Abstract
SMC3 is a chromatin binding factor that plays central roles in genome organization and in proper neurodevelopment. Mutations in SMC3 gene (SMC3) induce neurodevelopmental and behavioral phenotypes in humans, including changes in anxiety behavior and self-injury. However, it is not clear what are the exact roles of SMC3 in behavior in adulthood or if its effects are only developmental. Using an adult forebrain excitatory neuron specific Smc3 knockout mouse model, the current study determined specific sex-dependent effects of SMC3 ablation during adulthood. Behavioral tests identified anxiolytic effects of Smc3 knockout in females and anxiogenic effects in males four weeks after initiation of adult knockout. The prefrontal cortex, a regulator of anxiety behavior, also displayed sex-dependent effects in dendritic branching. Transcriptional analysis revealed gene expression effects of Smc3 knockout in males and females, including changes in anxiety-related genes and relevant transcriptional pathways. While effects on anxiety behavior was sex-specific, both males and females developed self-injury behavior at approximately ten weeks after induction of knockout. The current study suggests that neuronal SMC3 modulates anxiety during adulthood in a sex-specific manner.
Similar content being viewed by others
Introduction
SMC3 (Structural maintenance of chromosomes protein 3) is a core component of the cohesin complex. Cohesin is a highly conserved protein complex that is crucial for the cell cycle and is most known for its ability to mediate sister chromatid cohesion during cell replication [1, 2]. The capability of loading on DNA enables the cohesin complex to change the chromatin structure by preventing or promoting specific chromatin interactions, leading to its important regulatory role in gene expression [3, 4].
Human de novo mutation in cohesin members, including the SMC3 gene (SMC3), leads to cohesinopathies, of which Cornelia de Lange syndrome (CdLS) is the most common [5]. Behavioral phenotypes of CdLS include self-injury, intellectual disabilities, and anxiety together with physical abnormalities [6]. The syndrome affects both sexes [6,7,8]. The literature has limited specific information regarding sex differences in CdLS, despite the growing awareness of sex-specific contributions to biological and behavioral phenotypes [9]. The clinical presentation of CdLS remains highly variable, based on the gene and variant type involved or owing to yet unknown factors. Particularly, the clinical picture of SMC3 mutation is characterized by a mild CdLS phenotype [10, 11] including psychomotor retardation and postnatal growth deficiency [12]. The most in-depth comparison of individuals with Smc3 mutations found that among 16 individuals (11 of them children, and five adults), two showed self-injurious behavior and other behavioral phenotypes varied from aggression and autism to high sociability and agreeableness [13]. Therefore, there is need to understand what variables and mechanisms may underly different behavioral phenotypes induced by Smc3, and if these phenotypes will be present or changed in the adulthood.
Previous studies in a male mice model showed that homozygote knockout of Smc3 leads to embryonic lethality [14], while a heterozygous knockout male mice model displayed anxiety-like behavior in adulthood, abnormal dendritic harboring at six weeks of age, and transcriptional changes at developmental time points between newborns and four weeks of age [15]. However, the specific role of SMC3 in post developmental maintenance of neuronal function and sex-specific effects has not been determined.
To understand the function of SMC3 in the adult brain, a neuron-specific knockout of Smc3, specifically in adulthood, was established. Adult knockout of Smc3 revealed distinct effects in males as opposed to females, including anxiety-like behavior, morphological-neuronal changes in different brain regions, and transcriptional changes that were translated to biological pathways. These results suggest that genome organizational or epigenetic changes translate in different manner depending on sex, and demonstrate, while SMC3 protein has an important role in maintainingbiological process in the adulthood.
Results
Knockout of Smc3 gene in excitatory neurons
To understand the role of neuronal SMC3 protein in adult brain in behavior, we crossed Camk2a-cre/ERT2/floxed Smc3 (on exon 4) mice with floxed Smc3 mice [16]. Therefore, all offspring are homozygote for floxed Smc3, and knockout mice lack Smc3 in both alleles. Camk2a promoter promotes expression specifically in forebrain excitatory neurons, and Camk2a-cre/ERT2 mice have been extensively used to study the function of specific genes in these cell types [17,18,19]. Tamoxifen was given by gavage at eight weeks of age, followed by immunohistochemistry five weeks after treatment to determine knockdown of immunostaining of SMC3 (Fig. 1A–C). Immunohistochemistry in the dentate gyrus of the hippocampus demonstrates the knockout of SMC3 (Fig. 1A, B). We further performed immunohistochemistry in the cortex, where we see can see that SMC3 is present in NeuN positive (neuronal nuclei marker) cells in the wild type, but is absent in these cells in the Smc3 cKO. Nonetheless SMC3 is still found in many of the NeuN negative cells in the Smc3 cKO mice, as expected, demonstrating the cell-type specificity of the knockout.
A Representative pictures of hippocampus (dentate gyrus) in wild type and Smc3 knockout mice 5 weeks after tamoxifen treatment. Hippocampi were stained with antibodies for NeuN (neuronal marker), SMC3, and Hoechst (marker for nuclie) Scale bars present 100 μm. B quantification of SMC3 positive cells in dentate gyrus of both groups (two-sided t-test WT, n = 8; cKO, n = 12; ****P < 0.0001). C Representative pictures of Cortex cells in wild type and Smc3 knockout mice 5 weeks after tamoxifen treatment; Scale bars present 5μm. Arrows point to NeuN positive cells.
Alteration in exploratory pattern as result of Smc3 conditional knockout in adult brain
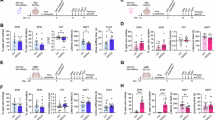
Initial behavioral experimentation was performed with a battery of anxiety-related tests. Open Field test revealed a decrease of visits to the center area in male Smc3 cKO mice compared to wild type (WT) controls (Fig. 2B). In addition, male Smc3 cKO mice displayed less distance traveled in the center area (Fig. 2C), despite no differences between genotypes in total distance traveled in the entire arena (Fig. 2D). In contrast, female Smc3 cKO mice displayed increased visits to the center of the arena, compared with WT controls (Fig. 2E). In addition, female Smc3 cKO mice displayed increased distance traveled in the center area, despite no differences between genotypes in total distance traveled (Fig. 2F, G). Together, these findings suggest increased anxiety in male Smc3 cKO mice and decreased anxiety in female Smc3 cKO mice.
A Graphical presentation of experimental timeline shows the time point of each test. Separate boxes represent separate groups of mice used for those specific experiments. B–G Open field test of male and female mice at 4 weeks after tamoxifen treatment. B Measurements of male mice, visiting the center in an Open field test, show a significant decrease in cKO group compared to WT (two-sided t-test WT, n = 14; cKO, n = 9; **P < 0.01, **P = 0.0093). C Measurements of distance traveled in the center region revealed a significant decrease in the male cKO group compared to the WT group (two-sided t-test WT, n = 14; cKO, n = 9; *P < 0.05, *P = 0.0182). D Analysis of total distance traveled by male mice in the Open Field Test displayed no significant differences between the WT and cKO groups (two-sided t-test WT, n = 14; cKO, n = 9; ns (not significant) P = 0.4972). E Measurements of female mice behavior in the Open Field Test revealed a significant increase in visits to the center in the cKO group compared to the WT group (two-sided t-test, WT, n = 11; cKO, n = 11; *P < 0.05, *P = 0.0402). F Measurements of distance traveled in the center region in female mice demonstrated a significant increase in the cKO group compared to the WT group (two-sided t-test WT, n = 11; cKO, n = 11; *P < 0.05, *P = 0.0121). G Analysis of total distance traveled by female mice in the Open Field Test displayed no significant differences between the WT and cKO groups (two-sided t-test WT, n = 14; cKO, n = 9; ns (not significant) P = 0.4550). H–L Elevated Plus Maze test of male and female mice at 4–5 weeks after tamoxifen treatment. H Quantification of the number of visits to the open arms during the Elevated Plus Maze Test in male mice. The cKO group show a significant decrease in the number of visits compared to the WT group (two-sided t-test WT, n = 14; cKO, n = 9; **P < 0.01, **P = 0.0016). I Quantification of time spent in open arms, closed arms, and center area during the Elevated Plus Maze Test in male mice. The cKO group show a significant reduction in open arms (two-sided t-test WT, n = 14; cKO, n = 9; **P < 0.01, **P = 0.001168) compared to the WT group. J Quantification of the number of visits to the open arms during the Elevated Plus Maze Test in female mice. The cKO group show a significant increase in the number of visits compared to the WT group (two-sided t-test WT, n = 11; cKO, n = 11; P < 0.05, *P = 0.0242). 1 mouse were taken out from cKO group. K Quantification of time spent in open arms, closed arms, and center area during the Elevated Plus Maze Test in female mice. The cKO group show a significant increase in center area (two-sided t-test WT, n = 11; cKO, n = 11; ***P < 0.001, ***P = 0.000486) and decrease in closed arms (two-sided t-test WT, n = 11; cKO, n = 11; **P < 0.01, **P = 0.007208) compared to the WT group. 1 mouse were taken out from cKO group. L Representative pictures and heatmap illustrating the behavior of both sexes cKO mice compared to their littermate WT in the Elevated Plus Maze. Heatmap representation of the spatial distribution of time spent in open arms. The color gradient indicates the frequency of visits, with warmer colors representing higher rates. Heatmaps are produced by the Ethovision tracking software (M) Comparison of contextual fear conditioning results, percentage of freezing time during tones in male mice between WT and cKO, 5 weeks after conditional knockout induced, revealed no significant differences in freezing behavior (two-sided t-test WT, n = 7; cKO, n = 11; ns (not significant) P = 0.6350). N Analysis of average freezing behavior during cue test in male mice revealed no significant differences between the WT and cKO groups (two-sided t-test WT, n = 7; cKO, n = 12; ns (not significant) P = 0.9895). O Analysis of the Rotarod test results show the latency to fall in male mice between WT and cKO groups demonstrating no significant differences as result of Smc3 knockout 4 weeks after knockout induced (two-sided t-test WT, n = 14; cKO, n = 9; ns (not significant) P = 0.2980). P Contextual fear conditioning results in female mice, for WT and cKO groups, 5 weeks after conditional knockout induced, revealed no significant differences in freezing behavior (two-sided t-test WT, n = 13; cKO, n = 12; ns (not significant), P = 0.17). Q Similarly, in female mice, the results of the Cue Test showed no significant differences in average freezing behavior between the WT and cKO groups (two-sided t-test WT, n = 13; cKO, n = 12; ns (not significant), P = 0.3022). R Analysis of the Rotarod test results show the latency to fall in female mice between WT and cKO groups demonstrating no significant differences as result of Smc3 knockout, 4 weeks after knockout induced (two-sided t-test WT, n = 12; cKO, n = 12; ns (not significant) P = 0.1983).
In the Elevated Plus Maze Test, male Smc3 cKO mice displayed a decrease in visits in the open arms and time spent in the open arms compared to WT controls (Fig. 2H, I). Conversely, female Smc3 cKO mice showed a significant increase in visits to the open arms, as well as a significant decrease in the amount of time in the closed arms. (Fig. 2J, K). Interestingly, female wild type mice displayed less time in open arms than male wild type mice (unpaired t test, p-value = 0.002). These results verify the anxiogenic behavior in male Smc3 cKO mice and anxiolytic behavior in female Smc3 mice. In order to determine if these effects are due to Smc3 deletion, or if these anxiety phenotypes may off-target effects of the tamoxifen-induced expression of Cre recombinase, we performed these experiments on a separate group of Camkiia-cre-ert positive and negative mice that are not floxed in the Smc3 alelle. There were no differences between genotypes, further suggesting that Smc3 deletion is responsible for sex-dependent changes in anxiety behavior (Supplementary Fig. 1).
In contextual fear conditioning test, there were no differences between in the genotypes in freezing in either males or females (Fig. 2M, P). In the Cue-dependent test, another component of fear conditioning memory, both sexes revealed no significant differences between the WT and cKO groups (Fig. 2N, Q). In the Rotarod test, an assessment of motor coordination and balance, indicate a lack of significant differences in the latency to fall between the WT and cKO groups in both male and female mice (Fig. 2O, R). Sociability was examined in the three-chambered social test (Supplementary Fig. 2), showed that there were no differences between genotypes in either males or females.
Progressive self-injury phenotype induced by excitatory cell-specific Smc3 knockout in adult brain
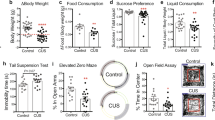
At approximately 9 weeks following tamoxifen administration, both male and female Smc3 cKO mice displayed injuries that were mainly on the head and upper back (Fig. 3A). To determine if these were self-injuries, we housed mice in single cages, and found that Smc3 cKO mice showed these injuries even when housed one mouse per cage. Therefore, these injuries are self-inflicted, similar to the self-injurious behavior that is often seen in humans with Cornelia De Lange syndrome. To understand if these injuries could be associated with dysregulated self-grooming, a classic repetitive behavior in mice, we tracked self-grooming. Both male and female Smc3 cKO mice, displayed increased self-grooming compared to WT mice (Fig. 3C, D). However, marble burying, a separate index of repetitive behavior in mice, was unchanged between the genotypes (Fig. 3C, E).
A Representative images of self-injury observed on the upper side of the head and around the eyes in mice from the cKO group at 12 weeks post-Smc3 knockout. B Grooming test in male mice revealed significantly elevated grooming behavior in the cKO group compared to WT littermates at 9 weeks post-Smc3 knockout (two-sided t-test WT, n = 11; cKO, n = 12; **P < 0.01, P = 0.0058). C No significant differences are observed between the cKO and WT groups in the marble burying test for male mice at 9 weeks post-knockout (two-sided t-test WT, n = 11; cKO, n = 12; ns (not significant) P > 0.05, P = 0.4197). D Grooming test in female mice revealed similar results to the male group, with significantly elevated grooming behavior in the cKO group compared to WT littermates at 9 weeks post-Smc3 knockout (two-sided t-test WT, n = 9; cKO, n = 11; *P < 0.05, P = 0.0287). 1 mouse were taken out from WT group and 1 mouse from cKO group due to being extreme outliers (three standard deviations from the average). E Marble burying test for female mice consistent with males, cKO group do not show significant differences in the buried marbles compared to WT at 9 weeks post-knockout (two-sided t-test WT, n = 10; cKO, n = 12; ns (not significant) P > 0.05, P = 0.4997).
Sex-specific change in dendritic branching in Smc3 cKO mice
To determine how Smc3 knockout may affect neuronal morphology, golgi staining was performed in multiple brain regions on behaviorally naïve mice. In the prefrontal cortex (PFC), male Smc3 cKO mice displayed a significant decrease in dendritic branching compared to WT, while female Smc3 cKO mice displayed an increase in dendritic branching compared to WT (Fig. 4A, B). The amygdala revealed an increase in dendritic intersections for Smc3 cKO mice compared to wild type controls in both sexes (Fig. 4C, D). In contrast, in the hippocampal regions CA1 and DG, Smc3 cKO mice displayed decreases in dendritic branching in both sexes (Fig. 4E–H). Therefore, the prefrontal cortex was the one region, among the tested regions, that displayed sex-dependent changes in neuronal morphology.
Morphological changes observed in different brain regions at 5 weeks post-Smc3 gene knockout, emphasizing the sex-specific alterations in dendritic intersections. Graphs show average dendritic interactions per neuron at every micron from the soma +/- Standard Error for each genotype. A Males’ prefrontal cortex (PFC) at 5 weeks after cKO show decreased dendritic intersections compared to WT (WT, n = 89 neurons from14 mice, R2 = 0.5138; cKO, n = 50 neurons from 5 mice, R2 = 0.3716; CI = 99%; ***P < 0.001, Non-linear regression). B In contrast, females in the cKO group demonstrate a significant increase in the prefrontal cortex compared to WT at 5 weeks post-knockout (WT, n = 50 neurons from 6 mice, R2 = 0.4308; cKO, n = 49 neurons from 8 mice, R2 = 0.5366; CI = 99%; ***P < 0.001, Non-linear regression). C Males’ amygdala at 5 weeks after cKO show increase dendritic intersections compared to WT (WT, n = 52 neurons from 14 mice, R2 = 0.4536; cKO, n = 33 neurons from 5 mice, R2 = 0.4456; CI = 99%; ***P < 0.001, Non-linear regression). D Similar to males, females in the cKO group display a significant increase in dendritic intersections in the amygdala compared to WT (WT, n = 29 neurons from 6 mice, R2 = 0.3242; cKO, n = 41 neurons from 8 mice, R2 = 0.3855; CI = 99%; ***P < 0.001, Non-linear regression). E Male cKO group showed a significant decrease in dendritic intersections in the hippocampal region CA1 compared to WT (WT, n = 51 neurons from 14 mice, R2 = 0.4344; cKO, n = 31 neurons from 5 mice, R2 = 0.4738; CI = 99%; ***P < 0.001, Non-linear regression). F Similar to males, females in the cKO group display a significant decrease in dendritic intersections in the hippocampal region CA1 compared to WT (WT, n = 20 neurons from 6 mice, R2 = 0.2716; cKO, n = 23 neurons from 8 mice, R2 = 0.4522; CI = 99%; ***P < 0.001, Non-linear regression). G In the hippocampal region DG, males in the cKO group show a significant decrease in dendritic intersections compared to WT (WT, n = 35 neurons from 14 mice, R2 = 0.1576; cKO, n = 29 neurons from 5 mice, R2 = 0.2032; CI = 99%; ***P < 0.001, Non-linear regression). H Similar to males, females in the cKO group display a significant decrease in dendritic intersections compared to WT (WT, n = 20 neurons from 6 mice, R2 = 0.2229; cKO, n = 22 neurons from 8 mice, R2 = 0.3037; CI = 99%; ***P < 0.001, Non-linear regression).
Transcriptomic analysis of prefrontal cortex in Smc3 knockout mice
Transcriptomic analysis of the prefrontal cortex in both behaviorally naïve male and female mice 6 weeks after Smc3 knockout was performed through RNA sequencing. At a statistical cutoff of FDR < 0.05, Male Smc3 cKO mice displayed 87 upregulated and 222 downregulated genes compared to male wild types (Fig. 5A, Supplementary Table 1). In contrast, female Smc3 cKO mice displayed 242 upregulated and 626 downregulated genes (Fig. 5B, Supplementary Fig. 2). Therefore, while Smc3 deletion induced a higher level of downregulated genes than upregulated genes in both sexes, there were more differentially expressed genes in females. Among downregulated genes, 171 overlapped between males and females (hypergeometric test p = 1.21e–220), however there were still 51 male-specific genes and 445 female specific genes, suggesting sex-specific regulation of gene expression (Fig. 5C). Among upregulated genes, 37 overlapped between males and females (hypergeometric test p = 1.609988735825237e–48), however there were 50 male-specific genes and 205 female specific genes. We further looked at what were the gene ontology categories in male-specific and female-specific differentially regulated genes (Fig. 5D). Male-specific gene-ontology categories for downregulated genes include a downregulation of the somatostatin receptor activity and neuropeptide Y receptor pathways (Fig. 5E), both of which are involved in anxiety response. In contrast, female-specific gene-ontology pathways include downregulation in the fear response pathway (including genes such as Crh and Crhr1) and in the dopamine and GABAergic pathways (Fig. 5F). Therefore, different fear-related cortical gene expression pathways were affected by SMC3 deletion between the sexes, which may partly explain the different morphological and behavioral phenotypes between male and female Smc3 cKO mice. The differentially expressed gene sets from the current study were then compared to gene sets from three separate studies of cohesin-depletion in neurons. There was an extremely significant overlap between our differentially expressed genes and the genes from the Calderon et. al. study [20] in both the downregulated genes and upregulated genes (Fig. 5G, H, Supplementary Table 3). There was a significant overlap between our differentially expressed genes and genes from the Weiss et. al. study [21] in downregulated genes, but not upregulated genes (Fig. 5G, H). Both those studies looked at genes that were effected by cohesin depletion in post-mitotic neurons. We also compared our genes to the differentially expressed genes in the Fujita et. al. study [15], who performed RNA-seq at several early developmental time points of Smc3 haploinsufficient mice. There was no overlap between our dataset and their dataset (Fig. 5G, H). This suggests that there are significant differences in the effects of Smc3 depletion in adulthood or postmitotic neurons compared to depletion in very early development.
At the 6-week mark post-induction of Smc3 knockout through tamoxifen treatment, RNA sequencing was performed from prefrontal cortex (PFC), uncovering differently gene expression profiles. A Volcano plot shows distribution of differently expressed genes of Smc3 cKO male mice compared to WT—87 upregulated genes and 222 downregulated genes. Threshold for differentially expressed genes is FDR < 0.05. B Volcano plot shows distribution of differently expressed genes of Smc3 cKO female mice compared to WT—242 upregulated genes and 616 downregulated genes. C Venn diagram shows overlap and unique sets of downregulated genes identified through RNA sequencing in the prefrontal cortex of male and female mice 6 weeks post-Smc3 knockout induction. D Venn diagram shows overlap and unique sets of upregulated genes identified through RNA sequencing in the PFC of male and female mice. E Gene ontology analysis of differentially expressed genes in males. F Gene ontology analysis of differentially expressed genes in females. G A comparison of differentially expressed genes from our dataset (X axis) and datasets of previous studies that performed RNA sequencing following the knockout of various cohesin components. The graph shows numbers of overlapping downregulated genes from each dataset. Each comparison was analyzed for statistical significance using hypergeometric distribution probability (p-value indicated in parentheses). H A comparison of differentially expressed genes from our dataset (X axis) and datasets of previous studies that performed RNA sequencing following the knockout of various cohesin components. The graph shows numbers of overlapping upregulated genes from each dataset. Each comparison was analyzed for statistical significance using hypergeometric distribution probability (p-value indicated in parentheses).
Discussion
The present study focuses on the role of SMC3 protein, a core component of the cohesin complex, in the adult brain, shedding light on its function on behavioral, morphological, and transcriptional effects. Fujita et al. previously found that depletion of Smc3 leads to defective synapse development and anxiety-related behavior [15]. Of importance, the previous study only looked at male mice. In the present study, we found that SMC3 in the adult brain has a sex-specific role in anxiety-like behavioral changes.
Behavior tests identified opposite behavior between male and female Smc3 cKO mice. To identify which anxiety-related brain regions may be responsible for the sex-dependent effects of Smc3 knockout on anxiety, Golgi staining was performed on multiple brain regions on behaviorally naïve mice. While several brain regions displayed dysregulated dendritic branching, the only brain region to show opposite effects between males and females was the prefrontal cortex. Since prefrontal cortex activity generally decreases anxiety-behavior, the increased dendritic branching in the prefrontal cortex of female Smc3 cKO mice may partially explain the decreased anxiety in these mice. In contrast, the decreased dendritic branching in the prefrontal cortex of the male Smc3 cKO mice may partially explain the increased anxiety in these mice. Fujita et. al. also found a decrease in dendritic branching in the cortex of male mice that were deficient in Smc3 in developmental time points. However, females were not examined in their study. It is also interesting to note the differences between female wild type and female Smc3 cKO mice were mostly in dendritic branching in areas proximal to the soma. In comparison, the differences between male wild type and male Smc3 cKO mice were mostly in dendritic branching in areas distal to the soma. Keil et. al. found that males and females have different dendritic branching due to an estrogen-dependent mechanism [22]. A separate study further found that female hippocampal neurons are more branched at proximal regions while male hippocampal neurons are more branched at distal regions, although no mechanism is known for this difference [23]. Therefore, SMC3, and downstream mechanisms may play a role in sex-specific dendritic branching.
Considering that the prefrontal cortex displayed sex-dependent morphological changes in Smc3 cKO mice, we further determined what molecular changes are present in the frontal cortex that may promote the sex-dependent differences. Gene expression analysis of Smc3 cKO mice revealed that both sexes display higher amounts of downregulated genes than upregulated genes. These findings correlate well with previous in-vitro neuronal studies, where deletion of cohesin-complex members induce gene expression changes with an emphasis on downregulation of genes [20, 21]. Females displayed a decrease in fear-related genes, including Crh, Crhr1, and Adra2a. While hypothalamic Crh is directly involved in anxiety behavior, it has also been shown that cortical Crh can increase anxiety [24]. Males did not display a significant decrease in this pathway, giving a possible explanation for why males did not display an anxiolytic effect. Female Smc3 cKO mice also displayed a downregulation of GABAergic-related genes in the frontal cortex. Decrease in GABAergic tone in the prefrontal cortex may lead to increased activity of the prefrontal cortex [25], which can also decrease levels of anxiety. Male mice displayed a decrease in genes related to the somatostatin signaling pathway. A previous study found that loss of function of somatostatin excitatory neuronal cells in mice is a trigger for repetitive self-grooming and anxiety elevation [26]. This may also partially explain the anxiety-like behavior that was seen in male mice.
By 9 weeks after induction of Smc3 knockout, both male and female mice manifest a phenotype of self-injury. The finding of self-injury in the current mouse model is especially important considering that self-injury is one of the primary behavioral phenotypes in individuals with Cornelia De Lange syndrome, and has previously been found in a subset of individuals with Smc3 mutations [13]. This phenotype has not been reported in previous models of this disorder, and therefore this model may serve well for future preclinical research. Although both sexes displayed an increased self-injury in this study it is not clear if this phenotype is the result of the same molecular pathway or which brain region is involved. It is possible that the decrease of the prefrontal GABAergic pathway in females is involved, considering that decreased GABAergic signaling in the frontal cortex has been associated with increased self grooming [27]. Males, in contrast displayed a decrease in Neuropeptide Y-related gene expression. It has been described previously that activation of neuropeptide Y neurons inhibit the mechanical itch as well as the chemical itch, and knockdown mice models of Npy1r present enhanced itching [28]. In a parallel study of these mice, we have found that Smc3 cKO mice display weight gain, starting at week five after tamoxifen treatment [29]. However, this weight gain is not sex-dependant, and both weight gain, and related hormonal and appetite phenotypes were found in both males and females. In the current study, we finished our behavioral analysis before five weeks, so that weight gain wouldn’t be a cofounding factor. Since metabolic phenotypes are the same in both males and females, they are also unlikely directly involved in the sex-dependant behavioral phenotypes. However, there could be some unknown involvement in how the two sexes respond to the metabolic findings.
There are several limitations to this study which should be kept in mind when interpreting the results. First, this is a complete adulthood knockout, and not a haploinsufficient model. Humans with the mutation are haploinsufficient, and therefore it is not clear if one working allele for Smc3 will compensate for some of the observed phenotypes. Secondly, while this work determines the role of Smc3 in excitatory neurons, it is still not clear what is the role of Smc3 in inhibitory neurons or glia. Furthermore, it is still not clear what is the mechanisms through which Smc3 in the postmitotic neurons are affecting gene expression. Future studies looking at genome conformation on these mice and similar models would be necessary to decipher these mechanisms.
In conclusion, our study demonstrates a role for Smc3 in regulating anxiety in a sex specific manner during adulthood, as well as regulating self-injury behavior in both sexes. The sex-specific effects underscore the importance of considering sex as a critical variable in studies exploring the molecular basis of complex behaviors. These findings contribute to our broader understanding of cohesinopathies, such as Cornelia De Lange syndrome, and provide a foundation for future investigations into the gender-specific mechanisms as part of brain function.
Materials and methods
Mouse model
All experimental procedures were conducted in accordance with the Federation of European Laboratory Animal Science Associations (FELASA) and the National Institutes of Health regulations and were approved by the Bar-Ilan University Institutional Animal Care and Use Committee (IACUC), protocols ID: BIU-MD-IL-2206-151-3 and BIU-MD-IL-2301-102-5.
The mice were bred and housed in a vivarium in Plexiglas cages, with constant temperature and humidity (22 °C, 50%) 12-h light/dark cycle, food (#1234 TPF, Altromin, Lage, Germany) and water provided ad libitum.
To generate post-mitotic neuronal-specific Smc3 knockout mice, C57/Bl6 Smc3 loxp/loxp (floxed Smc3) female mice with were crossed with Camk2a-cre/ERT2+ (Cre + ) floxed Smc3 C57/Bl6 males. Therefore, offspring are all floxed Smc3 (Smc3 loxp/loxp), and half are Camk2a-cre/ERT2+ (Cre+) while half are Camk2a-cre/ERT2− (Cre−). Mice with Cre+ named as cKO due the lack of Smc3 gene after tamoxifen treatment. Mice with Cre− served as WT. All mice were treated with tamoxifen (100 mg/kg). The CAMKIIa-Cre-ERT line was kindly provided by Prof. Alon Chen (Weizmann Institute of Science) [16]. Floxed Smc3 animals were purchased from Jackson Laboratory (stock: 030559) (Bar Harbor, Maine, USA) [30]. Supplementary Table 4 shows number of births from each genotype of several breedings, showing that genotypes were produced by the expected mendelian ratio.
Behavioral testing
All behavioral testing was performed at least 4 weeks after tamoxifen administration. All behavioral tests were recorded and were automatically scored and analyzed by the EthoVision XT-14 software (Noldus). All analysis was done blinded to genotype. Different mice were used for different behavioral tests as presented in Fig. 2A. Mice were excluded from an experiment if they left the experimental maze during the experiment or were extreme outliers (three standard deviations from the mean).
Open field test
Test was conducted by placing mice in a corner of a square arena made from Non-Glare Perspex (50 × 50 cm) under an illumination of 40 lux. The 10-min trial aimed to assess locomotion by measuring the total distance moved and to evaluate anxiety-like behavior by quantifying the time spent in the central region (25 × 25 cm).
Elevated plus maze test
This test was used to evaluate anxiety-related behaviors in mice, focusing on their exploration of the safe and unsafe zones of the maze. An X-shaped apparatus comprising two open arms (30 by 5 cm, white plastic) and two closed arms (15 cm high walls) was used for the test. The maze was positioned 55 cm above the floor, maintaining a light intensity of 15 lux. The mouse was initially placed in the central square (5 by 5 cm) of the maze and allowed to navigate among the arms for 5 min. Analysis included the frequency and cumulative duration of visits to the center, open, and closed arms.
Contextual and cued fear conditioning test
The cue fear conditioning test was utilized to study memory and learning abilities in mice. The initial day was allocated for training and habituation. Mice were introduced into the test chamber (10.5 × 10.5 × 10.5 cm) for five minutes. A 75 dB tone served as the conditioned stimulus for 30 s, followed by a brief, mild foot shock (0.7 mA) lasting two seconds as the unconditioned stimulus. After a one-minute interval, another tone-shock pair was administered, and subsequently, the mouse was returned to its home cage one minute after the second tone-shock pairing. The following day, mice were placed in a different chamber with novel sensory cues (odor, flooring, and lighting) for cue-dependent memory assessment. After a two-minute habituation period, the tone was presented three times for 30 s each, with one-minute intervals between tones. Freezing behavior during the three-tone presentations was recorded as a measure of cue-dependent memory recall.
Three-chambered social test
Test was conducted in a Non-Glare Perspex box measuring 60 × 40 cm, featuring two partitions dividing it into three chambers (left, center, and right, each 20 × 40 cm). The mouse underwent a 5-min habituation period in the middle chamber, with entry to the side chambers barred. Subsequently, during a 10-min test phase, the mouse had the freedom to explore the entire arena, choosing between interacting with a novel mouse in one chamber or remaining in an empty chamber. Following this period, a second stranger mouse was introduced to the empty chamber, and the test mouse was given an additional ten minutes to freely choose between interacting with the novel or familiar mouse.
Grooming test
Each mouse is placed individually into the open field chamber. After 10 min habituation period, each mouse was scored using parameters of cumulative time spent grooming during a 20 min session.
Marble buying test
Using a Non-Glare Perspex apparatus measuring 20 × 40 cm. A grid of twenty green glass marbles (15 mm in diameter) was arranged in a 4 × 5 pattern, covering 2/3 of the apparatus on top of 5 cm of clean bedding. Each mouse was placed in a marble-free corner and given a 30-min exploration period. Subsequently, the number of marbles buried, defined as being 2/3 covered by bedding, was counted. The test was conducted under dim light conditions (25 lux).
Rotarod test
Prior to the test, mice underwent a habituation session to familiarize them with the apparatus. During habituation, each mouse was placed on the stationary rod for 1–2 min to minimize stress and anxiety. Following habituation, mice underwent two training trials on two consecutive days, in which they were placed on the rod rotating (MedAssociates, St. Albans, VT) at a constant speed (40 rpm) for 60 s to allow them to adjust to the movement. For the test, each mouse was placed on the rotating rod, and the speed was fixed (40 rpm), the latency to fall was recorded. A cutoff time (ten minutes) was set to avoid excessive trial duration.
Immunohistochemistry
Mice were injected with veterinary Pentobarbital sodium - Pental 160 mg/kg (CTS Chemical Industries, Ltd, Hod-Hasharon, Israel, #077299195200), perfused with 4% paraformaldehyde followed by perfusion with saline. Brains were dissected and fixed overnight at 4 °C in 4% paraformaldehyde, then incubated with 30% sucrose. Brains were sectioned to 30 μm floating slices by sliding microtome. Slices were blocked for one hour in blocking solution (10% horse serum, 10% triton and TBS). Incubation with primary antibodies performed overnight at room temperature including αSMC3 (Bethyl, Montgomery, Texas, USA, #A300-060A) (1:1000) and NeuN (Merck Millipore, Darmstadt, Germany, #MAB377) (1:200). Incubation for one hour at room temperature was made with secondary antibody: Goat αRabbit Alexa flour 488 (Invitrogen Rhenium, Modi’in, Israel, #A-11008) (1:200) or Goat αMouse Cy-3 (Invitrogen, #A10521) (1:500). At the end Hoechst (Sigma-Aldrich, Rehovot, Israel, #94403) (1:1000) was used for 10 min, before covering with Shandon-mount (Thermo scientific, Rhenium, Modi’in, Israel, #1900331), as a nuclear dye to visualize cell nuclei. Immunohistochemistry images were taken with Zeiss AxioImager M2 with the apotome system or Axio Scan.Z1 microscope (Zeiss, Jena, Germany), processed with Zen pro software (version 3.3.89.0000, windows).
Golgi staining and sholl analysis
Fresh dissected brains of behaviorally naïve mice were stained according to instruction of SuperGolgi kit (Bioenno Tech, LLC, Santa Ana, California, USA, #003010). Briefly, brains were washed with PBS and placed for two weeks of impregnation. Coronal sections of the brains to 175 μm was performed using vibrotome Leica VT 1200 s. Sections were mounted to glass slides and staining using the kit reagents following manufacturer’s instructions.
Stack images of neurons from prefrontal cortex, amygdala and hippocampus were acquired using Zeiss AxioImager M2 (Zeiss, Jena, Germany) with apotome system (with ORCA-Flash 4.0 V3 camera). Images were acquired as Z-stack with 0.5 μm intervals at 40× magnification. Dendritic branching (sholl analysis) was calculated via automated filament reconstruction in Imaris (version 9.9 windows).
RNA-seq
RNA from frozen brain punches of behaviorally naïve mice was purified using the RNeasy mini kit (Quigen, Ilex, Petah-Tikva, Israel, # 74004) according to the manufacturer’s instructions. Quality of isolated RNA was tested using the Agilent RNA Pico 6000 kit and Bioanalyzer 2100 at the Genome Technology Center of the Azrieli Faculty of Medicine, Bar-Ilan University. 350 ng of total RNA were used for mRNA enrichment using NEBNext mRNA polyA isolation module (NEB, Ornat, Rehovot, Israel, #E7490L) and libraries for Illumina sequencing were prepared using the NEBNext Ultra II RNA kit (NEB, Ornat, Rehovot, Israel, #E7770L). Quantification of the library was performed using dsDNA HS assay kit and Qubit (Molecular Probes, Life Technologies, Rhenium, Modi’in, Israel) and quantification was performed using the Agilent DNA HS kit and Bioanalyzer 2100. 10 nM of each library were pooled together and were diluted to 4 nM according to NextSeq manufacturer’s instructions. 1.1 pM was loaded into the flow cell with 1% PhiX library control. Gene ontology analysis on differentially expressed genes was performed using the Toppgenetool (https://toppgene.cchmc.org/) [31]. Accession numbers for the raw data files for RNA-seq analysis reported in this paper is GEO: GSE270835.
Bioinformatics for RNA-seq
Poly-A/T stretches and Illumina adapters were trimmed from the reads using cutadapt [32]; resulting reads shorter than 30 bp were discarded. Reads were mapped to the M. musculus reference genome GRCm39 using STAR [33], supplied with gene annotations downloaded from Ensembl (and with EndToEnd option and outFilterMismatchNoverLmax was set to 0.04). Expression levels for each gene were quantified using htseq-count [34], using the gtf above. There was an average of 14,834,686 mapped reads per sample (minimum 12,777,895 and maximum 16,901,382). This represented approximately 75% of total reads. Differentially expressed genes were identified using DESeq2 [35] with the betaPrior, cooksCutoff and independentFiltering parameters set to False. Raw P values were adjusted for multiple testing using the procedure of Benjamini and Hochberg. We considered a gene significant at FDR < 0.05. Pipeline was run using snakemake [36].
Statistical analysis
Data were analyzed with GraphPad Prism (Windows, version 9.5). In-vivo data and Golgi staining for morphology are presented as mean ± standard error of the mean (SEM), when n values present biological repeats. Different statistical tests were used as appropriate for each experiment. The statistical significance was calculated using unpaired two-tailed t-test with confidence level of 95%. One-way ANOVA and Two-way ANOVA followed by Tukey-Kramer or Dunnett’s posthoc test. Third-order polynomial Non-linear regression curve was performed for dendritic branching of sholl analysis, with confidence level of 99%. Hypergeometric tests were used to determine the overlap of differentially expressed genes datasets in Fig. 5.
All values were considered as significant if P < 0.05 — For all the figures *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns P > 0.05 not significant.
Data availability
The RNA-seq data produced in this study is available at GSE270835. Any other data can be provided by request from the corresponding author.
References
Nasmyth K. How might cohesin hold sister chromatids together? Philos Trans R Soc Lond B Biol Sci. 2005;360:483–96.
Wong RW. An update on cohesin function as a ‘molecular glue’ on chromosomes and spindles. Cell Cycle. 2010;9:1754–8.
Peters JM, Tedeschi A, Schmitz J. The cohesin complex and its roles in chromosome biology. Genes Dev. 2008;22:3089–114.
Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet. 2009;43:525–58.
Piché J, Van Vliet PP, Pucéat M, Andelfinger G. The expanding phenotypes of cohesinopathies: one ring to rule them all! Cell Cycle. 2019;18:2828–48.
Cornelia de Lange Syndrome - StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK554584/ (2024). Accessed 5 Jun 2024.
Kline AD, Moss JF, Selicorni A, Bisgaard AM, Deardorff MA, Gillett PM, et al. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat Rev Genet. 2018;19:649–66.
Mariani M, Decimi V, Bettini LR, Maitz S, Gervasini C, Masciadri M, et al. Adolescents and adults affected by Cornelia de Lange syndrome: A report of 73 Italian patients. Am J Med Genet C Semin Med Genet. 2016;172:206–13.
Bölte S, Neufeld J, Marschik PB, Williams ZJ, Gallagher L, Lai MC. Sex and gender in neurodevelopmental conditions. Nat Rev Neurol. 2023;19:136–59.
Mannini L, Cucco F, Quarantotti V, Krantz ID, Musio A. Mutation spectrum and genotype-phenotype correlation in Cornelia de Lange syndrome. Hum Mutat. 2013;34:1589–96.
Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–94.
Rohatgi S, Clark D, Kline AD, Jackson LG, Pie J, Siu V, et al. Facial diagnosis of mild and variant CdLS: Insights from a dysmorphologist survey. Am J Med Genet A. 2010;152A:1641–53.
Gil-Rodríguez MC, Deardorff MA, Ansari M, Tan CA, Parenti I, Baquero-Montoya C, et al. De novo heterozygous mutations in SMC3 cause a range of cornelia de lange syndrome-overlapping phenotypes. Hum Mutat. 2015;36:454–62.
White JK, Gerdin AK, Karp NA, Ryder E, Buljan M, Bussell JN, et al. Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell. 2013;154:452.
Fujita Y, Masuda K, Bando M, Nakato R, Katou Y, Tanaka T, et al. Decreased cohesin in the brain leads to defective synapse development and anxiety-related behavior. J Exp Med. 2017;214:1431–52.
Erdmann G, Schütz G, Berger S. Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci. 2007;8:63 https://doi.org/10.1186/1471-2202-8-63.
Zha C, Gamache K, Hardt OM, Sossin WS. Behavioral characterization of Capn15 conditional knockout mice. Behav Brain Res. 2023;454:114635.
Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, et al. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 2012;32:10879–86.
Lipinski M, Muñoz-Viana R, del Blanco B, Marquez-Galera A, Medrano-Relinque J, Caramés JM, et al. KAT3-dependent acetylation of cell type-specific genes maintains neuronal identity in the adult mouse brain. Nat Commun. 2020;11:2588 https://doi.org/10.1038/S41467-020-16246-0.
Calderon L, Weiss FD, Beagan JA, Oliveira MS, Georgieva R, Wang YF, et al. Cohesin-dependence of neuronal gene expression relates to chromatin loop length. Elife. 2022;11:76539.
Weiss FD, Calderon L, Wang YF, Georgieva R, Guo Y, Cvetesic N, et al. Neuronal genes deregulated in Cornelia de Lange Syndrome respond to removal and re-expression of cohesin. Nat Commun. 2021;12:1–13.
Keil KP, Sethi S, Wilson MD, Chen H, Lein PJ. In vivo and in vitro sex differences in the dendritic morphology of developing murine hippocampal and cortical neurons. Sci Rep. 2017;7:8486 https://doi.org/10.1038/S41598-017-08459-Z.
Juraska JM. Changes in sex differences in neuroanatomical structure and cognitive behavior across the life span. Learn Mem. 2022;29:340–8.
Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res. 2007;1186:212–23.
Vargas RA. The GABAergic system: an overview of physiology, physiopathology and therapeutics. Int J Clin Pharmacol Pharmacother. 2018;2018:142.
Sun J, Yuan Y, Wu X, Liu A, Wang J, Yang S, et al. Excitatory SST neurons in the medial paralemniscal nucleus control repetitive self-grooming and encode reward. Neuron. 2022;110:3356–73.e8.
Wang X, Zhao Z, Guo J, Mei D, Duan Y, Zhang Y, et al. GABAB1 receptor knockdown in prefrontal cortex induces behavioral aberrations associated with autism spectrum disorder in mice. Brain Res Bull. 2023;202:110755 https://doi.org/10.1016/J.BRAINRESBULL.2023.110755.
Chen S, Chen J, Tang D, Yin W, Xu S, Gao P, et al. Mechanical and chemical itch regulated by neuropeptide Y-Y1 signaling. Mol Pain. 2024;20:17448069241242982 https://doi.org/10.1177/17448069241242982.
Saleev N, Getselter D, Bartman J, Gutman R, Marco A, Elliott E. Forebrain neuronal SMC3 regulates body weight and metabolic health partially through regulation of hypothalamic Melanocortin 4 receptor. bioRxiv [Preprint]. 2024. Available from: https://www.biorxiv.org/content/10.1101/2024.04.07.588459v1.
Viny AD, Ott CJ, Spitzer B, Rivas M, Meydan C, Papalexi E, et al. Dose-dependent role of the cohesin complex in normal and malignant hematopoiesis. J Exp Med. 2015;212:1819–32.
Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal. 2011;17:10.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Anders S, Pyl PT, Huber W. HTSeq-A python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550 https://doi.org/10.1186/S13059-014-0550-8.
Köster J, Rahmann S. Snakemake-a scalable bioinformatics workflow engine. Bioinformatics. 2012;28:2520–2.
Acknowledgements
We thank the Azrieli Faculty of Medicine Genome Center (Bar Ilan University) for help with sequencing and Dr. Amir Szitenberg from the Nancy and Stephen Grand Israel National Center for Personalized Medicine in the Weizmann Institute of Science for help with bioinformatic analysis. In addition, we thank Dr. Roey Lahav and the team at the Azrieli Faculty of Medicine Preclinical animal facility for assistance with metabolic cages and general animal care. This study was funded by grants from the Israel Science Foundation (898/17 and 1159/22).
Author information
Authors and Affiliations
Contributions
NS performed molecular, immunohistochemical, and behavioral experimentation described in this manuscript. DG performed some of the analysis on behavioral experimentation. EE designed and oversaw all of the experimentation. All authors were involved in writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimentation was approved by the Institutional Animal Care and Use Committee (IACUC) under protocol numbers BIU-MD-IL-2206-151-3 and BIU-MD-IL-2301-102-5 and experimentation was performed in accordance with the relevant guidelines and regulations (FELASA). There was no human experimentation that requires consent to participate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saleev, N., Getselter, D. & Elliott, E. Sex-specific modulation of anxiety-like behavior by forebrain neuronal SMC3 in mice. Transl Psychiatry 15, 266 (2025). https://doi.org/10.1038/s41398-025-03494-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03494-1