Abstract
Cognitive dysfunction is a prominent feature of psychotic spectrum disorders. Identifying neurocognitive subgroups and their neural underpinnings may help elucidate distinct pathophysiological mechanisms and inform targeted interventions. This study aimed to derive cognitive subtypes using latent profile analysis (LPA) of the Brief Assessment of Cognition in Schizophrenia (BACS) and investigate associated variations in resting-state functional connectivity among these cognitive profiles and biologically derived Biotypes. The BACS was administered to 1807 psychosis patients from the B-SNIP1 and 2 cohorts to perform LPA and identify cognitive subgroups. Regional homogeneity (ReHo), a measure of local functional connectivity, was computed from resting-state fMRI data in a subset (717 patients, 427 controls). Multivariate regression models examined associations between ReHo and cognitive LPA, Biotypes, and DSM diagnostic categories. LPA identified four cognitive profiles: cognitively comparable to controls (CCC), intermediate-1, intermediate-2, and severely impaired. These profiles showed unique dysconnectivity patterns, particularly within the striatal, default mode, salience, and executive control networks. The severely impaired group exhibited hyperconnectivity in basal ganglia and executive control networks. The intermediate groups showed default mode and salience network connectivity disruptions. The CCC group was the least impaired, with hyperconnectivity in sensory and auditory networks. Compared to Biotypes, LPA subgroups presented more domain-specific connectivity fingerprints. Psychosis patients exhibit heterogeneous cognitive profiles with divergent intrinsic functional dysconnectivity patterns. Cognitive LPA subgroups demonstrated more domain-localized neural signatures than DSM subtypes, potentially allowing for more targeted interventions. This approach highlights the utility of cognitive subtyping using standardized cognitive assessments in elucidating pathophysiological mechanisms in psychosis.
Similar content being viewed by others
Introduction
Psychosis presents a multifaceted clinical landscape characterized by diverse symptomatology and trajectories. Recent research efforts, including those from our group, have aimed to clarify this complexity using various subtyping approaches. Our group pioneered the Biotype classification system, which identifies neurobiologically distinct psychosis subgroups using multimodal biomarkers (e.g., EEG, eye tracking, structural MRI) that transcend traditional diagnostic boundaries. These Biotypes show differential patterns of cognitive deficits, neuroanatomical alterations, and functional outcomes, providing a framework for parsing biological heterogeneity in psychosis [1, 2]. Alongside traditional diagnostic classifications, which rely on symptomatology and disease course, newer methods have focused on subtyping based on biology and genetics [2, 3].
Cognitive-based subtyping is a data-driven approach that classifies individuals into distinct subgroups based on latent cognitive patterns, rather than classifying individuals based on traditional diagnostic criteria. This method has the potential to identify unique disease processes potentially related to distinct mechanisms that may have been overlooked by a priori hypotheses. Cognitive anomalies are an established hallmark of psychosis with considerable variability in cognitive ability existing in patients with psychotic spectrum disorders extending from severe to minimal dysfunction [4,5,6,7]. It is unclear whether these identifiable subgroups are characterized by unique neurocognitive profiles and possibly different disease mechanisms [4, 8].
Prior studies exploring cognitive heterogeneity in schizophrenia spectrum disorders using cluster analytic techniques consistently identify three to five cognitive subgroups regardless of amount and type of neuropsychological assessment. Findings typically indicate two extreme clusters, one displaying relatively intact cognitive functioning similar to healthy controls and another characterized by severe impairment [9,10,11]. Intermediate subgroups are often reported, characterized by moderate impairments differentiated by deficient psychomotor and executive function [9, 10]. However, additional studies using larger samples and more statistically objective approaches to subgroup determination are needed to clarify cognitive heterogeneity patterns in psychosis.
Cluster analytic techniques may be limited by the inability to compare different models to determine which model fits the data best. In contrast to the subjective a priori model selection in cluster analysis used in most prior studies, applying data-driven mixture model methodologies such as latent profile analysis (LPA) can provide more objective observations of potential factors impacting disorder characterization. LPA compares multiple model structures to quantitatively determine the best model fit [12]. Overall, such mixture model approaches are less vulnerable both to bias in identifying latent subgroups (i.e., over-extraction) and to bias in identifying latent subgroups that characterize a more representative profile solution [13], making them more generalizable.
Moreover, till date, very few studies have investigated the association between cognition and neurobiology to better understand disease mechanisms impacting heterogeneity. An emerging pattern of biological variability echoes the heterogeneity pattern seen among cognitive subgroups. Biologically focused batteries have detected three distinct subgroups. For example, in a sample of patients with psychotic spectrum disorders, electroencephalogram, eye tracking, and neuropsychological testing indicated distinct subgroups differing in terms of gray matter volume [1] cutting across clinical groups. Similarly, cluster analytic findings indicated three distinct groups (i.e., “morphologically impoverished”, “intermediate”, and “intact”) in schizophrenia patients based on structural MRI data. The “morphologically impoverished” group displayed increased cortical thinning compared to the other groups. This group was also characterized by more severe cognitive dysfunction and more positive symptoms [14].
Investigating functional neural correlates of such subtypes can help validate and refine cognitive subtyping approaches. In the current study, we chose resting state based regional homogeneity (ReHo) to capture local neural synchrony and provide insights into the functional organization of the brain in a robust, simple and standardized manner [15, 16]. This measure is widely utilized in psychiatric research due to its sensitivity to alterations in regional brain function associated with various psychiatric disorders [17,18,19]. While prior studies both from our group and others have examined neural underpinnings of cognition in psychosis [20] by leveraging ReHo as a measure of local neural synchrony, the current study aimed to uncover neural correlates of data driven cognitive subtypes in psychosis, to better understand the pathophysiological mechanisms underlying the disorder.
The primary objectives of the study were to: a) derive cognitive subtypes of psychosis based on the Brief Assessment of Cognition in Schizophrenia (BACS) using LPA, b) investigate the neural connectivity patterns among cognitive subtypes and c) compare such patterns among previously established biological subtypes (Biotypes) from the BSNIP group along with more traditional DSM diagnoses. We hypothesized that cognitive subtypes would exhibit distinct patterns of cognitive dysfunction, reflecting variations in underlying neural circuitry. Compared to previously established Biotypes, we expected to see both shared and divergent neural correlates among their respective subtypes. Further we hypothesized that biologically derived subgroups, being more homogeneous, would be more sensitive in uncovering compromised brain connectivity compared to symptom-based criteria.
Materials and methods
Participants
The study included N = 1807 idiopathic psychosis patients (schizophrenia, schizoaffective disorder, bipolar disorder with psychotic features) for the primary LPA. The male/female ratio was approximately 50% across patient groups. With mean age across groups ranging from 34.2–38.9 yrs. Subjects were ascertained from both BSNIP-1 and 2 cohorts, with procedures standardized across sites [1, 2]. Resting state functional magnetic resonance imaging (rs-fMRI) data were analyzed from a subset of 717 Psychosis Patients and 427 Healthy Controls, to probe underlying neural correlates of the corresponding cognitive, Biotype and DSM categorized subgroups. Biotype characteristics and subtyping are detailed elsewhere [1, 2]. Detailed demographics for the study samples are provided in Table 1a, b.
Psychiatric diagnoses were confirmed using the Structured Clinical Interview for DSM-IV-TR (SCID-IV). Exclusion criteria for all participants included serious medical or neurological illness, mental retardation (IQ < 65 on the Wide Range Achievement Test [21]), current substance abuse or dependence, and extensive history of drug dependence. Exclusion criteria for HC were a lifetime history of psychosis or recurrent mood syndromes. In addition, since psychotic illnesses are highly familial, and traits such as cognition and electrophysiological parameters are transmitted within families [22, 23], we decided to exclude first and second degree relatives of such individuals from the study’s healthy control subjects. Family history status was determined using the Family History of Psychosis screening form [24].
Cognitive assessment
The Brief Assessment of Cognition in Schizophrenia (BACS; [25]) was administered to all participants to assess cognitive functioning. The BACS evaluated cognitive abilities including verbal memory, working memory, motor speed, verbal fluency, attention, and executive functioning. Each BACS subtest, z-scored, stratified by age and sex, was used as an indicator variable in the LPA. Although much shorter and easier to administer, the BACS has shown to carry comparable reliability and validity to much larger and detailed batteries such as the MATRICS Consensus Cognitive Battery (MCCB), with strong correlations (r = 0.73–0.9) indicating they assess similar cognitive structures [26]. The BACS was used due to its efficiency in administration while still capturing key neurocognitive domains relevant for assessing general cognitive functioning in psychosis spectrum disorders [27].
Latent profile analysis
LPA is a data-driven mixture model approach that compares four different variance-covariance structures to identify the best-fitting model for the data [12]. The different variance-covariance structures could impact model selection. LPA computes simultaneous fit statistics within each set of assumptions for variance-covariance structures. Comparing the different constrained structures ensures that the optimal model is chosen quantitatively and objectively. To determine the optimal model and number of latent profiles, a one-profile model was initially estimated for each of the four different variance-covariance model structures. Subsequently, the number of profiles in each of the four model structures were increased by one until profiles were no longer identifiable (e.g., profiles with less than 5% of the sample and low levels of maximum log likelihood that indicated the solution did not replicate adequately). Issues related to model convergence were handled with random starts [12].
Within each set of model specification, several fit statistics were utilized to select the best-fitting model. The Bayesian Information Criterion (BIC) and Akaike Information Criterion (ACI) were prioritized, as lower values indicate improved model fit, provided that additional classes continue to reduce these values [12]. Likelihood ratio tests, including the Vuong-Lo-Mendell-Rubin (VLMR) likelihood, adjusted Lo-Mendell-Rubin (LMR; [28]), and bootstrapped likelihood ratio test (BLRT; [29]) were also applied to determine the improvement of fit between k and k-1 classes. The approximate Bayes factor (BF) was used to assess the relative strengths of the models [12]. For final model selection, the approximate correct model probability (cmPk) was used to choose the best fitting model (i.e. candidate model) within each set of model specifications. The cmPk provides a composite measure that integrates information from individual fit statistics. Unlike the individual fit statistics, which each capture unique aspects of model quality, cmPk aggregates multiple selection criteria into a single probability-based index, offering a more parsimonious evaluation of model adequacy within each model specification. To ensure robustness, the cmPk was computed first within each profile and then recalculated to determine the best model (cmP) across all profiles using the best, most parsimonious model structure [12].
Once the optimal model was identified, the resulting latent profiles were interpreted in terms of their characteristic patterns of BACS subtest scores. Descriptive statistics and graphical representations were used to characterize each latent profile and elucidate the cognitive profiles associated with different psychosis subgroups.
Imaging analysis
Resting state fMRI processing
A subset of subjects (427 Healthy Controls, 717 Psychosis Patients) underwent a 3D T1-weighted structural scan and a single resting state functional magnetic resonance imaging (rs-fMRI) scan on a 3 T scanner. Participants were instructed to keep their eyes open, focus on a crosshair displayed on a monitor, and to remain still during the entire scan. Head motion was minimized with a custom-built head-coil cushion. Scanning protocols across sites and studies are noted in Supplementary Table 1.
Data preprocessing was performed using the advanced DPARSF module within DPABI V6.0_210501 [30]. For each site separately, all volume slices were corrected for different signal acquisition times. Then, the time series of images for each subject were realigned. Individual structural images (T1-weighted MPRAGE) were co-registered to the mean functional image after realignment. The transformed structural images were then segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). Given that motion can be an issue with connectivity analyses, it was mitigated at different levels. Initially, as validated in prior studies, all subjects with movement exceeding 1 voxel in either of the 6° of freedom were excluded [31]. Following this, if subjects had greater than 30% of time points exceeding framewise displacement (FD) score of 0.5 [32], they were discarded. The Friston 24-parameter model [33] was then utilized to regress out head motion effects from the realigned data. To better control for physiological noise and motion, a combined WM/CSF mask was generated from which the top 5 principal components were extracted and signal adjusted using the aCompCor method [34]. Finally, FD was used in the statistical model to account for any residual motion. A recent study evaluating various denoising pipelines found that a combination of the Friston 24 model and aCompCor, similar to the approach used in the current study, ranked among the most effective methods for reducing motion artifacts in resting-state data [35]. In addition, linear trends were adjusted since the BOLD signal exhibits low-frequency drifts. A fast diffeomorphic registration algorithm [36, 37] was used to transform the functional data from individual native space to MNI space. Finally, temporal filtering (0.01–0.08 Hz) was performed on the time series [31, 35].
Regional homogeneity (ReHo) computation and harmonization
The preprocessed data mentioned above were utilized for the computation of Kendall’s coefficient of concordance (KCC), following the method outlined by Kendall and Gibbons 1990 (“Rank Correlation Methods: Kendall, the late Sir Maurice, Gibbons, Jean Dickinson: 9780195208375). This coefficient was employed to assess the Regional Homogeneity (ReHo) or the similarity of the time series of a specific voxel with its closest 26 neighboring voxels on a voxel-wise basis. Subsequently, a Z-standardized KCC map was generated for each participant. To minimize noise and account for residual anatomical differences, the resulting images underwent smoothing using a Gaussian kernel with a full width at half maximum (FWHM) of 6 mm.
An established ICA based functional resting state atlas [38] was used to extract ReHo estimates from the above preprocessed maps across 90 regional (ROI) nodes encompassing the whole brain derived from Shirer et al. [38]. We chose the Shirer atlas as it met three primary requirements in the context of the current study a) it was a data driven functional parcellation based on neurocognition (as opposed to prioritizing structural landmarks), b) provided whole brain coverage and c) has been validated in psychosis populations before. These 90 ROIs spanning 14 large-scale resting networks are shown in Supplementary Fig. 1. Regional estimates derived from the above were harmonized using NeuroCOMBAT. NeuroCOMBAT was selected for its ability to model site/scanner effects while conserving biological variance in non-Gaussian data. Unlike alternatives, it preserves diagnosis-related variability by explicitly adjusting for age/sex/group during harmonization, as validated in large neuroimaging consortia [39]. Batch effects across all 11 variants in site and scanner across both BSNIP cohorts were taken into account during this process, while simultaneously conserving variance related to biological variates of interest e.g. age, sex and group.
Statistical analyses
A multivariate general linear model (GLM) was employed to examine the associations between harmonized ReHo estimates and levels of LPA, BioT and DSM in separate analyses, adjusted for age, sex and framewise displacement. Omnibus F-scores were used to interpret the associative value with corresponding p-values subjected to a false discovery rate (FDR) correction. A FDR correction was chosen to effectively control false positive results while avoiding excessive stringency that could mask true findings. Regions surviving an alpha corrected level of 0.05 were statistically prioritized for further post-hoc testing for differences across LPA/BioT/DSM subgroups. Between-group post-hoc significance was further adjusted for multiple comparisons using Tukey’s method. Statistical analyses were performed in SPSS v18 (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.). The complete data processing pipeline is shown in Fig. 1.
Supplementary analysis
To assess real world utility, we explored associations of LPA subgroups with the Birchwood social functioning scale (SFS) [40]. Supplementary analyses assessed medication effects via: (1) chlorpromazine (CPZ) equivalents using the Andreasen 2010 standardized conversion method [41], (2) anticholinergic burden scores [42], and (3) binary on/off status for antipsychotics, antidepressants, mood stabilizers, lithium, and anticholinergics. Bivariate Pearlson correlations (adjusted for age, sex and mean FD) tested associations between medication variables and ReHo estimates for items 1 and 2. Simple independent t-tests were performed to assess associations between ReHo and binary medication statuses. Further, to assess if differences in clinical variables had any impact on ReHo metrics, we conducted partial Pearson correlations (adjusted for same variables as indicated above) with number of hospitalizations and PANSS scores within each LPA subgroup. All p-values surviving an FDR adjusted p < 0.05 were deemed significant.
Results
Latent profile analysis
The four-class, class-varying, diagonal model emerged as the optimal model based on the correct model probability metric. The modal class assignment proportions were close to the estimated class proportions, the average posterior probabilities for group membership were above 0.70, and the entropy value was above 0.80 (Supplementary Table 2). These indicators suggested that the four latent profiles were well-separated and that individuals were assigned to the appropriate profile with adequate accuracy [43]. Supplementary Table 3 presents the fit statistics for each model tested.
The four profiles differed both on cognitive performance level and performance consistency across subtests (Fig. 2). The mean subtest scores for the first profile were above the mean except for Token Motor test (mean = −0.66). Thus, Profile 1 (n = 257) was labeled “cognitively comparable to controls (CCC)” as overall cognitive performance was within the average range. The second profile was approximately 1 standard deviation below the mean on most tests except for higher order language and problem solving (i.e., verbal fluency and Tower of London). Therefore, profile 2 (n = 774) was labeled as “intermediate 1′′. Next, average subtest scores for those assigned to Profile 3 were approximately 1.5–2 standard deviations below the mean; profile 3 was labeled “intermediate 2′′. Finally, profile 4 (n = 143) scored 2–3 standard deviations below the mean on all subtests, and the level of performance was well into the impaired range. Thus, profile 4 was labeled as “severe impairment” (Fig. 1). To further validate the cognitive subgroups, control comparisons (n = 427) were included post-hoc to examine profile assignment probability. Using inverse logits of BACS subtest scores, control participants were probabilistically assigned to one of the cognitive profiles. Of note, control comparisions were not included as part of the main LPA analysis. Most controls were probabilistically assigned to the CCC controls (n = 234, 55%) and intermediate profiles (n = 150, 35%), with a less than 10% of participants probabilistically assigned to the intermediate 2 (n = 40, 9%) and severe impairment (n = 3, <1%) groups resembling a normal distribution. In addition, supplementary analyses revealed a significant association of the above cognitive subgroups with the employment (p < 0.001) and total scores (p < 0.001) assessed using the Birchwood SFS [40].
Neural correlates of psychosis subtypes
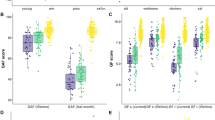
A total of 22 regional nodes spanning seven functional network domains, differentiated cognitive latent profile analysis (LPA) subgroups. Subsequent post-hoc examination delineated both distinct and shared connectivity patterns across groups. Notably, relative to controls, the CCC group exhibited the least disrupted connectivity, characterized by reduced sensorimotor and auditory connectivity, particularly in left superior temporal gyrus (L STG). Both Intermediate 1 and 2 groups displayed diminished auditory connectivity across bilateral STG and sensorimotor cortices (bilateral pre-post central gyrus). The Intermediate 1 cohort showcased elevated default mode connectivity coupled with diminished primary and higher order visual connectivity, contrasting with the Intermediate 2 group’s significant reductions in connectivity within various nodes of the anterior and posterior salience network. The severe group shared diminished sensorimotor and visuospatial connectivity with the intermediate groups, it however uniquely exhibited increased connectivity, particularly in the right executive control network and bilateral basal ganglia (thalamus-caudate). In addition, multiple nodes also showed significant group differences between cognitive based patient subtypes (See Fig. 3). In particular, differential connectivity in the anterior/posterior salience, executive control, dorsal DMN and higher order visual networks distinguished the two intermediate subtypes.
Line colors represent the four cognitive subgroups derived from the class-varying diagonal model. Depicted scores are Z-standardized and adjusted for age and sex. Significant between-proband differences in ReHo connectivity across A Cognitive B Biotype and C DSM subgroups. Curly brackets indicate significant effects at the p < 0.05 level, Sidak adjusted for multiple comparisons. Error bars refer to standard error of mean (SEM).
The Biotype analysis revealed connectivity abnormalities across 19 nodes spanning six resting-state networks. Compared to healthy controls all Biotype subgroups showed consistently, diminished connectivity in bilateral auditory (superior temporal gyri) and sensorimotor (precentral and postcentral gyri) networks. Both anterior and posterior salience nodes showed reduced connectivity in B2. In addition, B1 and B3 both demonstrated deficits transcending functional domains of executive control, visual, visuospatial. Interestingly, default mode nodal dysconnectivity was only observed in B3. In terms of between-patient differences, B1 and B3 showed differential connectivity across multiple DMN and ECN nodes. The B2 group differed in salience and visuospatial network connectivity compared to other patient groups (Fig. 3).
Similar analyses across DSM subtypes showed six functional nodes with compromised connectivity compared to controls. These included bilateral auditory, sensorimotor, R anterior salience and Visuospatial (R Middle temporal gyrus). Only one node (sensorimotor) showed differential connectivity between patient groups (SAD versus SZ). A detailed summary of post-hoc findings relative to controls is presented in Table 2.
Secondary analysis on regional homogeneity across the above nodes of interest revealed no associations with medication status, except for the primary visual (calcarine sulcus) node that associated with use of mood stabilizers (p < 0.002 FDR corrected). We also found no significant association between ReHo signal and clinical variables such as number of hospitalizations and PANSS (positive, negative, general and total) scores. In addition, after harmonization, we found no significant effect of site/protocol (p < 0.05 FDR corrected) on ReHo signal in the above nodes of interest.
Discussion
Cognitive impairment within psychosis is heterogeneous, and even within schizophrenia the existence of cognitively normal subgroups has long been recognized [6, 8] along with the observation that cognitive and structural brain status within psychosis may be allied [4, 8]. Similarly genetic liability for schizophrenia has also been associated with cognitive impairment [44, 45].Toulopoulou and colleagues estimate that around a third of the genetic risk of developing schizophrenia is mediated by influences on cognition. In the current study we tried to use cognition as a primary measure to tease apart the overlapping heterogeneity existing in psychoses and to better map them onto their underlying biological constructs.
Our findings from an LPA of BACS cognitive performance indicated four distinct psychosis subgroups in the current sample: a severely impaired, two intermediate, and one CCC subgroup. LPA fit statistics confirmed that the above groups were distinct and adequately separated, instead of merely being clustered on a continuum of cognitive ability. Overall, present findings corroborate previous reports that cognitive heterogeneity is characteristic of patients with psychosis. Consistent with our findings, a recent systematic review study found that 80% of studies that used mixed diagnostic groups found a four-cluster solution [7]. Previous studies report distinct profiles characterized by a range of cognitive function from global dysfunction to more selective deficits in executive functioning, attention, memory, and processing speed [46, 47]. Importantly and consistently, previous reports define a subset of patients with intact cognition [4, 46,47,48]. In schizophrenia, heterogeneity is often attributed to etiological and biological variability, suggesting the presence of subgroups with divergent pathophysiological mechanisms [5, 45].
The observed differences across the four cognitive subtypes in our current study, particularly in racial/ethnic variance, age, education, estimated premorbid ability, social functioning, and illness course/severity highlight the complex interplay between cognitive functioning, social determinants of health, and clinical course in psychotic spectrum disorders. Therefore, it is important to consider whether the cognitive subgroups derived from LPA represent distinct subtypes of psychotic spectrum disorders or instead, reflect the impact of chronic illness progression with co-occurring social disadvantages (please see Supplementary Methods for a more detailed discussion).
Neural correlates of cognitive subtypes
Importantly, our identified cognitive profiles showed differential functional connectivity patterns, suggesting a neurobiological basis for cognitive heterogeneity in psychosis. The CCC group demonstrated the least disrupted connectivity compared to controls, with notable reductions primarily in sensorimotor and auditory regions. This finding maybe consistent with the concept of cognitive resilience in psychosis, where some individuals maintain relatively preserved cognitive function and neural connectivity [4, 49]. In the severe impairment group, characterized by significantly lower global mean BACS Z scores, the unique caudate/thalamic dysconnectivity suggests specific disruptions in striatal and fronto-striatal (executive control) pathways, potentially contributing to the severity of cognitive deficits in these individuals. Recent clinical studies indicate that dopaminergic function changes in schizophrenia are associated with alterations in the associative striatum (caudate and ventral striatum) which is heavily involved in cognitive and decision-making tasks [50, 51].
The intermediate groups demonstrated complex, differentiated connectivity alteration patterns, revealing nuanced neural correlates of cognitive dysfunction. The Intermediate 1 subgroup, characterized by moderate cognitive performance deficits, exhibited elevated DMN connectivity coupled with reduced visual network connectivity, suggesting a neurocognitive shift favoring internally focused processes (e.g., self-referential thought) over external sensory integration. This aligns with fMRI studies linking DMN hyperconnectivity to introspective bias and attenuated visual-sensory engagement in psychosis [52, 53]. While potentially compensatory for maintaining executive function [54], this imbalance may underlie Intermediate-1’s suboptimal real-world functioning despite somewhat preserved test scores. Notably, while abnormal DMN connectivity has been a consistent finding in psychosis research [52, 55] and possibly a strong predictor of treatment response in schizophrenia [54], our study uniquely demonstrates that this alteration might be primarily applicable to a subset of psychosis individuals. In contrast, the Intermediate 2 cohort, with slightly lower cognitive performance than Intermediate 1, displayed reduced salience network connectivity indicating potential alterations in attentional and salience processing networks. This may influence cognitive flexibility and responses to environmental stimuli in this group. The salience network’s role as a dynamic switch between internally- and externally-oriented networks provides a mechanism for our Intermediate-2 group’s attention/executive deficits. Reduced salience network connectivity likely impairs rapid disengagement from default-mode processing during novel task demands, consistent with fMRI studies linking anterior insula-anterior cingulate hypoconnectivity to perseveration errors and delayed task-switching [56, 57]. Clinically, this suggests salience network-targeted interventions (e.g., dACC-rTMS or metacognitive strategy training could enhance cognitive flexibility in this subgroup [57]. The distinct connectivity patterns observed in the anterior/posterior salience, executive control, dorsal DMN, and higher-order visual networks between these two intermediate subtypes underscore that even within broadly similar cognitive profiles, there may be distinct underlying neural mechanisms. These results support the notion of a continuum of neural dysfunction in psychosis, corresponding to varying degrees of cognitive impairment [58]. Furthermore, the shared reduction in sensorimotor connectivity across all groups, irrespective of cognitive profile, highlights a common neural alteration that transcends specific cognitive profiles within a system frequently implicated across the psychosis spectrum [53].
Neural correlates of biotypes
The Biotype analysis offered a complementary perspective to the cognitive profile analysis. Approximately 60% of the nodes that validated Biotype differences overlapped with those differentiating cognitive subtypes, underscoring shared neural alterations across cognitive and Biotype categorizations. This overlap is not entirely unexpected, given that the BACS was a primary measure used in clustering the original Biotypes.
Reduced auditory and sensorimotor connectivity across all Biotypes suggests these may be core psychosis features, independent of specific symptom profiles. This finding is consistent with literature suggesting sensory integration deficits across the psychosis spectrum, not limited to a specific subtype (e.g. schizophrenia) [59]. The differential connectivity patterns observed in default mode, executive control, and salience networks across Biotypes align with previous studies highlighting the importance of these networks in psychosis pathophysiology [60, 61]. However, a closer examination of between-group effects reveals a more distinct picture between Biotypes and psychosis cognitive subtypes. In general, Biotype connectivity anomalies were more diffuse and transcended functional domains, in contrast to LPA results, which showed a more domain-specific pattern of subgroup-related disrupted connectivity. For instance, both B1 and B3 demonstrated widespread deficits transcending functional domains of executive control, visual, and visuospatial networks, while B2 showed distinctive reductions in anterior and posterior salience node connectivity (Table 2b).
The divergent patterns observed among these two approaches highlight the multifaceted nature of neurobiological heterogeneity in psychosis. While Biotypes capture broader biological disruptions, cognitive subtyping using standardized neuropsychological assessments like the BACS may offer a more refined delineation of neural correlates specific to cognitive dysfunction that appear to be functionally bounded.
DSM subtypes connectivity
Our analysis of DSM subtypes revealed fewer differentiating features compared to the LPA and Biotype approaches. The isolated SAD-SZ difference in sensorimotor connectivity may relate to SAD’s intermediate position on a psychosis continuum, sharing thalamocortical filtering deficits with SZ but lacking the pronounced cortical thinning seen in chronic schizophrenia [14]. This limited differentiation suggests that traditional DSM boundaries lack neurobiological specificity beyond sensorimotor integration differences, which may reflect residual diagnostic distinctions in sensory processing, further supporting the growing consensus that traditional diagnostic categories may not optimally capture the neurobiological heterogeneity of psychosis [1, 2].
Implications
These findings have important implications for understanding and treating psychosis. The cognitive subtypes are not merely variations in severity, as evidenced by their distinctly associated ReHo profiles, thus highlighting the neurobiological validity of a cognitive subtyping approach to psychosis. This suggests that cognitive profiles may be a useful target for both research and clinical intervention, via focused pharmacological or neurostimulation interventions to modulate specific circuits. For e.g. targeted rTMS in intermediate 1 group to rebalance DMN connectivity and reduce self referential thought processes [52]. Similarly, theta-burst stimulation (TBS) therapy to normalize salience network activity in intermediate 2 group might be a natural consideration [58]. In addition, the intermediate subgroups may also benefit from supplemental cognitive remediation strategies tailored to their specific domain deficits, (e.g. attention training for salience network dysfunction in the intermediate 2 group) [62]. The identification of shared connectivity abnormalities across psychosis subtypes (e.g., in auditory and sensorimotor regions) points to potential common therapeutic targets, regardless of specific symptom or cognitive profiles. This aligns with prior research highlighting sensory processing abnormalities as a fundamental aspect of psychosis pathophysiology predicting disruptions in global cognitive processing [53, 59]. Furthermore, cognitive subtyping via the BACS is a straightforward, standardized, clinically accessible approach, facilitating broader dissemination and translation compared to more extensive biomarker-based assessments required for B-SNIP Biotyping.
Our results seem to indicate that B-SNIP Biotypes capture broader neurobiological dysfunction that span across multiple subtypes and may therefore be more informative in guiding biomarker research. On the other hand, cognitive subtypes highlight more nuanced domain-specific circuits (e.g., DMN v Salience) and might be more readily used in scenarios where targeted interventions might be applicable (as mentioned above).
Finally, the absence of site/protocol effects post-harmonization underscores NeuroCOMBAT’s efficacy in removing technical confounds while preserving biological signals. While supplementary analyses of mood stabilizer use showed a marginal association with primary visual connectivity (r = 0.18), given the small effects it is likely reflective of medication-mediated neuroplasticity rather than a primary disease mechanism. Future studies should explore whether visual network stabilization represents a treatment target for psychotic patients with comorbid mood dysregulation.
While the study’s strengths lie in its large sample size, data-driven approach to parse cognitive heterogeneity through a straightforward, easy to administer assessment battery, we only focused on a single cognitive test battery. The BACS was designed as a brief measure of global cognition rather than a comprehensive assessment of specific cognitive domains. While the BACS effectively captures broad cognitive abilities [63] its brevity means it lacks the depth to assess multiple exemplars within cognitive and functional domains associated with deficits in psychosis spectrum disorders. Cognitive dysfunction in psychosis likely involves complex interactions across multiple neural systems, possibly not captured here. Future research could integrate complementary neuroimaging measures, e.g. structural, diffusion, or task-based functional MRI, to capture more comprehensively the neurobiology of cognitive heterogeneity. Finally, longitudinal studies can elucidate the temporal stability of these cognitive subtypes, as well as their predictive utility for clinical outcomes and treatment response.
Conclusions
This study provides evidence for the existence of distinct neurocognitive subgroups within the psychosis spectrum, characterized by unique patterns of intrinsic functional dysconnectivity. The integration of data-driven cognitive subtyping and biologically informed approaches offers a useful framework for parsing the complex neurobiological heterogeneity underlying cognitive dysfunction in these disorders. By elucidating the neural correlates of cognitive subtypes, this work suggests a path to future development of targeted, personalized interventions and improved understanding of the pathophysiological mechanisms contributing to the diverse cognitive manifestations of psychotic disorders.
Data availability
Original data will be made available upon appropriate request
References
Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84.
Clementz BA, Parker DA, Trotti RL, McDowell JE, Keedy SK, Keshavan MS, et al. Psychosis biotypes: replication and validation from the B-SNIP consortium. Schizophr Bull. 2022;48:56–68.
Bergen SE. Parsing psychosis subtypes through investigations of rare genetic variants. EBioMedicine. 2016;6:16–7.
Woodward ND, Heckers S. Brain structure in neuropsychologically defined subgroups of schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2015;41:1349–59.
Heinrichs RW, Pinnock F, Muharib E, Hartman L, Goldberg J, McDermid Vaz S. Neurocognitive normality in schizophrenia revisited. Schizophr Res Cogn. 2015;2:227–32.
Wexler BE, Zhu H, Bell MD, Nicholls SS, Fulbright RK, Gore JC, et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry. 2009;166:189–95.
Green M, Girshkin L, Kremerskothen K, Watkeys O, Quidé Y. A systematic review of studies reporting data-driven cognitive subtypes across the psychosis spectrum. Neuropsychol Rev. 2020; 30. https://doi.org/10.1007/s11065-019-09422-7.
Lee M, Cernvall M, Borg J, Plavén-Sigray P, Larsson C, Erhardt S, et al. Cognitive function and variability in antipsychotic drug-naive patients with first-episode psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:468–76.
Gilbert E, Mérette C, Jomphe V, Émond C, Rouleau N, Bouchard R-H, et al. Cluster analysis of cognitive deficits may mark heterogeneity in schizophrenia in terms of outcome and response to treatment. Eur Arch Psychiatry Clin Neurosci. 2014;264:333–43.
Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J Clin Exp Neuropsychol. 2002;24:765–80.
Lewandowski KE, Sperry SH, Cohen BM, Öngür D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med. 2014;44:3239–48.
Spurk D, Hirschi A, Wang M, Valero D, Kauffeld S. Latent profile analysis: a review and “how to” guide of its application within vocational behavior research. J Vocat Behav. 2020;120:103445.
Woo SE, Jebb AT, Tay L, Parrigon S. Putting the “person” in the center: review and synthesis of person-centered approaches and methods in organizational science. Organ Res Methods. 2018;21:814–45.
Pan Y, Pu W, Chen X, Huang X, Cai Y, Tao H, et al. Morphological profiling of schizophrenia: cluster analysis of MRI-based cortical thickness data. Schizophr Bull. 2020;46:623–32.
Jiang L, Zuo X-N. Regional homogeneity. Neuroscientist. 2016;22:486–505.
Zuo X-N, Xu T, Jiang L, Yang Z, Cao X-Y, He Y, et al. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65:374–86.
Cattarinussi G, Di Camillo F, Grimaldi DA, Sambataro F. Diagnostic value of regional homogeneity and fractional amplitude of low-frequency fluctuations in the classification of schizophrenia and bipolar disorders. Eur Arch Psychiatry Clin Neurosci. 2025;275:799–812.
Cai M, Wang R, Liu M, Du X, Xue K, Ji Y, et al. Disrupted local functional connectivity in schizophrenia: An updated and extended meta-analysis. Schizophrenia. 2022;8:1–9.
Pastrnak M, Simkova E, Novak T. Insula activity in resting-state differentiates bipolar from unipolar depression: a systematic review and meta-analysis. Sci Rep. 2021;11:16930.
Ji L, Meda SA, Tamminga CA, Clementz BA, Keshavan MS, Sweeney JA, et al. Characterizing functional regional homogeneity (ReHo) as a B-SNIP psychosis biomarker using traditional and machine learning approaches. Schizophr Res. 2020;215:430–8.
Jastak S. WRAT-R: wide range achievement test. 1984. https://search.library.wisc.edu/catalog/999634037702121. Accessed 4 Sep 2024.
Glahn DC, Paus T, Thompson PM. Imaging genomics: mapping the influence of genetics on brain structure and function. Hum Brain Mapp. 2007;28:461–3.
Calafato MS, Bramon E. The interplay between genetics, cognition and schizophrenia. Brain. 2019;142:236–8.
Ellman LM, Schiffman J, Mittal VA. Community psychosis risk screening: an instrument development investigation. J Psychiatr Brain Sci. 2020;5:e200019.
Keefe RSE, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, et al. Norms and standardization of the brief assessment of cognition in schizophrenia (BACS). Schizophr Res. 2008;102:108–15.
Kaneda Y, Ohmori T, Okahisa Y, Sumiyoshi T, Pu S, Ueoka Y, et al. Measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery: validation of the japanese version. Psychiatry Clin Neurosci. 2013;67:182–8.
Fett A-KJ, Viechtbauer W, Dominguez M-G, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–88.
Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–78.
McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023;28:1902–18.
Yan C-G, Wang X-D, Zuo X-N, Zang Y-F. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14:339–51.
Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014;84: https://doi.org/10.1016/j.neuroimage.2013.08.048.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54.
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101.
Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–36.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113.
Kendall MG, Gibbons JD. Rank correlation methods. Correlation (Statistics), 5th ed., (E. Arnold: London; Oxford University Press: New York, NY; 1990) pp. 238–55.
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65.
Fortin J-P, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20.
Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The social functioning scale the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1991;157:853–9.
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62.
Eum S, Hill SK, Rubin LH, Carnahan RM, Reilly JL, Ivleva EI, et al. Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr Res. 2017;190:129–35.
Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. J Classif. 1996;13:195–212.
Toulopoulou T, Picchioni M, Rijsdijk F, Hua-Hall M, Ettinger U, Sham P, et al. Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch Gen Psychiatry. 2007;64:1348–55.
Toulopoulou T, Zhang X, Cherny S, Dickinson D, Berman KF, Straub RE, et al. Polygenic risk score increases schizophrenia liability through cognition-relevant pathways. Brain. 2019;142:471–85.
Smucny J, Barch DM, Gold JM, Strauss ME, MacDonald AW, Boudewyn MA, et al. Cross-diagnostic analysis of cognitive control in mental illness: insights from the CNTRACS consortium. Schizophr Res. 2019;208:377–83.
Lim K, Smucny J, Barch DM, Lam M, Keefe RSE, Lee J. Cognitive subtyping in schizophrenia: a latent profile analysis. Schizophr Bull. 2021;47:712–21.
Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychol Rev. 2009;19:294–311.
Vargas T, Damme KSF, Ered A, Capizzi R, Frosch I, Ellman LM, et al. Neuroimaging markers of resiliency in clinical high risk (CHR) for psychosis youth: a qualitative review. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:166–77.
Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III-the final common pathway. Schizophr Bull. 2009;35:549–62.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84.
Ramsay IS, Schallmo M-P, Biagianti B, Fisher M, Vinogradov S, Sponheim SR. Deficits in auditory and visual sensory discrimination reflect a genetic liability for psychosis and predict disruptions in global cognitive functioning. Front Psychiatry 2020; 11. https://doi.org/10.3389/fpsyt.2020.00638.
Mehta UM, Ibrahim FA, Sharma MS, Venkatasubramanian G, Thirthalli J, Bharath RD, et al. Resting-state functional connectivity predictors of treatment response in schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;237:153–65.
Meda SA, Ruaño G, Windemuth A, O’Neil K, Berwise C, Dunn SM, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci USA. 2014;111:E2066–2075.
Kupis LB, Uddin LQ. Developmental neuroimaging of cognitive flexibility: update and future directions. Ann Rev Dev Psychol. 2023;5:263–84.
Tomiyama H, Nakao T, Murayama K, Nemoto K, Ikari K, Yamada S, et al. Dysfunction between dorsal caudate and salience network associated with impaired cognitive flexibility in obsessive-compulsive disorder: a resting-state fMRI study. Neuroimage Clin. 2019:24:102004.
Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, et al. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry. 2017;74:605–13.
Carter O, Bennett D, Nash T, Arnold S, Brown L, Cai RY, et al. Sensory integration deficits support a dimensional view of psychosis and are not limited to schizophrenia. Transl Psychiatry. 2017;7:e1118.
Meda SA, Clementz BA, Sweeney JA, Keshavan MS, Tamminga CA, Ivleva EI, et al. Examining functional resting-state connectivity in psychosis and its subgroups in the bipolar-schizophrenia network on intermediate phenotypes cohort. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:488–97.
Lizano P, Kiely C, Mijalkov M, Meda SA, Keedy SK, Hoang D, et al. Peripheral inflammatory subgroup differences in anterior default mode network and multiplex functional network topology are associated with cognition in psychosis. Brain Behav Immun. 2023;114:3–15.
De Marco M, Meneghello F, Pilosio C, Rigon J, Venneri A. Up-regulation of DMN connectivity in mild cognitive impairment via network-based cognitive training. Curr Alzheimer Res. 2018;15:578–89.
Hill SK, Sweeney JA, Hamer RM, Keefe RS, Perkins DO, Gu H, et al. Efficiency of the CATIE and BACS neuropsychological batteries in assessing cognitive effects of antipsychotic treatments in schizophrenia. J Int Neuropsychol Soc. 2008;14:209–21.
Acknowledgements
The current study was funded and supported by United Stated Public Health Service, National Institute of Health grants MH103366, MH096900, MH103368, MH077851, MH096913, MH078113, MH096942, MH077945, MH096957.
Author information
Authors and Affiliations
Contributions
SAM and MMD contributed to primary analysis design, computation and writing the first draft of the manuscript. Subsequent revisions were performed by SAM, MMD, SKH and GDP. BAC, SKK, JEM, EII, ESG, MSK, CT and GDP were responsible for design and securing funding for the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the ethical principles outlined in the Helsinki Declaration following all relevant guidelines and regulations. All procedures involving human participants were approved by the respective site’s institutional review board (IRB) and ethics committees (STU0702013-063, HHC-2014-0050, IRB14-0917, 2014P-000253). All participants provided their informed consent to take part in the study. In addition, written consent for publication of relevant imaging material was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meda, S.A., Dykins, M.M., Hill, S.K. et al. Neural fingerprints of data driven cognitive subtypes across the psychosis spectrum: a B-SNIP study. Transl Psychiatry 15, 224 (2025). https://doi.org/10.1038/s41398-025-03422-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03422-3