain. Carcinogenesis 38, 579–587 (2017).
Gross, A. & Katz, S. G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 24, 1348–1358 (2017).
Chong, S. J. F. et al. Noncanonical cell fate regulation by Bcl-2 proteins. Trends Cell Biol. 30, 537–555 (2020).
Tessoulin, B. et al. BCL2-family dysregulation in B-cell malignancies: from gene expression regulation to a targeted therapy biomarker. Front Oncol. 8, 645 (2018).
Pilling, A. B. & Hwang, C. Targeting prosurvival BCL2 signaling through Akt blockade sensitizes castration-resistant prostate cancer cells to enzalutamide. Prostate 79, 1347–1359 (2019).
Duan, L., Tadi, M. J., O’Hara, K. M. & Maki, C. G. Novel markers of MCL1 inhibitor sensitivity in triple-negative breast cancer cells. J. Biol. Chem. 300, 107375 (2024).
Casara, P. et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget 9, 20075–20088 (2018).
Tiran, A. L. et al. Abstract 1276: Identification of S65487/VOB560 as a potent and selective intravenous 2nd-generation BCL-2 inhibitor active in wild-type and clinical mutants resistant to Venetoclax. Cancer Res. 81, 1276 (2021).
Liu, J. et al. Sonrotoclax overcomes BCL2 G101V mutation-induced venetoclax resistance in preclinical models of hematologic malignancy. Blood 143, 1825–1836 (2024).
Guo, Y. et al. Discovery of the clinical candidate Sonrotoclax (BGB-11417), a highly potent and selective inhibitor for both WT and G101V mutant Bcl-2. J. Med. Chem. 67, 7836–7858 (2024).
Lee, H.-P. et al. BGB-11417-203: An ongoing, phase 2 study of sonrotoclax (BGB-11417), a next-generation BCL2 inhibitor, in patients with Waldenström macroglobulinemia. J. Clin. Oncol. 42, TPS7090 (2024).
Tam, C. S. et al. Combination treatment with Sonrotoclax (BGB-11417), a second-generation BCL2 Inhibitor, and Zanubrutinib, a Bruton Tyrosine Kinase (BTK) inhibitor, is well tolerated and achieves deep responses in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma (TN-CLL/SLL): Data from an ongoing Phase 1/2 Study. Blood 142, 327–327 (2023).
Shadman, M. et al. CELESTIAL-TNCLL: An ongoing, open-label, multiregional, phase 3 study of sonrotoclax (BGB-11417) + zanubrutinib vs venetoclax + obinutuzumab for treatment-naïve (TN) CLL. J. Clin. Oncol. 42, TPS7087 (2024).
Deng, J. et al. Lisaftoclax (APG-2575) is a novel BCL-2 inhibitor with robust antitumor activity in preclinical models of hematologic malignancy. Clin. Cancer Res. 28, 5455–5468 (2022).
Ailawadhi, S. et al. Novel BCL-2 Inhibitor Lisaftoclax in Relapsed or refractory chronic lymphocytic leukemia and other hematologic malignancies: first-in-human open-label trial. Clin. Cancer Res. 29, 2385–2393 (2023).
Zhai, Y. et al. Lisaftoclax in combination with Alrizomadlin overcomes Venetoclax resistance in acute myeloid leukemia and acute lymphoblastic leukemia: preclinical studies. Clin. Cancer Res. 29, 183–196 (2023).
Lin, S. et al. Abstract 2497: FCN-338, a novel and selective Bcl-2 inhibitor, exhibits potent anti-tumor activity in B-cell lymphoma. Cancer Res. 79, 2497 (2019).
Brandhuber, B. et al. Abstract 1258: Preclinical characterization of LOXO-338, a novel, oral and selective BCL2 inhibitor. Cancer Res. 81, 1258 (2021).
Kwiatek, M. et al. P636: A First-In-Human phase 1 study of oral LOXO-338, a selective BCL2 inhibitor, in patients with advanced hematologic malignancies. Hemasphere 7, e216785f (2023).
Jurczak, W. et al. P683: A First-In-Human phase 1 study of oral LOXO-338, a selective BCL2 inhibitor, in patients with advanced hematologic malignancies (Trial in progress). HemaSphere 6, 579–580 (2022).
Walker, A. R. et al. Phase 1 study of LP-108 as monotherapy and in combination with azacitidine in patients with relapsed or refractory myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), or acute myeloid leukemia (AML). J. Clin. Oncol. 40, TPS7071 (2022).
Koenig, K. et al. P492: safety and efficacy of LP-108 as monotherapy and combined with azacitidine in patients with relapsed/refractory myelodysplastic syndromes, chronic myelomonocytic leukemia, or acute myeloid leukemia. HemaSphere 7, e2546261 (2023).
Qi, J. et al. 800MO First report of BCL-2 inhibitor TQB3909 in pts with relapsed or refractory non-Hodgkin lymphoma (NHL) and acute myeloid leukemia (AML): Data from a phase I study. Ann. Oncol. 35, S596 (2024).
Lessene, G. et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat. Chem. Biol. 9, 390–397 (2013).
Karpel-Massler, G. et al. Inhibition of mitochondrial matrix Chaperones and antiapoptotic Bcl-2 family proteins empower antitumor therapeutic responses. Cancer Res. 77, 3513–3526 (2017).
Bessou, M. et al. The apoptosis inhibitor Bcl-xL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production. Oncogene 39, 3056–3074 (2020).
Pesch, A. M. et al. Bcl-xL inhibition radiosensitizes PIK3CA/PTEN wild-type triple negative breast cancers with low Mcl-1 expression. Cancer Res. Commun. 2, 679–693 (2022).
Tao, Z. F. et al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med. Chem. Lett. 5, 1088–1093 (2014).
Wang, L. et al. Discovery of A-1331852, a first-in-class, potent, and orally-bioavailable BCL-X(L) inhibitor. ACS Med. Chem. Lett. 11, 1829–1836 (2020).
Zhang, H. et al. Genomic analysis and selective small molecule inhibition identifies BCL-X(L) as a critical survival factor in a subset of colorectal cancer. Mol. Cancer 14, 126 (2015).
Ramesh, P. et al. BCL-XL inhibition induces an FGFR4-mediated rescue response in colorectal cancer. Cell Rep. 38, 110374 (2022).
Tao, Z. F. et al. Structure-based design of A-1293102, a potent and selective BCL-X(L) inhibitor. ACS Med. Chem. Lett. 12, 1011–1016 (2021).
Yi, H. et al. Bcl-2/Bcl-xl inhibitor APG-1252-M1 is a promising therapeutic strategy for gastric carcinoma. Cancer Med. 9, 4197–4206 (2020).
Yao, W. et al. Mcl-1 levels critically impact the sensitivities of human colorectal cancer cells to APG-1252-M1, a novel Bcl-2/Bcl-X(L) dual inhibitor that induces Bax-dependent apoptosis. Neoplasia 29, 100798 (2022).
Qian, L. et al. Therapeutic potential of the novel Bcl-2/Bcl-X(L) dual inhibitor, APG1252, alone or in combination against non-small cell lung cancer. Mol. Carcinog. 61, 1031–1042 (2022).
Lakhani, N. J. et al. First-in-human study with preclinical data of BCL-2/BCL-xL inhibitor pelcitoclax in locally advanced or metastatic solid tumors. Clin. Cancer Res. 30, 506–521 (2024).
Wuilleme-Toumi, S. et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 19, 1248–1252 (2005).
Xiang, W., Yang, C. Y. & Bai, L. MCL-1 inhibition in cancer treatment. Onco Targets Ther. 11, 7301–7314 (2018).
Li, X. X. et al. Increased MCL-1 expression predicts poor prognosis and disease recurrence in acute myeloid leukemia. Onco Targets Ther. 12, 3295–3304 (2019).
Kotschy, A. et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482 (2016).
Szlavik, Z. et al. Discovery of S64315, a potent and selective Mcl-1 inhibitor. J. Med Chem. 63, 13762–13795 (2020).
Yuda, J. et al. Selective MCL-1 inhibitor ABBV-467 is efficacious in tumor models but is associated with cardiac troponin increases in patients. Commun. Med. 3, 154 (2023).
Thomas, R. L. et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 27, 1365–1377 (2013).
Caenepeel, S. et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 8, 1582–1597 (2018).
Hargreaves, D. et al. Design of rigid protein-protein interaction inhibitors enables targeting of undruggable Mcl-1. Proc. Natl Acad. Sci. USA 120, e2221967120 (2023).
Caenepeel, S. et al. Abstract 6218: Discovery and preclinical evaluation of AMG 397, a potent, selective and orally bioavailable MCL1 inhibitor. Cancer Res. 80, 6218–6218 (2020).
Tsao, T. et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann. Hematol. 91, 1861–1870 (2012).
Falchook, G. et al. Abstract CT172: A phase 1, open-label, dose-escalation study of PRT1419, a selective induced myeloid leukemia cell differentiation protein (MCL-1) inhibitor, in patients (pts) with advanced/metastatic solid tumors. Cancer Res. 83, CT172–CT172 (2023).
Matson, C. K. et al. Abstract 3696: GS-9716: A potent, selective and orally bioavailable small molecule inhibitor of MCL1 for the treatment of cancer. Cancer Res. 82, 3696 (2022).
Daressy, F. et al. Drimane derivatives as the first examples of covalent BH3 mimetics that target MCL-1. ChemMedChem 16, 1788–1797 (2021).
Daressy, F. et al. NA1-115-7, from Zygogynum pancheri, is a new selective MCL-1 inhibitor inducing the apoptosis of hematological cancer cells but non-toxic to normal blood cells or cardiomyocytes. Biomed. Pharmacother. 154, 113546 (2022).
Seguy, L. et al. In vitro evaluation of NA1-115-7-loaded nanoemulsions, an MCL-1-specific inhibitor of natural origin, intended to treat B-cell lymphoproliferative disorders after oral administration. Int J. Pharm. 630, 122433 (2023).
Walensky, L. D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Walensky, L. D. et al. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell. 24, 199–210 (2006).
Hadji, A. et al. Preferential targeting of MCL-1 by a hydrocarbon-stapled BIM BH3 peptide. Oncotarget 10, 6219–6233 (2019).
LaBelle, J. L. et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. J. Clin. Invest. 122, 2018–2031 (2012).
Guerra, R. M. et al. Precision targeting of BFL-1/A1 and an ATM co-dependency in human cancer. Cell Rep. 24, 3393–3403 e3395 (2018).
Huhn, A. J. et al. Selective covalent targeting of anti-apoptotic BFL-1 by Cysteine-reactive stapled peptide inhibitors. Cell Chem. Biol. 23, 1123–1134 (2016).
Korshavn, K. J. et al. A redox switch regulates the structure and function of anti-apoptotic BFL-1. Nat. Struct. Mol. Biol. 27, 781–789 (2020).
Tang, S. X. et al. Dissecting the neuroprotective interaction between the BH4 domain of BCL-w and the IP3 receptor. Cell Chem. Biol. 31, 1815–1826 (2024).
Saleh, M. N. et al. Phase 1 trial of ALRN-6924, a dual inhibitor of MDMX and MDM2, in patients with solid tumors and lymphomas bearing wild-type TP53. Clin. Cancer Res. 27, 5236–5247 (2021).
Guerlavais, V. et al. Discovery of Sulanemadlin (ALRN-6924), the first cell-permeating, stabilized alpha-helical peptide in clinical development. J. Med Chem. 66, 9401–9417 (2023).
Zhong, F. et al. Induction of Ca(2)+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood 117, 2924–2934 (2011).
Akl, H. et al. IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis. 4, e632 (2013).
Bittremieux, M. et al. Constitutive IP(3) signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP(3) receptor disruptor BIRD-2. Cell Death Differ. 26, 531–547 (2019).
Vervloessem, T. et al. Reciprocal sensitivity of diffuse large B-cell lymphoma cells to Bcl-2 inhibitors BIRD-2 versus venetoclax. Oncotarget 8, 111656–111671 (2017).
Lavik, A. R. et al. A synthetic peptide targeting the BH4 domain of Bcl-2 induces apoptosis in multiple myeloma and follicular lymphoma cells alone or in combination with agents targeting the BH3-binding pocket of Bcl-2. Oncotarget 6, 27388–27402 (2015).
Greenberg, E. F. et al. Synergistic killing of human small cell lung cancer cells by the Bcl-2-inositol 1,4,5-trisphosphate receptor disruptor BIRD-2 and the BH3-mimetic ABT-263. Cell Death Dis. 6, e2034 (2015).
Xie, Q. et al. TAT‑fused IP3R‑derived peptide enhances cisplatin sensitivity of ovarian cancer cells by increasing ER Ca2+ release. Int J. Mol. Med. 41, 809–817 (2018).
Han, B. et al. Small-Molecule Bcl2 BH4 Antagonist for Lung Cancer Therapy. Cancer Cell. 27, 852–863 (2015).
Deng, J. et al. BCL2-BH4 antagonist BDA-366 suppresses human myeloma growth. Oncotarget 7, 27753–27763 (2016).
Vervloessem, T., La Rovere, R. & Bultynck, G. Antagonizing Bcl-2’s BH4 domain in cancer. Aging. 7, 748–749 (2015).
Vervloessem, T. et al. BDA-366, a putative Bcl-2 BH4 domain antagonist, induces apoptosis independently of Bcl-2 in a variety of cancer cell models. Cell Death Dis. 11, 769 (2020).
Birkinshaw, R. W. Challenges in small-molecule target identification: a commentary on “BDA-366, a putative Bcl-2 BH4 domain antagonist, induces apoptosis independently of Bcl-2 in a variety of cancer cell models. Cell Death Differ. 28, 1130–1132 (2021).
Lin, Y. et al. CYD0281, a Bcl-2 BH4 domain antagonist, inhibits tumor angiogenesis and breast cancer tumor growth. BMC Cancer 23, 479 (2023).
Zhou, J. Y. et al. Discovery and identification of a novel small molecule BCL-2 inhibitor that binds to the BH4 domain. Acta Pharm. Sin. 44, 475–485 (2023).
Casan, J. M. L. & Seymour, J. F. Degraders upgraded: the rise of PROTACs in hematological malignancies. Blood 143, 1218–1230 (2024).
Jevtic, P., Haakonsen, D. L. & Rape, M. An E3 ligase guide to the galaxy of small-molecule-induced protein degradation. Cell Chem. Biol. 28, 1000–1013 (2021).
Jiang, H., Xiong, H., Gu, S. X. & Wang, M. E3 ligase ligand optimization of Clinical PROTACs. Front Chem. 11, 1098331 (2023).
Bray, P. F. et al. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 14, 1 (2013).
Zhao, L. et al. Targeted protein degradation: mechanisms, strategies and application. Signal Transduct. Target Ther. 7, 113 (2022).
Khan, S. et al. A selective BCL-X(L) PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 25, 1938–1947 (2019).
He, Y. et al. DT2216-a Bcl-xL-specific degrader is highly active against Bcl-xL-dependent T cell lymphomas. J. Hematol. Oncol. 13, 95 (2020).
Thummuri, D. et al. Overcoming Gemcitabine resistance in pancreatic cancer using the BCL-X(L)-specific degrader DT2216. Mol. Cancer Ther. 21, 184–192 (2022).
Khan, S. et al. Co-targeting BCL-X(L) and MCL-1 with DT2216 and AZD8055 synergistically inhibit small-cell lung cancer growth without causing on-target toxicities in mice. Cell Death Discov. 9, 1 (2023).
Zhang, X. et al. Discovery of PROTAC BCL-X(L) degraders as potent anticancer agents with low on-target platelet toxicity. Eur. J. Med. Chem. 192, 112186 (2020).
Bharti, V. et al. BCL-xL inhibition potentiates cancer therapies by redirecting the outcome of p53 activation from senescence to apoptosis. Cell Rep. 41, 111826 (2022).
Zhang, S. et al. The PROTAC selectively degrading Bcl-x(L) represents a novel Hedgehog pathway inhibitor with capacity of combating resistance to Smoothened inhibitors while sparing bone growth. Theranostics 12, 7476–7490 (2022).
Zhang, X. et al. Sorafenib and SIAIS361034, a novel PROTAC degrader of BCL-x(L), display synergistic antitumor effects on hepatocellular carcinoma with minimal hepatotoxicity. Biochem. Pharmacol. 230, 116542 (2024).
Zhang, X. et al. Utilizing PROTAC technology to address the on-target platelet toxicity associated with inhibition of BCL-X(L). Chem. Commun. 55, 14765–14768 (2019).
Zhang, X. et al. Discovery of IAP-recruiting BCL-X(L) PROTACs as potent degraders across multiple cancer cell lines. Eur. J. Med. Chem. 199, 112397 (2020).
Pal, P. et al. Discovery of a novel BCL-X(L) PROTAC degrader with enhanced BCL-2 inhibition. J. Med Chem. 64, 14230–14246 (2021).
Jia, Y. et al. Co-targeting BCL-XL and BCL-2 by PROTAC 753B eliminates leukemia cells and enhances efficacy of chemotherapy by targeting senescent cells. Haematologica 108, 2626–2638 (2023).
Lv, D. et al. Development of a BCL-xL and BCL-2 dual degrader with improved anti-leukemic activity. Nat. Commun. 12, 6896 (2021).
Khan, S. et al. PROTAC-mediated dual degradation of BCL-xL and BCL-2 is a highly effective therapeutic strategy in small-cell lung cancer. Cells 13, 258 (2024).
Nayak, D. et al. Development and crystal structures of a potent second-generation dual degrader of BCL-2 and BCL-xL. Nat. Commun. 15, 2743 (2024).
Xie, Y. et al. Discovery of BCL-XL heterobifunctional degrader with potentially improved therapeutic window and minimal platelet toxicity for hematological malignancies. Blood 142, 117 (2023).
Wang, Z. et al. Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands. J. Med Chem. 62, 8152–8163 (2019).
Zhu, J. et al. Structure-based design, synthesis, and evaluation of Bcl-2/Mcl-1 dual inhibitors. Arch. Pharm. 353, e2000005 (2020).
Wang, J. et al. Thalidomide derivatives degrade BCL-2 by reprogramming the binding surface of CRBN. Cell Rep. Phys. Sci. 5, 101960 (2024).
Chang, M. et al. Bioorthogonal PROTAC prodrugs enabled by on-target activation. J. Am. Chem. Soc. 145, 14155–14163 (2023).
Advani, P. P. et al. Pharmacokinetic evaluation of oblimersen sodium for the treatment of chronic lymphocytic leukemia. Expert Opin. Drug Metab. Toxicol. 7, 765–774 (2011).
O’Brien, S. et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J. Clin. Oncol. 27, 5208–5212 (2009).
Hoogenboezem, E. N. et al. Structural optimization of siRNA conjugates for albumin binding achieves effective MCL1-directed cancer therapy. Nat. Commun. 15, 1581 (2024).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Schmid, D. et al. Nanoencapsulation of ABT-737 and camptothecin enhances their clinical potential through synergistic antitumor effects and reduction of systemic toxicity. Cell Death Dis. 5, e1454 (2014).
Bala Tannan, N. et al. Tumor-targeted nanoparticles improve the therapeutic index of BCL2 and MCL1 dual inhibition. Blood 137, 2057–2069 (2021).
Panwar, D. et al. Hyaluronic acid-engineered Bcl-2 inhibitor nanocrystals for site-specific delivery to breast tumor cells. Nanomedicine 18, 1005–1023 (2023).
Patterson, C. M. et al. Design and optimisation of dendrimer-conjugated Bcl-2/x(L) inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 4, 112 (2021).
Arulananda, S. et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 7, 122 (2021).
Arslan, S. et al. Safety and tolerability of AZD0466 as monotherapy for patients with advanced hematologic malignancies. results from a Phase I/II Trial. Blood 142, 5907–5907 (2023).
Carneiro, B. A. et al. Mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: Dose expansion results. J. Clin. Oncol. 41, 3027 (2023).
Tolcher, A. W. et al. A first-in-human study of mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: Dose escalation results. J. Clin. Oncol. 39, 3015 (2021).
Rotow, J. K. et al. 1318MO First-in-human study of ABBV-637, an EGFR-targeting BCL-XL–inhibiting antibody-drug conjugate combined with osimertinib (OSI) in relapsed/refractory, EGFR-mutated non-small cell lung cancer (NSCLC). Ann. Oncol. 34, S759 (2023).
Niu, X. et al. A small-molecule inhibitor of Bax and Bak oligomerization prevents genotoxic cell death and promotes neuroprotection. Cell Chem. Biol. 24, 493–506 e495 (2017).
D’Orsi, B. et al. Calpains are downstream effectors of bax-dependent excitotoxic apoptosis. J. Neurosci. 32, 1847–1858 (2012).
Garner, T. P. et al. Small-molecule allosteric inhibitors of BAX. Nat. Chem. Biol. 15, 322–330 (2019).
Kitamura, Y. et al. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 780, 260–269 (1998).
Vitale, I. et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 30, 1097–1154 (2023).
Paradis, E. et al. Amyloid beta peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates bax expression in human neurons. J. Neurosci. 16, 7533–7539 (1996).
Kudo, W. et al. Inhibition of Bax protects neuronal cells from oligomeric Abeta neurotoxicity. Cell Death Dis. 3, e309 (2012).
Pogmore, J. P., Uehling, D. & Andrews, D. W. Pharmacological targeting of executioner proteins: controlling life and death. J. Med Chem. 64, 5276–5290 (2021).
Rohn, T. T. et al. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J. Neurosci. 28, 3051–3059 (2008).
Karlnoski, R. et al. Up-regulation of Bcl-2 in APP transgenic mice is associated with neuroprotection. Neurobiol. Dis. 25, 179–188 (2007).
Kumasaka, D. K., Galvan, V., Head, E. & Rohn, T. T. Caspase cleavage of the amyloid precursor protein is prevented after overexpression of bcl-2 in a triple transgenic mouse model of Alzheimer’s disease. Int J. Physiol. Pathophysiol. Pharmacol. 1, 48–56 (2009).
Schrank, S., Barrington, N. & Stutzmann, G. E. Calcium-Handling Defects and Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol 12, a035212 (2020).
Webber, E. K., Fivaz, M., Stutzmann, G. E. & Griffioen, G. Cytosolic calcium: Judge, jury and executioner of neurodegeneration in Alzheimer’s disease and beyond. Alzheimers Dement. 19, 3701–3717 (2023).
Popugaeva, E., Chernyuk, D. & Bezprozvanny, I. Reversal of calcium dysregulation as potential approach for treating Alzheimer’s disease. Curr. Alzheimer Res. 17, 344–354 (2020).
Stutzmann, G. E. & Soboloff, J. Channelling calcium signals to therapeutics. J. Physiol. 602, 1445–1447 (2024).
Dahl, R. & Bezprozvanny, I. SERCA pump as a novel therapeutic target for treating neurodegenerative disorders. Biochem Biophys. Res Commun. 734, 150748 (2024).
Cheung, K. H. et al. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci. Signal. 3, ra22 (2010).
Muller, M., Cheung, K. H. & Foskett, J. K. Enhanced ROS generation mediated by Alzheimer’s disease presenilin regulation of InsP3R Ca2+ signaling. Antioxid. Redox Signal. 14, 1225–1235 (2011).
Shilling, D. et al. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J. Neurosci. 34, 6910–6923 (2014).
Goussakov, I., Miller, M. B. & Stutzmann, G. E. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J. Neurosci. 30, 12128–12137 (2010).
Bruno, A. M. et al. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 33, 1001,e1001–e1006 (2012).
Chen, X. et al. Dantrolene is neuroprotective in Huntington’s disease transgenic mouse model. Mol. Neurodegener. 6, 81 (2011).
Chakroborty, S. et al. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J. Neurosci. 32, 8341–8353 (2012).
Liu, J. et al. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels. 8, 230–242 (2014).
Zhang, H., Knight, C., Chen, S. R. W. & Bezprozvanny, I. A gating mutation in Ryanodine Receptor Type 2 rescues phenotypes of Alzheimer’s disease mouse models by upregulating neuronal autophagy. J. Neurosci. 43, 1441–1454 (2023).
Ivanova, H. et al. Bcl-2 and IP(3) compete for the ligand-binding domain of IP(3)Rs modulating Ca(2+) signaling output. Cell Mol. Life Sci. 76, 3843–3859 (2019).
Chernyuk, D. et al. Neuroprotective properties of anti-apoptotic BCL-2 proteins in 5xFAD mouse model of Alzheimer’s disease. IBRO Neurosci. Rep. 14, 273–283 (2023).
Tang, S. S. et al. Protective effect of pranlukast on Abeta(1)(-)(4)(2)-induced cognitive deficits associated with downregulation of cysteinyl leukotriene receptor 1. Int. J. Neuropsychopharmacol. 17, 581–592 (2014).
Lai, J. et al. Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Abeta1-42-induced memory impairment and neuroinflammatory and apoptotic responses in mice. Neuropharmacology 79, 707–714 (2014).
Wang, H. et al. Pretreatment with antiasthmatic drug ibudilast ameliorates Abeta 1-42-induced memory impairment and neurotoxicity in mice. Pharm. Biochem Behav. 124, 373–379 (2014).
Dehay, B. et al. Lysosomal impairment in Parkinson’s disease. Mov. Disord. 28, 725–732 (2013).
Oberle, C. et al. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ. 17, 1167–1178 (2010).
Karch, J. et al. Autophagic cell death is dependent on lysosomal membrane permeability through Bax and Bak. Elife 6, e30543 (2017).
Bove, J. et al. BAX channel activity mediates lysosomal disruption linked to Parkinson disease. Autophagy 10, 889–900 (2014).
Przedborski, S. et al. The parkinsonian toxin MPTP: action and mechanism. Restor. Neurol. Neurosci. 16, 135–142 (2000).
Vila, M. et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc. Natl Acad. Sci. USA 98, 2837–2842 (2001).
Offen, D. et al. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity. Proc. Natl Acad. Sci. USA 95, 5789–5794 (1998).
Yang, L. et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J. Neurosci. 18, 8145–8152 (1998).
Mu, X. et al. Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Ann. Neurol. 40, 379–386 (1996).
Vukosavic, S. et al. Delaying caspase activation by Bcl-2: A clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 20, 9119–9125 (2000).
Gould, T. W. et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J. Neurosci. 26, 8774–8786 (2006).
Hetz, C. et al. The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ. 14, 1386–1389 (2007).
Strasser, A. et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl Acad. Sci. USA 88, 8661–8665 (1991).
Liphaus, B. L., Kiss, M. H., Carrasco, S. & Goldenstein-Schainberg, C. Increased Fas and Bcl-2 expression on peripheral mononuclear cells from patients with active juvenile-onset systemic lupus erythematosus. J. Rheumatol. 34, 1580–1584 (2007).
Aringer, M. et al. High levels of bcl-2 protein in circulating T lymphocytes, but not B lymphocytes, of patients with systemic lupus erythematosus. Arthritis Rheum. 37, 1423–1430 (1994).
Gatenby, P. A. & Irvine, M. The bcl-2 proto-oncogene is overexpressed in systemic lupus erythematosus. J. Autoimmun. 7, 623–631 (1994).
Wang, W., Wang, X., Yang, K. & Fan, Y. Association of BCL2 polymorphisms and the IL19 single nucleotide polymorphism rs2243188 with systemic lupus erythematosus. J. Int. Med. Res. 49, 3000605211019187 (2021).
Kielbassa, K. et al. Differential expression pattern of Bcl-2 family members in B and T cells in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 25, 225 (2023).
Bardwell, P. D. et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J. Immunol. 182, 7482–7489 (2009).
Nader, A., Minocha, M. & Othman, A. A. Exposure-response analyses of the effects of venetoclax, a selective BCL-2 inhibitor, on B-Lymphocyte and total lymphocyte counts in women with systemic Lupus Erythematosus. Clin. Pharmacokinet. 59, 335–347 (2020).
Minocha, M., Zeng, J., Medema, J. K. & Othman, A. A. Pharmacokinetics of the B-Cell Lymphoma 2 (Bcl-2) inhibitor venetoclax in female subjects with systemic Lupus Erythematosus. Clin. Pharmacokinet. 57, 1185–1198 (2018).
Lu, P. et al. Safety and pharmacodynamics of venetoclax (ABT-199) in a randomized single and multiple ascending dose study in women with systemic lupus erythematosus. Lupus 27, 290–302 (2018).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
Ina, K. et al. Resistance of Crohn’s disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J. Immunol. 163, 1081–1090 (1999).
Johnson, L. A. et al. Effect of ABT-263 on intestinal fibrosis in human myofibroblasts, human intestinal organoids, and the mouse Salmonella typhimurium Model. Inflamm. Bowel Dis. 28, 161–175 (2022).
Weder, B. et al. BCL2 regulates differentiation of intestinal fibroblasts. Inflamm. Bowel Dis. 24, 1953–1966 (2018).
Gurzov, E. N. & Eizirik, D. L. Bcl-2 proteins in diabetes: mitochondrial pathways of beta-cell death and dysfunction. Trends Cell Biol. 21, 424–431 (2011).
Thompson, P. J. et al. Targeted elimination of senescent beta cells prevents Type 1 Diabetes. Cell Metab. 29, 1045–1060.e1010 (2019).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Jeyapalan, J. C. & Sedivy, J. M. Cellular senescence and organismal aging. Mech. Ageing Dev. 129, 467–474 (2008).
Campisi, J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11, S27–S31 (2001).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Campisi, J. Aging, cellular senescence, and cancer. Annu Rev. Physiol. 75, 685–705 (2013).
Kirkland, J. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518–536 (2020).
Dhokia, V. et al. A second generation of senotherapies: the development of targeted senolytics, senoblockers and senoreversers for healthy ageing. Biochem. Soc. Trans. 52, 1661–1671 (2024).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Anantram, A. & Degani, M. Targeting cancer’s Achilles’ heel: role of BCL-2 inhibitors in cellular senescence and apoptosis. Future Med. Chem. 11, 2287–2312 (2019).
Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016).
Victorelli, S. et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 622, 627–636 (2023).
Sharma, A. K. et al. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front Cell Dev. Biol. 8, 354 (2020).
Moncsek, A. et al. Targeting senescent cholangiocytes and activated fibroblasts with B‐cell lymphoma‐extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2−/−) mice. Hepatology 67, 247–259 (2018).
Gonzales, M. M. et al. Senolytic therapy in mild Alzheimer’s disease: a phase 1 feasibility trial. Nat. Med. 29, 2481–2488 (2023).
Agostini, A. et al. Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew. Chem. Int Ed. 51, 10556–10560 (2012).
Muñoz‐Espín, D. et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 10, e9355 (2018).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Tiong, I. S. et al. BAX mutated clonal hematopoiesis arises following treatment with the BCL2 inhibitor class of therapeutics across a range of hematological and non-hematological neoplasms. Blood 142, 2688–2688 (2023).
Bisaillon, R. et al. Genetic characterization of ABT-199 sensitivity in human AML. Leukemia 34, 63–74 (2020).
Zhang, H. et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat. Cancer 1, 826–839 (2020).
Li, X., Dou, J., You, Q. & Jiang, Z. Inhibitors of BCL2A1/Bfl-1 protein: Potential stock in cancer therapy. Eur. J. Med. Chem. 220, 113539 (2021).
Araujo, A. D. et al. Bicyclic helical peptides as dual inhibitors selective for Bcl2A1 and Mcl-1 proteins. J. Med. Chem. 61, 2962–2972 (2018).
Lucas, S. C. C. et al. Identification and Evaluation of Reversible Covalent Binders to Cys55 of Bfl-1 from a DNA-Encoded Chemical Library Screen. ACS Med Chem. Lett. 15, 791–797 (2024).
Lou, J. et al. Discovery of a covalent inhibitor that overcame resistance to Venetoclax in AML cells overexpressing BFL-1. J. Med. Chem. 67, 10795–10830 (2024).
Lucas, S. C. C. et al. Structure-based optimization of a series of covalent, cell active Bfl-1 inhibitors. J. Med. Chem. 67, 16455–16479 (2024).
Nechiporuk, T. et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 9, 910–925 (2019).
Thijssen, R. et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood 137, 2721–2735 (2021).
Pollyea, D. A. et al. Outcomes in patients with poor-risk cytogenetics with or without TP53 mutations treated with Venetoclax and Azacitidine. Clin. Cancer Res. 28, 5272–5279 (2022).
Diepstraten, S. T. et al. Putting the STING back into BH3-mimetic drugs for TP53-mutant blood cancers. Cancer Cell. 42, 850–868.e859 (2024).
Kehr, S. et al. Targeting BCL-2 proteins in pediatric cancer: Dual inhibition of BCL-X(L) and MCL-1 leads to rapid induction of intrinsic apoptosis. Cancer Lett. 482, 19–32 (2020).
Cidado, J. et al. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin. Cancer Res. 26, 922–934 (2020).
Barlaam, B. et al. Discovery of AZD4573, a potent and selective inhibitor of CDK9 that enables short duration of target engagement for the treatment of hematological malignancies. J. Med. Chem. 63, 15564–15590 (2020).
Melani, C. et al. Combination targeted therapy in relapsed diffuse large B-cell lymphoma. N. Engl. J. Med. 390, 2143–2155 (2024).
Dyer, M. J. S. et al. Precision medicines for B-cell leukaemias and lymphomas; progress and potential pitfalls. Br. J. Haematol. 160, 725–733 (2013).
Goldstein, J. S. & Alizadeh, A. A. ViPOR’s venom - rationally targeting DLBCL with precision. N. Engl. J. Med. 390, 2209–2211 (2024).
Tron, A. E. et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 9, 5341 (2018).
He, Y. et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun. 11, 1996 (2020).
de Bruijn, I. et al. Analysis and visualization of longitudinal genomic and clinical data from the AACR Project GENIE biopharma collaborative in cBioPortal. Cancer Res. 83, 3861–3867 (2023).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Zehir, A. et al. Erratum: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 1004 (2017).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52, 1219–1226 (2020).
Wu, L. et al. Landscape of somatic alterations in large-scale solid tumors from an Asian population. Nat. Commun. 13, 4264 (2022).
Nguyen, B. et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 185, 563–575.e511 (2022).
Acknowledgements
This study was supported by the Deutsche Krebshilfe (to M.V.), the Wilhelm Sander Stiftung (to M.V.), the Deutsche Kinderkrebsstiftung (to M.V.), the Deutsche José Carreras Leukämie-Stiftung (to M.V and S.M.), and research funding from LOXO (to V.M.S). Y.B. is supported by a Junior Clinician Scientist grant of the Goethe-University Frankfurt and by funds of the Rudolf-Geißendörfer-Stiftung. Work in MJSD lab is supported by funds from the Scott-Waudby Trust, the Hope Against Cancer charity, Cancer Research UK in conjunction with the UK Department of Health on an Experimental Cancer Medicine Center grant [MRC] and by Leicester Drug Discovery and Diagnostics under the University of Leicester Institute for Precision Health [MRC - Impact Acceleration AccountMR/X502777/1] and is carried out at the National Institute for Health and Care Research (NIHR) Leicester Biomedical Research Center (BRC). Additional funding was obtained from Beigene and LOXO pharma. Work in the G.B. lab is funded by grants from the Research Foundation Flanders (G0E7520N, G081821N and G094522 N), from the Research Council, KU Leuven (C14/19/099) and the Central European Leuven Strategic Alliance (CELSA/23/031 and CELSA/23/032). GB is member of the FWO Scientific Research Network CaSign (W0.014.22N). MC (application number 11K7122N) and TV (application number: 12ZG121N) were supported by fellowships from the Research Foundation Flanders. Work in SM lab is supported by project PI20/00328 from the Instituto de Salud Carlos III (Spain) and the M. C. Andreu Memorial Fund.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Meike Vogler (MV), Yannick Braun (YB), Victoria M Smith (VMS), Mike-Andrew Westhoff, Raquel S Pereira, Nadja M Pieper (NMP), Marius Anders, Manon Callens, Tim Vervliet, Maha Abbas, Salvador Macip, Ralf Schmid (RS), Geert Bultynck and Martin JS Dyer (MJSD) were involved in writing of the original draft as well as reviewing and editing. MV and MJSD were involved in the conceptualization of the article. MV, YB and VMS were involved in data curation. MV, RS, NMP, VMS and YB were involved in visualization. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
MJSD has received research funding from LOXO and Beigene and financial assistance with meeting attendance from Beigene. GB is involved in a fee-for-service agreement with ReMynd. All other authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vogler, M., Braun, Y., Smith, V.M. et al. The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy. Sig Transduct Target Ther 10, 91 (2025). https://doi.org/10.1038/s41392-025-02176-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-025-02176-0
This article is cited by
-
A new era in cancer therapy: targeting the Proteasome-Bcl-2 axis
Journal of Experimental & Clinical Cancer Research (2025)
-
Aging-related signature stratifies LUAD prognosis and uncovers senescence-induced chemoresistance via NF-κB/RELA activation
Respiratory Research (2025)
-
High content-imaging drug synergy screening identifies specific senescence-related vulnerabilities of mesenchymal neuroblastomas
Cell Death & Disease (2025)
-
Bcl-2 modifying factor (Bmf): “a mysterious stranger” in the Bcl-2 family proteins
Cell Death & Differentiation (2025)
-
Stress granules and cell death: crosstalk and potential therapeutic strategies in infectious diseases
Cell Death & Disease (2025)