Abstract
The excitation/inhibition (E/I) ratio has been shown to be imbalanced in individuals diagnosed with autism (AT) or schizophrenia (SZ), relative to neurotypically developed controls (TD). However, the degree of E/I imbalance overlap between SZ and AT has not been extensively compared. In this project, we used resting state fMRI data to estimate the E/I ratio via the Hurst (H) exponent. Our main objectives were (1) to quantify group differences in the E/I ratio between TD, AT, and SZ, (2) to assess the potential of the E/I ratio for differential diagnosis, and (3) to verify the replicability of our findings in an independently acquired dataset. For each participant, we computed the Hurst exponent (H), an indicator of the E/I ratio, from the time courses of 53 independent components. Using a random forest classifier (RF), we ran a classification analysis using the larger of the two datasets (exploratory dataset; 519 TD, 200 AT, 355 SZ) to determine which of the 53 H would yield the highest performance in classifying SZ and AT. Next, taking the ten most important H based on the exploratory dataset, in combination with phenotypic information collected in the replication dataset (55 TD, 30 AT, 39 SZ), we used RF to compare the classification performance using five feature sets: (a) H only; (b) Positive and Negative Syndrome Scale (PANSS) and the Autism Diagnostic Observation Schedule (ADOS) only; (c) PANSS, ADOS, Bermond–Vorst Alexithymia Questionnaire (BVAQ), Empathy Quotient (EQ), and IQ; (d) H, PANSS and ADOS; (e) H, PANSS, ADOS, BVAQ, EQ and IQ. Classification performance using H only was higher in the exploratory dataset (AUC = 84%) compared to the replication dataset (AUC = 72%). In the replication dataset, the highest classification performance was obtained when combining H with PANSS, ADOS, BVAQ, EQ and IQ (i.e., model e; AUC = 83%). These results illustrate the added value of E/I to typical phenotypic data in differentiating AT and SZ.
Similar content being viewed by others
Introduction
Autism (AT) and schizophrenia (SZ) have been recognized as independent diagnoses since the 1970s [1]. While AT is primarily characterized by distinct patterns of social communication, and repetitive behaviors, SZ are characterized by positive (e.g., hallucinations, delusions), negative (e.g., social withdrawal), and cognitive symptoms (e.g., executive functions deficits) [2]. They also show different onset trajectories (i.e., AT becomes apparent in early childhood, while SZ onset usually occurs in late adolescence or early adulthood), albeit a progression from AT to SZ cannot be excluded [3].
However, the heterogeneity of both diagnostic categories [4, 5] and their phenotypic overlap [6,7,8] can hinder accurate psychiatric diagnosis. For example, a review [9] reported that catatonic features, commonly associated with psychosis, were prevalent in up to 20% of autistic samples. Social functioning patterns, such as emotion processing, have also been shown to bear similarities in AT and SZ [10]. In addition, AT and SZ co-occur in approximately 4% of cases [11], and AT has been found to be diagnosed in up to 11% of people at clinical high risk for psychosis [12]. Differential diagnosis is additionally hindered by the fact that the common clinical observational and interviews to assess diagnosis-specific symptoms, such as the Autism Diagnostic Observation Schedule (ADOS) and the Positive and Negative Syndrome Scale (PANSS), do not have good specificity [13, 14]. Specifically [13], reported a higher degree of overlap between AT and SZ with respect to negative symptoms, while [14] reported that positive symptoms can better discriminate between the two groups. This symptom overlap between SZ and AT has led us to inquire about the underlying neural mechanisms, and whether these might aid in differential diagnosis [15]. A recent international machine learning competition aimed to classify AT and typically developed (TD) showed that fMRI data can yield a classification accuracy of ~ 80% [16]. Classification accuracies based on structural or functional MRI data can be just as high or higher when distinguishing SZ from TD [17, 18]. However, even though some previous attempts have been made [19], the challenge is greater when attempting to discriminate between heterogeneous nosological categories, such as AT and SZ, that share both genetic variants and neuroimaging patterns [20]. For example [21], computed functional connectivity based on resting state fMRI data from 2980 subjects (1665 TD, 537 SZ, and 778 AT). They revealed an astounding level of heterogeneity, with both extensively overlapping connectivity patterns between AT and SZ, especially in the default Mode Network, as well as opposing patterns, such as increased connectivity between sensorimotor and default mode areas in SZ, but decreased connectivity between these areas in AT.
One potential discriminatory brain-based marker is the excitation/inhibition (E/I) ratio, which has been shown to be different in TD compared to both AT [22] and SZ [23]. The E/I ratio is based on the concerted activity of mostly glutamatergic (i.e., excitatory) and GABAergic (i.e., inhibitory) neurons. The former are the most numerous and project throughout the entire brain, while the latter are fewer and synapse locally (for a comprehensive account of excitatory and inhibitory activity balance in the human brain, see [24]). A way to estimate the E/I ratio in humans based on non-invasive measures, such as resting state fMRI (rsfMRI), is by computing the Hurst (H) exponent from the acquired timeseries; an increased H values indicates a decreased E/I ratio, and vice-versa (for a review see [25]).
In AT [26], first hypothesized that observed sensory processing patterns may result from an increased E/I ratio. Among the evidence they cite is the fact that parietal and cerebellar areas show ~50% less glutamic acid decarboxylase (GAD), the enzyme that synthesizes the inhibitory neurotransmitter γ -aminobutyric acid (GABA) in AT compared to TD [27]. Additionally, cortical mini columns, which are functional units composed of GABAergic and glutamatergic neurons processing thalamic inputs, are smaller and more numerous in AT compared to TD [28]. A more recent summary specifically points towards the impact of reduced inhibition on cortical and hippocampal functioning in AT [29]. Whether this E/I imbalance is mainly due to excessive excitatory activity, or deficient inhibitory activity, is not entirely clear [30, 31], but recent evidence points to the E/I imbalance in AT being caused by concomitant effects [32]. Finally, direct evidence for the contribution of an E/I imbalance in AT comes from a study using bumetanide (i.e., a selective NKCC1 chloride importer antagonist, which decreases depolarizing GABA action, to reduce the E/I ratio) in a large cohort of AT children [33]. These authors reported a decrease in repetitive behaviors following a 91-day bumetanide trial. Another direct link between sensory processing and GABAergic activity in AT has been provided by [34]. These authors used arbaclofen, a GABA type B receptor agonist, to show that auditory repetition suppression was negatively impacted by the drug in TD, but improved in AT.
In SZ, the E/I ratio has also been reported to be imbalanced compared to TD. Post-mortem and genetic evidence [35], and computational modeling revealed that this imbalance causes hyperconnectivity in association brain areas [36]. In addition, research has also shown a link between an E/I imbalance and aberrant internal sensory processing in SZ [37], such as hallucinations [23]. Finally, dopamine appears to be crucial in maintaining the E/I balance by modulating the excitability of glutamatergic and GABAergic neurons, thus contributing crucially to the E/I ratio in SZ [38]. Dopaminergic activity, in concerted action with glutamatergic and GABAergic activity, when disrupted, can directly impact memory function and prefrontal connectivity in SZ (for an extended account please see [39]).
It has been proposed that an E/I imbalance characterizes both AT and SZ [40, 41], and that this relies in turn on shared genotype [42]. However, given the substantial heterogeneity in both AT and SZ [5], it is difficult to ascertain to which extent there is overlap between the brain areas that display this imbalance in these populations.
In recent years, various machine learning approaches have been employed to improve differential diagnosis of mental disorders [43]. Among these, interpretable models, such as Random Forest (RF), have become increasingly popular due to their transparency, as opposed to the traditional “black box” methods, such as support vector machine [44, 45]. A trade-off between high interpretability and high accuracy is usually considered when opting for a particular classification approach, as highly accurate classifiers tend to provide less interpretability (but see also [45], for a different account). Among interpretable approaches, RF stands out as an algorithm that can provide both high accuracy and reasonable interpretability [46]. In addition, it can also be used for feature selection based on feature importance in an out-of-sample classification, as we did in the current project [47].
Considering the current state of knowledge regarding the E/I ratio in different populations, we had three main objectives: (1) to quantify group differences in the E/I ratio between TD, AT, and SZ, (2) to assess whether the E/I ratio could support differential diagnosis, and (3) to verify the replicability of our findings in an independently acquired dataset.
To assess the role of clinical vs. E/I ratio data in classifying AT and SZ, we used two independent datasets and five distinct sets of features (i.e., classification models) comprising either phenotypic assessment only, the E/I ratio (as indexed by the H exponent) of multiple brain areas only, or all these together. To quantify the E/I ratio based on rsfMRI timeseries, we computed the H of 53 predefined functional brain areas, based on the Neuromark templates [48]. The H exponent has been refined as a reliable computational approximation of synaptic E/I based on extensive physiological and in silico studies [22]. For the phenotypic features we focused on core symptoms assessments, namely: ADOS — measuring AT-related social and communication patterns, and PANSS — measuring SZ-related positive (e.g. delusions, hallucinations) and negative (e.g., social withdrawal) symptoms, and general psychopathology (e.g., attention deficits). The total ADOS scores were calculated using the original ADOS-2 algorithm, thus including the following items: A-4, 8, 9 and 10, and B-1, 2, 6, 8, 9, 11 and 12. In addition, we used an IQ estimate, and two social cognitive measures: Empathizing Quotient (EQ) — measuring empathy, and the Bermond–Vorst Alexithymia Questionnaire (BVAQ) — measuring alexithymia, both of which have been shown to be different in AT and SZ compared to TD [49,50,51]. Two rsfMRI datasets were used to test replicability. The first dataset included data from publicly available fMRI repositories of either AT or SZ data, and included a relatively large dataset [21]. The second, smaller, dataset was collected on-site from both AT, SZ, and NT. We believe the use of both datasets holds important advantages. The replication dataset, while consisting of fewer participants, contains both rsfMRI and phenotypic data. In addition, the AT, SZ and TD in this dataset were collected in the same setting, which precludes the risk of site-related confounds. The larger, exploratory dataset was obtained by sourcing datasets from different online repositories. These datasets had been acquired at various sites with different scanning parameters, and phenotypical data was not uniformly available across sites and clinical groups. While this prevented us for doing a full exploration and replication of all the classification models that we were able to test using the smaller dataset, it allowed us to: (1) reduce model complexity and increase model stability [52] in the smaller replication dataset by using only the most important H features (i.e., brain regions) from the larger exploratory dataset, and (2) illustrate the replicability of the results of the H only classification model.
Methods
Participants
Two independent datasets were used in the current project. An exploratory dataset (Exploratory), based on several publicly-available online datasets (described below), and an internally-collected replication dataset (Replication).
For the Exploratory dataset, we analyzed 519 TD (362 males & 157 females; mean age = 28.49 ± 7.68), 200 AT (180 males & 20 females; mean age = 24.74 ± 6.6), and 355 SZ (245 males & 110 females; mean age = 30.91 ± 7.95) from the previously preprocessed and harmonized dataset used in [21]. The participants in [21] had been selected from several data repositories: the AT from the Autism Brain Imaging Data Exchange (ABIDE I and II), and the SZ from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), the Center for Biomedical Research Excellence (COBRE), the Maryland Psychiatric Research Center (MPRC), and the Function Biomedical Informatics Research Network (FBIRN). From the dataset of [21], we chose a subset of participants that closely matched the age (18–35 y.o.) and intelligence quotient (IQ) of the Replication dataset. Note that an estimated IQ > 75 criterion was chosen because it was the inclusion threshold in the BSNIP dataset, where no IQ values were recorded. Because some of the data from the Replication dataset had been previously submitted to data repositories (e.g., ABIDE II) from which the dataset of [21] had been drawn, we ensured that no participants were included in both the Exploratory and Replication datasets, by excluding repeated participants from the Exploratory dataset.
For the Replication dataset, participants were recruited via the Olin Neuropsychiatry Research Center (ONRC) and the Yale University School of Medicine and underwent rsfMRI scanning for the current study. We discarded participants with head motion > 10 mm, and those with incomplete phenotypic assessment information, resulting in a final dataset consisting of 55 TD (26 males & 29 females; mean age = 23.86 ± 3.65), 30 AT (25 males & 5 females, mean age = 22.33 ± 3.78), and 39 SZ (31 males & 8 females, mean age = 25.66 ± 3.53). The Replication dataset has been previously used by [53, 54] and [19], and the exclusion criteria we used here were the same: intellectual disability (i.e., estimated IQ < 80), neurological disorders (e.g., epilepsy), current drug use as indicated by pre-scanning interview and urine test, incompatibility with MRI safety measures (e.g., metal implants), and a history of psychiatric diagnoses in TD.
Phenotypic assessment in the replication dataset
Below we describe the phenotypic data that were collected for the Replication dataset. These were not consistently available for the Exploratory dataset. In addition, we also recorded the chlorpromazine equivalent for participants from the Replication dataset who took antipsychotic medication (i.e., eight AT, and 37 SZ).
Diagnostic assessment
The severity of psychotic symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS; [55]) in both AT and SZ. The PANSS scores can be interpreted along three subscales: (1) positive symptoms, reflecting the severity of hallucinations and delusions; (2) negative symptoms, reflecting the severity of blunted affect and anhedonia, and (3) a general subscale quantifying other psychopathology such as poor attention and lack of insight.
The ADOS, module 4 [56] was administered to all participants and the total score was used in this study to confirm or rule out an autism diagnosis and quantify autistic social and communication characteristics.
The Structured clinical interview for DSM-IV-TR Axis I disorders (SCID; [57]) was used to confirm/rule out the diagnosis of SZ and the absence of any Axis I diagnoses in TD.
Social cognition and estimated IQ
Estimated IQ was calculated for the entire dataset using the Vocabulary and Block Design subtests of the Wechsler Scale of Adult Intelligence-III (WAIS-III; [58, 59]). Additionally, all participants completed: (1) the Empathizing Quotient (EQ; [60]) which measures general empathy including both the affective and cognitive empathy components; (2) the Bermond–Vorst Alexithymia Questionnaire (BVAQ; [61]), whose sub-scores are computed along five distinct dimensions: “verbalizing”, reflecting one’s propensity to talk about one’s feelings; “identifying”, capturing the extent to which one is able to accurately define one’s emotional states; “analyzing”, quantifying the extent to which one seeks to understand the reason for one’s emotions; “fantasizing”, quantifying one’s tendency to day-dream, and “emotionalizing”, reflecting the extent to which a person is emotionally aroused by emotion-inducing events. Descriptive statistics and group comparisons of phenotypic data are given in Table 1.
Imaging data acquisition and preprocessing
For the Exploratory dataset, the rsfMRI data was preprocessed using the SPM toolbox, as described extensively in [21]. In short, the first few volumes were discarded, then rigid-body motion correction and slice-timing correction were performed. Finally, the data were normalized, resampled to 3 mm3 isotropic voxels, and smoothed with a 6 mm FWHM Gaussian kernel. Prior to preprocessing, the effects of age, gender, site acquisition, and interactions between age and site, and gender and site were regressed from the gray matter volumes of each voxel, to ensure between-site harmonization.
For the Replication dataset, rsfMRI scans lasted 7.5 min and were collected using a Siemens Skyra 3 T scanner at the ONRC. Participants lay still, with eyes open, while fixating a centrally presented cross. Blood oxygenation level dependent (BOLD) signal was obtained with a T2*-weighted echo planar imaging (EPI) sequence: TR = 475 ms, TE = 30 ms, flip angle = 60 deg, 48 slices, multiband (8), interleaved slice order, 3 mm3 voxels. Neuroimaging data were preprocessed using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8/). Each dataset was realigned to the first T2* image using the INRIAlign toolbox (https://www-sop.inria.fr/epidaure/Collaborations/IRMf/INRIAlign.html), coregistered to their corresponding high signal-to-noise single-band reference image (sbREF; [62]), spatially normalized to the Montreal Neurological Institute (MNI) standard template [63], and spatially smoothed (6 mm3). Finally, framewise displacement (FD) motion parameters were computed according to the FSL library algorithm [64], and the mean FD value for each run was used as a covariate in group analyses.
Data analysis
For both datasets, we ran a fully automated independent component analysis (ICA) on the preprocessed rsfMRI data using the Group ICA for fMRI Toolbox (GIFT v4.0c; https://trendscenter.org/software/gift/; [65]) to define functional brain regions. The 53 replicable independent component (IC) templates from the NeuroMark pipeline with the neuromark_fMRI_1.0 templates [48] were used to estimate participant-specific, spatially-independent components using a spatially-constrained ICA algorithm [66]. A complete list of the NeuroMark IC templates, arranged into seven functional domains, and peak MNI coordinates for each IC template are given in Table 2 and illustrated in Supplementary Fig. 1. After detrending and despiking using 3dDespike [67], we extracted one Hurst exponent (H), an estimate of the E/I ratio, from each of the resulting 53 IC time courses of each participant.
We estimated H using the nonfractal MATLAB toolbox (https://github.com/wonsang/nonfractal; [68]). Specifically, we used the function bfn_mfin_ml.m with the “filter” argument set to “haar” and the “ub” (upper bound) and “lb” (lower bound) arguments set to [1.5, 10] and [−0.5, 0], respectively, as previously recommended by [22]. This is a wavelet-based maximum likelihood estimation using a discrete wavelet transform using volumes in power of 2 and a filter of type Haar. Similar to [22], for the replication dataset, this resulted in the first 512 volumes being utilized.
All the other statistical analyses were performed with R 4.1.1. These included a one-way analysis of covariance (ANCOVA), Tuckey post-hoc, two-sided two-sample Welch t tests, and a Spearman correlation analysis.
Classification analyses
A crucial aspect of classification algorithms in neuroimaging is sample size. It has been shown that larger sample sizes lead to more accurate estimates when brain-behavior relationships are investigated via traditional statistical approaches [69]. While this seems to be generally true also for machine learning [52], the relationship between sample size and classification accuracy does not appear to be entirely linear [70]. For this reason, in the current project, we established the initial classification accuracy of our brain-based classification model based on the largest of the two datasets.
First, using the Exploratory dataset, we classified the AT and SZ participants using a random forest (RF) algorithm, as implemented in the Interpretable Artificial Intelligence (IAI) toolbox (https://www.interpretable.ai/) and accessed through R 4.2.1 [71]. The RF algorithm was optimized using a grid search with 100 iterations of 100 trees of maximum 10 leaves per tree. The features were normalized before being used by the classifier, and the optimum sample split was determined using the “gini” criterion, which was preferred because it is less computationally expensive and gives comparable results to other criteria (e.g., “entropy”). The feature set consisted of the 53 H exponents of each participant. We ran 100 randomized sample splits and averaged the model performance metrics that we obtained for each of the splits to obtain three final classification performance indices (i.e., area under the curve/AUC, sensitivity and specificity). From each group, with each new split, 50% of the data were randomly allocated to the test, and the rest to the train group. We used the AT sample as reference for calculating sensitivity and specificity. Following the classification, we selected the ten ICs with the highest feature importance of H (Supplementary Fig. 2) as a simplified feature set for use in RF classification of the Replication dataset. Next, using the Replication dataset, we classified the AT and SZ participants using the same algorithm and toolbox, with 100 splits and 50% randomized allocation of data into the train and test groups. Five models were used for the RF classification in this case, containing the following features: (a) E/I model: the H only values of the 10 ICs ranked as most important by the RF classification in the Exploratory dataset; (b) symptoms only model: PANSS 3 factor scores and ADOS total scores; (c) symptoms and cognitive model: PANSS, ADOS, EQ, BVAQ, and IQ scores; (d) E/I and symptoms model: the ten H from model (a) plus the PANSS and ADOS scores, and (e) E/I, symptoms and cognitive model: the ten H from model (a) plus the PANSS, ADOS, EQ, BVAQ, and IQ scores. Like in the previous step, we calculated three classification performance indices (i.e., AUC, sensitivity and specificity), and used the AT sample as reference for sensitivity and specificity. A misclassification index for each participant and each model was calculated as the ratio between the misclassification instances of each participant and the total number of times they were allocated to a test set.
Results
Group differences in demographic and phenotypic assessment
In the Exploratory dataset, there were significant group differences in age (F (2) = 42.45, p < 0.001), and sex (χ2 (2) = 35.156, p < 0.001).
Data for the Replication dataset, including statistical tests, are presented in Table 1. There were significant differences in estimated IQ, age, sex and FD, and therefore these parameters were used as covariates in further group analyses. Regarding symptom assessments, the AT and SZ groups did not significantly differ in their social and communication skills, as indicated by the ADOS scores, but the PANSS scores on all three domains (i.e., positive and negative symptoms, and general psychopathology) were significantly elevated in SZ compared to AT. For social functioning, BVAQ-Fantasizing was significantly decreased in AT compared to SZ, while Empathy was significantly decreased in both AT and SZ compared to TD, and in AT compared to SZ.
Group differences in H in the exploratory dataset
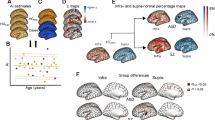
The ANOVA results testing group differences in H values are given in Table 2. While all areas showed significant group differences, the largest effect size (i.e., η2 > = 0.06) was found for: the paracentral lobule (i.e., ICs no. 10 and 13 from the Sensorimotor domain), the calcarine gyrus, middle occipital gyrus, fusiform gyrus, and inferior occipital gyrus (i.e., ICs no. 17, 18, 20, 23 from the Visual network), the insula, and the superior, middle and right inferior frontal gyrus (i.e., ICs no. 27, 30, 31, 35 from the Cognitive control domain), the precuneus and anterior cingulate cortex (i.e., IC no. 43, 44, 47 from the Default Mode network), and one area of the Cerebellar domain (i.e., IC no. 50).
Group differences in H in the replication dataset
The ANCOVA results showing group differences in H values from each component are given in Table 3. The areas most sensitive to overall group differences, after controlling for age, sex, IQ, and FD, were the left and right postcentral gyrus and paracentral lobule (i.e., IC no. 9, 11, and 10, from the Sensorimotor network), and the calcarine gyrus, middle occipital gyrus, middle temporal gyrus, inferior occipital gyrus, and lingual gyrus (i.e., IC no. 17, 18, 19, 23 and 24 from the Visual network). The supplementary motor area (i.e., IC no. 34), associated with the Cognitive Control domain, also yielded significant group differences.
Classification accuracy and feature importance in the exploratory dataset
Using the complete 53 H feature set, the classification performance was: AUC = 84%, Sensitivity = 65%, and Specificity = 83% (Fig. 1A; please note that sensitivity and specificity are calculated in relation to AT).
A Classification performance for each model and dataset. AUC area under the curve; a Expl: model with all 53 H values in the Exploratory dataset; a Repl: model with the ten H values in the Replication dataset; b: model with ADOS and PANSS; c: model with ADOS, PANSS, IQ, EQ, BVAQ; d: model with the ten H, ADOS and PANSS; e: model with the ten H, ADOS, PANSS, IQ, EQ, BVAQ. B Misclassification of participants. AT autism, SZ schizophrenia.
Next, we used the ten ICs with the highest feature importance from the RF classification of the Exploratory dataset to simplify the feature set for the RF classification of the Replication dataset. These ten ICs were: the precuneus and the anterior cingulate cortex (i.e., ICs 43, 44, 47, and 48, from the Default Mode Network), the superior frontal gyrus (i.e., IC 35 of the Cognitive Control domain), the paracentral lobule and the precentral gyrus (ICs 10 and 14, from the Sensorimotor domain), and the middle occipital gyrus, the inferior occipital gyrus, and the lingual gyrus (i.e., ICs 18, 23, and 24, from the Visual domain) (Supplementary Fig. 2).
Classification results in the replication dataset
In the Replication dataset, we obtained the following classification performance, outlined in Fig. 1A: model (a), using the reduced H feature set (ten ICs): AUC = 72%, Sensitivity = 64%, and Specificity = 67%; model (b), using the PANSS and ADOS as features: AUC = 78%, Sensitivity = 65%, and Specificity = 73%; model (c), using the PANSS, ADOS, EQ, IQ, and BVAQ as features: AUC = 76%, Sensitivity = 62%, and Specificity = 76%; model (d), using the ten H plus PANSS and ADOS: AUC = 81%, Sensitivity = 67%, and Specificity = 76%; model (e), using the ten H plus PANSS, ADOS, EQ, IQ, and BVAQ: AUC = 83%, Sensitivity = 68%, and Specificity = 79%.
In the Replication dataset, for each classification instance, we inspected the scaled contribution of each feature included in each of the five models. In order to account for the different number of features included in each classification model, we scaled the raw feature importance values (Supplementary Fig. 3) by multiplying them by the number of features included in that respective model (Supplementary Fig. 4). As can be seen in Supplementary Fig. 4, the precentral gyrus (IC 14), the inferior occipital gyrus (IC 23), the lingual gyrus (IC 24), and the precuneus (IC 48) are the most important H features overall. The most important symptom scores are the ADOS total, followed by PANSS positive and negative. However, the most important feature overall was the estimated IQ, which prompted our concern that the IQ might bias our classification output and misleadingly appear as being more important than other features in model (e), given that our groups were not matched for IQ (Table 1). We therefore repeated the classification for models (c) and (e) without IQ, and found that performance remained virtually unchanged (i.e., model (c): AUC = 0.75, Sensitivity = 0.6, Specificity = 0. 76, and model (e): AUC = 0.83, Sensitivity = 0.68, Specificity = 0. 81). Feature hierarchy also remained otherwise unchanged following IQ elimination.
Finally, inspection of misclassified participants (Fig. 1B), showed that on average, SZ were increasingly more accurately classified as we moved from models (a) through (e), while AT were most accurately classified when H features were included (i.e., models a, d, and e).
Antipsychotic medication effect
Finally, we verified whether medication (i.e., chlorpromazine equivalent) in the SZ group of the Replication dataset might have influenced the H values that we observed. We ran a Spearman correlation between the chlorpromazine equivalent and each of the 53 H values and found no significant correlations (all p > 0.05).
Discussion
In the current project, we assessed the feasibility of using the E/I ratio, as estimated by the H exponent, to distinguish between AT and SZ. We had two main goals: (1) to compare the classification accuracy when different sets of clinical, social and non-social cognition, and imaging features were combined, and (2) to perform an out-of-sample replication of the classification model using a reduced set of brain-based features (i.e., the H exponent from the ten most important brain components).
We first explored group differences between the three groups, in each independent dataset (Tables 2 and 3). The most consistent findings across both datasets reflected a significantly reduced H (i.e., increased E/I ratio) in SZ compared to TD in most areas of the cerebellar domain, the bilateral postcentral gyrus and paracentral lobule (from the Sensorimotor domain), and all but one area of the Visual domain. Given that levels of glutamate and GABA have been reported to vary inconsistently across SZ, and moreover, to be impacted by both medication and disease progression (see [41] for an in-depth overview), we believe that the replicability of these group differences are even more notable. What is more, this may suggest that despite the aforementioned medication and disease progression related neurotransmitter variations in SZ, a persistently elevated E/I ratio remains, predominantly in visual processing brain areas. In addition, we also checked whether antipsychotic medication in the SZ group from the Replication dataset correlated with H; we found that it did not, which suggests that at least in this sample, medication did not substantially bias our results. Nevertheless, the effects of medication and condition cannot be completely disentangled in the current study.
Group differences were less consistent between TD and AT, and the effects mostly displayed opposite directions in the two independent datasets. Namely, in the Exploratory dataset, significantly larger H (i.e., reduced E/I ratio) were found in AT compared to TD in the precuneus, the cerebellum, the frontal cortex, the left inferior parietal lobule, and some areas of the visual domain (i.e., calcarine, fusiform, inferior occipital, and middle temporal gyrus). In the Replication dataset, the supplementary motor area showed significantly reduced H (i.e., increased E/I ratio) in AT compared to TD. We believe that one potential explanation that could account for this reduced replicability in this case could be the increased heterogeneity of AT which may simply not have been sufficiently captured given the limited size of the Replication dataset. Indeed [41], also suggest that E/I variations appear to be more heterogenous in AT than SZ. Another aspect that could contribute to these inconsistent findings might be due to sex differences, which we were unable to assess in the current project given insufficient female AT participants. Previously [22], reported that the H of the ventromedial prefrontal cortex was significantly elevated in female AT compared to female TD, but significantly decreased in male AT compared to male TD. Thus, a more extensive exploration of sex differences in larger samples of AT could clarify this aspect.
A direct comparison of H in AT v. SZ revealed no significant differences in the Replication dataset but showed a consistent pattern of significantly increased H (i.e., decreased E/I ratio) in AT compared to SZ, in all but six of the 53 brain areas. These findings require further validation given the heterogeneity in data collection between these two groups in the Exploratory dataset.
Classification performance of AT and SZ, using the H values of all 53 brain areas, in the Exploratory dataset, was very good (AUC = 84%), with especially high specificity for SZ (83%). A similar trend was maintained when using a reduced classification model comprising the ten most important H-based features in the Replication dataset (Fig. 1A), though classification performance was overall reduced, as was to be expected in an out-of-sample replication, and given the limited size of the Replication dataset. We further explored how augmenting the feature set in the Replication dataset could improve classification performance. We found that while using PANSS and ADOS alone resulted in increased classification performance compared to using H alone, performance increased further when combining H, PANSS and ADOS, and was the highest when using H, PANSS, ADOS, as well as social and non-social cognitive measures (i.e., IQ, EQ and BVAQ). This increase in performance appeared to be mostly driven by a steady decrease in misclassified SZ (Fig. 1B). Due to some concerns that unmatched IQ between AT and SZ might be biasing these results, we re-ran these classification models without IQ as a feature and obtained similar results. In both the Exploratory and the Replication dataset, sensitivity was < 70% for all models, indicating that our models retained substantial error when identifying true positives. The specificity was however constantly > 70% in both datasets and for all models except for model (a) in the Replication dataset. This indicates that using the reduced set of H features alone is insufficient to correctly identify true negatives.
We may therefore conclude that: (1) classification performance based on E/I, as estimated by H, is substantial and replicable across independent datasets, and (2) classification performance is the highest when H and phenotypic assessment are combined, resulting in notably decreased misclassification instances in both AT and SZ (Fig. 1B). Interestingly, AT were more frequently misclassified when only phenotypic assessment was used, compared to both H only and H combined with phenotypic assessment (Fig. 1B). Given that both AT and SZ in the Replication dataset were acquired using the same protocol and testing environment, we surmise that inherent AT heterogeneity could best explain these different trajectories in classification performance.
Using the H to estimate the E/I ratio based on fMRI data has long been proposed as a valuable biomarker, and this approach has seen a substantial increase in recent years, likely due to the availability of less computationally expensive implementations. In addition, toolboxes equipped with algorithms that are better suited to estimate H based on biological signals, such as the nonfractal toolbox [72], have likely also contributed to the neuroimaging community’s increased attention to the multiple applications of the H exponent. Thus, recent studies have investigated the longitudinal developmental trajectories of AT children [73] using the H and have shown that E/I imbalances can already be identified in childhood. Also using H based on rsfMRI to compute non-fractal connectivity in a sample of adult AT and TD participants [74], obtained a classification performance of > 90%. The H exponent has been further shown to correlate positively with molecular indices of E/I balance, such as parvalbumin mRNA expression in human children and adults, as well as parvalbumin-positive cell counts in mice [75]. Thus, the H appears to be an ever-stronger contender among the biomarkers that have so far been proposed for the study of AT and E/I. These latest findings also add further support to the results of our current study, as they indicate that a pervasive E/I imbalance is characteristic of AT, and that H can be used as a robust estimator.
Limitations
While the Exploratory dataset that we used in the first step of our classification analysis offered a satisfactory amount of data, it does have the limitation that these data were collected using a variety of protocols and acquisition sites. On the other hand, while the Replication dataset was acquired uniformly and offered us the possibility to test different classification models, given that identical clinical assessment protocols were used for both AT and SZ, it contained few participants. Nevertheless, we believe that especially given these limitations, the replicable results using the brain-based data are noteworthy. Another limitation which we were unable to address using the currently available datasets were sex differences. This is especially true for AT, given the historic bias which has resulted in currently available samples being overwhelmingly dominated by male participants. A limitation regarding the replication sample was that interviewers were not blind to participants’ diagnoses, which could have biased the on-site assessments. Finally, we wish to note that in this project, the E/I ratio was estimated indirectly based on rsfMRI BOLD signal through the H exponent. While rsfMRI has the advantage of being noninvasive and only requires a few minutes of acquisition, the shortcoming is that H is a computational proxi of the E/I ratio and therefore it is unclear whether it captures the underlying fluctuations of excitatory and inhibitory neurotransmitters. We therefore caution readers to interpret these results with this limitation in mind.
Conclusion
In conclusion, in the current study, we probed the ability of the E/I ratio, as estimated indirectly by the H exponent, to discriminate between AT and SZ. We showed that this has a good discriminatory power, on its own as well as in combination with additional phenotypic measures. In fact, this neurophysiological measure augmented the discriminative effect of traditional phenotypic measures. Notably, when using brain-based classification features only (i.e., H), we obtained a good classification performance, which speaks for the distinct possibility that different etiologic mechanisms underlie the observed E/I ratio alterations in AT and SZ. Given how E/I ratio alterations are closely linked to impaired sensory processing, these results may offer an avenue for future clinical research to explore potential therapeutic interventions specific for each group. Our results point to incorporating the H as a valuable discriminant criterion in differential diagnosis, with especially encouraging results for SZ, since misclassification instances of SZ were minimized when H was added to all the available phenotypic measures. In addition, for AT, even using H only led to fewer misclassifications compared to when the standard ADOS and PANSS were used, but combining H with phenotypic measures did not lead to significantly better discriminatory effect. We believe that this stresses the potential for the E/I ratio to contribute to differential diagnosis.
Data availability
The exploratory dataset is available through multiple open-access repositories mentioned in the Methods section. The replication dataset can be made available following reasonable request.
Code availability
All the codes used in this project are available upon request.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). 2013. https://doi.org/10.1176/appi.books.9780890425596
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—an overview. JAMA Psychiatry. 2020;77:201.
Hsu T, Chu C, Tsai S, Hsu J, Huang K, Cheng C, et al. Diagnostic progression to schizophrenia: a nationwide cohort study of 11 170 adolescents and young adults with autism spectrum disorder. Psychiatry Clin Neurosci. 2022;76:644–51.
Benkarim O, Paquola C, Park BY, Kebets V, Hong SJ, Vos de Wael R, et al. Population heterogeneity in clinical cohorts affects the predictive accuracy of brain imaging. PLoS Biol. 2022;20:e3001627.
Segal A, Parkes L, Aquino K, Kia SM, Wolfers T, Franke B, et al. Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders. Nat Neurosci. 2023;26:1613–29.
Kästner A, Begemann M, Michel TM, Everts S, Stepniak B, Bach C, et al. Autism beyond diagnostic categories: characterization of autistic phenotypes in schizophrenia. BMC Psychiatry. 2015;15:1–12.
Kwok TT, Chan MM, Mo FY, Hung SF, Leung PW, Lai KY, et al. Prevalence of autism in first-episode psychosis in two Hong Kong teaching hospitals. Autism. 2024;28:2412–21.
Schalbroeck R, Foss-Feig JH, Jutla A, Ziermans TB. Integrating neuropsychological research on autism and psychosis to improve clinical outcomes. Nat Rev Psychol. 2023;2:723–39.
Vaquerizo-Serrano J, Salazar De Pablo G, Singh J, Santosh P. Catatonia in autism spectrum disorders: a systematic review and meta-analysis. Eur Psychiatry. 2021;65:e4.
Oliver LD, Moxon-Emre I, Lai MC, Grennan L, Voineskos AN, Ameis SH. Social cognitive performance in schizophrenia spectrum disorders compared with autism spectrum disorder: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2021;78:281–92.
Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:819–29.
Vaquerizo-Serrano J, Salazar de Pablo G, Singh J, Santosh P. Autism spectrum disorder and clinical high risk for psychosis: a systematic review and meta-analysis. J Autism Dev Disord. 2022;52:1568–86.
Bastiaansen JA, Meffert H, Hein S, Huizinga P, Ketelaars C, Pijnenborg M, et al. Diagnosing autism spectrum disorders in adults: the use of autism diagnostic observation schedule (ADOS) module 4. J Autism Dev Disord. 2011;41:1256–66.
Trevisan DA, Foss-Feig JH, Naples AJ, Srihari V, Anticevic A, McPartland JC. Autism spectrum disorder and schizophrenia are better differentiated by positive symptoms than negative symptoms. Front Psychiatry. 2020;11:548.
Horien C, Floris DL, Greene AS, Noble S, Rolison M, Tejavibulya L, et al. Functional connectome-based predictive modelling in autism. Biol Psychiatry. 2022;92:626–42.
Traut N, Heuer K, Lemaître G, Beggiato A, Germanaud D, Elmaleh, et al. Insights from an autism imaging biomarker challenge: promises and threats to biomarker discovery. Neuroimage. 2022;255:119171.
de Filippis R, Carbone EA, Gaetano R, Bruni A, Pugliese V, Segura-Garcia C, et al. Machine learning techniques in a structural and functional MRI diagnostic approach in schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2019;15:1605–27.
Meng X, Iraji A, Fu Z, Kochunov P, Belger A, Ford JM, et al. Multi-model order spatially constrained ICA reveals highly replicable group differences and consistent predictive results from resting data: a large N fMRI schizophrenia study. Neuroimage Clin. 2023;38:103434.
Rabany L, Brocke S, Calhoun VD, Pittman B, Corbera S, Wexler BE, et al. Dynamic functional connectivity in schizophrenia and autism spectrum disorder: convergence, divergence and classification. Neuroimage Clin. 2019;24:101966.
Moreau CA, Raznahan A, Bellec P, Chakravarty M, Thompson PM, Jacquemont S. Dissecting autism and schizophrenia through neuroimaging genomics. Brain. 2021;144:1943–57.
Du Y, He X, Kochunov P, Pearlson G, Hong LE, van Erp TGM, et al. A new multimodality fusion classification approach to explore the uniqueness of schizophrenia and autism spectrum disorder. Hum Brain Mapp. 2022;43:3887–903.
Trakoshis S, Rocchi F, Canella C, You W, Chakrabarti B, Ruigrok AN, et al. Intrinsic excitation-inhibition imbalance affects medial prefrontal cortex differently in autistic men versus women. eLife. 2020;9:e55684.
Jardri R, Hugdahl K, Hughes M, Brunelin J, Waters F, Alderson-Day B, et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr Bull. 2016;42:1124–34.
Tatti R, Haley MS, Swanson OK, Tselha T, Maffei A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol Psychiatry. 2017;81:821–31.
Campbell OL, Weber AM. Monofractal analysis of functional magnetic resonance imaging: an introductory review. Hum Brain Mapp. 2022;43:2693–706.
Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67.
Fatemi S, Halt A, Stary J, Kanodia R, Schulz S, Realmuto G. Glutamic acid decarboxylase 65 and 67kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805.
Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–32.
Sohal VS, Rubenstein JL. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry. 2019;24:1248–57.
Dickinson A, Jones M, Milne E. Measuring neural excitation and inhibition in autism: different approaches, different findings and different interpretations. Brain Res. 2016;1648:277–89.
Ford TC, Crewther DP. A comprehensive review of the 1H-MRS metabolite spectrum in autism spectrum disorder. Front Mol Neurosci. 2016;9:14.
Canitano R, Palumbi R. Excitation/inhibition modulators in autism spectrum disorder: current clinical research. Front Neurosci. 2021;15:753274.
Juarez-Martinez EL, Sprengers JJ, Cristian G, Oranje B, van Andel DM, Avramiea A-E, et al. Prediction of behavioral improvement through resting-state electroencephalography and clinical severity in a randomized controlled trial testing bumetanide in autism spectrum disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8:251–61.
Huang Q, Velthuis H, Pereira AC, Ahmad J, Cooke SF, Ellis CL, et al. Exploratory evidence for differences in GABAergic regulation of auditory processing in autism spectrum disorder. Transl Psychiatry. 2023;13:320.
Anticevic A, Lisman J. How can global alteration of excitation/inhibition balance lead to the local dysfunctions that underlie schizophrenia? Biol Psychiatry. 2017;81:818–20.
Yang GJ, Murray JD, Wang XJ, Glahn DC, Pearlson G, Revops G, et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci USA. 2016;113:E219–E228.
Abbasi S, Wolff A, Çatal Y, Northoff G. Increased noise relates to abnormal excitation-inhibition balance in schizophrenia: a combined empirical and computational study. Cereb Cortex. 2023;33:10477–91.
Purves-Tyson TD, Brown AM, Weissleder C, Rothmond DA, Weickert CS. Reductions in midbrain GABAergic and dopamine neuron markers are linked in schizophrenia. Mol Brain. 2021;14:1–19.
Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–90.
Canitano R, Pallagrosi M. Autism spectrum disorders and schizophrenia spectrum disorders: excitation/inhibition imbalance and developmental trajectories. Front Psychiatry. 2017;8:69.
Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, et al. Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol Psychiatry. 2017;81:848–61.
Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–67.
Cho G, Yim J, Choi Y, Ko J, Lee SH. Review of machine learning algorithms for diagnosing mental illness. Psychiatry Investig. 2019;16:262–9.
Murdoch WJ, Singh C, Kumbier K, Abbasi-Asl R, Yu B. Definitions, methods, and applications in interpretable machine learning. Proc Natl Acad Sci USA. 2019;116:22071–80.
Rudin C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell. 2019;1:206–15.
Bhattacharya A Applied machine learning explainability techniques: make ML models explainable and trustworthy for practical applications using LIME, SHAP, and more. Packt Publishing Ltd. p. 18. 2022.
Speiser JL, Miller ME, Tooze J, Ip E. A comparison of random forest variable selection methods for classification prediction modeling. Expert Syst Appl. 2019;134:93–101.
Du Y, Fu Z, Sui J, Gao S, Xing Y, Lin D, et al. NeuroMark: an automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders. Neuroimage. 2020;28:102375.
van’t Wout M, Aleman A, Bermond B, Kahn RS. No words for feelings: alexithymia in schizophrenia patients and first-degree relatives. Compr Psychiatry. 2007;48:27–33.
Warrier V, Toro R, Chakrabarti B, Børglum AD, Grove J, Hinds DA, et al. Genome-wide analyses of self-reported empathy: correlations with autism, schizophrenia, and anorexia nervosa. Transl Psychiatry. 2018;8:1–10.
Kinnaird E, Stewart C, Tchanturia K. Investigating alexithymia in autism: a systematic review and meta-analysis. Eur Psychiatry. 2019;55:80–89.
Schulz MA, Bzdok D, Haufe S, Haynes JD, Ritter K. Performance reserves in brain-imaging-based phenotype prediction. Cell reports. 2024;43:113597.
Hyatt CJ, Calhoun VD, Pittman B, Corbera S, Bell MD, Rabany L, et al. Default mode network modulation by mentalizing in young adults with autism spectrum disorder or schizophrenia. Neuroimage. 2020;27:102343.
Hyatt CJ, Wexler BE, Pittman B, Nicholson, Pearlson GD, Corbera S, et al. Atypical dynamic functional network connectivity state engagement during social–emotional processing in schizophrenia and autism. Cereb Cortex. 2021;32:3406–22.
Kay SR, Opler A, Fiszbein A, Ramirez PM, White L. The positive and négative syndrome scale for schizophrenia. Schizophr Bull. 1987;3:26–76.
Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23.
First MB, Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). In: Hilsenroth MJ & Segal DL, (Eds.). Comprehensive handbook of psychological assessment. John Wiley & Sons, Inc; 2004. Vol. 2. Personality assessment. pp. 134-43.
Wechsler DS III. WAIS-III, wechsler adult intelligence scale - administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997.
Sattler JM, Ryan JJ. Assessment of children: WAIS-III supplement. 3rd ed. La Mesa, CA: Jerome M. Sattler Publisher, Inc; 1999. Rev. and Updated
Wakabayashi A, Baron-Cohen S, Wheelwright S. Individual and gender differences in empathizing and systemizing: measurement of individual differences by the empathy quotient (EQ) and the systemizing quotient (SQ). JPN J Psychol. 2006;77:271–7.
Vorst HC, Bermond B. Validity and reliability of the bermond–vorst alexithymia questionnaire. Pers Individ Dif. 2001;30:413–34.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–24.
Friston KJ, Ashburner J, Frith CD, Poline, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3:165–89.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51.
Du Y, Fryer SL, Lin D, Sui J, Yu Q, Chen J, et al. Identifying functional network changing patterns in individuals at clinical high-risk for psychosis and patients with early illness schizophrenia: a group ICA study. Neuroimage. 2018;17:335–46.
AFNI. http://afni.nimh.nih.gov/afni. 1995.
You W, Achard S, Stadler J, Brückner B, & Seiffert U Fractal analysis of resting state functional connectivity of the brain. In: The 2012 international joint conference on neural networks (IJCNN). 2012. pp. 1–8.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Flint C, Cearns M, Opel N, Redlich R, Mehler DMA, Emdem D, et al. Systematic misestimation of machine learning performance in neuroimaging studies of depression. Neuropsychopharmacology. 2021;46:1510–7.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org/ URL
You W, Achard S, Stadler J, Brückner B, Seiffert U. (2012, June). Fractal analysis of resting state functional connectivity of the brain. In The 2012 International Joint Conference on Neural Networks (IJCNN) (pp. 1–8). IEEE.
Linke AC, Chen B, Olson L, Cordova M, Wilkinson M, Wang T, et al. Altered development of the hurst exponent in the medial prefrontal cortex in preschoolers with autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2024. https://www.sciencedirect.com/journal/biological-psychiatry-cognitive-neuroscience-and-neuroimaging/articles-in-press
Rakshe C, Kunneth S, Sundaram S, Murugappan M, Agastinose Ronickom JF. Autism spectrum disorder diagnosis using fractal and non-fractal-based functional connectivity analysis and machine learning methods. Neural Comput Appl. 2024;36:12565–85.
Nishio M, Ellwood-Lowe ME, Woodburn M, McDermott CL, Park AT, Tooley UA, et al. The Hurst exponent as a marker of inhibition in the developing brain. bioRxiv 2024.09.29.615675.
Funding
Funding for this work has been provided by the National Institutes of Health (R01 MH095888, R01 MH119069 to M.A.); the National Alliance for Research in Schizophrenia and Affective Disorders (Young Investigator Award17525 to S.C.
Author information
Authors and Affiliations
Contributions
Author LCU proposed the hypotheses and approach, performed the analyses and wrote the manuscript; author CJU assisted with the analysis code and with the conceptual development; author JD created the R toolbox that was used in the classification analysis; author MK contributed to the conceptual development of this project; author VC supplied the preprocessed exploratory sample; author SC was actively involved in recruiting and testing the replication dataset; author KP provided support with the statistical analyses; authors BP and GP were actively involved in recruiting and testing the replication dataset; author MA was actively involved in recruiting and testing the replication sample, contributed to the conceptual development and analysis plan, and oversaw every step of the project. All authors contributed to the manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Participants provided written informed consent after the study had been explained to them and were paid for their time. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the Institutional Review Boards of Hartford Hospital and Yale University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Uscătescu, L.C., Hyatt, C.J., Dunn, J. et al. Using the excitation/inhibition ratio to optimize the classification of autism and schizophrenia. Transl Psychiatry 15, 234 (2025). https://doi.org/10.1038/s41398-025-03455-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03455-8