Abstract
Postpartum depression (PPD) significantly impacts women’s mental health and social functioning, yet effective therapies remain limited. This study investigates the preventive effects of music therapy on PPD-like behaviors and the underlying neurobiological mechanisms in a mouse model subjected to ovarian hormone withdrawal (HW). Mice exposed to daily music sessions exhibited markedly reduced depression- and anxiety-like behaviors, as evidenced by enhanced performance in behavioral tests such as the open field test (OFT), forced swim test (FST), elevated plus maze test (EPM), sucrose preference test (SPT), novelty-suppressed feeding (NSF) test, and tail suspension test (TST). Furthermore, music therapy normalized oxidative stress indicators (NO, MDA, SOD, CAT, GSH-Px, T-AOC, ATP, and glutamate) in the serum, hippocampus, and prefrontal cortex. Additionally, music exposure reduced levels of proinflammatory factors (IL-6, IL-1β, iNOS, TNF-α, and TGF-β) and the activation of microglia and astrocytes in these brain regions. Notably, music therapy preserved neuronal integrity, promoted neurogenesis, and maintained synaptic plasticity, evidenced by the restoration of dendritic spines. Transcriptome sequencing identified differential gene expression in pathways related to synaptic plasticity, inflammation, and oxidative stress. These findings suggest that music therapy prevents PPD by modulating oxidative stress, inflammation, and synaptic integrity, providing robust preclinical evidence for its potential as a natural preventive intervention for PPD. This study underscores the need for further clinical research to validate the therapeutic efficacy of music in preventing PPD in humans, highlighting its promise as a non-invasive and accessible treatment modality.
Similar content being viewed by others
Introduction
Global economic growth and technological advancements continue to strengthen, intensifying competition. In modern society, individuals often face significant psychological pressure due to multiple conflicting identities and roles, particularly women [1]. During pregnancy, women frequently struggle to balance family and professional roles, leading to severe psychological stress and eventual somatization, estrogen imbalance, and increased risk of postpartum depression.
PPD not only severely impacts women’s psychological and social functioning but also increases the overall societal health burden [2]. Clinical manifestations include anxiety, anhedonia, mood disturbances, and potential suicidal tendencies, which pose significant challenges for patients and their families [3]. Although the etiology is complex, numerous studies have highlighted the pivotal role of neurotrophic factors, inflammatory cytokines, and hormonal imbalances in its pathogenesis. Current treatments, including pharmacotherapy and psychotherapy, are ineffective for approximately one-third of patients [4]. Moreover, due to the blood‒brain barrier, many drugs exhibit poor therapeutic efficacy and potential adverse effects. Long-term consumption of psychological therapies further increases life burdens. Therefore, prevention of postpartum depression is crucial, necessitating the development of more effective, side effect-free prevention strategies to enhance women’s quality of life.
Fortunately, our era highly esteems art and its expressive forms. In a globalized context, art serves not only as a reflection of culture and history but also as a vital medium connecting diverse social groups and reflecting individual emotions and resonance. Throughout history, music has been recognized as a healer of physiology, emotions, and psyche [5]. In 1947, Edgar Cayce prophesied and asserted that “music is the medicine of the future,” having restored many from trance states [6]. As a language that directly expresses emotions and aesthetics, music offers noninvasiveness, affordability, and a lack of adverse reactions [7], making it widely applicable in treating psychiatric disorders, particularly as an adjunct therapy for postpartum depression. Through its unique rhythms, melodies, and tones, music aids women in enhancing self-control, reducing pain perception, promoting endorphin secretion, and achieving emotional balance and release [8, 9]. Research indicates that actively listening to music for at least 30 min daily significantly reduces stress and anxiety in pregnant women while improving sleep quality, demonstrating the pleasant, noninvasive nature of music therapy in creating a supportive environment for maternal health [10].
Despite the recognized potential of music in preventing and treating postpartum depression, its mechanism in animal models remains unclear. By employing biomedical approaches, we analyzed the effects of music on PPD mice to explore the potential mechanisms underlying the preventive role of music, paving the way for novel therapeutic strategies in the future.
Materials and methods
Animal preparation
Female BALB/c mice, aged 11 weeks and weighing 22 ± 1.5 g, were acquired from the Fang Yuanyuan Breeding Farm, located in Beijing, China. These animals were housed under controlled environmental conditions, maintaining a temperature of 23 ± 1 °C and a relative humidity of 50 ± 1%, within a regimen that included a 12-h light/dark cycle (lights on from 8 a.m. to 8 p.m.). The rats had unrestricted access to a standard diet and water ad libitum.
Modeling of postpartum depression and music listening strategies
HW models are widely used as PPD mouse models for scientific research [11]. A hormone-induced PPD model was developed in female BALB/c mice at 12 weeks of age [12]. These mice were systematically allocated into four distinct groups, each consisting of ten individuals. To establish the PPD model, the experimental group of mice first underwent bilateral ovariectomy to eliminate the endogenous source of hormones, followed by a seven-day recovery period. After the recovery phase, the mice entered the induction phase, during which they received daily intraperitoneal injections of β-estradiol (E2, 0.5 μg/50 g/day) and progesterone (P4, 0.8 mg/50 g/day) for 16 consecutive days, with E2 and P4 dissolved in a plant-based solution. On day 17, the dose of E2 was gradually increased to 10 μg/50 g/day, and P4 injection was discontinued. The E2 escalation phase lasted for 7 days. Following this phase, E2 injections were stopped, and the mice underwent a two-day hormone withdrawal period. Behavioral assessments were performed two days after cessation of E2 injections to confirm the establishment of the PPD model. During the 23-day model establishment phase, the mice listened to music prepared by us for 2 h per day, with a music volume of approximately 70 decibels. To ensure minimal disturbance during music exposure, the mice were placed in a sealed room. This process involved moving the mice, which could have some impact, so we also moved the control groups an equal distance, despite them not needing to listen to the music.
Regarding music selection, in the research conducted by the music-focused team, a carefully curated collection of 25 musical pieces was planned to facilitate auditory stimulation experiments involving murine subjects (Table S1). This collection showcases a comprehensive range of musical styles spanning various historical periods, including the Baroque, Classical, and Romantic eras. For musical genres, the collection presents a diverse array of instrumental and vocal compositions from both Eastern and Western traditions, incorporating instruments such as the piano, flute, harp, violin, guqin, and Cucurbit flute. The vocal repertoire includes operatic arias, indigenous folk songs, and contemporary popular tunes, with a particular emphasis on the rich traditional Chinese ethnic music legacy originating from diverse cultural groups, including Han, Tibetan, Mongolian, Dai, and Uighur.
The mice were arbitrarily divided into four groups, each containing ten mice, as follows:
-
(1)
SHAM: Subjects underwent sham surgery without ovariectomy and were administered 0.1 ml/day of vegetable oil intraperitoneally.
-
(2)
PPD: Bilateral ovariectomy was conducted to establish the PPD model using E2 and P4.
-
(3)
PPD + Music: This group employed the same PPD model and listened to music.
-
(4)
Music: Sham surgery group, only listened to music.
Behavioral assessments
Two days after the final E2 injection, the mice were subjected to a comprehensive set of behavioral assessments, accompanied by daily music exposure until euthanasia. These assessments were carefully performed in a calm environment to minimize potential external stressors that might influence the results. To prepare for these tests, the mice were moved to the testing area at least three hours before the test. This acclimatization period was essential for allowing the animals sufficient time to become comfortable with the new setting, ensuring that their reactions genuinely represented their natural behaviors.
Sucrose preference test
The SPT is designed to assess anhedonia by measuring a mouse’s preference for a sweet solution, reflecting their pursuit of pleasure [13]. Initially, the mice were acclimated to a 1% sucrose solution for three days to familiarize them with the taste. Then, they were given access to both tap water and the sucrose solution for 24 h to determine their baseline preferences. The actual 12-h SPT followed, during which the mice were provided with both liquids in identical bottles. The positions of these bottles were alternated every 6 h to guarantee accurate evaluation. We measured sucrose consumption to calculate sucrose preference as follows: percentage preference = [(sucrose intake/total intake) × 100]. All tests were conducted blindly to maintain the integrity of the data.
Open field test
The OFT is a widely used method to evaluate the locomotor and exploratory behaviors of mice [14]. The apparatus for this test is divided into 16 equal squares. Mice were initially placed in the center of the apparatus and allowed to acclimate for 2 min, which allowed them to adjust to the new environment and ensured that their responses were natural. Following this habituation period, key metrics such as the total distance moved, time spent in the central zone, and the frequency of entries into the center were meticulously quantified over a 3-min duration. The execution of the test by an investigator who was unaware of the treatment status of the subjects ensured the integrity and impartiality of the analysis.
Tail suspension test
The TST is an established method for detecting despair and depression-like behaviors in mice [15]. In this test, mice are suspended by their tails using adhesive tape positioned approximately 1 cm from the tip of the tail within a designated enclosure. A 1-min habituation period was provided before the start of the measurement to allow the mice to adjust to being suspended. The duration of immobility was then carefully recorded over a 5-min period. To maintain consistency in the data, durations that exceeded this time were recorded as 5 min. The procedures were carried out by an investigator who was blinded to the treatment conditions of the animals, ensuring the objectivity and reliability of the behavioral assessments.
Novelty-suppressed feeding
The NSF test was designed to assess the motivational state and response of mice to environmental novelty following a 24-h fasting period to increase their sensitivity to food cues [16]. In a 50 × 50 × 45 cm testing apparatus, a piece of white filter paper with food particles was placed at the center to encourage exploratory behavior. Mice were individually positioned at a corner grid, allowing them to freely explore the new surroundings. The latency to initiate feeding behavior was closely observed and recorded for each mouse, serving as a measure of their anxiety and motivation levels [17].
Elevated plus maze
The EPM test is a widely recognized method for evaluating anxiety-like behavior in rodents and was conducted in accordance with protocols from previous studies [18]. The apparatus consisted of two open arms (50 × 10 cm) and two enclosed arms (50 × 10 × 40 cm), which were arranged in a plus shape and elevated 50 cm above the floor. To reduce stress and allow animals to adjust to the test conditions, a 30–45 min acclimatization period in the testing room was provided before starting the experiment. Each mouse was gently placed on the central platform facing an open arm, marking the beginning of the trial. An observer, unaware of the animals’ treatment groups, accurately recorded the number of entries and the time spent in each arm over a 5-min period. After each trial, the maze was cleaned with 30% isopropanol to remove olfactory cues and ensure unbiased results for subsequent subjects.
Forced swimming test
The FST is a critical method in behavioral research, particularly for evaluating the efficacy of antidepressant treatments [19]. In this test, the mice were gently placed into cylindrical containers filled with water (40 cm in diameter and 80 cm in height) at a controlled temperature of 23 ± 1 °C. This setting forces mice to swim in an inescapable environment, tapping into their natural aversion to water. Initially, mice make vigorous attempts to escape, demonstrating their instincts to avoid such problems. However, as people recognize the futility of escape, they exhibit a state of “behavioral despair,” characterized by a significant reduction in active movements and passive floating behavior. The test lasted for 6 min, with the first minute dedicated to acclimatization and the following 5 min focused on observing and recording the duration of immobility.
Quantitative real-time polymerase chain reaction analysis
To accurately determine gene expression levels in the hippocampus and prefrontal cortex of mice, we employed quantitative real-time polymerase chain reaction (qRT‒PCR) analysis. Total RNA was extracted from these brain regions using TRIzol reagent (Invitrogen, Catalog #15596026) according to the manufacturer’s instructions [20]. The extracted RNA was then subjected to qRT‒PCR analysis using 2×SYBR Green qPCR Master Mix (catalog #Q341; Vazyme), which facilitates the precise detection and quantification of mRNA levels. A StepOnePlus Real-Time PCR System (Applied Biosystems) was used for the analysis, which offers high accuracy in measuring gene expression. The specific sequences of primers used for qPCR are detailed in Table S2, providing a clear guide to the genetic targets analyzed in this study.
Assessment of oxidative stress markers
The assessment of oxidative stress markers was conducted with precision using enzymatic colorimetric assays, in strict accordance with the manufacturer’s guidelines. Peripheral serum samples were initially collected from the retroorbital plexus of mice, yielding approximately 0.8 mL per sample. Following centrifugation at 4 °C and 4000 rpm for 20 min, 300 μL of serum was isolated from each sample. This serum preparation was critical for the accurate measurement of oxidative stress markers.
To assess oxidative stress comprehensively, both blood serum and hippocampal tissue samples were collected from each mouse. Hippocampal tissues were processed into homogenates, and the levels of key oxidative stress markers, including malondialdehyde (MDA) (No. A003-1-2), glutathione peroxidase (GSH-Px) (No. A005-1-2), catalase (CAT) (No. A007-1-1), nitric oxide (NO) (No. A012-1-2), superoxide dismutase (SOD) (No. A001-3-2), and total antioxidant capacity (T-AOC) (No. A015-2-1), were quantified using commercially available assay kits.
For the detection of oxidative stress markers, tissue homogenates were processed following the manufacturer’s protocols. The specific kits for each marker provided clear guidelines on the experimental procedure. The absorbance of each marker was measured using a spectrophotometer, and the data were processed using the formulas provided in the respective kit instructions. Detailed protocols can be found on the manufacturer’s website: http://www.njjcbio.com/.
Immunofluorescence analysis
Following behavioral assessments and euthanasia via intracardial perfusion with saline, the brains were extracted and preserved in 4% paraformaldehyde. The fixed specimens were dehydrated in sucrose solutions (20% followed by 30%) prepared in phosphate-buffered saline (PBS). Coronal brain sections of 35 μm thickness were then prepared and washed with PBS.
To reduce nonspecific binding, the sections were blocked using a solution containing 1% bovine serum albumin (BSA), 0.3% Triton X-100, and 10% goat serum in PBS for one hour at room temperature, followed by three PBS washes. These sections were then incubated with the following primary antibodies: rabbit anti-doublecortin (DCX, Cell Signaling Technology, #14082, 1:400), rabbit anti-glial fibrillary acidic protein (GFAP, Cell Signaling Technology, #14082, 1:400), mouse anti-ionized calcium-binding adapter molecule 1 (IBA-1, Cell Signaling Technology, #14082, 1:400), and rabbit anti-microtubule-associated protein 2 (MAP2, Cell Signaling Technology, #14082, 1:400). This incubation occurred overnight at 4 °C, followed by an additional incubation period of two hours at room temperature with fluorophore-conjugated secondary antibodies: goat anti-rabbit IgG (Alexa Fluor® 594 conjugate, Invitrogen, #A11008, 1:1000) and goat anti-mouse IgG (Alexa Fluor® 594 conjugate, Invitrogen, #A21422, 1:1000).
After three final washes, the sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Imaging was performed using a Leica TCS SP8 confocal microscope (Leica Microsystems, Germany), allowing for the detailed observation and documentation of labeled cellular and subcellular structures [21].
Golgi-Cox impregnation
Golgi-Cox impregnation, a time-honored histological technique, was precisely utilized to explore the complex architecture of neuronal structures and their dendritic trees within the brain [22]. This method offers unparalleled insights into neuronal morphology and synaptic connections, which are crucial for understanding the neural foundations of behavior and disease processes. After euthanasia, the brains were promptly removed and submerged in Golgi-Cox solution. This process initiated a critical 14-day impregnation phase, which was conducted in darkness to ensure comprehensive staining of neural components.
After impregnation, the brain specimens were dehydrated in a 30% sucrose solution until saturation, a marker of sufficient infiltration. Coronal sections of 100 μm were then prepared using a vibratome, facilitating intricate morphological analysis. These slices were mounted on gelatin-coated slides and left to air-dry at room temperature. After drying, the slides were rinsed in distilled water to eliminate excess potassium dichromate and silver nitrate, thereby improving the visual contrast of the stained neurons against the tissue background.
The dehydration of the mounted sections was followed by the addition of a series of increasing concentrations of alcohol, after which the sections were cleared in xylene and finally sealed with a coverslip using mounting medium. Bright-field microscopy, equipped with a high-resolution imaging system, was employed to visualize the Golgi-stained sections, which captured detailed images of neuronal and synaptic formations. The use of Golgi-Cox impregnation sheds light on the complex structural dynamics and adaptability of neuronal circuits, greatly enriching our understanding of the structural underpinnings of neural functionality and pathology.
Western blot
Approximately 20 mg of mPFC tissue and 15 mg of Hip tissue were collected. RIPA lysis buffer (Solarbio, Beijing, China, R0020) along with phosphatase and protease inhibitors (Bimake, Texas, USA, B14001, B15001) were added for tissue homogenization. Total protein concentrations were quantified using the BCA assay with a BCA kit (Beyotime, Shanghai, China, P0009). Proteins were separated using sulfate-polyacrylamide gel electrophoresis and incubated overnight with primary antibodies at 4 °C. The membrane was then incubated with a secondary antibody (1:5000, ZB-2301, Zhongshan Jin Qiao, Beijing) for 1 h at room temperature, and protein bands were detected using an enhanced chemiluminescence solution. The antibodies used for Western blotting included: inducible nitric oxide synthase (iNOS, 1:1000, Wanleibio, Shenyang, China, WL0992a), tumor necrosis factor-alpha (TNF-α, 1:1500, Wanleibio, Shenyang, China, WL01581), interleukin-1 beta (IL-1β, 1:1000, Beyotime, Shanghai, China, AF7209), and GAPDH (1:2000, Abcam, Cambridge, UK, ab8245).
Transcriptome sequencing of hippocampal and prefrontal cortex tissue
RNA isolation and qualification
To investigate how music influences the prevention of postpartum depression, we conducted transcriptome sequencing (RNA-Seq) on hippocampal and prefrontal cortex samples from mice. Our research involved three distinct groups: a sham-operated control (SHAM), a model for PPD, and a group treated with music (PPD+Music), with three biological replicates per group to guarantee data reliability and reproducibility.
RNA extraction was performed via the TRIzol method (Invitrogen, CA, USA) with subsequent purification to eliminate DNA contamination by employing RNase-free DNase I (Takara, Kusatsu, Japan). RNA quality assessment involved visualization on 1% agarose gels and determination of RNA concentration and purity using a NanoDrop spectrophotometer (Thermo Scientific, DE, USA). The integrity of the RNA samples was further confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA).
Library preparation and sequencing
For library preparation, 1.5 μg of RNA per sample was utilized with the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA). Index codes were incorporated into each sample, and mRNA was isolated from total RNA using poly-T oligo-attached magnetic beads, fragmented, and subjected to first-strand cDNA synthesis employing random hexamer primers and M-MuLV reverse transcriptase. Subsequent steps included second-strand cDNA synthesis utilizing DNA Polymerase I and RNase H, followed by purification of cDNA fragments to select for sizes ranging from 200–250 bp. PCR am plification was performed, and the libraries were sequenced on an Illumina NovaSeq 6000 platform, generating paired-end reads of 150 bp.
Data processing and analysis
The raw FASTQ data were preprocessed to remove adapter-containing, poly-N, and low-quality reads. The quality assessment included evaluating the Q20 and Q30 scores, GC content, and sequence duplication levels. Clean reads were aligned to the reference genome using STAR, focusing on reads with perfect matches or single mismatches. For SNP calling, Picard tools, SAMtools were used for BAM file processing, and GATK2 was used for SNP identification and filtering. Gene expression levels were quantified using HTSeq, with DESeq used for differential expression analysis in samples with replicates and edgeR and DEGseq used for those without replicates. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses identified affected biological processes and molecular functions. Protein‒protein interactions among DEGs were analyzed using STRING and visualized with Cytoscape to visualize molecular networks.
Statistical analysis was performed with GraphPad Prism (version 5.0). Data are presented as mean ± standard error of the mean (SEM). The normality of the data was assessed using the Shapiro-Wilk test to determine if the data followed a normal distribution. For normally distributed data, a one-way analysis of variance (ANOVA) was performed to compare differences among multiple groups, followed by Tukey’s post-hoc test for pairwise comparisons. If the data failed the normality test, non-parametric tests were applied as appropriate. The homogeneity of variances between groups was tested using the F test. Differences were considered statistically significant at p < 0.05.
Results
Music prevents depression and anxiety-like behavior in HW-treated mice
Behavioral tests are the most effective means of assessing visual responses to depression- and anxiety-like behavior in mice to verify whether music can prevent depression- and anxiety-like behaviors induced by ovarian hormone withdrawal in female mice. We established the “PPD+music” mouse model in which mice exposed to ovarian hormone withdrawal listened to music for 2 h daily to explore whether they displayed diminished postpartum depression-like behaviors.
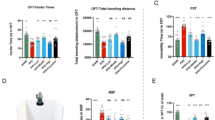
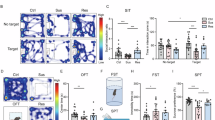
Figure 1A illustrates the experimental timeline. PPD mice treated exclusively with HW exhibited reduced exploration time in the central area during the open field test and shorter total movement distances (Fig. 1B), indicating heightened anxiety-like behavior. In contrast, PPD+Music mice exposed to both HW and music relaxation demonstrated increased activity.
A Experimental design: schematic representation of music prevention. B Music prevents the decrease in exploration central area time and total travel distance induced by PPD in the open field test. C Music prevents the increase in immobility time induced by PPD in the forced swim test. D Music inhibited the decrease in the number of open arm entries induced by PPD in the elevated plus maze test. E Music prevents the decreased consumption of sucrose solution induced by PPD in the sucrose preference test. F Music prevents the increase in preparation time to eat the food induced by PPD in the novelty-suppressed feeding test. G Music prevented the increase in immobility time induced by PPD in the TST. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 10 per group. *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. (OFT: open field test, FST: forced swim test, NSF: novelty-suppressed feeding test, EPM: elevated plus maze test, TST: tail suspension test, SPT: sucrose preference test).
In enclosed and inescapable water spaces (that is, the FST), rodents exhibit floating behavior, which is indicative of depression and anxiety-like behaviors. PPD mice subjected to HW treatment exhibited longer periods of immobility (Fig. 1C). Conversely, PPD mice that consistently listen to music attempt to escape adversity by swimming and struggling, leading to shorter periods of immobility.
The EPM test is utilized to evaluate anxiety responses in rodents. When mice are exposed to a novel environment (open arm), they exhibit curiosity and exploration, while being confined to a dark environment (closed arm) induces anxiety-like behavior. Faced with adversity, PPD mice subjected to HW tend to curl up in the dark environment of the closed arm, showing a reluctance to explore. Fortunately, mice that listened to music appeared braver and spent more time exploring the open arm (Fig. 1D).
Rodents have a strong innate desire for sweet foods and will preferentially consume sweetened solutions when given the choice between sucrose solution and plain water. In this experiment, all groups except the PPD group subjected to HW treatment showed a preference for the sucrose solution without exhibiting depressive-like behavior (Fig. 1E). Notably, mice in the PPD + Music group, which listened to the music daily, demonstrated a preference for the sugar water, suggesting that music can prevent depressive-like behavior in postpartum mice.
Animals with postpartum depression often exhibit reduced food intake and appetite. NSF experiments can be utilized to observe changes in animals devoid of euphoria. Postpartum depression mice take longer to reach food in the central area. Conversely, mice that listened to music spent less time autonomously accessing food and did not exhibit depressive-like behavior in NSF experiments (Fig. 1F).
In the tail suspension experiment, the animals were suspended with their heads facing downward. To overcome their abnormal body position, they initially struggle to escape. When they cannot break free from this situation, they intermittently cease movement, displaying “BEHAVIORAL DESPAIR”. Music stimulation protected PPD+Music mice from exhibiting despair, while PPD mice demonstrated severe behavioral despair (Fig. 1G).
Music prevents oxidative stress in the serum and brain tissues of HW-treated mice
The excessive accumulation of oxidative stress and reactive oxygen species (ROS) is a primary pathological feature of postpartum depression, leading to abnormal neuronal signal transmission and brain dysfunction [23]. To verify whether music can regulate antioxidant capacity and ROS levels, we measured the expression levels of NO, MDA, SOD, CAT, GSH-Px, T-AOC, ATP and glutamate in the serum and in two brain regions, the hippocampus and prefrontal cortex, of mice [24].
NO is a redox-active molecule with roles in oxidative and antioxidative pathways and is implicated in neurotransmitter release, neuronal development, gene expression modulation, and synaptic plasticity, suggesting its relevance in depressive disorders [25]. Elevated levels of MDA, the ultimate product of lipid peroxidation reactions, have been implicated in depression [26, 27]. The quantification of NO (Fig. 2A, H) and MDA (Fig. 2B, I) levels is frequently employed to assess the extent of oxidative stress-induced damage [28]. We assessed the expression levels of NO and MDA in the serum, hippocampus, and prefrontal cortex of the mice. The results revealed that the expression levels of SOD and CAT were abnormally elevated in PPD mice subjected to HW treatment. Interestingly, mice receiving long-term music stimulation (PPD+Music) did not show this abnormal elevation.
A–N Music prevents the abnormal expression of oxidative stress-related factors such as NO, MDA, GSH, SOD, CAT, T-AOC, ATP and glutamate in the blood, hippocampus and prefrontal cortex of mice subjected to CUMS. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group. *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. (NO: nitric oxide, MDA: malondialdehyde, GSH: glutathione peroxidase, SOD: superoxide dismutase, CAT: catalase, T-AOC: total antioxidant capacity, ATP: adenosine triphosphate).
SOD and CAT are antioxidant enzymes [29]. SOD catalyzes the conversion of superoxide anion radicals (O2–) into hydrogen peroxide and molecular oxygen, playing a crucial role in controlling intracellular ROS levels [30]. Several studies have reported a significant decrease in SOD activity in patients with depression [31]. CAT’s primary function is to decompose hydrogen peroxide into water and oxygen, thereby preventing cells from experiencing oxidative stress [32]. A decrease in CAT leads to increased oxidative stress, accelerating the progression of depression [33]. We observed a downward trend in SOD (Fig. 2C, J) and CAT (Fig. 2D, K) expression in the serum, hippocampus, and prefrontal cortex of PPD mice subjected to HW treatment. However, even after prolonged exposure to music stimulation (PPD+Music), SOD and CAT expression did not significantly decrease compared to that in the SHAM group but did significantly differ from that in the PPD group. GSH-Px is also an antioxidant in the brain that neutralizes excessive ROS and prevents oxidative damage [34, 35]. Depression has been associated with decreased levels of GSH [36]. We observed a decrease in GSH levels in the hippocampus, prefrontal cortex, and serum of PPD mice subjected to HW. However, mice receiving music intervention (PPD+Music) did not show a significant decrease in GSH expression (Fig. 2E, L). The T-AOC represents the overall antioxidant levels of various substances and enzymes and serves as a crucial indicator of oxidative stress [36]. In PPD mice, the T-AOC was significantly reduced, whereas mice in the music intervention group did not show abnormal T-AOC (Fig. 2F, M). Patients with depression typically have lower levels of ATP production in the brain [37]. This may be related to impaired hippocampal neurogenesis, which is believed to play a role in depression, as neurogenesis is a metabolically demanding process [38]. Indeed, we observed a significant decrease in ATP levels in the hippocampal and prefrontal cortex tissues of PPD mice, while no abnormalities occurred with music prevention (Fig. 2G).
Glutamate-induced excitotoxicity is linked to oxidative stress, which promotes ROS generation [39, 40]. In patients with depression, there is a significant increase in serum glutamate levels. The accumulation of ROS leads to a reduction in glutamate-mediated excitatory synaptic transmission in the brain, resulting in impaired learning and memory efficiency [41]. The experimental findings demonstrated a marked elevation in glutamate levels within the blood and cerebral tissues of the HW-treated PPD mice, which is indicative of a pronounced surge in ROS. Mice subjected to long-term music stimulation did not exhibit abnormalities (Fig. 2N).
Overall, compared to those in the SHAM group, the PPD group showed abnormalities in NO, MDA, SOD, CAT, GSH-Px, T-AOC, ATP, and glutamate levels in the serum, prefrontal cortex, and hippocampal tissue, while no abnormalities were observed in the PPD+Music group.
Music prevents elevated levels of inflammatory factors in the brain tissues of mice
Neuroinflammation and oxidative stress dysregulation are commonly present in PPD, where neuroinflammation can impair mitochondrial function, weaken antioxidant defenses, and lead to oxidative stress, thereby promoting the onset and progression of PPD. Both are considered causal factors and consequences of the disease. Therefore, we further evaluate the pathological characteristics of neuroinflammation [42, 43]. We examined the RNA expression levels of TNF-α (Fig. 3A), IL-1β (Fig. 3B), iNOS (Fig. 4A), interleukin 6 (IL-6) (Fig. 4B), interleukin 10 (IL-10) (Fig. 4C) and transforming growth factor-β (TGF-β) (Fig. 4D) in mouse cortical and hippocampal tissues. Additionally, we also measured the protein expression levels of iNOS, TNF-α, and IL-1β in the cortical and hippocampal tissues of mice(Fig. 3C). The results showed that PPD mice had abnormal levels of inflammatory factors compared to SHAM mice, whereas PPD+Music mice had largely similar levels of inflammatory factors to controls.
A–C Music intervention protects against the aberrant expression of inflammation-related factors (such as TNF-α, IL-1β, and iNOS) in the Hip and mPFC of PPD mice. D Representative fluorescence micrographs showing the morphology and density of microglia in the DG, CA1, CA2, CA3, and mPFC regions. E Morphological data analysis of microglia in the DG region (left) and analysis of microglia numbers in the DG, CA1, CA2, CA3, and mPFC regions (right). All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. (TNF-α: tumor necrosis factor-α, IL-1β: interleukin 1 beta, DAPI: 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride, IBA1: ionized calcium binding adapter molecule 1, DG: dentate gyrus, CA1: cornu ammonis 1 of the hippocampus, CA2: cornu ammonis 2 of the hippocampus, CA3: cornu ammonis 3 of the hippocampus, mPFC: medial prefrontal cortex).
A–D Music prevents the aberrant expression of inflammation-related factors such as iNOS, TGF-β, IL-6, and IL-10 in the hippocampus and prefrontal cortex of PPD mice. E Representative fluorescence micrographs showing the morphology and density of astrocytes in the DG, CA1, CA2, and CA3 regions. F Music prevented the abnormal increase in the number of astrocytes in the DG, CA1, CA2 and CA3 of PPD mice. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. iNOS: inducible nitric oxide, IL-6: interleukin 6, IL-10: interleukin 10, TGF-β: transforming growth factor-β, DAPI: 2 - (4 - amidinophenyl) – 6 - indolecarbamidine dihydrochloride, GFAP: glial fibrillary acidic protein, DG: dentate gyrus, CA1: cornu ammonis 1 of the hippocampus, CA2: cornu ammonis 2 of the hippocampus, CA3: cornu ammonis 3 of the hippocampus).
Astrocytes release inflammatory mediators during neuroinflammation, promoting the inflammatory response and influencing synaptic plasticity by modulating the synaptic environment [44]. Microglia, as immune cells in the brain, regulate neuroinflammatory processes, affecting neuronal survival and the synaptic microenvironment, thereby influencing synaptic plasticity and neural network function [45]. We examined the number of microglia and astrocytes in the hippocampus and prefrontal cortex of mice. The results revealed a significant increase in the number of microglia in the DG, CA1, CA2, CA3, and mPFC regions of PPD mice. Additionally, the size of microglial cell bodies relative to total cell area was increased, and the total length of all microglial projections was longer (Fig. 3D, E). There was also a tendency for an increase in the number of astrocytes in the DG, CA1, CA2, and CA3 regions. However, this abnormality was not observed in the PPD+Music group (Fig. 4E-F).
Music prevents neuronal death and promotes neurogenesis
PPD patients exhibit significant hippocampal neuronal loss and degeneration [46]. To investigate whether music is associated with neuronal death, we immunofluorescently labeled MAP2 to quantify neurons. Results showed a marked reduction in neuron count in the DG and mPFC of PPD mice, whereas PPD+Music mice showed no significant decrease (Fig. 5A-C). Additionally, Golgi staining of the hippocampus revealed a notable decrease in dendritic spine density in the DG of PPD mice, which was absent in PPD+Music mice (Fig. 5D, F). Bax, a pro-apoptotic protein, showed elevated mRNA levels in the cortex and hippocampus of PPD mice, alongside decreased Bcl-2 mRNA levels (Fig. 5H, I), findings absent in PPD+Music mice, suggesting that music may inhibit hippocampal neuronal apoptosis. Immunofluorescence results also indicated reduced DCX expression in the DG of PPD mice compared to controls, while PPD+Music mice showed no significant difference in DCX expression compared to controls (Fig. 5E, G).
A–C Music prevents the loss of MAP2 cells induced by CUMS in the DG and mPFC. D, F Music prevents the reduction of synapse number. E, G Music intervention prevents the reduction of DCX-positive newborn neurons in the DG region. H, I Music prevents the abnormal expression of apoptosis-related factors such as Bax and Bcl-2 in the hippocampus and prefrontal cortex after CUMS. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group. *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. (DAPI: 2 - (4 - Amidinophenyl) – 6 - indolecarbamidine dihydrochloride, DCX: doublecortin, MAP2: microtubule association protein-2, Bax: B-cell lymphoma-2 associated X protein, Bcl-2: B-cell lymphoma-2).
Music prevents synaptic plasticity damage
To investigate how music influences gene regulation, we conducted transcriptome sequencing of the hippocampus and prefrontal cortex of three groups of mice, SHAM, PPD, and PPD+Music, with three mice per group. Differential gene expression analysis revealed that in the hippocampus, compared to the SHAM group, the PPD group had 1095 DEGs, with 780 upregulated and 204 downregulated genes. In contrast, the PPD+Music group exhibited 523 differentially expressed genes compared to the PPD group, with 406 upregulated and 117 downregulated genes (Fig. 6A). Further analysis of these DEGs revealed that the PPD and PPD+Music groups shared 50 genes with the SHAM and PPD groups, as depicted in a Venn diagram (Fig. 6B). Cluster analysis of these genes was subsequently conducted to explore their potential functions (Fig. 6C). GO analysis indicated that these genes may be involved in biological processes such as “cell communication,” “signal transduction,” “regulation of cell communication,” “positive regulation of multicellular organismal process,” “cell‒cell signaling,” “positive regulation of developmental process,” “positive regulation of neuron differentiation,” “G protein-coupled receptor signaling pathway,” and “positive regulation of angiogenesis” (Fig. 6D). KEGG analysis suggested that these processes and molecular functions could involve pathways such as the “P53 signaling pathway,” “ECM-receptor interaction,” “Long-term potentiation,” “Apelin signaling pathway,” “cAMP signaling pathway,” “Inflammatory mediator regulation of TRP channels,” “Wnt signaling pathway,” “Neuroactive ligand‒receptor interaction,” and “Rap1 signaling pathway” (Fig. 6E). Four DEGs identified via transcriptome sequencing—Wnt6 (Fig. 6F), Sele (Fig. 6G), Sfrp5 (Fig. 6H), and Nupr1 (Fig. 6I)—were validated via PCR and found to be consistent with the transcriptome sequencing results.
A Volcano plot of differentially expressed genes. B Venn diagram of differentially expressed genes. C Heatmaps of the cluster analysis data. D GO analysis. E KEGG analysis. F–I Expression levels of Wnt6, Sele, Sfrp5 and Nupr1 mRNA in the Hip. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group. All significant differences were compared with those in the PPD group. *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. (Hip: hippocampus, GO: Gene Ontology, KEGG: Kyoto Encyclopedia of Genes and Genomes).
In the prefrontal cortex, transcriptome sequencing revealed 430 genes that were differentially expressed between the PPD group and the SHAM group, with 217 upregulated and 213 downregulated genes. Conversely, the PPD+Music group exhibited 3733 DEGs compared to the PPD group, with 1462 upregulated and 2271 downregulated genes (Fig. 7A). Analysis of these DEGs revealed that the PPD and PPD+Music groups shared 186 genes with the SHAM and PPD groups, as illustrated in a Venn diagram (Fig. 7B). Similarly, cluster analysis of these genes was conducted (Fig. 7C). KEGG analysis indicated that the affected pathways might involve “long-term depression,” “cholinergic synapse,” “oxytocin signaling pathway,” “glutamatergic synapse,” “dopaminergic synapse,” “cGMP-PKG signaling pathway,” “calcium signaling pathway,” “long-term potentiation,” “axon guidance,” “HIF-1 signaling pathway,” and “Ras signaling pathway” (Fig. 7E). PCR validation of two DEGs, Eya4 (Fig. 7F) and Lhx8 (Fig. 7G), revealed abnormal expression in the PPD group, which was consistent with the transcriptome sequencing results.
A Volcano plot of differentially expressed genes. B Venn diagram of differentially expressed genes. C Heatmaps of the cluster analysis data. D GO analysis. E KEGG analysis. F, G Expression levels of Eya4 and Lhx8 mRNA in the mPFC. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group. All significant differences were compared with those in the PPD group. *(#,^)P < 0.05; **(##,^^)P < 0.01; ***(###,^^^)P < 0.001. (mPFC: medial prefrontal cortex, GO: Gene Ontology, KEGG: Kyoto Encyclopedia of Genes and Genomes).
Wnt6, Sele, Sfrp5, Nupr1, Eya4, and Lhx8 are significantly differentially expressed genes in our RNA sequencing data, and these genes are closely related to the known pathogenic mechanisms of postpartum depression (PPD), including inflammation, oxidative stress, and synaptic plasticity. Specifically, Wnt6 is involved in neurodevelopment and synaptic plasticity, and it has been shown to play a role in neuroinflammation [47]; Sele (E-selectin) is an important molecule involved in the adhesion of immune cells to endothelial cells, and it plays a significant role in neuroinflammation [48]; Sfrp5 is an inhibitor of the Wnt signaling pathway, which participates in synaptic plasticity and neuronal repair [49]; Nupr1 is a stress-response gene that provides protective effects against oxidative stress and neuroinflammation [50]; Eya4 plays an important role in synaptic repair, neurodevelopment, and synaptic plasticity [51]; Lhx8 is a transcription factor that regulates neurodevelopment and synaptic function, particularly in neuroplasticity and inflammation [52]. PCR verification confirmed that their differential expression is consistent with the RNA sequencing results.
Discussion
In recent years, research on music therapy has expanded significantly. Previous animal studies have demonstrated that music can alleviate pain and improve social behaviors under various pathological conditions [14,15,16, 18]. Zhou et al. reported that exposure to music at approximately 50 dB can increase the pain threshold in mice with injured hind limbs [53]. Additionally, music-assisted therapy has been reported to enhance the efficacy of antiepileptic drugs in various temporal lobe epilepsy models [14]. Interestingly, Semyachkina et al. showed that music can induce the opening of the blood‒brain barrier in mice, suggesting its ability to modulate interactions between the brain and peripheral systems [54]. Moreover, our previous research indicated that music prevented depressive and anxiety-like behaviors in CUMS mice induced by stress [32]. Postpartum depression is a severe psychiatric disorder that significantly affects women’s psychological and social functioning, yet there is limited research on its neural mechanisms at the animal level. Here, we provide in-depth insights through mouse experiments into how music can prevent postpartum depression-like behaviors caused by hormone imbalances. Music achieves this by safeguarding and enhancing synaptic plasticity, as well as regulating oxidative stress and inflammation levels to protect neurons from damage and maintain normal homeostasis. While mice communicate using ultrasonic frequencies, music falls within both mouse and human ranges (below 20 kHz). Furthermore, despite certain sounds and vocalizations inducing aversion in rodents, a study by Yan et al. demonstrated that mice do not exhibit aversion or fear responses to different types of music, whether fast-paced, slow-paced, or heavy metal [21]. Hence, these findings contribute to the translation of these preclinical research outcomes into human applications.
The use of music therapy for the treatment and prevention of postpartum depression has gained recognition [55, 56]. Researchers have found positive indications of reduced trait anxiety among pregnant women listening to music during nonstress tests [9, 57]. A meta-analysis involving 392 primiparous women revealed that music application correlated with alleviated pain and anxiety compared to controls [58]. Another study highlighted significant reductions in anxiety indices and increased β-endorphin levels following music interventions during pregnancy [59]. Obstetric examinations indicated significantly lower levels of adrenaline, noradrenaline, and angiotensin II in the music observation group, contributing to greater emotional stability [9]. Recommending therapeutic music as an effective method for alleviating trait anxiety during pregnancy is thus suggested. Music therapy for preterm infants in neonatal intensive care units (NICUs) has proven effective for physiological and psychological outcomes, including sucking behaviors, behavior regulation, stress reduction, neurodevelopment, and the fostering of emotional bonds [60]. However, the potential of music for preventing postpartum depression has not been extensively researched. Here, we established a mouse model (HW mice) exposed daily to 1.5 h of music to prevent postpartum depression, simulating how mothers might prevent postpartum depression by listening to music, followed by behavioral testing after 28 days. In behavioral experiments assessing depression-like behaviors, including the OFT, NSF, EPM, TST and SPT, mice stimulated with and exposed to music (PPD+Music) did not exhibit depression-like behaviors. This finding suggested that music can prevent the development of depression-like behaviors in mice and may enhance their activity levels.
Hormonal imbalances lead to chronic inflammation and oxidative stress disorders that impair synaptic plasticity, contributing to postpartum depression [53, 61]. Haowen Wang et al. reported that high-decibel noise affects ROS abnormalities in chickens by disrupting oxidative stress indicators such as SOD, CAT, and GSH-Px [62]. Our experimental results demonstrated that melodic music stimuli protect mouse brain tissue and blood ROS from abnormalities. This maintains oxidative stress indicators such as SOD, CAT, GSH-Px, NO, MDA, T-AOC, ATP, and glutamate. Transcriptome sequencing of the mouse hippocampus revealed that differentially expressed genes, such as NUPR1, are associated with the regulation of ROS; moreover, knockdown of NUPR1 reduced mitophagy induced by fascaplysin, promoting ROS production [50]. This finding is consistent with our results, which were further validated by PCR, suggesting that music protects mice from oxidative stress disorders caused by hormonal imbalances. Estrogen disruption not only disrupts oxidative stress but also elevates inflammatory factor levels. Kari Johnson et al. reported that music reduces postoperative patient inflammation [63, 64]. Similarly, our animal-level study revealed that music prevents increased levels of the proinflammatory factors IL-6, IL-1β, iNOS, TNF-α, and TGF-β in the hippocampus and prefrontal cortex of PPD mice. Astrocytes regulate synaptic occurrence, while microglia function as brain immune cells, reshaping synapses [65]. Moreover, the activation/quantity of microglia and astrocytes indirectly reflects the level of inflammation in the brain [66]. The experimental results demonstrated that music prevents abnormal expression of microglia and astrocytes in the hippocampus and prefrontal cortex of mice in the PPD group. Elevated inflammation leads to neuronal death, as observed in the hippocampus and prefrontal cortex of PPD mice. Importantly, studies have shown that music promotes neuroplasticity and neurodevelopment [67,68,69], protecting neurons from death and promoting the growth of regenerative neurons in the DG of mice. These results indicate that music effectively prevents chronic inflammation caused by hormonal imbalances, oxidative stress disorders, and abnormal neuronal death.
Wenjie Zhou et al. reported that music alleviates pain related to Glu in the auditory cortex (ACx) and Glu synaptic transmission in the medial geniculate body (MGB) [53]. Transcriptome sequencing of mouse hippocampal tissue revealed that music may exert its effects through various biological pathways related to synaptic plasticity, such as cholinergic synapses, glutamatergic synapses, and dopaminergic synapses, similar to findings by Wenjie Zhou et al. Moreover, Schmitz et al. reported that long-term enhancement of glutamatergic synapses is reduced in Shisa7 KO mice [70] and that Shisa7 phosphorylation regulates GABAergic transmission and neuronal developmental behaviors [71]. Similarly, our transcriptome sequencing revealed that the expression of differentially expressed genes, such as Shisa7, was significantly reduced in the prefrontal cortex of PPD mice, yet music preserved Shisa7 expression. Sebastian R et al. reported that NRXN1 loss in multipotent stem cells disrupts selective splicing and synaptic signaling pathway disorders, impairing synaptic plasticity [72]. Similarly, NRXN1 was differentially expressed; Pdlim4 plays a critical role in dendritic cell (DC) migration, dendritic formation, and subsequent functional T-cell responses mediated by CCR7 (C-C chemokine receptor type 7)-JNK. Under Pdlim4 knockout conditions, mature DC dendritic formation significantly decreased [73]. Correspondingly, music protects against abnormal reductions in Pdlim4 in the prefrontal cortex of PPD mice. In conclusion, music may prevent PPD by safeguarding/enhancing synaptic plasticity, thereby preventing disruption caused by hormonal imbalances and increasing oxidative stress and inflammation levels, ultimately protecting neurons from damage and maintaining homeostasis. Therefore, we continue to demonstrate the effectiveness of music in preventing postpartum depression in animal studies, providing critical evidence for clinical research. In the future, music should be further explored as a more natural method for preventing postpartum depression.
Although we provide robust preclinical evidence for the use of music in preventing postpartum depression, this study has several limitations. The first limitation is that PPD mice only simulate human postpartum depression patients, and experimental results may not translate directly to pregnant mice. The experiment utilized bilateral ovariectomy plus hormone regulation to establish a postpartum depression model, but postpartum depression can also occur during pregnancy [74]. Experiments with pregnant mice are also needed, but ethical considerations prevent the killing of many pregnant mice for strong experimental results. This restriction requires future research for verification. Second, the mice were nocturnal. Ideally, mice listen to music at night to simulate relaxation from music during the day. However, it is well known that most researchers conduct experiments on rodents during the day, mainly due to the inconvenience of conducting experiments at night and the need for researchers to rest. PPD establishment and music intervention experiments require long daily completion, making it very difficult for researchers to conduct experiments at night. Nevertheless, under appropriate control conditions, this is unlikely to affect the soundness of the conclusions of this study.
In summary, the neurogenic mechanism of music in preventing postpartum depression may be that music prevents synaptic plasticity from being destroyed by protecting it, protects against the increase in oxidative stress and inflammation levels caused by estrogen imbalances, and ultimately protects neurons from damage and maintains stability. Therefore, we continue to demonstrate the effectiveness of music in preventing postpartum depression in animal studies, providing critical evidence for clinical research. In the future, music should be further explored as a more natural method for preventing postpartum depression.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Sakurai T. Social processes and social environment during development. Semin Cell Dev Biol. 2022;129:40–46. https://doi.org/10.1016/j.semcdb.2021.09.016
Oyetunji A, Chandra P. Postpartum stress and infant outcome: a review of current literature. Psychiatry Res. 2020;284:112769. https://doi.org/10.1016/j.psychres.2020.112769
Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165–80. https://doi.org/10.1016/j.yfrne.2018.12.001
Stewart DE, Vigod SN. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu Rev Med. 2019;70:183–96. https://doi.org/10.1146/annurev-med-041217-011106
Fukui H, Toyoshima K. Music facilitate the neurogenesis, regeneration and repair of neurons. Med Hypotheses. 2008;71:765–9. https://doi.org/10.1016/j.mehy.2008.06.019
Yinger OS, Gooding L. Music therapy and music medicine for children and adolescents. Child Adolesc Psychiatr Clin N Am. 2014;23:535–53. https://doi.org/10.1016/j.chc.2013.03.003
Tang Q, Zhou Y, Yang S, Thomas WKS, Smith GD, Yang Z, et al. Effect of music intervention on apathy in nursing home residents with dementia. Geriatr Nurs. 2018;39:471–6. https://doi.org/10.1016/j.gerinurse.2018.02.003
Allen KA. Music therapy in the NICU: is there evidence to support integration for procedural support? Adv Neonatal Care. 2013;13:349–52. https://doi.org/10.1097/ANC.0b013e3182a0278b
Ji C, Zhao J, Nie Q, Wang S. The role and outcomes of music therapy during pregnancy: a systematic review of randomized controlled trials. J Psychosom Obstet Gynaecol. 2024;45:2291635. https://doi.org/10.1080/0167482x.2023.2291635
Liu YH, Lee CS, Yu CH, Chen CH. Effects of music listening on stress, anxiety, and sleep quality for sleep-disturbed pregnant women. Women Health. 2016;56:296–311. https://doi.org/10.1080/03630242.2015.1088116
Luo F, Liu L, Guo M, Liang J, Chen L, Shi X, et al. Deciphering and targeting the ESR2–miR-10a-5p–BDNF axis in the prefrontal cortex: advancing postpartum depression understanding and therapeutics. Research. 2024;7:0537. https://doi.org/10.34133/research.0537
Levin G, Ein-Dor T. A unified model of the biology of peripartum depression. Transl Psychiatry. 2023;13:138. https://doi.org/10.1038/s41398-023-02439-w
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–98. https://doi.org/10.1038/s41596-018-0011-z
Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–60. https://doi.org/10.1016/s0149-7634(01)00011-2
Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. https://doi.org/10.1016/j.neubiorev.2005.03.009
Ramaker MJ, Dulawa SC. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry. 2017;22:656–65. https://doi.org/10.1038/mp.2017.36
Liu S, Chen L, Guo M, Li Y, Liu Q, Cheng Y. Targeted delivery of engineered RVG-BDNF-exosomes: a novel neurobiological approach for ameliorating depression and regulating neurogenesis. Research. 2024;7:0402. https://doi.org/10.34133/research.0402
Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. https://doi.org/10.1016/j.neubiorev.2005.04.017
Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–86. https://doi.org/10.1016/s0149-7634(98)00029-3
Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010;2010:pdb.prot5439. https://doi.org/10.1101/pdb.prot5439
Im K, Mareninov S, Diaz MFP, Yong WH. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299–311. https://doi.org/10.1007/978-1-4939-8935-5_26
Boros BD, Greathouse KM, Gentry EG, Curtis KA, Birchall EL, Gearing M, et al. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann Neurol. 2017;82:602–14. https://doi.org/10.1002/ana.25049
Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. https://doi.org/10.1016/s0165-0327(00)00199-3
Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–52. https://doi.org/10.1016/j.arcmed.2006.10.005
Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, et al. Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev. 2014;45:46–62. https://doi.org/10.1016/j.neubiorev.2014.05.007
Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A. Increased plasma levels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res. 2008;161:59–66. https://doi.org/10.1016/j.psychres.2007.07.019
Islam MR, Islam MR, Ahmed I, Moktadir AA, Nahar Z, Islam MS, et al. Elevated serum levels of malondialdehyde and cortisol are associated with major depressive disorder: a case-control study. SAGE Open Med. 2018;6:2050312118773953. https://doi.org/10.1177/2050312118773953
Hashimoto M, Fujimoto M, Konno K, Lee ML, Yamada Y, Yamashita K, et al. Ubiquitin-specific protease 2 in the ventromedial hypothalamus modifies blood glucose levels by controlling sympathetic nervous activation. J Neurosci. 2022;42:4607–18. https://doi.org/10.1523/jneurosci.2504-21.2022
Kwon K, Jung J, Sahu A, Tae G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J Control Rel. 2022;344:160–72. https://doi.org/10.1016/j.jconrel.2022.02.033
Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19:41. https://doi.org/10.1186/s12974-022-02400-6
Shi ZM, Jing JJ, Xue ZJ, Chen WJ, Tang YB, Chen DJ, et al. Stellate ganglion block ameliorated central post-stroke pain with comorbid anxiety and depression through inhibiting HIF-1α/NLRP3 signaling following thalamic hemorrhagic stroke. J Neuroinflammation. 2023;20:82. https://doi.org/10.1186/s12974-023-02765-2
Fu Q, Qiu R, Chen L, Chen Y, Qi W, Cheng Y. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl Psychiatry. 2023;13:317. https://doi.org/10.1038/s41398-023-02606-z
Yao C, Zhang Y, Sun X, Pei H, Wei S, Wang M, et al. Areca catechu L. ameliorates chronic unpredictable mild stress-induced depression behavior in rats by the promotion of the BDNF signaling pathway. Biomed Pharmacother. 2023;164:114459. https://doi.org/10.1016/j.biopha.2023.114459
Mohammed AM, Khardali IA, Oraiby ME, Hakami AF, Shaheen ES, Ageel IM, et al. Anxiety, depression-like behaviors and biochemistry disorders induced by cannabis extract in female mice. Saudi J Biol Sci. 2021;28:6097–111. https://doi.org/10.1016/j.sjbs.2021.08.085
Murrough JW, Huryk KM, Mao X, Iacoviello B, Collins K, Nierenberg AA, et al. A pilot study of minocycline for the treatment of bipolar depression: effects on cortical glutathione and oxidative stress in vivo. J Affect Disord. 2018;230:56–64. https://doi.org/10.1016/j.jad.2017.12.067
Steenkamp LR, Hough CM, Reus VI, Jain FA, Epel ES, James SJ, et al. Severity of anxiety- but not depression- is associated with oxidative stress in major depressive disorder. J Affect Disord. 2017;219:193–200. https://doi.org/10.1016/j.jad.2017.04.042
Moretti A, Gorini A, Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–85. https://doi.org/10.1038/sj.mp.4001353
Caruncho HJ, Brymer K, Romay-Tallón R, Mitchell MA, Rivera-Baltanás T, Botterill J, et al. Reelin-related disturbances in depression: implications for translational studies. Front Cell Neurosci. 2016;10:48. https://doi.org/10.3389/fncel.2016.00048
Xie D, Xiong K, Su X, Wang G, Ji Q, Zou Q, et al. Identification of an endogenous glutamatergic transmitter system controlling excitability and conductivity of atrial cardiomyocytes. Cell Res. 2021;31:951–64. https://doi.org/10.1038/s41422-021-00499-5
Gu Y, Huang P, Cheng T, Yang J, Wu G, Sun Y, et al. A multiomics and network pharmacological study reveals the neuroprotective efficacy of Fu-Fang-Dan-Zhi tablets against glutamate-induced oxidative cell death. Comput Biol Med. 2022;148:105873. https://doi.org/10.1016/j.compbiomed.2022.105873
Li Y, Cui R, Liu S, Qin Z, Sun W, Cheng Y, et al. The efficacy and safety of post-stroke cognitive impairment therapies: an umbrella review. Front Pharmacol. 2023;14:1207075. https://doi.org/10.3389/fphar.2023.1207075
Simpson DSA, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 2020;9:743. https://doi.org/10.3390/antiox9080743
Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA, et al. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. 2009;115:287–92. https://doi.org/10.1016/j.jad.2008.07.008
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Zusso M, Lunardi V, Franceschini D, Pagetta A, Lo R, Stifani S, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory respon se via TLR4/NF-kB pathway. J Neuroinflammation. 2019;16:148. https://doi.org/10.1186/s12974-019-1538-9
Berger T, Lee H, Young AH, Aarsland D, Thuret S. Adult hippocampal neurogenesis in major depressive disorder and Alzheimer’s disease. Trends Mol Med. 2020;26:803–18. https://doi.org/10.1016/j.molmed.2020.03.010
Wei M, Zhang C, Tian Y, Du X, Wang Q, Zhao H. Expression and function of WNT6: from development to disease. Front Cell Dev Biol. 2020;8:558155. https://doi.org/10.3389/fcell.2020.558155
Rodrigues RM, He Y, Hwang S, Bertola A, Mackowiak B, Ahmed YA, et al. E-selectin-dependent inflammation and lipolysis in adipose tissue exacerbate steatosis-to-NASH progression via S100A8/9. Cell Mol Gastroenterol Hepatol. 2022;13:151–71. https://doi.org/10.1016/j.jcmgh.2021.08.002
Carstensen M, Herder C, Kempf K, Erlund I, Martin S, Koenig W, et al. Sfrp5 correlates with insulin resistance and oxidative stress. Eur J Clin Invest. 2013;43:350–7. https://doi.org/10.1111/eci.12052
Zhan Y, Zhang Z, Liu Y, Fang Y, Xie Y, Zheng Y, et al. NUPR1 contributes to radiation resistance by maintaining ROS homeostasis via AhR/CYP signal axis in hepatocellular carcinoma. BMC Med. 2022;20:365. https://doi.org/10.1186/s12916-022-02554-3
de la Peña Avalos B, Tropée R, Duijf PHG, Dray E. EYA4 promotes breast cancer progression and metastasis through its role in replication stress avoidance. Mol Cancer. 2023;22:158. https://doi.org/10.1186/s12943-023-01861-4
Wang Z, Liu CY, Zhao Y, Dean J. FIGLA, LHX8 and SOHLH1 transcription factor networks regulate mouse oocyte growth and differentiation. Nucleic Acids Res. 2020;48:3525–41. https://doi.org/10.1093/nar/gkaa101
Zhou W, Ye C, Wang H, Mao Y, Zhang W, Liu A, et al. Sound induces analgesia through corticothalamic circuits. Science. 2022;377:198–204. https://doi.org/10.1126/science.abn4663
Semyachkina-Glushkovskaya O, Esmat A, Bragin D, Bragina O, Shirokov AA, Navolokin N, et al. Phenomenon of music-induced opening of the blood-brain barrier in heal thy mice. Proc Biol Sci. 2020;287:20202337. https://doi.org/10.1098/rspb.2020.2337
Ji C, Li J, Nie Q, Wang S. Effect of music therapy on anxiety in full-term pregnant women. Front Psychiatry. 2024;15:1429999. https://doi.org/10.3389/fpsyt.2024.1429999
Cordoba-Silva J, Maya R, Valderrama M, Giraldo LF, Betancourt-Zapata W, Salgado-Vasco A, et al. Music therapy with adult burn patients in the intensive care unit: short-term analysis of electrophysiological signals during music-assisted relaxation. Sci Rep. 2024;14:23592. https://doi.org/10.1038/s41598-024-73211-3
Garcia-Gonzalez J, Ventura-Miranda MI, Requena-Mullor M, Parron-Carreño T, Alarcon-Rodriguez R. State-trait anxiety levels during pregnancy and foetal parameters following intervention with music therapy. J Affect Disord. 2018;232:17–22. https://doi.org/10.1016/j.jad.2018.02.008
Chuang CH, Chen PC, Lee CS, Chen CH, Tu YK, Wu SC. Music intervention for pain and anxiety management of the primiparous women during labour: a systematic review and meta-analysis. J Adv Nurs. 2019;75:723–33. https://doi.org/10.1111/jan.13871
Salafas E, Lestari P, Listiyaningsih M. The effectiveness of music therapy in reducing anxiety in third trimes ter of pregnancy. Siklus. 2020;9:39–44. https://doi.org/10.30591/siklus.v9i1.1634
Jaschke AC, Mitra S, Bos AF. Music therapy in tertiary neonatal intensive care: a matter of unlikely allies? Acta paediatrica. 2024;113:1772–7. https://doi.org/10.1111/apa.17297
Hung PL, Wu KLH, Chen CJ, Siu KK, Hsin YJ, Wang LJ, et al. Music-based intervention ameliorates Mecp2-loss-mediated sociability repression in mice through the prefrontal cortex FNDC5/BDNF pathway. Int J Mol Sci. 2021;22:7174. https://doi.org/10.3390/ijms22137174
Wang H, Chai Y, Xu Y, Wang Y, Li J, Zhang R, et al. Long-term music stimulating alleviated the inflammatory responses caused by acute noise stress on the immune organs of broilers by NF-κB signaling pathway. Ecotoxicol Env Saf. 2024;273:116131. https://doi.org/10.1016/j.ecoenv.2024.116131
Johnson K, Fleury J, McClain D. Music intervention to prevent delirium among older patients admitted to a trauma intensive care unit and a trauma orthopaedic unit. Intensive Crit Care Nurs. 2018;47:7–14. https://doi.org/10.1016/j.iccn.2018.03.007
Khan SH, Kitsis M, Golovyan D, Wang S, Chlan LL, Boustani M, et al. Effects of music intervention on inflammatory markers in critically ill and post-operative patients: a systematic review of the literature. Heart Lung. 2018;47:489–96. https://doi.org/10.1016/j.hrtlng.2018.05.015
Keeler JL, Kan C, Treasure J, Himmerich H. Novel treatments for anorexia nervosa: Insights from neuroplasticity research. Eur Eat Disord Rev. 2023;32:1069–84. https://doi.org/10.1002/erv.3039
Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. https://doi.org/10.1186/s40035-020-00221-2
Chorna O, Filippa M, De Almeida JS, Lordier L, Monaci MG, Hüppi P, et al. Neuroprocessing mechanisms of music during fetal and neonatal development: a role in neuroplasticity and neurodevelopment. Neural Plast. 2019;2019:3972918. https://doi.org/10.1155/2019/3972918
Schlaug G. Musicians and music making as a model for the study of brain plasticity. Prog Brain Res. 2015;217:37–55. https://doi.org/10.1016/bs.pbr.2014.11.020
James CE, Altenmüller E, Kliegel M, Krüger THC, Van De Ville D, Worschech F, et al. Train the brain with music (TBM): brain plasticity and cognitive benefits induced by musical training in elderly people in Germany and Switzerland, a study protocol for an RCT comparing musical instrumental practice to sensitization to music. BMC Geriatr. 2020;20:418. https://doi.org/10.1186/s12877-020-01761-y
Schmitz LJM, Klaassen RV, Ruiperez-Alonso M, Zamri AE, Stroeder J, Rao-Ruiz P, et al. The AMPA receptor-associated protein Shisa7 regulates hippocampal synaptic function and contextual memory. Elife. 2017;6:e24192. https://doi.org/10.7554/eLife.24192
Wu K, Shepard RD, Castellano D, Han W, Tian Q, Dong L, et al. Shisa7 phosphorylation regulates GABAergic transmission and neurodevelopmental behaviors. Neuropsychopharmacology. 2022;47:2160–70. https://doi.org/10.1038/s41386-022-01334-0
Sebastian R, Jin K, Pavon N, Bansal R, Potter A, Song Y, et al. Schizophrenia-associated NRXN1 deletions induce developmental-timing- and cell-type-specific vulnerabilities in human brain organoids. Nat Commun. 2023;14:3770. https://doi.org/10.1038/s41467-023-39420-6
Yoo JY, Jung NC, Lee JH, Choi SY, Choi HJ, Park SY, et al. Pdlim4 is essential for CCR7-JNK-mediated dendritic cell migration and F-actin-related dendrite formation. FASEB J. 2019;33:11035–44. https://doi.org/10.1096/fj.201901031
Nayyer MA, Khan SM, Umer M, Imran H, Khalid S, Murtaza H, et al. Efficacy and safety of peri-partum Esketamine for prevention of post-p artum depression in women undergoing caesarian section: a meta-analysi s and systematic review of randomized controlled trials. Asian J Psychiatr. 2024;97:104090. https://doi.org/10.1016/j.ajp.2024.104090
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82471560) and the Guangdong Provincial Fund for Basic and Applied Basic Research (2025A1515012427).
Author information
Authors and Affiliations
Contributions
YC conceived the study; WQ and YWC designed the study; QF, RQ, TY, LL, YL and XL performed the experiments; All the authors analyzed and interpreted the data; QF drafted the manuscript with critical revisions from YC, WQ and YWC.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments within this investigation adhered strongly to the guidelines outlined by the National Institutes of Health for the Care and Use of Laboratory Animals (NIH Publication No. 80-23), receiving full endorsement from the Animal Care and Use Committee at Minzu University of China.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, Q., Qiu, R., Yao, T. et al. Music therapy as a preventive intervention for postpartum depression: modulation of synaptic plasticity, oxidative stress, and inflammation in a mouse model. Transl Psychiatry 15, 143 (2025). https://doi.org/10.1038/s41398-025-03370-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03370-y