Abstract
This study examines α-klotho levels in depressed American adults and their association with cardiovascular disease and all-cause mortality, utilizing data from the National Health and Nutrition Examination Survey (2007–2016) and mortality details from the National Death Index up to December 31, 2019. Including 3329 participants with depression, findings revealed 485 all-cause and 113 cardiovascular deaths. To investigate the nonlinear association between α-klotho and mortality, the Cox proportional hazards regression model, restricted cubic splines, and two-piecewise Cox proportional hazards model were developed. Analyzes indicated an “L-shaped” relationship between ln-transformed α-klotho levels and all-cause mortality, with a significant threshold effect at 6.53 ln(pg/ml). Below this threshold, ln-transformed α-klotho levels were inversely related to all-cause mortality (adjusted HR 0.33, 95%CI = 0.19–0.56), with no significant association above it (adjusted HR 1.41, 95%CI = 0.84–2.36). Cardiovascular mortality showed no link to α-klotho levels. Subgroup analysis shown that, the association between ln-transformed α-klotho concentration and all-cause mortality was consistent in subgroups according to gender, age, BMI, race, and depression(adjusted P > 0.05). The study uncovers a non-linear “L-shaped” association between ln-transformed α-klotho levels and all-cause mortality in depressed individuals, suggesting α-klotho assessment as a tool for identifying high-risk patients and guiding preventive strategies to enhance survival.
Similar content being viewed by others
Introduction
Depression is a widespread mental condition that affects a significant number of older individuals, with a global prevalence of 28.4% among this population [1]. This poses a substantial public health concern on a global scale. In addition to the physical obstacles and economic difficulties commonly associated with depression [2], there is also substantial evidence linking it to cardiovascular illnesses and mortality. Depression is not only a separate component that increases the risk of cardiovascular illnesses [3], but it also independently increases the risk of mortality in older adults. Those who experience severe levels of depression have a 25–43% higher risk of mortality [4]. Research has demonstrated that depression in older adults is linked to a higher likelihood of all-cause and cardiovascular disease mortality [5].
The klotho gene, which is associated with the process of aging, produces the klotho protein. This protein is recognized for its antioxidative and anti-apoptotic properties, as well as its capacity to suppress inflammation and fibrosis [6, 7]. Serum klotho serves as a reliable indicator of the likelihood of all-cause mortality in older adults living in the community. Those with lower levels of klotho are at a higher risk of mortality [8]. Chuang, M. H., et al. discovered a non-linear correlation between soluble α-klotho and the risk of all-cause mortality in the middle-aged and senior population in the USA. They found that persons in both the lowest and highest septile of soluble α-klotho levels had an increased probability of all-cause mortality [9]. A study conducted by Lanzani C and colleagues discovered a correlation between circulating klotho and cardiovascular illnesses. It was observed that a lack of klotho may result in cardiovascular events, but maintaining elevated levels of serum klotho can decrease cardiovascular mortality [7]. In fact, α-klotho is not only implicated in the aging process, but also has a role in regulating psychological functioning in cognitive and mental disorders [10]. A new study discovered a relationship between middle-aged and older people’s serum α-klotho and depression, especially in women [11]. Another study found that plasma α-klotho levels decline with the increased severity of major depressive disorder (MDD) in the elderly, suggesting that α-klotho plays a key role in older MDD patients [12]. While previous research has shown a connection between the klotho protein and depression as well as mortality, there is less information available on the association between α-klotho and all-cause or cardiovascular mortality in adults with depression. It is yet unclear what the independent prognostic value of klotho is for mortality in depressed individuals, both from cardiovascular and all-cause mortality, which calls for more investigation.
An analysis was conducted using data from the representative National Health and Nutrition Examination Survey (NHANES) and mortality-related information provided by the National Center for Health Statistics (NCHS) to investigate the connection between klotho and both all-cause and cardiovascular mortality rates among middle-aged and elderly individuals with depression in the United States. Furthermore, to determine if α-klotho is an independent predictor of mortality in middle-aged and elderly adults with depression, several risk regression models were created to account for any factors that could potentially influence the results. Furthermore, the study conducted stratified analyses based on characteristics including gender, age, body mass index (BMI), race, and the presence or absence of clinical symptoms of depression.
Methods
Participants and study design
This study is conducted using the NHANES, a prominent research program that investigates the health and nutritional well-being of individuals in the United States, encompassing both adults and children [13]. The Centers for Disease Control and Prevention (CDC) was charged with supplying crucial health data to the country, and the NHANES study was granted official approval from the research ethics review committee of the NCHS. All participants were required to provide explicit informed permission to safeguard their rights. The dataset utilized in this investigation is conveniently obtainable on the official NHANES website [13]. This study examined data from four NHANES cycles spanning from 2007 to 2016. The diagnosis of depression was assessed using the Patient Health Questionnaire-9 (PHQ-9), a screening instrument consisting of nine items that assess the frequency of depressed symptoms experienced in the past 2 weeks [14]. This test incorporates the diagnostic criteria for depression established by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [15]. The PHQ-9 has a total score range of 0–27. A score of 5 or higher indicates depression, and a score of 10 or higher indicates clinically symptomatic depression [16]. Using a commercial enzyme-linked immunosorbent assay (ELISA) test made by IBL International, Japan, the concentration of serum soluble α-klotho was detected [17]. Serum α-klotho samples were stored at −80 °C and examined twice for concentration measurement. The average of the two values was used for the final computation. If the value of a quality control sample did not fall within two standard deviations of the specified value, the analysis was repeated. 114 donor samples that appeared healthy were also examined for the reference range; the average α-klotho level was 698.0 pg/ml, and the levels ranged from 285.8 to 1638.6 pg/ml.
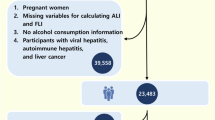
The study initially comprised a total of 50,588 participants. After removing instances with incomplete depression survey questionnaire data (24,067 individuals), missing plasma soluble α-klotho concentration measurements (13,798 individuals), or no mortality follow-up data (14 individuals), the analysis proceeded with 12,709 participants. Out of the total number of participants, 3329 individuals were diagnosed with depression (PHQ-9 ≥ 5) and were considered for the final analysis, as depicted in Fig. 1.
Covariates
During the household interview phase of NHANES, a range of demographic and health-related data were collected, encompassing age, gender, race, poverty income ratio (PIR, a ratio of family income to poverty threshold), education level, marital status, smoking status, health status, medication usage and so on. Race was categorized into five groups: Mexican American, Non-Hispanic White, Non-Hispanic Black, other Hispanic, and other. BMI was calculated using weight (kg) divided by height (m)^2 and classified according to the World Health Organization (WHO) international standards into four categories: underweight (<18.5), normal weight (18.5–25), overweight (25–30), and obese (≥30). Educational levels were divided into less than high school, high school graduate or GED, and some college or above. Marital status was categorized as married and a partner, widowed, divorced or separated, and never married.
Smoking statuses were classified into never smoker, former smoker, and current smoker. Alcohol consumption status was determined by asking, “In any one year, have you had at least 12 drinks of any type of alcoholic beverage?” (yes/no). Hypertension was defined as systolic blood pressure greater than 140 mmHg, diastolic blood pressure greater than 90 mmHg, self-reported history of hypertension, or current use of antihypertensive medication. Diabetes was defined as random blood glucose greater than 11.1 mmol/l, glycated hemoglobin greater than 6.5%, self-reported history of diabetes, or current use of antidiabetic medication. A history of cardiovascular diseases (CVD) was determined by asking, “Have you ever been told by a doctor or health professional that you have congestive heart failure, coronary heart disease, angina, heart attack, or stroke?” (yes/no). Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m^2, calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [18].
Additionally, NHANES laboratories measured various clinical markers such as serum creatinine (mg/dL), uric acid (UA, umol/L), calcium-phosphorus levels (mmol/L), serum albumin (g/L), serum iron (umol/L), serum 25-hydroxyvitamin D [25 (OH) D, nmol/L], glycohemoglobin (%), total cholesterol (TC, mmol/L), high-density lipoprotein cholesterol (HDL-C, mmol/L), lactate dehydrogenase (LDH, U/L), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), gamma-glutamyl transferase (GGT, U/L), and total bilirubin (μmol/L).
Ascertainment of mortality
To determine the mortality status of the follow-up population, we utilized the NHANES public mortality linkage file, updated as of December 31, 2019. This file was linked to the National Death Index (NDI) by the NCHS using a probabilistic matching algorithm. All-cause mortality was classified according to the International Classification of Diseases, Tenth Revision (ICD-10) codes. Cardiovascular mortality was defined based on ICD-10 codes I00-I09, I11, I13, and I20-I51.
Statistical analysis
All statistical analyses were conducted in R software (version 4.3.2). We used the Mobile Examination Center (MEC) exam weights as the weighting variable for the analysis. Study participants were divided into four groups based on the quartiles (Q1-Q4) of α-klotho concentrations. Continuous variables were summarized using mean and standard deviation (SD), while categorical variables were presented as frequencies and percentages. For continuous variables, one-way analysis of variance (ANOVA) was used to compare baseline characteristics across different α-klotho quartile groups; for categorical variables, Pearson’s chi-square test was utilized. Incidence rates of all-cause mortality and cardiovascular mortality during the entire follow-up period were calculated for each α-klotho quartile group. To assess the independent predictive value of α-klotho, multivariate Cox proportional hazards regression models were established, including three models to control for confounding factors. Model 1 was unadjusted; Model 2 adjusted for age, gender, and race; Model 3 adjusted for age, gender, race, BMI, PIR, education, marital status, smoking status, alcohol, hypertension, diabetes, CVD, sleep disorder, self-reported cancer, self-reported liver diseases, CKD, antidepressant drug, year cycle, and depressive symptom severity. Random forest imputation was used for covariates with missing values [19], and variables with more than 20% missing values were deleted. The distribution of missing values is shown in Supplementary Fig. 1. Utilizing restricted cubic splines and smoothing curve fitting (penalized spline method) to investigate the nonlinear association between α-klotho and mortality. Considering that the skewed distribution might affect the results of restricted cubic splines, we conducted a normality test on the α-klotho distribution before the analysis. The results indicated that α-klotho did not follow a normal distribution. Therefore, we performed a normality transformation on the α-klotho protein using the natural logarithm (Ln) transformation. We estimated the threshold by trying all values and chose the threshold point with the highest likelihood as the inflection point. On both sides of the inflection point, a two-piecewise Cox proportional hazards model was used to investigate the association between α-klotho and risks of all-cause mortality and cardiovascular disease mortality. Additionally, stratified analyses were performed across variables such as gender, age, BMI, race, and asymptomatic or symptomatic of depression. A p-value less than 0.05 was considered statistically significant. The P for trend assessed the linear relationship between α-klotho quartiles and mortality, with P < 0.05 indicating a significant trend.
Results
Baseline characteristics of study participants
Table 1 presents the baseline characteristics of the cohort study participants (n = 3329) stratified by the quartiles of α-klotho. The average age of the participants was 55.63 ± 10.16 years, with 62.16% being female. The average α-klotho concentration among enrolled patients was 851 ± 327 pg/ml. Based on the quartiles of α-klotho, baseline laboratory characteristics are displayed in Table 2. Participants with higher α-klotho levels were more likely to be younger, Non-Hispanic Black, and of normal weight compared to those in the lowest quartile. Significant differences in biochemical markers observed between groups include lower levels of serum creatinine, uric acid (UA), and albumin in the highest quartile group compared to the lowest quartile group.
Relationships of α-klotho concentration with mortality
Table 3 shows a total of 485 all-cause deaths and 113 cardiovascular disease-related deaths that occurred during the follow-up period. We constructed three Cox regression models to study the independent association between α-klotho concentration levels and mortality risk. After adjusting for age, gender, race, body mass index (BMI), poverty income ratio (PIR), education, marital status, smoking status, alcohol, hypertension, diabetes, cardiovascular disease (CVD), sleep disorder, self-reported cancer, self-reported liver diseases, chronic kidney disease (CKD), antidepressant drug, year cycle, and depressive symptom severity in Model 3, the multivariable-adjusted HRs and 95% CIs for all-cause mortality for quartiles of α-klotho concentration from lowest to highest were 1.00 (reference), 0.74 (0.51, 1.09), 0.79 (0.56, 1.13), and 0.91 (0.65, 1.27) (P for trend = 0.70). For cardiovascular mortality, the HRs were 1.00 (reference), 0.55 (0.27, 1.11), 0.73 (0.35, 1.50), and 0.69 (0.31, 1.53) (P for trend = 0.52).
The testing of non-linear relationships
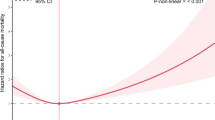
Our previous multivariate analysis showed no nonlinear relationship between ln-transformed α-klotho concentration and cardiovascular mortality risk (adjusted P for nonlinear >0.05, Fig. 2B). However, a nonlinear relationship was observed between ln-transformed α-klotho concentration and all-cause mortality risk (adjusted P for nonlinear <0.01, Fig. 3B). Therefore, we further investigated this correlation using a Cox proportional hazards regression model with restricted cubic splines and smoothing curve fitting (penalized spline method). Interestingly, the adjusted smooth curve demonstrated an “L” shaped relationship between ln-transformed α-klotho concentration and all-cause mortality (Fig. 3B). The association between ln-transformed α-klotho concentration and all-cause mortality was fitted using standard Cox proportional hazards regression model and two-piecewise Cox proportional hazards model. Based on the two-piecewise Cox proportional hazard model, we identified an inflection point for all-cause mortality at 6.53[ln(pg/ml)] (685.40 pg/ml) (P-value for log-likelihood ratio <0.001, in Table 4). After adjusting for age, gender, race, BMI, PIR, education, marital status, smoking status, alcohol, hypertension, diabetes, CVD, sleep disorder, self-reported cancer, self-reported liver diseases, CKD, antidepressant drug, year cycle, and depressive symptom severity, the risk of all-cause mortality decreased by 67% (HR 0.33, 95%CI = 0.19–0.56), with each unit increase in the ln-transformed α-klotho concentration[ln(pg/ml)] up to the inflection point (Table 4 and Fig. 3B). However, there was no significant association between baseline ln-transformed α-klotho concentration and all-cause mortality if it exceeded 6.53[ln(pg/ml)] (HR 1.41, 95%CI = 0.84–2.36) (Table 4).
A Model 1 for CVD mortality: non-adjusted; B Model 3 for CVD mortality: adjusted for age, gender, race, BMI, PIR, education, marital status, smoking status, alcohol, hypertension, diabetes, CVD, sleep disorder, self-reported cancer, self-reported liver diseases, CKD, antidepressant drug, year cycle, depressive symptom severity. The solid line and red area represent the estimated values and their corresponding 95% CIs. Abbreviations: PIR poverty income ratio, CVD cardiovascular disease, CKD chronic kidney disease, HR hazard ratio, CI confidence interval.
A Model 1 for all-cause mortality: non-adjusted; B Model 3 for all-cause mortality: adjusted for age, gender, race, BMI, PIR, education, marital status, smoking status, alcohol, hypertension, diabetes, CVD, sleep disorder, self-reported cancer, self-reported liver diseases, CKD, antidepressant drug, year cycle, depressive symptom severity. The solid line and red area represent the estimated values and their corresponding 95% CIs. Each hazard ratio was computed with a ln-transformed α-klotho level of 6.53[ln(pg/ml)] as the reference in Fig. 3B. Abbreviations: PIR poverty income ratio, CVD cardiovascular disease, CKD chronic kidney disease, HR hazard ratio, CI confidence interval.
Stratified analyses
As shown in Table 5, the association between ln-transformed α-klotho concentration and all-cause mortality was consistent in subgroups according to gender, age, BMI, race, and depression(adjusted P > 0.05). And there was no significant interaction between ln-transformed α-klotho concentration and stratified variables (adjusted P for interaction >0.05).
Discussion
This study utilized data from the NHANES to thoroughly investigate the correlation between α-klotho levels and both all-cause mortality and cardiovascular mortality in adult patients diagnosed with depression. The results suggest a notable non-linear relationship between ln-transformed α-klotho levels and all-cause mortality in individuals with depression, displaying a trend that resembles the shape of the letter “L”. However, there is no significant correlation observed with cardiovascular mortality. Furthermore, the α-klotho protein is able to independently predict all-cause mortality in depressed individuals, with a threshold occurring at 6.53 ln(pg/ml). This discovery implies that lower serum ln-transformed α-klotho levels are significantly associated with higher all-cause mortality within a certain range. These findings hold great importance in the realm of biomarker investigation for depression, providing new perspectives on the survival prognosis of individuals suffering from depression.
Klotho protein, known as an anti-aging protein, has been extensively studied since its discovery, with some experts referring to it as “the elephant in the room” of aging-related research [20]. Despite more than 20 years passing since the first publication of the klotho protein [21], our comprehension of this “elephant” may still be lacking. Lower levels of Klotho have been associated with a number of diseases, and preceding studies have partially shown the connections between the klotho protein and cardiovascular diseases [22], kidney diseases [23, 24], and neurodegenerative disorders [25], among other conditions. The klotho protein is thought to have various functions, including the inhibition of lipid peroxidation and inflammation, the prevention of endothelial damage and vascular calcification, the reduction of vascular stiffness, and the suppression of cardiac fibrosis development. As a result, it plays a role in protecting the heart [26]. Nevertheless, the investigation of the klotho protein’s function in individuals with depression is still a nascent field of study. Our study extends this understanding by identifying a significant non-linear relationship between ln-transformed α-klotho levels and all-cause mortality in individuals with depression, which aligns with the known protective roles of klotho protein.
Zhang et al.’s research revealed a negative correlation between serum α-klotho levels and the prevalence of depression in middle-aged and elderly women [11]. Similarly, the research conducted by Wu et al. showed that knocking down the klotho gene in the nucleus accumbens of mice induced changes in behavior associated with depression. Conversely, increasing the expression of Klotho had an antidepressant effect [27]. This indicates a potential involvement of klotho in the pathogenesis of depression. Additionally, it has been observed that individuals suffering from MDD often have a decrease within the volume of their hippocampal [28]. Klotho, acting as a neuroprotective protein, can potentially alleviate depressed symptoms or slow the progression of the disease by activating antioxidant enzymes to shield hippocampal neurons from the harmful effects of amyloid and glutamate [29]. Furthermore, research suggests that klotho plays a role in the intricate mechanisms by which elderly people with MDD respond to treatment with selective serotonin reuptake inhibitors (SSRIs) [30]. This effect of klotho might be through its anti-inflammatory and anti-oxidative stress functions, impacting the hypothalamic-pituitary-adrenal (HPA) axis, as well as the synthesis and reuptake of neurotransmitters like serotonin and dopamine [11, 31]. Our study highlights the importance of klotho in depression, as lower levels of α-klotho are not only significantly associated with a higher prevalence of depression but also with increased all-cause mortality in depressed individuals. Based on previous research findings, aberrant expression of klotho protein may impact the physiological condition of depressed individuals, hence influencing their all-cause mortality risk. It is necessary for additional research to investigate the precise biological mechanisms of klotho and its potential therapeutic uses.
This study uncovered a noteworthy discovery: there exists a correlation between ln-transformed α-klotho levels and the all-cause mortality in individuals with depression, which follows a “L” shape pattern, suggesting the presence of a threshold. Below this threshold, patients with depression who have higher levels of α-klotho demonstrate a decreased risk of all-cause mortality. This indicates that α-klotho has a protective impact against mortality risk in these patients. However, there is no such association seen with cardiovascular mortality. This discovery is similar to the findings of Chuang, M. H., et al. [9], who, while they did not determine a specific threshold, analyzed the risk of mortality over several ranges of klotho concentration. Prior research has additionally discovered that higher levels of klotho in the blood are linked to lower all-cause mortality in individuals with hypertension, while there is no correlation with cardiovascular mortality [32]. In CKD patients, researchers found a correlation between serum klotho levels and all-cause mortality. The relationship followed a “L” shape, with a turning point at 760 pg/ml [33]. Our research demonstrates that once the ln-transformed α-klotho level above the threshold of 6.53[ln(pg/ml)], its correlation with all-cause mortality becomes insignificant. Strategies for public health and clinical practice may be significantly impacted by this finding. While the exact mechanisms via which the klotho protein influences depression remain unclear, its correlation with all-cause mortality indicates that measuring klotho protein levels could be a valuable biomarker for evaluating the health and mortality risk of individuals with depression. Due to the widespread prevalence of depression as a matter of public health, it is imperative to implement accurate risk evaluation and timely intervention to enhance the quality of life and prognosis for patients.
The results of this study should be interpreted with caution. Firstly, due to its cross-sectional nature, causality cannot be established. Fluctuations in klotho protein levels may be a consequence of alterations in the health condition of individuals with depression, rather than being a direct factor contributing to their all-cause mortality. Secondly, even though NHANES data covers a broad spectrum of the adult population in the United States, there may still be some selection bias, and the sample might not fully represent all patients with depression. Therefore, future studies should be conducted in a broader and more diverse population to validate and deepen our findings.
Conclusion
In conclusion, our study found a non-linear “L-shaped” relationship between ln-transformed α-klotho levels and all-cause mortality in patients with depression. This finding suggests that α-klotho levels could potentially serve as a biomarker for assessing survival prognosis in depressed patients, and monitoring α-klotho levels may help in the early detection of individuals at higher risk of mortality. Although α-klotho levels are not routinely assessed in clinical practice for depression, if this finding is further validated, it would be helpful to implement preventive measures to decrease all-cause mortality associated with depression and prolong patient survival. Additionally, further research is needed to explore the correlation between α-klotho levels and different subtypes of depression, as well as its impact on diverse populations with varying ages, genders, and ethnic backgrounds, to gain a more comprehensive understanding of its clinical application value.
Data availability
You can get the data used for this study at NHANES website: https://www.cdc.gov/nchs/nhanes.
Code availability
The code used in this study can be found in “Supplementary Software-code”.
References
Hu T, Zhao X, Wu M, Li Z, Luo L, Yang C, et al. Prevalence of depression in older adults: a systematic review and meta-analysis. Psychiatry Res. 2022;311:114511.
Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98:99–143.
Fiedorowicz JG. Depression and cardiovascular disease: an update on how course of illness may influence risk. Curr Psychiatry Rep. 2014;16:492.
Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–8.
Wei J, Lu Y, Li K, Goodman M, Xu H. The associations of late-life depression with all-cause and cardiovascular mortality: the NHANES 2005-2014. J Affect Disord. 2022;300:189–94.
Xu Y, Sun Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev. 2015;36:174–93.
Lanzani C, Citterio L, Vezzoli G. Klotho: a link between cardiovascular and non-cardiovascular mortality. Clin Kidney J. 2020;13:926–32.
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800.
Chuang MH, Wang HW, Huang YT, Jiang MY. Association between soluble α-klotho and mortality risk in middle-aged and older adults. Front Endocrinol. 2023;14:1246590.
Gao X, Li Y, Sun Z, Xu H, Ma G, Deng Q, et al. Could α-klotho unlock the key between depression and dementia in the elderly: from animal to human studies. Mol Neurobiol. 2021;58:2874–85.
Zhang Y, Lu J, Huang S, Chen Y, Fang Q, Cao Y. Sex differences in the association between serum α-klotho and depression in middle-aged and elderly individuals: a cross-sectional study from NHANES 2007–2016. J Affect Disord. 2023;337:186–94.
Gao X, Sun Z, Ma G, Li Y, Liu M, Zhang G, et al. Reduced plasma levels of α-klotho and their correlation with klotho polymorphisms in elderly patients with major depressive disorders. Front Psychiatry. 2021;12:682691.
Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS).National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). http://www.cdc.gov/nchs/nhanes.htm (2006).
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13.
Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44.
Iranpour S, Sabour S. Inverse association between caffeine intake and depressive symptoms in US adults: data from National Health and Nutrition Examination Survey (NHANES) 2005-2006. Psychiatry Res. 2019;271:732–9.
Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-klotho measurement: age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–8.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Stekhoven DJ, Bühlmann P. MissForest-non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–8.
Cheikhi A, Barchowsky A, Sahu A, Shinde SN, Pius A, Clemens ZJ, et al. Klotho: an elephant in aging research. J Gerontol A Biol Sci Med Sci. 2019;74:1031–42.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51.
Liu Y, Chen M. Emerging role of α-klotho in energy metabolism and cardiometabolic diseases. Diabetes Metab Syndr. 2023;17:102854.
Li SS, Sheng MJ, Sun ZY, Liang Y, Yu LX, Liu QF. Upstream and downstream regulators of klotho expression in chronic kidney disease. Metabolism. 2023;142:155530.
Cuarental L, Ribagorda M, Ceballos MI, Pintor-Chocano A, Carriazo SM, Dopazo A, et al. The transcription factor Fosl1 preserves Klotho expression and protects from acute kidney injury. Kidney Int. 2023;103:686–701.
Zhao Y, Zeng CY, Li XH, Yang TT, Kuang X, Du JR. Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell. 2020;19:e13239.
Tyurenkov IN, Perfilova VN, Nesterova AA, Glinka Y.Klotho protein and cardio-vascular system.Biochemistry. 2021;86:132–45.
Wu HJ, Wu WN, Fan H, Liu LE, Zhan JQ, Li YH, et al. Life extension factor klotho regulates behavioral responses to stress via modulation of GluN2B function in the nucleus accumbens. Neuropsychopharmacology. 2022;47:1710–20.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–50.
Abraham CR, Mullen PC, Tucker-Zhou T, Chen CD, Zeldich E. Klotho is a neuroprotective and cognition-enhancing protein. Vitam Horm. 2016;101:215–38.
Paroni G, Seripa D, Fontana A, D’Onofrio G, Gravina C, Urbano M, et al. Klotho gene and selective serotonin reuptake inhibitors: response to treatment in late-life major depressive disorder. Mol Neurobiol. 2017;54:1340–51.
Paroni G, Panza F, De Cosmo S, Greco A, Seripa D, Mazzoccoli G. Klotho at the edge of Alzheimer’s disease and senile depression. Mol Neurobiol. 2019;56:1908–20.
Yan Y, Chen J. Association between serum klotho concentration and all-cause and cardiovascular mortality among American individuals with hypertension. Front Cardiovasc Med. 2022;9:1013747.
Han S, Zhang X, Wang X, Wang Y, Xu Y, Shang L. Association between serum klotho and all-cause mortality in chronic kidney disease: evidence from a prospective cohort study. Am J Nephrol. 2024;55:273–83.
Acknowledgements
The authors thank the participants of the NHANES databases.
Funding
This work was supported by the Funding Project of the Bureau of Science and Technology and Intellectual Property of Nanchong City (No. 23YYJCYJ0163). The views stated in this work are of the authors only.
Author information
Authors and Affiliations
Contributions
Jiayu Zhao: conceptualization, data curation, methodology, writing original draft, writing—review & editing, validation, supervision. Tong Zhou: conceptualization, data curation, methodology, formal analysis, writing original draft, writing—review & editing, software. Yang Jing: conceptualization, data curation, validation, writing original draft. Jiarui Shao: data curation, investigation, validation. Cailin Xie: data curation, investigation, formal analysis. Yingying Huang: data curation, methodology, investigation. Tian Long: data curation, methodology, funding acquisition, project administration. Jiaming Luo: conceptualization, funding acquisition, project administration, supervision, resources. All authors approved the submitted and final versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The NHANES project has been formally approved by the National Center for Health Statistics’ research ethics review committee. NHANES has obtained written informed consent from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, J., Zhou, T., Jing, Y. et al. Association of α-klotho concentrations with cardiovascular and all-cause mortality in American adults with depression: a national prospective cohort study. Transl Psychiatry 14, 505 (2024). https://doi.org/10.1038/s41398-024-03215-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-024-03215-0