Abstract
Vascular endothelial growth factor (VEGF) signaling is a critical regulator of vasculogenesis, angiogenesis, and lymphangiogenesis, processes that are vital for the development of vascular and lymphatic systems, tissue repair, and the maintenance of homeostasis. VEGF ligands and their receptors orchestrate endothelial cell proliferation, migration, and survival, playing a pivotal role in dynamic vascular remodeling. Dysregulated VEGF signaling drives diverse pathological conditions, including tumor angiogenesis, cardiovascular diseases, and ocular disorders. Excessive VEGF activity promotes tumor growth, invasion, and metastasis, while insufficient signaling contributes to impaired wound healing and ischemic diseases. VEGF-targeted therapies, such as monoclonal antibodies and tyrosine kinase inhibitors, have revolutionized the treatment of diseases involving pathological angiogenesis, offering significant clinical benefits in oncology and ophthalmology. These therapies inhibit angiogenesis and slow disease progression, but they often face challenges such as therapeutic resistance, suboptimal efficacy, and adverse effects. To further explore these issues, this review provides a comprehensive overview of VEGF ligands and receptors, elucidating their molecular mechanisms and regulatory networks. It evaluates the latest progress in VEGF-targeted therapies and examines strategies to address current challenges, such as resistance mechanisms. Moreover, the discussion includes emerging therapeutic strategies such as innovative drug delivery systems and combination therapies, highlighting the continuous efforts to improve the effectiveness and safety of VEGF-targeted treatments. This review highlights the translational potential of recent discoveries in VEGF biology for improving patient outcomes.
Similar content being viewed by others
Introduction
Vascular endothelial growth factors (VEGF) and their receptors (VEGFR) are critical factors of angiogenesis, a process critical for physiological and pathological conditions.1,2 Specifically, the signaling axis of VEGF-VEGFR controls endothelial cell (EC) proliferation, migration, and survival, thereby facilitating the organized development of new blood vessels. Vascular development is indispensable for embryonic growth, wound healing, and the female reproductive cycle.1 However, when VEGF signaling is dysregulated, various disorders such as cancer, diabetic retinopathy (DR, diabetes-induced retinal vascular disorder), and age-related macular degeneration (AMD, retinal disease characterized by macular degeneration) develop.3 Extensive research on VEGF-VEGFR signaling has advanced the development of therapeutic inhibitors that disrupt pathological angiogenesis. As a result, this therapeutic intervention has achieved significant milestones, especially in cancer therapy and ocular disease treatment. Despite this progress with anti-angiogenic drugs, therapeutic resistance and the complex regulation of angiogenesis remain major challenges, highlighting the critical need to target the VEGF-VEGFR pathway across various indications effectively.
The concept of angiogenesis has been acknowledged as a fundamental biological process since the 18th century (Fig. 1). British surgeon John Hunter first observed vascular growth in rabbit ears exposed to cold, with his 1794 publication laying the foundation for vascular research.4,5 Carl Thiersch later linked angiogenesis to tumor biology by demonstrating that new vessels in carcinoma originate from preexisting capillaries.6 Further expanding this understanding, the role of angiogenesis in cancer was emphasized by Rudolf Virchow and Ernst Goldmann, who proposed its dual function in tumor progression.4,6 This connection was further strengthened by Algire and Chalkley’s studies, which culminated in Judah Folkman’s 1971 hypothesis that tumor growth is angiogenesis-dependent, thereby establishing angiogenesis as a therapeutic target.7
Timeline of key discoveries in angiogenesis research. The timeline begins with John Hunter’s observation of blood vessel growth and follows major advancements, including Folkman’s hypothesis, which links tumor growth to angiogenesis (1971). The significant milestones include the discovering the vascular permeability factor in 1983 and identifying vascular endothelial growth factor (VEGF), its receptors, and other VEGF family members between 1989 and 1997. It also highlights the approval of several anti-angiogenic drugs: pegaptanib for age-related macular degeneration (AMD) in 2004, bevacizumab for metastatic colorectal cancer in 2004, ranibizumab for AMD in 2006, aflibercept for AMD in 2011, ramucirumab for advanced gastric cancer in 2014, brolucizumab for AMD in 2019, the combination of atezolizumab and bevacizumab for hepatocellular carcinoma (HCC) in 2020, and faricimab for AMD in 2022. Created in BioRendender.com
A major breakthrough occurred in 1983 when Harold Dvorak and colleagues identified vascular permeability factor (VPF), a protein that significantly increased vascular permeability.8 Subsequent purification and sequencing confirmed that VPF was identical to VEGF,8,9 with Napoleone Ferrara successfully isolating VEGF from bovine pituitary follicular cells in 1989.10 This identification revealed VEGF as a potent endothelial mitogen with high specificity, further confirmed by studies on its role in vascular permeability and EC proliferation.11,12 The discovery of VEGF catalyzed the identification of additional family members, including VEGF-B, VEGF-C, and VEGF-D, and their receptors VEGFR1, VEGFR2, and VEGFR3.13,14,15,16,17,18 Moreover, alternative splicing of VEGF-A generated isoforms such as VEGF-A121, VEGF-A165, and VEGF-A189, which differ in receptor binding and bioavailability.11,19
The identification of VEGF as a central regulator of angiogenesis established its importance in both physiological and pathological contexts. Targeting VEGF has since become a cornerstone of anti-angiogenic therapies, profoundly impacting oncology and ophthalmology. This review examines VEGF family members and their receptors in angiogenesis and lymphangiogenesis, therapeutic interventions, and emerging research directions to advance precision medicine.
Structural diversity of VEGF family members with their receptors
The VEGF family, comprising VEGF-A, VEGF-B, VEGF-C, and VEGF-D, regulates vascular and lymphatic development through isoforms generated by alternative splicing20 or proteolytic cleavage.21 These proteins share a cystine-knot motif critical for receptor binding and dimerization,22,23 interacting with VEGFR1, VEGFR2, VEGFR3, and neuropilin (NRP) as a co-receptor to modulate diverse functions.24
VEGF-A
VEGF-A exists in multiple isoforms (Fig. 2a), including VEGF-A111, 121, 145, 165, 183, 189, and 206, generated through alternative splicing of exons such as 6A, 6B, 7A, and 7B, which tailor their lengths and interactions with extracellular matrix (ECM) components to specific tissues and contexts.24,25,26 All isoforms share a conserved N-terminal region responsible for binding to VEGFRs and initiating signaling.24
Schematic representation of alternative splicing variants and proteolytic processing of vascular endothelial growth factors (VEGFs). a VEGF-A isoforms generated by alternative splicing including VEGF-A111, VEGF-A121, VEGF-A145, VEGF-A165, VEGF-A183, VEGF-A189, VEGF-A206, and VEGF165b. Each isoform is represented by its exon composition, highlighting the variations in exons 6A, 6B, 7, and 8 across the isoforms contributing to differences in receptor-binding affinities and functional properties. b Alternative splicing generates two VEGF-B isoforms, VEGF-B167 and VEGF-B186. These isoforms differ in their use of either exon 6A or 6B, which determines their specific molecular characteristics. c VEGF-C and VEGF-D exist in unprocessed dimerized forms, and their proteolytic processing occurs through cleavage by the protein convertases, kallikrein 3 (KLK3), cathepsin D (CTSD), or thrombin, resulting in mature, active forms with modified functional properties. Created in BioRendender.com
VEGF-A111, excluding exons 5–7, diffuses easily, resists proteolysis, and is induced by DNA damage,27 while VEGF-A121, lacking exons 6A and 7, is soluble, forming stable homodimers resilient to oxidative stress.19,25,28 In contrast, VEGF-A145, missing exon 7, strongly binds ECM but exhibits reduced binding with its receptor and NRP1 due to missing motifs.29,30 VEGF-A165, the predominant isoform, features an N-terminal receptor-binding domain (RBD) and a C-terminal heparin-binding domain (HBD), enabling ECM retention and systemic distribution.31,32,33,34,35 This isoform binds VEGFR2 with a dissociation constant (Kd) of 1–10 nM, ensuring rapid receptor engagement at physiological levels,24,36 and forms a homodimer (Fig. 4a), with each monomer stabilized by hydrogen bonds and hydrophobic contacts, notably Glu64 and Asp63, interacting with the VEGFR2 D2 domain.37,38,39 The HBD of VEGF-A165 anchors it to the ECM, prolonging receptor activation and enhancing vascular stability.40
However, VEGF-A121, lacking this HBD (Fig. 2a), disperses widely but is unable to bind co-receptor, NRP1, resulting in lower VEGFR2 activation and approximately 10-100 times reduced mitogenic activity, often leading to leakier vessels due to limited tissue retention.30,41,42 Similarly, VEGF-A145 retains partial ECM-binding capacity but has reduced heparin affinity compared with VEGF-A189, forming intermediate gradients that support vascularization when VEGF-A165 is insufficient.29 VEGF-A183, with a truncated exon 6A, exhibits intermediate heparin-binding affinity, remaining ECM-associated,43,44 whereas VEGF-A189 and VEGF-A206, both retaining exons 6 and 7 (Fig. 2a), tightly bind heparan sulfate proteoglycans (HSPGs), restricting diffusion and forming steep VEGF gradients that promote dense, structured vascular networks.25,45 Furthermore, VEGF-A189’s interaction with NRP1 enhances VEGFR2 signaling, although proteolytic cleavage modulates its bioavailability.46 Lastly, VEGF-A206, the longest isoform, contains an extended HBD, strongly binding the ECM to localize angiogenic activity, particularly in fetal and pathological tissues.20,25,47 Thus, the diverse isoforms of VEGF-A, through their unique structural properties and receptor interactions, finely tune angiogenesis across physiological and pathological contexts.
VEGF-B
VEGF-B exists in two isoforms, VEGF-B167 and VEGF-B186 (Fig. 2b), arising from alternative splicing and distinguished by their C-terminal domains.14 Both share an identical N-terminal domain containing a VEGF homology domain (VHD) and eight cysteine residues crucial for forming intra- and intermolecular disulfide bonds, predominantly resulting in homodimers with a molecular weight of 44–54 kDa, widely expressed in normal and tumor tissues.14,48 Specifically, VEGF-B167, featuring a heparin-binding C-terminal domain (CTD), attaches to cell surface heparan sulfate proteoglycans (HSPGs) and constitutes >80% of VEGF-B levels in most tissues.49 In contrast, VEGF-B186, with a hydrophobic O-glycosylated CTD, is freely soluble, upregulated in several primary tumors and tumor cell lines, and prone to proteolytic cleavage that generates biologically active fragments.50 Additionally, both isoforms can form disulfide-linked heterodimers with VEGF-A, which remain cell-associated, unlike the secreted VEGF-A homodimers, suggesting diverse functions across physiological contexts.14,51 Functionally, VEGF-B binds exclusively to VEGFR1 (Fig. 4a), engaging the same region as VEGF-A but with slightly lower affinity (Kd 1–3 nM for VEGF-B167), and plays a specialized role in tissue protection and metabolic regulation rather than promoting angiogenesis.52,53,54,55,56,57 However, despite this high-affinity binding, VEGF-B167 induces minimal signaling due to low kinase activity of VEGFR1, constrained by an inhibitory sequence in the juxtamembrane region, thus limiting its ability to transduce angiogenic signals.52,58,59 Consequently, unique structural and binding properties of VEGF-B underscore its distinct contributions to tissue homeostasis and disease.
VEGF-C
VEGF-C stands out in the VEGF family for its structural complexity and intricate post-translational modifications.15 It is a 419-amino acid protein with 30% sequence identity to VEGF-A and 27% to VEGF-B.15,60 VEGF-C is initially synthesized as an inactive precursor sequestered in the ECM (Fig. 2c) and undergoes sequential proteolytic cleavages to achieve full activation.61
Proprotein convertases (PC) including furin generate a partially active intermediate, while the final cleavage by ADAMTS3 produces the mature form (~21 kDa) with high affinity for VEGFR2 and VEGFR3, driving lymphangiogenesis and vascular remodeling.62,63 Additionally, proteases, such as plasmin, thrombin, KLK3, and cathepsin D, modify VEGF-C, altering its receptor-binding affinities and activity profiles.61,64 These modifications, particularly in the tumor microenvironment (TME), fine-tune the role of VEGF-C in cancer progression, metastasis, and tumor-associated lymphangiogenesis.
VEGF-D
VEGF-D was identified from a human EST sequence, resulting in a full-length cDNA encoding a 354-amino acid protein with 23% identity to VEGF-C.16 It is structurally similar to VEGF-C but features unique N- and C-terminal extensions. VEGF-D undergoes proteolytic processing (Fig. 2c) to generate isoforms with distinct receptor affinities.65,66 The unprocessed precursor (~50 kDa) is inactive until N- and C-terminal propeptides are cleaved. Intermediate forms (31–35 kDa) bind VEGFR3 with high affinity but exhibit lower activity than the fully processed form (~21 kDa), which binds VEGFR2 and VEGFR3, promoting angiogenesis and lymphangiogenesis. This maturation, regulated by extracellular proteases such as PC and plasmin, modulates receptor specificity and bioactivity.65,67 VEGF-D differs from VEGF-C in expression patterns, predominantly found in the lungs, heart, skeletal muscle, and intestines.67,68 VEGF-C and VEGF-D regulate lymphangiogenesis via VEGFR3 and contribute to angiogenesis through VEGFR2 under specific conditions.69,70 This dual functionality highlights its role in coordinating vascular and lymphatic growth, particularly in cancer metastasis, underscoring its significance in tissue homeostasis and disease progression.
VEGF-C and VEGF-D primarily drive lymphangiogenesis by binding to VEGFR3 (Fig. 4a), a receptor predominantly expressed in lymphatic endothelial cells (LECs).71 Both ligands require proteolytic processing for full activity. Initially, full-length VEGF-C exhibits low affinity for VEGFR3, but cleavage by enzymes such as ADAMTS3 (Fig. 2c) enhances its binding strength, potently activating VEGFR3 to promote LEC proliferation and migration.15,72 Similarly, VEGF-D binds both VEGFR2 and VEGFR3 (Fig. 4a), with its affinity increasing post-processing, enabling the mature form to strongly support angiogenesis and lymphangiogenesis, while the unprocessed form binds VEGFR3 less effectively.66,67,73 Additionally, VEGF-D can activate VEGFR2/VEGFR3 heterodimers, contributing to lymphatic dilation and vascular remodeling, particularly in cancer.74
VEGFxxxb
VEGF-A isoforms are primarily pro-angiogenic; however, specific inhibitory variants, known as VEGFxxxb isoforms,75 such as VEGF165b, arise through alternative splicing at the distal splice site of exon 8 (Fig. 2a). These isoforms feature a C-terminal sequence (SLTRKD) that replace the pro-angiogenic CDKPRR sequence in VEGF-Axxx isoforms,76 allowing them to bind VEGFR2 competitively without initiating complete receptor phosphorylation and signaling, which reduce angiogenic responses.77
The existence and role of VEGFxxxb isoforms in angiogenic balance are debated. Some studies suggest they may be methodological artifacts, as large-scale RNA-seq often fails to detect them in human tissues, and antibody specificity is questioned.78,79,80,81 This inconsistency fuels skepticism, implying VEGFxxxb isoforms might only appear under specific experimental conditions. However, VEGF-A165b, known for its anti-angiogenic properties, is elevated in peripheral artery disease (PAD) and linked to reduced vascularization despite higher total VEGF-A levels.82 In a PAD mouse model, metabolic dysfunction and Wnt5a signaling increased VEGF-A165b, impairing angiogenesis, while its neutralization restored revascularization. These results indicate VEGF-A165b may suppress pathological angiogenesis in PAD, suggesting potential as a therapeutic target. While the physiological relevance of VEGFxxxb remains controversial, the evidence of VEGF-A165b’s inhibitory effect in PAD highlights their potential clinical significance.
Placenta growth factor (PlGF)
PlGF is a VEGF family glycoprotein sharing ~42% sequence homology with VEGF-A.83,84 It features a conserved VHD essential for dimerization and receptor binding. Alternative splicing of PlGF generates four isoforms: PlGF-1, PlGF-2, PlGF-3, and PlGF-4, distinguished by C-terminal variations that affect their receptor affinities, spatial distribution, and biological properties.85,86,87
PlGF-1 (131 amino acids) is the most studied isoform, lacking HBD and circulating as a soluble protein. It forms homo- and heterodimers with VEGF family members to modulate VEGF-A activity.83,88 PlGF-2 (152 amino acids) contains an additional 21-amino acid HBD, enabling interactions with the ECM and cell surface, confining its distribution and enhancing localized paracrine signaling.85,89 PlGF-3 (203 amino acids) includes a unique 72-amino acid C-terminal region, with tissue-specific roles yet to be fully defined, suggesting potential regulatory functions in specific pathological contexts.86 In addition, PlGF-4 (224 amino acids) is the longest isoform, similar to PlGF-2 in HBD-mediated ECM and cell interactions, although expressed at lower levels.90 Notably, the VEGF-A/PlGF heterodimer primarily binds to VEGFR1, which leads to enhanced receptor activation compared with the individual factor alone,88 thereby promoting EC migration, permeability, particularly in pathological conditions, such as cancer and ischemia, where it facilitates neovascularization and vascular inflammation.53
PlGF binds exclusively to VEGFR1 (Fig. 4a), playing a significant role in pathological angiogenesis.91 Through high-affinity binding, it promotes VEGFR1 dimerization and activation,88 triggering unique angiogenic and inflammatory signaling pathways despite the lower kinase activity of VEGFR1.53 PlGF modulates VEGF-A signaling by competing for VEGFR1 binding, reducing VEGF-A sequestration.92 This competition shifts receptor dynamics, freeing more VEGF-A to engage VEGFR2, thereby amplifying VEGF-A-driven angiogenesis. Furthermore, PlGF enhances vascular remodeling and inflammation through VEGFR1 signaling, extending its effect beyond direct receptor activation.
VEGF receptors
VEGFR1
VEGFR1, also known as Fms-like tyrosine kinase-1 (Flt1),93 regulates various aspects of angiogenesis through its complex multi-domain structure, serving as a decoy receptor.94 Its extracellular domain (ECD) comprises seven immunoglobulin-like (Ig) domains, with D2 and D3 forming the primary ligand-binding interface that confers high affinity for VEGF-A (Fig. 3a). This interaction sequesters VEGF-A, reducing its availability for VEGFR2, and thus modulates angiogenic signaling.54,95 The remaining Ig-like domains (D4-D7) contribute to structural integrity and facilitate receptor dimerization, supporting interactions with other receptors and stabilizing the receptor complex.96,97,98,99
Structural domains of vascular endothelial growth factor receptors (VEGFRs) and isoforms of soluble VEGFR1. a Domain organization of VEGFR1, VEGFR2, and VEGFR3. Each receptor has key structural features, including Ig-like extracellular domains (D1-D7), transmembrane domain (TMD), juxtamembrane domain (JMD), kinase domain (KD), and C-terminal domain (CTD). b Alternative splicing generates five distinct splice variants of VEGFR1: the full-length VEGFR1, VEGFR1 with intron 13 retention (sVEGFR1-i13), VEGFR1 with intron 14 retention (sVEGFR1-i14), and the soluble isoforms sVEGFR1-e15a and sVEGFR1-e15b with alternative terminal exons. The schematic highlights the differences in domain configurations between full-length receptors and their soluble forms, illustrating the structural diversity resulting from alternative splicing. Created in BioRendender.com
The transmembrane domain (TMD) is crucial for anchoring the receptor to the cell membrane and facilitating its function in VEGF signaling.100 Notably, VEGFR1 is expressed in two isoforms: a membrane-bound full-length form and a soluble variant lacking the TMD.94 The TMD anchors VEGFR1 to the cell surface, enabling it to modulate the spatial distribution of VEGF and regulate downstream signaling.101 The intracellular domain (ICD) of VEGFR1 (Fig. 3a) comprises the juxtamembrane domain (JMD), split tyrosine kinase domain (KD), and C-terminal tail. VEGFR1 exhibits low kinase activity compared with VEGFR2, which is linked to unique structural features. The JMD includes a repressor sequence with three serine residues that inhibit downstream signaling, such as PI3K activation, even in the presence of VEGF.59 Additionally, an “electrostatic latch” in the JMD maintains VEGFR1 in an auto-inhibitory conformation, preventing ligand-independent activation.102 Moreover, VEGFR1 catalytic function is impaired by a critical asparagine in its activation loop, replacing a conserved aspartic acid, which disrupts transphosphorylation of essential tyrosine residues, further limiting its activity. These structural elements collectively define its unique role in signaling.103,104 Collectively, the complex architecture and regulatory features of VEGFR1 enable it to fine-tune angiogenesis by modulating VEGF availability and selectively suppressing signaling.
Soluble VEGFR1 (sVEGFR1)
The sVEGFR1 isoforms, sVEGFR1-i13, sVEGFR1-i14, sVEGFR1-e15a, and sVEGFR1-e15b (Fig. 3b), arise from alternative splicing and retain VEGF-binding capacity without downstream signaling.105 Produced by various cells, including endothelial and melanoma cells, sVEGFR1 often circulates bound to VEGF, playing a key role in regulating angiogenesis, particularly in pathological states such as pre-eclampsia.106
sVEGFR1-i13, the most common isoform, is generated by intron 13 retention107 and acts as a VEGF scavenger, inhibiting VEGF-A-induced angiogenesis by forming non-signaling complexes with VEGFR2.108 sVEGFR1-i14, generated via intron 14 retention (Fig. 3b), is primarily expressed in the brain and testes,109 where it regulates local VEGF activity and vascular stability. sVEGFR1-e15a, formed through exon 15a inclusion, is abundant in the placenta, playing a crucial role in vascular development.105,109 Elevated levels are linked to pre-eclampsia, disrupting angiogenesis. Moreover, sVEGFR1-e15b, produced by exon 15b inclusion (Fig. 3b), features matrix-binding properties and is expressed in the placenta and vascular tissues, contributing to abnormal angiogenesis in cancers and ocular diseases.105 Functioning as decoy receptors, these isoforms regulate VEGF ligand distribution, limiting VEGFR2 activation and attenuating pro-angiogenic signaling. Collectively, these variants provide a dynamic regulatory mechanism that ensures tissue-specific vascular stability and growth control.
VEGFR2
VEGFR2, also known as kinase insert domain receptor (KDR) in humans and fetal liver kinase-1 (Flk1) in mice, is a receptor tyrosine kinase (RTK).110,111,112 The ECD comprises seven Ig-like domains (D1-D7), each with D2 and D3 primarily responsible for high-affinity VEGF binding (Fig. 3a). D2 forms hydrophobic interactions as the main binding site, while D3 stabilizes the receptor-ligand complex through hydrophilic interactions.113,114 Ligand binding induces conformational changes in D2 and D3, essential for receptor dimerization and activation.113,115 D4 and D5 support receptor dimerization and allosteric regulation, ensuring proper alignment of the intracellular kinase domains,116 while D7 facilitates receptor dimerization via charged interactions in its E-F loop.116,117 Ligand binding also reorients TMD helices, a prerequisite for the kinase domain activation.118 Although VEGFR2 can dimerize, ligand binding ensures correct helix alignment downstream signaling.119
The JMD functions as a regulatory gate, keeping the receptor in an inactive state until ligand-induced conformational changes activate the KD.100 The KD phosphorylates specific tyrosine residues, such as Tyr1059 (Y1059) in the activation loop, transitioning the kinase to an active state.120 The kinase insert domain (KID) serves as a docking platform for downstream signaling molecules, with phosphorylation at sites such as Y951 enabling interactions with effectors such as TSAd, which mediate vascular permeability and remodeling.121 The CTD stabilizes the receptor in an inactive state but undergoes structural rearrangements upon ligand binding to facilitate full activation. Phosphorylation of key residues within the CTD, such as Y1175, recruits signaling proteins including PLCγ1, driving EC proliferation and migration.122 Moreover, the KID and CTD are integral to intrinsic receptor dimerization, which is essential for full kinase activation.123 Deletion of these domains diminishes dimerization efficiency and compromises signaling. Together, these structural features ensure precise regulation of VEGFR2 activity, supporting its pivotal role in vascular development and pathological angiogenesis.
VEGFR3
VEGFR3, also known as Fms-related tyrosine kinase-4, is an RTK essential for vascular development but is primarily involved in lymphangiogenesis. A unique feature of VEGFR3 is the proteolytic cleavage of its ECD (Fig. 3a), resulting in a mature form linked by disulfide bonds.124 D1-D3 in the ECD are critical for VEGF-C binding, particularly through interactions with D2, while D1 stabilizes the ligand-receptor complex. D4-D7 mediate receptor dimerization and activation.67,124 Proteolytic cleavage at D5 divides the ECD into two subunits, which remain connected by disulfide bonds. This cleavage is essential for effective ligand binding, signaling, and receptor activation, facilitated by homotypic interactions between D5 and D7.124 The TMD and ICD are structurally similar to those of VEGFR1 and VEGFR2, featuring a single-pass transmembrane region and split tyrosine KD, enabling ligand-induced dimerization and autophosphorylation.125 VEGFR3 primarily mediates lymphangiogenesis through VEGF-C and VEGF-D signaling in lymphatic endothelial cells (LECs). It also modulates VEGFR2 activity via heterodimerization, indirectly supporting blood vessel development while maintaining its distinct role in lymphatic growth.
VEGFR dimerization
VEGFR homodimers are formed when two identical receptors pair in response to ligand binding. VEGFR2 homodimers (Fig. 4) are mainly activated by VEGF-A, which triggers a series of key processes in ECs.126,127 Upon activation, these receptors undergo autophosphorylation, initiating intracellular signaling cascades, which drive angiogenesis.126 In contrast, VEGFR1 homodimers, which are capable of binding to VEGF-A and other ligands, play a regulatory role. They regulate VEGFR2 signaling and inhibit excessive angiogenic responses.127 VEGFR2 can exist in a monomeric or dimeric state without ligand binding.128,129 VEGFR2 forms dimers without ligands at physiological levels, showing low levels of basal phosphorylation.129 This pre-formed, partially active state enables rapid angiogenic signaling, offering a refined model of RTK activation beyond traditional ligand-induced dimerization.
Vascular endothelial growth factor (VEGF) family members and their receptors. Binding of VEGF-A, -B, -C, -D, and PlGF with their respective receptors, VEGFR1, VEGFR2, and VEGFR3, highlighting the primary receptor specificities. VEGF-A binds VEGFR1 and VEGFR2, VEGF-B and PlGF selectively interact with VEGFR1. VEGFR2 is the primary mediator of VEGF-A-driven angiogenesis, also binding VEGF-C. VEGFR3 predominantly binds VEGF-C and VEGF-D, regulating lymphangiogenesis. This binding schematic illustrates the selective affinities of VEGF family members for their receptors and their distinct roles in vascular and lymphatic regulation. Additionally, VEGF-B is depicted as a metabolic regulator that acts through VEGFR1, while PlGF is shown to modulate inflammation and vascular homeostasis. Created in BioRendender.com
Heterodimerization fine-tunes VEGF signaling by modulating receptor activity in response to ligand concentrations and receptor abundances. VEGFR1-VEGFR2 heterodimers (Fig. 4) regulate VEGF-A activity by inhibiting VEGFR2, primarily through reduced PI3K-mediated phosphorylation, balancing EC migration and angiogenic processes.130 VEGFR2-VEGFR3 heterodimers, induced by VEGF-C and VEGF-D (Fig. 4), are critical for blood and lymphatic vessel formation, particularly in angiogenic tip cells where they control sprouting and branching.131,132 Modeling studies predict that VEGFR1-VEGFR2 heterodimers constitute 10–50% of active signaling complexes, depending on receptor expression levels. High VEGFR2 abundance suppresses VEGFR1 homodimer formation, favoring VEGFR2 signaling.128 Notably, VEGFR1-VEGFR2 heterodimers are more prevalent in neuronal cells, whereas VEGFR2 homodimers dominate in ECs.133 This differential dimerization may explain VEGF’s distinct roles in nerve regeneration versus blood vessel growth, underscoring the various regulatory functions of receptor interactions across physiological contexts.
Heteromeric complexes between VEGFRs and non-VEGFRs generate diverse signaling outcomes, enhancing functional flexibility. VEGFR1 forms heterodimers with NRP1 (Fig. 5a), increasing VEGF binding and modulating signaling.134 Additionally, VEGFR1 also forms a complex with NRP2 (Fig. 5a), enabling NRP2 to bind VEGF-A121.135 Moreover, VEGFR1 interacts with integrin α5β1 (Fig. 5a) to mediate endothelial adhesion to the ECM136 and binds FGFR1, suppressing FGF2-induced angiogenesis.137
Interactions of vascular endothelial growth factor receptors (VEGFR) with co-receptors and structural features of Neuropilin. a Interaction of VEGFR1 with co-receptors NRP1 and NRP2, α5β1 integrin, and FGFR1. The VEGFR1-NRP2 interaction facilitates VEGF-A121 binding, whereas VEGFR1 engagement with α5β1 promotes EC adhesion to the extracellular matrix. Additionally, the interaction of VEGFR1 with FGFR1 blocks FGF2-induced angiogenesis, highlighting the role of the VEGFR1 complex in modulating angiogenic signaling pathways and EC behavior. b Association of VEGFR2 with various co-receptors and adhesion molecules, including NRPs (NRP1 and NRP2), PDGFRβ, EGFR, CD44, EphA4, VE-Cadherin, and c-Met. These interactions contribute to the specificity and complexity of VEGF signaling, enabling crosstalk between VEGF receptors and other signaling pathways. Such an association with co-receptors and adhesion molecules enhances cellular responses, including EC migration, adhesion, and survival, supporting key physiological processes such as angiogenesis, vascular maturation, and cellular adhesion dynamics within the VEGF pathway. c Interaction between VEGFR3 and the co-receptor NRP2, which plays a critical role in promoting lymphangiogenesis and supporting EC survival. d NRP is shown with additional structural motifs, including the CUB, FV/VIII, MAM, and SEA domains. These domains contribute to receptor interactions and enhance signaling specificity within the VEGF pathway, potentially influencing binding affinities and receptor-ligand selectivity. NRP neuropilin, FGFR fibroblast growth factor receptor, PDGFR platelet-derived growth factor receptor, EGFR epidermal growth factor receptor. Created in BioRendender.com
VEGFR2 similarly forms complexes with non-VEGFRs, notably NRP1 and NRP2,138 which act as co-receptors (Fig. 5b). VEGFR2-NRP1 heterodimers enhance VEGF binding and signaling potential. VEGF facilitates these interactions by bridging VEGFR2 and NRP1, forming complexes in cis (within the same cell) or trans (between cells), with the cis configuration being kinetically favored.139,140 The VEGF-NRP1 interaction relies on the C-terminal tail of VEGF binding to the b1 domain of NRP1, offering structural flexibility that allows these complexes to adapt to diverse cellular contexts.
αvβ3 integrin binds vitronectin to amplify VEGFR2 phosphorylation and angiogenic signaling. Similarly, α4β1 integrin forms a complex with VEGFR2 in chronic lymphocytic leukemia cells (Fig. 5b), enhancing VEGF signaling and inducing apoptosis when disrupted,141 underscoring a therapeutic target. VEGFR2-platelet-derived growth factor receptor β (PDGFRβ) heterodimers (Fig. 5b) link angiogenesis to vessel maturation via pericyte recruitment, stabilizing tumor vasculature.142 VEGFR2-epidermal growth factor receptor (EGFR) heterodimers, formed in the presence of both EGF and VEGF, offer a ligand-dependent mechanism for diversifying cancer signaling.143 Moreover, the CD44v6 isoform acts as a co-receptor for VEGFR2 in ECs,144 enhancing ERK signaling (Fig. 5b). EphA4, a receptor tyrosine kinase involved in neuronal development, forms a kinase-dependent complex with VEGFR2 (Fig. 5b), promoting neuronal differentiation in neural stem and progenitor cells when activated by ephrin A1 and VEGF-A165.145
VEGFR2 forms a complex with VE-cadherin (VE-cad) at cell-cell junctions (Fig. 5b), limiting VEGFR2 phosphorylation by recruiting phosphatases.146 This interaction reduces VEGF-induced proliferation while promoting EC survival. Additionally, VEGFR2 interacts with c-Met (hepatocyte growth factor receptor) in a heterocomplex (Fig. 5b), where VEGF-A recruits protein tyrosine phosphatase 1B (PTP1B) to dephosphorylate c-Met, inhibiting its signaling and reducing tumor cell migration and invasiveness.147 VEGFR3 also forms a complex with NRP2 (Fig. 5c), enhancing lymphangiogenesis and supporting EC survival.148 VEGF-C stimulation strengthens this interaction, leading to increased VEGFR3 phosphorylation and activation of downstream signaling. This association with non-VEGFRs highlights the complex regulation of angiogenesis and lymphangiogenesis, offering new avenues for therapeutic intervention for abnormal blood and lymphatic vessel formation.
Neuropilin
NRP1 and NRP2 are non-tyrosine kinase co-receptors that enhance VEGF signaling by binding to the VEGF family in conjunction with VEGFRs.149 Although they lack catalytic activity, their ECD enhance VEGF-VEGFR signaling, playing critical roles in angiogenesis and lymphangiogenesis.
Both NRP1 and NRP2 feature a structured ECD comprising two complement-binding (CUB) domains (a1/a2) and two coagulation factor homology domains (b1/b2), which enable their binding ligands such as VEGFs and semaphorins (Fig. 5d). The a1/a2 domains are essential for binding semaphorins, such as sema3A, involved in axonal guidance, while the b1 domain allows high-affinity binding to VEGF-A165 in NRP1 and to VEGF-C in NRP2, with the b2 domain enhancing these interactions.140 Additionally, both NRPs contain a MAM domain (c) that supports homodimerization and receptor interactions, strengthening their signaling roles.150 Although both have short ICDs without catalytic activity, they feature PDZ-binding motifs (SEA) that allow interactions with intracellular proteins, such as synectin, to facilitate downstream signaling.151 NRP2 uniquely includes soluble splice variants (sNRP2) that act as decoy receptors by sequestering VEGF-C and VEGF-D, thus modulating their bioavailability and lymphangiogenic functions.152 Together, the structural motifs of NRP1 and NRP2 enable their versatile roles across physiological and pathological processes.
VEGFR and NRP signaling pathways
VEGFR1 signaling
Specific tyrosine phosphorylation sites on VEGFR1 are crucial for recruiting adaptor molecules and mediating key signaling pathways to regulate cell migration, proliferation and survival. In particular, Y794 is located in the JMD region (Fig. 6a) and is an important site for PLCγ1 binding.153 Additionally, activation of endothelial nitric oxide synthase (eNOS) depends on Y794, resulting in nitric oxide (NO) release, which is essential for forming capillary-like networks in vitro.154 Y1169 was identified as a major phosphorylation site,153,155 critical for recruiting PLCγ1. Y1213, Y1242, and Y1333 were identified as key phosphorylation sites for VEGFR1’s biological functions (Fig. 6a). Phosphorylation at Y1213 enables the binding of several SH2 domain-containing proteins, such as PLCγ1, SHP2, and GRB2. Specifically, PLCγ1 and SHP2 were found to be directly associated with Y1213 in a phosphotyrosine-dependent manner.156 They also facilitate the binding of PI3K, which play an important role in cell proliferation and survival in response to VEGF.157 Although Y1242 and Y1333 retain kinase activity and support PLCγ1 phosphorylation, they cannot mediate downstream mitogenic signals.156,158 Y1333 also binds important signaling proteins such as NCK and CRK, while it allows for PLCγ1 recruitment, it is insufficient for full signal transduction and biological responses such as cell proliferation.156,158 Interestingly, phosphorylation at Y1333 was inhibited by VEGF165b in ischemic muscle and blocking VEGF165b restored VEGFR1 phosphorylation and enhanced the VEGFR1-STAT3 pathway, promoting angiogenesis and perfusion recovery in PAD.159
Signal transduction of vascular endothelial growth factor receptors. a VEGFR1: Schematic representation of critical phosphorylation sites and their roles in signaling. Y794 in the juxtamembrane domain facilitates PLCγ1 binding and eNOS activation, promoting nitric oxide (NO) release and tubular structure formation. Y1169 recruits PLCγ1, while Y1213 enables binding of SH2 domain proteins such as PLCγ1, SHP2, and GRB2, supporting PI3K-mediated cell survival and proliferation. Y1242 and Y1333 support PLCγ1 phosphorylation but are insufficient for full mitogenic signaling. VEGF165b inhibits Y1333 phosphorylation, suppressing VEGFR1-STAT3 signaling, which can be restored to enhance angiogenesis in peripheral artery disease. b VEGFR2: Key phosphorylation sites and their roles in vascular functions. Y801 and Y1214 activate GAB1, promoting PI3K/AKT signaling, endothelial survival, migration, and NO production. Y951 (Y949 in mice) binds TSAd, regulating vascular permeability via the VEGFR2-TSAd-SRC complex. Y1054 and Y1059 are essential for full kinase activity, while Y1175 recruits adaptor proteins such as SHB and PLCγ1. Serine phosphorylation at S1183 and S1188 in mice (S1185 and S1190 in humans) promotes VEGFR2 degradation via β-TRCP1-mediated ubiquitination, regulating receptor stability. c VEGFR3: Schematic representation of essential phosphorylation sites and their roles in cell signaling. Phosphorylation at Y1063 recruits CRK I/II, activating the JNK pathway to support cell survival. Y1230 and Y1231 facilitate GRB2 recruitment, initiating ERK and AKT signaling pathways that promote cell proliferation and migration. Y1337 acts as a docking site for the GRB2-SHC complex, driving RAS-mediated signaling. PLCγ1 Phospholipase C gamma 1, GAB1 GRB2-associated binding protein 1, SHP2 Src homology-2 domain-containing protein tyrosine phosphatase-2, GRB2 growth factor receptor-bound protein 2, PI3K Phosphoinositide 3-kinase, TSad T cell-specific adapter protein, SHC SH2 domain protein C1, SHB SH2 domain-containing adapter protein B, SCK SHC-like protein, CRK I/II CT10 regulator of kinase I and II. Created in BioRendender.com
Although VEGF-B binds to VEGFR1 with high affinity,160 it does not induce tyrosine phosphorylation.55 However, in human retinal ECs, it leads to only a modest increase in VEGFR1 phosphorylation.161 This contrasts with other VEGF family ligands such as VEGF-A, which robustly activate VEGFR1, triggering extensive phosphorylation at multiple tyrosine residues. Mass spectrometry analysis revealed that stimulation with human PlGF-2 resulted in Y1309 phosphorylation of VEGFR1 whereas Y1213 remained unphosphorylated,162 highlighting a selective phosphorylation pattern in response to different ligands that could lead to the differential signaling potential of VEGF-B and PlGF on VEGFR1.
sVEGFR1 signaling
sVEGFR1 is highly expressed in trophoblasts, particularly under hypoxic conditions, it reduces VEGF-mediated EC migration and angiogenesis. This upregulation, linked to conditions such as pre-eclampsia, correlates with impaired placental angiogenesis.163 Additionally, VEGF-A stimulates its own negative regulator, sVEGFR1, through the VEGFR2-PKC-MEK pathway, establishing a feedback loop that limits VEGF-A activity and angiogenesis.164
However, in squamous cell lung carcinoma, sVEGFR1-i13 regulates a β1 integrin/VEGFR autocrine loop, promoting tumor proliferation and resistance to anti-angiogenic therapies.165 Elevated sVEGFR1-i13 levels are associated with advanced disease stages and poor outcomes. Moreover, sVEGFR1 interacts with α5β1 integrins, modulating cytoskeletal dynamics, and activating RAC1 signaling, thereby driving EC migration and motility through the phosphorylation of proteins such as MARCKS and RADIXIN. Notably, sVEGFR1 is essential for podocyte function and glomerular barrier integrity, interacting with lipid microdomains to regulate cytoskeletal organization and actin dynamics.166 Moreover, loss of sVEGFR1 disrupts the cytoskeleton, leading to proteinuria and glomerular dysfunction. These findings highlight the dual functions of sVEGFR1, acting as both an inhibitor and promoter of angiogenesis depending on the biological context and its molecular interactions.
VEGFR2 signaling
VEGFR2 dimerizes and autophosphorylates upon VEGF binding, activating pathways that regulate EC proliferation, migration, and vascular permeability. Activation of VEGFR2 is characterized by significant heterogeneity at the single-molecule level, with receptor mobility and interactions varying across the EC surface.167 VEGF binding triggers diverse activation mechanisms, including ligand-induced dimerization and engagement with pre-formed dimers. Even without VEGF, VEGFR2 exists as dimers with low phosphorylation levels, with ligand binding enhancing kinase activity through conformational change.129 Structural studies emphasize inter-domain contacts in stabilizing dimer formation, but pathogenic mutations such as C482R and R1051Q disrupt regulation,168 causing ligand-independent activation and altered membrane dynamics, underscoring the complexity of VEGFR2 regulation and its implications for signaling and disease progression.
Kinase assays and phosphopeptide mapping analysis reveal VEGFR2 contains 19 tyrosine residues, with 11 potential phosphorylation sites in non-catalytic regions.169,170 Key phosphorylation sites include Y951 in the KID and Y1054, Y1059, Y1175, and Y1214 in the C-terminal tail (Fig. 6b), while other residues, such as Y801, Y822, Y938, and Y996 remain unphosphorylated. Low-level phosphorylation at Y1305, Y1309, and Y1319 suggest minor role or transient roles. Phosphorylation at Y801 and Y1214, activates GAB1,170 promoting PI3K/AKT signaling for EC survival, migration, and NO production. Y951 (Y949 in mice) phosphorylation recruits TSAd, essential for vascular permeability by destabilizing VE-cad via the VEGFR2-TSAd-SRC complex.169 Mice with the Y949 mutation (Flk1Y949F/Y949F) show reduced VEGFA-induced vascular leakage and metastasis, offering therapeutic potential for conditions such as oxygen-induced retinopathy.171
Y1054 and Y1059 in the kinase activation loop are critical for full VEGFR2 activity, with mutations at these sites impairing downstream signaling.172 Y1175 recruits adaptor proteins such as SHB (SRC homology 2 domain-containing adaptor protein B), SCK (Shc-like protein), PLCγ1, and Y1173F mutations (Flk1Y1173F/+; Y1175 in humans) in mice reduce vascular leakage and improve chemotherapy responses while preserving normal vessel development.153,173,174,175 Y1214 phosphorylation activates CDC42, leading to SAPK2/p38 activation, stress fiber formation, and cell migration via NCK and FYN recruitment.176,177 Y1212F mutations in mice (Flk1Y1212F/Y1212F;Y1214 in humans) show strain-specific effects, with C57Bl/6 mutants exhibiting partial embryonic lethality and reduced EC proliferation whereas FVB mutants display delayed retinal vascular development and vessel instability, suggesting the essential role of Y1212 in vascular integrity.178
Serine phosphorylation at S1183 and S1188 (S1185 and S1190 in humans) in the proline, glutamic acid, serine and threonine (PEST) domain (K1171-K1209) of mouse VEGFR2 has been reported.179,180 These phosphorylation recruit β-TRCP1 E3 ligase (Fig. 6b), promoting VEGFR2 ubiquitination and proteasomal degradation. Mutation of these serine residues reduces ubiquitination, whereas phosphomimetic mutations enhance it, underscoring the role of serine phosphorylation in VEGFR2 stability. While tyrosine phosphorylation is well-studied and crucial for VEGFR2 signaling, serine phosphorylation remains less explored and threonine phosphorylation has yet to be identified.
VEGFR3 signaling
VEGFR3 phosphorylation occurs at several tyrosine residues upon VEGF-C binding with different patterns depending on whether VEGFR3 forms a homodimer or heterodimer with VEGFR2. In VEGFR3 homodimers, five key tyrosine residues are phosphorylated: Y1230, Y1231, Y1265, Y1337, and Y1363 (Fig. 6c). When VEGFR3 forms a heterodimer with VEGFR2, only Y1230, Y1231, and Y1265 are phosphorylated.131 Phosphorylation at Y1063 recruits adapter proteins, CRK I/II (Fig. 6c), activating the JNK pathway, which is vital for promoting cell survival. Similarly, phosphorylation at Y1230 and Y1231 plays a key role in recruiting GRB2, triggering the ERK and AKT pathways that drive cell proliferation and migration.181 Y1337 has also been identified as a critical phosphorylation site and serves as a docking site for the GRB2-SHC complex (Fig. 6c), which participates in RAS signaling.182 In the analysis of families with primary lymphedema, it was found that VEGFR3 proteins carrying mutations such as G857R, R1041P, and L1044P are tyrosine kinase-negative.183 Additionally, these mutant VEGFR3 receptors exhibit a longer half-life compared with wild-type receptors. Consequently, they accumulate on the cell surface, potentially contributing to the dominant-negative effects observed in individuals harboring with these mutations.
NRP signaling
NRPs utilize a conserved binding site structured by the b1 coagulation factor loop, which specifically interacts with the C-terminal arginine motif present in certain VEGF ligands.184,185 The interaction of VEGF-A165 with NRP1 is facilitated through specific residues in the b1 domain, where the ligand C-terminal arginine forms a salt bridge with Asp320 in NRP1.186 Additional hydrogen bonds between other amino acids stabilize this interaction, allowing for precise isoform-specific binding, which is critical for the selective signaling roles of NRP1 and NRP2.186 Structural studies have demonstrated that VEGF-A isoforms bind exclusively to NRP1, while VEGF-C and its related isoforms target NRP2. This distinction enables NRP1 to dominate blood vessel formation and NRP2 to dominate lymphatic vessel development.
NRP1 lacks intrinsic signaling activity. Instead, it relies on its activity to enhance VEGFR2 signaling by recruiting VEGF-A165 and positioning VEGFR2 to amplify downstream pathways.187 When VEGF-A165 binds to VEGFR2 with NRP1 assistance, it initiates a cascade of phosphorylation events in VEGFR2. Importantly, NRP1-mediated VEGF-A165/VEGFR2 signaling is also involved in vascular permeability, allowing for better tumor infiltration by blood vessels.188,189 NRP2, structurally similar to NRP1, is more selective in lymphangiogenesis, particularly through its interactions with VEGF-C and VEGFR3.138,190 NRP2 enhances VEGF-C binding to VEGFR3, facilitating the LEC responses required for lymphangiogenesis. Tumors often induce high expression levels of VEGF-C, which, through the VEGF-C/VEGFR3/NRP2 axis, promotes the proliferation, migration, and survival of LECs.191,192 This mechanism is especially relevant in lymphatic metastasis, as lymphatic vessels serve as conduits for tumor cells to reach and colonize distant sites.
Crosstalk with angiopoietin-TIE receptor
Angiopoietin (Ang) ligands and TIE receptors dynamically interact with the VEGF-VEGFR system to regulate vascular homeostasis and adapt to physiological and pathological changes. Ang1, via TIE2 activation, stabilizes blood vessels, supporting EC survival, vascular barrier integrity, and quiescence. In contrast, Ang2 acts as a context-dependent modulator, often destabilizing vessels under conditions such as inflammation, ischemia, and tumor angiogenesis.193,194 By increasing vessel permeability, Ang2 sensitizes ECs to VEGF, enhancing angiogenic sprouting.195,196 Notably, VEGF signaling upregulates Ang2, creating a feedback loop where VEGF-induced Ang2 expression counteracts TIE2 stabilization by Ang1.197
Additionally, vascular endothelial protein tyrosine phosphatase (VE-PTP) modulates TIE2 and VEGFR2, stabilizing endothelial junctions by dephosphorylating these receptors.198,199 Its inhibition enhances TIE2 signaling and strengthens the endothelial barrier, emphasizing the interplay between the VEGF and Ang-TIE pathways in regulating vascular stability. In pathological conditions, such as cancer and retinal diseases, this balance is disrupted. Combined inhibition of VEGF and Ang2, as seen with faricimab, normalizes tumor vessels, reduces vascular permeability, and improves outcomes in diseases such as diabetic macular edema (DME, a complication of diabetes characterized by the accumulation of fluid in the macula due to damaged blood vessels) and AMD.200,201,202 This Ang-TIE-VEGF interplay is critical for vascular stability, and its imbalance contributes to diseases such as cancer.
Multi-level regulatory mechanisms of VEGF and VEGFR
The VEGF family is tightly regulated at transcriptional and translational levels in response to factors such as hypoxia and metabolic changes. Disruption of this delicate regulation can lead to diseases such as cancer and lymphedema in which abnormal blood vessel growth or lymphatic function becomes a problem.
Transcriptional and post-transcriptional regulation of VEGF family
The expression of VEGF genes is highly controlled by a complex interplay between transcription factors, signaling pathways, and external stimuli. This network reflects the essential role of VEGFs in physiological processes, as well as in pathological conditions such as cancer.
Hypoxia
One of the most significant regulatory mechanisms of VEGF-A transcription is hypoxia, which is mediated by hypoxia-inducible factor 1 (HIF-1).203 Under normoxic conditions, HIF-1α is hydroxylated by prolyl hydroxylases, marking it for degradation via the von Hippel-Lindau tumor suppressor protein.204 However, under low oxygen conditions, HIF-1α is stabilized, allowing it to dimerize with HIF-1β and bind to the hypoxia response element located in the VEGF-A promoter region.205 This binding leads to enhanced transcription of VEGF-A and drives angiogenesis in oxygen-deprived tissues, such as growing tumor. Interestingly, HIF-1-mediated activation is not the only factor involved in VEGF-A regulation under hypoxic conditions. Additional regions upstream of the HIF-1 binding site also contribute to transcriptional activation. For example, the AP1 transcription factor binds to the region between -1168 and -1015 of the promoter,206 which is essential for full transcriptional activation under hypoxic conditions, particularly in glioblastoma cells.
Hypoxia-independent regulation
Several studies reveal VEGF regulation mechanisms independent of the HIF pathway, highlighting alternative angiogenesis routes. Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) promotes VEGF expression and angiogenesis via estrogen-related receptor α (ERRα) binding to the VEGF gene, identified in muscle cells aiding ischemic recovery.207 Oxidative stress also drives VEGF production, as seen in hypertrophied adipocytes releasing VEGF-A120 through the PI3K pathway without hypoxia.208 Similarly, cold-induced angiogenesis in adipose tissue, mediated by sympathetic activation and VEGFR2 signaling, further demonstrates hypoxia-independent regulation.209 Notably, a nuclear VEGF fragment (N-VEGF) upregulates VEGF-A and hypoxia-related genes independently, offering a novel HIF-bypassing mechanism.210
Promoters of VEGF family members exhibit unique regulatory features enabling differential responses to stimuli. VEGF-A and VEGF-B promoters contain Sp1 and AP2 transcription factor binding sites and a CpG island, but VEGF-B uniquely includes Egr-1 sites and lacks AP1 and HIF-1 binding sites, suggesting distinct regulatory pathways.48,211 The VEGF-C promoter lacks a TATA box but contains Sp1, AP2, and NF-κB binding sites with a long 5’-untranslated region potentially affecting post-transcriptional regulation.212 Additionally, VEGF-D regulation involves a critical direct repeat element in its proximal region, where orphan nuclear receptors HNF-4A and COUP-TF1/TF2 bind coactivators such as CBP and GRIP-1 to enhance expression. The AP1 pathway, particularly c-Fos/c-Jun-dependent, regulates VEGF-D in lung cancer,213 with IL-7/IL-7R signaling upregulating VEGF-D via AP1 binding, promoting lymphangiogenesis through enhanced c-Fos/c-Jun heterodimers.
N6-methyladenosine modification
N6-methyladenosine (m6A) is a prevalent modification in eukaryotic mRNA that plays a critical role in post-transcriptional regulation. m6A modifications occur at specific adenine residues in a consensus sequence and are dynamically regulated by m6A writers (methyltransferases, such as METTL3 and METTL14), erasers (demethylases, such as FTO and ALKBH5), and readers (binding proteins, such as YTH domain family proteins).214 m6A modification in the 5′UTR of VEGF-A mRNA enhances cap-independent translation, driven by METTL3-mediated methylation and YTHDC2/eIF4GI interactions, which promotes VEGF-A expression and tumor progression. Knockdown of METTL3 reduced VEGF-A translation and angiogenesis, demonstrating the therapeutic potential of targeting the m6A/VEGF-A axis in lung cancer.215 In addition, Wilms tumor 1-associated protein (WTAP), a key component of the m6A methyltransferase complex, promotes colorectal cancer progression by enhancing VEGF-A through m6A modification mediated by YTHDC1.216 Interestingly, YTHDF2, an m6A reader protein, promotes hepatocellular carcinoma (HCC) progression by enhancing ETV5 translation, which upregulates PD-L1 and VEGF-A, driving immune evasion and angiogenesis. Targeting YTHDF2 with siRNA-loaded liposomes effectively reduces tumor growth and restores immune activity, highlighting its therapeutic potential.217 m6A modification is a dynamic regulatory mechanism that enhances VEGF-A expression, contributing to tumor progression and angiogenesis in various cancers. Targeting key components of the m6A machinery, such as METTL3, WTAP, and YTHDF2, offers promising therapeutic strategies for inhibiting VEGF-A-driven oncogenic pathways.
MicroRNAs and non-coding RNAs
MicroRNAs (miRNAs) are small, evolutionarily conserved non-coding RNAs, approximately twenty-two nucleotides in length, first discovered in C. elegans.218 Typically, miRNAs act as negative regulators by binding to complementary sequences in the 3′-UTR of mRNAs, promoting mRNA degradation or inhibiting translation.219 This post-transcriptional regulation is essential for controlling homeostatic and developmental events.
miR-126 is a key regulator of VEGF signaling,220 which elevates angiogenesis by suppressing negative regulators such as SPRED1 and PIK3R2, affecting the MAPK and PI3K pathways.221 miR-126 downregulation elevates VEGF-A expression, driving tumor angiogenesis. Epigenetic silencing of miR-126 increases VEGF-A levels, enhancing neovascularization and metastasis.222 Conversely, miR-16 directly targets VEGF-A in cancers such as multiple myeloma and lung cancer, reducing tumor growth and angiogenesis.223,224 Similarly, miR-205 is a key regulator in various cancers, affecting angiogenesis, metastasis, and chemoresistance. In breast cancer, miR-205 enhances chemosensitivity by targeting VEGF-A and FGF2, reducing resistance to drugs, such as doxorubicin and paclitaxel, and promoting apoptosis through the PI3K/AKT pathway.225 In HCC, miR-205 inhibits tumor growth and metastasis by directly targeting VEGF-A, suggesting its potential as a therapeutic target.226 Additionally, in breast cancer-associated fibroblasts, miR-205 mediates VEGF-independent angiogenesis through the YAP1-IL-11/IL-15-STAT3 axis, providing new strategies to combat resistance to anti-VEGF treatments.227 These findings underscore the dual role of miRNAs in regulating VEGF-A expression and angiogenesis, either promoting or inhibiting tumor growth and metastasis. The activity of specific miRNAs, such as miR-126 and miR-205, to modulate key pathways associated with tumor progression and angiogenesis highlights their significance in disease mechanisms and their potential as therapeutic targets.
Long non-coding RNAs (lncRNAs), RNA molecules over 200 nucleotides long, regulate gene expression without encoding proteins.228,229 They function through various mechanisms such as chromatin remodeling or acting as decoys for proteins or miRNAs. Maternally expressed gene 3 (MEG3), a key lncRNA, regulates angiogenesis and vascular function by exerting anti-proliferative and pro-apoptotic effects, partly through p53 accumulation.229,230,231 In DR, MEG3 downregulation exacerbates microvascular damage by increasing VEGF and inflammatory cytokines.232,233 MEG3 also regulates VEGFR2 expression in ECs, and its reduction impairs VEGF-driven angiogenesis.234 Overexpression of MEG3 can mitigate high glucose-induced VEGF and TGFβ1 levels, suggesting therapeutic potential for pathological angiogenesis.233 In contrast, the lncRNA MALAT1 plays a pro-angiogenic role. In DR, MALAT1 supported EC growth and migration via the YAP1/miR-200b-3p/VEGF-A pathway.235 In retinopathy of prematurity (ROP, eye disorder of abnormal retinal blood vessel development in premature infants), MALAT1 inhibition with siRNA reduced retinal neovascularization and lowered VEGF and inflammatory markers, highlighting its role in abnormal retinal vessel development.236 Together, MEG3 and MALAT1 illustrate the diverse and opposing roles of lncRNAs in angiogenesis and vascular pathology.
Post-translational regulation
Post-translational modifications enhance protein diversity and help cells maintain homeostasis by altering the protein structure, stability, and interactions.
Glycosylation
Glycosylation of VEGF and VEGFR family members plays a subtle yet key role in their stability, secretion, and interactions, although it is not always essential for core functions, such as receptor binding. For VEGF-A, glycosylation at Asn74 (N74) is not essential for receptor binding or EC proliferation,237 although it enhances protein stability and binding to GAGs. Similarly, VEGF-B186 is glycosylated in its C-terminal region, increasing its molecular weight and solubility compared with the non-glycosylated VEGF-B167, facilitating its secretion and extracellular stability.14 N-glycosylation is critical for VEGF-D, as glycosylation at N155 and N185 ensures proper folding, solubility, and secretion.238 Similarly, for VEGFR2, glycosylation at N247 regulates receptor activation, specifically, removing sialylated N-glycans at this site increases ligand-induced activation, which, in turn, regulates receptor dimerization and downstream signaling.239 Moreover, glycosylation and sialylation of VEGFR2 regulate its stability, trafficking, and receptor-ligand interactions, underscoring their critical role in regulating VEGF/VEGFR signaling and function.
SUMOylation
SUMOylation is a reversible process in which small ubiquitin-like modifier (SUMO) proteins are attached to target proteins, and regulate key functions, such as DNA repair.240,241 This modification involves a series of enzymes (E1, E2, and E3) that attach SUMO to specific lysine residues, while SUMO-specific proteases (SENPs) remove it, allowing for dynamic control of protein activity. SUMOylation of Lys1270 of VEGFR2 leads to its retention in the Golgi, reducing its expression on the cell surface and impairing VEGFR2 signaling.242 SENP1, a SUMO-specific protease, removes this modification, allowing VEGFR2 to reach the cell membrane and activate angiogenesis.242,243 In SENP1-deficient cells, VEGFR2 remains hyper-SUMOylated and trapped in the Golgi, leading to impaired angiogenesis. Moreover, SENP6 deSUMOylates VEGFR2, promoting its transport from the Golgi to the cell membrane and enhancing VEGFR2-mediated angiogenesis.244 This process is particularly important in conditions such as diabetic microangiopathy, where altered SUMOylation dynamics can affect pathological angiogenesis.244 Similarly, SUMOylation at Lys1270 inhibits VEGFR2 activity by reducing its phosphorylation and downstream signaling pathways, such as the AKT and ERK pathways. This modification also impairs cell proliferation and migration while promoting apoptosis in non-small cell lung carcinoma cells. Conversely, deSUMOylation of VEGFR2 by enzymes such as SENP1 can enhance VEGFR2 activity and restore angiogenic signaling.245
Ubiquitination
VEGFR2 ubiquitination is a critical post-translational modification that regulates its signaling, stability, and degradation, and directly affects angiogenesis. Among the key regulators, various E3 ubiquitin ligases, such as c-CBL, β-TRCP, and RNF121, activate VEGFR2 with ubiquitin, marking it for degradation through the endosomal-lysosomal system. This process is particularly important because ligand-dependent ubiquitination is necessary for regulating receptor expression on the membrane and preventing overstimulation of VEGF-A signaling.246,247 In addition, the CUL3-SPOP-DAXX axis has been identified as a key regulator of VEGFR2 expression.248,249 This complex facilitates ubiquitination of VEGFR2, reducing its expression and subsequently limiting angiogenic signaling. Moreover, E2 ubiquitin-conjugating enzymes such as UBE2D1 and UBE2D2 play crucial roles in modulating VEGFR2 ubiquitination. Notably, their depletion leads to increased VEGFR2 levels, enhancing VEGF-A-stimulated signaling pathways, such as MAPK, AKT, and PLCγ1, and promoting EC migration and tubulogenesis.250 Thus, the balance of ubiquitination, recycling, and degradation orchestrates the intensity and duration of VEGFR2 signaling in physiological and pathological angiogenesis. Conversely, USP8, a deubiquitinating enzyme, removes ubiquitin chains from VEGFR2, facilitating its recycling from early endosomes back to the membrane and preventing its degradation in the lysosome.251 However, when USP8 is depleted, VEGFR2 accumulates in early endosomes, leading to the generation of a novel 120 kDa proteolytic fragment. As a result, this accumulation also impairs VEGF-A-induced signaling, particularly through the AKT and ERK pathways.
Regulatory mechanisms of VEGFR activity
VEGFR activity is tightly regulated by various molecular processes that maintain vascular homeostasis and modulate the angiogenic response. These regulatory mechanisms include both positive and negative modulators, which fine-tune VEGFRs signaling to prevent excessive or insufficient angiogenesis.
Protein phosphatase
VE-PTP, a receptor-type phosphatase, is crucial for regulating endothelial junctions and blood vessel development.252,253 VE-PTP is highly expressed in ECs, especially during vascular development, VE-PTP interacts with TIE2 (Fig. 7a), dephosphorylating it to regulate Ang/TIE2 signaling.254 It also negatively regulates VEGFR2 phosphorylation at endothelial junctions, controlling VEGFR2 activity and preventing excessive angiogenic signaling.255 VE-PTP ensures proper EC polarity and lumen formation with its deficiency leading to increased VEGFR2 phosphorylation, abnormal vessel sprouting and endothelial dysfunction.256,257 Recent studies showed that activin A, a member of the TGFβ family, reduces VEGF-induced vascular permeability by increasing VE-PTP expression which dephosphorylates VEGFR2 and dampens VEGF signaling,258 suggesting therapeutic potential for conditions such as DR (Fig. 7a).
Negative regulation and fine-tuning of VEGFR2-mediated signaling pathways. a VE-PTP regulates TIE2 and VEGFR2 dephosphorylation to balance angiogenic signaling and support vessel integrity. Activin A enhances VE-PTP expression and reduces VEGF-induced permeability, which may help control excessive vessel leakage in conditions such as diabetic retinopathy. b PTP1B regulates VEGFR2 signaling by binding to its cytoplasmic domain and dephosphorylating it, thereby acting as a negative regulator. c SHP1 acts as a negative regulator of VEGFR2 by dephosphorylating key tyrosine residues (Y996, Y1059, and Y1175) essential for endothelial cell proliferation. d TSP-1 modulates VEGFR2 activity by binding to CD36 and recruiting SHP1, which dephosphorylates VEGFR2 at Y1175. This action suppresses VEGF signaling and inhibits angiogenesis. e Notch signaling coordinates angiogenesis by balancing the roles of tip and stalk cells. Dll4 on tip cells activates Notch in stalk cells, reducing VEGFR expression and VEGF sensitivity to stabilize newly formed vessels. Fringe, a glycosyltransferase in stalk cells, enhances Dll4-Notch signaling, further reducing VEGFR levels and reinforcing stalk cell quiescence. Meanwhile, Jagged1 counteracts Dll4 by modulating Notch in stalk cells, allowing some cells to remain responsive to VEGF, supporting sprouting. This interplay ensures selective sprouting while maintaining vessel stability. Notably, the Notch-VEGFR3 pathway can also promote angiogenesis independently of VEGF-A/VEGFR2. VE-PTP vascular endothelial protein tyrosine phosphatase, VEGFR vascular endothelial growth factor receptor, PTP1B protein tyrosine phosphatase 1B, SHP-1 Src homology 2 domain-containing phosphatase-1, TSP-1 Thrombospondin-1. Created in BioRendender.com
PTP1B, another phosphatase, binds to the cytoplasmic domain of VEGFR2 and directly dephosphorylates it (Fig. 7b), acting as a negative regulator.259 Overexpression of PTP1B reduces VEGF-induced VEGFR2 autophosphorylation, inhibiting downstream signaling ERK signaling and EC proliferation. PTP1B also dephosphorylates VEGFR2 at Y1175 site during endocytic trafficking, suppressing VEGF signaling and the PLCγ1-MAPK pathway essential for arterial development.260 Similarly, SHP1 negatively regulates VEGFR2 by dephosphorylating key tyrosine residues (Y996, Y1059, Y1175) (Fig. 7c) upon VEGF stimulation via a c-SRC-dependent mechanism.261 In addition, thrombospondin-1 (TSP-1) enhances this regulation by binding to CD36 and recruiting SHP1 to dephosphorylate VEGFR2 at Y1175 (Fig. 7d), further suppressing VEGF signaling and angiogenesis.262 While this study focuses on tyrosine phosphatases, serine/threonine phosphatases also play distinct roles in VEGFR2 regulation and downstream signaling.
Negative feedback regulator
Negative feedback regulation maintains homeostasis by reducing or turning off a signaling pathway once the desired response is achieved.263 Key regulators such as Sprouty, suppressor of cytokine signaling (SOCS), and ErbB receptor feedback inhibitor-1 (ERRFI1) inhibit excessive signaling by targeting pathways such as RTKs, JAK/STAT, and RAS/MAPK pathways, ensuring tight control over signaling cascades.264,265
Vasohibin (VASH), identified as a VEGF-inducible gene in ECs, acts as a negative regulator of angiogenesis.266,267 VASH inhibits EC migration, proliferation, and network formation, specifically targeting ECs without affecting other cell types.266,268 Its expression is downregulated by hypoxia and inflammatory cytokines, such as TNF-α, potentially impairing its anti-angiogenic activity in conditions such as cancer. VASH has two isoforms: VASH1 and VASH2. VASH1, with two variants (VASH1A and VASH1B), primarily functions as an anti-angiogenic factor.269 VASH1A normalizes tumor vessels, improving perfusion and chemotherapy efficacy, while VASH1B induces autophagic cell death in ECs, leading to vascular pruning and increased hypoxia.270 In contrast, VASH2 promotes angiogenesis and is highly expressed in cancers, supporting tumor growth and metastasis by facilitating new blood vessel formation.271,272,273 This dual role of VASH, acting as both an inhibitor and a promoter of angiogenesis, highlights its dynamic function in tumor development and therapeutic outcomes.
The VEGF/VEGFR and Notch/Dll4 pathways function together in a finely tuned negative feedback loop.274,275 Indeed, Notch signaling is essential for angiogenesis, coordinating blood vessel formation.276,277 When Dll4 on endothelial tip cells binds to Notch receptors on neighboring stalk cells, it triggers the release of Notch intracellular domain (NICD), which moves to the nucleus to affect gene expression. This Notch/Dll4 signaling balances tip and stalk cell roles during vessel sprouting and acts closely with VEGF signaling to maintain blood vessel formation (Fig. 7e). Tip cells, which lead to new vessel sprouting, are highly affected by VEGF-A/VEGFR2 activation, which triggers strong ERK signaling and upregulates tip cell-specific genes such as Dll4.278,279,280 In turn, Dll4 interacts with Notch receptors in nearby stalk cells, reducing their expression of VEGFR2 and VEGFR3 and making them less responsive to VEGF.281,282 As a result, these cells adopt the role of stabilizing newly formed vessels rather than participating in further sprouting. This feedback loop ensures that stalk cells stabilize new vessels rather than participating in further sprouting, maintaining a balance between sprouting and stabilization.281,282 This tightly regulated interaction is vital for proper vascular development, as it ensures that sprouting occurs in a controlled manner.
In contrast, Jagged1, another Notch ligand, promotes angiogenesis by antagonizing Dll4-Notch signaling (Fig. 7e), especially in cells expressing the Fringe glycosyltransferase.283 Fringe enhances Dll4-Notch signaling while diminishing the Jagged1 effect, allowing Jagged1 to support tip cell formation and sprouting. This interplay between Dll4 and Jagged1 finely balances angiogenesis. Interestingly, Notch signaling can drive angiogenesis independently of VEGF-A and VEGFR2 by upregulating VEGFR3, enabling vessel sprouting even when VEGFR2 is inhibited.284 Blocking Notch signaling increased sprouting and vessel density in the absence of VEGFR2, revealing a VEGF-A-independent mechanism. This challenges the traditional reliance on VEGF-A/VEGFR2 in angiogenesis and suggests that targeting Notch and VEGFR3 could offer new strategies, particularly for tumors resistant to anti-VEGF treatment. Overall, this complex interplay between Notch, Dll4, Jagged1, VEGF, and VEGFR signaling pathways forms a multilayered feedback system that precisely controls angiogenic sprouting and vessel stability, highlighting a sophisticated regulatory network essential for balanced vascular growth.
Physiological roles of VEGF/VEGFR signaling
In multicellular animals, the slow efficiency of oxygen and nutrient diffusion limits their reach to short distances.285 To address the high demand for oxygen and nutrients driven by body size expansion during evolution, the development of an internal transport and exchange system became essential for substance delivery and waste removal. Invertebrates evolved circulatory systems - both open and closed - with vessels lined by ECM.286 Vertebrates, however, developed a closed circulatory system featuring vessels with a true endothelial lining.287 This endothelial lining, composed of tightly interconnected ECs anchored to a basement membrane (BM) with basoapical polarity, represents an evolutionary innovation. Beyond forming a functional vasculature for oxygen and nutrient delivery, ECs interact with perivascular cells to regulate blood flow, modulate immune cell trafficking, and produce signaling molecules to maintain tissue homeostasis.
Angiogenesis
Angiogenesis has been recognized since ancient times, with early descriptions by the Greek physician Galen, who compared embryonic growth along umbilical veins to plant growth.288,289 While its morphological features were detailed in the 1960s,290,291 the molecular mechanisms were not elucidated until the 1970s by Judah Folkman and others.292
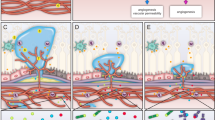
Angiogenesis begins (Fig. 8a) with (1) the degradation of BM and loss of pericyte coverage in response to angiogenic stimuli. (2) ECs then migrate toward the stimulus with stalk cells aligning under the control of tip cells. (3) tip cells survey the microenvironment and connect to sprouts. Following this, (4) vessel maturation occurs, involving new BM formation and coverage by perivascular cell types, (5) blood flow is established, supporting the survival of new vessels.293,294 Angiogenesis is tightly regulated by a balance of pro-angiogenic and anti-angiogenic factors. Among these, VEGF/VEGFR signaling is the best-defined key stimulator, demonstrated in models such as chicken embryos,11 and primate,295 and plays a critical role in every step of angiogenesis.
VEGF/VEGFR signaling controls angiogenesis and vascular permeability. a Role of VEGF/VEGFR signaling pathway in angiogenesis. (1) The vascular stability of quiescent microvessels covered by pericytes and basement membrane (BM) is maintained by non-VEGF/VEGFR signaling pathways such as Ang/TIE signaling. (2) Upon VEGF ligand stimulation, the BM degrades and releases bio-unavailable VEGF ligands. Endothelial cells (ECs) lose structural support and key adhesion molecules such as VE-cadherin and undergo tip cell selection. (3) VEGF signaling strongly induces the tip cell phenotype and metabolic characteristics, providing guidance cue for tip cells, whereas VEGFR2 and VEGFR3 are highly expressed in tip cells. Tip cells inhibit VEGFR2 and VEGFR3 and increase VEGFR1 and sVEGFR1 in stalk cells. VEGF supports perpendicular proliferation of stalk cells and lumen formation. (4) Tip cells recognize each other and induce sprout anastomosis. sVEGFR1, possibly produced by macrophages, regulates this process. VEGF negatively regulates vessel maturation. b Role of VEGF/VEGFR signaling in vascular permeability. The vascular permeability of quiescent microvessels is maintained by pores in ECs, glycocalyx, and EC junctions. VEGFR2 Y949 phosphosite in mice downregulates VE-cadherin via SRC. VEGFR2 also promotes nitric oxide (NO) production to reduce the expression of adhesion molecules required for paracellular permeability. VEGF has also been shown to stimulate vesicovacuolar organelles (VVO) and fenestrae formation for transcellular permeability. However, whether the glycocalyx can be cleaved by VEGF stimulation is not fully understood. Created in BioRendender.com
VEGF in BM degradation
During BM degradation step, ECs lose structural support and tissues undergo decompartmentalization, enabling sprouting. The BM, composed of collagen,296 laminin,297 and elastin,298 and inter-EC adhesion molecules such as VE-cad,299 claudin,300 and junctional adhesion molecules,301 separates blood from underlying tissue. BM degradation, mediated by the proteolytic process of urokinase plasminogen activator (uPA) and matrix metalloproteinases (MMPs), facilitates angiogenesis.302 ECM-degrading enzymes regulate VEGF signaling through:
-
(1)
releasing bio-unavailable ligands: due to the varying heparin-binding and heparan sulfate-binding capacities of VEGF isoforms, some VEGF ligands are sequestered in the ECM. These bio-unavailable VEGF ligands are released following proteoglycan degradation, promoting angiogenesis303;
-
(2)
directly cleaving matrix-bound VEGF isoforms to modulate their angiogenic properties;304 and
-
(3)
stimulating VEGF expression via membrane-type MMP and SRC tyrosine kinases.305,306 Following degradation, ECM fragments such as endorepellin,307 endostatin,308 thrombospondin-1,309 and tumstatin,310 often act as endogenous inhibitors, balancing pro-angiogenic and anti-angiogenic signals.
Accompanying BM degradation, VEGF also disrupts inter-EC adhesion in quiescent vessels by:
-
(1)
adherens junction dissociation: VEGF induces rapid dissociation of the EC-specific tyrosine phosphatase VE-PTP from VE-cad (Fig. 8b), thereby disrupting the endothelial barrier;311
-
(2)
endocytosis of adhesion molecules: VEGF stimulation promotes endocytosis of VE-cad, enabling EC migration and proliferation;312 and
-
(3)
regulating adherens junctions to control tight junctions: VE-cad promotes intracellular signaling that upregulates claudin-5, a major component of tight junction component, ensuring coordinated regulation of endothelial permeability.313
Thus, VEGF downregulates both junction types and promotes angiogenesis. Conversely, adherens junction proteins, including VE-cad and PECAM1 (also known as CD31) can be phosphorylated by mechanical forces, activating VEGFR-related signaling.314,315 This integrates mechanical and chemical stimuli in ECs, driving angiogenesis.316
VEGF in tip cell formation and EC migration
Once the barrier is breached, a small subset of ECs undergoes an angiogenic switch, enabling migration and proliferation. At this stage, tip cells emerge from this EC subpopulation, becoming the leading cells of sprouting vessels (Fig. 8a). Morphologically distinct, tip cells function as sensors, using dynamic filopodia to survey the environment for guidance cues and direct migration. This process resembles the insect tracheal system, where specialized cells guide migration and determine cell fate.317 Interestingly, although tip cells were observed in the 1970s,318 their molecular understanding emerged in the 2000s.293
VEGF signaling strongly drives the tip cell phenotype. During retinal development, tip cells exhibit high VEGFR2 expression compared with stalk or quiescent ECs.319 VEGF ligands guide tip cell migration and are essential for tip cell filopodia formation, extension, and orientation.319 In addition to VEGF/VEGFR2 signaling, VEGFR3 is highly expressed in tip cells and sustains angiogenesis even when VEGFR2 is inhibited.320 VEGFR2/3 heterodimers often found on tip cell filopodia,132 likely mediate responses to VEGF-A and VEGF-C in vivo. Beyond regulating tip cell behavior, VEGF also affects its metabolic phenotype. While ECs in perfused vessels rely on oxygen, VEGF-driven tip cells depend on glycolysis, mediated by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB).321,322
Uncontrolled tip cell formation results in dysfunctional vessels, so tip cells must regulate neighboring ECs to maintain the stalk cell phenotype. In zebrafish, VEGFR1 is highly expressed in stalk cells, and its loss leads to excessive tip cells and hyperbranching, indicating that VEGFR1 negatively regulates the tip cell phenotype.323 Furthermore, through Notch signaling, tip cells suppress VEGFR2 and VEGFR3 expression whereas increasing VEGFR1 and sVEGFR1 in stalk cells, reducing their sensitivity to VEGF and maintain the stalk phenotype.277,324 Tip cells also produce various immune and angiogenic modulators that facilitate tip-stalk cell communication.325 Importantly, tip and stalk cells are not fixed phenotypes. Therefore, continuous communication is essential to sustain their distinct roles during angiogenesis.326
Stalk cells follow tip cells, proliferating perpendicular to their guidance to elongate vessels and form lumens for tubular morphogenesis. Disrupted VEGF signaling misorients stalk cell division, causing vessel dysmorphogenesis, indicating a flow-independent regulatory mechanism.327 During lumen formation, cell-cell contact is crucial for EC shape changes. In mouse aortic development, VEGF recruits myosin to apical cell surfaces at EC contact sites, driving morphological alterations.328
VEGF in sprout anastomosis
The mechanism of tip cell fusion during sprout anastomosis remains unclear but appears to involve tip cell recognition, EC polarization, and apical membrane fusion. VE-cad and filopodia are essential for this process.329,330 Interestingly, the spatial distribution of VEGFR1 regulates anastomosis, as in vitro studies show that tip cells make transient contacts before stable vessel connection, while VEGFR1 reduces contact frequency and alters vessel connection site.331 Perivascular cells may act as chaperones332 and resident macrophages, which secrete sVEGFR1 via a non-canonical pathway,333 could play a regulatory role in sprout anastomosis.
VEGF in vessel maturation
After sprouting, vessels undergo remodeling and maturation, a critical step for establishing a functional vascular network.334 Blood flow delivers oxygen and mechanical forces that shape vascular remodeling.335,336 As vessels mature, ECs form a new BM to stabilize and pattern the vasculature.337 Perivascular cells, including pericytes, are recruited and stabilize vessels through non-VEGF signals such as PDGF, TGFβ, Ang1, and Notch.338,339 Notably, VEGF signaling inhibits vessel maturation, highlighting its role in early angiogenesis rather than stabilization.142
Interplay between VEGF and other pro-angiogenic factors
Several angiogenic factors, including FGFs and Ang, stimulate angiogenesis under physiological conditions, differing from VEGF pathways.340,341 For example, FGFs are widely expressed, promoting EC proliferation,342 whereas VEGF is mainly EC-specific, exerting diverse regulatory effects. During development, FGF1 and FGF2 are less critical,343 whereas TIE2, expressed in ECs, interacts with Ang1 and Ang2. Ang1 stabilizes quiescent blood vessels,344 whereas Ang2 modulates the Ang1/TIE2 axis, sensitizing vessels to VEGF and other factors.341 Ang1 and VEGF complement in each other in early vascular development, with VEGF initiating vessel formation and Ang1 supporting subsequent maturation.345 Ang2 is required for post-natal angiogenesis.346 These factors, along with VEGF, synergistically orchestrate angiogenesis to meet tissue-specific vascular needs.347,348
VEGF signaling is central to every step of angiogenesis (Fig. 8a), yet its exact role in processes such as sprout anastomosis remain unclear due to EC heterogeneity and dynamic phenotypic changes. The involvement of other cell types also requires further investigation. Recent single-cell studies may provide deeper insights.349 While the molecular mechanisms of VEGF signaling in angiogenesis are well understood, angiogenesis relies on precise spatiotemporal regulation to ensure proper vascular development. A comprehensive understanding of these dynamic processes may open new avenues for therapeutic strategies targeting pathological angiogenesis.
Vasculogenesis and other types of vascular formation
Vasculogenesis involves the de novo formation of blood vessels from endothelial progenitor cells (EPC), occurring during early embryonic development or rapid vascularization of avascular tissues.350,351 Endothelial and hematopoietic progenitor cells arise from the embryonic mesoderm, yolk sac, allantois, and placenta, migrating to blood islands where they differentiate into angioblasts (forming ECs) and hematopoietic precursors (forming blood cells).352,353 Differentiated ECs polarize and form a vascular lumen, through intracellular vesicle fusion or slit formation,354 eventually forming major vessels such as aorta or middle cerebral artery after BM deposition.355
Morphogens including Wnt, Hedgehog, TGFβ, and FGFs, collectively regulate blood island formation, angioblast induction, and yolk sac vasculogenesis.356 Additionally, VEGF, expressed in the extra-embryonic endoderm and mesoderm,357 is crucial for vasculogenesis; for instance, embryos lacking VEGF, VEGFR2, or VEGFR3 exhibit severe defect (Table 1), including failed blood island formation, aortic abnormalities, or large vessel defects,358,359,360,361 Moreover, VEGFR1 inhibits EC overgrowth and thereby tightly regulates VEGF signaling during vasculogenesis.362
Intussusceptive angiogenesis involves vessels splitting to form new vessels, observed in developing organs and pathological vascular remodeling.363,364 During this process, transluminal tissue pillars develop and fuse, creating new vascular structures. While VEGF and Notch signaling are implicated in intussusceptive angiogenesis,365,366 the detailed molecular mechanisms remain unclear. Alternatively, another mode of vascular formation involves the segregation of cells from a common precursor vessel into discrete arterial and venous vessels, as seen in zebrafish embryo development. Specifically, VEGF-induced Ephb2a directs angioblast migration, determining vessel identity.367 Compared with angiogenesis, vasculogenesis and other types of vascular formation are less studied with most descriptive studies, offering limited insights into molecular mechanisms. This is partly due to the transient nature of these processes and their overlapping occurrence in vivo, which complicates understanding. To address this, models that can specifically induce distinct forms of vascular formation are needed to clarify whether and how VEGF signaling affects these processes.
Lymphangiogenesis
Approximately 400 years ago, lymphatic vessels were first described by Gaspare Aselli in Italy and their route was correctly identified by Jean Pecquet in France.368 Functioning as a parallel circulatory system to blood vessels, lymphatic vessels maintain tissue homeostasis by draining interstitial fluid and transporting antigens and immune cells to lymph nodes for immune surveillance. During early development, lymphatic vessels originate from the cardinal,369 as demonstrated by genetic tracing of Prox1 cells in mice.370
Since the 1990s, lymphangiogenic factors have been identified with the VEGF-C/D-VEGFR3 axis emerging as the most critical signaling pathway regulating lymphangiogenesis.371,372 Notably, VEGF-C transgenic mice exhibited overgrowth of lymphatic vessels but not blood vessels, underscoring its specific role in lymphangiogenesis.373 Subsequently, VEGF-D was identified and shown to also promote lymphangiogenesis.73,374 However, while Vegfc-deficient mouse embryos failed to form lymphatic vessels, this defect could be rescued by external VEGF-C or -D supplementation.375 Interestingly, although VEGF-D is a potent lymphangiogenic factor, it is not essential for lymphatic development.376 VEGFR3 is crucial for blood and lymphatic vascular formation during early developmental stages. Studies involving mutations in the ligand-binding domain or tyrosine KD of VEGFR3 revealed that ligand binding is essential for lymphatic vessel development but not for blood vessel development. This supports a regulatory model where VEGFR3 modulates VEGF-A/VEGFR2 signaling through VEGFR2/3 heterodimers for blood vascular formation. Thus, the VEGF-C/D-VEGFR3 axis plays a pivotal role in lymphangiogenesis, distinct from its tole in blood vessel development.377 Furthermore, other members of the VEGF/VEGFR family also contribute significantly to lmphangiogenesis. LEC-specific knockout of Vegfr2 (Flk1) leads to hypoplastic yet functional lymphatic vessels, indicating that VEGFR2, activated by both VEGF-A and VEGF-C, plays a role in this process. Additionally, VEGF-A alone has been shown to induce lymphangiogenesis,378,379 although this effect is partially mediated by macrophage recruitment.380,381 Thus, while VEGF-C/D-VEGFR3 remains central to lymphangiogenesis, VEGFR2 and VEGF-A also play supportive roles, often involving indirect mechanisms such as macrophage involvement.
Ang-TIE signaling,346,382 FGF signaling,342,383 and other factors including HGF,384 PDGF,385 and IGF386 have been shown to promote lymphangiogenesis.372,387 However, despite the identification of numerous lymphangiogenic factors, it remains unclear whether the mechanisms of lymphatic vessel sprouting and guidance resemble those of angiogenesis. The current model divides lymphangiogenesis into four key steps: (1) guidance/alignment, (2) proliferation, (3) sprouting, and (4) redirection.372 In this process, the BM stabilizes lymphatic vessels,388 LEC tip cells guiding sprouting,389 and tissue macrophages provide VEGF-C guidance.390 Future studies should focus on dissecting each step of lymphangiogenesis and uncovering the molecular mechanisms underlying these processes.
Embryonic development
Blood vessels are present in nearly all tissues, making VEGF signaling crucial for regulating angiogenesis and maintaining tissue homeostasis, including vascular permeability. To highlight its physiological significance, VEGF signaling plays key roles in (1) embryonic development by regulating angiogenesis, (2) maintaining endocrine organ homeostasis through vascular permeability, and (3) exerting neurotrophic functions independent of ECs.
Mouse embryos lacking VEGF ligands or their receptors exhibit embryonic lethality due to vascular defects (Table 1).358,359,360,361,362,375 Specifically, VEGF-A, VEGFR2, and VEGFR3 promote hematopoietic and EC development, while VEGFR1 ensures precise blood vessel assembly. In contrast, PlGF and VEGF-B have minimal roles in development87,391 whereas VEGF-C drives lymphatic vascular formation. Even slight alterations in VEGF levels, such as deletion of a single allele or modest overexpression, lead to abnormal blood vessel development and embryonic lethality,358,359 emphasizing the need for precise VEGF signaling regulation during embryonic development. Postnatally, VEGF signaling is critical for organ development, as its disruption causes glomerular defects in neonates,392 but not in adult mice.393 Although VEGF/VEGFR signaling is vital for embryonic development, it remains active in adults, affecting both pathological and physiological conditions. For instance, modestly elevated circulating VEGF levels from early adulthood have been shown to preserve a youthful microvascular phenotype and extend lifespan in mice.394 Thus, while VEGF signaling is indispensable during development, its regulation continues to play a beneficial role in maintaining physiological health throughout life.
Vascular permeability
Vascular permeability is a selective mechanism that regulates material exchange between blood vessels and surrounding tissues through small and large pores,395 glycocalyx, and tight junctions on ECs (Fig. 8b).396 Under normal conditions, the endothelium allows the passage of solutes and small molecules while restricting larger molecules. However, in response to stimuli, the endothelium can become more permeable, enabling the leakage of larger molecules or cells. This permeability varies across organs based on physiological needs; for example, fenestrated endothelial in endocrine glands and kidney peritubular capillaries facilitate rapid transport, while the blood–brain barrier features tight junctions and perivascular coverage to restrict permeability. This plasticity is tightly controlled by various extracellular signaling molecules, including VEGF, Ang, histamine, platelet-activating factor, as well as intracellular mediators, such as NO, focal adhesion kinase (FAK), and GTPases.397,398
VEGF-A/VEGFR2 signaling is widely recognized for promoting vascular permeability through multiple mechanisms. Specifically, the Y949 (Y951 in humans) in VEGFR2 in mice binds to TSad, regulating c-SRC signaling at the EC junction.399 Disruption of Y949 signaling stabilizes adherens junctions, blocking extravasation.171 In contrast, the Y1173 in mice (Y1175 in humans) binds to PLCγ1, driving EC proliferation,400 and its disruption halts developmental processes.401 Notably, the role of VEGFR2 in permeability depends on its co-receptor NRP1, as SRC signaling in EC is largely NRP1-dependent.189 Beyond direct junction regulation, VEGFR2 activates AKT and eNOS to promote NO synthesis,402 indirectly enhancing vascular permeability, a process regulated by caveolin-1.403,404 These findings highlight the critical role of VEGF-A/VEGFR2 in paracellular permeability, surpassing even certain inflammatory cytokines in potency.171 Additionally, VEGF stimulates the formation of vesiculo-vacuolar organelles (VVO) in EC,405 interconnected structures spanning the EC lumen, which support transcellular permeability and macromolecular extravasation. Thus, VEGF signaling plays a dual role in regulating both paracellular and transcellular permeability, underscoring its importance in vascular function.406 VEGF/VEGFR signaling primarily regulates endothelial permeability through VEGFR2, facilitating the rapid extravasation of macromolecules (Fig. 8b). Furthermore, the glycocalyx, a key structural component for vascular permeability, can be cleaved by inflammatory cytokines.407 While it remains unclear whether VEGF signaling directly regulates the glycocalyx in EC, the glycocalyx itself inhibits VEGFR2 internalization, reducing VEGF-induced vessel permeability.408 Overall, these interactions highlight the complex relationship between VEGF signaling and the glycocalyx in regulating vascular function.
Under physiological conditions, most tissues require a certain degree of vascular permeability for efficient substance exchange. Particularly, endocrine organs exhibit high permeability to facilitate hormone delivery, maintained by both general and organ-specific factors.409 For example, the thyroid gland, the largest endocrine organ,410 features a dense capillary network with abundant endothelial fenestrae,410 which are VEGF-dependent for their maintenance.411 Recent studies highlight that VEGF/VEGFR signaling, rather than Ang/TIE signaling molecules, is prominently expressed in thyroid organs.412 Blocking VEGF or VEGFR2 in the thyroid leads to thickened cytoplasmic membrane, loss of EC fenestration, and accumulation of caveolae, modestly reducing circulating hormone levels.412,413 Importantly, VEGF-VEGFR2 inhibition not only alters vascular permeability but also causes significant vascular regression in thyroid, more pronounced than tumors.413,414 Clinically, this is evident as bevacizumab rapidly reduces thyroid perfusion.415 Even in healthy adults, autocrine VEGF is essential for blood vessel homeostasis, as endothelial-specific deletion of Vegf results in progressive endothelial degeneration.416 Thus, endocrine organs uniquely depend on VEGF-VEGFR2 signaling not only for vascular permeability but also for maintaining vascular homeostasis.
Animal models of VEGF and VEGFR
Animal models, including various VEGF and VEGFR knockout and conditional knockout mouse lines, have provided pivotal insights into the complexities of VEGF signaling in endothelial and lymphatic cells. These models reveal how genetic alterations in the VEGF/VEGFR pathways affect vascular development, lymphangiogenesis, and pathological processes, offering a detailed view of the VEGF regulatory network in both physiological and pathological contexts. These distinct phenotypes underscore the crucial roles of VEGF signaling in various biological processes (Table 1).
VEGF and VEGFR signaling in diseases
The discovery of VEGF has been a pivotal milestone and has significantly advanced our understanding of angiogenesis. Recent studies have investigated the specific roles of different VEGF isoforms under various conditions. These isoforms have also been found to contribute to several cardiovascular, ocular, metabolic, and reproductive disease. Despite this advancement in our knowledge, the intricate relationship between hyperglycemia, oxidative stress, inflammation, and VEGF expression remains a challenging area of research.
Cancer
Cancer, the second leading cause of death worldwide, is projected to account for approximately 618,120 fatalities and 2 million new cases in the US in 2025, according to the American Cancer Society’s latest estimates.417 Globally, the burden continues to escalate, with projections suggesting new cancer cases could surpass 35 million by 2050. This complex disease can originate in nearly any part of the body, characterized by the uncontrolled proliferation of abnormal cells that invade neighboring tissues and metastasize to distant sites through blood vessels, reflecting its developmental intricacy and diverse manifestations.
Tumor angiogenesis
Dr. Folkman proposed the existence of a tumor angiogenic factor (TAF) critical for tumor growth, suggesting that inhibiting TAF could suppress tumor progression.7 Angiogenesis, essential for solid tumor growth and metastasis, is regulated by a balance of pro- and anti-angiogenic factors.418 Tumor cells promote angiogenesis by secreting VEGFs, stabilized by HIF-1α in a hypoxic TME,84,419 while oncogenes such as RAS and RAF (Fig. 9) further affect VEGF expression.420,421,422 In turn, VEGF from tumor and stromal cells drives pathological angiogenesis by activating VEGFR1 and VEGFR2 on tumor ECs.423
VEGF/VEGFR signaling under pathological conditions. The diverse roles of VEGF ligands and their receptors drive pathological processes, including tumor angiogenesis, cardiovascular diseases, ocular diseases, and metabolic/immune-related/reproductive disorders. Key molecular interactions and downstream signaling pathways are upregulated or dysregulated in disease states, thereby promoting cell survival, migration, and proliferation. These pathways highlight the therapeutic potential of targeting VEGF/VEGFR signaling across various disease contexts. ATH atherosclerosis, MI myocardial ischemia, DR diabetic retinopathy, AMD age-related macular degeneration, FA fatty acid, NAFLD non-alcoholic fatty liver disease, RA rheumatoid arthritis, PE pre-eclampsia, EM endometriosis. Created in BioRendender.com
Recent studies show that most VEGF family members, except PlGF, correlate with angiogenesis and lymphangiogenesis in cancer.424 However, the role of PlGF remains complex, as it can either promote425,426 or inhibit tumor growth427,428 depending on the context. Similarly, VEGF-B exhibits varying effects on tumor angiogenesis and survival, with its dual functions linked to FGF-2/FGFR activity.137 Likewise, VEGF-C, activating VEGFR2 and VEGFR3, promotes blood vessel growth in breast cancer, while its overexpression enhances lymphangiogenesis and lymphatic metastasis.61,429,430,431 In addition, VEGF-A also supports lymphangiogenesis by activating VEGFR2, forming heterodimers with VEGFR3, and recruiting macrophages and mast cells to secrete VEGF-C and VEGF-D, thus promoting lymphatic vessel growth.33,432 Furthermore, inhibition of VEGFR3 signaling blocks VEGF-C-induced lymphangiogenesis and tumor cell infiltration into lymphatic vessels.433 Notably, VEGF-D overexpression can drive tumor growth and angiogenesis, enabling to anti-VEGF-A therapy and highlighting its critical role in cancer progression.434,435 These findings underscore the complex and pivotal roles of VEGF family members in tumor angiogenesis, lymphangiogeneis, and therapeutic evasion.
Importantly, a recent study presents a single-cell atlas of tumor vasculature, analyzing around 200,000 cells from various cancers.349 Specifically, tumor angiogenesis is traced from venous ECs to capillary and arterial ECs through angiogenic stages, with apelin+ tip cells identified as biomarkers for poor prognosis and anti-VEGF therapy response. Furthermore, LECs show two lineages, one driving lymphangiogenesis and another enhancing antigen presentation. Additionally, BASP1+ matrix-producing pericytes linked to endoplasmic reticulum stress were shown to play a pro-angiogenic role. Moreover, neovascular ECs also contribute to an immunosuppressive TME through stromal and immune cell interactions. In summary, these findings highlight the interplay between vascular cells and the TME, offering insights into vascular heterogeneity and novel anti-angiogenic therapeutic targets.
Cardiovascular diseases
Cardiovascular diseases (CVDs) encompass a range of disorders affecting the heart and blood vessels, rooted in vascular dysfunction, including atherosclerosis, myocardial infarction, and hypertension.
Atherosclerosis
VEGF plays a complex role in atherosclerosis, with both beneficial and detrimental effects. Systemic VEGF gene delivery does not induce atherosclerosis in hypercholesterolemic mice,436 but local administration of VEGF-A and VEGF-D promotes angiogenesis and intimal hyperplasia in rabbits.437 Moreover, VEGF can recruit macrophages and monocytes, potentially exacerbating plaque formation,438 as demonstrated by intracoronary VEGF-A164 delivery, which worsened arteriosclerotic injury in rabbits. Similarly, macrophages in human plaques express VEGF-A via HIF-1α,439 highlighting its inflammatory role. Additionally, PlGF-2 gene transfer increases VEGF-A levels, driving inflammatory angiogenesis,440 while PlGF itself promotes atherosclerosis in hypercholesterolemic models despite reducing macrophage content.441,442 Furthermore, VEGF facilitates macrophage infiltration through VEGFR1 and mobilizes endothelial and smooth muscle progenitor cells via VEGFR2, contributing to coronary arteriosclerosis.443,444 Additionally, VEGF-A accelerates vascular remodeling by enhancing T cell recruitment to vessels.445 Therefore, while VEGF supports angiogenesis and vascular repair, its role in plaque formation and progression underscores its context-dependent and multifaceted impact on atherosclerosis (Fig. 9).
Low VEGF-C levels are linked to higher mortality in patients with coronary artery disease and may enhance plaque stability, positioning VEGF-C as a promising biomarker and therapeutic target for CVD. Specifically, its protective effects on smooth muscle cells, promotion of cholesterol efflux, and reduction of cellular stress underscore its role in maintaining vascular health and mitigating the progression of atherosclerosis.446,447 Thus, further investigation is warranted to explore its clinical applications for risk stratification and innovative treatments. Similarly, VEGF-D is expressed at all stages of human atherosclerotic lesion development, implicating its involvement in the disease.448 For example, adenovirus-mediated VEGF-D gene transfer has been shown to reduce neointimal thickening and macrophage infiltration, suggesting its therapeutic potential.449 Moreover, elevated VEGF-D levels also independently predict mortality in patients with coronary artery disease undergoing elective angiography.450 Collectively, these findings emphasize multifaceted role of VEGF-D in atherosclerosis, highlighting its potential as both a therapeutic target and a prognostic marker in CVD. Together, VEGF-C and VEGF-D represent critical factors in understanding and managing cardiovascular diseases.
Myocardial ischemia (MI)
The roles of VEGFs and VEGFRs in MI are complex, significantly impacting cardiac function and recovery. Specifically, ischemia triggers VEGF release from cardiomyocytes, binding to VEGFR1 and VEGFR2 on microvascular ECs,17,451,452 promoting angiogenesis and vascular remodeling. However, recent studies show mild hypoxia in developing mouse hearts does not induce VEGF-A or coronary angiogenesis,453 suggesting other factors. Moreover, Vegfa deletion in cardiomyocytes impairs blood vessel formation and worsens heart contractions.454
Inflammation drives MI, with Annexin A1 promoting cardiac repair and angiogenesis via macrophage-derived VEGF-A.455 Similarly, VEGFR2 signaling also aids barrier function with losartan treatment reduces ischemic reperfusion injury in mice.456 Intracoronary VEGF-A gene transfer improves myocardial perfusion, offering a strategy for therapeutic angiogenesis in ischemic heart disease.457 Furthermore, VEGF-B or PlGF overexpression enhances coronary vasculature and stimulates cardiomyocyte hypertrophy, yet long-term hypertrophy effects remain unclear.56,458,459 Combining PlGF and VEGF-A enhances angiogenesis in ischemic myocardial tissue resistant to VEGF-A alone, suggesting synergistic benefits in certain clinical scenarios.162 Notably, VEGF-B promotes coronary blood vessel development without permeability or inflammation, making it an attractive therapeutic target.56,460
Post-MI, VEGF-C enhances lymphangiogenesis, reduces fluid retention and inflammation (Fig. 9), and improves cardiac function.461,462 Additionally, VEGF-C protects cardiomyocytes by activating AKT signaling through VEGFR2, thereby reducing apoptosis.463 Similarly, VEGF-D increases vascular permeability, stimulates angiogenesis, and improves blood flow, yet it also contributes cardiac fibrosis, underscoring the need to balance its therapeutic benefits and adverse effects.464,465 In summary, the VEGFs and their receptors diversely affect MI through angiogenesis, inflammation, cardiac repair, necessitating deeper understanding for targeted therapies.
Hypertension
Hypertension is the most common comorbidity associated with anti-VEGF therapy.466,467 Under hypertensive conditions, disrupted VEGF signaling impairs endothelial dysfunction and alters hemodynamics. Specifically, VEGF-A binding to VEGFR2 or shear stress activates the PI3K/AKT pathway, stimulating eNOS to produce vasodilators such as NO and prostacyclin (PGI2), highlighting the critical role of VEGF signaling in vascular tone regulation.468,469
Studies in animal models and patients treated with VEGF signaling pathway (VSP) inhibitors show reduced urinary nitrite/nitrate excretion and serum NO metabolites, impairing NO-dependent microvasodilation.470,471 Additionally, VSP inhibitors upregulate endothelin-1 (ET-1), a potent vasoconstrictor, contributing to hypertensive effects. These findings reveal the complex interplay between VEGF signaling, NO production, and blood pressure regulation.472,473 In patients with gastrointestinal stromal tumors, regorafenib-induced ET-1 fluctuations correlated with therapy cycles and blood pressure changes.474 In contrast, sorafenib did not significantly alter ET-1 levels in renal cell carcinoma patients, despite hypertension.475 Similarly, preclinical studies in rats showed that sunitinib increased ET-1 and blood pressure, effects reversed by the endothelin receptor antagonist macitentan.472 These results suggest a variable interplay between VSP inhibitors, ET-1, and blood pressure, depending on the inhibitor and cancer type. Moreover, VSP inhibition may trigger hypertension via microvascular rarefaction, reducing vessel density, or functional rarefaction, heightening vasomotor tone and limiting blood flow.476 Recent studies also link VSP inhibitors to salt-sensitive hypertension through interstitial sodium buildup and osmotic stress, especially in the skin.477,478,479 This complex interplay between VEGF signaling, endothelial function, and blood pressure highlights challenges in managing anti-VEGF therapy-induced hypertension, warranting further research for effective strategies.
Ocular diseases
Ocular diseases encompass a range of conditions that target the eye and its visual system, significantly impacting various retinal cell types and structures. Notably, the prevalence of ocular microvascular diseases, such as DR and AMD has risen considerably. These conditions pose significant threats to ocular health and visual acuity, often leading to visual impairment or blindness if not treated promptly.
Diabetic retinopathy
The global rise in diabetes has intensified microvascular complications, notably, DR, a leading cause of vision.480,481 Specifically, hyperglycemia forms AGEs, driving neovascularization, permeability, and macular edema in DR.482 As a result, retinal cells such as ganglion cells,483 astrocytes,484 ECs,484 pericytes,485 microglia,486 Müller glia,487 and retinal pigment epithelial (RPE) cells488 secrete VEGFs. Moreover, ischemia worsens inflammation and endothelial damage, upregulating VEGFs, VEGFRs, and HIF-1α to regulate neovascularization.489,490,491
VEGF-A plays a dual role in DR, both promoting vascular permeability and protecting retinal cells.492 Initially, it increases edema, and disrupts blood-retinal barrier,493,494 fueling endothelial proliferation and proliferative diabetic retinopathy (PDR), marked by abnormal retinal vessel growth.495 Yet, it also guards neurons and Müller cells against apoptosis,483,496 reflecting complex regulation.497
Hyperglycemia-induced reactive oxygen species (ROS) abnormally phosphorylate VEGFR2 independently of VEGF-A,498 with elevated VEGFR2 expression in diabetic retinal microvascular ECs, especially in the macula.499 Consequently, VEGF-A phosphorylates tight junction proteins such as occludin, increasing permeability,500,501,502 and activates pathways such as phospholipase A2,503 PKC,504 and PI3K/AKT,505 driving endothelial dysfunction and plasma extravasation. Additionally, VEGF-A level variations affect DR risk,506,507 with elevated VEGF-A, PlGF, and VEGF-B in vitreous and serum promoting angiogenesis and PDR.508,509 However, their roles in progression and treatment response remain unclear.
In contrast, VEGF-B, VEGFR1, and NRP1 provide neuroprotection via AKT and ERK pathways, reducing apoptosis,510 while low VEGF-B impairs vascular integrity by downregulating VE-cad, ZO-1, and CDC42.511 Similarly, PlGF rises in diabetic macular edema (DME) and PDR,512,513,514 disrupting RPE barrier integrity via MEK/ERK under hypoxia.515 For instance, PlGF deletion in diabetic mice reduces apoptosis and microcapillary damage by inhibiting HIF-1α-induced VEGF signaling through AKT,516 yet its overexpression increases microaneurysms, leakage, and inflammation via IL-6.517 Additionally, dysfunctional lipid metabolism upregulates VEGF-A, VEGF-C, VEGF-D, and PlGF, advancing DR.518 Notably, VEGFR2 localizes to leaky microvessels, while VEGFR3 is limited to specific PAL-E-positive vessels, indicating altered receptor expression.519 Although VEGFs’ roles in DR are clear, isoform-specific contributions and receptor crosstalk require further study to refine targeted therapies.
Age-related macular degeneration
AMD is a complex, multifactorial disease influenced by aging, environmental factors, and genetic predisposition. Its pathogenesis is associated with chronic inflammation, lipid accumulation, oxidative stress, and ECM dysfunction.520 In neovascular (wet) AMD (nAMD), immune cell recruitment to the macula leads to the release of pro-inflammatory and pro-angiogenic cytokines, including VEGF, driving pathological neovascularization.
VEGF-A is the primary factor mediating this process, making it a key therapeutic target.521 Additionally, VEGF-B overexpression disrupts the outer blood-retinal barrier and promotes inflammatory angiogenesis by upregulating cell survival factors.522 Notably, patients with wet AMD and polypoidal choroidal vasculopathy exhibit elevated VEGF-B levels, suggesting its involvement in disease progression, particularly after VEGF-A inhibition.523
Beyond VEGF-A and VEGF-B, VEGF-C and VEGF-D, traditionally recognized for their roles in lymphangiogenesis via VEGFR-3, have recently been implicated in retinal and choroidal angiogenesis.524,525 These factors can activate VEGFR-2, contributing to vascular permeability and neovascularization independently of VEGF-A. Importantly, VEGF-C and VEGF-D are upregulated following VEGF-A inhibition with agents such as aflibercept or bevacizumab, which may limit the efficacy of anti-VEGF-A therapy and lead to persistent disease activity.526 RPE cells from wet AMD patients express VEGF-C and VEGF-D, further promoting neovascularization despite the absence of lymphatic vessels.524 Inflammatory cytokines in AMD also upregulate VEGF-A and VEGF-C, with VEGFR-2 and VEGFR-3 detected in choroidal neovascularization (CNV) membranes, reinforcing the role of VEGF family members in disease progression.527,528 Given the limitations of VEGF-A inhibition alone, novel approaches targeting VEGF-C and VEGF-D have emerged. A phase 2b clinical trial of OPT-302, a VEGF-C/D inhibitor, demonstrated that adding VEGF-C/D blockade to standard anti-VEGF-A therapy (ranibizumab) significantly improved visual outcomes in nAMD patients.529 These findings suggest that dual VEGF-A and VEGF-C/D inhibition may enhance therapeutic efficacy by targeting alternative angiogenic pathways, although further studies are needed to confirm long-term benefits.
Meanwhile, VEGFR1, highly expressed by infiltrating monocytes and macrophages in AMD, modulates angiogenesis by suppressing VEGF-A-induced migration when neutralized.530 Blocking PlGF and VEGFR1 reduces monocyte recruitment after laser-induced injury, highlighting VEGFR1’s role in immune cell infiltration.531,532 Additionally, excessive VEGF-A disrupts RPE barrier function, exacerbating choroidal neovascularization.533 However, while VEGF-A/VEGFR-2 inhibition reduces vascular permeability, RPE-derived VEGF-A is essential for choriocapillaris maintenance,534,535 underscoring the need for a delicate balance in anti-VEGF therapies to suppress pathological signaling without compromising retinal function.
A comprehensive understanding of VEGF signaling in AMD, particularly the interplay between VEGF-A, VEGF-B, VEGF-C, and VEGF-D, is essential for optimizing therapeutic strategies and refining treatment approaches to improve long-term patient outcomes.
Metabolic diseases
Metabolic disorders arise from disruptions in energy utilization and storage, driven by hormonal, enzymatic, and cellular imbalances. In this context, VEGFs play a dual role in metabolic diseases, influencing vascular complications in diabetes mellitus, diabetic nephropathy, and non-alcoholic fatty liver disease with both protective and detrimental effects.
Type 2 diabetes mellitus (T2DM)
T2DM is a metabolic disorder driven by insulin resistance or β-cell dysfunction, leading to vascular alterations in insulin-sensitive tissues.536 Notably, VEGF-A, primarily produced by pancreatic α and β cells, plays a key role in islet microvasculature.537 Deleting Vegf in insulin-producing cells reduces islet vascularization and impairs insulin secretion, while VEGF-A deficiency leads to islet hypoxia and elevated blood glucose levels.537,538,539
Glucose homeostasis and VEGF-A secretion are tightly linked. Lower glucose levels reduce VEGF-A secretion, whereas prolonged hypoglycemia induces apoptosis in ECs and β cells.540 Exogenous VEGF-A mitigates hypoglycemia-induced damage, suggesting potential therapeutic applications.541 However, chronic VEGF upregulation may be detrimental, contributing to β-cell dysfunction and PKC-mediated endothelial abnormalities in diabetic vasculopathy.542,543 In the inflammatory context, VEGF-A exerts complex effects. Diabetic patients exhibit impaired monocyte responses to VEGF-A, hindering inflammatory cell infiltration in ischemic tissues and compromising wound healing.544 Conversely, VEGF-A promotes macrophage recruitment, supporting β-cell proliferation and regeneration.545,546,547 Blocking VEGF-B improves glucose tolerance (Fig. 9) and insulin sensitivity,548 while VEGF-C accelerates wound healing in diabetic conditions.549
Ultimately, VEGF signaling acts as a double-edged sword in T2DM. While essential for islet function, dysregulated VEGF expression may worsen β-cell dysfunction and vascular complications. Future research should clarify the roles of VEGF isoforms to develop targeted therapies that optimize VEGF levels, improving glycemic control and preventing diabetic complications.
Diabetic nephropathy (DN)
DN, a major renal complication of diabetes, arises from chronic hyperglycemia-induced oxidative stress, inflammation, and vascular dysfunction.550,551 In the kidney, VEGF-A, mainly produced by podocytes and detected in distal tubules and collecting ducts,552,553,554 is expressed in glomerular and tubular cells such as pericapillary ECs, mesangial cells, and interstitial fibroblasts, to maintain vascular integrity.519,555,556 Initially, in early DN, rising VEGF-A levels in renal and urinary systems increase permeability and endothelial dysfunction, driving glomerular damage,557,558 with elevated VEGF and VEGFR2 expression in diabetic models.559,560 For example, in eNOS (Nos3) knockout mice, higher VEGF-A correlates with glomerular hypervascularization, worsening DN.561 However, anti-VEGF therapy reduces albuminuria and glomerular dysfunction in diabetic mice,562,563 although excessive suppression risks endothelial injury and increased permeability.564
VEGF-B contributes to DN by inducing podocyte insulin resistance via lipid accumulation,565 with elevated levels in T2DM patients linked to renal decline.566 Notably, an anti-VEGF-B/IL-22 fusion protein mitigates oxidative stress, inflammation, and lipid deposition, offering therapeutic potential.567 Similarly, VEGF-C and VEGF-D, signaling through VEGFR3, regulate glomerular permeability despite the absence of lymphatics in glomeruli,568 as podocyte-derived VEGF-C enhances permeability via VEGFR3 and VEGFR2.569,570 In contrast, podocyte-specific VEGF-C overexpression in diabetic mice improves albuminuria and endothelial function by lowering VEGFR2,571,572 while elevated VEGF-D correlates with renal impairment, albuminuria, and proteinuria.573
Nevertheless, excessive VEGF manipulation can be detrimental. Complete Vegfa loss in podocytes causes neonatal death, and a single allele leads to proteinuria and endotheliosis,392 while its deficiency disrupts complement regulation, increasing C3 accumulation.574 Likewise, bevacizumab-induced VEGF inhibition in humans causes endothelial injury and plasma buildup,575 and Vegfr1 deletion in podocytes triggers severe proteinuria, although kinase-deficient VEGFR1 mitigates this, suggesting sVEGFR1’s protective role.166
Thus, VEGF signaling in DN balances vascular protection and pathological remodeling. The complex interplay of VEGF isoforms and receptors highlights the need for precise therapies to modulate VEGF activity, preserve renal function, and minimize harm, necessitating further research.576,577,578
Non-alcoholic fatty liver disease (NAFLD)
NAFLD is a major public health concern characterized by excessive hepatic lipid accumulation in individuals with minimal or no alcohol consumption.579 It encompasses a spectrum from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to fibrosis, cirrhosis, and HCC.580 The advancement of liver fibrosis is marked by increased vascular growth, as observed in both human and animal models.581 Recent findings suggest a potential role for VEGF in NAFLD pathogenesis and progression.582,583 Notably, VEGF and VEGFR1 mRNA levels are elevated in steatotic livers compared with NASH, indicating an early induction of angiogenesis in NAFLD.584 However, clinical studies have shown conflicting results regarding VEGF-A and VEGFR alterations in NAFLD progression.584,585
Hepatocytes play a key role in NAFLD progression by synthesizing VEGF-A, which promotes fibrosis and endothelial dysfunction, contributing to disease severity.586 In a murine model of diet-induced NASH, VEGFR2 inhibition reduced steatosis and inflammation, highlighting VEGF signaling as a potential therapeutic target.587 Beyond hepatic angiogenesis, adipose tissue metabolism affects NAFLD development. VEGF-B signaling enhances fatty acid uptake in the liver, driving hepatic steatosis in diabetic models.588 Additionally, VEGF-B has been identified in the subcutaneous white adipose tissue of NAFLD patients, emphasizing its translational relevance in human disease.589 Patients with NAFLD also exhibit elevated serum levels of angiogenic markers, including VEGF, sVEGFR1, and sVEGFR2, which correlate with hepatic fibrosis severity and may serve as biomarkers.584
Emerging evidence suggests VEGF-C as a therapeutic target for NAFLD, with flavonoid-based interventions showing potential in modulating VEGF-C activity.590 The interplay between VEGF-A, VEGF-B, and VEGF-C contributes to hepatic lipid accumulation, fibrosis, and carcinogenesis, highlighting VEGF signaling as a critical target for therapeutic strategies in NAFLD.
Immune-related diseases
Immune-related diseases are characterized by dysregulation of the immune system, resulting in inflammation and tissue damage. VEGFs are critical in these conditions by promoting angiogenesis and modulating immune responses.
Rheumatoid arthritis (RA)
RA is a chronic autoimmune disorder characterized by persistent synovial inflammation, leading to joint damage and functional impairment.591 A key driver of RA progression is angiogenesis, which facilitates inflammatory cell infiltration and pannus formation, accelerating joint destruction.592 Notably, HIF-1 and HIF-2 are highly expressed in the RA synovium, promoting VEGF production and excessive vascularization, which exacerbate inflammation and synovial hyperplasia.593
VEGF-A plays dual roles as both a pro-inflammatory mediator and an angiogenesis promoter within synovial tissue.594 Synovial macrophages and fibroblasts produce VEGF, while osteoclasts and their precursors express VEGF receptors, forming a complex network that drives disease progression.595 Elevated serum VEGF levels correlate with c-reactive protein (CRP), highlighting its diagnostic and prognostic value in RA.596,597 Furthermore, VEGF supports rheumatoid synoviocyte survival and proliferation through IL-6/JAK2/STAT3 and Notch signaling, reinforcing its role in RA pathogenesis.598
Other VEGF family members contribute to RA pathology.599 VEGF-B deficiency reduces synovial angiogenesis and inflammation, whereas VEGF-C promotes lymphangiogenesis, improving lymphatic drainage and reducing tissue damage.600 Given the central role of VEGF signaling, targeted therapies such as soluble VEGFRs, anti-VEGF antibodies, and the VEGFR2 inhibitor ramucirumab have shown promise in reducing inflammation and disease severity.601,602,603 Their efficacy is further enhanced in combination with methotrexate, presenting a potential therapeutic strategy for managing RA.
Psoriasis
Psoriasis is a chronic inflammatory skin disorder that significantly impacts the quality of life.604 A key but underappreciated factor in its pathogenesis is aberrant angiogenesis, which increases vascular permeability, capillary dilation, and elongation, contributing to epidermal hyperplasia and disease progression.605,606 Elevated VEGF-A levels in psoriatic epidermis and plasma promote EC proliferation, migration, and survival while enhancing vasodilation and permeability.607 VEGF-A also regulates keratinocyte proliferation and differentiation, with VEGFR1, VEGFR2, and NRPs expressed in keratinocytes, indicating autocrine VEGF-A signaling.608,609
Non-lesional psoriatic skin overexpresses VEGF isoforms such as VEGF-A, VEGFR3, and sNRP1.610,611 VEGFR1, VEGFR2, and VEGFR3 are upregulated in psoriatic lesions, while VEGF-C, in conjunction with nerve growth factor (NGF), has been implicated in disease development.612 Preclinical models highlight the role of VEGF in psoriasis pathogenesis (Fig. 9). Transgenic VEGF delivery induces chronic inflammation with hyperplasia and vascular abnormalities resembling psoriasis.613 Excess VEGF-A increases vascular density and permeability, exacerbating inflammation. In psoriasis mouse models, anti-VEGF therapies reduce inflammation, normalize epidermal structure, and decrease vascular density and immune infiltration.614
Targeting angiogenesis through anti-VEGF therapies holds potential for psoriasis treatment, yet further clinical studies are required to assess their efficacy and safety. A better understanding of the angiogenesis-inflammation interplay may lead to improved therapeutic strategies and better patient outcomes.
Reproductive disorders
Reproductive disorders, a broad spectrum of conditions that affect male and female reproductive health, significantly affect hormone production, gamete formation, and reproductive capacity. VEGFs play a crucial role in the development and maintenance of reproductive tissues, including follicular development, corpus luteum formation, and endometrial vascularization.
Pre-eclampsia (PE)
PE is a significant hypertensive disorder arising after the 20th week of gestation, often accompanied by complications such as proteinuria, maternal organ dysfunction, or uteroplacental dysfunction.615 A key pathological feature is the disruption of angiogenic balance due to trophoblast dysfunction and incomplete spiral artery remodeling, leading to placental hypoxia, oxidative stress, and endothelial dysfunction.616
Endovascular trophoblasts and decidual leukocytes regulate VEGF and PlGF production in normal pregnancies.617 Free VEGF helps counteract endothelial shear stress and inflammation, maintaining vascular quiescence.618 However, PE is marked by elevated plasma sVEGFR1 levels before clinical diagnosis.619 Placental hypoxia triggers VEGF secretion,205 which in turn increases sVEGFR1 expression, disrupting angiogenic balance.620 Experimental models confirm that sVEGFR1 infusion induces PE-like features, including hypertension and proteinuria.621 Similarly, adenoviral sVEGFR1 overexpression reduces VEGF and PlGF, impairing placental and fetal growth.622 Clinically, placental sVEGFR1 levels are markedly elevated in PE, antagonizing VEGF and PlGF, which leads to systemic endothelial dysfunction.622 The severity of PE correlates with sVEGFR1 elevation, emphasizing the critical VEGF-sVEGFR1 balance.623 The sVEGFR1:PlGF ratio serves as a predictive marker for PE risk.105,624 Additionally, the sVEGFR1-e15a variant predominates in PE circulation, highlighting its role in disease pathogenesis.625
Targeting VEGF and PlGF have been explored in experimental PE models. In sVEGFR1-induced PE, recombinant VEGF-A121 administration lowered blood pressure without affecting proteinuria.626 Similarly, in the reduced uterine perfusion pressure model (widely used animal model of pre-eclampsia), rhVEGF-A121 and recombinant PlGF reduced sVEGFR1 levels, improved renal function, and mitigated oxidative stress.627,628,629 Furthermore, adenoviral VEGF expression in hypertensive mouse models decreased blood pressure and proteinuria.630,631 Notably, modified VEGF-B conjugated with an elastin-like polypeptide showed therapeutic potential without inducing angiogenesis.632
Despite promising results, VEGF-based therapies raise safety concerns. Excess VEGF can cause in utero mortality due to cardiac failure, and its placental transfer remains unclear.633 The potential fetal risks highlight the need for rigorous safety evaluations before clinical application. Careful translation of VEGF-targeted therapies from preclinical models to clinical practice is essential for effective PE management.
Endometriosis
Endometriosis is a persistent gynecological disorder characterized by the ectopic growth of endometrial-like tissue, primarily in the pelvic region.634 Its pathogenesis involves endocrine, inflammatory, and pro-angiogenic factors, although their precise role—whether causal or secondary—remains debated.635 Notably, angiogenesis is a key driver of lesion progression.636
VEGF is regulated by multiple signaling pathways, with IL-1β playing a significant role.637 Normal endometrial stromal cells express VEGF at baseline, but its levels rise in response to estrogen and progesterone.637,638 Patients with endometriosis exhibit increased VEGF concentrations in peritoneal fluid and ectopic lesions, fostering a pro-angiogenic environment.639,640 VEGFR1/VEGF signaling in macrophages and fibroblasts further promotes lesion growth and lymphangiogenesis.641,642
Hypoxia plays a crucial role in endometriosis pathogenesis. HIF-1α levels are significantly elevated in ovarian endometriomas, correlating with increased VEGF mRNA expression under hypoxic conditions.643,644,645 Additionally, VEGF-C and VEGF-D contribute to lymphangiogenesis in endometriotic lesions, as observed in patients undergoing laparoscopic surgery.646 VEGF-C levels are consistently elevated, with recent findings linking COUP-TFII deficiency in ectopic stromal cells to excessive VEGF-C production. This imbalance enhances immune cell migration and lymphatic vessel formation, driving disease progression.647,648,649
Elevated VEGF-A, VEGFR2, and MMP9 levels correlate with lesion development in animal models.650 Anti-angiogenic therapies such as pazopanib and sorafenib show potential in treating endometriosis. Pazopanib reduces lesion size by at least 45%, while sorafenib more effectively modulates VEGF levels, suggesting distinct therapeutic roles.651 Given the cyclical regeneration of the endometrium, angiogenesis should remain precisely regulated. Further research is essential to refine targeted interventions while preserving normal reproductive function.
Therapeutic targeting of VEGF/VEGFR signaling
Current anti-VEGF/VEGFR therapies
The development of VEGF-targeting therapies has transformed treatment strategies for angiogenesis-driven diseases. Bevacizumab, a humanized anti-VEGF-A Ab, the first FDA-approved VEGF inhibitor (Table 2), marked a breakthrough in VEGF/VEGFR research.652 It has shown improved survival in metastatic colorectal cancer and other malignancies.652,653 Additionally, decoy receptors such as aflibercept sequester VEGF family members have further enhanced therapeutic efficacy.654,655
Recent clinical trials continue to highlight the importance of anti-VEGF therapies. For instance, the PAOLA-1 trial (NCT02477644) demonstrated that combining olaparib, a PARP (poly ADP-ribose polymerase) inhibitor targeting DNA damage repair, with bevacizumab provides a significant survival benefit in ovarian cancer patients with homologous recombination deficiency-positive tumors.656 Similarly, the BEACON CRC trial (NCT02928224) showed that the combination of encorafenib (a BRAF inhibitor specifically targeting the BRAF V600E mutation), cetuximab (an anti-EGFR antibody), and bevacizumab substantially improves overall survival in BRAF-mutated metastatic colorectal cancer.657 Likewise, the RELAY study (NCT02411448) demonstrated that ramucirumab (anti-VEGFR2 Ab) plus erlotinib (EGFR TKI) significantly improved progression-free survival in EGFR-mutated metastatic non-small cell lung cancer (NSCLC), regardless of baseline mutations or resistance-associated alterations.658 Liquid biopsy revealed worse outcomes in patients with detectable circulating EGFR mutations, but VEGFR2 inhibition still provided benefits, supporting its role in overcoming EGFR-TKI resistance. The VEGFR inhibition has also demonstrated strong clinical benefits, particularly in HCC. The REACH-2 trial (NCT02435433) validated that ramucirumab provides significant survival benefits in patients with high levels of alpha-fetoprotein, a biomarker commonly used in HCC.659 Furthermore, combining VEGF inhibition with immune checkpoint and epigenetic regulators has emerged as a promising strategy to enhance VEGF-targeted therapy, potentially offering synergistic benefits.660 The CAPability-01 trial (NCT04724239) showed that addition of bevacizumab to sintilimab (anti-PD-1 Ab) and chidamide (histone deacetylase inhibitor) significantly improved survival and response rates in microsatellite (MSS)/proficient mismatch repair (pMMR) colorectal cancer, further supporting the rationale for combinatorial treatment strategies.
In ophthalmology, novel anti-VEGF approaches are redefining treatment paradigms. Smaller molecules designed for rapid ocular penetration have revolutionized AMD management. Ranibizumab, a humanized anti-VEGF-A Ab fragment, and brolucizumab, a single-chain anti-VEGF-A Ab, continue to improve retinal disease outcomes.661,662 The NORSE-EIGHT clinical trial validated ranibizumab’s superior efficacy over bevacizumab in improving best-corrected visual acuity.663 Notably, bevacizumab, under the brand name LYTENAVA™, has received regulatory approval for wet AMD treatment in the EU and UK.664
Collectively, these advancements in anti-VEGF/VEGFR therapies reinforce their integral role across oncology and ophthalmology. As combination approaches and biomarker-driven treatments continue to emerge, optimizing dosing strategies and overcoming resistance remain key priorities in the field.
Biomarkers for VEGF/VEGFR pathway activity
Identifying biomarkers for VEGF/VEGFR activity is critical for optimizing therapy and predicting patient outcomes. Circulating VEGF levels, sVEGFRs, and Ang2 are commonly studied biomarkers. Elevated VEGF levels correlate with poor prognosis in cancers such as breast and lungs,665 whereas sVEGFR1 has been linked to pre-eclampsia and various cancers. Ang2 complements VEGF as a marker of vascular instability and disease severity.666 Additionally, epigenetic markers such as DNA methylation and circulating miRNAs provide minimally invasive tools for monitoring pathway activity.667,668
Advanced imaging techniques, including VEGF-specific PET tracers, enable real-time assessment of VEGF pathway activity, enhancing treatment precision.669 Additionally, transcriptomic and proteomic analyses have identified unique biomarker signatures, establishing a foundation for personalized therapy. Ongoing research continues to explore novel biomarkers, aiming to refine therapeutic strategies and improve patient outcomes. Furthermore, microvessel density (MVD) in tumor tissues indirectly reflects VEGF activity and correlates with poor outcomes in cancers such as breast and colorectal cancer.670,671 Circulating endothelial progenitor cells (EPCs) also serve as indicators of vascular repair and VEGF activity, with reduced EPC levels linked to impaired angiogenesis in cardiovascular diseases.672,673,674
Emerging paradigms in anti-VEGF/VEGFR therapies and innovative strategies
The multifaceted biology of VEGF presents challenges for anti-VEGF/VEGFR therapies. Several mechanisms contribute to their enhanced efficacy: (1) anti-angiogenesis, targeting immature and leaky tumor vasculature to inhibit growth.7 (2) sensitization to chemotherapy, enhancing the anti-EC effects of low-dose chemotherapy by blocking EC survival.675 (3) vessel normalization, improving blood flow and enhancing delivery of therapeutics.351 (4) direct anti-tumor effects, interfering with tumor cell survival.676 (5) off-tumor benefits, improving cancer-associated systemic syndromes.677,678 (6) immune modulation, countering tumor immune privilege by enhancing dendritic cell (DC) differentiation.679,680 (7) reduced toxicity, mitigating chemotherapy-induced adverse effects such as bone marrow suppression.681
Resistance arises from alternative angiogenic pathways (e.g., FGF2, PDGF), vessel co-option, vasculogenic mimicry, or recruitment of myeloid-derived suppressor cells (MDSCs).682,683,684 Moreover, tumor heterogeneity and hypoxic/inflammatory microenvironments select resistant clones, necessitating novel approaches. To tackle resistance, combination therapies are actively explored. One strategy pairs anti-VEGF agent with FGF or Ang2 inhibitors. Another integrates anti-VEGF therapies with immune checkpoint inhibitors (ICIs), such as anti-PD-1/PD-L1 therapies, where tumor vessel normalization enhances immune cell infiltration.351 Consequently, FDA-approved regimens include atezolizumab (anti-PD-L1 Ab) plus bevacizumab for HCC,685 pembrolizumab (anti-PD-1 Ab) plus axitinib (TKI) for renal cell carcinoma,686 and atezolizumab (anti-PD-L1 Ab) plus bevacizumab (anti-VEGF-A Ab) with chemotherapy for NSCLC.687 Yet, efforts persist to optimize patient selection and minimize toxicity.688,689
Importantly, the theoretical framework for combining anti-VEGF/VEGFR therapies with ICIs involves two key mechanisms: (1) immune modulation, where VEGF inhibition reduces immunosuppression and transforms tumors into a “hot” phenotype more responsive to ICIs,690,691 and (2) vessel normalization, which enhances immune infiltration by improving vascular perfusion.692 Yet, critical issues remain. Considering the anti-tumor effects observed in immunodeficient models, it remains unclear to what degree VEGF/VEGFR inhibition relies on immunomodulation. Additionally, in “non-angiogenic” tumors resistant to these therapies,693 can the immunomodulatory benefits of combination therapy provide an advantage? While vessel normalization improves immune infiltration, excessive vascular reduction may impair it, underscoring the need to balance normalization with maintaining adequate vascular density. Moreover, unraveling the interplay between TME dynamics and VEGF signaling could unlock new strategies to overcome these limitations (Fig. 10).
Mechanisms of action of anti-VEGF/VEGFR therapies in combination therapy. In the tumor microenvironment (TME), the mechanisms of action of anti-VEGF/VEGFR therapies combined with chemotherapy or immune checkpoint inhibitors (ICIs) include reducing angiogenicity of vessels for tumor inhibition, inducing vessel normalization for better chemo-drug or immune cell delivery, directly inhibiting tumor cell proliferation, increasing anti-endothelial cell effect of chemotherapy, and modulating immune cells for better dendritic cell (DC) differentiation and T-cell activation. Outside the TME, the anti-VEGF/VEGFR therapies may reduce chemotoxicity and improve cancer-associated systemic syndromes, thereby improving therapeutic efficacy. Created in BioRendender.com
To address these challenges, proteolysis-targeting chimeras (PROTACs) have emerged as a novel modality. Additionally, antibody-drug conjugates (ADC) targeting VEGF-VEGFR pathways represent another promising modality, combining precise targeting of angiogenic signaling with the delivery of cytotoxic agents to inhibit tumor growth and angiogenesis.694 Concurrently, artificial intelligence (AI) is revolutionizing drug discovery by identifying dual-target VEGFR inhibitors with greater precision.424 Coupled with genomic and transcriptomic profiling, AI provides critical insights into oncogenic pathways, enabling biomarker development and rational combination therapies. Despite progress, toxicity and biomarker gaps hinder adoption, necessitating refined patient selection. These advances signal a shift toward personalized, multi-modal approaches, tackling resistance and expanding therapeutic horizons.
Conclusion and perspective
The VEGF/VEGFR signaling pathway remains central to angiogenesis, lymphangiogenesis, and vascular remodeling, affecting diverse physiological and pathological processes. Over the past few decades, the development of VEGF-targeted therapies has transformed the treatment landscape for cancer, ocular diseases, and inflammatory disorders. Monoclonal antibodies, tyrosine kinase inhibitors, and VEGF-trap fusion proteins have significantly improved patient survival and quality of life. However, therapeutic resistance, systemic toxicities, and limitations in predictive biomarkers continue to present challenges.
One of the major challenges in anti-VEGF therapy is the emergence of compensatory pro-angiogenic pathways. The upregulation of FGF2, Ang, and the induction of vasculogenic mimicry - where tumor cells adopt endothelial-like characteristics - contribute to tumor escape from VEGF inhibition. Similarly, VEGF-C and VEGF-D-mediated lymphangiogenesis facilitates cancer progression and metastasis, emphasizing the need for combinatorial targeting strategies. In wet AMD and DR, VEGF-C/D upregulation has been implicated in resistance to anti-VEGF-A monotherapies. The ongoing development of VEGF-C/D inhibitors, such as OPT-302,529 represents a promising approach to overcoming this resistance.
The role of VEGFs extends beyond angiogenesis, impacting fibrosis, metabolic syndromes, and neurodegeneration. VEGF-B, traditionally considered minimally angiogenic, has emerged as a critical player in neuroprotection, with potential implications for neurodegenerative disorders such as Alzheimer’s disease.695,696 In addition, VEGF signaling contributes to endothelial dysfunction in diabetes and cardiovascular disease, highlighting its potential as a therapeutic target in metabolic disorders. Moreover, recent findings show VEGF-B inhibits angiogenesis by suppressing FGF2/FGFR1 signaling and forming VEGFR1/FGFR1 complexes that block ERK activation.137 This raises concerns about therapies including aflibercept, which targets both VEGF-A and VEGF-B, as blocking VEGF-B may disrupt its regulatory function in pathological angiogenesis.
Advancements in precision medicine have introduced new opportunities for optimizing VEGF-targeted therapies. AI-driven biomarker identification and spatial transcriptomics hold promises for improving patient stratification and treatment personalization. However, the standardization of predictive biomarkers remains a challenge due to tumor heterogeneity and the dynamic nature of VEGF signaling. For instance, VEGF-A+ fibroblasts have been proposed as a biomarker in HCC,697 yet their clinical validation across diverse patient cohorts is still lacking. Future efforts should focus on refining biomarker discovery approaches to enhance their clinical utility. Additionally, identifying non-invasive biomarkers, such as circulating exosomal VEGF or imaging-based vascular signatures, could improve real-time monitoring of treatment responses.
To address systemic toxicities associated with VEGF inhibition, emerging drug modalities offer alternative solutions. Long-acting gene therapy, nanoparticle-encapsulated siRNA, and CRISPR-based VEGF modulation represent promising strategies to mitigate hypertension, proteinuria, and impaired wound healing. Additionally, newer modalities such as bi-specific antibodies (e.g., faricimab, targeting both VEGF and Ang2) and VEGF-trap fusion proteins (e.g., conbercept in ocular diseases) provide enhanced specificity and efficacy. Recent advances in ADCs and PROTACs have further expanded the landscape of VEGF-related interventions, enabling more selective and potent therapeutic strategies. These approaches warrant further clinical exploration to determine their safety, efficacy, and long-term benefits.
Furthermore, research into the interplay between VEGF and other angiogenic factors is essential for refining combination therapies. Dual inhibition of VEGF and HIF-1α has shown promise in overcoming hypoxia-induced resistance, while VEGF and PDGFRβ co-targeting strategies aim to enhance vascular normalization and drug delivery in tumors.698,699 Additionally, integrating VEGF-targeted approaches with ICIs continues to gain momentum, particularly in cancers with immunosuppressive microenvironments. A deeper mechanistic understanding of these interactions will be critical for optimizing treatment regimens and improving patient outcomes.
Future research should also explore the impact of VEGF inhibition in aging and regenerative medicine. As the aging population grows, understanding the consequences of long-term VEGF suppression on vascular integrity, wound healing, and neurovascular health is crucial. VEGF plays a pivotal role in tissue repair and homeostasis, and prolonged inhibition may contribute to frailty, cognitive decline, and impaired organ regeneration. Balancing VEGF suppression for disease treatment while preserving its physiological functions will be essential for minimizing unintended side effects.
Additionally, continued investigation into the role of VEGFs in inflammatory and autoimmune diseases is warranted. VEGF has been implicated in conditions such as RA, psoriasis, and multiple sclerosis, where its modulation could provide therapeutic benefits. Exploring VEGF-targeting strategies in these diseases could expand the clinical applications of anti-angiogenic therapies beyond oncology and ophthalmology. Furthermore, understanding the interplay between VEGF signaling and the gut microbiome may offer novel insights into angiogenesis-related metabolic disorders, paving the way for innovative treatment approaches.
In conclusion, VEGF-targeted therapies have fundamentally reshaped disease management. However, their future success depends on overcoming resistance, enhancing treatment precision, and exploring novel applications beyond angiogenesis. Continued investigation into VEGF signaling complexity, combined with cutting-edge technological innovations, will be essential in maintaining VEGF inhibitors as a cornerstone of therapeutic intervention. As these efforts progress, VEGF-targeted therapies will continue to hold transformative potential across cancer, ocular diseases, and other fields, offering new hope to patients.
References
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Stephenson, J. A., Goddard, J. C., Al-Taan, O., Dennison, A. R. & Morgan, B. Tumour angiogenesis: a growth area—from John Hunter to Judah Folkman and beyond. J. Cancer Res. 2013, 895019 (2013).
Lenzi, P., Bocci, G. & Natale, G. John Hunter and the origin of the term “angiogenesis”. Angiogenesis 19, 255–256 (2016).
Bikfalvi, A. History and conceptual developments in vascular biology and angiogenesis research: a personal view. Angiogenesis 20, 463–478 (2017).
Folkman, J. Tumor angiogenesis: therapeutic implications. New Engl. J. Med. 285, 1182–1186 (1971).
Senger, D. R. et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983).
Senger, D. R., Connolly, D. T., Van de Water, L., Feder, J. & Dvorak, H. F. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 50, 1774–1778 (1990).
Ferrara, N. & Henzel, W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161, 851–858 (1989).
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
Keck, P. J. et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246, 1309–1312 (1989).
Grimmond, S. et al. Cloning and characterization of a novel human gene related to vascular endothelial growth factor. Genome Res. 6, 124–131 (1996).
Olofsson, B. et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc. Natl. Acad. Sci. USA 93, 2576–2581 (1996).
Joukov, V. et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 290–298 (1996).
Yamada, Y., Nezu, J., Shimane, M. & Hirata, Y. Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics 42, 483–488 (1997).
Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9, 225–230 (2006).
Jussila, L. & Alitalo, K. Vascular growth factors and lymphangiogenesis. Physiol. Rev. 82, 673–700 (2002).
Tischer, E. et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 266, 11947–11954 (1991).
White, A. L. & Bix, G. J. VEGFA isoforms as pro-angiogenic therapeutics for cerebrovascular diseases. Biomolecules 13, 702 (2023).
Kunnapuu, J., Bokharaie, H. & Jeltsch, M. Proteolytic cleavages in the VEGF family: generating diversity among angiogenic VEGFs, essential for the activation of lymphangiogenic VEGFs. Biology 10, 167 (2021).
Holmes, D. I. R. & Zachary, I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 6, 209 (2005).
Iyer, S. & Acharya, K. R. Tying the knot: the cystine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J. 278, 4304–4322 (2011).
Robinson, C. J. & Stringer, S. E. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell. Sci. 114, 853–865 (2001).
Houck, K. A. et al. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 5, 1806–1814 (1991).
Perez-Gutierrez, L., Li, P. & Ferrara, N. Endothelial cell diversity: the many facets of the crystal. FEBS J. 291, 3287–3302 (2022).
Mineur, P. et al. Newly identified biologically active and proteolysis-resistant VEGF-A isoform VEGF111 is induced by genotoxic agents. J. Cell Biol. 179, 1261–1273 (2007).
Gaspar, N. J. et al. Cysteine 116 participates in intermolecular bonding of the human VEGF(121) homodimer. Arch. Biochem. Biophys. 404, 126–135 (2002).
Poltorak, Z. et al. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J. Biol. Chem. 272, 7151–7158 (1997).
Peach, C. J. et al. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Int. J. Mol. Sci. 19, 1264 (2018).
Cao, Y. et al. Forty-year journey of angiogenesis translational research. Sci. Transl. Med. 3, 114rv113 (2011).
Moens, S., Goveia, J., Stapor, P. C., Cantelmo, A. R. & Carmeliet, P. The multifaceted activity of VEGF in angiogenesis - Implications for therapy responses. Cytokine Growth Factor Rev. 25, 473–482 (2014).
Simons, M., Gordon, E. & Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 17, 611–625 (2016).
Fairbrother, W. J., Champe, M. A., Christinger, H. W., Keyt, B. A. & Starovasnik, M. A. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure 6, 637–648 (1998).
Jeong, K. W., Jeong, M. C., Jin, B. & Kim, Y. Relationship between structural flexibility and function in the C-terminal region of the heparin-binding domain of VEGF165. Biochemistry 52, 8823–8832 (2013).
Huang, X., Gottstein, C., Brekken, R. A. & Thorpe, P. E. Expression of soluble VEGF receptor 2 and characterization of its binding by surface plasmon resonance. Biochem. Biophys. Res. Commun. 252, 643–648 (1998).
Muller, Y. A. et al. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl. Acad. Sci. USA 94, 7192–7197 (1997).
Takahashi, H. & Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 109, 227–241 (2005).
Leppänen, V. M. et al. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc. Natl. Acad. Sci. USA 107, 2425–2430 (2010).
Park, J. E., Keller, G. A. & Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 4, 1317–1326 (1993).
von Wronski, M. A., Tweedle, M. F. & Nunn, A. D. Binding of the C-terminal amino acids of VEGF121 directly with neuropilin-1 should be considered. FASEB J. 21, 1292 (2007).
Ogawa, S. et al. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem. 273, 31273–31282 (1998).
Lei, J., Jiang, A. & Pei, D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim. Biophys. Acta 1443, 400–406 (1998).
Jingjing, L., Xue, Y., Agarwal, N. & Roque, R. S. Human Müller cells express VEGF183, a novel spliced variant of vascular endothelial growth factor. Invest. Ophthalmol. Vis. Sci. 40, 752–759 (1999).
Stevens, M. & Oltean, S. Modulation of receptor tyrosine kinase activity through alternative splicing of ligands and receptors in the VEGF-A/VEGFR axis. Cells 8, 288 (2019).
Mac Gabhann, F. & Popel, A. S. Differential binding of VEGF isoforms to VEGF receptor 2 in the presence of neuropilin-1: a computational model. Am. J. Physiol. Heart Circ. Physiol. 288, H2851–H2860 (2005).
Anthony, F. W., Wheeler, T., Elcock, C. L., Pickett, M. & Thomas, E. J. Short report: identification of a specific pattern of vascular endothelial growth factor mRNA expression in human placenta and cultured placental fibroblasts. Placenta 15, 557–561 (1994).
Nash, A. D., Baca, M., Wright, C. & Scotney, P. D. The biology of vascular endothelial growth factor-B (VEGF-B). Pulm. Pharmacol. Ther. 19, 61–69 (2006).
Makinen, T. et al. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J. Biol. Chem. 274, 21217–21222 (1999).
Li, X., Aase, K., Li, H., von Euler, G. & Eriksson, U. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors 19, 49–59 (2001).
Olofsson, B. et al. Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J. Biol. Chem. 271, 19310–19317 (1996).
Olofsson, B. et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 95, 11709–11714 (1998).
Cao, Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci. Signal. 2, re1 (2009).
Iyer, S., Darley, P. I. & Acharya, K. R. Structural insights into the binding of VEGF-B by VEGFR-1D2: recognition and specificity. J. Biol. Chem. 285, 23779 (2010).
Anisimov, A. et al. The basis for the distinct biological activities of vascular endothelial growth factor receptor–1 ligands. Sci. Signal. 6, ra52 (2013).
Bry, M., Kivelä, R., Leppänen, V. M. & Alitalo, K. Vascular endothelial growth factor-B in physiology and disease. Physiol. Rev. 94, 779–794 (2014).
Chen, R., Lee, C., Lin, X., Zhao, C. & Li, X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol. Res. 143, 33–39 (2019).
Rahimi, N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp. Eye Res. 83, 1005–1016 (2006).
Gille, H. et al. A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits VEGF-dependent PI 3 kinase activation and endothelial cell migration. EMBO J. 19, 4064–4073 (2000).
Lee, J. et al. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc. Natl. Acad. Sci. USA 93, 1988–1992 (1996).
Joukov, V. et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 (1997).
Siegfried, G. et al. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Invest. 111, 1723–1732 (2003).
Rauniyar, K., Jha, S. K. & Jeltsch, M. Biology of vascular endothelial growth factor C in the morphogenesis of lymphatic vessels. Front. Bioeng. Biotechnol. 6, 7 (2018).
Jha, S. K. et al. KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D. eLife 8, e44478 (2019).
Baldwin, M. E. et al. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J. Biol. Chem. 276, 44307–44314 (2001).
Stacker, S. A. et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 274, 32127–32136 (1999).
Leppänen, V.-M. et al. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood 117, 1507–1515 (2011).
Achen, M. G. et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 95, 548–553 (1998).
McColl, B. K. et al. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J. Exp. Med. 198, 863–868 (2003).
Lohela, M., Bry, M., Tammela, T. & Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 21, 154–165 (2009).
Monaghan, R. M., Page, D. J., Ostergaard, P. & Keavney, B. D. The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases. Cardiovasc. Res. 117, 1877–1890 (2021).
Jeltsch, M. et al. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129, 1962–1971 (2014).
Achen, M. G. et al. The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer. Growth Factors 20, 99–107 (2002).
Achen, M. G. & Stacker, S. A. Vascular endothelial growth factor-D: signaling mechanisms, biology, and clinical relevance. Growth Factors 30, 283–296 (2012).
Bates, D. O. et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 62, 4123–4131 (2002).
Catena, R. et al. VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Mol. Cancer 9, 320 (2010).
Nowak, D. G. et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J. Cell. Sci. 121, 3487–3495 (2008).
Harris, S. et al. Do anti-angiogenic VEGF (VEGFxxxb) isoforms exist? A cautionary tale. PLoS ONE 7, e35231 (2012).
Lomet, D., Piégu, B., Wood, S. H. & Dardente, H. Anti-angiogenic VEGFAxxxb transcripts are not expressed in the medio-basal hypothalamus of the seasonal sheep. PLoS ONE 13, e0197123 (2018).
Bridgett, S., Dellett, M. & Simpson, D. A. RNA-sequencing data supports the existence of novel VEGFA splicing events but not of VEGFA(xxx)b isoforms. Sci. Rep. 7, 58 (2017).
Qiu, Y., Hoareau-Aveilla, C., Oltean, S., Harper, S. J. & Bates, D. O. The anti-angiogenic isoforms of VEGF in health and disease. Biochem. Soc. Trans. 37, 1207–1213 (2009).
Kikuchi, R. et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 20, 1464–1471 (2014).
Maglione, D., Guerriero, V., Viglietto, G., Delli-Bovi, P. & Persico, M. G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA 88, 9267–9271 (1991).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Hauser, S. & Weich, H. A. A heparin-binding form of placenta growth factor (PlGF-2) is expressed in human umbilical vein endothelial cells and in placenta. Growth Factors 9, 259–268 (1993).
Cao, Y., Ji, W. R., Qi, P., Rosin, A. & Cao, Y. Placenta growth factor: identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem. Biophys. Res. Commun. 235, 493–498 (1997).
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7, 575–583 (2001).
Cao, Y. et al. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J. Biol. Chem. 271, 3154–3162 (1996).
Hoffmann, D. C. et al. Proteolytic processing regulates placental growth factor activities. J. Biol. Chem. 288, 17976–17989 (2013).
Yang, W., Ahn, H., Hinrichs, M., Torry, R. J. & Torry, D. S. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J. Reprod. Immunol. 60, 53–60 (2003).
Dewerchin, M. & Carmeliet, P. Placental growth factor in cancer. Expert Opin. Ther. Targets 18, 1339–1354 (2014).
Mac Gabhann, F. & Popel, A. S. Model of competitive binding of vascular endothelial growth factor and placental growth factor to VEGF receptors on endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 286, H153–H164 (2004).
Shibuya, M. et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5, 519–524 (1990).
Kendall, R. L. & Thomas, K. A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 90, 10705–10709 (1993).
Papadopoulos, N. et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15, 171–185 (2012).
Tanaka, K., Yamaguchi, S., Sawano, A. & Shibuya, M. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase). Jpn. J. Cancer Res. 88, 867–876 (1997).
Shaik, F. et al. Structural basis for vascular endothelial growth factor receptor activation and implications for disease therapy. Biomolecules 10, 1673 (2020).
Stuttfeld, E. & Ballmer-Hofer, K. Structure and function of VEGF receptors. IUBMB Life 61, 915–922 (2009).
Markovic-Mueller, S. Structure of the full-length VEGFR-1 extracellular domain in complex with VEGFA. Structure 25, 341–352 (2017).
Olsson, A. K., Dimberg, A., Kreuger, J. & Claesson-Welsh, L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 (2006).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Chakraborty, M. P. et al. Molecular basis of VEGFR1 autoinhibition at the plasma membrane. Nat. Commun. 15, 1346 (2024).
Meyer, R. D., Mohammadi, M. & Rahimi, N. A single amino acid substitution in the activation loop defines the decoy characteristic of VEGFR-1/FLT-1. J. Biol. Chem. 281, 867–875 (2006).
Rahimi, N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front. Biosci. 11, 818–829 (2006).
Wazan, L. E., Widhibrata, A. & Liu, G. S. Soluble FLT-1 in angiogenesis: pathophysiological roles and therapeutic implications. Angiogenesis 27, 641–661 (2024).
Hornig, C. et al. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab. Invest. 80, 443–454 (2000).
Thomas, C. P., Raikwar, N. S., Kelley, E. A. & Liu, K. Z. Alternate processing of Flt1 transcripts is directed by conserved cis-elements within an intronic region of FLT1 that reciprocally regulates splicing and polyadenylation. Nucleic Acids Res. 38, 5130–5140 (2010).
Abou Faycal, C., Gazzeri, S. & Eymin, B. A VEGF-A/SOX2/SRSF2 network controls VEGFR1 pre-mRNA alternative splicing in lung carcinoma cells. Sci. Rep. 9, 336 (2019).
Heydarian, M. et al. Novel splice variants of sFlt1 are upregulated in preeclampsia. Placenta 30, 250–255 (2009).
Terman, B. I. et al. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene 6, 1677–1683 (1991).
Millauer, B. et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72, 835–846 (1993).
Matthews, W. et al. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc. Natl. Acad. Sci. USA 88, 9026–9030 (1991).
Brozzo, M. S. et al. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood 119, 1781–1788 (2012).
Wang, X., Bove, A. M., Simone, G. & Ma, B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front. Cell Dev. Biol. 8, 599281 (2020).
Shinkai, A. et al. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J. Biol. Chem. 273, 31283–31288 (1998).
Hyde, C. A. et al. Targeting extracellular domains D4 and D7 of vascular endothelial growth factor receptor 2 reveals allosteric receptor regulatory sites. Mol. Cell. Biol. 32, 3802–3813 (2012).
King, C., Wirth, D., Workman, S. & Hristova, K. Cooperative interactions between VEGFR2 extracellular Ig-like subdomains ensure VEGFR2 dimerization. Biochim. Biophys. Acta Gen. Subj. 1861, 2559–2567 (2017).
Mineev, K. S. et al. NMR-based approach to measure the free energy of transmembrane helix-helix interactions. Biochim. Biophys. Acta 1838, 164–172 (2014).
Dosch, D. D. & Ballmer-Hofer, K. Transmembrane domain-mediated orientation of receptor monomers in active VEGFR-2 dimers. FASEB J. 24, 32–38 (2010).
Parast, C. V. et al. Characterization and kinetic mechanism of catalytic domain of human vascular endothelial growth factor receptor-2 tyrosine kinase (VEGFR2 TK), a key enzyme in angiogenesis. Biochemistry 37, 16788–16801 (1998).
Koch, S., Tugues, S., Li, X., Gualandi, L. & Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 (2011).
Meyer, R. D., Dayanir, V., Majnoun, F. & Rahimi, N. The presence of a single tyrosine residue at the carboxyl domain of vascular endothelial growth factor receptor-2/FLK-1 regulates its autophosphorylation and activation of signaling molecules. J. Biol. Chem. 277, 27081–27087 (2002).
Manni, S., Kisko, K., Schleier, T., Missimer, J. & Ballmer-Hofer, K. Functional and structural characterization of the kinase insert and the carboxy terminal domain in VEGF receptor 2 activation. FASEB J. 28, 4914–4923 (2014).
Leppänen, V. M. et al. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc. Natl. Acad. Sci. USA 110, 12960–12965 (2013).
Wang, J. et al. Pathway-related molecules of VEGFC/D-VEGFR3/NRP2 axis in tumor lymphangiogenesis and lymphatic metastasis. Clin. Chim. Acta 461, 165–171 (2016).
Domigan, C. K., Ziyad, S. & Iruela-Arispe, M. L. Canonical and noncanonical vascular endothelial growth factor pathways: new developments in biology and signal transduction. Arterioscler. Thromb. Vasc. Biol. 35, 30–39 (2015).
King, C. & Hristova, K. Direct measurements of VEGF–VEGFR2 binding affinities reveal the coupling between ligand binding and receptor dimerization. J. Biol. Chem. 294, 9064–9075 (2019).
Mac Gabhann, F. & Popel, A. S. Dimerization of VEGF receptors and implications for signal transduction: a computational study. Biophys. Chem. 128, 125–139 (2007).
Sarabipour, S., Ballmer-Hofer, K. & Hristova, K. VEGFR-2 conformational switch in response to ligand binding. eLife 5, e13876 (2016).
Cudmore, M. J. et al. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat. Commun. 3, 972 (2012).
Dixelius, J. et al. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J. Biol. Chem. 278, 40973–40979 (2003).
Nilsson, I. et al. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 29, 1377–1388 (2010).
Sarkar, J. et al. VEGF receptor heterodimers and homodimers are differentially expressed in neuronal and endothelial cell types. PLoS ONE 17, e0269818 (2022).
Fuh, G., Garcia, K. C. & de Vos, A. M. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J. Biol. Chem. 275, 26690–26695 (2000).
Gluzman-Poltorak, Z., Cohen, T., Shibuya, M. & Neufeld, G. Vascular endothelial growth factor receptor-1 and neuropilin-2 form complexes. J. Biol. Chem. 276, 18688–18694 (2001).
Orecchia, A. et al. Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the alpha 5 beta 1 integrin. J. Cell. Sci. 116, 3479–3489 (2003).
Lee, C. et al. VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway. Signal Transduct. Target. Ther. 8, 305 (2023).
Zachary, I. Neuropilins: role in signalling, angiogenesis and disease. Chem. Immunol. Allergy 99, 37–70 (2014).
Koch, S. et al. NRP1 presented in trans to the endothelium arrests VEGFR2 endocytosis, preventing angiogenic signaling and tumor initiation. Dev. Cell 28, 633–646 (2014).
Soker, S., Miao, H. Q., Nomi, M., Takashima, S. & Klagsbrun, M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J. Cell. Biochem. 85, 357–368 (2002).
Gutiérrez-González, A. et al. α4β1 integrin associates with VEGFR2 in CLL cells and contributes to VEGF binding and intracellular signaling. Blood Adv. 3, 2144–2148 (2019).
Greenberg, J. I. et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456, 809–813 (2008).
Paul, M. D. & Hristova, K. Interactions between Ligand-Bound EGFR and VEGFR2. J. Mol. Biol. 433, 167006 (2021).
Tremmel, M. et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood 114, 5236–5244 (2009).
Chen, Q. et al. Possible role of EphA4 and VEGFR2 interactions in neural stem and progenitor cell differentiation. Exp. Ther. Med. 19, 1789–1796 (2020).
Lampugnani, M. G., Orsenigo, F., Gagliani, M. C., Tacchetti, C. & Dejana, E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174, 593–604 (2006).
Lu, K. V. et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21–35 (2012).
Favier, B. et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 108, 1243–1250 (2006).
Soker, S., Takashima, S., Miao, H. Q., Neufeld, G. & Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 (1998).
Nakamura, F. & Goshima, Y. Structural and functional relation of neuropilins. Adv. Exp. Med. Biol. 515, 55–69 (2002).
Prahst, C. et al. Neuropilin-1-VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J. Biol. Chem. 283, 25110–25114 (2008).
Parker, M. W. et al. Structural basis for VEGF-C binding to neuropilin-2 and sequestration by a soluble splice form. Structure 23, 677–687 (2015).
Cunningham, S. A., Stephan, C. C., Arrate, M. P., Ayer, K. G. & Brock, T. A. Identification of the extracellular domains of Flt-1 that mediate ligand interactions. Biochem. Biophys. Res. Commun. 231, 596–599 (1997).
Ahmad, S. et al. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ. Res. 99, 715–722 (2006).
Sawano, A., Takahashi, T., Yamaguchi, S. & Shibuya, M. The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCγ. Biochem. Biophys. Res. Commun. 238, 487–491 (1997).
Ito, N., Wernstedt, C., Engström, U. & Claesson-Welsh, L. Identification of vascular endothelial growth factor receptor-1 tyrosine phosphorylation sites and binding of SH2 domain-containing molecules. J. Biol. Chem. 273, 23410–23418 (1998).
Yu, Y. et al. Direct identification of a major autophosphorylation site on vascular endothelial growth factor receptor Flt-1 that mediates phosphatidylinositol 3’-kinase binding. Biochem. J. 358, 465–472 (2001).
Ito, N., Huang, K. & Claesson-Welsh, L. Signal transduction by VEGF receptor-1 wild type and mutant proteins. Cell. Signal. 13, 849–854 (2001).
Ganta, V. C., Choi, M., Kutateladze, A. & Annex, B. H. VEGF165b modulates endothelial VEGFR1-STAT3 signaling pathway and angiogenesis in human and experimental peripheral arterial disease. Circ. Res. 120, 282–295 (2017).
Scotney, P. D. et al. Human vascular endothelial growth factor B: characterization of recombinant isoforms and generation of neutralizing monoclonal antibodies. Clin. Exp. Pharmacol. Physiol. 29, 1024–1029 (2002).
Singh, N. K., Hansen, D. E. 3rd, Kundumani-Sridharan, V. & Rao, G. N. Both Kdr and Flt1 play a vital role in hypoxia-induced Src-PLD1-PKCγ-cPLA(2) activation and retinal neovascularization. Blood 121, 1911–1923 (2013).
Autiero, M. et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat. Med. 9, 936–943 (2003).
Ahmad, S. & Ahmed, A. Antiangiogenic effect of soluble vascular endothelial growth factor receptor-1 in placental angiogenesis. Endothelium 12, 89–95 (2005).
Saito, T. et al. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. FEBS Lett. 587, 2179–2185 (2013).
Abou Faycal, C. et al. The sVEGFR1-i13 splice variant regulates a β1 integrin/VEGFR autocrine loop involved in the progression and the response to anti-angiogenic therapies of squamous cell lung carcinoma. Br. J. Cancer 118, 1596–1608 (2018).
Jin, J. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151, 384–399 (2012).
da Rocha-Azevedo, B. et al. Heterogeneity in VEGF receptor-2 mobility and organization on the endothelial cell surface leads to diverse models of activation by VEGF. Cell Rep. 32, 108187 (2020).
Corsini, M., Ravelli, C., Grillo, E., Domenichini, M. & Mitola, S. Mutation in the kinase domain alters the VEGFR2 membrane dynamics. Cells 13, 1346 (2024).
Matsumoto, T. et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 24, 2342–2353 (2005).
Caron, C. et al. Non-redundant roles of the Gab1 and Gab2 scaffolding adapters in VEGF-mediated signalling, migration, and survival of endothelial cells. Cell. Signal. 21, 943–953 (2009).
Li, X. et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat. Commun. 7, 11017 (2016).
Dougher, M. & Terman, B. I. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 18, 1619–1627 (1999).
Holmqvist, K. et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J. Biol. Chem. 279, 22267–22275 (2004).
Warner, A. J., Lopez-Dee, J., Knight, E. L., Feramisco, J. R. & Prigent, S. A. The Shc-related adaptor protein, Sck, forms a complex with the vascular-endothelial-growth-factor receptor KDR in transfected cells. Biochem. J. 347, 501–509 (2000).
Sjöberg, E. et al. Endothelial VEGFR2-PLCγ signaling regulates vascular permeability and antitumor immunity through eNOS/Src. J. Clin. Invest. 133, e161366 (2023).
Lamalice, L., Houle, F., Jourdan, G. & Huot, J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 23, 434–445 (2004).
Lamalice, L., Houle, F. & Huot, J. Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J. Biol. Chem. 281, 34009–34020 (2006).
Testini, C. et al. Myc-dependent endothelial proliferation is controlled by phosphotyrosine 1212 in VEGF receptor-2. EMBO Rep. 20, e47845 (2019).
Meyer, R. D. et al. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol. Cell. Biol. 31, 2010–2025 (2011).
Rahimi, N. & Costello, C. E. Emerging roles of post-translational modifications in signal transduction and angiogenesis. Proteomics 15, 300–309 (2015).
Salameh, A., Galvagni, F., Bardelli, M., Bussolino, F. & Oliviero, S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood 106, 3423–3431 (2005).
Fournier, E., Dubreuil, P., Birnbaum, D. & Borg, J. P. Mutation at tyrosine residue 1337 abrogates ligand-dependent transforming capacity of the FLT4 receptor. Oncogene 11, 921–931 (1995).
Karkkainen, M. J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Teesalu, T., Sugahara, K. N., Kotamraju, V. R. & Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 106, 16157–16162 (2009).
Parker, M. W., Xu, P., Li, X. & Vander Kooi, C. W. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J. Biol. Chem. 287, 11082–11089 (2012).
Parker, M. W., Xu, P., Guo, H.-F. & Vander Kooi, C. W. Mechanism of selective VEGF-A binding by neuropilin-1 reveals a basis for specific ligand inhibition. PLoS ONE 7, e49177 (2012).
Klagsbrun, M., Takashima, S. & Mamluk, R. The role of neuropilin in vascular and tumor biology. Adv. Exp. Med. Biol. 515, 33–48 (2002).
Wang, L., Zeng, H., Wang, P., Soker, S. & Mukhopadhyay, D. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J. Biol. Chem. 278, 48848–48860 (2003).
Fantin, A. et al. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J. Exp. Med. 214, 1049–1064 (2017).
Kärpänen, T. et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 20, 1462–1472 (2006).
Zeng, Y., Opeskin, K., Goad, J. & Williams, E. D. Tumor-induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor-2 is critical for prostate cancer lymphatic metastasis. Cancer Res. 66, 9566–9575 (2006).
Su, J. L. et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 96, 541–545 (2007).
Papapetropoulos, A. et al. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Investig. 79, 213–223 (1999).
London, N. R., Whitehead, K. J. & Li, D. Y. Endogenous endothelial cell signaling systems maintain vascular stability. Angiogenesis 12, 149–158 (2009).
Nicolini, G., Forini, F., Kusmic, C., Iervasi, G. & Balzan, S. Angiopoietin 2 signal complexity in cardiovascular disease and cancer. Life Sci. 239, 117080 (2019).
Nguyen, Q. D. et al. The Tie2 signaling pathway in retinal vascular diseases: a novel therapeutic target in the eye. Int. J. Retin. Vitreous 6, 48 (2020).
Tsiamis, A. C., Morris, P. N., Marron, M. B. & Brindle, N. P. Vascular endothelial growth factor modulates the Tie-2:Tie-1 receptor complex. Microvasc. Res. 63, 149–158 (2002).
Winderlich, M. et al. VE-PTP controls blood vessel development by balancing Tie-2 activity. J. Cell Biol. 185, 657–671 (2009).
Frye, M. et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J. Exp. Med. 212, 2267–2287 (2015).
Hashizume, H. et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 70, 2213–2223 (2010).
Gerald, D., Chintharlapalli, S., Augustin, H. G. & Benjamin, L. E. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 73, 1649–1657 (2013).
Cao, Y., Langer, R. & Ferrara, N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 22, 476–495 (2023).
Semenza, G. L. HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 19, 59–65 (2000).
Maxwell, P. H. & Ratcliffe, P. J. Oxygen sensors and angiogenesis. Semin. Cell Dev. Biol. 13, 29–37 (2002).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1996).
Damert, A., Ikeda, E. & Risau, W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 327, 419–423 (1997).
Arany, Z. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451, 1008–1012 (2008).
Takahashi, K. et al. Endogenous oxidative stress, but not ER stress, induces hypoxia-independent VEGF120 release through PI3K-dependent pathways in 3T3-L1 adipocytes. Obesity 21, 1625–1634 (2013).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 9, 99–109 (2009).
Katsman, M., Azriel, A., Horev, G., Reizel, Y. & Levi, B. Z. N.-V. E. G. F. The autoregulatory arm of VEGF-A. Cells 11, 1289 (2022).
Silins, G., Grimmond, S., Egerton, M. & Hayward, N. Analysis of the promoter region of the human VEGF-related factor gene. Biochem. Biophys. Res. Commun. 230, 413–418 (1997).
Chilov, D. et al. Genomic organization of human and mouse genes for vascular endothelial growth factor C. J. Biol. Chem. 272, 25176–25183 (1997).
Ming, J., Zhang, Q., Qiu, X. & Wang, E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: a mechanism of lymphangiogenesis in lung cancer. Eur. J. Cancer 45, 866–873 (2009).
Chen, K., Li, W. D. & Li, X. Q. The role of m6A in angiogenesis and vascular diseases. iScience 27, 110082 (2024).
Zhang, H. et al. N6-methyladenosine promotes translation of VEGFA to accelerate angiogenesis in lung cancer. Cancer Res. 83, 2208–2225 (2023).
Ye, M. et al. WTAP activates MAPK signaling through m6A methylation in VEGFA mRNA-mediated by YTHDC1 to promote colorectal cancer development. FASEB J. 37, e23090 (2023).
Wen, J. et al. YTHDF2 is a therapeutic target for HCC by suppressing immune evasion and angiogenesis through ETV5/PD-L1/VEGFA axis. Adv. Sci. 11, e2307242 (2024).
Kato, M. & Slack, F. J. microRNAs: small molecules with big roles—C. elegans to human cancer. Biol. Cell 100, 71–81 (2008).
Fabian, M. R., Sonenberg, N. & Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 (2010).
Jalil, A. T. et al. The emerging role of microRNA-126 as a potential therapeutic target in cancer: a comprehensive review. Pathol. Res. Pract. 248, 154631 (2023).
Wang, S. et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 15, 261–271 (2008).
Zhang, Y. et al. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol. Rep. 30, 1976–1984 (2013).
Sun, C. Y. et al. miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis 34, 426–435 (2013).
Tung, Y. T. et al. Lung tumorigenesis induced by human vascular endothelial growth factor (hVEGF)-A165 overexpression in transgenic mice and amelioration of tumor formation by miR-16. Oncotarget 6, 10222–10238 (2015).
Hu, Y. et al. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis. 7, e2291 (2016).
Zhao, X. et al. MicroRNA-205 is downregulated in hepatocellular carcinoma and inhibits cell growth and metastasis via directly targeting vascular endothelial growth factor A. Oncol. Lett. 16, 2207–2214 (2018).
Du, Y. E. et al. MiR-205/YAP1 in activated fibroblasts of breast tumor promotes VEGF-independent angiogenesis through STAT3 signaling. Theranostics 7, 3972–3988 (2017).
Fontemaggi, G. Non-coding RNA regulatory networks in post-transcriptional regulation of VEGFA in cancer. IUBMB Life 75, 30–39 (2023).
Gandhi, P., Wang, Y., Li, G. & Wang, S. The role of long noncoding RNAs in ocular angiogenesis and vascular oculopathy. Cell Biosci. 14, 39 (2024).
Zhou, Y. et al. Activation of p53 by MEG3 non-coding RNA. J. Biol. Chem. 282, 24731–24742 (2007).
Zhou, Y., Zhang, X. & Klibanski, A. MEG3 noncoding RNA: a tumor suppressor. J. Mol. Endocrinol. 48, R45–R53 (2012).
Qiu, G. Z., Tian, W., Fu, H. T., Li, C. P. & Liu, B. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem. Biophys. Res. Commun. 471, 135–141 (2016).
Zhang, D. et al. LncRNA MEG3 overexpression inhibits the development of diabetic retinopathy by regulating TGF-β1 and VEGF. Exp. Ther. Med. 16, 2337–2342 (2018).
Ruan, W. et al. Knockdown of long noncoding RNA MEG3 impairs VEGF-stimulated endothelial sprouting angiogenesis via modulating VEGFR2 expression in human umbilical vein endothelial cells. Gene 649, 32–39 (2018).
Han, N., Tian, W., Yu, N. & Yu, L. YAP1 is required for the angiogenesis in retinal microvascular endothelial cells via the inhibition of MALAT1-mediated miR-200b-3p in high glucose-induced diabetic retinopathy. J. Cell. Physiol. 235, 1309–1320 (2020).
Wang, Y., Wang, X., Wang, Y. X., Ma, Y. & Di, Y. Effect and mechanism of the long noncoding RNA MALAT1 on retinal neovascularization in retinopathy of prematurity. Life Sci. 260, 118299 (2020).
Claffey, K. P., Senger, D. R. & Spiegelman, B. M. Structural requirements for dimerization, glycosylation, secretion, and biological function of VPF/VEGF. Biochim. Biophys. Acta 1246, 1–9 (1995).
Davydova, N. et al. Preparation of human vascular endothelial growth factor-D for structural and preclinical therapeutic studies. Protein Expr. Purif. 82, 232–239 (2012).
Chandler, K. B. et al. N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2). J. Biol. Chem. 294, 13117–13130 (2019).
Yeh, E. T. SUMOylation and De-SUMOylation: wrestling with life’s processes. J. Biol. Chem. 284, 8223–8227 (2009).
Flotho, A. & Melchior, F. Sumoylation: a regulatory protein modification in health and disease. Annu. Rev. Biochem. 82, 357–385 (2013).
Zhou, H. J. et al. SUMOylation of VEGFR2 regulates its intracellular trafficking and pathological angiogenesis. Nat. Commun. 9, 3303 (2018).
Xu, Y. et al. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J. Biol. Chem. 285, 36682–36688 (2010).
He, Q. et al. SENP6-mediated deSUMOylation of VEGFR2 enhances its cell membrane transport in angiogenesis. Int. J. Mol. Sci. 24, 2544 (2023).
Wang, M. & Jiang, X. SUMOylation of vascular endothelial growth factor receptor 2 inhibits the proliferation, migration, and angiogenesis signaling pathway in non-small cell lung cancer. Anticancer Drugs 31, 492–499 (2020).
Smith, G. A. et al. Ubiquitination of basal VEGFR2 regulates signal transduction and endothelial function. Biol. Open 6, 1404–1415 (2017).
Shaik, S. et al. SCF(β-TRCP) suppresses angiogenesis and thyroid cancer cell migration by promoting ubiquitination and destruction of VEGF receptor 2. J. Exp. Med. 209, 1289–1307 (2012).
Sakaue, T., Maekawa, M., Nakayama, H. & Higashiyama, S. Prospect of divergent roles for the CUL3 system in vascular endothelial cell function and angiogenesis. J. Biochem. 162, 237–245 (2017).
Sakaue, T. et al. The CUL3-SPOP-DAXX axis is a novel regulator of VEGFR2 expression in vascular endothelial cells. Sci. Rep. 7, 42845 (2017).
Critchley, W. R., Smith, G. A., Zachary, I. C., Harrison, M. A. & Ponnambalam, S. The E2 ubiquitin-conjugating enzymes UBE2D1 and UBE2D2 regulate VEGFR2 dynamics and endothelial function. J. Cell. Sci. 136, jcs260657 (2023).
Smith, G. A. et al. VEGFR2 trafficking, signaling and proteolysis is regulated by the ubiquitin isopeptidase USP8. Traffic 17, 53–65 (2016).
Corti, F. & Simons, M. Modulation of VEGF receptor 2 signaling by protein phosphatases. Pharmacol. Res. 115, 107–123 (2017).
Drexler, H. C. A. et al. Vascular endothelial receptor tyrosine phosphatase: identification of novel substrates related to junctions and a ternary complex with EPHB4 and TIE2. Mol. Cell. Proteom. 18, 2058–2077 (2019).
Fachinger, G., Deutsch, U. & Risau, W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene 18, 5948–5953 (1999).
Nawroth, R. et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 21, 4885–4895 (2002).
Hayashi, M. et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat. Commun. 4, 1672 (2013).
Mellberg, S. et al. Transcriptional profiling reveals a critical role for tyrosine phosphatase VE-PTP in regulation of VEGFR2 activity and endothelial cell morphogenesis. FASEB J. 23, 1490–1502 (2009).
Baccouche, B., Lietuvninkas, L. & Kazlauskas, A. Activin A limits VEGF-induced permeability via VE-PTP. Int. J. Mol. Sci. 24, 8698 (2023).
Nakamura, Y. et al. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ. Res. 102, 1182–1191 (2008).
Lanahan, A. A. et al. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell 18, 713–724 (2010).
Bhattacharya, R., Kwon, J., Wang, E., Mukherjee, P. & Mukhopadhyay, D. Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration. J. Mol. Signal. 3, 8 (2008).
Chu, L.-Y., Ramakrishnan, D. P. & Silverstein, R. L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 122, 1822–1832 (2013).
Chandarlapaty, S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2, 311–319 (2012).
Mason, J. M., Morrison, D. J., Basson, M. A. & Licht, J. D. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell. Biol. 16, 45–54 (2006).
Anastasi, S., Lamberti, D., Alemà, S. & Segatto, O. Regulation of the ErbB network by the MIG6 feedback loop in physiology, tumor suppression and responses to oncogene-targeted therapeutics. Semin. Cell Dev. Biol. 50, 115–124 (2016).
Watanabe, K. et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J. Clin. Invest. 114, 898–907 (2004).
Kerbel, R. S. Vasohibin: the feedback on a new inhibitor of angiogenesis. J. Clin. Invest. 114, 884–886 (2004).
Shen, J. et al. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 20, 723–725 (2006).
Nimmagadda, S. et al. Expression pattern of Vasohibin during chick development. Dev. Dyn. 236, 1358–1362 (2007).
Horie, S. et al. Distinctive role of vasohibin-1A and its splicing variant vasohibin-1B in tumor angiogenesis. Cancer Gene Ther. 23, 133–141 (2016).
Kimura, H. et al. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood 113, 4810–4818 (2009).
Takahashi, Y. et al. Vasohibin-2 expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol. Cancer Res. 10, 1135–1146 (2012).
Sato, Y. Double-face of vasohibin-1 for the maintenance of vascular homeostasis and healthy longevity. J. Atheroscler. Thromb. 25, 461–466 (2018).
Lobov, I. B. et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219–3224 (2007).
Jakobsson, L., Bentley, K. & Gerhardt, H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem. Soc. Trans. 37, 1233–1236 (2009).
Hellström, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Siekmann, A. F. & Lawson, N. D. Notch signalling and the regulation of angiogenesis. Cell Adh. Migr. 1, 104–105 (2007).
Hellström, M., Phng, L.-K. & Gerhardt, H. VEGF and notch signaling. Cell Adh. Migr. 1, 133–136 (2007).
Gerhardt, H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4, 241–246 (2008).
Shin, M. et al. Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development 143, 3796–3805 (2016).
Eilken, H. M. & Adams, R. H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 22, 617–625 (2010).
Blanco, R. & Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 3, a006569 (2013).
Benedito, R. et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135 (2009).
Benedito, R. et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 484, 110–114 (2012).
Monahan-Earley, R., Dvorak, A. M. & Aird, W. C. Evolutionary origins of the blood vascular system and endothelium. J. Thromb. Haemost. 11(Suppl 1), 46–66 (2013).
Hartenstein, V. & Mandal, L. The blood/vascular system in a phylogenetic perspective. Bioessays 28, 1203–1210 (2006).
Farmer, C. G. Evolution of the vertebrate cardio-pulmonary system. Annu. Rev. Physiol. 61, 573–592 (1999).
Patan, S. Vasculogenesis and angiogenesis. Cancer Treat. Res. 117, 3–32 (2004).
Littman, R. J. The heart and the vascular system in ancient-Greek medicine— from Alcmaeon to Galen - Harris,Crs. Classical World 69, 143–144 (1975).
Schoefl, G. I. Studies on inflammation. Iii. Growing capillaries: their structure and permeability. Virchows Arch. Pathol. Anat. Physiol. Klin. Med 337, 97–141 (1963).
Cliff, W. J. Observations on healing tissue—a combined light and electron microscopic investigation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 246, 305 (1963).
Folkman, J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 46, 467–473 (1986).
Herbert, S. P. & Stainier, D. Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 551–564 (2011).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Tolentino, M. J. et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch. Ophthalmol. 114, 964–970 (1996).
Abdelrahim, M., Smith, R. III, Burghardt, R. & Safe, S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 64, 6740–6749 (2004).
Hallmann, R. et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 85, 979–1000 (2005).
Mecham, R. P. & Ramirez, F. Extracellular determinants of arterial morphogenesis, growth, and homeostasis. Curr. Top. Dev. Biol. 130, 193–216 (2018).
Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Lal-Nag, M. & Morin, P. J. The claudins. Genome Biol. 10, 235 (2009).
Mandell, K. J. & Parkos, C. A. The JAM family of proteins. Adv. Drug Deliv. Rev. 57, 857–867 (2005).
van Hinsbergh, V. W., Engelse, M. A. & Quax, P. H. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 26, 716–728 (2006).
Houck, K. A., Leung, D. W., Rowland, A. M., Winer, J. & Ferrara, N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 267, 26031–26037 (1992).
Lee, S., Jilani, S. M., Nikolova, G. V., Carpizo, D. & Iruela-Arispe, M. L. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 169, 681–691 (2005).
Sounni, N. E. et al. Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J. Biol. Chem. 279, 13564–13574 (2004).
Sounni, N. E. et al. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 16, 555–564 (2002).
Mongiat, M., Sweeney, S. M., San Antonio, J. D., Fu, J. & Iozzo, R. V. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 278, 4238–4249 (2003).
O’Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Good, D. J. et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA 87, 6624–6628 (1990).
Maeshima, Y. et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 295, 140–143 (2002).
Nottebaum, A. F. et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J. Exp. Med. 205, 2929–2945 (2008).
Gavard, J. & Gutkind, J. S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8, 1223–1234 (2006).
Taddei, A. et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 10, 923–934 (2008).
Coon, B. G. et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 208, 975–986 (2015).
Bordeleau, F. et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA 114, 492–497 (2017).
Zanotelli, M. R. & Reinhart-King, C. A. Mechanical forces in tumor angiogenesis. Adv. Exp. Med. Biol. 1092, 91–112 (2018).
Zelzer, E. & Shilo, B. Z. Cell fate choices in Drosophila tracheal morphogenesis. Bioessays 22, 219–226 (2000).
Bar, T. & Wolff, J. R. The formation of capillary basement membranes during internal vascularization of the rat’s cerebral cortex. Z. Zellforsch. Mikros Anat. 133, 231–248 (1972).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Tammela, T. et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 (2008).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Li, X., Sun, X. & Carmeliet, P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 30, 414–433 (2019).
Krueger, J. et al. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138, 2111–2120 (2011).
Tammela, T. et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 13, 1202–1213 (2011).
Strasser, G. A., Kaminker, J. S. & Tessier-Lavigne, M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115, 5102–5110 (2010).
Jakobsson, L. et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943–953 (2010).
Zeng, G. et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood 109, 1345–1352 (2007).
Strilic, B. et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 17, 505–515 (2009).
Lenard, A. et al. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev. Cell 25, 492–506 (2013).
Phng, L. K., Stanchi, F. & Gerhardt, H. Filopodia are dispensable for endothelial tip cell guidance. Development 140, 4031–4040 (2013).
Nesmith, J. E., Chappell, J. C., Cluceru, J. G. & Bautch, V. L. Blood vessel anastomosis is spatially regulated by Flt1 during angiogenesis. Development 144, 889–896 (2017).
Fantin, A. et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840 (2010).
Stefater, J. A. et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474, 511–515 (2011).
Furtado, J. & Eichmann, A. Vascular development, remodeling and maturation. Curr. Top. Dev. Biol. 159, 344–370 (2024).
Claxton, S. & Fruttiger, M. Oxygen modifies artery differentiation and network morphogenesis in the retinal vasculature. Dev. Dyn. 233, 822–828 (2005).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014).
Davis, G. E. & Kemp, S. S. Extracellular matrix regulation of vascular morphogenesis, maturation, and stabilization. Cold Spring Harb. Perspect. Med. 13, a041156 (2023).
Jain, R. K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Li, G., Gao, J., Ding, P. & Gao, Y. The role of endothelial cell-pericyte interactions in vascularization and diseases. J. Adv. Res. 67, 269–288 (2024).
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 (2009).
Saharinen, P., Eklund, L. & Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 16, 635–661 (2017).
Cao, R. et al. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 94, 664–670 (2004).
Miller, D. L., Ortega, S., Bashayan, O., Basch, R. & Basilico, C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol. Cell. Biol. 20, 2260–2268 (2000).
Thurston, G. et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286, 2511–2514 (1999).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996).
Gale, N. W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 3, 411–423 (2002).
Lobov, I. B., Brooks, P. C. & Lang, R. A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 99, 11205–11210 (2002).
Kano, M. R. et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J. Cell. Sci. 118, 3759–3768 (2005).
Pan, X. et al. Tumour vasculature at single-cell resolution. Nature 632, 429–436 (2024).
Risau, W. & Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 11, 73–91 (1995).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Caprioli, A. et al. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev. Biol. 238, 64–78 (2001).
De Val, S. & Black, B. L. Transcriptional control of endothelial cell development. Dev. Cell 16, 180–195 (2009).
Xu, K. & Cleaver, O. Tubulogenesis during blood vessel formation. Semin. Cell Dev. Biol. 22, 993–1004 (2011).
Lawson, N. D. & Weinstein, B. M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 (2002).
Patel-Hett, S. & D’Amore, P. A. Signal transduction in vasculogenesis and developmental angiogenesis. Int. J. Dev. Biol. 55, 353–363 (2011).
Patan, S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 50, 1–15 (2000).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996).
Ferrara, N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 (1996).
Shalaby, F. et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 (1995).
Dumont, D. J. et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282, 946–949 (1998).
Fong, G. H., Rossant, J., Gertsenstein, M. & Breitman, M. L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376, 66–70 (1995).
Djonov, V. G., Kurz, H. & Burri, P. H. Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev. Dyn. 224, 391–402 (2002).
Peebo, B. B., Fagerholm, P., Traneus-Rockert, C. & Lagali, N. Cellular level characterization of capillary regression in inflammatory angiogenesis using an in vivo corneal model. Angiogenesis 14, 393–405 (2011).
Makanya, A. N., Stauffer, D., Ribatti, D., Burri, P. H. & Djonov, V. Microvascular growth, development, and remodeling in the embryonic avian kidney: the interplay between sprouting and intussusceptive angiogenic mechanisms. Microsc. Res. Tech. 66, 275–288 (2005).
Dill, M. T. et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology 142, 967–977 e962 (2012).
Herbert, S. P. et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326, 294–298 (2009).
Ribatti, D. Historical overview of lymphangiogenesis. Curr. Opin. Immunol. 53, 161–166 (2018).
Sabin, F. R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–389 (1902).
Srinivasan, R. S. et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422–2432 (2007).
Zheng, W., Aspelund, A. & Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Invest. 124, 878–887 (2014).
Vaahtomeri, K., Karaman, S., Makinen, T. & Alitalo, K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev. 31, 1615–1634 (2017).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Orlandini, M., Marconcini, L., Ferruzzi, R. & Oliviero, S. Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc. Natl. Acad. Sci. USA 93, 11675–11680 (1996).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Baldwin, M. E. et al. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol. Cell. Biol. 25, 2441–2449 (2005).
Zhang, L. et al. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 20, 1319–1331 (2010).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Hong, Y. K. et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 18, 1111–1113 (2004).
Cursiefen, C. et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050 (2004).
Murakami, M. et al. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler. Thromb. Vasc. Biol. 28, 658–664 (2008).
Tammela, T. et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 105, 4642–4648 (2005).
Kubo, H. et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. USA 99, 8868–8873 (2002).
Cao, R. et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood 107, 3531–3536 (2006).
Cao, R. et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6, 333–345 (2004).
Bjorndahl, M. et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc. Natl. Acad. Sci. USA 102, 15593–15598 (2005).
Sainz-Jaspeado, M. & Claesson-Welsh, L. Cytokines regulating lymphangiogenesis. Curr. Opin. Immunol. 53, 58–63 (2018).
Detry, B. et al. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood 119, 5048–5056 (2012).
Zheng, W. et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118, 1154–1162 (2011).
Schoppmann, S. F. et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161, 947–956 (2002).
Bellomo, D. et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ. Res. 86, E29–E35 (2000).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Gerber, H. P. et al. VEGF is required for growth and survival in neonatal mice. Development 126, 1149–1159 (1999).
Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 373, eabc8479 (2021).
Grotte, G. Passage of dextran molecules across the blood-lymph barrier. Acta Chir. Scand. Suppl. 211, 1–84 (1956).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Claesson-Welsh, L., Dejana, E. & McDonald, D. M. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol. Med. 27, 314–331 (2021).
Nagy, J. A., Benjamin, L., Zeng, H., Dvorak, A. M. & Dvorak, H. F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11, 109–119 (2008).
Sun, Z. et al. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J. Exp. Med. 209, 1363–1377 (2012).
Takahashi, T., Yamaguchi, S., Chida, K. & Shibuya, M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20, 2768–2778 (2001).
Sakurai, Y., Ohgimoto, K., Kataoka, Y., Yoshida, N. & Shibuya, M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 102, 1076–1081 (2005).
Ackah, E. et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J. Clin. Invest. 115, 2119–2127 (2005).
Bauer, P. M. et al. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc. Natl. Acad. Sci. USA 102, 204–209 (2005).
Labrecque, L. et al. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol. Biol. Cell 14, 334–347 (2003).
Matsunaga, Y., Yamazaki, Y., Suzuki, H. & Morita, T. VEGF-A and VEGF-F evoke distinct changes in vascular ultrastructure. Biochem. Biophys. Res. Commun. 379, 872–875 (2009).
Kohn, S., Nagy, J. A., Dvorak, H. F. & Dvorak, A. M. Pathways of macromolecular tracer transport across venules and small veins. Structural basis for the hyperpermeability of tumor blood vessels. Lab. Investig. 67, 596–607 (1992).
Yang, J. et al. ADAM10 and ADAM17 proteases mediate proinflammatory cytokine-induced and constitutive cleavage of endomucin from the endothelial surface. J. Biol. Chem. 295, 6641–6651 (2020).
LeBlanc, M. E. et al. Glycocalyx regulation of vascular endothelial growth factor receptor 2 activity. FASEB J. 33, 9362–9373 (2019).
LeCouter, J. et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412, 877–884 (2001).
Colin, I. M., Denef, J. F., Lengele, B., Many, M. C. & Gerard, A. C. Recent insights into the cell biology of thyroid angiofollicular units. Endocr. Rev. 34, 209–238 (2013).
Kamba, T. et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560–H576 (2006).
Jang, J. Y. et al. VEGFR2 but not VEGFR3 governs integrity and remodeling of thyroid angiofollicular unit in normal state and during goitrogenesis. EMBO Mol. Med. 9, 750–769 (2017).
Yang, Y. et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc. Natl. Acad. Sci. USA 110, 12018–12023 (2013).
Zhang, Y. et al. Endocrine vasculatures are preferable targets of an antitumor ineffective low dose of anti-VEGF therapy. Proc. Natl. Acad. Sci. USA 113, 4158–4163 (2016).
van der Veldt, A. A., Lammertsma, A. A. & Smit, E. F. Reduction in thyroid perfusion after bevacizumab treatment. Thyroid 23, 1329–1330 (2013).
Lee, S. et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 (2007).
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H. & Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 75, 10–45 (2025).
Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3), 4–10 (2005).
Krock, B. L., Skuli, N. & Simon, M. C. Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2, 1117–1133 (2011).
Rak, J. et al. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 14, 263–277 (1995).
Rak, J. et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 55, 4575–4580 (1995).
Khromova, N. V., Kopnin, P. B., Stepanova, E. V., Agapova, L. S. & Kopnin, B. P. p53 hot-spot mutants increase tumor vascularization via ROS-mediated activation of the HIF1/VEGF-A pathway. Cancer Lett. 276, 143–151 (2009).
Nagy, J. A., Dvorak, A. M. & Dvorak, H. F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2, 251–275 (2007).
Nayarisseri, A. et al. Potential inhibitors of VEGFR1, VEGFR2, and VEGFR3 developed through deep learning for the treatment of cervical cancer. Sci. Rep. 14, 13251 (2024).
Fischer, C. et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131, 463–475 (2007).
Marcellini, M. et al. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am. J. Pathol. 169, 643–654 (2006).
Schomber, T. et al. Placental growth factor-1 attenuates vascular endothelial growth factor-A-dependent tumor angiogenesis during beta cell carcinogenesis. Cancer Res. 67, 10840–10848 (2007).
Bais, C. et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell 141, 166–177 (2010).
Valtola, R. et al. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am. J. Pathol. 154, 1381–1390 (1999).
Karpanen, T. et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786–1790 (2001).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7, 192–198 (2001).
Tammela, T. & Alitalo, K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140, 460–476 (2010).
He, Y. et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 65, 4739–4746 (2005).
Bokhari, S. M. Z. & Hamar, P. Vascular endothelial growth factor-D (VEGF-D): an angiogenesis bypass in malignant tumors. Int. J. Mol. Sci. 24, 13317 (2023).
Yang, Z. et al. Changes in serum growth factors during resistance to atezolizumab plus bevacizumab treatment in patients with unresectable hepatocellular carcinoma. Cancers 15, 593 (2023).
Holm, P. W., Slart, R. H., Zeebregts, C. J., Hillebrands, J. L. & Tio, R. A. Atherosclerotic plaque development and instability: a dual role for VEGF. Ann. Med. 41, 257–264 (2009).
Bhardwaj, S., Roy, H., Heikura, T. & Yla-Herttuala, S. VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur. J. Clin. Invest. 35, 669–676 (2005).
Celletti, F. L. et al. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat. Med. 7, 425–429 (2001).
Guo, L. et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Invest. 128, 1106–1124 (2018).
Roy, H. et al. Adenovirus-mediated gene transfer of placental growth factor to perivascular tissue induces angiogenesis via upregulation of the expression of endogenous vascular endothelial growth factor-A. Hum. Gene Ther. 16, 1422–1428 (2005).
Dewerchin, M. & Carmeliet, P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb. Perspect. Med. 2, a011056 (2012).
Khurana, R. et al. Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation 111, 2828–2836 (2005).
Barleon, B. et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87, 3336–3343 (1996).
Yamashita, J. et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92–96 (2000).
Zhang, J. et al. VEGF blockade inhibits lymphocyte recruitment and ameliorates immune-mediated vascular remodeling. Circ. Res. 107, 408–417 (2010).
Wada, H. et al. VEGF-C and mortality in patients with suspected or known coronary artery disease. J. Am. Heart Assoc. 7, e010355 (2018).
Silvestre-Roig, C. et al. Arterial delivery of VEGF-C stabilizes atherosclerotic lesions. Circ. Res. 128, 284–286 (2021).
Rutanen, J. et al. Vascular endothelial growth factor-D expression in human atherosclerotic lesions. Cardiovasc. Res. 59, 971–979 (2003).
Rutanen, J. et al. Gene transfer using the mature form of VEGF-D reduces neointimal thickening through nitric oxide-dependent mechanism. Gene Ther. 12, 980–987 (2005).
Wada, H. et al. Distinct characteristics of VEGF-D and VEGF-C to predict mortality in patients with suspected or known coronary artery disease. J. Am. Heart Assoc. 9, e015761 (2020).
Kuwabara, M. et al. Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. J. Physiol. Sci. 56, 95–101 (2006).
Braile, M. et al. VEGF-A in cardiomyocytes and heart diseases. Int. J. Mol. Sci. 21, 5294 (2020).
Cai, L. X., Alkassis, F. F. & Kasahara, H. Defective coronary vessel organization and reduction of VEGF-A in mouse embryonic hearts with gestational mild hypoxia. Dev. Dyn. 249, 636–645 (2020).
Giordano, F. J. et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc. Natl. Acad. Sci. USA 98, 5780–5785 (2001).
Ferraro, B. et al. Pro-angiogenic macrophage phenotype to promote myocardial repair. J. Am. Coll. Cardiol. 73, 2990–3002 (2019).
Li, Y. et al. Losartan protects against myocardial ischemia and reperfusion injury via vascular integrity preservation. FASEB J. 33, 8555–8564 (2019).
Hedman, M. et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 107, 2677–2683 (2003).
Jaba, I. M. et al. NO triggers RGS4 degradation to coordinate angiogenesis and cardiomyocyte growth. J. Clin. Invest. 123, 1718–1731 (2013).
Bry, M. et al. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation 122, 1725–1733 (2010).
Kivelä, R. et al. VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Mol. Med. 6, 307–321 (2014).
Klotz, L. et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522, 62–67 (2015).
Henri, O. et al. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation 133, 1484–1497 (2016).
Chen, X. G. et al. Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol. Cell. Biochem. 413, 9–23 (2016).
Rutanen, J. et al. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation 109, 1029–1035 (2004).
Zhao, T. et al. Vascular endothelial growth factor-D mediates fibrogenic response in myofibroblasts. Mol. Cell. Biochem. 413, 127–135 (2016).
Piccirillo, J. F., Tierney, R. M., Costas, I., Grove, L. & Spitznagel, E. L. Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 291, 2441–2447 (2004).
Meadows, K. L. & Hurwitz, H. I. Anti-VEGF therapies in the clinic. Cold Spring Harb. Perspect. Med. 2, a006577 (2012).
Facemire, C. S., Nixon, A. B., Griffiths, R., Hurwitz, H. & Coffman, T. M. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54, 652–658 (2009).
Hood, J. D., Meininger, C. J., Ziche, M. & Granger, H. J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol. 274, H1054–H1058 (1998).
Robinson, E. S. et al. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors. Hypertension 56, 1131–1136 (2010).
Mayer, E. L. et al. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension 58, 85–92 (2011).
Kappers, M. H. et al. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 58, 295–302 (2011).
Kappers, M. H. et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 56, 675–681 (2010).
de Jesus-Gonzalez, N. et al. Regorafenib induces rapid and reversible changes in plasma nitric oxide and endothelin-1. Am. J. Hypertens. 25, 1118–1123 (2012).
Veronese, M. L. et al. Mechanisms of hypertension associated with BAY 43-9006. J. Clin. Oncol. 24, 1363–1369 (2006).
Levy, B. I., Ambrosio, G., Pries, A. R. & Struijker-Boudier, H. A. Microcirculation in hypertension: a new target for treatment?. Circulation 104, 735–740 (2001).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Wiig, H. et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Invest. 123, 2803–2815 (2013).
Lankhorst, S. et al. Salt sensitivity of angiogenesis inhibition-induced blood pressure rise: role of interstitial sodium accumulation?. Hypertension 69, 919–926 (2017).
Wong, T. Y., Cheung, C. M., Larsen, M., Sharma, S. & Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Prim. 2, 16012 (2016).
Kaur, A., Kumar, R. & Sharma, A. Diabetic retinopathy leading to blindness—a review. Curr. Diab. Rev. 20, e240124225997 (2024).
Jhaveri, C. D. et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. New Engl. J. Med. 387, 692–703 (2022).
Froger, N. et al. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci. Rep. 10, 12409 (2020).
Stone, J. et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 15, 4738–4747 (1995).
Darland, D. C. et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 264, 275–288 (2003).
Ding, X. et al. Microglia enhanced the angiogenesis, migration and proliferation of co-cultured RMECs. BMC Ophthalmol. 18, 249 (2018).
Bai, Y. et al. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 219, 446–454 (2009).
Saint-Geniez, M., Maldonado, A. E. & D’Amore, P. A. VEGF expression and receptor activation in the choroid during development and in the adult. Invest. Ophthalmol. Vis. Sci. 47, 3135–3142 (2006).
Kim, I. et al. Constitutive expression of VEGF, VEGFR-1, and VEGFR-2 in normal eyes. Invest. Ophthalmol. Vis. Sci. 40, 2115–2121 (1999).
Mammadzada, P., Corredoira, P. M. & Andre, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: a gene therapy perspective. Cell Mol. Life Sci. 77, 819–833 (2020).
Kim, Y. et al. Methylation-dependent regulation of HIF-1alpha stability restricts retinal and tumour angiogenesis. Nat. Commun. 7, 10347 (2016).
Qaum, T. et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest. Ophthalmol. Vis. Sci. 42, 2408–2413 (2001).
Aiello, L. P. et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New Engl. J. Med. 331, 1480–1487 (1994).
Ahuja, S. et al. Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int. J. Retin. Vitreous 5, 29 (2019).
Adamis, A. P. et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 118, 445–450 (1994).
Jin, K. L., Mao, X. O. & Greenberg, D. A. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. USA 97, 10242–10247 (2000).
Kida, T., Oku, H., Osuka, S., Horie, T. & Ikeda, T. Hyperglycemia-induced VEGF and ROS production in retinal cells is inhibited by the mTOR inhibitor, rapamycin. Sci. Rep. 11, 1885 (2021).
Warren, C. M., Ziyad, S., Briot, A., Der, A. & Iruela-Arispe, M. L. A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci. Signal. 7, ra1 (2014).
Sun, D. et al. Molecular imaging reveals elevated VEGFR-2 expression in retinal capillaries in diabetes: a novel biomarker for early diagnosis. FASEB J. 28, 3942–3951 (2014).
Zhao, B., Smith, G., Cai, J., Ma, A. & Boulton, M. Vascular endothelial growth factor C promotes survival of retinal vascular endothelial cells via vascular endothelial growth factor receptor-2. Br. J. Ophthalmol. 91, 538–545 (2007).
Harhaj, N. S. et al. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest. Ophthalmol. Vis. Sci. 47, 5106–5115 (2006).
Murakami, T., Felinski, E. A. & Antonetti, D. A. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem. 284, 21036–21046 (2009).
Lupo, G. et al. Role of phospholipases A2 in diabetic retinopathy: in vitro and in vivo studies. Biochem. Pharmacol. 86, 1603–1613 (2013).
Murakami, T., Frey, T., Lin, C. & Antonetti, D. A. Protein kinase cbeta phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes 61, 1573–1583 (2012).
Lu, J. M., Zhang, Z. Z., Ma, X., Fang, S. F. & Qin, X. H. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp. Eye Res. 190, 107886 (2020).
Abhary, S. et al. Common sequence variation in the VEGFA gene predicts risk of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 50, 5552–5558 (2009).
Tetikoglu, M., Yuksel, Z., Aktas, S., Sagdik, H. M. & Ozcura, F. VEGF-A gene polymorphisms and responses to intravitreal ranibizumab treatment in patients with diabetic macular edema. Int. Ophthalmol. 38, 2381–2388 (2018).
Wu, F. et al. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 61, 26 (2020).
Mesquita, J. et al. Evaluation of the growth factors VEGF-a and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors 36, 48–57 (2018).
Huang, D. et al. VEGF-B inhibits hyperglycemia- and Macugen-induced retinal apoptosis. Sci. Rep. 6, 26059 (2016).
Xu, Y. et al. VEGF-B prevents chronic hyperglycemia-induced retinal vascular leakage by regulating the CDC42-ZO1/VE-cadherin pathway. FASEB J. 38, e70019 (2024).
Mitamura, Y. et al. Vitreous levels of placenta growth factor and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diab. Care 25, 2352 (2002).
Nguyen, Q. D. et al. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol. 96, e1–e9 (2018).
Van Bergen, T. et al. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog. Retin. Eye Res. 69, 116–136 (2019).
Miyamoto, N. et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia 50, 461–470 (2007).
Huang, H. et al. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha-VEGF pathway inhibition. Diabetes 64, 200–212 (2015).
Kowalczuk, L. et al. Placental growth factor contributes to micro-vascular abnormalization and blood-retinal barrier breakdown in diabetic retinopathy. PLoS ONE 6, e17462 (2011).
Zhang, X. et al. Dysregulated serum lipid metabolism promotes the occurrence and development of diabetic retinopathy associated with upregulated circulating levels of VEGF-A, VEGF-D, and PlGF. Front. Med. 8, 779413 (2021).
Witmer, A. N. et al. Altered expression patterns of VEGF receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Invest. Ophthalmol. Vis. Sci. 43, 849–857 (2002).
Fleckenstein, M. et al. Age-related macular degeneration. Nat. Rev. Dis. Prim. 7, 31 (2021).
Aiello, L. P. Targeting intraocular neovascularization and edema–one drop at a time. New Engl. J. Med. 359, 967–969 (2008).
Zhang, F. et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 106, 6152–6157 (2009).
Zhou, H., Zhao, X., Wang, S. & Chen, Y. Determination of vascular endothelial growth factor-B concentrations in aqueous humor and plasma of neovascular age-related macular degeneration and polypoidal choroidal vasculopathy patients before and after anti-VEGF therapy. Ophthalmol. Ther. 12, 827–837 (2023).
Ikeda, Y. et al. The regulation of vascular endothelial growth factors (VEGF-A, -C, and -D) expression in the retinal pigment epithelium. Exp. Eye Res. 83, 1031–1040 (2006).
Leitch, I. M. et al. Vascular endothelial growth factor C and D signaling pathways as potential targets for the treatment of neovascular age-related macular degeneration: a narrative review. Ophthalmol. Ther. 13, 1857–1875 (2024).
Cabral, T. et al. Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmol. Retin. 2, 31–37 (2018).
Nagineni, C. N., Kommineni, V. K., William, A., Detrick, B. & Hooks, J. J. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J. Cell. Physiol. 227, 116–126 (2012).
Zhou, H., Zhao, X., Yuan, M. & Chen, Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmol. 20, 15 (2020).
Jackson, T. L. et al. A randomized controlled trial of OPT-302, a VEGF-C/D inhibitor for neovascular age-related macular degeneration. Ophthalmology 130, 588–597 (2023).
Sawano, A. et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97, 785–791 (2001).
Crespo-Garcia, S. et al. Inhibition of placenta growth factor reduces subretinal mononuclear phagocyte accumulation in choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 58, 4997–5006 (2017).
Huang, H., Parlier, R., Shen, J. K., Lutty, G. A. & Vinores, S. A. VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser-induced CNV. PLoS ONE 8, e71808 (2013).
Marneros, A. G. NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 4, 945–958 (2013).
Saint-Geniez, M., Kurihara, T., Sekiyama, E., Maldonado, A. E. & D’Amore, P. A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 106, 18751–18756 (2009).
Kurihara, T., Westenskow, P. D., Bravo, S., Aguilar, E. & Friedlander, M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Invest. 122, 4213–4217 (2012).
Howangyin, K. Y. & Silvestre, J. S. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler. Thromb. Vasc. Biol. 34, 1126–1135 (2014).
Brissova, M. et al. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes 55, 2974–2985 (2006).
Watada, H. Role of VEGF-A in pancreatic beta cells. Endocr. J. 57, 185–191 (2010).
Iwashita, N. et al. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia 50, 380–389 (2007).
Jansson, L., Andersson, A., Bodin, B. & Kallskog, O. Pancreatic islet blood flow during euglycaemic, hyperinsulinaemic clamp in anaesthetized rats. Acta Physiol. 189, 319–324 (2007).
Xiao, X. et al. Hypoglycemia reduces vascular endothelial growth factor A production by pancreatic beta cells as a regulator of beta cell mass. J. Biol. Chem. 288, 8636–8646 (2013).
Agudo, J. et al. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of beta-cell mass. Diabetes 61, 2851–2861 (2012).
Kizub, I. V., Klymenko, K. I. & Soloviev, A. I. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int. J. Cardiol. 174, 230–242 (2014).
Waltenberger, J., Lange, J. & Kranz, A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation 102, 185–190 (2000).
Brissova, M. et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab. 19, 498–511 (2014).
De Leu, N. et al. Short-term overexpression of VEGF-A in mouse beta cells indirectly stimulates their proliferation and protects against diabetes. Diabetologia 57, 140–147 (2014).
Staels, W., Heremans, Y., Heimberg, H. & De Leu, N. VEGF-A and blood vessels: a beta cell perspective. Diabetologia 62, 1961–1968 (2019).
Hagberg, C. E. et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490, 426–430 (2012).
Brunner, L. M. et al. Promotion of lymphangiogenesis by targeted delivery of VEGF-C improves diabetic wound healing. Cells 12, 472 (2023).
Natesan, V. & Kim, S. J. Diabetic nephropathy—a review of risk factors, progression, mechanism, and dietary management. Biomol. Ther. 29, 365–372 (2021).
Li, Y. et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 8, 152 (2023).
Simon, M. et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am. J. Physiol. 268, F240–F250 (1995).
Brown, L. F. et al. Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 42, 1457–1461 (1992).
Breier, G., Albrecht, U., Sterrer, S. & Risau, W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 114, 521–532 (1992).
Thomas, S. et al. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J. Am. Soc. Nephrol. 11, 1236–1243 (2000).
Kanellis, J., Fraser, S., Katerelos, M. & Power, D. A. Vascular endothelial growth factor is a survival factor for renal tubular epithelial cells. Am. J. Physiol. Ren. Physiol. 278, F905–F915 (2000).
Kim, N. H. et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. 67, 167–177 (2005).
Kanesaki, Y. et al. Vascular endothelial growth factor gene expression is correlated with glomerular neovascularization in human diabetic nephropathy. Am. J. Kidney Dis. 45, 288–294 (2005).
Huang, H. et al. Effect of fosinopril on chemerin and VEGF expression in diabetic nephropathy rats. Int. J. Clin. Exp. Pathol. 8, 11470–11474 (2015).
Cha, D. R. et al. Vascular endothelial growth factor is increased during early stage of diabetic nephropathy in type II diabetic rats. J. Endocrinol. 183, 183–194 (2004).
Nakagawa, T. et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 18, 539–550 (2007).
Vriese, A. S. et al. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J. Am. Soc. Nephrol. 12, 993–1000 (2001).
Sung, S. H. et al. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J. Am. Soc. Nephrol. 17, 3093–3104 (2006).
Weis, S. M. & Cheresh, D. A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437, 497–504 (2005).
Falkevall, A. et al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 25, 713–726 (2017).
Wei, Y. et al. Increased serum VEGF-B level is associated with renal function impairment in patients with type 2 diabetes. Front. Endocrinol. 13, 862545 (2022).
Shen, Y. et al. VEGF-B antibody and interleukin-22 fusion protein ameliorates diabetic nephropathy through inhibiting lipid accumulation and inflammatory responses. Acta Pharmacol. Sin. 11, 127–142 (2021).
Zarjou, A. et al. Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab. Investig. 99, 1376–1388 (2019).
Foster, R. R. et al. VEGF-C promotes survival in podocytes. Am. J. Physiol. Ren. Physiol. 291, F196–F207 (2006).
Foster, R. R. et al. Vascular endothelial growth factor-C, a potential paracrine regulator of glomerular permeability, increases glomerular endothelial cell monolayer integrity and intracellular calcium. Am. J. Pathol. 173, 938–948 (2008).
Onions, K. L. et al. VEGFC reduces glomerular albumin permeability and protects against alterations in VEGF receptor expression in diabetic nephropathy. Diabetes 68, 172–187 (2019).
Tanabe, K., Wada, J. & Sato, Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat. Rev. Nephrol. 16, 289–303 (2020).
Nguyen, T. T. U., Kim, H., Chae, Y. J., Jung, J. H. & Kim, W. Serum VEGF-D level is correlated with renal dysfunction and proteinuria in patients with diabetic chronic kidney disease. Medicine 101, e28804 (2022).
Keir, L. S. et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Invest. 127, 199–214 (2017).
Person, F. et al. Bevacizumab-associated glomerular microangiopathy. Mod. Pathol. 32, 684–700 (2019).
Iruela-Arispe, L. et al. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am. J. Pathol. 147, 1715–1727 (1995).
Masuda, Y. et al. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am. J. Pathol. 159, 599–608 (2001).
Guan, F., Villegas, G., Teichman, J., Mundel, P. & Tufro, A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am. J. Physiol. Ren. Physiol. 291, F422–F428 (2006).
Maurice, J. & Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 18, 245–250 (2018).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Yoshiji, H. et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut 52, 1347–1354 (2003).
Lei, L., Ei Mourabit, H., Housset, C., Cadoret, A. & Lemoinne, S. Role of angiogenesis in the pathogenesis of NAFLD. J. Clin. Med. 10, 1338 (2021).
Li, Y. Q., Xin, L., Zhao, Y. C., Li, S. Q. & Li, Y. N. Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value. World J. Hepatol. 15, 786–796 (2023).
Coulon, S. et al. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine 59, 442–449 (2012).
Yilmaz, Y. et al. Circulating levels of vascular endothelial growth factor A and its soluble receptor in patients with biopsy-proven nonalcoholic fatty liver disease. Arch. Med. Res. 42, 38–43 (2011).
Shen, H. et al. Hepatocyte-derived VEGFA accelerates the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma via activating hepatic stellate cells. Acta Pharmacol. Sin. 43, 2917–2928 (2022).
Coulon, S. et al. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology 57, 1793–1805 (2013).
Falkevall, A. et al. Inhibition of VEGF-B signaling prevents non-alcoholic fatty liver disease development by targeting lipolysis in the white adipose tissue. J. Hepatol. 78, 901–913 (2023).
Osorio-Conles, O. et al. A distinctive NAFLD signature in adipose tissue from women with severe obesity. Int. J. Mol. Sci. 22, 10541 (2021).
Hong, W. et al. Prediction of VEGF-C as a key target of pure total flavonoids from citrus against NAFLD in mice via network pharmacology. Front. Pharmacol. 10, 582 (2019).
Harris, E. D. Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. New Engl. J. Med. 322, 1277–1289 (1990).
Marrelli, A. et al. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation?. Autoimmun. Rev. 10, 595–598 (2011).
Giatromanolaki, A. et al. Upregulated hypoxia inducible factor-1alpha and -2alpha pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 5, R193–R201 (2003).
Yoo, S. A., Kwok, S. K. & Kim, W. U. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators Inflamm. 2008, 129873 (2008).
Kasama, T. et al. Expression of vascular endothelial growth factor by synovial fluid neutrophils in rheumatoid arthritis (RA). Clin. Exp. Immunol. 121, 533–538 (2000).
Lee, S. S. et al. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 19, 321–324 (2001).
Harada, M. et al. Vascular endothelial growth factor in patients with rheumatoid arthritis. Scand. J. Rheumatol. 27, 377–380 (1998).
Cheng, W. X. et al. Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signalling pathway. J. Orthop. Transl. 22, 92–100 (2020).
Mould, A. W. et al. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 48, 2660–2669 (2003).
Zhou, Q. et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum. 63, 2318–2328 (2011).
Miotla, J., Maciewicz, R., Kendrew, J., Feldmann, M. & Paleolog, E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab. Investig. 80, 1195–1205 (2000).
Sone, H. et al. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem. Biophys. Res. Commun. 281, 562–568 (2001).
Abdel-Maged, A. E., Gad, A. M., Wahdan, S. A. & Azab, S. S. Efficacy and safety of Ramucirumab and methotrexate co-therapy in rheumatoid arthritis experimental model: involvement of angiogenic and immunomodulatory signaling. Toxicol. Appl. Pharmacol. 380, 114702 (2019).
Armstrong, A. W. & Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 323, 1945–1960 (2020).
Heidenreich, R., Rocken, M. & Ghoreschi, K. Angiogenesis drives psoriasis pathogenesis. Int. J. Exp. Pathol. 90, 232–248 (2009).
Vybohova, D., Adamicova, K., Mellova, Y. & Heskova, G. Microvascular changes in relation to inflammation and epidermal hyperplasia in chronic cutaneous lesions of psoriasis vulgaris. Histol. Histopathol. 32, 461–470 (2017).
Watanabe, A. et al. Serum levels of angiogenesis-related factors in patients with psoriasis. J. Dermatol. 50, 222–228 (2023).
Wilgus, T. A. et al. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am. J. Pathol. 167, 1257–1266 (2005).
Man, X. Y., Yang, X. H., Cai, S. Q., Yao, Y. G. & Zheng, M. Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol. Med. 12, 127–136 (2006).
Detmar, M. The role of VEGF and thrombospondins in skin angiogenesis. J. Dermatol. Sci. 24(Suppl 1), S78–S84 (2000).
Henno, A. et al. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. Br. J. Dermatol. 160, 581–590 (2009).
Liew, S. C. et al. Differential expression of the angiogenesis growth factors in psoriasis vulgaris. BMC Res. Notes 5, 201 (2012).
Xia, Y. P. et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 102, 161–168 (2003).
Schonthaler, H. B., Huggenberger, R., Wculek, S. K., Detmar, M. & Wagner, E. F. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc. Natl. Acad. Sci. USA 106, 21264–21269 (2009).
Dimitriadis, E. et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 9, 8 (2023).
Velegrakis, A., Kouvidi, E., Fragkiadaki, P. & Sifakis, S. Predictive value of the sFlt‑1/PlGF ratio in women with suspected preeclampsia: an update (Review). Int. J. Mol. Med. 52, 89 (2023).
Gamliel, M. et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity 48, 951–962 (2018).
Chaiworapongsa, T. et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am. J. Obstet. Gynecol. 190, 1541–1547 (2004).
Chaiworapongsa, T. et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J. Matern. Fetal Neonatal Med. 17, 3–18 (2005).
Nagamatsu, T. et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145, 4838–4845 (2004).
Bridges, J. P. et al. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am. J. Hypertens. 22, 564–568 (2009).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003).
Zeisler, H. et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. New Engl. J. Med. 374, 13–22 (2016).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. New Engl. J. Med. 350, 672–683 (2004).
Palmer, K. R. et al. Placental-specific sFLT-1 e15a protein is increased in preeclampsia, antagonizes vascular endothelial growth factor signaling, and has antiangiogenic activity. Hypertension 66, 1251–1259 (2015).
Li, Z. et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50, 686–692 (2007).
Gilbert, J. S., Babcock, S. A. & Granger, J. P. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50, 1142–1147 (2007).
Gilbert, J. S. et al. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55, 380–385 (2010).
Spradley, F. T. et al. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension 67, 740–747 (2016).
Davisson, R. L. et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 39, 337–342 (2002).
Woods, A. K. et al. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 57, 94–102 (2011).
Waller, J. P. et al. Elastin-like polypeptide: VEGF-B fusion protein for treatment of preeclampsia. Hypertension 78, 1888–1901 (2021).
Hastie, R. et al. EGFR (Epidermal growth factor receptor) signaling and the mitochondria regulate sFlt-1 (soluble FMS-like tyrosine kinase-1) secretion. Hypertension 73, 659–670 (2019).
Benagiano, G., Brosens, I. & Lippi, D. The history of endometriosis. Gynecol. Obstet. Invest. 78, 1–9 (2014).
Zondervan, K. T., Becker, C. M. & Missmer, S. A. Endometriosis. New Engl. J. Med. 382, 1244–1256 (2020).
Taylor, R. N., Lebovic, D. I. & Mueller, M. D. Angiogenic factors in endometriosis. Ann. NY Acad. Sci. 955, 89–100 (2002).
Lebovic, D. I. et al. Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum. Reprod. 15(Suppl 3), 67–77 (2000).
Iruela-Arispe, M. L., Rodriguez-Manzaneque, J. C. & Abu-Jawdeh, G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation 6, 127–140 (1999).
Donnez, J., Smoes, P., Gillerot, S., Casanas-Roux, F. & Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 13, 1686–1690 (1998).
Bourlev, V. et al. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction 132, 501–509 (2006).
Hattori, K. et al. Lymphangiogenesis induced by vascular endothelial growth factor receptor 1 signaling contributes to the progression of endometriosis in mice. J. Pharmacol. Sci. 143, 255–263 (2020).
Sekiguchi, K. et al. VEGF Receptor 1-expressing macrophages recruited from bone marrow enhances angiogenesis in endometrial tissues. Sci. Rep. 9, 7037 (2019).
Filippi, I. et al. Different expression of hypoxic and angiogenic factors in human endometriotic lesions. Reprod. Sci. 23, 492–497 (2016).
Dai, W. et al. Hypoxia and the endometrium: an indispensable role for HIF-1alpha as therapeutic strategies. Redox Biol. 73, 103205 (2024).
Li, J. et al. HIF1A and VEGF regulate each other by competing endogenous RNA mechanism and involve in the pathogenesis of peritoneal fibrosis. Pathol. Res. Pract. 215, 644–652 (2019).
Keichel, S. et al. Lymphangiogenesis in deep infiltrating endometriosis. Hum. Reprod. 26, 2713–2720 (2011).
Takehara, M. et al. Vascular endothelial growth factor A and C gene expression in endometriosis. Hum. Pathol. 35, 1369–1375 (2004).
Xu, H. et al. Vascular endothelial growth factor C is increased in endometrium and promotes endothelial functions, vascular permeability and angiogenesis and growth of endometriosis. Angiogenesis 16, 541–551 (2013).
Li, W. N. et al. Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis. Proc. Natl. Acad. Sci. USA 117, 25859–25868 (2020).
Machado, D. E., Berardo, P. T., Palmero, C. Y. & Nasciutti, L. E. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J. Exp. Clin. Cancer Res. 29, 4 (2010).
Yildiz, C. et al. Effects of pazopanib, sunitinib, and sorafenib, anti-VEGF agents, on the growth of experimental endometriosis in rats. Reprod. Sci. 22, 1445–1451 (2015).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl. J. Med. 350, 2335–2342 (2004).
Ellis, L. M. & Hicklin, D. J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer 8, 579–591 (2008).
Holash, J. et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 99, 11393–11398 (2002).
Van Cutsem, E. et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 30, 3499–3506 (2012).
Ray-Coquard, I. et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. New Engl. J. Med. 381, 2416–2428 (2019).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. New Engl. J. Med. 381, 1632–1643 (2019).
Garon, E. B. et al. Ramucirumab plus erlotinib versus placebo plus erlotinib in previously untreated EGFR-mutated metastatic non-small-cell lung cancer (RELAY): exploratory analysis of next-generation sequencing results. ESMO Open 8, 101580 (2023).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296 (2019).
Wang, F. et al. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat. Med. 30, 1035–1043 (2024).
Ferrara, N., Damico, L., Shams, N., Lowman, H. & Kim, R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26, 859–870 (2006).
Dugel, P. U. et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 127, 72–84 (2020).
Therapeutics, O. Outlook Therapeutics® Announces Preliminary Topline Results of NORSE EIGHT Clinical Trial (New Jersey, 2024).
Therapeutics, O. Outlook Therapeutics® Announces NICE Recommendation of LYTENAVA™ (bevacizumabgamma) for the Treatment of Wet AMD (New Jersey, 2024).
Caine, G. J., Stonelake, P. S., Lip, G. Y. & Blann, A. D. Changes in plasma vascular endothelial growth factor, angiopoietins, and their receptors following surgery for breast cancer. Cancer Lett. 248, 131–136 (2007).
Chi, Y. et al. Role of Angiopoietin/Tie2 system in sepsis: a potential therapeutic target. Clin. Appl. Thromb. Hemost. 30, 10760296241238010 (2024).
Ha, T. Y. MicroRNAs in human diseases: from cancer to cardiovascular disease. Immune Netw. 11, 135–154 (2011).
Gorenjak, V. et al. Epigenome-wide association study in healthy individuals identifies significant associations with DNA methylation and PBMC extract VEGF-A concentration. Clin. Epigenetics 12, 79 (2020).
Murukesh, N., Dive, C. & Jayson, G. C. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br. J. Cancer 102, 8–18 (2010).
Mohammed, R. A. et al. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br. J. Cancer 96, 1092–1100 (2007).
Des Guetz, G. et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br. J. Cancer 94, 1823–1832 (2006).
Werner, N. et al. Circulating endothelial progenitor cells and cardiovascular outcomes. New Engl. J. Med. 353, 999–1007 (2005).
Lee, P. S. & Poh, K. K. Endothelial progenitor cells in cardiovascular diseases. World J. Stem Cells 6, 355–366 (2014).
Liu, X. et al. Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. Am. J. Pathol. 176, 504–515 (2010).
Klement, G. et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin. Invest. 105, R15–R24 (2000).
Bachelder, R. E. et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 61, 5736–5740 (2001).
Xue, Y. et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc. Natl. Acad. Sci. USA 105, 18513–18518 (2008).
Cao, Y. Off-tumor target–beneficial site for antiangiogenic cancer therapy?. Nat. Rev. Clin. Oncol. 7, 604–608 (2010).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Ohm, J. E. et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101, 4878–4886 (2003).
Zhang, D. et al. Antiangiogenic agents significantly improve survival in tumor-bearing mice by increasing tolerance to chemotherapy-induced toxicity. Proc. Natl. Acad. Sci. USA 108, 4117–4122 (2011).
Pinto, M. P., Sotomayor, P., Carrasco-Avino, G., Corvalan, A. H. & Owen, G. I. Escaping antiangiogenic therapy: strategies employed by cancer cells. Int. J. Mol. Sci. 17, 1489 (2016).
Belotti, D., Pinessi, D. & Taraboletti, G. Alternative vascularization mechanisms in tumor resistance to therapy. Cancers 13, 1912 (2021).
Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 221, 1428–1438 (2022).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl. J. Med. 382, 1894–1905 (2020).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl. J. Med. 380, 1116–1127 (2019).
Reck, M. et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 7, 387–401 (2019).
Pal, S. K. et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): a multicentre, randomised, open-label, phase 3 trial. Lancet 402, 185–195 (2023).
Kuo, H. Y., Khan, K. A. & Kerbel, R. S. Antiangiogenic-immune-checkpoint inhibitor combinations: lessons from phase III clinical trials. Nat. Rev. Clin. Oncol. 21, 468–482 (2024).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 15, 310–324 (2018).
Wu, B., Zhang, B., Li, B., Wu, H. & Jiang, M. Cold and hot tumors: from molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 9, 274 (2024).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Paulsen, E. E. et al. Impact of microvessel patterns and immune status in NSCLC: a non-angiogenic vasculature is an independent negative prognostic factor in lung adenocarcinoma. Front. Oncol. 13, 1157461 (2023).
Li, Y. et al. Discovery of novel antibody-drug conjugates bearing tissue protease specific linker with both anti-angiogenic and strong cytotoxic effects. Bioorg. Chem. 137, 106575 (2023).
Caballero, B., Sherman, S. J. & Falk, T. Insights into the mechanisms involved in protective effects of VEGF-B in dopaminergic neurons. Parkinsons Dis. 2017, 4263795 (2017).
Ceci, C. et al. The VEGFs/VEGFRs system in Alzheimer’s and Parkinson’s diseases: pathophysiological roles and therapeutic implications. Pharmacol. Res. 201, 107101 (2024).
Liu, Y., Dong, G., Yu, J. & Liang, P. Integration of single-cell and spatial transcriptomics reveals fibroblast subtypes in hepatocellular carcinoma: spatial distribution, differentiation trajectories, and therapeutic potential. J. Transl. Med. 23, 198 (2025).
McIntyre, A. & Harris, A. L. Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality. EMBO Mol. Med. 7, 368–379 (2015).
Hosaka, K. et al. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat. Commun. 11, 3704 (2020).
Stalmans, I. et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 109, 327–336 (2002).
Nilsson, I. et al. Thrombolysis exacerbates cerebrovascular injury after ischemic stroke via a VEGF-B dependent effect on adipose lipolysis. Preprint at http://www.biorxiv.org/content/10.1101/2024.10.11.617532v1.full.pdf (2024).
Ning, F. C. et al. VEGF-B ablation in pancreatic β-cells upregulates insulin expression without affecting glucose homeostasis or islet lipid uptake. Sci. Rep. 10, 923 (2020).
He, J. et al. Autocrine VEGF-B signaling maintains lipid synthesis and mitochondrial fitness to support T cell immune responses. J. Clin. Invest. 134, e176586 (2024).
Lim, L. et al. Hemostasis stimulates lymphangiogenesis through release and activation of VEGFC. Blood 134, 1764–1775 (2019).
Ho, V. C., Duan, L. J., Cronin, C., Liang, B. T. & Fong, G. H. Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation 126, 741–752 (2012).
Hiratsuka, S., Minowa, O., Kuno, J., Noda, T. & Shibuya, M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. USA 95, 9349–9354 (1998).
Okabe, K. et al. Neurons limit angiogenesis by titrating VEGF in retina. Cell 159, 584–596 (2014).
Dellinger, M. T., Meadows, S. M., Wynne, K., Cleaver, O. & Brekken, R. A. Vascular endothelial growth factor receptor-2 promotes the development of the lymphatic vasculature. PLoS ONE 8, e74686 (2013).
Ferrara, N., Hillan, K. J., Gerber, H. P. & Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 3, 391–400 (2004).
Mésange, P. et al. Intrinsic bevacizumab resistance is associated with prolonged activation of autocrine VEGF signaling and hypoxia tolerance in colorectal cancer cells and can be overcome by nintedanib, a small molecule angiokinase inhibitor. Oncotarget 5, 4709–4721 (2014).
Casak, S. J. et al. FDA’s approval of the first biosimilar to bevacizumab. Clin. Cancer Res. 24, 4365–4370 (2018).
Javle, M., Smyth, E. C. & Chau, I. Ramucirumab: successfully targeting angiogenesis in gastric cancer. Clin. Cancer Res. 20, 5875–5881 (2014).
Fuchs, C. S. et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br. J. Cancer 115, 974–982 (2016).
Tiwari, P. Ramucirumab: boon or bane. J. Egypt. Natl. Cancer Inst. 28, 133–140 (2016).
Blick, S. K., Keating, G. M. & Wagstaff, A. J. Ranibizumab. Drugs 67, 1199–1206 (2007).
Shirley, M. Faricimab: first approval. Drugs 82, 825–830 (2022).
Stewart, M. W., Grippon, S. & Kirkpatrick, P. Aflibercept. Nat. Rev. Drug Discov. 11, 269–270 (2012).
Chung, C. & Pherwani, N. Ziv-aflibercept: a novel angiogenesis inhibitor for the treatment of metastatic colorectal cancer. Am. J. Health Syst. Pharm. 70, 1887–1896 (2013).
Ng, E. W. et al. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 5, 123–132 (2006).
Bukowski, R. M., Yasothan, U. & Kirkpatrick, P. Pazopanib. Nat. Rev. Drug Discov. 9, 17–18 (2010).
Yokoyama, N. et al. Activation of ERK1/2 causes pazopanib resistance via downregulation of DUSP6 in synovial sarcoma cells. Sci. Rep. 7, 45332 (2017).
Kane, R. C. et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 12, 7271–7278 (2006).
Goodman, V. L. et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer Res. 13, 1367–1373 (2007).
Morais, C. Sunitinib resistance in renal cell carcinoma. J. Kidney Cancer VHL 1, 1–11 (2014).
Makhov, P. et al. Resistance to systemic therapies in clear cell renal cell carcinoma: mechanisms and management strategies. Mol. Cancer Ther. 17, 1355–1364 (2018).
Makhov, P. B. et al. Modulation of Akt/mTOR signaling overcomes sunitinib resistance in renal and prostate cancer cells. Mol. Cancer Ther. 11, 1510–1517 (2012).
Gross-Goupil, M., François, L., Quivy, A. & Ravaud, A. Axitinib: a review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin. Med. Insights Oncol. 7, 269–277 (2013).
Li, W. et al. Knockdown of LINC00467 contributed to Axitinib sensitivity in hepatocellular carcinoma through miR-509-3p/PDGFRA axis. Gene Ther. 28, 634–645 (2021).
Singh, H. et al. U.S. Food and Drug Administration Approval: cabozantinib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 23, 330–335 (2017).
Hoy, S. M. Cabozantinib: a review of its use in patients with medullary thyroid cancer. Drugs 74, 1435–1444 (2014).
Park, K. Y. et al. Immune cell mediated cabozantinib resistance for patients with renal cell carcinoma. Integr. Biol. 13, 259–268 (2021).
Scott, L. J. Lenvatinib: first global approval. Drugs 75, 553–560 (2015).
Bo, W. & Chen, Y. Lenvatinib resistance mechanism and potential ways to conquer. Front. Pharmacol. 14, 1153991 (2023).
Heo, Y. A. & Syed, Y. Y. Regorafenib: a review in hepatocellular carcinoma. Drugs 78, 951–958 (2018).
Kehagias, P. et al. Regorafenib induces senescence and epithelial-mesenchymal transition in colorectal cancer to promote drug resistance. Cells 11, 3663 (2022).
Wei, N., Chu, E., Wu, S. Y., Wipf, P. & Schmitz, J. C. The cytotoxic effects of regorafenib in combination with protein kinase D inhibition in human colorectal cancer cells. Oncotarget 6, 4745–4756 (2015).
Frampton, J. E. Vandetanib: in medullary thyroid cancer. Drugs 72, 1423–1436 (2012).
Nakaoku, T. et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat. Commun. 9, 625 (2018).
Ichihara, E. et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res. 69, 5091–5098 (2009).
Tan, F. H., Putoczki, T. L., Stylli, S. S. & Luwor, R. B. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. OncoTargets Ther. 12, 635–645 (2019).
Eide, C. A. et al. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell 36, 431–443.e435 (2019).
Li, H. et al. Apatinib: a novel antiangiogenic drug in monotherapy or combination immunotherapy for digestive system malignancies. Front. Immunol. 13, 937307 (2022).
Zhou, X. et al. Activated amino acid response pathway generates apatinib resistance by reprograming glutamine metabolism in non-small-cell lung cancer. Cell Death Dis. 13, 636 (2022).
Teng, F. et al. DUSP1 induces apatinib resistance by activating the MAPK pathway in gastric cancer. Oncol. Rep. 40, 1203–1222 (2018).
Fala, L. Ofev (nintedanib): first tyrosine kinase inhibitor approved for the treatment of patients with idiopathic pulmonary fibrosis. Am. Health Drug Benefits 8, 101–104 (2015).
Englinger, B. et al. Acquired nintedanib resistance in FGFR1-driven small cell lung cancer: role of endothelin-A receptor-activated ABCB1 expression. Oncotarget 7, 50161–50179 (2016).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean Government (MSIP) (RS-2023-00268276 to C.L., NRF-2023R1A2C3007266, and NRF-2021R1A6A1A03038890 to Y.K.). This research was partially supported by the Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (No. 2021R1A6C101A564 and RS-2024-00436674), Industrial Technology Innovation Program (RS-2024-00403190) funded by the Ministry of Trade, Industry & Energy of the Republic of Korea, and Korea Drug Development Fund (RS-2024-00463605). We thank Dr. Ruibo Chen and Na-Young Oh for their contribution to the schematic diagrams.
Author information
Authors and Affiliations
Contributions
C.L. and Y.K. conceptualized and wrote the manuscript, prepared figures, secured funding, and reviewed and finalized the manuscript. M.-J.K., A.K., and Y.Y. contributed to manuscript writing, figure and table preparation. H.-W.L. participated in conceptual discussions and reviewed the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, C., Kim, MJ., Kumar, A. et al. Vascular endothelial growth factor signaling in health and disease: from molecular mechanisms to therapeutic perspectives. Sig Transduct Target Ther 10, 170 (2025). https://doi.org/10.1038/s41392-025-02249-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-025-02249-0
This article is cited by
-
Recent advances in engineered exosome-based therapies for ocular vascular disease
Journal of Nanobiotechnology (2025)
-
TGF-β1-triggered maladaptive bone marrow endothelium impedes hematopoietic recovery
Signal Transduction and Targeted Therapy (2025)
-
In silico discovery of alkaloids as VEGFR-2 inhibitors with favorable toxicity profiles: insights from molecular dynamics and MM/GBSA
In Silico Pharmacology (2025)