Abstract
Malaria, caused by Plasmodium parasites and transmitted by Anopheles mosquitoes, greatly impacts public health and socioeconomic development, particularly in sub-Saharan African countries. Despite advances in malaria treatment and prevention, the number of clinical cases and deaths have increased in recent years. The complex life cycle and genetic diversity of Plasmodium parasites pose significant challenges in drug and vaccine development, particularly due to the emerging partial resistance of parasites to artemisinin. With the availability and application of state-of-the-art biotechnology in recent years, knowledge in terms of parasite biology, pathogenicity, host–parasite interactions and pathogenesis has advanced tremendously. This review highlights the most recent research progress and understanding in Plasmodium biology, with a primary focus on P. falciparum and associated pathogenesis. The therapeutic targets and progress in the clinical application of anti-malaria drugs have also been summarized. The FDA-approved regimens like Artemether-Lumefantrine, Atovaquone-Proguanil, and Primaquine are discussed, and their benefits and limitations are highlighted, especially in terms of drug resistance. Perspectives in the development of novel vaccines and new drugs, such as Sevuparin, Imatinib, and Cipargamin, and combination therapies with promise in overcoming resistance has been proposed. Overall, this review provides a detailed summary of the latest progress in malaria research and emphasizes the need for continuous monitoring and innovation in malaria treatment.
Similar content being viewed by others
Introduction
Malaria, a disease caused by several Plasmodium species, namely P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi, profoundly impacts human health.1,2,3,4,5,6 Mixed infections of P. falciparum with P. malariae and/or P. ovale in Africa7 and P. falciparum with P. vivax and/or P. knowlesi in Southeast Asia have posed challenges in disease control.8,9 In contrast to earlier optimistic predictions of malaria eradication by 2030, the number of cases has been increasing in recent years.10 According to the latest WHO’s World malaria report, global malaria cases in 2023 continually increased. An estimated 263 million cases occurred in 83 malaria-endemic countries, with Nigeria (25.9%), the Democratic Republic of the Congo (12.6%), Uganda (4.8%), Ethiopia (3.6%) and Mozambique (3.5%) accounting for over half of all cases. Malaria also leads to great economic losses of approximately $12 billion US dollars per year in sub-Saharan Africa.11
Once injected into the human host by the female Anopheles mosquito, the sporozoites travel to the liver for differentiation.8,12 After multiplication and adaptation in hepatocytes for 6–7 days, thousands of merozoites egress from hepatic cells to infect red blood cells (RBCs).13 A recent study revealed that P. falciparum transmission is disproportionately driven by infected school-aged boys who receive a high number of mosquito bites, with infectious mosquitoes preferentially biting already infected individuals, highlighting the importance of targeted interventions for interrupting malaria transmission.14 The invasion and intracellular development of malaria parasites in RBCs results in various pathologies in the host, with clinical symptoms including periodic fever episodes (a cyclical occurrence of sudden coldness followed by shivering and then fever and sweating), headache, chills, and vomiting.15 Without prompt treatment, P. falciparum malaria can progress to severe illness and death, with symptoms such as severe anemia, respiratory distress, or cerebral malaria (CM).16
Malaria treatment regimens are based on the parasite type, symptom severity, and patient age.17 Classical antimalarial drugs such as chloroquine, quinine, pyronaridine, pyrimethamine, primaquine, and piperaquine have been widely applied in clinics to for decades. However, with the emergence of classical antimalarial drug resistance, especially in P. falciparum, artemisinin (ART)-based combination therapy (ACT) has been recommended as the first-line treatment. In this respect, Chinese scientists have made important contributions. Professor Tu Youyou was the pioneer who discovered a rational method for extracting the active ingredient, artemisinin, from the Artemisia annua plant and conducted the first clinical trial in patients.18 Professors Zhou Weishan and Mrs. Luo Zeyuan resolved the structural determination and synthesized the structures of artemisinin.18 Later, Professor Li Guoqiao’s team developed the ACT regimen with an aim to overcome resistance to single-drug treatments.18 However, ART-resistant strains of P. falciparum have now been frequently detected in African and Southeast Asian countries, presenting a great challenge for disease control.19 The underlying mechanisms of the emergence of drug-resistant P. falciparum have attracted tremendous attention, and gene mutations and duplication have been regarded as the main causes, whereas the specific mechanism of ART resistance is a debated issue that will be discussed later.
This review will explore various aspects of malaria, starting with its epidemiology, where will examine the global distribution and prevalence of the disease, as well as trends in malaria incidence and mortality. Next, this review will delve into parasite genomics, providing an overview of Plasmodium species genotypes and discussing recent advances in genomic research, including the use of single-cell RNA sequencing. The clinical features of malaria will be discussed in detail, focusing on symptoms such as fever, cerebral, and placenta-associated malaria. We will then explore signaling pathways and crosstalk, highlighting the regulatory mechanisms that govern parasite development and invasion, as well as key pathways involved in Plasmodium development. The pathogenic mechanisms will be addressed next, with a focus on the molecular mechanisms of host cell recognition and cytoadherence, along with the immune response strategies employed by the parasites. Finally, the review will conclude with an overview of therapeutic targets and clinical research progress, covering the challenges of drug resistance, emerging therapeutic targets, and recent developments in malaria treatment, including promising clinical trials and FDA-approved drugs. This review will provide a comprehensive understanding of the current state of malaria research and its future
The research history and milestone events in studies on malaria
Malaria research has evolved through a series of landmark discoveries and milestone events, each contributing to our understanding of the disease and its control (Fig. 1). The modern study of malaria began in 1880, when the French military doctor Charles Louis Alphonse Laveran discovered Plasmodium parasites in the blood of infected patients,20 earning him the 1907 Nobel Prize in Physiology or Medicine.21 This established malaria as a parasitic disease, laying the foundation for further exploration.22 In 1897, British physician Sir Ronald Ross demonstrated that Anopheles mosquitoes are the vectors of malaria and elucidated the developmental stages of Plasmodium in the mosquito.23 For this pivotal work, Ross was awarded the 1902 Nobel Prize in Physiology or Medicine. These findings revolutionized malaria control efforts, enabling vector management strategies that remain integral to modern malaria prevention.24 The 20th century saw several groundbreaking advancements. In 1927, the Austrian psychiatrist Julius Wagner-Jauregg received the Nobel Prize in Physiology or Medicine for his innovative use of Plasmodium infection to treat neurosyphilis-induced paralysis.25 While controversial, this method highlighted malaria’s potential for therapeutic applications in a pre-antibiotic era.26,27 In 1965, American chemist Robert Burns Woodward was awarded the Nobel Prize in Chemistry for the first total synthesis of quinine,28,29 one of the earliest and most effective antimalarial drugs.30,31 His work underscored the role of chemistry in developing treatments for malaria and other diseases.32,33 The mid-20th century was also marked by the introduction of synthetic antimalarials such as chloroquine (1934)34 and the first modern insecticide Dichloro-diphenyl-trichloroethane (DDT, 1939),35 which became central to the World Health Organization’s (WHO) global malaria eradication campaign launched in 1955.36 Despite early successes, the emergence of resistance to both chloroquine and DDT revealed the need for sustained innovation and comprehensive strategies.37,38
Milestones in malaria. The key milestones in the history of malaria research and control are depicted in this timeline. It highlights major discoveries, the development of treatments and vaccines, and significant global initiatives from the identification of Plasmodium parasites in 1880 to the approval of the RTS, S/AS01 (Mosquirix) malaria vaccine in 2018. This figure was created with BioRender.com
A major breakthrough came in 1972, when Chinese scientist Tu Youyou discovered a simple technique and extracted the potent anti-malaria component, artemisinin,39 from the traditional Chinese medicinal plant Artemisia annua.18 She was awarded the Nobel Prize in Physiology or Medicine for the astonishing discovery in 2015. Artemisinin and its derivatives form the basis of ACT, which are currently the most adapted regimen for treating drug-resistant Plasmodium falciparum.40,41 Her work, inspired by ancient Chinese medical texts and validated through modern pharmacological research, has saved millions of lives and remains a cornerstone of global malaria treatment. In the year 2000, the WHO launched the Global Technical Strategy for Malaria 2016–2030,42 setting ambitious goals to reduce malaria incidence and mortality rates by at least 90% by 2030 compared to 2015 levels. This goal is unlikely to be achieved, due to the fact that the 2023 global malaria incidence was nearly three times higher than that of the WHO’s aim. In the meantime, malaria vaccines have been pursued by various approaches including attenuation of the sporozoites by radiation.43 Experiments in rodent models propelled scientists to identify the “target antigen” on the sporozoite surface, which led to the cloning of the gene coding for the circumsporozoite surface protein (CSP) in Pf malaria.44,45,46 CSP has, since then, been regarded as the primary malaria vaccine candidate.47 Later, the central repetitive region of CSP was selected and biosynthesized (expressed) in a fused form with the S-antigen of the Hepatitis B virus. The product of the recombinant fusion protein was named RTS,S.48 After several rounds of clinical trials in African adults and children, RTS,S/AS01E (Mosquirix) became the first malaria vaccine approved by the WHO,49 representing a milestone in prevention strategies.50,51 The vaccine’s pilot implementation in sub-Saharan Africa offered hope for reducing the disease burden in high-risk populations.52 The completion of the genome sequencing of several Plasmodium species marked a new era in research on malaria, which has provided innovative pathways accelerating the process of both drug mining and vaccine development.53,54 From 2016 to 2024, several countries achieved malaria-free certification by WHO, including Algeria and Argentina in 2019,55 China in 2021,56 Azerbaijan and Tajikistan in 2023, and Belize and Egypt in 2024, showcasing the success of elimination campaigns. Another major development occurred in 2024 with the WHO approval of the R21/Matrix-M malaria vaccine, which meets the efficacy target of 75% in young African children.57
Epidemiology of malaria
Malaria remains a significant global health challenge, with an estimated 263 million cases reported in 83 endemic countries across five WHO regions in 2023, reflecting a slight increase from 11 million cases in 2022, according to WHO’s World Malaria Report 2024 (www.who.int/teams/global-malaria-programme). Of the 93 countries that were malaria endemic in 2015, 26% (including those that are now certified malaria free) met the GTS morbidity milestone for 2023, 34% made progress in reducing malaria case incidence but by less than the expected target, 15% had similar incidence to 2015 and 26% experienced an increase in case incidence. Despite some progress in malaria control, several factors, including funding gaps, poverty, and climate change, have contributed to setbacks in global efforts to reduce malaria transmission.
Sub-Saharan Africa remains the region most affected by malaria, accounting for ~94% of global cases in 2023, with the highest burden concentrated in countries such as Nigeria (30.9%), the Democratic Republic of the Congo (11.3%), Niger (5.9%), and United Republic of Tanzania (4.3%). In 2023, the region reported 246 million cases and 569,000 deaths. The overwhelming prevalence of P. falciparum, the most virulent malaria species, exacerbates the disease burden, especially among vulnerable groups such as young children58 and pregnant women.59 While adults in endemic areas often develop partial immunity,60 young children continue to face the greatest risk of severe disease.61 The high transmission rates are largely driven by favorable environmental factors, including the tropical climate, which supports year-round breeding of Anopheles mosquitoes.62,63,64 Despite this, significant challenges persist in controlling malaria, such as weak health infrastructure, limited access to diagnostic tools, and the high cost of prevention measures (such as insecticide-treated bed nets and antimalarial medications).65 These barriers hinder the effectiveness of malaria control efforts and contribute to the ongoing high burden of the disease. The rapid spread of artemisinin partial resistance (ART-R) in Africa also poses a serious threat to malaria control efforts, with potential economic and health impacts. Urgent regional initiatives are required to address ART-R through coordinated cross-border actions, enhanced surveillance, diversified treatments, and strengthened health systems, similar to the successful approaches in Southeast Asia, to prevent the further spread of resistance and safeguard malaria elimination goals.66
South-East Asia exhibits a mixed malaria burden, reporting 4 million cases in 2023 according to World Malaria Report 2024. While some countries, such as India (51%), Indonesia (27%), and Myanmar (21%), experience high transmission rates, others, such as Viet Nam (only 370 cases), have made considerable strides in malaria elimination. India remains one of the largest contributors to malaria cases in the region, reporting 48% of all cases in the region were due to P. vivax. A major concern for Asia is the growing problem of drug resistance, particularly to ART. Resistance has been detected in several countries, including Cambodia, Thailand, Myanmar, and Vietnam, raising alarm about the future effectiveness of ACTs.67 In recent years, P. knowlesi infection has become an increasingly significant issue in malaria cases, particularly in Southeast Asia, with prevalence most prominent in Indonesia, Malaysia, and Thailand, and more recently observed in Cambodia. On a global scale, 3,290 cases of P. knowlesi infection were documented in 2023, reflecting an 18.9% rise from the 2768 cases reported in 2022. Similarly, indigenous cases of P. knowlesi showed a 22% increase, growing from 2682 in 2022 to 3274 in 2023.
Malaria in the Americas is primarily confined to Brazil (33%), the Bolivarian Republic of Venezuela (26%), Colombia (21%), Guyana (6%), and Peru (4%) reporting the highest burden according to World Malaria Report 2024. In 2023, the region recorded ~505,642 cases. All indigenous malaria cases reported by Guatemala and Mexico were attributed to P. vivax. In the Bolivarian Republic of Venezuela, Brazil, Colombia, Ecuador, French Guiana, Guyana, Honduras, Nicaragua, Panama, Peru, and the Plurinational State of Bolivia, P. vivax accounted for 60% to 99% of the documented indigenous cases. Conversely, all indigenous cases reported by the Dominican Republic and Haiti, along with 92% of the indigenous cases recorded in Costa Rica in 2023, were attributed to P. falciparum. Colombia reported the highest number of P. falciparum cases in the region. Although malaria transmission is less intense than that in sub-Saharan Africa, challenges remain in remote and rural areas where healthcare access is limited, and migratory movements increase the risk of malaria transmission. Efforts to control malaria in South America include interventions such as indoor residual spraying and mass drug administration (MDA) programs. However, regional differences in program effectiveness highlight the need for tailored approaches. Resistance to chloroquine, the traditional first-line treatment for P. vivax, remains a concern in certain areas, further complicating control efforts.
Malaria transmission in Western Pacific Region is mainly concentrated in Papua New Guinea (88%), which continues to experience a high burden of both P. falciparum (71%) and P. vivax (29%) according to World Malaria Report 2024. In 2023, the region recorded an estimated 1.7 million malaria cases and 3360 deaths. This represents a 5% increase in cases and a 3% reduction in deaths compared to 2010. Papua New Guinea remains one of the few countries outside sub-Saharan Africa with significant malaria transmission, with the disease contributing to considerable morbidity. In contrast, the Pacific islands have largely succeeded in eliminating indigenous malaria transmission, with countries such as Australia, New Zealand, and several island nations achieving malaria-free status.
Malaria cases in the WHO Eastern Mediterranean Region were estimated to have decreased by 37.7% between 2000 and 2015, dropping from 6.9 million to 4.3 million according to World Malaria Report 2024. However, this trend reversed, with cases rising by 137% between 2015 and 2023, reaching an estimated 10.2 million. Notably, there was a significant increase of 62% between 2021 and 2023, largely driven by a malaria outbreak in Pakistan, which saw a rise of 3.7 million cases following catastrophic flooding that affected over 30 million people. Several countries experienced notable increases in malaria cases, with Afghanistan seeing a rise in estimated cases from 288,000 in 2022 to 424,000 in 2023. In the same year, P. vivax accounted for 35.2% of the cases in the region, primarily in Afghanistan and Pakistan. However, due to ongoing instability and significant security challenges in Sudan, as well as incomplete reporting in Yemen, comprehensive data collection remains a challenge. As a result, recent estimates of malaria burden in these countries should be interpreted with caution. To address this, WHO is supporting subnational burden estimation efforts in these nations to improve decision-making and guide malaria control strategies in regions with unstable conditions.
In addition to the regional trends, several global challenges have complicated efforts to control malaria. The World Malaria Report 2024 highlights the substantial risk posed by climate change, which can alter the behavior of malaria vectors and increase the areas at risk of transmission. Extreme weather events, such as floods68 and heatwaves,69 have been linked to increased malaria outbreaks, though the precise relationship between climate change and malaria transmission remains unclear. The COVID-19 pandemic has also significantly disrupted malaria control efforts,70,71 leading to delays in the distribution of mosquito nets,72 diagnostic tools,73,74 and antimalarial treatments.75 Many countries reported a decline in malaria-related services, exacerbating the disease burden in already high-risk areas.
Despite some notable progress in malaria control and the introduction of new interventions, including the RTS, S/AS01E malaria vaccine and the recommendation of the R21/Matrix-M vaccine, the global malaria burden remains high. The increase in malaria cases in 2024 compared to pre-pandemic levels underscores the ongoing need for comprehensive and sustained malaria control efforts. The emergence of drug resistance, climate change, and the lingering effects of the COVID-19 pandemic present significant challenges and addressing these issues will be crucial for meeting the global malaria elimination targets. Continued investment in research, surveillance, and the development of innovative tools and strategies is essential for reducing the global burden of malaria and ultimately achieving its eradication.
Plasmodium genomics
Ancestors of the Plasmodium parasite clade may have been free-living protozoa with chloroplasts that adapted to living in the intestines of aquatic invertebrates.76 The evolution of Plasmodium species involved a shift from an ancestor that performed photosynthesis to a complex parasite with a crucial apicoplast for host adaptation.77 DNA sequence comparisons suggest that the origins of Plasmodium parasites are closely linked to their hosts.78,79,80 This is supported by a comprehensive analysis of the mitochondrial and nuclear genomes of P. falciparum, P. vivax, and P. malariae from 16 countries spanning ~5500 years of human history.81 This section will explore the evolution of Plasmodium parasites, tracing their origins from free-living protozoa with chloroplasts to the complex parasites that depend on the apicoplast for host adaptation. It delves into the genomic characteristics of various Plasmodium species, highlighting differences in genome size, organization, and GC/AT content variations, as well as the extensive genome sequencing efforts listed in PlasmoDB. Comparative genomic analyses of different Plasmodium strains have been explored to reveal insights into genomic diversity, parasite evolution, and population genetics. Additionally, the section will review rodent malarial parasite models, such as P. chabaudi, P. yoelii, and P. berghei, emphasizing their conserved core genomes and the unique subtelomeric gene families that facilitate immune evasion. Finally, it highlights the advancements in single-cell biology techniques applied to Plasmodium research, showcasing significant findings from single-cell RNA sequencing studies that enhance researcher understanding of parasite development, transmission-blocking strategies, and host-parasite interactions.
In terms of DNA sequences, Plasmodium species have compact genomes of 18–30 megabases (Mb) packaged into 14 chromosomes,82 with multigene families commonly found near the telomeric ends of each chromosome, which are organized as heterochromatin in distinct clusters at the periphery of the nucleus.83 The P. falciparum 3D7 genome was the first malaria parasite genome to be fully sequenced and the sequencing results revealed that it has an exceptionally low GC content of under 20%.84 Moreover, the genomes of avian malaria parasites such as P. relictum and P. gallinaceum, which are similar to that of P. falciparum, have high AT contents.85,86 Polychromophilus parasites, which infect bats, have compact genomes with a small number of protein-coding and RNA genes, highlighting their unique evolutionary adaptations.87 By 2022, many Plasmodium genomes had been sequenced and deposited in the public database PlasmoDB (https://plasmodb.org/).
Comparative analyses of the genomic sequences from the field isolates of various Plasmodium species revealed features in genomic diversity, parasite evolution, population genetics, and drug resistance possibilities.54 For example, P. falciparum NF54, which was isolated from a patient in the Netherlands, was one of the first strains used in clinical trials for malaria vaccine study.88,89 Its genome size is ~23.40 Mb, with ~5273 protein-coding genes (PCGs), 229 noncoding RNA (ncRNA) genes, and 107 pseudogenes. The P. falciparum 3D7 strain, a parent clone of P. falciparum NF54, is the most widely used strain in laboratories worldwide.53 Its genome is ~23.33 Mb, comprising ~5318 PCGs, 244 ncRNA genes, and 158 pseudogenes. The P. falciparum HB3 strain is a well-characterized Honduran chloroquine-sensitive strain.90,91 Its genome is approximately 22.81 Mb, with ~5186 PCGs, 141 ncRNA genes, and 134 pseudogenes. The P. falciparum 7G8, a Brazilian isolate and genetically distinct from the West African parasite P. falciparum NF54,92 its genome is ~22.83 Mb, containing ~5183 PCGs, 161 ncRNA genes, and 161 pseudogenes. Collectively, the genomic sequences of these strains provide valuable insights into the diversity and evolution of P. falciparum, aiding in vaccine development and drug resistance studies.
Rodent malarial parasite species serve as valuable models for studying issues that are challenging to address with human-infecting species such as P. falciparum and P. vivax.93 Three commonly used laboratory species are P. chabaudi, P. yoelii, and P. berghei.93 Both human and animal malarial parasites share a highly conserved core genome.82 This includes essential genes for fundamental biological processes, such as replication, transcription, and basic metabolic pathways.94,95 In addition, both human and animal Plasmodium species have chromosomal subtelomeric regions that contain large gene families involved in host‒pathogen interactions and antigenic variation. These regions are prone to a high rate of recombination, aiding in gene diversity and immune evasion. For example, the P. vivax (human) and P. yoelii (rodent) genomes both feature variable gene families in subtelomeric regions. However, P. falciparum has a unique gene family, the var gene family, encoding P. falciparum erythrocyte membrane protein 1 (PfEMP1) proteins involved in cell adhesion and pathogenesis, which are absent in rodent and other primate malarial parasites. Similarly, rodent malarial parasites have their own unique gene families, such as the CIR/BIR/YIR families, which are absent in human malarial parasites.94,96
Research on the Plasmodium genome has entered an exciting era with the development and application of single-cell biology (Table 1). In 1998, single-cell reverse transcription PCR was first applied to amplify var transcripts encoding PfEMP1 with degenerate primers (Fig. 2a), leading to the discovery of multiple transcription events of var genes in a single P. falciparum parasite.97 In 2019, Howick et al. utilized single-cell RNA sequencing (scRNA-seq) and identified 20 transcriptional modules among 5,156 key genes, revealing a high-resolution transcriptional atlas during the life cycle of P. berghei. The application of this atlas led to the possibility of defining all Plasmodium developmental stages on the basis of stage-specific transcription markers (Fig. 2b).98 In the ookinete stage, Witmer et al. utilized scRNA-seq to profile transcriptional variation in P. berghei ookinetes across different vector species and within individual midguts.99 The findings revealed significant clonal variation, which is crucial for understanding how ookinetes adapt to different environmental cues and how this adaptation impacts transmission-blocking strategies. Additionally, an scRNA-seq analysis revealed that hepatocyte zonation affects the development of the rodent malaria parasite P. berghei ANKA in the liver stage, with parasites developing more rapidly in pericentral lobule zones; moreover, this study revealed a subpopulation of periportally biased hepatocytes with abortive infections that promote immune cell recruitment.100
Clinical features of malaria
Malaria presents with a wide spectrum of clinical manifestations, ranging from uncomplicated forms to severe, life-threatening complications.101 The clinical features of malaria are primarily influenced by the species of Plasmodium responsible for the infection, and timing of the diagnosis and treatment.61 This section will provide an in-depth analysis of the clinical manifestations primarily associated with P. falciparum infection, which mainly focuses on severe symptoms. It begins by outlining the range of symptoms, highlighting the complex pathologies that necessitate comprehensive management strategies. The section will then focus on CM, detailing its definition. Additionally, the section will explore pregnancy-associated malaria (PAM), emphasizing the unique mechanisms of placental sequestration and its detrimental effects on both maternal and fetal health. Pulmonary complications such as pulmonary edema and acute respiratory distress syndrome (ARDS) are also examined, with an emphasis on their pathophysiological mechanisms, differences in prevalence and presentation between adults and children, and the underlying immune responses. Finally, the multifaceted clinical features of severe malaria are summarized, integrating both direct parasite-induced effects and indirect immune-mediated processes, and potential therapeutic interventions aimed at mitigating these severe outcomes are reviewed.
In its uncomplicated form, malaria typically begins with a combination of nonspecific symptoms such as fever, chills, sweats, headaches, nausea, vomiting, muscle aches, and general malaise.102 These symptoms can often be mistaken for common viral infections like influenza, especially in regions where malaria is rare. However, in malaria-endemic areas, these symptoms are frequently recognized as indicative of malaria, leading to self-treatment or presumptive diagnosis. On physical examination, signs such as elevated temperature, sweating, weakness, splenomegaly, mild jaundice, hepatomegaly, and an increased respiratory rate may be observed. Diagnosis of uncomplicated malaria is confirmed through the identification of Plasmodium parasites in blood samples, typically using microscopy. Laboratory findings often include mild anemia, thrombocytopenia (low platelet count), elevated bilirubin, and elevated liver enzymes (aminotransferases).102,103 In clinics with the availability of the rapid diagnostic test, malaria can be determined.104
Severe malaria occurs when the infection leads to serious complications, often involving organ failure or abnormalities in the blood or metabolism.105 This progression typically follows delayed diagnosis or inadequate treatment. Criteria for severe malaria can vary, but in the US commonly include high parasitemia (≥5%), impaired consciousness, seizures, circulatory collapse or shock, acute respiratory distress syndrome (ARDS), acidosis, acute kidney injury, disseminated intravascular coagulation (DIC), jaundice (accompanied by at least one other sign), severe anemia (hemoglobin <7 g/dL).102
In P. vivax and P. ovale infections, patients who have recovered from an initial episode may experience relapses months or even years later.106 These relapses are caused by the dormant liver-stage parasites, known as hypnozoites, which can reactivate and initiate new cycles of infection.106
Malaria can lead to a variety of other complications. Neurological deficits,107,108 such as ataxia, palsies, speech difficulties, hearing loss, cognitive impairments, and blindness, may persist after cerebral malaria, particularly in children. Recurrent infections with P. falciparum may result in severe anemia,109 especially in young children in tropical regions. Pregnancy-related malaria,110 particularly caused by P. falciparum, can lead to severe disease in the mother, premature delivery, or low birth weight infants. Rare complications include splenic rupture in P. vivax infections and nephrotic syndrome due to chronic P. malariae infections.111
Periodic fever, a hallmark of falciparum and vivax malaria, is linked to erythrocyte rupture accompanied by the release of hemozoin after each erythrocytic cycle and the host’s inflammatory response.112 The periodicity of fever is determined by the replication cycle of the parasite within RBC.112,113,114 In P. falciparum115 and P. vivax,116 the fever cycle is typically 48 h (known as the tertian cycle), while in P. malariae, the fever cycle extends to 72 h (fever regularly occurs again on the fourth day in many patients, quartan cycle).117 The synchronized rupture of RBCs at these intervals leads to the periodic nature of fever, which typically follows a “chill-fever-sweat” pattern (An attack usually starts with shivering and chills, followed by a high fever, sweating, and a return to normal temperature). In addition to the characteristic periodic fever, anemia in malaria is primarily due to the destruction of both iRBCs and uninfected RBCs.118 Thrombocytopenia, often observed in individuals with malaria, results from both the direct destruction of platelets and splenic sequestration.119 Renal impairment, including acute kidney injury (AKI), can occur due to systemic inflammation and direct effects of the parasitic infection on the kidneys.120 These complex pathologies underscore the need for comprehensive management strategies in severe malaria patients to address the multifaceted pathological effects.
CM is the most severe form of P. falciparum infection and mostly occurs in children under 5 years of age in malaria-endemic areas.108 It is defined as a microscopically confirmed P. falciparum infection and a Blantyre coma score ≤2, with no other known cause of coma.121 The sequestration of iRBCs in brain capillaries and postcapillary venules is the cause of cerebral hypoxia and coma.122 Additionally, the defining factor of CM is the formation of rosettes by P. falciparum erythrocyte membrane protein 1 (PfEMP1) binding to uninfected erythrocytes.123 A study showed that P. falciparum isolates from children with CM consistently form erythrocyte rosettes and lack anti-rosette antibodies, whereas isolates from children with mild malaria exhibit reduced or no rosettes and are disrupted by anti-rosette antibodies, thereby supporting the role of erythrocyte rosetting in the pathogenesis of CM and the protective effect of anti-rosette antibodies.124 Another study showed that specific PfEMP1-duffy binding-like domain 1 (DBL1α) motifs are correlated with rosetting and severe malaria, suggesting that P. falciparum strains with particular PfEMP1 sequences cause severe malaria.125 The pathophysiological processes underlying CM involve substantial microvascular changes, including ring hemorrhages, microthrombi, and fibrin deposits, predominantly in the white matter and border zones between the major cerebral arteries (Fig. 3a, b).126 These structural changes have been found to be caused by coagulation defects in both murine experimental cerebral malaria (ECM) and human CM.127 Brain swelling, associated with cerebral vasculature sequestration, is a leading cause of death in CM.128 Among 348 children admitted with CM (as defined by the WHO), 168 met the inclusion criteria and were included in a correlation analysis. Of these, 25 children (15%) died, 21 of whom (84%) had severe brain swelling on magnetic resonance imaging (MRI) at admission, whereas only 27% (39 of 143) of the survivors had similar swelling.129 Serial MRI scans of survivors initially presenting with brain swelling revealed a decrease in brain volume postinfection.129 The mechanisms proposed for this swelling include cytotoxic edema caused by cellular injury and swelling and vasogenic edema resulting from disruption of the blood‒brain barrier (BBB) and leakage of plasma into the brain.130,131 High-resolution MRI studies suggest that vasogenic edema is a predominant feature of CM that can be rapidly alleviated with treatment.132,133 These findings align with the characteristics of posterior reversible encephalopathy syndrome,134 highlighting potential endothelial dysfunction and impaired autoregulation in CM.
Pathophysiology of CM. a Subcortical petechial hemorrhages and microthrombi formation in the brain that occur during CM often result in ring hemorrhages and microvascular damage. b The interaction between iRBCs and endothelial cells in the cerebral vasculature. This figure is created with BioRender.com
Pregnancy-associated malaria (PAM), also known as placental malaria, is caused by P. falciparum parasites that express a specific PfEMP1 variant (VAR2CSA) only in pregnant women, enabling placental sequestration by the parasites through binding to the placental ligand chondroitin sulfate A (CSA).135,136,137 This sequestration leads to damage to the placenta, as well as adverse effects on both the fetus and the mother. PAM is a significant public health concern, particularly in malaria-endemic regions.138 Adult residents of malaria-endemic regions typically develop immunity to malaria through repeated exposures to malaria parasites. However, malaria poses a unique and heightened risk to pregnant women, especially to those experiencing the first pregnency.139 The majority of malaria infections during pregnancy remain asymptomatic or paucisymptomatic yet are a major cause of severe maternal anemia and preventable adverse outcomes for both mothers and infants, especially in the first and second pregnancies.139 Despite the implementation of preventive measures such as intermittent preventive treatment with sulfadoxine-pyrimethamine (SP), many pregnant women are unaware of these preventative treatments, and patient adherence to these interventions can be poor. Studies have shown that even with high attendance at antenatal care clinics, the prevalence of asymptomatic P. falciparum infections among pregnant women remains high, contributing to maternal anemia and low birth weight in newborns.140,141 The structural basis for the interaction between Var2CSA and CSA has been elucidated through advanced techniques such as cryo-electron microscopy, revealing that Var2CSA has a unique architecture that facilitates its binding to CSA.142,143 Specifically, Var2CSA interacts with CSA by binding within two distinct channels that traverse the core domain. Importantly, binding to CSA does not induce significant conformational changes in the Var2CSA protein, maintaining its structural integrity during the adhesion process. Furthermore, the phosphorylation of Var2CSA has been identified as a critical factor that enhances its adhesive properties to CSA, indicating that posttranslational modifications can influence the virulence of the parasite.144
Pulmonary complications in P. falciparum malaria patients primarily manifest as pulmonary edema and acute respiratory distress syndrome (ARDS).145 Pneumonia, often caused by bacterial or viral infections, is also common in malaria patients. However, few clinical or histopathological studies have focused specifically on lung complications. ARDS is characterized by diffuse lung inflammation, alveolar damage (Fig. 4), as evidenced by poor oxygenation and radiological images of diffuse lung involvement.146 It is well recognized in adults with severe malaria, although its incidence varies widely.147 ARDS often occurs late in the disease course, even after antimalarial treatment has begun.148 Ultrastructural analysis of the lungs of Asian adults with severe malaria and ARDS revealed classic features, such as hyaline membranes and neutrophil and monocyte infiltration, accompanied by significant fibrin formation (Fig. 4).149 Furthermore, postmortem studies in Vietnamese adults with fatal severe malaria revealed marked loss of EPCR and thrombomodulin in the lungs, similar to findings in children with CM, indicating a shared pathophysiological mechanism150 Pulmonary edema is typically linked to fluid overload from excessive intravenous fluids, heart failure, or renal failure and may be exacerbated by increased vascular permeability (Fig. 4).151 ARDS and pulmonary edema occur less frequently in children than in adults.152 Data from the Fluid Expansion as Supportive Therapy (FEAST) study indicate that fluid administration in children can increase mortality, with post hoc analysis suggesting respiratory deterioration as a key mechanism.153 This implies that children with CM may have an increased, although lower than that of adults, risk of capillary leakage in the lungs. Other studies supported this result. In children, respiratory distress is often associated with acidosis rather than hypoxia, but ARDS154 and pulmonary edema155 are rare, indicating compensatory hyperventilation rather than lung pathology. The absence of hyaline membranes or alveolar damage in pediatric autopsy studies suggests that lung pathology in children may be subclinical and detectable only postmortem, indicating greater lung vulnerability in adults than in children.156
Comparison of healthy and injured alveoli in malaria-induced acute lung injury. The healthy alveoli show intact epithelial and endothelial barriers, clear alveolar air spaces, and functional type I and II cells. In contrast, the injured alveoli display sloughing of the bronchial epithelium, protein-rich edema fluid, and necrotic type I cells. This figure was created with BioRender.com
Overall, the pathophysiology of malaria is multifaceted and involves both direct and indirect mechanisms. The direct effects of P. falciparum include the sequestration of iRBCs in the pulmonary microvasculature, leading to microvascular obstruction, endothelial activation, and subsequent inflammatory responses.157,158 This sequestration is also mediated by the interaction of parasite-derived proteins such as PfEMP1 with endothelial receptors such as ICAM-1 and EPCR, resulting in endothelial cell activation and disruption of the endothelial barrier.125 Importantly, depolymerized glycosaminoglycans (dGAGs) lacking anticoagulant activity have been identified as promising candidates for adjunct therapy in severe malaria.159 These dGAGs effectively disrupt rosette formation, inhibit merozoite invasion and endothelial binding, and reduce sequestration of P. falciparum-infected erythrocytes in the nonhuman primate Macaca fascicularis.159 The indirect effects involve systemic inflammatory responses, where cytokines such as TNF-α and IFN-γ may play critical roles in exacerbating endothelial permeability and promoting leukocyte recruitment to the lungs.160 Neutrophils, monocytes, and other immune cells are recruited to the lungs, where they release inflammatory mediators and proteolytic enzymes that contribute to tissue damage. The formation of neutrophil extracellular traps (NETs) and the release of reactive oxygen species further damage the alveolar‒capillary barrier, promoting edema and impairing gas exchange.161 Thus, understanding these mechanisms is crucial for developing targeted interventions to mitigate lung damage and improve outcomes in severe malaria patients.
Crosstalks between Plasmodium and host red blood cells
Regulatory mechanisms governing Plasmodium development
The complex life cycle of Plasmodium species involves repeated transmission between mosquitoes and vertebrate hosts (Fig. 5).162 This section outlines the complex lifecycle of the parasites, from gametocyte formation in the human host to sporozoite development in mosquitoes and subsequent infection of hepatocytes in mammalian hosts. It emphasizes the critical roles of various transcription factors in regulating stages of gametocytogenesis, ookinete formation, and sporozoite development. Additionally, this section explores host invasion mechanisms, detailing the multistep process of erythrocyte invasion by merozoites, the involvement of merozoite surface proteins, erythrocyte binding antigens, and the formation of tight junctions mediated by the apical membrane antigen 1 (AMA1) -RON complex.
Gametocytes are the first life forms of the sexual phase in the Plasmodium parasite life cycle,163 which are critical for parasite dissemination (Fig. 5). The transcription factor (TF), P. falciparum apetala2 gametocyte (PfAP2-G), is involved in the regulation of gametocytogenesis and sexual commitment in P. falciparum,164 which orchestrates the gametocyte stage.165 Moreover, conditional expression of PfAP2-G enables the characterization of the early sexual stages of the parasite, including sexually committed schizonts and sexual rings, and reveals key changes, such as the downregulation of genes involved in solute transport upon sexual commitment.166 Additionally, PfAP2-G2 significantly modulates the production and maturation of gametocytes by regulating the expression of P. falciparum male development gene 1 (MDV-1).167 Furthermore, P. falciparum apetala2 ookinetes 3 (PfAP2-O3) acts as a repressor in female gametocytes, ensuring sex-specific gene expression.168 AP2-O3-deficient parasites produce apparently normal female gametocytes, but these gametocytes fail to differentiate, leading to developmental arrest after fertilization.168 In P. berghei, a rodent malarial parasite, apetala2-female specific (AP2-FG) has been reported as a TF for female-specific gene regulation, emphasizing its role in the development of female gametocytes.169 In addition to the AP2 transcription factor, histone variants and histone modifications also play roles in sex specification in parasites.170,171 Specifically, in female gametocytes, the P. falciparum histone variants PfH2A.Z/H2B. Z are highly enriched in histone H3 lysine 9 trimethylation (H3K9me3)-associated heterochromatin.172
These mature gametocytes are taken up by mosquitoes during a blood meal, leading to the mosquito stage of the parasite’s life cycle. Gametogony and sporogeny are the most important stages of Plasmodium development in mosquitoes (Fig. 5). In this stage, PbAP2-FG2 and AP2R-2 form a transcriptional repressor complex essential for female gametocyte development, with disruptions in the formation of this complex leading to developmental arrest during ookinete formation.173 AP2-O is a TF that is expressed in several ookinete stages, from retort ookinetes to mature ookinetes, and activates the majority of known ookinete genes.174,175 Thus, disruption of AP2-O results in the impaired development of ookinetes. Moreover, P. falciparum apetala2 zygote (AP2-Z) is a novel TF crucial for ookinete development, with AP2-Z-mediated transcription in zygotes essential for ookinete formation; additionally, the targets of AP2-Z overlap with those of AP2-O.176
The development of ookinetes and oocysts in mosquitoes leads to the production of sporozoites, which are the parasite form that infects mammalian hosts.177 Four TFs, including AP2-sporozoite (Sp),178,179 AP2-Sp2,178 and AP2-Sp3,178 which are asparagine-rich proteins (SLARPs),180 have been reported to play important roles in gene regulation during this stage. AP2-Sp maintains its own expression via a transcriptional autoactivation mechanism (positive-feedback loop) and activates other transcription factors, including AP2-Sp2, AP2-Sp3, and SLARP, at this stage.181
Upon entering the mammalian host, sporozoites infect hepatocytes, eventually leading to the release of thousands of merozoites that invade RBCs. The asexual replication cycle of P. falciparum in erythrocytes is characterized by sequential transformations from rings (0–10 h) to trophozoites (10–40 h) and schizonts (40–48 h)182 (Fig. 5). As the parasite develops inside RBCs, it alters host erythrocyte biomechanical properties, notably reducing iRBC deformability.183 Our previous findings indicate that P. falciparum remodels the erythrocyte cytoskeleton through P. falciparum phosphoinositide 3-kinase (PfPI3K)-regulated ubiquitination and degradation of α-spectrin, a process that facilitates egress of mature parasites from iRBCs.184 In addition, many proteins and posttranslational modifications have been shown to be involved in regulating the asexual replication cycle.185 For example, Pf DNA/RNA-binding protein (ALBA1) can bind to four mRNA transcripts encoding erythrocyte invasion-associated proteins, including rhoptry-associated protein 1 (Rap1), rhoptry neck protein 3 (RhopH3), calcium-dependent protein kinase 1 (CDPK1), and apical membrane antigen 1 (AMA1), which are important regulators of the translational timing and asexual proliferation of P. falciparum.186 Pfactin1187 and Pfformin-2188 are actin-related proteins that are essential for proper and efficient segmentation in iRBCs and involve the structural organization necessary for cell division. PfCyc1, a cyclin H homolog, along with its potential partners PfMAT1 and MO15-related protein kinase PfMRK, are critical for merozoite formation and development.189 Parasites lacking PfCyc1 can still form nuclei and apical organelles but fail to produce merozoites.189,190 In addition, the PfAP2-invasion (PfAP2-I) factor, which belongs to the Apicomplexan AP2 family, is responsible for regulating the expression of genes involved in RBC invasion.191 Furthermore, PfAP2-EXP2 regulates genes associated with parasite virulence and host‒parasite interactions.192 A recent study revealed that the expression of the essential TF PfAP2- pathogenesis (P), which critically regulates the parasite transition from trophozoites to schizonts, peaks during two phases of the blood-stage development of P. falciparum. The underlying mechanism involves PfAP2-P binding to the promoters of genes controlling trophozoite development and host cell remodeling.193 Additionally, the inhibition of N-myristoyl transferase (NMT) in P. falciparum disrupts parasite development and growth at multiple stages, including schizogony, rhoptry formation, merozoite egress, and erythrocyte invasion, highlighting NMT as a critical drug target due to the pleiotropic effects of its inhibition.194 Moreover, the critical role of IMC1g proteins in the Plasmodium parasite life cycle, specifically PbIMC1g in P. berghei195 and its functional counterpart, PfIMC1g,196 in P. falciparum has been recognized. PbIMC1g is involved in asexual replication, gametogenesis, ookinete motility, and mosquito midgut invasion, confirming its role in maintaining structural integrity and facilitating parasite motility during invasion. In P. falciparum, PfIMC1g is essential for the asexual replication stage, as its deficiency leads to parasite death shortly after red blood cell invasion. The evolutionary conservation of IMC1g proteins across Plasmodium species also indicates that these proteins could be key targets for therapeutic interventions. Overall, understanding these regulatory mechanisms across life cycle stages is crucial for developing effective malaria control and treatment strategies.
Host cell invasion mechanisms of malarial parasites
Once released from schizonts, merozoites may take several seconds or minutes before establishing contact with the surface of an RBC and commencing invasion. Plasmodium merozoites, previously thought not to exhibit gliding motility, can indeed undergo this movement in vitro, a crucial step for successful invasion.197 After primary attachment of the merozoite to the RBC surface, invasion occurs within ~30 s.198,199 The invasion of RBCs by P. falciparum merozoites is a complex, multistep process involving numerous parasite proteins and host RBC surface receptors. This invasion process can be achieved through two distinct pathways: (1) the sialic acid (SA)-dependent pathway, where proteins such as erythrocyte binding antigen 175 (EBA-175), erythrocyte binding ligand 1 (EBL-1) bind to glycophorin A and EBA-140 bind to glycophorin C200,201 on the RBC surface; and (2) the SA-independent pathway, where proteins such as P. falciparum reticulocyte binding protein homolog 5 (RH5) and PfRh4 interact with receptors such as complement receptor 1 (CR1), basigin (also known as CD147), and glycophorin A (GYPA), enabling invasion without requiring SA.
The initial attachment to the RBC surface is mediated by merozoite surface proteins (MSPs), such as MSP1,202 MSP2, MSP6,203 and MSP9 (orthologous to p101/ABRA of P. falciparum),204 along with other glycosyl phosphatidylinositol (GPI)-anchored MSPs (Figs. 6 and 7). MSP1 is the most abundant merozoite surface protein anchored on the merozoite surface (Fig. 6).205 It essentially mediates erythrocyte invasion via interactions with host glycophorin A206 and heparin-like molecules.207 Recently, a study revealed that t a highly basic region within the central cavity of MSP1 may promote weak adhesion to erythrocytes via long-range electrostatic interactions, specifically targeting negatively charged heparin-like polysaccharides abundant on the erythrocyte surface.208 The posttranslational modification and processing of MSP1 by the parasite protease SUB1, which is released from dense granules, are necessary steps in merozoite maturation.185,209 Initially, expressed as a high-molecular-weight protein (∼200 kDa), MSP1 undergoes primary proteolytic processing, resulting in four fragments (83 kDa, 30 kDa, 38 kDa, and a C-terminal 42 kDa segment) that form a noncovalent complex on the merozoite surface.210 This complex mediates the initial attachment of the merozoite to RBCs through interactions with heparin-like proteoglycans or Band 3 proteins, facilitating successful invasion.211 Following egress from the host cell, MSP1 is further cleaved at a juxtamembrane site by P. falciparum subtilisin-like protease 2 (Pf SUB2), leading to shedding of the majority of the MSP1 complex, with only a 19 kDa C-terminal region (MSP119) attached to the merozoite surface.212 The precise timing and spatial regulation of these processing events are governed by the discharge of subtilisin-like protease 1 (SUB1),209 which is activated by plasmepsin X through the cleavage of SUB1 inhibitory segments.213 Recently, a study shown that membrane-bound protease SUB2 is essential for the shedding of surface proteins during Plasmodium merozoite invasion into RBCs. Genetic depletion of SUB2 disrupts this process, leading to abortive invasion or developmental arrest.214 Heparin and heparan sulfate (HS), members of the glycosaminoglycan (GAG) family and consist of repeating disaccharide units of β-glucuronic acid (GlcA) and α-N-acetylglucosamine (GlcNAc),207 can interact with MSP133. They inhibit P. falciparum growth and merozoite invasion by interacting with a variety of merozoite-derived proteins, and the use of structurally defined modified K5 polysaccharides enables the investigation of the specific structural requirements of antimalarial drugs to exert a robust therapeutic effect.207,215 Furthermore, heparin-like GAGs, such as heparan sulfate, are receptors in parasite rosettes,216 and rosettes may assist newly egressed merozoites in invading surrounding RBCs.217 Our laboratory used two-dimensional liquid chromatography‒mass spectrometry to identify 811 schizont-derived proteins that bind strongly to heparin, and those exhibited most affinity to heparin are merozoite-derived proteins.215 Heparin-like GAGs are likely common receptors for Plasmodium parasites, as numerous merozoite proteins from P. berghei have also been found to interact with these GAGs218 Therefore, heparin can be developed as an antimalarial drug or as a carrier for the targeted delivery of other antimalarial agents.219 Additionally, although its receptor‒ligand interaction199 remains further exploration, another MSP member, MSP2, is essential for invasion and is characterized by its dimorphic nature and propensity to form fibrils.220
Merozoite proteins involved in erythrocyte invasion of P. falciparum parasites. The various protein groups associated with different organelles in the Plasmodium parasite were shown. It categorizes key proteins into distinct groups, including rhoptry proteins, dense granule proteins, surface proteins, GPI-anchored surface proteins, peripheral proteins, and microneme proteins. Each protein group is color-coded for clarity and shown in association with the relevant organelle or cellular structure. This figure was created with BioRender.com
Mechanisms of Plasmodium invasion in erythrocytes. In the upper panel, the sequence of invasion begins with the attachment of the merozoite to the erythrocyte surface, followed by the discharge of the microneme and rhoptry contents, leading to apical reorientation, tight junction formation and erythrocyte membrane invagination, and the eventual entry of the merozoite into the erythrocyte. The lower panel highlights the key protein interactions during this process, revealing two distinct invasion pathways. HBP heparin-binding proteins, HS heparan sulfate, SA sialic acid, SA sialic acid, GYPA glycophorin A, GYPA glycophorin C, CR1 complement receptor 1, MSPs merozoite surface proteins, EBA erythrocyte binding antigen, EBL erythrocyte binding ligand, Rh reticulocyte binding protein homolog, AMA1 apical membrane antigen 1. This figure was created with BioRender.com
Following initial attachment, the merozoite reorients itself so that its apical end faces the erythrocyte membrane. This reorientation is crucial for successful invasion and is mediated by microneme proteins such as erythrocyte binding antigen 175 (EBA175).221,222 Moreover, Plasmodium erythrocyte binding antigen (EBA) families are generally thought to play a role in the later stages of invasion, but some members may be presented on the merozoite surface in a regulated manner after the initial merozoite–erythrocyte contact has occurred (Figs. 6 and 7).223 Low potassium ion concentrations trigger an increase in cytosolic calcium levels in P. falciparum merozoites, leading to the sequential secretion of EBA-175.224 The crystal structure of the erythrocyte-binding domain of EBA-175 revealed its dimeric organization with critical glycan binding sites, highlighting the significant role of the F2 domain in cytoadherence (Fig. 7).225 Furthermore, EBA-175 protein shed from P. falciparum promoted the clustering of RBCs through a glycophorin A-dependent mechanism (Fig. 7), facilitating parasite growth by providing daughter merozoites with access to uninfected RBCs and protecting the invasion machinery from immune recognition.226 Recent studies have shown that P. falciparum utilizes CD44 as a coreceptor during erythrocyte invasion, with EBA-175 and EBA-140 binding to CD44 and inducing CD44-dependent phosphorylation of host cytoskeletal proteins, which enhances parasite entry by altering erythrocyte deformability.227 However, different malaria parasite clones utilize distinct invasion pathways, including the utilization of a glycophorin B-dependent, sialic acid-dependent pathway that operates independently by EBA-175.228 P. falciparum also employs multiple polymorphic ligands, including JESEBL/EBA-181 and EBA-140, to recognize various receptors on the erythrocyte surface, demonstrating a high level of invasion adaptability that contrasts with the single-pathway invasion strategy of P. vivax and contributes to its success in endemic regions.229,230 EBA-140 specifically binds to glycophorin C through its binding region (Region II) (Fig. 7), highlighting the role of specific glycophorin C regions and glycans in this interaction.230 The inactivation (pseudogenization) of the EBA165 gene in P. falciparum, which originally encoded an erythrocyte invasion protein specific to ape erythrocytes, was a key evolutionary step that allowed the parasite to adapt to human hosts by avoiding incompatibility with human erythrocytes.231 Moreover, PfRH2a/2b proteins are critical for P. falciparum erythrocyte invasion through distinct sialic acid-dependent and independent pathways, with their conserved N-terminal receptor-binding domain being a promising target for malaria vaccine development.232 Other studies investigated the prevalence of a 0.58 kbp deletion in the PfRh2b gene in P. falciparum populations, which is linked to immune evasion. The deletion is widespread across various transmission areas in Ghana and globally, with a significant frequency in hyper-endemic regions, and its presence correlates with lower immune recognition, as shown by antibody levels similar to those against PfRh5.233
Following reorientation, tight junctions are formed through high-affinity interactions between apical membrane antigen 1 (AMA1) and the rhoptry neck protein complex, thereby linking the merozoite surface with the erythrocyte membrane (Fig. 7).234 The AMA1-RON complex is also crucial for the invasion of Plasmodium sporozoites into mosquito salivary glands and mammalian host hepatocytes, with its absence leading to impaired colonization and altered entry junction morphology.235 Research on the related parasite T. gondii suggests that RON2 integrates into the host membrane, where it acts as the receptor for AMA1, a mechanism used by all apicomplexan parasites to facilitate invasion through their own ligand‒receptor interactions.236 Interestingly, blocking the AMA1-RON2 interaction inhibited tight junction formation but still resulted in erythrocyte echinocytosis, suggesting that tight junction formation follows the engagement of reticulocyte binding protein homolog 5 and the signaling events triggered by rhoptry release.237
With tight junctions established, the merozoite invades the erythrocyte through a process involving rhoptry proteins and the formation of a parasitophorous vacuole (PV). Rhoptry neck proteins such as (reticulocyte binding protein) Rh1, Rh2b, and rhoptry neck protein 2 (RON2), as well as rhoptry associated protein 1 (RAP1) and RhopH3, contribute to the establishment of the parasitophorous vacuole and subsequent modification of the host cell for parasite development.238
Pathogenetic mechanisms
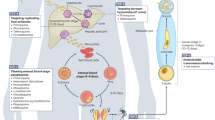
The pathogenesis of malaria, particularly P. falciparum infection, involves intricate molecular mechanisms that lead to severe clinical outcomes. This section highlights the role of cytokines like TNF-α and IFN-γ in activating endothelial cells, leading to the sequestration of infected red blood cells (iRBCs) via the PfEMP1 protein, a key factor in CM. It concludes how the PfEMP1 family enables the parasite to evade the immune system through antigenic variation, allowing it to adhere to host receptors such as CD36, ICAM-1, PECAM-1, and EPCR, which are associated with severe malaria. The section also covers the immune response, noting the roles of innate immune cells like macrophages and dendritic cells in producing inflammatory cytokines, and adaptive immune components such as CD4⁺ T cells, CD8⁺ T cells, and antibodies. It further describes the challenges of antigenic variation and the difficulty in achieving long-term immunity and vaccine development.
The primary processes of sequestration of P. falciparum-infected erythrocytes in the microvasculature involve the activation of endothelial cells mediated by various cytokines and the adherence of iRBCs to multiple host receptors via PfEMP1 (Fig. 8a). Tumor necrosis factor-alpha (TNF-α)239,240,241 and interferon-gamma (IFN-γ)242,243,244 play critical roles in endothelial activation by upregulating the expression of endothelial adhesion molecules, thereby facilitating the sequestration of iRBCs (Fig. 8a). Additionally, the release of cytokines by immune effector cells contributes to the procoagulant state of the brain observed in patients with CM.245 A recent study revealed that CD8+ T cells adhere to the endothelium and that their interaction with perivascular macrophages leads to the release of cytotoxic cytokines, further damaging the BBB and contributing to brain edema.246 Mechanistically, the NH2-terminal head structure containing the duffy binding-like domain 1 (DBL1α), cysteine-rich interdomain region (CIDR1α) and DBL2δ of PfEMP1 mediates iRBC adherence to multiple host receptors (Fig. 8a),247 including cluster of differentiation 36 (CD36), intercellular adhesion molecule 1 (ICAM-1), platelet endothelial cell adhesion molecule-1 (PECAM-1), and endothelial cell protein c receptor (EPCR), which are closely associated with the occurrence of CM.3,245,248 This is discussed in more detail in the following paragraph. The binding of iRBCs to these receptors triggers a cascade of inflammatory responses and endothelial activation, contributing to the pathophysiological changes observed in CM.249,250
Endothelial cell activation and P. falciparum erythrocyte membrane protein 1 (PfEMP1)-mediated sequestration of iRBCs. a Mechanistic overview of the process by which activated endothelial cells express receptors that mediate the rolling and eventual sequestration of iRBCs. b PfEMP1 variants interact with distinct endothelial cell receptors. The PfEMP1 variants have been reviewed by Mats Wahlgren.509
The sequestration of P. falciparum-infected iRBCs in the microvasculature has been recognized as the main cause of organ failure in patients with severe malaria.251 As previously discussed, PfEMP1, encoded by the ~60 var gene family, is the principal molecule implicated in CM and has been extensively characterized in the context of malaria pathogenesis.252 After synthesis, PfEMP1 is exported to the surface of infected red blood cells, where it forms knob structures that facilitate iRBC attachment.253 Although multiple distinct var gene transcripts can be detected simultaneously in bulk cultures and in individual infected erythrocytes, only one var transcript is virtually expressed and translocated on the surface of an iRBC. Moreover, frequent expression switching of these transcripts, which is mutually exclusive,254 results in almost unlimited strategies for the parasite to escape immune recognition and clearance.255
On the basis of sequence homology in the upstream regions, the var genes can be categorized into five subgroups: UpsA, UpsB, UpsC, UpsD, and UpsE.256 These subgroups are distributed across different locations on P. falciparum chromosomes. The UpsA subgroup var genes are located in the subtelomeric regions of the chromosomes; UpsB subgroup genes can be found in either telomeric or central regions; and UpsC subgroup genes are located primarily in the central regions of the chromosomes.257 Severe malaria is frequently associated with the expression of A or B subgroup var genes,258 whereas mild or asymptomatic malaria is linked to the expression of C subgroup var genes.259 In the protein structure (Fig. 8b), PfEMP1 contains multiple Duffy-binding-like (DBL) domains and a cysteine-rich interdomain region (CIDR) in its extracellular sequence, along with a shorter acidic terminal sequence in its cytoplasmic tail. CD36 is a receptor for most N-terminal DBL–CIDR domain cassettes across various PfEMP1 variants, a common feature of the majority of PfEMP1 variants (types B and C).260,261 Another receptor common to the PfEMP1 A and B types is ICAM-1.250,262 Antibodies against the PfEMP1 NTS-DBL1α domain can inhibit rosette formation and cytoadherence of iRBCs.263 Moreover, antibodies against the PfEMP1 head structure DBL-CIDR domain are more indicative of malaria exposure than are those against the DBL-α tag,264 offering insights into exposure and immunity dynamics. Moreover, the binding of PfEMP1 to nonimmune IgM and α2-macroglobulin (α2M) on the surface of immune cells hinders immune recognition of iRBCs, manipulates host responses, and aids in immune evasion.265 Additionally, experimental vaccines using virus-like particles (VLPs) conjugated to PfEMP1 domains have shown promise in inducing inhibitory antibodies, offering a potential pathway for developing effective malaria vaccines.266 Recently, the breadth of antibody responses to P. falciparum variant surface antigens on iRBCs, not to specific PfEMP1 antigens, has also been implicated as a predictive factor for protection against malaria in controlled human malaria infection.267
Host immune responses to malaria
The pathogenesis of malaria is closely linked to the host immune response, which affects the severity and outcome of the infection. The immune response to malaria is complex and involves both innate and adaptive responses. Initially, the innate immune system mounts a nonspecific defense,268 primarily through macrophages and dendritic cells, which identify infected cells and produce inflammatory cytokines such as TNF-α and IL-6.269 These cytokines are critical for early parasite control but also contribute to clinical symptoms, such as fever and malaise.269,270 Following this, the adaptive immune response is activated, characterized by the production of malaria-specific antibodies targeting parasite proteins.271 CD8⁺ T cells have been reported to eliminate parasite-infected hepatocytes,272,273 whereas CD4⁺ T-cell-dependent antibodies prevent sporozoite invasion of hepatocytes.274 During intraerythrocytic development, CD4+ T helper cells and potentially γδ T cells exert antiparasitic effects (Fig. 9).275 However, our recent study revealed increased expression of host SOD3, which is bound to T cells and is negatively associated with host immunity to malaria.276 T cells also play a crucial role in supporting B-cell-mediated antibody production.277 However, the high variability of Plasmodium antigens and the parasite’s ability to suppress certain immune functions pose significant challenges for the development of an effective immune response in the host.278 Recently, the immune landscape established via scRNA-seq revealed that, during P. falciparum infection, the proportions of immunosuppressive monocytes, IL-10-producing Tr1 CD4 T cells and IL-10-producing regulatory B cells increased, and tolerogenic markers in natural killer (NK) and γδ T cells were upregulated.279
CD4+ T cells
CD4+ T helper (TH) cells, particularly TH1 cells, play an important role in immunity against malaria by producing IFN-γ, which activates macrophages.280,281 Both experimental and clinical studies have shown the importance of early IFN-γ production in controlling Plasmodium replication,282,283 although the precise protective mechanisms are still not fully understood. IFN-γ-producing TH1 cells are linked to resistance during liver-stage Plasmodium infection.284,285 In addition, IFN-γ-expressing CSP-specific TH1 cells reduce parasite burdens.286 However, CD4+ T-cell responses can also impair humoral immunity and expand self-reactive B cells.287 Within the first four days of infection, a dominant and phenotypically stable CXCR5+ TFH population emerges, resulting in a persistent CXCR5+ CCR7+ TFH/central memory T-cell response. Notably, CD4+ T-cell priming by B cells is both essential and sufficient for the establishment of this TFH-dominant response. TH2 cells, characterized by GATA3 and IL-4 production, play a limited role in malaria but are essential for robust CD8+ T-cell responses through IL-4-mediated CD4/CD8 cross-talk.288 CD8+ T-cell activity is significantly diminished without CD4+ T-cell support, highlighting their synergy in generating effector cells during immunization with radiation-attenuated sporozoites. Memory CD8+ T-cell populations are particularly dependent on CD4+ T-cell assistance to control liver-stage parasites.289
T follicular helper (TFH) cells, marked by BCL-6, CXCR5, and PD-1 expression, are critical for antibody production and the generation of long-lived plasma cells and memory B cells during Plasmodium infection.290,291 TFH and TH1 differentiation pathways diverge early in blood-stage infection, influenced by inflammatory monocytes and galectin-1.292 Despite this, IL-21 from IFN-γ+ TFH cells is crucial for resolving P. chabaudi infections by promoting specific IgG responses and immunity to reinfection.293
Regulatory T (Treg) cells, characterized by FOXP3 expression, modulate immune responses in malaria. In high-transmission areas, individuals show increased proportions of CD4+FOXP3+CD127lo/− Tregs with an effector memory phenotype that suppress malaria antigen-induced cytokine production, maintaining immune homeostasis.294 Acute infections with P. vivax and P. falciparum induce expanded Treg populations and altered dendritic cell ratios, correlating with parasite load but not clinical severity.295 Increased Treg numbers are also associated with lethal P. berghei and P. yoelii infections.296
CD8+ T cells
CD8+ T cells play a critical role in recognizing pathogen-derived peptides presented by MHC class I molecules on APCs or infected cells, contributing to the clearance of intracellular pathogens and the development of immune memory.277 Malaria-specific CD8+ T cells have been identified in endemic populations and vaccinated individuals,297,298,299,300,301 with the HLA-B*53:01 and HLA-C*06:02, that were associated with a higher prevalence of P. falciparum infection.302 Studies in rodent models further corroborate CD8+ T-cell-mediated protection, particularly after immunization with irradiated sporozoites.303 These cells target sporozoites, liver-stage, and blood-stage antigens of Plasmodium, though their role in primary malaria infection remains contentious due to limited hepatocyte infection and a narrow response window.304,305,306,307,308,309,310,311 Vaccines that elicit robust CD8+ T-cell responses, such as the PfSPZ vaccine, prevent malaria progression and establish long-lived tissue-resident T cells in the liver, underscoring their importance in durable immunity.308,312 Attenuated malaria sporozoite vaccines induce protective CD8+ T cells in primates, as demonstrated by the finding that CD8+ T-cell depletion via cM-T807 leads to malaria infection in previously protected monkeys, whereas those with intact CD8+ T cells remain protected.309 Although radiation-attenuated sporozoite (RAS) immunization can generate high proportions of CD8 + T cells, this may still not be sufficient for establishing sterile immunity, emphasizing the complex role of CD8+ T-cell responses in malaria vaccine efficacy.311
CD11c+ dendritic cells play a key role in priming CD8+ T cells against pre-erythrocytic parasites via cross-presentation of sporozoite antigens in skin-draining lymph nodes.311,313,314 Immunization with irradiated sporozoites induces robust protective CD8+ T-cell responses, with dendritic cells in cutaneous lymph nodes initiating these responses after mosquito bites. Once activated, CD8+ T cells migrate to systemic sites, such as the liver, in an S1P-dependent manner and subsequently recognize antigens on hepatocytes rather than relying on bone marrow-derived antigen-presenting cells.314 Another study revealed that sporozoites are directly taken up by lymph node-resident CD8α+ dendritic cells, which then form clusters with CD8+ T cells, facilitating antigen presentation and priming.315 However, genetically attenuated parasites that are arrested in the late liver stage elicit stronger CD8+ T-cell responses than those arrested earlier.316 Live attenuated vaccines generate robust CD8+ T-cell-mediated immunity, but the precise dynamics of CD8+ T-cell priming in natural infections or vaccination contexts remain an area of active investigation. Immunization with genetically attenuated P. berghei sporozoites lacking the microneme protein P36p provides extended protection lasting 12 to 18 months in mice, with efficacy maintained even with reduced dosages and alternative routes of administration.317 CD8+ T-cell responses may be primed not only in liver-draining lymph nodes but also in the spleen,318 with the generation and maintenance of these responses influenced by additional immune cells such as NK cells, helper T cells, and regulatory T cells, underscoring the need for a deeper understanding of these dynamics to develop strategies for robust and enduring immunity against malaria.318,319,320,321
CD8+ T cells may contribute to the pathogenesis of CM,322 a severe complication of malaria, by targeting infected reticulocytes and endothelial cells, leading to BBB disruption.323,324,325 H-2Kb and H-2Db class I molecules on brain endothelial cells uniquely influence disease progression, CD8 + T-cell activation, and BBB disruption; their ablation significantly mitigates ECM pathology and preserves BBB integrity.326 scRNA-seq revealed extensive infiltration and high activation of CD8+ T cells in the brainstem during ECM, with a subset of Ki-67+ CD8+ T cells exhibiting elevated levels of activation- and proliferation-related genes, suggesting antigen exposure by brain parenchyma cells; these CD8+ T cells were the sole source of IFN-γ, and their activity was modulated by astrocyte-mediated cross-presentation and upregulation of the immune checkpoint molecules PD-1 and PD-L1.327 Further research is needed to understand the full scope of the functions of CD8+ T cells and their potential in the development of effective malaria vaccines and treatments.
Memory CD8+ T-cell-mediated immunity against liver-stage Plasmodium infection involves IFN-γ and TNF-α as crucial noncytolytic factors, with perforin playing a species-specific role. While IFN-γ is essential for protection against both P. berghei and P. yoelii, perforin is critical only for P. yoelii, and TNF-alpha neutralization significantly impairs memory CD8+ T-cell-mediated protection across both parasite species.328 Consistent with the above findings, natural and recombinant human interferons, particularly Hu IFN-γ, effectively inhibited hepatic schizogony of P. falciparum at low concentrations, with postinoculation application showing significant inhibitory effects beyond parasitostasis, whereas Hu IFN-α, -β, and IL-1 also had inhibitory effects but at relatively high concentrations or when administered prior to inoculation.329 Compared with other tissues, effector memory CD8+ T cells rapidly infiltrate the liver within 6 h of malaria infection, mediating pathogen clearance through LFA-1 and liver phagocyte-dependent mechanisms, with a shorter recruitment time (within 6 h) compared to other cells.330 Interestingly, CD8+ T cells expressing inhibitory molecules such as PD-1 and LAG-3 exhibit suppressive, rather than exhausted, features.331
CD8+ T cells are integral to malaria immunity, particularly in vaccine-induced protection and liver-stage infection control. However, their role in primary infection and pathogenesis, especially in CM, underscores their complexity. Further research is essential to fully elucidate their functions and optimize strategies for malaria vaccine development.
γδ T cells
γδ T cells are a subgroup of T cells characterized by distinct TCRγ and TCRδ chains, accounting for approximately 4% of all T cells in healthy adults.332,333,334,335 Their contributions to host immunity are complex and varied due to their wide range of effector functions, which are influenced by tissue microenvironments.335 In malaria, the role of γδ T cells, particularly those expressing Vγ9+Vδ2+ chains, remains poorly understood. These cells expand during primary P. falciparum336,337 infections and are correlated with protection.337 Studies in endemic regions indicate that recurrent malaria challenges might influence γδ T-cell expansion, potentially aiding in clinical malaria control as individuals age.338,339 In African children with P. falciparum malaria, the majority of the perturbing γδT cells expressed V delta 1 and exhibited a highly activated phenotype, with TCR analysis revealing that the expanded V delta 1+ population was highly polyclonal, used various V gamma chains, and predominantly produced IFN-g, although fewer V delta 1+ T cells produced TNF-α than the overall CD3+ T-cell population.336 Interestingly, Vγ9+Vδ2+ γδ T cells expand during acute infections but tend to contract with subsequent exposures, despite reactivating each time.337,338,340 Recently, scRNA sequencing revealed an increase in immunosuppressive monocytes and the upregulation of tolerogenic markers in NK and γδ T cells.279 And placental P. falciparum infection represented altered γδ T-cell proportions, with increased Vδ1+ subsets and decreased Vδ2+ proportions. These changes, along with altered activation and exhaustion in marker expression, correlate negatively with maternal hemoglobin levels and birth weight.341
In rodent malaria models, γδ T cells expand clonally during the blood stage and support TFH cell responses by producing IL-21. They help to control recrudescence via TCR-dependent mechanisms, potentially involving M-CSF production. Their presence correlates with the efficacy of RAS vaccines, as their depletion impairs CD11c+ DC influx into the liver and hinders optimal CD8+ T-cell responses, reducing sterile immunity.342,343,344 In studies with a murine model, separating liver and blood stages of infection, it was revealed that liver stage-dependent activation of Vγ4+ γδ T cells was crucial for mouse survival. Whereas blood-stage parasite loads were associated with cytokine profiles, where low parasite loads promoted IL-17-producing γδ T cells. These cells drive extramedullary erythropoiesis and reticulocytosis, protecting mice from ECM. This protection can be replicated through adoptive transfer of erythroid precursors.339
Humoral immunity and malaria vaccines
Humoral immunity, which is mediated by antibodies, is crucial in controlling Plasmodium infections and mitigating malaria severity.345 Antibodies target various antigens of parasites in different life cycle stages, particularly blood-stage antigens such as PfEMP1,250,346 MSP1,347,348 and circumsporozoite protein (CSP).349,350,351 These antibodies facilitate parasite clearance through mechanisms such as opsonization,352 neutralization,353 and complement activation.354,355 However, naturally acquired humoral immunity against malaria tends to be inefficient and short-lived due to parasite antigenic variation and immune evasion strategies.356 Recently, immunization with the single-component SBD1 immunogen, which retains the structure of the AMA1-RON2L complex, was found to elicit more potent strain-transcending neutralizing antibody responses against P. falciparum than did immunization with the AMA1 or AMA1-RON2L complex alone, highlighting its potential for advancing malaria vaccine development.357
Plasmodium infections induce robust B-cell responses,358,359 but the maintenance of these responses is hindered by factors such as the parasite-derived metabolic product, hemozoin, which activates inflammasomes and restricts long-term antibody production and memory B-cell formation.360 The latest results from our laboratory show that B-cell differentiation into IL-35+ Bregs during Plasmodium infection, driven by TLR9 activation and distinct signaling via IRF3 pathways, plays a critical role in malaria pathology, with IL-35+ Bregs contributing to the development of ECM and influencing parasitemia levels. The generation of durable immunity is further complicated by the need for continuous exposure to the parasite to maintain antibody levels, as well as the parasite’s ability to undergo antigenic variation, which challenges the immune system’s capacity to form effective memory responses. During malaria infection, the rapid development of short-lived plasmablasts disrupts the formation of long-lasting humoral immunity by impairing germinal center responses, as these plasmablasts exhibit metabolic hyperactivity that deprives the germinal center of necessary nutrients.361 However, therapeutic interventions targeting metabolic constraints can enhance parasite clearance and promote the development of protective immune memory. Additionally, cytokines such as GM-CSF and IL-3, produced by IgM+ and IgG+ B1b B cell plasmablasts, play an important role in the immune response.362 Early in the infection, these cytokines are primarily produced by IgM+ B1b B cells, with a later shift to IgG+ plasmablasts, suggesting an isotype switch and highlighting the functional plasticity and phenotypic heterogeneity of innate B1 B cell subsets.362
Current malaria vaccines aim to elicit strong humoral and cellular immune responses (Table 2).363 The RTS,S/AS01E (Mosquirix) vaccine, which targets the CSP, is the most advanced malaria vaccine and has been approved for use in endemic regions.364 RTS,S/AS01E primarily induces antibody and CD4+ T-cell responses that target preerythrocytic-stage parasites.365 Despite its limited efficacy, studies have shown that delayed fractional dosing of RTS,S/AS01E can enhance the quality and longevity of the humoral response by promoting a balanced production of polyfunctional antibodies against CSP and Pf16 antigens.366 Antibody responses to a three-dose primary vaccination series were significantly greater observed in Ghana than in Malawi and Gabon. However, neither antibody levels nor vaccine efficacy against initial malaria cases were influenced by background incidence or parasitemia during the vaccination series.367 A phase 1 clinical trial demonstrated that the combination of full-length P. falciparum MSP1 with the GLA-SE adjuvant is safe, well tolerated, and immunogenic, inducing lasting MSP1-specific IgG and IgM responses and memory T-cell responses, making it a promising candidate for further efficacy evaluation in malaria vaccine development (EudraCT 2016-002463-33).368 AMA1 has been identified as a conserved and essential malaria vaccine target. A human monoclonal antibody targeting AMA1 domain II, which effectively inhibits P. falciparum growth through a novel mechanism independent of RON2 binding, was successfully isolated and optimized, demonstrating the potential of phage display libraries for developing potent blood-stage malaria interventions.369 Additionally, a plant-based vaccine incorporating the AMA1 and MSP119 proteins induced specific immune responses in test animals, showing promise as a subunit vaccine.370 Compared with vaccines targeting the F2 domain and full region II, vaccines targeting the EBA-140 F1 domain, which includes the crucial SA-binding pocket, present significantly better parasite neutralization, highlighting the importance of targeting functionally relevant epitopes for enhancing malaria vaccine efficacy.371
The two pre-erythrocytic vaccines, R21/Matrix-M and RTS,S/AS01, do not elicit protection against blood-stage parasites. Rh5.1/Matrix-M is a blood-stage P. falciparum vaccine candidate. In a phase 1b trial, the RH5.1/Matrix-M malaria vaccine candidate exhibited good safety and immunogenicity in both adults and children in a malaria-endemic area, with sera from all children in the delayed third-dose regimen displayed a growth inhibition activity (GIA) previously linked to protective immunity. The vaccine induced strong anti-RH5.1 antibody responses and showed promising results for further efficacy trials against clinical malaria in young African children.372 In a phase 2b trial, the RH5.1/Matrix-M vaccine also demonstrated good safety and immunogenicity in Burkinabe children, with a 55% vaccine efficacy in the delayed third-dose regimen, alongside strong antibody responses and significant P. falciparum growth inhibition activity in vitro.373 A recent study investigated the potential of enhanced vaccine efficacy by immunization with a cocktail of the RCR-complex, consisting of RH5, CyRPA, and RIPR, compared to RH5 alone. Despite the identification of additive or synergistic effects of monoclonal antibodies targeting different antigens, vaccination with the RCR-complex in rats did not outperform RH5 alone due to RIPR immuno-dominance; however, combining RH5 with a fusion protein (R78C) improved parasite growth inhibition, supporting the advancement of the RH5.1 + R78C/Matrix-M™ vaccine to clinical trials.374
Another promising approach is to block malaria transmission by targeting antigens expressed during the mosquito stage of the parasites, such as the AnAPN1 vaccine.375 This vaccine induces functional antibodies that prevent the development of the parasite within the mosquito vector, thus curbing transmission.375 Immunology-based strategies have been employed to increase the efficacy of AnAPN1, resulting in more potent and durable antibody responses.375 Ongoing studies are aiming to develop vaccines that not only provide short-term protection but also induce long-lasting immunity.
Vaccine studies on P. vivax and other malarial species are also progressing. A study characterized the sequence and structural diversity of P. vivax merozoite surface protein 3 (PvMSP3γ) by analyzing 118 complete pvmsp3γ sequences from Thailand and 9 reported sequences, revealed 86 distinct haplotypes. The findings suggest that polymorphism in PvMSP3γ is driven by recombination and natural selection, with structural variations potentially complicating vaccine development due to alterations in immunogenic epitopes among variants.376 Another study analyzed natural IgG antibody responses in 246 symptomatic P. vivax malaria patients to PvMSP3γ recombinant proteins revealed widespread seropositivity and a strong correlation with previous malaria episodes. The findings highlighted the presence of B-cell epitopes across PvMSP3γ, with predominant IgG1 and IgG3 responses.377 Moreover, other studies investigated the immunogenicity of P. ovale merozoite surface protein 4 (PoMSP4), a potential vaccine candidate. The findings exhibited that both P. ovale curtisi (PocMSP4) and P. ovale wallikeri (PowMSP4) protein sequences are highly conserved, and the recombinant proteins could induce strong humoral and cellular immune responses in mice, including high antibody titers and significant splenocyte proliferation, suggesting its potential as a vaccine target for malaria.378
Naturally, acquired immunity to malaria develops gradually after repeated exposure to the parasites, leading to the accumulation of antibodies that target various Plasmodium antigens.356 Studies in endemic regions have shown that individuals frequently infected with malaria parasites eventually develop a repertoire of antibodies that can confer partial protection against clinical malaria.356,379 However, this immunity is often incomplete and can wane in the absence of continuous exposure.380 Children in high-transmission areas are at greater risk because of their less mature immune systems and lower antibody titers.381 Protective immunity requires a threshold concentration of antibodies against merozoite antigens, such as MSP1 and AMA1, which are crucial for inhibiting parasite invasion of red blood cells.381 Recently, the development of an engineered SpyCatcher-mi3 nanoparticle has shown significant potential in the field of nanobiotechnology and vaccine development. This nanoparticle efficiently binds to malaria antigens, eliciting strong immune responses and demonstrating its versatility as a future platform for medical advancements.382
Understanding the dynamics of naturally acquired immunity to malaria will provide valuable insights for vaccine development. For example, insights into how the immune system responds to repeated infections and how memory B cells are generated and maintained can facilitate the design of vaccines that mimic natural exposure and increase long-term immunity.383 Additionally, the identification of correlates of protection, such as specific antibody profiles that confer immunity, is essential for evaluating vaccine efficacy and guiding the development of more effective immunization strategies.384
In conclusion, while significant progress has been made in understanding the mechanisms of humoral and cellular immunity and the development of malaria vaccines, challenges remain in achieving durable and broadly protective immunity. Continued research into the molecular and cellular pathways involved in immune responses to Plasmodium infection, as well as innovative vaccine strategies, will be critical in the global effort to control and eventually eradicate malaria.
Therapeutic targets of anti-malaria drugs and progress in clinical application
Malaria remains a significant global health challenge, necessitating ongoing research on therapeutic targets and clinical interventions. This section is divided into three parts. The first part provides information on malaria drugs currently approved by the Food and Drug Administration (FDA) (Fig. 10). The second part discusses frontline antimalarial treatments and their mechanisms of resistance (Fig. 10). The third part summarizes the conditions and objectives that must be met in the development of new drugs to counteract resistance and the new drug list in malaria treatment.
The drugs and structures of anti-malaria drugs were approved by FDA. The drugs and structures shown include well-known treatments such as Artemisinin, Artemether, Dihydroartemisinin, Artesunate, and others like Atovaquone, Pyrimethamine, and Chloroquine, which are currently used in the treatment of malaria
Malaria can be effectively treated when therapy is initiated promptly, but delayed treatment can lead to severe or even fatal outcomes.105,385 The choice of treatment depends on several factors, including the parasite species, the severity of the infection, the risk of drug resistance based on the region where the infection was contracted,386 as well as the patient’s age,387 pregnancy status,388 or breastfeeding considerations.389 Current FDA-approved malaria treatments, including Artemether-Lumefantrine (Coartem®),390 Atovaquone-Proguanil (Malarone™),391 and Primaquine,392 play crucial roles in combating malaria but face notable limitations in clinical settings. Artemether-Lumefantrine is widely used for uncomplicated P. falciparum malaria and is effective even during pregnancy393,394; however, it requires precise dosing schedules and administration with food to optimize absorption,395,396 which can be challenging in resource-limited or emergency contexts. Atovaquone-Proguanil, favored for its shorter treatment duration and ease of administration,397,398 is not recommended during pregnancy,399 in infants weighing less than 5 kg,400 or for breastfeeding mothers of such infants,401 restricting its use in some vulnerable populations. Primaquine is useful for eliminating P. vivax and P. ovale hypnozoites to prevent relapses106 but poses significant risks of hemolytic anemia in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency.402 This necessitates quantitative G6PD testing before administration, a resource often unavailable in endemic areas, further complicating its deployment. Additionally, Primaquine is contraindicated during pregnancy and also poses risks for breastfeeding infants without confirmed normal G6PD activity.403 Drug resistance further confounds these issues, as resistance to chloroquine and other classical antimalarials has necessitated the adoption of combination therapies, which, while being effective, increase costs404 and logistical complexities. Other challenges include ensuring safe and effective treatment for pregnant women and children, both are highly vulnerable groups, as well as achieving patient adherence to the often complex and prolonged treatment regimens.
The genetic variability of the human malarial parasite P. falciparum is suggested to be the main cause of its resistance to drug treatments.405 The rapid evolution of P. falciparum genomes in response to drug pressure necessitates the continuous monitoring and updating of antimalarial strategies.406 Frontline antimalarial therapeutics primarily encompass ART and its derivatives, chloroquine, and sulfadoxine–pyrimethamine.407 Consequently, this paragraph will systematically review drug resistance according to the aforementioned drug classes. ART and its derivatives are most effective against Plasmodium species, but their efficacy is threatened by the emergence of ART-resistant parasites.408 Clinically, partial resistance to ART is characterized by a delayed clearance phenomenon, with a parasite clearance half-life >5 h409 or parasites persist up to a standard 3-day ACT treatment regimen. However, by day 28, the clinical and parasitic response rates remain unaffected if the partner drug retains its efficacy.410 This suggests that initial delays in parasite clearance do not necessarily indicate treatment failure.410 In P. falciparum, resistance to ART is often quantified in vitro as more than 1% of early ring-stage parasites surviving a 6-h exposure to 700 nM ART derivative dihydroartemisinin (DHA).411 P. falciparum partial resistance mechanisms to ART and DHA have been suggested to primarily involve genetic mutations in the P. falciparum kelch 13 (PfK13), alterations in parasite metabolic pathways, and epitranscriptomic and epigenetic mechanisms. Consequently, this paragraph will systematically explore resistance to ART and DHA through these aforementioned mechanisms. PfK13 is a protein encoded by the kelch13 gene,412 which is involved in hemoglobin trafficking by parasites during the asexual blood stage.412 Mutations in PfK13, including Y493H, R539T, I543T, and C580Y mutations (Fig. 11a, b), have been linked to delayed parasite clearance following ART treatment.413,414 C580Y mutation in the PfK13 protein has become dominant in parasite strains common in regions such as the eastern Greater Mekong Subregion.415 This mutation spreads in specific parasite sublineages that may also possess secondary factors that increase resistance or mitigate the adverse effects of mutations on parasite physiology.415 While there is a possibility that the C580Y mutation can enhance parasite transmissibility, further research is needed to verify this phenomenon. There are significant differences in the resistance conferred by PfK13 mutations in African isolates,416 with some mutations resulting in little to no resistance in vitro.417 The competitive fitness of these mutated strains against wild-type strains has also been questioned, and the slower spread of ART-resistant strains in Africa may be due to various ecological and biological factors.416
PfKelch13 mutations. a The global emergence of ART resistance-associated mutations in the P. falciparum K13 protein from 2001 to 2024.The references are shown in [a],67 [b],510 [c],511 [d],512 [e],513 [f],514 [g],415 [h],515 [i],516 [j],517 [k],518 [l],519 [m],520 [n],521 [o],522 [p],523 [q],524 [r],525 [s],416 [t],526 [u],527 [v].528 b Markers of artemisinin resistance in pfkelch13 and commonly observed mutations in clinical studies. The data was from WHO (https://www.who.int/news-room/questions-and-answers/item/artemisinin-resistance). This figure was created with BioRender.com
Upon ART treatment, parasites exhibit various responses, including activation of the unfolded protein response, altered mitochondrial physiology, and changes in developmental stages that contribute to resistance.418,419,420,421,422,423 One mechanism underlying ART resistance associated with mutations in the kelch13 gene involves a reduction in hemoglobin endocytosis, which subsequently leads to decreased levels of Fe2+ heme. This reduction is believed to be critical for the activation of ART through the cleavage of its endoperoxide bridge.424 Another mechanism is that PfK13 mutations (Y493H and 580Y) may be associated with alterations in parasite metabolic pathways in ring stage, including the tricarboxylic acid cycle, glycolysis, and amino acid metabolism, in response to DHA exposure.425 Furthermore, an epitranscriptomic mechanism involving tRNA hypomodification and codon-biased translation, particularly the modification of mcm⁵s²U on tRNA resulting in tRNA s²U reprogramming, may also regulate PfK13 function to enabled the survival of parasites under ART-induced stress.426 Moreover, emerging evidence suggests that multicopy Pfpm2 may compensate for the fitness impacts of various PfCRT isoforms by increasing hemoglobin degradation, potentially contributing to DHA + piperaquine (PPQ) resistance in P. falciparum.427 The evidence of epidemiology is that following the introduction of DHA-piperaquine in 2010, newly emerged PfCRT mutations rapidly increased in prevalence, reaching more than 98% by 2017 in northern Cambodia. In contrast, after artesunate-mefloquine treatment, the prevalence of parasites with increased copy numbers of plasmepsin II (pfpm2) decreased, with nearly half of the piperaquine-resistant strains carrying a single copy of pfpm2.428 Additionally, a recent study revealed that brief exposure to other ART derivative artesunate (AS) stimulates rosette formation in P. falciparum, especially in ART-resistant isolates, enabling infected erythrocytes to survive drug exposure and evade phagocytosis, indicating that AS-mediated rosette formation in late-stage parasites contributes to ART resistance by allowing parasites to persist in less drug-susceptible environments.429 Overall, the mechanisms underlying resistance to ART predominantly encompass alterations in heme uptake, metabolic adaptations, epigenetic modifications, and rosette formation.
Chloroquine (CQ) was once an effective antimalarial drug, but resistance mediated by mutations in the P. falciparum CQ resistance transporter (PfCRT) has significantly reduced its efficacy against P. falciparum. PfCRT is a protein encoded by the pfcrt gene in P. falciparum.430 This protein is located in the digestive vacuole membrane of the parasite.430 Mutations in PfCRT lead to a reduction in drug accumulation within the parasite’s digestive vacuole, which is essential for its antimalarial effect.431 To date, no fewer than 30 variant residues in PfCRT have been identified, rendering PfCRT an extraordinarily polymorphic protein. Notably, in all resistant parasites, lysine 76 (K76) in PfCRT is replaced with an uncharged amino acid, either a threonine (76T) in the case of virtually all field isolates (with one reported exception of a 76A variant)432 or an asparagine or isoleucine (76 N/I) in laboratory-adapted lines exposed to CQ.433 The 3.2 Å resolution structure of the PfCRT protein from CQ-resistant but PPQ-sensitive P. falciparum 7G8 parasites revealed that mutations contributing to resistance occur in different helices lining a central negatively charged cavity-the primary interaction site for the positively charged drugs. Functional analyses demonstrated that the newly emerging PfCRT mutations, namely F145I and C350R, enable PPQ transport and confer resistance, providing insights into the distinct mechanisms by which PfCRT mediates CQ and piperaquine resistance.434 Overall, these results indicate that drug resistance is an ongoing battle. P. falciparum can utilize gene mutations or amplifications that confer resistance to other antimalarial drugs to resist new antimalarial medications. Furthermore, another vacuolar protein, the amino acid transporter (PfAAT1), was also found to be associated with CQ resistance. This may be a compensatory evolution in P. falciparum. A longitudinal genomic analysis of Gambian P. falciparum isolates revealed the PfAAT1 variant S258L, which increased in frequency alongside the PfCRT K76T mutation, and gene editing confirmed that this variant enhances CQ resistance at the cost of fitness, with other regional variants, such as F313S, mitigate resistance while restoring fitness.435 Moreover, the nondrug-related function of PfCRT in P. falciparum was determined by generating a conditional knockdown mutant, revealing its potential role in oligopeptide transport.
SP is used throughout Africa for intermittent preventive treatment of malaria, but parasite resistance to SP threatens its efficacy. PfDHFR (P. falciparum dihydrofolate reductase) and PfDHPS (P. falciparum dihydropteroate synthetase) are functionally critical enzymes in P. falciparum that are linked to SP resistance.436 Mutations in the PfDHFR (N51I, C59R, S108N, and I164L)437,438 and PfDHPS (I431V, S436 A/F, A437G, K540 E/N, A581G, and A613T)439,440 genes are key contributors to SP resistance. The combination of triple pfdhfr (N51I/C59R/S108N) and double pfdhps (A437G/K540E) mutations is a strong predictor of SP treatment failure and reduces the efficacy of SP-based interventions, especially in areas where the prevalence of dhps K540E exceeds 50%.441,442 In 2019, a study conducted on Bioko Island revealed a high prevalence of these mutations, underscoring the necessity for ongoing molecular monitoring and control efforts to manage SP resistance effectively.443 The drug CID 10476801 has emerged as a potent inhibitor in docking studies of pyrimethamine derivatives, suggesting another potential avenue for treatment.444 Further research using pharmacophore modeling and docking has identified several natural products as potential PfDHFR inhibitors.445 These candidates show promise for development against both WT and mutant PfDHFR strains, which could lead to new, effective treatments for malaria.445
The criteria for substitution of existing therapies with novel treatments are rigorously defined. Moreover, the development of new treatments for severe malaria remains necessary, especially in cases where oral medications are not suitable. Additional efforts are also required to create drugs capable of managing asymptomatic infections and eliminating dormant parasites in P. vivax malaria. The second target focuses on chemoprevention and prophylaxis, driven by the absence of a fully protective vaccine. Chemoprevention involves the administration of a full treatment dose to individuals in highly endemic areas to control transmission, as some individuals may be asymptomatic carriers. Prophylaxis entails medication for individuals who are at risk of infection. The third objective is to develop new antimalarial drugs that encompass several key features. The first is stability, particularly under conditions of high temperature and humidity. The second is the consideration of specific needs for children and pregnant women, including safety and appropriate drug formulation. The third is to ensure that the cost is kept as low as possible. Additionally, in malaria, as with many infectious diseases, drug development is more complex because an optimal medicine is often combined of two or more active drugs.410
Recent advancements in antimalarial drug development have introduced a diverse array of novel therapeutics and combination therapies aimed at enhancing efficacy, overcoming drug resistance, and improving patient outcomes (Fig. 12). Sevuparin, a modified heparin derivative, disrupts the sequestration of Plasmodium-infected erythrocytes, thereby reducing microvascular obstruction in severe malaria cases.446,447 Imatinib, originally for cancer treatment, has been repurposed to inhibit essential kinases in the malaria parasite, showing promise in reducing parasitemia.448,449,450,451 Rosiglitazone, a PPAR-γ agonist, is being evaluated as an adjunctive therapy to improve clinical outcomes in severe malaria.452,453,454,455 In a phase IIa randomized, double-blind, placebo-controlled trial in Mozambique, adjunctive rosiglitazone was found to be safe and well-tolerated in children with uncomplicated malaria, supporting its further evaluation as an adjunctive therapy for severe malaria.453 But in another randomized, double-blind, placebo-controlled trial involving 180 Mozambican children with severe malaria, adjunctive rosiglitazone treatment did not significantly reduce circulating angiopoietin-2 levels or improve clinical outcomes compared to placebo when administered alongside artesunate.452 Cipargamin, a spiroindolone compound targeting the PfATP4 protein, disrupts parasite ion homeostasis, leading to rapid clearance.456,457,458,459 Combination therapies such as ZY19489 (sutidiazine) with ferroquine410,460 and Fosmidomycin with piperaquine461,462 offer synergistic effects by targeting multiple parasite pathways, enhancing efficacy against resistant strains. M5717 (DDD107498), an inhibitor of the P. falciparum translation elongation factor 2, demonstrates potent antimalarial activity in clinical trials.463,464,465,466 Pyronaridine is under optimization in combination with artesunate to prevent resistance development,467,468,469,470 while (+)-SJ733, another PfATP4 inhibitor, is undergoing clinical evaluation for its effectiveness in both uncomplicated and severe malaria.471,472,473,474 AQ-13, a novel quinoline-based antimalarial, shows efficacy against multiple Plasmodium species,475,476,477, and L9LS mAb, a monoclonal antibody, aims to neutralize the parasite and prevent its proliferation.478,479 New drugs such as Ivermectin (LYN-163)480,481,482 and combination therapies such as methylene blue with amodiaquine leverage unique mechanisms, including redox-active properties and traditional antimalarial action, to enhance parasite killing.483,484,485,486,487 Moreover, recent Phase II data indicate that the novel antimalarial ganaplacide is effective and well-tolerated for treating uncomplicated P. falciparum malaria in adults, adolescents, and children. Ganaplacide targets the parasite’s internal protein secretory pathway, and its reduced susceptibility is linked to mutations in the P. falciparum genes CARL, UDP-galactose, and Acetyl-CoA transporters. When combined with lumefantrine, which inhibits the parasite’s conversion of toxic heme to non-toxic hemozoin, this combination enhances the treatment’s efficacy.488,489,490,491 Moreover, in a randomized, double-blind, placebo-controlled clinical trial conducted in Gabon and Mozambique, intermittent preventive treatment with DHA-piperaquine for pregnant women with HIV receiving co-trimoxazole prophylaxis was found to be safe and effective, significantly reducing the incidence of clinical malaria and overall P. falciparum infection.492 These innovative approaches collectively represent a comprehensive strategy to combat malaria, addressing critical challenges such as drug resistance and treatment efficacy, and hold significant promise for advancing global malaria control and eradication efforts.
The structures of new anti-malaria drugs. The drugs presented include novel and experimental compounds that have shown potential against Plasmodium infections, such as Imatinib, Rosiglitazone, Cipargamin, ZY19489, and Ferroquine, among others. These structures represent different classes of anti-malaria agents currently under investigation or development, encompassing a range of mechanisms of action aimed at combating malaria
These findings underscore that drug resistance in P. falciparum is an ongoing battle, as the parasite continuously evolves through gene mutations and amplifications to resist new antimalarial medications. Future efforts must focus on enhanced surveillance, a deeper understanding of resistance mechanisms, the development of new drugs effective against resistant strains, the optimization of combination therapies, and global collaboration to adapt to the evolving challenge posed by P. falciparum.
Conclusion and future perspectives
Malaria, caused by Plasmodium parasites transmitted by Anopheles mosquitoes, continues to be a major global health challenge, particularly in sub-Saharan Africa, where most malaria cases and malaria-related deaths occur. The life cycle of Plasmodium involves complex interactions between the parasite and its mosquito and vertebrate hosts. The parasite undergoes various developmental stages, including the liver and blood stages, each of which contributes to disease pathogenesis and transmission dynamics. The emergence of drug-resistant strains as a result of the genetic mutagenesis of P. falciparum poses challenges in treatment and disease control. Mutations in genes such as PfK13 (associated with ART resistance) and PfCRT (associated with CQ resistance) highlight the need for ongoing surveillance and the development of new therapeutic strategies.
Advances in genomic and molecular biology have provided deeper insights into Plasmodium pathogenesis, immune evasion, and parasite‒host interactions. Genomic studies have revealed significant genetic diversity among Plasmodium species, influencing parasite evolution, population genetics, and drug resistance mechanisms. ScRNA-seq analyses have further revealed the transcriptional landscapes of Plasmodium parasites at various stages and in different environmental settings, aiding in the identification of potential targets for vaccine development and therapeutic interventions. Future research should focus on expanding these technologies to unravel the molecular mechanisms underlying parasite ability in adaptation to different environments and evade the host immune system. Additionally, a more comprehensive understanding of gene regulation in both human and rodent species, as well as the role of genomic diversity in drug resistance, will be crucial for developing more effective therapeutic and preventive measures. Moving forward, the integration of cutting-edge technologies, such as scRNA-seq and spatial transcriptomics, with advanced computational tools, will be essential for advancing our knowledge of parasite biology and overcoming the challenges in malaria control.
Host immune responses to malaria are complex and involve both innate and adaptive immunity, which includes the activation of macrophages and dendritic cells and the production of proinflammatory cytokines crucial for controlling parasite replication and clinical symptoms. Adaptive immune responses, particularly the production of specific antibodies and the activation of T cells, play essential roles in long-term immunity and protection against malaria. However, the development of effective and durable immunity is hindered by the parasite’s ability to evade the immune system through antigenic variation and immune suppression mechanisms.
Efforts to develop malaria vaccines have yielded some success, with the RTS,S/AS01E (Mosquirix) vaccine showing certain protection efficiency after several immunization. Ongoing research aims to improve vaccine design and efficacy by targeting multiple stages of the parasite’s life cycle and increasing the quality and longevity of immune responses. Innovative approaches, such as transmission-blocking vaccines, hold promise for advancing malaria control and eradication strategies.
Developing a comprehensive strategy for future malaria research requires focused attention on several critical areas. First, in-depth studies of parasite biology and genetics are essential, including genomic sequencing to identify genetic variations linked to drug resistance and transmission dynamics. Dissection of the molecular mechanisms at each stage of the parasite’s life cycle will help identify potential intervention points. A thorough understanding of the host immune response and genetics is also crucial. This includes exploring how the parasite evades immune detection, identifying host genetic factors influencing susceptibility or resistance, and developing strategies to enhance immune reactivity for more effective parasite clearance.
Vector biology and control are key research areas, particularly the genetic study of Anopheles mosquitoes to uncover factors that contribute to vector competence and insecticide resistance. Innovative vector control strategies, such as genetically modified mosquitoes and biological control agents, should also be explored. Additionally, vaccine development through antigen discovery, optimizing antigen delivery platforms, and rigorous clinical trials is critical. Improving diagnostics and surveillance, through the development of rapid diagnostic tests, molecular diagnostic tools, and leveraging geospatial technologies for more effective monitoring, will also be essential components of future malaria research efforts.
The control of malaria requires a comprehensive and integrated approach. Integrated Vector Management combines chemical, biological, and environmental strategies to effectively control mosquito populations while addressing insecticide resistance through measures such as rotating insecticides and incorporating non-chemical controls. Community engagement is crucial for ensuring the sustainability and acceptance of these initiatives. Universal access to effective treatment demands widespread distribution of antimalarial drugs, particularly ACTs, alongside regular updates to treatment guidelines based on evolving resistance patterns. Strengthening health infrastructure is essential for providing timely diagnosis and treatment, especially in remote areas. Preventive measures, including the use of insecticide-treated nets (ITNs), indoor residual spraying (IRS) in high-transmission zones, and chemoprophylaxis for vulnerable populations, are critical to reducing mosquito bites and preventing malaria transmission. Furthermore, enhancing surveillance and response systems through early detection, data integration, and adaptive management allows for rapid identification and containment of outbreaks.
Future drug development may aim to disrupt the malaria life cycle by focusing on both the pathogen and the host, as well as their interactions. Parasite targets include novel enzymes and metabolic pathways unique to Plasmodium, such as kinases and energy-associated pathways, to inhibit parasite development and replication. Strategies to block transmission involve targeting gametocyte development and preventing the parasite from invading the mosquito midgut. On the host side, enhancing the immune response to more effectively clear the parasite and reducing immunopathology are key strategies. Targeting host metabolic pathways to deprive the parasite of necessary nutrients or manipulating iron metabolism can inhibit parasite growth. Additionally, disrupting host-pathogen interactions with adhesion inhibition molecules can prevent complications like severe anemia and dysfunction of critical organs. Emphasizing multi-target and combination therapies through polypharmacology and optimized combination regimens can reduce the likelihood of resistance development and improve treatment efficacy. By integrating these approaches, future drug development can achieve more effective and sustainable malaria control.
In conclusion, while significant progress has been made in understanding the molecular and genetic underpinnings of malarial parasites, challenges remain in achieving comprehensive and sustainable disease control. Continued research into parasite biology, host‒parasite interactions, and immune responses, coupled with the development of novel therapeutic and preventive measures, is essential for overcoming the persistent burden of malaria.
References
Kwiatkowski, D. P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77, 171–192 (2005).
Miller, L. H., Good, M. F. & Milon, G. Malaria pathogenesis. Science 264, 1878–1883 (1994).
Cowman, A. F., Healer, J., Marapana, D. & Marsh, K. Malaria: biology and disease. Cell 167, 610–624 (2016).
White, N. J. Severe malaria. Malar. J. 21, 284 (2022).
Phillips, R. S. Current status of malaria and potential for control. Clin. Microbiol. Rev. 14, 208–226 (2001).
Sato, S. Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 40, 1 (2021).
Mayxay, M., Pukrittayakamee, S., Newton, P. N. & White, N. J. Mixed-species malaria infections in humans. Trends Parasitol. 20, 233–240 (2004).
Bousema, T. & Drakeley, C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24, 377–410 (2011).
Fornace, K. M. et al. Environmental risk factors and exposure to the zoonotic malaria parasite Plasmodium knowlesi across northern Sabah, Malaysia: a population-based cross-sectional survey. Lancet Planet Health 3, e179–e186 (2019).
Paintain, L. et al. Using donor funding to catalyse investment in malaria prevention in Ghana: an analysis of the potential impact on public and private sector expenditure. Malar. J. 21, 203 (2022).
Nonvignon, J. et al. Economic burden of malaria on businesses in Ghana: a case for private sector investment in malaria control. Malar. J. 15, 454 (2016).
Vaughan, A. M. & Kappe, S. H. J. Malaria parasite liver infection and exoerythrocytic biology. Cold Spring Harb. Perspect. Med. 7, a025486 (2017).
Ménard, R. et al. Looking under the skin: the first steps in malarial infection and immunity. Nat. Rev. Microbiol. 11, 701–712 (2013).
Markwalter, C. F. et al. Plasmodium falciparum infection in humans and mosquitoes influence natural Anopheline biting behavior and transmission. Nat. Commun. 15, 4626 (2024).
Milner, D. A. Jr Malaria Pathogenesis. Cold Spring Harb. Perspect. Med. 8, a025569 (2018).
Mousa, A. et al. The impact of delayed treatment of uncomplicated P. falciparum malaria on progression to severe malaria: A systematic review and a pooled multicentre individual-patient meta-analysis. PLOS Med. 17, e1003359 (2020).
Ashley, E. A., Pyae Phyo, A. & Woodrow, C. J. Malaria. Lancet 391, 1608–1621 (2018).
Su, X. Z. & Miller, L. H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. sci. China Life sci. 58, 1175–1179 (2015).
Blasco, B., Leroy, D. & Fidock, D. A. Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 23, 917–928 (2017).
Nye, E. R. Alphonse Laveran (1845-1922): discoverer of the malarial parasite and Nobel laureate, 1907. J. Med. Biogr. 10, 81–87 (2002).
Sundberg, C. Alphonse Laveran: the Nobel Prize for Medicine 1907. Parassitologia 49, 257–260 (2007).
Sequeira, J. H. Alphonse Laveran And His Work. Br. Med. J. 1, 1145–1147 (1930).
Malpe, M., Choudhari, S. G., Nagtode, N. & Muntode Gharde, P. The legacy of Sir Ronald Ross: from malaria research to multifaceted achievements. Cureus 16, e65999 (2024).
Pande, V., Bahal, M., Dua, J. & Gupta, A. Ronald Ross: pioneer of malaria research and nobel laureate. Cureus 16, e65993 (2024).
Tsay, C. J. J. ulius Wagner-Jauregg and the legacy of malarial therapy for the treatment of general paresis of the insane. Yale J. Biol. Med. 86, 245–254 (2013).
Raju, T. N. Hot brains: manipulating body heat to save the brain. Pediatrics 117, e320–e321 (2006).
Vogel, G. Malaria as lifesaving therapy. Science 342, 686 (2013).
Woodward, R. B. & Doering, W. The total synthesis of quinine. J. Am. Chem. Soc. 67, 860–874 (1945).
Benfey, O. T. & Morris, P. J. T. Robert Burns Woodward: Architect and Artist in the World of Molecules (Chemical Heritage Foundation, 2001).
Achan, J. et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 10, 144 (2011).
Kaufman, T. S. & Rúveda, E. The quest for quinine: those who won the battles and those who won the war. Angew. Chem. Int. Ed. Engl. 44, 854–885 (2005).
Liles, N. W. et al. Diversity and severity of adverse reactions to quinine: a systematic review. Am. J. Hematol. 91, 461–466 (2016).
Cortegiani, A., Ingoglia, G., Ippolito, M., Giarratano, A. & Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 57, 279–283 (2020).
Wellems, T. E. & Plowe, C. Chloroquine-resistant malaria. J. Infect. Dis. 184, 770–776 (2001).
Buxton, P. & Hygiene. The use of the new insecticide DDT in relation to the problems of tropical medicine. Trans. R. Soc. Trop. Med. Hyg. 38, 367–400 (1945).
World Health Organization. Global Malaria Control and Elimination: Report of a Technical Review (2008).
Rasmussen, C., Alonso, P. & Ringwald, P. Current and emerging strategies to combat antimalarial resistance. Expert Rev. Anti Infect. Ther. 20, 353–372 (2022).
Thellier, M., Gemegah, A. & Tantaoui, I. Global fight against malaria: goals and achievements 1900–2022. J. Clin. Med. 13, 5680 (2024).
Zhai, X., Wang, Q. & Li, M. Tu Youyou’s Nobel Prize and the academic evaluation system in China. Lancet 387, 1722 (2016).
White, N. Qinghaosu (artemisinin): the price of success. Science 320, 330–334 (2008).
Ma, N. et al. The birth of artemisinin. Pharm. Ther. 216, 107658 (2020).
World Health Organization. Global Technical Strategy for Malaria 2016–2030 (2015).
Richie, T. L. et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33, 7452–7461 (2015).
Triller, G. et al. Natural parasite exposure induces protective human anti-malarial antibodies. Immunity 47, 1197–1209 (2017).
Dame, J. B. et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science 225, 593–599 (1984).
Zavala, F. et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228, 1436–1440 (1985).
Ballou, W. R. et al. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet 1, 1277–1281 (1987).
Cohen, J., Nussenzweig, V., Nussenzweig, R., Vekemans, J. & Leach, A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccin. 6, 90–96 (2010).
Laurens, M. R. T. S. S/AS01 vaccine (Mosquirix™): an overview. Hum. Vaccin. Immunother. 16, 480–489 (2020).
RTS,S Clinical Trials Partnership, Agnandji, S. T. et al. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N. Engl. J. Med 365, 1863–1875 (2011).
RTS,S Clinical Trials Partnership Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015).
Lubanga, A. F. et al. Beyond RTS, S malaria vaccine piloting to adoption and historic introduction in sub-Saharan Africa: a new hope in the fight against the vector-borne disease. Front. Trop. Dis. 5, 1387078 (2024).
Moser, K. A. et al. Strains used in whole-organism Plasmodium falciparum vaccine trials differ in genome structure, sequence, and immunogenic potential. Genome Med. 12, 6 (2020).
Su, X. Z., Lane, K. D., Xia, L., Sá, J. M. & Wellems, T. E. Plasmodium genomics and genetics: new insights into malaria pathogenesis, drug resistance, epidemiology, and evolution. Clin. Microbiol. Rev. 32, e00019 (2019).
Sharma, S. et al. What India can learn from globally successful malaria elimination programmes. BMJ Glob. Health 7, e008431 (2022).
Cao, J. et al. Achieving malaria elimination in China. Lancet Public Health 6, e871–e872 (2021).
Datoo, M. S. et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect. Dis. 22, 1728–1736 (2022).
Eisele, T. Are investments in malaria control saving the lives of children? Challenges in using all-cause child mortality for measuring the impact of malaria control programs. Malar. J. 13, O27 (2014).
Adam, I., Khamis, A. H. & Elbashir, M. I. Prevalence and risk factors for Plasmodium falciparum malaria in pregnant women of eastern Sudan. Malar. J. 4, 18 (2005).
Kayiba, N. K. et al. Malaria infection among adults residing in a highly endemic region from the Democratic Republic of the Congo. Malar. J. 23, 82 (2024).
Phillips, M. A. et al. Malaria. Nat. Rev. Dis. Prim. 3, 17050 (2017).
Ashton, R. A. et al. Why does malaria transmission continue at high levels despite universal vector control? Quantifying persistent malaria transmission by Anopheles funestus in Western Province, Zambia. Parasites Vectors 17, 429 (2024).
Andagalu, B. et al. Malaria Transmission Dynamics in a High-Transmission Setting of Western Kenya and the Inadequate Treatment Response to Artemether-Lumefantrine in an Asymptomatic Population. Clin. Infect. Dis. 76, 704–712 (2023).
Kokwaro, G. Ongoing challenges in the management of malaria. Malar. J. 8, S2 (2009).
Hossain, M. S., Ahmed, T. S., Sultana, N., Chowdhury, M. A. B. & Uddin, M. J. Examining the disparities of anti-malarial drug consumption among children under the age of five: a study of 5 malaria-endemic countries. Malar. J. 22, 370 (2023).
Martinez-Vega, R. et al. Regional action needed to halt antimalarial drug resistance in Africa. Lancet 405, 7–10 (2024).
Amato, R. et al. Origins of the current outbreak of multidrug-resistant malaria in Southeast Asia: a retrospective genetic study. Lancet Infect. Dis. 18, 337–345 (2018).
Boyce, R. et al. Severe flooding and malaria transmission in the western ugandan highlands: implications for disease control in an era of global climate change. J. Infect. Dis. 214, 1403–1410 (2016).
Patz, J. A. & Olson, S. H. Malaria risk and temperature: Influences from global climate change and local land use practices. Proc. Natl Acad. Sci. USA 103, 5635–5636 (2006).
Gao, L., Shi, Q., Liu, Z., Li, Z. & Dong, X. Impact of the COVID-19 pandemic on malaria control in Africa: a preliminary analysis. Trop. Med Infect. Dis. 8, 67 (2023).
Weiss, D. J. et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect. Dis. 21, 59–69 (2021).
Aguma, H. B. et al. Mass distribution campaign of long-lasting insecticidal nets (LLINs) during the COVID-19 pandemic in Uganda: lessons learned. Malar. J. 22, 310 (2023).
Kerr, G., Robinson, L. J., Russell, T. L. & Macdonald, J. Lessons for improved COVID-19 surveillance from the scale-up of malaria testing strategies. Malar. J. 21, 223 (2022).
Dittrich, S. et al. Diagnosing malaria and other febrile illnesses during the COVID-19 pandemic. Lancet Glob. Health 8, e879–e880 (2020).
Zawawi, A. et al. The impact of COVID-19 pandemic on malaria elimination. Parasite Epidemiol. Control. 11, e00187 (2020).
Dorrell, R. G., Drew, J., Nisbet, R. E. & Howe, C. J. Evolution of chloroplast transcript processing in Plasmodium and its chromerid algal relatives. PLoS Genet. 10, e1004008 (2014).
McFadden, G. I. & Yeh, E. The apicoplast: now you see it, now you don’t. Int. J. Parasitol. 47, 137–144 (2017).
Siao, M. C., Borner, J., Perkins, S. L., Deitsch, K. W. & Kirkman, L. A. Evolution of host specificity by malaria parasites through altered mechanisms controlling genome maintenance. mBio 11, e03272 (2020).
Evans, A. G. & Wellems, T. E. Coevolutionary genetics of Plasmodium malaria parasites and their human hosts. Integr. Comp. Biol. 42, 401–407 (2002).
Su, X. Z., Zhang, C. & Joy, D. A. Host-malaria parasite interactions and impacts on mutual evolution. Front. Cell Infect. Microbiol 10, 587933 (2020).
Michel, M. et al. Ancient Plasmodium genomes shed light on the history of human malaria. Nature 631, 125–133 (2024).
Otto, T. D. et al. A comprehensive evaluation of rodent malaria parasite genomes and gene expression. BMC Biol. 12, 86 (2014).
Omelianczyk, R. I. et al. Rapid activation of distinct members of multigene families in Plasmodium spp. Commun. Biol. 3, 351 (2020).
Gardner, M. J. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 (2002).
Martinsen, E. S., Perkins, S. L. & Schall, J. J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 47, 261–273 (2008).
Bensch, S., Hellgren, O. & Pérez-Tris, J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353–1358 (2009).
Schaer, J. et al. High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa. Proc. Natl. Acad. Sci. USA 110, 17415–17419 (2013).
Walk, J. et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med. 15, 168 (2017).
Bojang, K. A. et al. Efficacy of RTS, S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358, 1927–1934 (2001).
Sanchez, C. P., Wünsch, S. & Lanzer, M. Identification of a chloroquine importer in Plasmodium falciparum: differences in import kinetics are genetically linked with the chloroquine-resistant phenotype. J. Biol. Chem. 272, 2652–2658 (1997).
Valderramos, S. G. et al. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PLoS Pathog. 6, e1000887 (2010).
Drakeley, C. J. et al. Geographical distribution of a variant epitope of Pfs4845, a Plasmodium falciparum transmission-blocking vaccine candidate. Mol. Biochem. Parasitol. 81, 253–257 (1996).
De Niz, M. & Heussler, V. T. Rodent malaria models: insights into human disease and parasite biology. Curr. Opin. Microbiol. 46, 93–101 (2018).
Frech, C. & Chen, N. Genome comparison of human and non-human malaria parasites reveals species subset-specific genes potentially linked to human disease. PLoS Comput. Biol. 7, e1002320 (2011).
Kooij, T. W. A. et al. A plasmodium whole-genome synteny map: indels and synteny breakpoints as foci for species-specific genes. PLoS Pathog. 1, e44 (2005).
Hall, N. & Carlton, J. Comparative genomics of malaria parasites. Curr. Opin. Genet. Dev. 15, 609–613 (2005).
Chen, Q. et al. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J. Exp. Med. 187, 15–23 (1998).
Howick, V. M. et al. The Malaria Cell Atlas: single parasite transcriptomes across the complete Plasmodium life cycle. Science 365, eaaw2619 (2019).
Witmer, K. et al. Using scRNA-seq to identify transcriptional variation in the malaria parasite Ookinete stage. Front. Cell Infect. Microbiol. 11, 604129 (2021).
Afriat, A. et al. A spatiotemporally resolved single-cell atlas of the Plasmodium liver stage. Nature 611, 563–569 (2022).
Severe malaria. Trop. Med. Int. Health 19, 7–131 (2014).
Daily, J. P., Minuti, A. & Khan, N. Diagnosis, treatment, and prevention of malaria in the US: a review. JAMA 328, 460–471 (2022).
Lalloo, D. G. et al. UK malaria treatment guidelines 2016. J. Infect. 72, 635–649 (2016).
McMorrow, M. L., Aidoo, M. & Kachur, S. P. Malaria rapid diagnostic tests in elimination settings-can they find the last parasite?. Clin. Microbiol. Infect. 17, 1624–1631 (2011).
Trampuz, A., Jereb, M., Muzlovic, I. & Prabhu, R. M. Clinical review: severe malaria. Crit. Care 7, 315–323 (2003).
Wångdahl, A. et al. Relapse of plasmodium vivax and plasmodium ovale malaria with and without primaquine treatment in a nonendemic area. Clin. Infect. Dis. 74, 1199–1207 (2022).
Rosa-Gonçalves, P., Ribeiro-Gomes, F. L. & Daniel-Ribeiro, C. T. Malaria-related neurocognitive deficits and behavioral alterations. Front. Cell Infect. Microbiol. 12, 829413 (2022.
Idro, R., Marsh, K., John, C. C. & Newton, C. R. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 68, 267–274 (2010).
Haldar, K. & Mohandas, N. Malaria, erythrocytic infection, and anemia. Hematol. Am. Soc. Hematol. Educ. Program, 87–93 (2009).
Schantz-Dunn, J. & Nour, N. M. Malaria and pregnancy: a global health perspective. Rev. Obstet. Gynecol. 2, 186–192 (2009).
Kim, K. M., Bae, B. K. & Lee, S. B. Spontaneous splenic rupture in Plasmodium vivax malaria. Ann. Surg. Treat. Res. 87, 44–46 (2014).
Rouzine, I. M. & McKenzie, F. E. Link between immune response and parasite synchronization in malaria. Proc. Natl. Acad. Sci. USA 100, 3473–3478 (2003).
Su, Y., Ruan, S. & Wei, J. Periodicity and synchronization in blood-stage malaria infection. J. Math. Biol. 63, 557–574 (2011).
Kwiatkowski, D. & Greenwood, B. M. Why is malaria fever periodic? A hypothesis. Parasitol. Today 5, 264–266 (1989).
Smith, L. M. et al. An intrinsic oscillator drives the blood stage cycle of the malaria parasite Plasmodium falciparum. Science 368, 754–759 (2020).
Karunaweera, N. D., Grau, G. E., Gamage, P., Carter, R. & Mendis, K. N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. USA 89, 3200–3203 (1992).
Collins, W. E. & Jeffery, G. M. Plasmodium malariae: parasite and disease. Clin. Microbiol. Rev. 20, 579–592 (2007).
White, N. J. Anaemia and malaria. Malar. J. 17, 371 (2018).
Lampah, D. A. et al. Severe malarial thrombocytopenia: a risk factor for mortality in Papua, Indonesia. J. Infect. Dis. 211, 623–634 (2015).
Silva, G. B. D. J., Pinto, J. R., Barros, E. J. G., Farias, G. M. N. & Daher, E. F. Kidney involvement in malaria: an update. Rev. Inst. Med. Trop. Sao Paulo 59, e53 (2017).
Nunes-Silva, S. et al. Beninese children with cerebral malaria do not develop humoral immunity against the IT4-VAR19-DC8 PfEMP1 variant linked to EPCR and brain endothelial binding. Malar. J. 14, 493 (2015).
Wassmer, S. C., de Koning-Ward, T. F., Grau, G. E. R. & Pai, S. Unravelling mysteries at the perivascular space: a new rationale for cerebral malaria pathogenesis. Trends Parasitol. 40, 28–44 (2024).
Rowe, J. A., Moulds, J. M., Newbold, C. I. & Miller, L. H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388, 292–295 (1997).
Carlson, J. et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336, 1457–1460 (1990).
Normark, J. et al. PfEMP1-DBL1alpha amino acid motifs in severe disease states of Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 104, 15835–15840 (2007).
Hadjilaou, A., Brandi, J., Riehn, M., Friese, M. A. & Jacobs, T. Pathogenetic mechanisms and treatment targets in cerebral malaria. Nat. Rev. Neurol. 19, 688–709 (2023).
Wilson, K. D. et al. Elimination of intravascular thrombi prevents early mortality and reduces gliosis in hyper-inflammatory experimental cerebral malaria. J. Neuroinflamm. 15, 173 (2018).
Wilson, K. J. et al. Predicting acute and post-recovery outcomes in cerebral malaria and other comas by Optical Coherence Tomography (OCT in CM)—a protocol for an observational cohort study of Malawian children. Wellcome Open Res 8, 172 (2023).
Seydel, K. B. et al. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 372, 1126–1137 (2015).
Rosenblum, W. I. Cytotoxic edema: monitoring its magnitude and contribution to brain swelling. J. Neuropathol. Exp. Neurol. 66, 771–778 (2007).
Riggle, B. A. et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J. Clin. Investig. 130, 1128–1138 (2020).
Mohanty, S. et al. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. mSphere 2, e00193 (2017).
Coughlan, C. et al. Adult cerebral malaria: acute and subacute imaging findings, long-term clinical consequences. Clin. Infect. Dis. 78, 457–460 (2024).
Sudulagunta, S. R., Sodalagunta, M. B., Kumbhat, M. & Settikere Nataraju, A. Posterior reversible encephalopathy syndrome (PRES). Oxf. Med. Case Rep. 2017, omx011 (2017).
Salanti, A. et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell 28, 500–514 (2015).
Srivastava, A. et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. USA 107, 4884–4889 (2010).
Fried, M. & Duffy, P. E. Adherence of Plasmodium falciparum to Chondroitin sulfate A in the human placenta. Science 272, 1502–1504 (1996).
Odorizzi, P. M. & Feeney, M. E. Impact of in utero exposure to malaria on fetal T cell immunity. Trends Mol. Med. 22, 877–888 (2016).
Berhe, A. D. & Doritchamou, J. Y. Malaria in pregnancy: adverse pregnancy outcomes and the future of prevention. Front. Trop. Dis. 4, 1229735 (2023).
Dellicour, S., Tatem, A. J., Guerra, C. A., Snow, R. W. & ter Kuile, F. O. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 7, e1000221 (2010).
Kamga, S. L. S. et al. Uptake of intermittent preventive treatment of malaria in pregnancy and risk factors for maternal anaemia and low birthweight among HIV-negative mothers in Dschang, West region of Cameroon: a cross-sectional study. Malar. J. 23, 6 (2024).
Ma, R. et al. Structural basis for placental malaria mediated by Plasmodium falciparum VAR2CSA. Nat. Microbiol. 6, 380–391 (2021).
Wang, K. et al. Cryo-EM reveals the architecture of placental malaria VAR2CSA and provides molecular insight into chondroitin sulfate binding. Nat. Commun. 12, 2956 (2021).
Dorin-Semblat, D. et al. Phosphorylation of the VAR2CSA extracellular region is associated with enhanced adhesive properties to the placental receptor CSA. PLoS Biol. 17, e3000308 (2019).
Elzein, F. et al. Pulmonary manifestation of Plasmodium falciparum malaria: case reports and review of the literature. Respir. Med. Case Rep. 22, 83–86 (2017).
Vandermosten, L. et al. Experimental malaria-associated acute respiratory distress syndrome is dependent on the parasite-host combination and coincides with normocyte invasion. Malar. J. 17, 102 (2018).
Hoffmeister, B. Respiratory distress complicating falciparum malaria imported to berlin, germany: incidence, burden, and risk factors. Microorganisms 11 (2023).
Van den Steen, P. E. et al. Pathogenesis of malaria-associated acute respiratory distress syndrome. Trends Parasitol. 29, 346–358 (2013).
MacPherson, G. G., Warrell, M. J., White, N. J., Looareesuwan, S. & Warrell, D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119, 385–401 (1985).
Maknitikul, S., Luplertlop, N., Grau, G. E. R. & Ampawong, S. Dysregulation of pulmonary endothelial protein C receptor and thrombomodulin in severe falciparum malaria-associated ARDS relevant to hemozoin. PLoS One 12, e0181674 (2017).
Taylor, W. R., Cañon, V. & White, N. J. Pulmonary manifestations of malaria : recognition and management. Treat. Respir. Med. 5, 419–428 (2006).
Graça, L. et al. Descriptive Acute Respiratory Distress Syndrome (ARDS) in adults with imported severe Plasmodium falciparum malaria: a 10 year-study in a Portuguese tertiary care hospital. PLoS One 15, e0235437 (2020).
Maitland, K. et al. Mortality after fluid bolus in African children with severe infection. N. Engl. J. Med. 364, 2483–2495 (2011).
Zaki, S. A. & Shanbag, P. Atypical manifestations of malaria. Res Rep. Trop. Med. 2, 9–22 (2011).
Taylor, W. R. J., Hanson, J., Turner, G. D. H., White, N. J. & Dondorp, A. M. Respiratory manifestations of malaria. Chest 142, 492–505 (2012).
Moxon, C. A., Gibbins, M. P., McGuinness, D., Milner, D. A. Jr & Marti, M. New Insights into malaria pathogenesis. Annu. Rev. Pathol. 15, 315–343 (2020).
Bernabeu, M., Howard, C., Zheng, Y. & Smith, J. D. Bioengineered 3D microvessels for investigating Plasmodium falciparum pathogenesis. Trends Parasitol. 37, 401–413 (2021).
Pettersson, F. et al. Whole-body imaging of sequestration of Plasmodium falciparum in the rat. Infect. Immun. 73, 7736–7746 (2005).
Vogt, A. M. et al. Release of sequestered malaria parasites upon injection of a glycosaminoglycan. PLoS Pathog. 2, e100 (2006).
Claser, C. et al. Lung endothelial cell antigen cross-presentation to CD8+T cells drives malaria-associated lung injury. Nat. Commun. 10, 4241 (2019).
Du, X. et al. PRL2 regulates neutrophil extracellular trap formation which contributes to severe malaria and acute lung injury. Nat. Commun. 15, 881 (2024).
Venugopal, K., Hentzschel, F., Valkiūnas, G. & Marti, M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat. Rev. Microbiol 18, 177–189 (2020).
Dash, M., Sachdeva, S., Bansal, A. & Sinha, A. Gametogenesis in Plasmodium: delving deeper to connect the dots. Front. Cell Infect. Microbiol 12, 877907 (2022).
Kafsack, B. F. et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507, 248–252 (2014).
Josling, G. A. et al. Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nat. Commun. 11, 1503 (2020).
Llorà-Batlle, O. et al. Conditional expression of PfAP2-G for controlled massive sexual conversion in Plasmodium falciparum. Sci. Adv. 6, eaaz5057 (2020).
Xu, Y. et al. PfAP2-G2 is associated to production and maturation of gametocytes in Plasmodium falciparum via regulating the expression of PfMDV-1. Front. Microbiol. 11, 631444 (2020).
Li, Z. et al. Plasmodium transcription repressor AP2-O3 regulates sex-specific identity of gene expression in female gametocytes. EMBO Rep. 22, e51660 (2021).
Yuda, M., Kaneko, I., Iwanaga, S., Murata, Y. & Kato, T. Female-specific gene regulation in malaria parasites by an AP2-family transcription factor. Mol. Microbiol. 113, 40–51 (2020).
Bechtsi, D. P. & Waters, A. P. Genomics and epigenetics of sexual commitment in Plasmodium. Int. J. Parasitol. 47, 425–434 (2017).
Shrestha, S. et al. Distinct histone post-translational modifications during Plasmodium falciparum Gametocyte development. J. Proteome Res. 21, 1857–1867 (2022).
Jeninga, M. D. et al. Plasmodium falciparum gametocytes display global chromatin remodelling during sexual differentiation. BMC Biol. 21, 65 (2023).
Nishi, T., Kaneko, I., Iwanaga, S. & Yuda, M. PbAP2-FG2 and PbAP2R-2 function together as a transcriptional repressor complex essential for Plasmodium female development. PLoS Pathog. 19, e1010890 (2023).
Kaneko, I., Iwanaga, S., Kato, T., Kobayashi, I. & Yuda, M. Genome-wide identification of the target genes of AP2-O, a Plasmodium AP2-family transcription factor. PLoS Pathog. 11, e1004905 (2015).
Yuda, M. et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol. Microbiol. 71, 1402–1414 (2009).
Nishi, T., Kaneko, I., Iwanaga, S. & Yuda, M. Identification of a novel AP2 transcription factor in zygotes with an essential role in Plasmodium ookinete development. PLoS Pathog. 18, e1010510 (2022).
Frischknecht, F. & Matuschewski, K. Plasmodium sporozoite biology. Cold Spring Harb. Perspect. Med. 7 (2017).
Modrzynska, K. et al. A knockout screen of ApiAP2 genes reveals networks of interacting transcriptional regulators controlling the plasmodium life cycle. Cell Host Microbe 21, 11–22 (2017).
Yuda, M., Iwanaga, S., Shigenobu, S., Kato, T. & Kaneko, I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Mol. Microbiol. 75, 854–863 (2010).
Silvie, O., Goetz, K. & Matuschewski, K. A sporozoite asparagine-rich protein controls initiation of plasmodium liver stage development. PLoS Pathog. 4, e1000086 (2008).
Yuda, M., Kaneko, I., Murata, Y., Iwanaga, S. & Nishi, T. Targetome analysis of malaria sporozoite transcription factor AP2-Sp reveals its role as a master regulator. mBio 14 (2023).
Grüring, C. et al. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun. 2, 165 (2011).
Suwanarusk, R. et al. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis. 189, 190–194 (2004).
Zheng, K. et al. Plasmodium falciparum selectively degrades α-spectrin of infected erythrocytes after invasion. mBio 15, e0351023 (2024).
Wang, J. et al. Protein modification characteristics of the malaria parasite Plasmodium falciparum and the infected erythrocytes. Mol. Cell Proteom. 205, 100001 (2021).
Vembar, S. S., Macpherson, C. R., Sismeiro, O., Coppée, J. Y. & Scherf, A. The PfAlba1 RNA-binding protein is an important regulator of translational timing in Plasmodium falciparum blood stages. Genome Biol. 16, 212 (2015).
Das, S., Lemgruber, L., Tay, C. L., Baum, J. & Meissner, M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 15, 70 (2017).
Stortz, J. F. et al. Formin-2 drives polymerisation of actin filaments enabling segregation of apicoplasts and cytokinesis in Plasmodium falciparum. ELife 8, e49030 (2019).
Robbins, J. A., Absalon, S., Streva, V. A. & Dvorin, J. D. The malaria parasite cyclin H homolog Pfcyc1 is required for efficient cytokinesis in blood-stage Plasmodium falciparum. mBio 8, 3 (2017).
Voß, Y., Klaus, S., Guizetti, J. & Ganter, M. Plasmodium schizogony, a chronology of the parasite’s cell cycle in the blood stage. PLoS Pathog. 19, 3 (2023).
Santos, J. M. et al. Red blood cell invasion by the malaria parasite is coordinated by the PfAP2-I transcription factor. Cell Host Microbe 21, 731–741 (2017).
Shang, X. et al. Genome-wide landscape of ApiAP2 transcription factors reveals a heterochromatin-associated regulatory network during Plasmodium falciparum blood-stage development. Nucleic Acids Res. 50, 3413–3431 (2022).
Subudhi, A. K. et al. DNA-binding protein PfAP2-P regulates parasite pathogenesis during malaria parasite blood stages. Nat. Microbiol. 8, 2154–2169 (2023).
Schlott, A. C. et al. Inhibition of protein N-myristoylation blocks Plasmodium falciparum intraerythrocytic development, egress and invasion. PLoS Biol. 19, e3001408 (2021).
Liu, Y. et al. An inner membrane complex protein IMC1g in Plasmodium berghei is involved in asexual stage schizogony and parasite transmission. mBio e02652–02624 (2023).
Cepeda Diaz Ana, K., Rudlaff Rachel, M., Farringer, M. & Dvorin Jeffrey, D. Essential function of alveolin PfIMC1g in the Plasmodium falciparum asexual blood stage. mBio 14, 5 (2023).
Yahata, K. et al. Gliding motility of Plasmodium merozoites. Proc. Natl. Acad. Sci. USA 118, e2114442118 (2021).
Gilson, P. R. & Crabb, B. S. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 39, 91–96 (2009).
Beeson, J. G. et al. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 40, 343–372 (2016).
Shakya, B., Patel, S. D., Tani, Y. & Egan, E. Erythrocyte CD55 mediates the internalization of Plasmodium falciparum parasites. ELife 10, e61516 (2021).
Mohandas, N. & Blanc, L. Parasite hijacks red cell membrane proteins. Blood 142, 1942–1944 (2023).
Gilson, P. R. et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol. Cell Proteom. 5, 1286–1299 (2006).
López, R. et al. Plasmodium falciparum merozoite surface protein 6 (MSP-6) derived peptides bind erythrocytes and partially inhibit parasite invasion. Peptides 27, 1685–1692 (2006).
Vargas-Serrato, E. et al. Merozoite surface protein-9 of Plasmodium vivax and related simian malaria parasites is orthologous to p101/ABRA of P. falciparum. Mol. Biochem. Parasitol. 120, 41–52 (2002).
Lin, C. S. et al. The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by Plasmodium falciparum. J. Biol. Chem. 289, 25655–25669 (2014).
Baldwin, M. R., Li, X., Hanada, T., Liu, S. C. & Chishti, A. H. Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells. Blood 125, 2704–2711 (2015).
Boyle, M. J., Richards, J. S., Gilson, P. R., Chai, W. & Beeson, J. G. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood 115, 4559–4568 (2010).
Dijkman, P. M. et al. Structure of the merozoite surface protein 1 from Plasmodium falciparum. Sci. Adv. 7, eabg0465 (2021).
Das, S. et al. Processing of Plasmodium falciparum merozoite surface protein MSP1 activates a spectrin-binding function enabling parasite Egress from RBCs. Cell Host Microbe 18, 433–444 (2015).
Blackman, M. J. & Holder, A. A. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50, 307–315 (1992).
Lin, C. S. et al. Multiple Plasmodium falciparum merozoite surface protein 1 complexes mediate merozoite binding to human erythrocytes. J. Biol. Chem. 291, 7703–7715 (2016).
Harris, P. K. et al. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 1, e29 (2005).
Mukherjee, S., Nasamu, A. S., Rubiano, K. C. & Goldberg, D. E. Activation of the Plasmodium Egress effector subtilisin-like protease 1 is mediated by Plasmepsin X destruction of the prodomain. mBio 14, e00673–00623 (2023).
Collins, C. R. et al. The malaria parasite sheddase SUB2 governs host red blood cell membrane sealing at invasion. ELife 9, e61121 (2020).
Zhang, Y. et al. Proteomic Analysis of Plasmodium falciparum schizonts reveals heparin-binding merozoite proteins. J. Proteome Res. 12, 2185–2193 (2013).
Vogt, A. M. et al. Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1alpha domain of PfEMP1. Blood 101, 2405–2411 (2003).
Coppi, A. et al. Heparan sulfate proteoglycans provide a signal to plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2, 316–327 (2007).
Gao, J. et al. A heparin-binding protein of Plasmodium berghei is associated with merozoite invasion of erythrocytes. Parasites Vectors 16, 277 (2023).
Wang, X. et al. Enhanced antimalarial efficacy obtained by targeted delivery of artemisinin in heparin-coated magnetic hollow mesoporous nanoparticles. ACS Appl. Mater. Interfaces 13, 287–297 (2021).
Adda, C. G. et al. Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils. Mol. Biochem. Parasitol. 166, 159–171 (2009).
Lopaticki, S. et al. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect. Immun. 79, 1107–1117 (2011).
Duraisingh, M. T., Maier, A. G., Triglia, T. & Cowman, A. F. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl Acad. Sci. USA 100, 4796–4801 (2003).
Wright, G. J. & Rayner, J. C. Plasmodium falciparum erythrocyte invasion: combining function with immune evasion. PLoS Pathog. 10, e1003943 (2014).
Singh, S., Alam, M. M., Pal-Bhowmick, I., Brzostowski, J. A. & Chitnis, C. E. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 6, e1000746 (2010).
Tolia, N. H., Enemark, E. J., Sim, B. K. & Joshua-Tor, L. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122, 183–193 (2005).
Paing, M. M. et al. Shed EBA-175 mediates red blood cell clustering that enhances malaria parasite growth and enables immune evasion. ELife 7, e43224 (2018).
Baro, B. et al. Plasmodium falciparum exploits CD44 as a coreceptor for erythrocyte invasion. Blood 142, 2016–2028 (2023).
Dolan, S. A. et al. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol. 64, 55–63 (1994).
Mayer, D. C. G. et al. Polymorphism in the Plasmodium falciparum erythrocyte-binding ligand JESEBL/EBA-181 alters its receptor specificity. Proc. Natl. Acad. Sci. USA 101, 2518–2523 (2004).
Rydzak, J. et al. The baculovirus-expressed binding region of Plasmodium falciparum EBA-140 ligand and its glycophorin C binding specificity. PLoS One 10, e0115437 (2015).
Proto, W. R. et al. Adaptation of Plasmodium falciparum to humans involved the loss of an ape-specific erythrocyte invasion ligand. Nat. Commun. 10, 4512 (2019).
Sahar, T. et al. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS One 6, e17102 (2011).
Aniweh, Y. et al. Analysis of Plasmodium falciparum Rh2b deletion polymorphism across different transmission areas. Sci. Rep. 10, 1498 (2020).
Lamarque, M. et al. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 7, e1001276 (2011).
Fernandes, P. et al. The AMA1-RON complex drives Plasmodium sporozoite invasion in the mosquito and mammalian hosts. PLoS Pathog. 18, e1010643 (2022).
Krishnamurthy, S. et al. Not a simple tether: binding of Toxoplasma gondii AMA1 to RON2 during invasion protects AMA1 from rhomboid-mediated clvikeavage and leads to dephosphorylation of its Cytosolic Tail. mBio 7 (2016).
Weiss, G. E. et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 11 (2015).
Richard, D. et al. Identification of Rhoptry Trafficking Determinants and Evidence for a Novel Sorting Mechanism in the Malaria Parasite Plasmodium falciparum. PLoS Pathog. 5 (2009).
Mita-Mendoza, N. K. et al. Dimethyl fumarate reduces TNF and Plasmodium falciparum-induced brain endothelium activation in vitro. Malar. J. 19, 376 (2020).
Fiedler, U. et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat. Med. 12, 235–239 (2006).
Tchinda, V. H. M. et al. Severe malaria in Cameroonian children: correlation between plasma levels of three soluble inducible adhesion molecules and TNF-α. Acta Trop. 102, 20–28 (2007).
Pais, T. F. et al. Brain endothelial STING1 activation by Plasmodium-sequestered heme promotes cerebral malaria via type I IFN response. Proc. Natl. Acad. Sci. USA 119, e2206327119 (2022).
He, X. et al. RTP4 inhibits IFN-I response and enhances experimental cerebral malaria and neuropathology. Proc. Natl. Acad. Sci. USA 117, 19465–19474 (2020).
Belnoue, E. et al. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol. 30, 544–553 (2008).
Chen, Q., Schlichtherle, M. & Wahlgren, M. Molecular aspects of severe malaria. Clin. Microbiol. Rev. 13, 439–450 (2000).
Wang, Y. et al. Neurons upregulate PD-L1 via IFN/STAT1/IRF1 to alleviate damage by CD8+ T cells in cerebral malaria. J. Neuroinflamm. 21, 119 (2024).
Chen, Q. et al. The Semiconserved Head Structure of Plasmodium falciparum Erythrocyte Membrane Protein 1 Mediates Binding to Multiple Independent Host Receptors. J. Exp. Med. 192, 1–10 (2000).
Smith, J. D. et al. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA 97, 1766–1771 (2000).
Wassmer, S. C. et al. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am. J. Trop. Med. Hyg. 93, 42–56 (2015).
Jensen, A. R., Adams, Y. & Hviid, L. Cerebral Plasmodium falciparum malaria: the role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it. Immunol. Rev. 293, 230–252 (2020).
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002).
Albrecht, L. et al. Var gene transcription and PfEMP1 expression in the rosetting and cytoadhesive Plasmodium falciparum clone FCR3S1. 2. Malar. J. 10, 1–9 (2011).
Joergensen, L. M. et al. The kinetics of antibody binding to Plasmodium falciparum VAR2CSA PfEMP1 antigen and modelling of PfEMP1 antigen packing on the membrane knobs. Malar. J. 9, 1–12 (2010).
Chen, Q. et al. Developmental selection of var gene expression in Plasmodium falciparum. Nature 394, 392–395 (1998).
Pasternak, N. D. & Dzikowski, R. PfEMP1: an antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum. Int. J. Biochem. Cell Biol. 41, 1463–1466 (2009).
Kraemer, S. M. & Smith, J. D. J. C. o. i. m. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9, 374–380 (2006).
Lavstsen, T., Salanti, A., Jensen, A. T., Arnot, D. E. & Theander, T. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 2, 1–14 (2003).
Jensen, A. T. et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199, 1179–1190 (2004).
Rottmann, M. et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect. Immun. 74, 3904–3911 (2006).
Smith, J. D. et al. Analysis of adhesive domains from the A4VAR Plasmodium falciparum erythrocyte membrane protein-1 identifies a CD36 binding domain. Mol. Biochem. Parasitol. 97, 133–148 (1998).
Baruch, D. I. et al. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood 90, 3766–3775 (1997).
Ockenhouse, C. F. et al. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-l. J. Infect. Dis. 164, 163–169 (1991).
Quintana, M. D. P., Angeletti, D., Moll, K., Chen, Q. & Wahlgren, M. Phagocytosis-inducing antibodies to Plasmodium falciparum upon immunization with a recombinant PfEMP1 NTS-DBL1α domain. Malar. J. 15, 416 (2016).
Stucke, E. M. et al. Serologic responses to the PfEMP1 DBL-CIDR head structure may be a better indicator of malaria exposure than those to the DBL-α tag. Malar. J. 18, 273 (2019).
Hviid, L. & Lopez-Perez, M. Analysis by flow cytometry of α(2)-macroglobulin and nonimmune IgM-binding to Plasmodium falciparum-infected erythrocytes. Methods Mol. Biol. 2470, 435–444 (2022).
Harmsen, C. et al. Immunization with virus-like particles conjugated to CIDRα1 domain of Plasmodium falciparum erythrocyte membrane protein 1 induces inhibitory antibodies. Malar. J. 19, 132 (2020).
Kinyua, A. W. et al. Antibodies to PfEMP1 and variant surface antigens: protection after controlled human malaria infection in semi-immune Kenyan adults. J. Infect. 106252 (2024).
Kwiatkowski, D. Malaria: becoming more specific about non-specific immunity. Curr. Opin. Immunol. 4, 425–431 (1992).
Urban, B. C. & Roberts, D. J. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr. Opin. Immunol. 14, 458–465 (2002).
Mawson, A. R. The pathogenesis of malaria: a new perspective. Pathog. Glob. Health 107, 122–129 (2013).
Long, C. A. & Zavala, F. Immune responses in malaria. Cold Spring Harb. Perspect. Med. 7, a025577 (2017).
Overstreet, M. G., Cockburn, I. A., Chen, Y. C. & Zavala, F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 225, 272–283 (2008).
Hassert, M., Arumugam, S. & Harty, J. T. Memory CD8+ T cell-mediated protection against liver-stage malaria. Immunol. Rev. 316, 84–103 (2023).
Charoenvit, Y. et al. CD4+ T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect. Immun. 67, 5604–5614 (1999).
Wykes, M. N. & Lewin, S. R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 18, 91–104 (2018).
Li, Q. et al. SOD3 suppresses early cellular immune responses to parasite infection. Nat. Commun. 15, 4913 (2024).
Sun, L., Su, Y., Jiao, A., Wang, X. & Zhang, B. T cells in health and disease. Signal Transduct. Target Ther. 8, 235 (2023).
Hou, N. et al. Low-complexity repetitive epitopes of Plasmodium falciparum are decoys for humoural immune responses. Front Immunol. 11, 610 (2020).
Dooley, N. L. et al. Single-cell transcriptomics shows that malaria promotes unique regulatory responses across multiple immune cell subsets. Nat. Commun. 14, 7387 (2023).
Drewry, L. L., Pewe, L. L., Hancox, L. S., Van de Wall, S. & Harty, J. T. CD4 T cell-dependent and -independent roles for IFN-γ in blood-stage malaria. J. Immunol. 210, 1305–1313 (2023).
Deng, S., Graham, M. L. & Chen, X. M. The complexity of interferon signaling in host defense against protozoan parasite infection. Pathogens 12, 319 (2023).
D’Ombrain, M. C. et al. Association of early interferon-γ production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin. Infect. Dis. 47, 1380–1387 (2008).
Mitchell, A. J. et al. Early cytokine production is associated with protection from murine cerebral malaria. Infect. Immun. 73, 5645–5653 (2005).
Chelimo, K., Sumba, P. O., Kazura, J. W., Ofula, A. V. & John, C. C. Interferon-gamma responses to Plasmodium falciparum liver-stage antigen-1 and merozoite-surface protein-1 increase with age in children in a malaria holoendemic area of western Kenya. Malar. J. 2, 37 (2003).
Ibraheem, Y., Bayarsaikhan, G. & Inoue, S.-I. Host immunity to plasmodium infection: contribution of Plasmodium berghei to our understanding of T cell-related immune response to blood-stage malaria. Parasitol. Int 92, 102646 (2023).
Ndungu, F. M. et al. A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTS,S/AS01E. PLoS One 7, e52870 (2012).
Arroyo, E. N. & Pepper, M. B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection. J. Exp. Med. 217, e20190849 (2020).
Carvalho, L. H. et al. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8, 166–170 (2002).
Overstreet, M. G., Chen, Y. C., Cockburn, I. A., Tse, S. W. & Zavala, F. CD4+ T cells modulate expansion and survival but not functional properties of effector and memory CD8+ T cells induced by malaria sporozoites. PLoS One 6, e15948 (2011).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Lönnberg, T. et al. Single-cell RNA-seq and computational analysis using temporal mixture modeling resolves TH1/TFH fate bifurcation in malaria. Sci. Immunol. 2, eaal2192 (2017).
Pérez-Mazliah, D. et al. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog. 11, e1004715 (2015).
Finney, O. C., Nwakanma, D., Conway, D. J., Walther, M. & Riley, E. M. Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur. J. Immunol. 39, 1288–1300 (2009).
Gonçalves, R. M. et al. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses?. Infect. Immun. 78, 4763–4772 (2010).
Chauhan, R. et al. CD4+ICOS+Foxp3+: a sub-population of regulatory T cells contribute to malaria pathogenesis. Malar. J. 21, 32 (2022).
Sedegah, M. et al. Naturally acquired CD8+ cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. J. Immunol. 149, 966–971 (1992).
Doolan, D. L. et al. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 7, 97–112 (1997).
Romero, P. et al. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341, 323–326 (1989).
Doolan, D. L. et al. HLA-DR-promiscuous T cell epitopes from Plasmodium falciparum pre-erythrocytic-stage antigens restricted by multiple HLA class II alleles. J. Immunol. 165, 1123–1137 (2000).
Doolan, D. L. et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. USA 100, 9952–9957 (2003).
Digitale, J. C. et al. HLA alleles B(*)53:01 and C(*)06:02 are associated with higher risk of P. falciparum Parasitemia in a cohort in Uganda. Front. Immunol. 12, 650028 (2021).
Doolan, D. L. & Hoffman, S. L. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165, 1453–1462 (2000).
Epstein, J. E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⁺ T cell immunity. Science 334, 475–480 (2011).
Hafalla, J. C. et al. Identification of targets of CD8⁺ T cell responses to malaria liver stages by genome-wide epitope profiling. PLoS Pathog. 9, e1003303 (2013).
Van Braeckel-Budimir, N. & Harty, J. T. CD8 T-cell-mediated protection against liver-stage malaria: lessons from a mouse model. Front. Microbiol 5, 272 (2014).
Villarino, N. & Schmidt, N. W. CD8+ T cell responses to Plasmodium and Intracellular Parasites. Curr. Immunol. Rev. 9, 169–178 (2013).
Ishizuka, A. S. et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 22, 614–623 (2016).
Weiss, W. R. & Jiang, C. G. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One 7, e31247 (2012).
Weiss, W. R., Sedegah, M., Beaudoin, R. L., Miller, L. H. & Good, M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl Acad. Sci. USA 85, 573–576 (1988).
Schmidt, N. W., Butler, N. S., Badovinac, V. P. & Harty, J. T. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 6, e1000998 (2010).
Mouwenda, Y. D. et al. Immune responses associated with protection induced by chemoattenuated PfSPZ vaccine in malaria-naive Europeans. JCI insight 9 (2024).
Zhang, N. & Bevan, M. J. CD8+ T cells: foot soldiers of the immune system. Immunity 35, 161–168 (2011).
Chakravarty, S. et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 13, 1035–1041 (2007).
Radtke, A. J. et al. Lymph-node resident CD8α+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS Pathog. 11, e1004637 (2015).
Butler, N. S. et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011).
Douradinha, B. et al. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int. J. Parasitol. 37, 1511–1519 (2007).
Holz, L. E., Fernandez-Ruiz, D. & Heath, W. R. Protective immunity to liver-stage malaria. Clin. Transl. Immunol. 5, e105 (2016).
Ryg-Cornejo, V. et al. NK cells and conventional dendritic cells engage in reciprocal activation for the induction of inflammatory responses during Plasmodium berghei ANKA infection. Immunobiology 218, 263–271 (2013).
Morrot, A., Hafalla, J. C., Cockburn, I. A., Carvalho, L. H. & Zavala, F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J. Exp. Med. 202, 551–560 (2005).
da Silva, H. B. et al. Early skin immunological disturbance after Plasmodium-infected mosquito bites. Cell Immunol. 277, 22–32 (2012).
Kyriacou, H. M. et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol. Biochem. Parasitol. 150, 211–218 (2006).
Bertin, G. I. et al. Proteomic analysis of Plasmodium falciparum parasites from patients with cerebral and uncomplicated malaria. Sci. Rep. 6, 26773 (2016).
Mazier, D., Nitcheu, J. & Idrissa-Boubou, M. Cerebral malaria and immunogenetics. Parasite Immunol. 22, 613–623 (2000).
Mackey, L. J., Hochmann, A., June, C. H., Contreras, C. E. & Lambert, P. H. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin. Exp. Immunol. 42, 412–420 (1980).
Fain, C. E. et al. Discrete class I molecules on brain endothelium differentially regulate neuropathology in experimental cerebral malaria. Brain 147, 566–589 (2023).
Wang, J. et al. CD8+ T cell infiltration and proliferation in the brainstem during experimental cerebral malaria. CNS Neurosci. Ther. 30, e14431 (2024).
Butler, N. S., Schmidt, N. W. & Harty, J. T. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P. yoelii sporozoites. J. Immunol. 184, 2528–2538 (2010).
Mellouk, S. et al. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J. Immunol. 139, 4192–4195 (1987).
Lefebvre, M. N. et al. Expeditious recruitment of circulating memory CD8 T+ cells to the liver facilitates control of malaria. Cell Rep. 37, 109956 (2021).
Brandi, J. et al. T cells expressing multiple co-inhibitory molecules in acute malaria are not exhausted but exert a suppressive function in mice. Eur. J. Immunol. 52, 312–327 (2022).
Chien, Y. H., Meyer, C. & Bonneville, M. γδ T cells: first line of defense and beyond. Annu. Rev. Immunol. 32, 121–155 (2014).
Holtmeier, W. & Kabelitz, D. gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 86, 151–183 (2005).
Hayday, A. C. γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 (2000).
Carding, S. R. & Egan, P. J. γδT cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 (2002).
Hviid, L. et al. Perturbation and proinflammatory type activation of V delta 1+ γδ T cells in African children with Plasmodium falciparum malaria. Infect. Immun. 69, 3190–3196 (2001).
Roussilhon, C., Agrapart, M., Ballet, J. J. & Bensussan, A. T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparum malaria. J. Infect. Dis. 162, 283–285 (1990).
Goodier, M. et al. Γδ T cells in the peripheral blood of individuals from an area of holoendemic Plasmodium falciparum transmission. Trans. R. Soc. Trop. Med. Hyg. 87, 692–696 (1993).
Schwartz, E., Sadetzki, S., Murad, H. & Raveh, D. Age as a risk factor for severe Plasmodium falciparum malaria in nonimmune patients. Clin. Infect. Dis. 33, 1774–1777 (2001).
Teirlinck, A. C. et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 7, e1002389 (2011).
Nana, C. M. M. et al. Phenotypic changes of γδ T cells in Plasmodium falciparum placental malaria and pregnancy outcomes in women at delivery in Cameroon. Front. Immunol. 15 (2024).
Inoue, S. I., Niikura, M., Asahi, H., Kawakami, Y. & Kobayashi, F. γδ T cells modulate humoral immunity against Plasmodium berghei infection. Immunology 155, 519–532 (2018).
Mamedov, M. R. et al. A macrophage colony-stimulating-factor-producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity 48, 350–363 (2018).
Zaidi, I. et al. γδ T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J. Immunol. 199, 3781–3788 (2017).
Moormann, A. M., Nixon, C. E. & Forconi, C. S. Immune effector mechanisms in malaria: an update focusing on human immunity. Parasite Immunol. 41, e12628 (2019).
Beeson, J. G., Chan, J.-A. & Fowkes, F. PfEMP1 as a target of human immunity and a vaccine candidate against malaria. Expert Rev. Vaccines 12, 105–108 (2013).
Holder, A. J. P. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology 136, 1445–1456 (2009).
Woehlbier, U., Epp, C., Hackett, F., Blackman, M. J. & Bujard, H. J. M. j. Antibodies against multiple merozoite surface antigens of the human malaria parasite Plasmodium falciparum inhibit parasite maturation and red blood cell invasion. Malar. J. 9, 77 (2010).
Wang, L. T., Idris, A. H., Kisalu, N. K., Crompton, P. D. & Seder, R. Monoclonal antibodies to the circumsporozoite proteins as an emerging tool for malaria prevention. Nat. Immunol. 25, 1–16 (2024).
Wang, L. T. et al. A potent anti-malarial human monoclonal antibody targets circumsporozoite protein minor repeats and neutralizes sporozoites in the liver. Immunity 53, 733–744. e738 (2020).
Chatterjee, D. & Cockburn, I. The challenges of a circumsporozoite protein-based malaria vaccine. Vaccines 20, 113–125 (2021).
Chaudhury, S. et al. The biological function of antibodies induced by the RTS, S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar. J. 15, 1–12 (2016).
Douglas, A. D. et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun. 2, 601 (2011).
Kurtovic, L. et al. Complement in malaria immunity and vaccines. Immunol. Rev. 293, 38–56 (2020).
Hill, D. L., Schofield, L. & Wilson, D. W. IgG opsonization of merozoites: multiple immune mechanisms for malaria vaccine development. Int. J. Parasitol. 47, 585–595 (2017).
Doolan, D. L., Dobaño, C. & Baird, J. K. Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–36 (2009).
Patel, P. N. et al. Structure-based design of a strain-transcending AMA1-RON2L malaria vaccine. Nat. Commun. 14, 5345 (2023).
Achtman, A. H., Khan, M., MacLennan, I. & Langhorne, J. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J. Immunol. 171, 317–324 (2003).
Donati, D. et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect. Immun. 72, 5412–5418 (2004).
Pack, A. D. et al. Hemozoin-mediated inflammasome activation limits long-lived anti-malarial immunity. Cell Rep. 36 (2021).
Vijay, R. et al. Infection-induced plasmablasts are a nutrient sink that impairs humoral immunity to malaria. Nat. Immunol. 21, 790–801 (2020).
Chin, S. S., Chorro, L., Chan, J. & Lauvau, G. Splenic innate B1 B cell plasmablasts produce sustained granulocyte-macrophage colony-stimulating factor and interleukin-3 cytokines during murine malaria infections. Infect. Immun. 87 (2019).
Cockburn, I. A. & Seder, R. A. Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 19, 1199–1211 (2018).
Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015).
Kurtovic, L. et al. Multifunctional antibodies are Iinduced by the RTS,S malaria vaccine and associated with protection in a phase 1/2a trial. J. Infect. Dis. 224, 1128–1138 (2021).
Das, J. et al. Delayed fractional dosing with RTS,S/AS01 improves humoral immunity to malaria via a balance of polyfunctional NANP6- and Pf16-specific antibodies. Medicines 2, 1269–1286.e1269 (2021).
Bell, G. J. et al. Background malaria incidence and parasitemia during the three-dose RTS,S/AS01 vaccination series do not reduce magnitude of antibody response nor efficacy against the first case of malaria. BMC Infect. Dis. 23, 716 (2023).
Blank, A. et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. npj Vaccines 5, 10 (2020).
Seidel-Greven, M. et al. Isolation and light chain shuffling of a Plasmodium falciparum AMA1-specific human monoclonal antibody with growth inhibitory activity. Malar. J. 20, 37 (2021).
Milán-Noris, E. M. et al. An AMA1/MSP1(19) adjuvanted malaria transplastomic plant-based vaccine induces immune responses in test animals. Mol. Biotechnol. 62, 534–545 (2020).
Salinas, N. D., Paing, M. M., Adhikari, J., Gross, M. L. & Tolia, N. Moderately neutralizing epitopes in nonfunctional regions dominate the antibody response to Plasmodium falciparum EBA-140. Infect. Immun. 87, e00716 (2019).
Silk, S. E. et al. Blood-stage malaria vaccine candidate RH5.1/Matrix-M in healthy Tanzanian adults and children; an open-label, non-randomised, first-in-human, single-centre, phase 1b trial. Lancet Infect. Dis. 24, 1105–1117 (2024).
Natama, H. M. et al. Safety and efficacy of the blood-stage malaria vaccine RH5.1/Matrix-M in Burkina Faso: interim results of a double-blind, randomised, controlled, phase 2b trial in children. Lancet Infect. Dis.S1473-3099(24)00752-7.
Williams, B. G. et al. Development of an improved blood-stage malaria vaccine targeting the essential RH5-CyRPA-RIPR invasion complex. Nat. Commun. 15, 4857 (2024).
Bender, N. G. et al. Immunofocusing humoral immunity potentiates the functional efficacy of the AnAPN1 malaria transmission-blocking vaccine antigen. NPJ Vaccines 6, 49 (2021).
Kuamsab, N., Putaporntip, C., Pattanawong, U. & Jongwutiwes, S. Insights into the molecular diversity of Plasmodium vivax merozoite surface protein-3γ (pvmsp3γ), a polymorphic member in the msp3 multi-gene family. Sci. Rep. 10, 10977 (2020).
Kuamsab, N. et al. Anti-Plasmodium vivax merozoite surface protein 3γ (PvMSP3 γ) antibodies upon natural infection. Sci. Rep. 14, 9595 (2024).
Uwase, J. et al. Immunogenicity analysis of conserved fragments in Plasmodium ovale species merozoite surface protein 4. Malar. J. 19, 126 (2020).
Kanoi, B. N. et al. Global repertoire of human antibodies against Plasmodium falciparum RIFINs, SURFINs, and STEVORs in a malaria-exposed population. Front. Immunol. 11, 893 (2020).
Langhorne, J., Ndungu, F. M., Sponaas, A.-M. & Marsh, K. Immunity to malaria: more questions than answers. Nat. Immunol. 9, 725–732 (2008).
Sagara, I. et al. The anti-circumsporozoite antibody response of children to seasonal vaccination with the RTS,S/AS01E malaria vaccine. Clin. Infect. Dis. 75, 613–622 (2022).
Bruun, T. U. J., Andersson, A. C., Draper, S. J. & Howarth, M. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination. ACS Nano 12, 8855–8866 (2018).
McGrath, J. J. C., Li, L. & Wilson, P. C. Memory B cell diversity: insights for optimized vaccine design. Trends Immunol. 43, 343–354 (2022).
Britto, C. & Alter, G. The next frontier in vaccine design: blending immune correlates of protection into rational vaccine design. Curr. Opin. Immunol. 78, 102234 (2022).
Seringe, E. et al. Severe imported Plasmodium falciparum malaria, France, 1996-2003. Emerg. Infect. Dis. 17, 807–813 (2011).
Haldar, K., Bhattacharjee, S. & Safeukui, I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 16, 156–170 (2018).
Ashley, E. A. & Poespoprodjo, J. A. Treatment and prevention of malaria in children. Lancet Child Adolesc. Health 4, 775–789 (2020).
Kovacs, S. D., Rijken, M. J. & Stergachis, A. Treating severe malaria in pregnancy: a review of the evidence. Drug Saf. 38, 165–181 (2015).
Ojara, F. W., Kawuma, A. N. & Waitt, C. Systematic review on maternal-to-infant transfer of drugs through breast milk during the treatment of malaria, tuberculosis, and neglected tropical diseases. PLoS Negl. Trop. Dis. 17, e0011449 (2023).
Derbie, A. et al. Therapeutic Efficacy of Artemether-Lumefantrine (Coartem®) for the treatment of uncomplicated falciparum malaria in Africa: a systematic review. J. Parasitol. Res 2020, 7371681 (2020).
Blanshard, A. & Hine, P. Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst. Rev. 1, CD004529 (2021).
Commons, R. J. et al. Effect of primaquine dose on the risk of recurrence in patients with uncomplicated Plasmodium vivax: a systematic review and individual patient data meta-analysis. Lancet Infect. Dis. 24, 172–183 (2024).
Castro, L., Ridpath, A., Mace, K. & Gutman, J. R. Have you heard the news? Artemether-lumefantrine is now recommended for ALL uncomplicated malaria in the United States, including in pregnancy. Clin. Infect. Dis. 78, 245–247 (2023).
McGready, R. et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med. 5, e253 (2008).
Borrmann, S. et al. The effect of food consumption on lumefantrine bioavailability in African children receiving artemether-lumefantrine crushed or dispersible tablets (Coartem) for acute uncomplicated Plasmodium falciparum malaria. Trop. Med. Int. Health 15, 434–441 (2010).
Ashley, E. A. et al. How much fat is necessary to optimize lumefantrine oral bioavailability?. Trop. Med. Int. Health 12, 195–200 (2007).
Leshem, E., Meltzer, E., Stienlauf, S., Kopel, E. & Schwartz, E. Effectiveness of short prophylactic course of atovaquone-proguanil in travelers to sub-Saharan Africa. J. Travel. Med. 21, 82–85 (2014).
McKeage, K. & Scott, L. Atovaquone/proguanil: a review of its use for the prophylaxis of Plasmodium falciparum malaria. Drugs 63, 597–623 (2003).
Mayer, R. C., Tan, K. R. & Gutman, J. R. Safety of atovaquone-proguanil during pregnancy. J. Travel. Med. 26, tay138 (2019).
McKinney, K. L., Wu, H. M., Tan, K. R. & Gutman, J. R. Malaria in the pregnant traveler. J. Travel. Med. 27, taaa074 (2020).
Chen, L. H. et al. Breastfeeding travelers: precautions and recommendations. J. Travel. Med. 17, 32–47 (2009).
Ashley, E. A., Recht, J. & White, N. J. Primaquine: the risks and the benefits. Malar. J. 13, 418 (2014).
Gilder, M. E. et al. Primaquine pharmacokinetics in lactating women and breastfed infant exposures. Clin. Infect. Dis. 67, 1000–1007 (2018).
Yeung, S., Van Damme, W., Socheat, D., White, N. J. & Mills, A. Cost of increasing access to artemisinin combination therapy: the Cambodian experience. Malar. J. 7, 84 (2008).
Apinjoh, T. O., Ouattara, A., Titanji, V. P. K., Djimde, A. & Amambua-Ngwa, A. Genetic diversity and drug resistance surveillance of Plasmodium falciparum for malaria elimination: is there an ideal tool for resource-limited sub-Saharan Africa?. Malar. J. 18, 217 (2019).
Duffey, M. et al. Assessing risks of Plasmodium falciparum resistance to select next-generation antimalarials. Trends Parasitol. 37, 709–721 (2021).
Buyon, L. E., Elsworth, B. & Duraisingh, M. T. The molecular basis of antimalarial drug resistance in Plasmodium vivax. Int. J. Parasitol. Drugs Drug Resist. 16, 23–37 (2021).
Li, J. W. & Vederas, J. C. Drug discovery and natural products: end of an era or an endless frontier?. Science 325, 161–165 (2009).
Watts, R. E. et al. Safety and parasite clearance of artemisinin-resistant Plasmodium falciparum infection: a pilot and a randomised volunteer infection study in Australia. PLoS Med. 17, e1003203 (2020).
Siqueira-Neto, J. L. et al. Antimalarial drug discovery: progress and approaches. Nat. Rev. Drug Discov. 22, 807–826 (2023).
Yang, T. et al. Decreased K13 abundance reduces hemoglobin catabolism and proteotoxic stress, underpinning artemisinin resistance. Cell Rep. 29, 2917–2928 (2019).
Ariey, F. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505, 50–55 (2014).
Ghorbal, M. et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat. Biotechnol. 32, 819–821 (2014).
Straimer, J. et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347, 428–431 (2015).
Imwong, M. et al. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study. Lancet Infect. Dis. 20, 1470–1480 (2020).
Stokes, B. H., Ward, K. E. & Fidock, D. A. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 386, 1385–1386 (2022).
Stokes, B. H. et al. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. ELife 10, e66277 (2021).
Mok, S. et al. Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 347, 431–435 (2015).
Reyser, T. et al. Identification of compounds active against quiescent artemisinin-resistant Plasmodium falciparum parasites via the quiescent-stage survival assay (QSA). J. Antimicrob. Chemother. 75, 2826–2834 (2020).
Xie, S. C., Ralph, S. A. & Tilley, L. K13, the cytostome, and artemisinin resistance. Trends Parasitol. 36, 533–544 (2020).
Hott, A. et al. Artemisinin-resistant Plasmodium falciparum parasites exhibit altered patterns of development in infected erythrocytes. Antimicrob. Agents Chemother. 59, 3156–3167 (2015).
Connelly et al. Restructured mitochondrial-nuclear interaction in Plasmodium falciparum dormancy and persister survival after artemisinin exposure. mBio 12, e0075321 (2021).
Mok, S. et al. Artemisinin-resistant K13 mutations rewire Plasmodium falciparum’s intra-erythrocytic metabolic program to enhance survival. Nat. Commun. 12, 530 (2021).
Posner, G. H. et al. Mechanism-based design, synthesis, and in vitro antimalarial testing of new 4-methylated trioxanes structurally related to artemisinin: the importance of a carbon-centered radical for antimalarial activity. J. Med. Chem. 37, 1256–1258 (1994).
Yu, X. et al. Ring-stage growth arrest: metabolic basis of artemisinin tolerance in Plasmodium falciparum. iScience 26, 105725 (2023).
Small-Saunders, J. L. et al. tRNA modification reprogramming contributes to artemisinin resistance in Plasmodium falciparum. Nat. Microbiol. 9, 1483–1498 (2024).
Ross, L. S. et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 9, 3314 (2018).
Shrestha, B. et al. Distribution and temporal dynamics of Plasmodium falciparum chloroquine resistance transporter mutations associated with piperaquine resistance in Northern Cambodia. J. Infect. Dis. 224, 1077–1085 (2021).
Lee, W. C. et al. Plasmodium falciparum rosetting protects schizonts against artemisinin. EBioMedicine 73, 103680 (2021).
Ecker, A., Lehane, A. M., Clain, J. & Fidock, D. A. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 28, 504–514 (2012).
Fidock, D. A. et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6, 861–871 (2000).
Chaijaroenkul, W. et al. Sequence and gene expression of chloroquine resistance transporter (pfcrt) in the association of in vitro drugs resistance of Plasmodium falciparum. Malar. J. 10, 42 (2011).
Sidhu, A. B., Verdier-Pinard, D. & Fidock, D. A. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298, 210–213 (2002).
Kim, J. et al. Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature 576, 315–320 (2019).
Amambua-Ngwa, A. et al. Chloroquine resistance evolution in Plasmodium falciparum is mediated by the putative amino acid transporter AAT1. Nat. Microbiol. 8, 1213–1226 (2023).
Aubouy, A. et al. DHFR and DHPS genotypes of Plasmodium falciparum isolates from Gabon correlate with in vitro activity of pyrimethamine and cycloguanil, but not with sulfadoxine-pyrimethamine treatment efficacy. J. Antimicrob. Chemother. 52, 43–49 (2003).
Cowman, A. F., Morry, M. J., Biggs, B. A., Cross, G. A. & Foote, S. J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85, 9109–9113 (1988).
Peterson, D. S., Walliker, D. & Wellems, T. E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA 85, 9114–9118 (1988).
Brooks, D. R. et al. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur. J. Biochem. 224, 397–405 (1994).
Triglia, T., Menting, J. G., Wilson, C. & Cowman, A. F. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 94, 13944–13949 (1997).
Picot, S. et al. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar. J. 8, 89 (2009).
Sutherland, C. J. et al. Novel pfdhps haplotypes among imported cases of Plasmodium falciparum malaria in the United Kingdom. Antimicrob. Agents Chemother. 53, 3405–3410 (2009).
Jiang, T. et al. High prevalence of Pfdhfr–Pfdhps quadruple mutations associated with sulfadoxine–pyrimethamine resistance in Plasmodium falciparum isolates from Bioko Island, Equatorial Guinea. Malar. J. 18, 101 (2019).
Singh, I. V. & Mishra, S. Molecular docking analysis of pyrimethamine derivatives with Plasmodium falciparum dihydrofolate reductase. Bioinformation 14, 232–235 (2018).
Pethrak, C. et al. New insights into antimalarial chemopreventive activity of antifolates. Antimicrob. Agents Chemother. 66, e015382 (2022).
Maitland, K. et al. SEVUparin as a potential Adjunctive Treatment in children with severe malaria: A phase I trial safety and dose finding trial (SEVUSMAART). Wellcome Open Res. 8, 484 (2023).
Leitgeb, A. M. et al. Inhibition of merozoite invasion and transient de-sequestration by sevuparin in humans with Plasmodium falciparum malaria. PLos One 12, e0188754 (2017).
Kesely, K. R., Pantaleo, A., Turrini, F. M., Olupot-Olupot, P. & Low, P. S. Inhibition of an erythrocyte tyrosine kinase with imatinib prevents Plasmodium falciparum Egress and terminates parasitemia. PLoS One 11, e0164895 (2016).
Iqbal, N. & Iqbal, N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother. Res Pr. 2014, 357027 (2014).
Ong, H. W., Adderley, J., Tobin, A. B., Drewry, D. H. & Doerig, C. Parasite and host kinases as targets for antimalarials. Expert Opin. Ther. Targets 27, 151–169 (2023).
Chien, H. D. et al. Imatinib augments standard malaria combination therapy without added toxicity. J. Exp. Med. 218 (2021).
Varo, R. et al. Adjunctive rosiglitazone treatment for severe pediatric malaria: a randomized placebo-controlled trial in Mozambican children. Int. J. Infect. Dis. 139, 34–40 (2024).
Varo, R. et al. Safety and tolerability of adjunctive rosiglitazone treatment for children with uncomplicated malaria. Malar. J. 16, 215 (2017).
Varo, R. et al. Adjunctive therapy for severe malaria: a review and critical appraisal. Malar. J. 17, 47 (2018).
Serghides, L. et al. PPARγ agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathog. 10, e1003980 (2014).
Goldgof, G. M. et al. Comparative chemical genomics reveal that the spiroindolone antimalarial KAE609 (Cipargamin) is a P-type ATPase inhibitor. Sci. Rep. 6, 27806 (2016).
Schmitt, E. K. et al. Efficacy of cipargamin (KAE609) in a randomized, phase II dose-escalation study in adults in sub-Saharan Africa with uncomplicated Plasmodium falciparum malaria. Clin. Infect. Dis. 74, 1831–1839 (2022).
Ndayisaba, G. et al. Hepatic safety and tolerability of cipargamin (KAE609), in adult patients with Plasmodium falciparum malaria: a randomized, phase II, controlled, dose-escalation trial in sub-Saharan Africa. Malar. J. 20, 478 (2021).
Bouwman, S. M. et al. The early preclinical and clinical development of cipargamin (KAE609), a novel antimalarial compound. Travel Med. Infect. Dis. 36, 101765 (2020).
Barber, B. E. et al. Safety, pharmacokinetics, and antimalarial activity of the novel triaminopyrimidine ZY-19489: a first-in-human, randomised, placebo-controlled, double-blind, single ascending dose study, pilot food-effect study, and volunteer infection study. Lancet Infect. Dis. 22, 879–890 (2022).
Mombo-Ngoma, G. et al. Efficacy and safety of fosmidomycin-piperaquine as nonartemisinin-based combination therapy for uncomplicated falciparum malaria: a single-arm, age de-escalation proof-of-concept study in Gabon. Clin. Infect. Dis. 66, 1823–1830 (2018).
Ghyslain, M.-N. et al. Fosmidomycin-piperaquine as non-artemisinin-based combination for acute uncomplicated plasmodium falciparum malaria. BMJ Glob. Health 2, (2017).
McCarthy, J. S. et al. Safety, pharmacokinetics, and antimalarial activity of the novel plasmodium eukaryotic translation elongation factor 2 inhibitor M5717: a first-in-human, randomised, placebo-controlled, double-blind, single ascending dose study and volunteer infection study. Lancet Infect. Dis. 21, 1713–1724 (2021).
Khandelwal, A. et al. Translation of liver stage activity of M5717, a Plasmodium elongation factor 2 inhibitor: from bench to bedside. Malar. J. 21, 151 (2022).
Parkyn Schneider, M. et al. The delayed bloodstream clearance of Plasmodium falciparum parasites after M5717 treatment is attributable to the inability to modify their red blood cell hosts. Front. Cell Infect. Microbiol. 13, 1211613 (2023).
Rottmann, M. et al. Preclinical antimalarial combination study of M5717, a Plasmodium falciparum elongation factor 2 inhibitor, and pyronaridine, a hemozoin formation inhibitor. Antimicrob. Agents Chemother. 64, e02181–19 (2020).
Chu, W. Y. & Dorlo, T. P. C. Pyronaridine: a review of its clinical pharmacology in the treatment of malaria. J. Antimicrob. Chemother. 78, 2406–2418 (2023).
Pryce, J., Taylor, M., Fox, T. & Hine, P. Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst. Rev. 6, Cd006404 (2022).
Croft, S. L. et al. Review of pyronaridine anti-malarial properties and product characteristics. Malar. J. 11, 2700 (2012).
Stone, W. et al. Pyronaridine artesunate or dihydroartemisinin piperaquine combined with single low-dose primaquine to prevent Plasmodium falciparum malaria transmission in a four-arm, single-blind, phase 2/3, randomised trial. Lancet Microbe 3, e41–e51 (2022).
Jiménez-Díaz, M. B. et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. USA 111, E5455–E5462 (2014).
Gaur, A. H. et al. Combining SJ733, an oral ATP4 inhibitor of Plasmodium falciparum, with the pharmacokinetic enhancer cobicistat: An innovative approach in antimalarial drug development. EBioMedicine 80, 104065 (2022).
Gaur, A. H. et al. Safety, tolerability, pharmacokinetics, and antimalarial efficacy of a novel Plasmodium falciparum ATP4 inhibitor SJ733: a first-in-human and induced blood-stage malaria phase 1a/b trial. Lancet Infect. Dis. 20, 964–975 (2020).
SheelaNair, A. et al. Similarly efficacious anti-malarial drugs SJ733 and pyronaridine differ in their ability to remove circulating parasites in mice. Malar. J. 21, 49 (2022).
Koita, O. A. et al. AQ-13, an investigational antimalarial, versus artemether plus lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria: a randomised, phase 2, non-inferiority clinical trial. Lancet Infect. Dis. 17, 1266–1275 (2017).
Nardella, F. et al. Cross-resistance of the chloroquine-derivative AQ-13 with amodiaquine in Cambodian Plasmodium falciparum isolates. J. Antimicrob. Chemother. 76, 2565–2568 (2021).
Mengue, J. B., Held, J. & Kreidenweiss, A. AQ-13—an investigational antimalarial drug. Expert Opin. Investig. Drugs 28, 217–222 (2019).
Wu, R. L. et al. Low-dose subcutaneous or intravenous monoclonal antibody to prevent malaria. N. Engl. J. Med. 387, 397–407 (2022).
Kayentao, K. et al. Subcutaneous administration of a monoclonal antibody to prevent malaria. N. Engl. J. Med. 390, 1549–1559 (2024).
Slater, H. C. et al. Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: a modelling study. Lancet Infect. Dis. 20, 498–508 (2020).
Alout, H. & Foy, B. D. Ivermectin: a complimentary weapon against the spread of malaria?. Expert Rev. Anti Infect. Ther. 15, 231–240 (2017).
Foy, B. D. et al. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet 393, 1517–1526 (2019).
Dormoi, J., Amalvict, R., Gendrot, M. & Pradines, B. Methylene blue-based combination therapy with amodiaquine prevents severe malaria in an experimental rodent model. Pharmaceutics 14, 2031 (2022).
Coulibaly, B. et al. Efficacy and safety of triple combination therapy with artesunate-amodiaquine–methylene blue for Falciparum malaria in cildren: a randomized controlled trial in Burkina Faso. J. Infect. Dis. 211, 689–697 (2014).
Zoungrana, A. et al. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. Plos One 3, e1630 (2008).
Dicko, A. et al. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. Lancet Infect. Dis. 18, 627–639 (2018).
Fabbri, C. et al. The activity of methylene blue against asexual and sexual stages of Plasmodium vivax. Front. Cell Infect. Microbiol. 13, 1108366 (2023).
Sangana, R. et al. Pharmacokinetics of ganaplacide and lumefantrine in adults, adolescents, and children with Plasmodium falciparum malaria treated with ganaplacide plus lumefantrine solid dispersion formulation: analysis of data from a multinational phase 2 study. J. Clin. Pharmacol. 65, 179–189 (2024).
Ogutu, B. et al. Ganaplacide (KAF156) plus lumefantrine solid dispersion formulation combination for uncomplicated Plasmodium falciparum malaria: an open-label, multicentre, parallel-group, randomised, controlled, phase 2 trial. Lancet Infect. Dis. 23, 1051–1061 (2023).
Manaranche, J. et al. In vitro evaluation of ganaplacide/lumefantrine combination against Plasmodium falciparum in a context of artemisinin resistance. J. Antimicrob. Chemother. 79, 2877–2886 (2024).
LaMonte, G. M. et al. Pan-active imidazolopiperazine antimalarials target the Plasmodium falciparum intracellular secretory pathway. Nat. Commun. 11, 1780 (2020).
González, R. et al. Safety and efficacy of dihydroartemisinin piperaquine for intermittent preventive treatment of malaria in pregnant women with HIV from Gabon and Mozambique: a randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 24, 476–487 (2024).
Nair, S. et al. Single-cell genomics for dissection of complex malaria infections. Genome Res. 24, 1028–1038 (2014).
Trevino, S. G. et al. High-resolution single-cell sequencing of malaria parasites. Genome Biol. Evol. 9, 3373–3383 (2017).
Poran, A. et al. Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites. Nature 551, 95–99 (2017).
Reid, A. J. et al. Single-cell RNA-seq reveals hidden transcriptional variation in malaria parasites. ELife 7, e33105 (2018).
Ngara, M. et al. Exploring parasite heterogeneity using single-cell RNA-seq reveals a gene signature among sexual stage Plasmodium falciparum parasites. Exp. Cell Res. 371, 130–138 (2018).
Walzer, K. A., Kubicki, D. M., Tang, X. & Chi, J. T. Single-cell analysis reveals distinct gene expression and heterogeneity in male and female Plasmodium falciparum gametocytes. mSphere 3, e00130 (2018).
Brancucci, N. M. B. et al. Probing Plasmodium falciparum sexual commitment at the single-cell level. Wellcome Open Res. 3, 70 (2018).
Bancells, C. et al. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol. 4, 144–154 (2019).
Walzer, K. A., Fradin, H., Emerson, L. Y., Corcoran, D. L. & Chi, J. T. Latent transcriptional variations of individual Plasmodium falciparum uncovered by single-cell RNA-seq and fluorescence imaging. PLOS Genet. 15, e1008506 (2019).
Dia, A. et al. Single-genome sequencing reveals within-host evolution of human malaria parasites. Cell Host Microbe 29, 1496–1506 (2021).
Rawat, M., Srivastava, A., Johri, S., Gupta, I. & Karmodiya, K. Single-cell RNA sequencing reveals cellular heterogeneity and stage transition under temperature stress in synchronized Plasmodium falciparum cells. Microbiol. Spectr. 9, e0000821 (2021).
Real, E. et al. A single-cell atlas of Plasmodium falciparum transmission through the mosquito. Nat. Commun. 12, 3196 (2021).
Tripathi, J., Zhu, L., Nayak, S., Stoklasa, M. & Bozdech, Z. Stochastic expression of invasion genes in Plasmodium falciparum schizonts. Nat. Commun. 13, 3004 (2022).
Mohammed, M. et al. Single-cell transcriptomics to define Plasmodium falciparum stage transition in the mosquito midgut. Microbiol. Spectr. 11, e03671–03622 (2023).
Pollenus, E. et al. Single-cell RNA sequencing reveals endothelial cell killing and resolution pathways in experimental malaria-associated acute respiratory distress syndrome. PLoS Pathog. 20, e1011929 (2024).
Dogga, S. K. et al. A single cell atlas of sexual development in Plasmodium falciparum. Science 384, eadj4088 (2024).
Wahlgren, M., Goel, S. & Akhouri, R. R. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 15, 479–491 (2017).
Hamilton, W. L. et al. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect. Dis. 19, 943–951 (2019).
Rovira-Vallbona, E. et al. Molecular surveillance of Plasmodium falciparum drug-resistance markers in Vietnam using multiplex amplicon sequencing (2000–2016). Sci. Rep. 13, 13948 (2023).
Talundzic, E. et al. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog. 11, e1004789 (2015).
Chenet, S. M. et al. Independent emergence of the Plasmodium falciparum Kelch propeller domain mutant allele C580Y in Guyana. J. Infect. Dis. 213, 1472–1475 (2016).
Tun, K. M. et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect. Dis. 15, 415–421 (2015).
Uwimana, A. et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 26, 1602–1608 (2020).
Uwimana, A. et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect. Dis. 21, 1120–1128 (2021).
Bergmann, C. et al. Increase in Kelch 13 polymorphisms in Plasmodium falciparum, Southern Rwanda. Emerg. Infect. Dis. 27, 294–296 (2021).
Straimer, J., Gandhi, P., Renner, K. C. & Schmitt, E. K. High Prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J. Infect. Dis. 225, 1411–1414 (2022).
Bayih, A. G. et al. A Unique Plasmodium falciparum K13 gene mutation in Northwest Ethiopia. Am. J. Trop. Med. Hyg. 94, 132–135 (2016).
Mihreteab, S. et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in Eritrea. N. Engl. J. Med. 389, 1191–1202 (2023).
Fola, A. A. et al. Plasmodium falciparum resistant to artemisinin and diagnostics have emerged in Ethiopia. Nat. Microbiol. 8, 1911–1919 (2023).
Wasakul, V. et al. Malaria outbreak in Laos driven by a selective sweep for Plasmodium falciparum kelch13 R539T mutants: a genetic epidemiology analysis. Lancet Infect. Dis. 23, 568–577 (2023).
Conrad, M. D. et al. Evolution of partial resistance to artemisinins in malaria parasites in Uganda. N. Engl. J. Med. 389, 722–732 (2023).
Asua, V. et al. Changing molecular markers of antimalarial drug sensitivity across Uganda. Antimicrob. Agents Chemother. 63, e01818 (2019).
Asua, V. et al. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J. Infect. Dis. 223, 985–994 (2021).
Adam, M. et al. Antimalarial drug efficacy and resistance in malaria-endemic countries in HANMAT-PIAM_net countries of the Eastern Mediterranean Region 2016–2020: clinical and genetic studies. Trop. Med. Int. Health 28, 817–829 (2023).
Bakari, C. et al. Trends of Plasmodium falciparum molecular markers associated with resistance to artemisinins and reduced susceptibility to lumefantrine in Mainland Tanzania from 2016 to 2021. Malar. J. 23, 71 (2024).
Jeang, B. et al. Molecular surveillance of Kelch 13 polymorphisms in Plasmodium falciparum isolates from Kenya and Ethiopia. Malar. J. 23, 36 (2024).
Acknowledgements
This research was supported by a grant from the National Natural Science Foundation of China (grant number 82030060 to Q.C.).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the review. Q.C. conceptualized and supervised the study. QL wrote the manuscript, designed the figures, and collected the related references. F.L., J.W., and Y.T. conceived, provided guidance, and revised this manuscript. T.L. and K.L. prepared the figures and tables. Q.C. reviewed and prepared the final manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Q., Liu, T., Lv, K. et al. Malaria: past, present, and future. Sig Transduct Target Ther 10, 188 (2025). https://doi.org/10.1038/s41392-025-02246-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-025-02246-3