Abstract
All medications currently used to treat schizophrenia, which exert their therapeutic effects by inhibiting dopaminergic neurotransmission, have their greatest efficacy against the positive symptoms of schizophrenia but have limited impact on negative symptoms and cognitive deficits, core symptoms that robustly predict outcome. Recent research, which has implicated glutamatergic neuronal dysfunction in a subgroup of subjects with schizophrenia, has given rise to the development of several experimental glutamatergic medications. While Phase III clinical trials have not shown significant group effectiveness of these drugs, some subjects were reported to exhibit substantial reductions of symptoms. Identifying such a subgroup prior to drug testing would permit more targeted design of Phase III clinical trials and could lead to more personalized prescription of drugs to treat schizophrenia, especially its core symptoms. Using data from two failed Phase III clinical trials (N = 163 and N = 235) of the experimental glutamatergic drug pomaglumetad methionil (an mGluR2/3 agonist) and applying a gradient-boosted machine learning algorithm, we identified novel, pre-treatment EEG biomarkers that predicted responders with accuracy rates over ninety percent. These constellations of EEG markers predicted pomaglumetad responders prior to treatment in comparison to standard-of-care antipsychotic treatment, indicating that they are specific to pomaglumetad and do not represent a marker for response to antipsychotic treatment generically. The effects were seen with positive and negative symptoms as well as cognitive deficits. The method described could be applied to identify likely responders to other mechanistically novel psychotropic medications in schizophrenia and other neuropsychiatric disorders.
Similar content being viewed by others
Introduction
Very few mechanistically novel psychiatric medications have been approved over the last 50-plus years since the serendipitous discovery of the antipsychotic drugs, biogenic amine potentiating antidepressants, the mood stabilizer lithium and the GABAergic anxiolytic drugs. The dearth of novel and more effective psychiatric medications is remarkable given the tremendous advances in neuroscience and human genetics that have occurred in the 21st century. This research—including recent highly powered Genome Wide Association Studies (GWAS) [1]—has shed light on underlying pathophysiology and has demonstrated that several psychiatric disorders are the product of complex genetics, indicating that current psychiatric diagnoses encompass many etiologically distinct conditions. We believe that this heterogeneity underlies the large number of failures of recent clinical trials of neuropsychiatric drugs. These Phase III clinical trials included all patients with a given Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnosis, rather than more neurobiologically homogenous subgroups who might preferentially respond to a particular agent. This underlying heterogeneity results in non-responders and placebo responders obscuring the drug’s efficacy in the sub-group of potential true responders in these clinical trials.
It has been suggested that risk genes could define such subgroups and thus might be better predictors of drug response. In fact, polygenic risk scores, which aggregate risk genes to better identify potentially responsive subjects, have a poor record of identifying responders and predicting outcomes in neuropsychiatric illness [2]. A likely explanation is that it is not the risk genes themselves but rather that final common pathways of dysfunction that are responsible for symptoms of the illnesses.
One way to understand and quantify such common final pathways is by analysis of brain electrical activity, as measured by electroencephalography (EEG). We note that EEG abnormalities have been reported in several psychiatric illnesses, including autism spectrum disorder [3], Alzheimer’s Disease [4, 5], bipolar disorder [6], and schizophrenia [7, 8]. While psychiatric diagnoses are made based on clinical signs and symptoms rather than EEG findings, historically many studies have demonstrated a range of EEG abnormalities for a given condition, possibly reflecting differing neurobiologically defined subgroups [9]. EEG patterns could therefore be endophenotypic measures, lying between genetic risk factors and clinically observed behavior. Discerning complex EEG patterns associated with such subgroups is not straightforward for conventional statistical methods but is one that is ideally suited for artificial intelligence (AI) approaches [10].
Schizophrenia (SZ) is a case-in-point for the aforementioned challenges. Clinically, a diagnosis of schizophrenia is based upon a checklist of symptoms codified in the DSM-5 [11]. However, considerable research supports the notion that SZ primarily affects three somewhat independent domains: positive symptoms (e.g., delusions, hallucinations, disorganization of thinking and behavior), negative symptoms (e.g., poverty of speech and thought, constricted affect, lack of motivation, anhedonia), and a significant decline in occupational or academic functioning (cognitive symptoms). Deficits in cognition are a core feature of SZ, including deficits in working memory, problem solving, and social cognition [12]. Negative symptoms and cognitive deficits, which until recently were not appreciated as an important feature of the illness, are the primary drivers of functional disability and poor life outcomes in schizophrenia rather than the severity of positive symptoms [13].
While more than a score of antipsychotic drugs have been introduced since the discovery of chlorpromazine in the early 1950’s, all—with the exception of the recently released Cobenfy (xanomeline/trospium chloride)—exert their effect by acting on the dopamine system, generally by inhibiting dopamine D2 receptors [14]. With the exception of clozapine [15, 16], currently available dopamine-acting antipsychotic medications have greatest efficacy against the positive symptoms of SZ and have negligible efficacy against negative symptoms and cognitive deficits [17]. Thus, most antipsychotic drug treated patients remain severely disabled. Additionally, these traditional antipsychotics have well-documented noxious side effects [18].
The development of medications for SZ with fundamentally new (non-dopaminergic) mechanisms of action has been limited by the fact that, until recently, researchers have not had a good understanding of the underlying neurobiological causes of SZ. This has changed in the past 15 years, with research on the biological basis of SZ that has implicated the glutamate system [19]. As an example of this research, recent GWAS have identified ~130 genes that confer risk for SZ [20]. About 25% of these risk genes encode proteins that cluster around the pre- and post-synaptic components of the glutamatergic synapse [19]. Glutamate is the most prevalent neurotransmitter in the human brain, especially in the cerebral cortex where it is utilized by approximately eighty percent of synapses. Its effects are mediated by two families of receptors: glutamate-gated excitatory cation channels and glutamate activated G-protein coupled receptors (GPCRs) designated metabotropic glutamate receptors (mGluRs). mGluRs modulate glutamatergic neurotransmission with the mGluR2/3 receptor acting pre-synaptically to inhibit glutamate release. Thus, disruptions in glutamatergic neurotransmission are consistent with the cognitive and motivational deficits in SZ. Dysfunction of cortico-hippocampal glutamatergic neurons may account for subcortical dopaminergic disinhibition responsible for positive symptoms of the illness [21,22,23].
Several experimental glutamatergic psychotropic drugs have been developed [24]. These drugs act through a variety of direct and indirect mechanisms, all having the ultimate effect of altering signaling at the glutamatergic synapse. While multiple clinical trials of these drugs have been conducted, none has yet gained FDA approval because all failed Phase III clinical trials. Notably, in some instances, while there is not a uniform response, a subgroup of patients appears to show a unique benefit to the tested glutamatergic agent [25]. A pre-treatment method to identify responders to these agents, likely corresponding to the subset of patients with glutamatergic dysfunction, would be very useful in designing successful Phase III clinical trials as well identifying likely responders in clinical practice.
We hypothesized that a combination of EEG biomarkers—that is, patterns of electrical activity across the cortex—measured in the pre-treatment state could predict responders to glutamatergic drugs. To examine this, we used data from two Phase III clinical trials of the experimental drug pomaglumetad methionil (POMA), an agonist at mGluR2/3 glutamate metabotropic receptors. Applying a gradient-boosted machine learning (ML) algorithm to a number of novel EEG biomarkers that we identified, we were able to select patients with SZ who would respond to POMA with accuracy rates over ninety percent. Proposed applications of this method as well as potential usage for other neuropsychiatric conditions affected by other neurotransmitter systems are discussed.
Methods
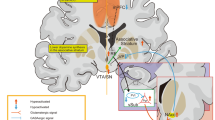
Data are from two Phase III clinical trials of subjects with SZ treated with pomaglumetad methionil (LY-2140023; POMA). In Trial 1, patients were treated with POMA plus standard-of-care (SOC) antipsychotic drugs as compared to standard-of-care antipsychotic drugs alone (N = 163). In Trial 2 [26], patients were treated with POMA alone as compared with SOC (N = 235). EEG recordings were taken pre-treatment using a standard 19-electrode montage (Fig. 1A) both in the resting state and when patients were exposed to photic stimulation, which was operationalized via flashing light at frequencies ranging from 1 to 30 Hz. Additional details can be found in the Supplementary Methods. A preliminary version of this work has been previously published [27].
A EEG electrode locations. Electroencephalographic recording was carried out using a 19-electrode montage, arranged according to the International 10–20 system [72]. Front of head is upward in image. Fp, prefrontal; F, frontal; T, temporal; P, parietal; O, occipital; C, Central. B Power law exponent (PLE). Example of a power spectrum (in black) of a resting EEG signal, displayed on a log-log plot. Slope of line (in red) fitted to curve is the power law exponent. Inset shows close-up of quality of fit (at higher frequency range). From Miller et al. [73].
Ethics approval and consent to participate
All subjects received a description of the study and provided written informed consent. Both trials were conducted in accordance with consensus ethics and principles as detailed in international guidelines (e.g., the Declaration of Helsinki), the International Conference on Harmonisation (ICH) of Good Clinical Practices (GCP) Guideline, and appliable laws and regulations. Protocols for these multi-site trials were approved by Ethics Review Boards under the supervision of the Eli Lilly Bioethics Advisory Committee. Full details for both Trial 1 (Registration Number NCT00845026) and Trial 2 (Registration Number NCT01052103) can be found at ClinicalTrials.gov [28, 29].
Pre-treatment EEG biomarkers
Previous attempts to use EEG activity to diagnose SZ and other mental illnesses or to predict treatment response have often relied on examination of a single frequency band at a particular electrode, frequently in the resting state (i.e., when the patient is not engaged in a cognitive or sensory task) [30]. More sophisticated measures of brain electrical activity are likely necessary to tap into underlying cognitive functioning [31]. We employed several EEG metrics designed to capture the cognitive complexity embodied in EEG recordings. For example, while patients with SZ consistently show an inability to attune to gamma (30 – 80 Hz) stimuli compared to controls, research that we have conducted has shown that some patients with SZ may show greater attunement to beta band (approximately 20 Hz) stimuli compared with controls. We found that the ratio of beta to gamma (β/γ) oscillations may correlate with the degree of SZ illness or serve as a marker for a particular subgroup of patients with SZ [32]. Another example is fractal behavior in the EEG signals. The power spectrum of resting EEG signals, when viewed on a log-log plot (that is, with log of frequency of oscillations on the horizontal axis and log of power on the vertical axis) forms roughly a straight line (Fig. 1B). This is referred to as “scale invariant” or “fractal” behavior, as the slope of the fitted line is a constant, over a broad range of frequencies. The slope of the line is termed the fractal exponent or the power law exponent (PLE). While the precise physical interpretation is still a matter of debate, the PLE may carry functional significance—it is possible that higher values (that is, steeper slopes) reveal a higher degree of “structure” or “memory” in underlying brain interactions [33].
EEG acquisition and preprocessing
For the predictive measures for resting state EEG data, we calculated power in the traditional frequency bands of delta, theta, alpha, beta, and gamma, as well as PLE. For the photically stimulated state, we calculated power at the driven frequency (10, 15, 20 and 30 Hz) as well as β/γ. To calculate power, we used both fast Fourier transform and wavelet analysis. As there can be considerable redundancy in human electrode-level EEG data, we also performed independent component analyses (ICA), a process that decomposes the recorded EEG data into functionally and spatially separated signals. This produced a set of 6 “auxiliary electrodes”, each of which consists of a trace that may more accurately represent an actual brain source of electrical activity; these were labeled ic1 – ic6. All measures were calculated at each EEG electrode, both original and ICA-derived.
Statistical analysis
Preliminary statistical analysis that used all outcome measures of the original clinical trials (Positive and Negative Symptom Scale [PANSS] [34], Clinical Global Impression Severity Scale [CGI-S], 16-Item Negative Symptoms Assessment [NSA-16] [35], and Personal and Social Performance Scale [PSP], as well as cognitive measures) indicated that POMA had a particularly marked effect on cognitive outcomes, which are unaffected by traditional antipsychotic drugs. Therefore, for the initial analysis, we focused on these measures. Specifically, outcome measures were taken as percent change from baseline on an average of the MATRICS Consensus Cognitive Battery (MCCB) [36, 37] subscales: working memory, verbal learning, visual learning, reasoning-problem solving, attention-vigilance, speed of processing, social cognition, and a composite cognitive measure. Additionally, we looked at two other outcome measures: decrease in negative symptoms, and decrease in positive symptoms. Our study was performed as three separate statistical analyses, one for each definition of treatment response.
Cognitive outcomes
The method for the cognitive outcomes case is described in detail here, followed by the positive and negative symptoms cases (which are methodologically analogous to the cognitive case). In the initial step, we determined whether a correlation existed between any of our calculated EEG metrics at the electrode or independent component level in the pretreatment condition and treatment outcome measures, the purpose of which was to arrive at a set of potential inputs, or features, for the ML predictive model. Steps described below apply to both Trial 1 and Trial 2, as these had identical predictor and outcome variables. All statistical analyses and machine learning modeling were carried out in R, version 4.0.4.
Given the large number of independent variables, and the fact that these differed between experimental conditions, for each Trial, data for the resting state condition and the photically stimulated condition were analyzed separately. For the resting state, there were eight outcome variables for cognitive symptoms, as described above. As predictors, there were nine FFT-based and nine wavelet-based metrics (eight for oscillatory bands and one for PLE); each was calculated for each EEG electrode and independent component. Thus, there were (9 + 9) × (17 + 6) = 414 potential pretreatment predictor variables and 414 × 8 = 3,312 possible correlations with outcome measures. To arrive at statistically meaningful correlations, bearing in mind the possible confounds resulting from multiple comparisons, we followed the four-step statistical algorithm detailed in the Supplementary Methods. For photic stimulation data, we also used this statistical protocol (though of course predictive measures were different) and similarly arrived at a set of retained prediction variables. These were pooled with those of the resting EEG case to serve as potential features for the machine learning model.
The ML model was implemented using XGBoost [38], a gradient boosted decision tree machine learning algorithm, run utilizing the R package xgboost [39, 40] version 1.4.1.1, following the approach of Kuhn et al. [41, 42]. Initial input features were EEG predictors, as identified above. As an outcome measure, we averaged over all subscales of the MATRICS Consensus Cognitive Battery. Those with improvement of 40% or greater were considered responders. This defined a group of responders that was 12.3% of the study subjects for Trial 1 and defined a group of responders that was 16% of the subjects for Trial 2.
Model tuning and feature pruning were carried out as follows. We identified the best performing parameters using a 50,000-iteration random search of the parameter space. For each iteration, we trained and tested the model using 10-fold cross-validation, implemented with the function xgb.cv of the xgboost package. Performance was evaluated using the binary classification error rate (number of incorrect predictions/total number of predictions). For the best performing iteration, the relative contribution of each feature was evaluated using function xgb.plot.importance, and the feature set was adjusted accordingly. Parameter search was again undertaken, and the above process was repeated until error rate no longer showed improvement. The optimal case cross validation performance was taken as a measure of model accuracy, in terms of binary classification rate; performance was also calculated using area under the curve analysis (AUC-ROC). (Given the relatively small Ns of our clinical trials, cross validation would produce a more accurate estimate of actual predictive performance compared to the training set-validation set-holdout (test) set that might be employed in more data-rich scenarios [43]). The parameters of the optimal case were used to create the predictive ML model. (Full details of model development process can be found in the Supplementary Methods). Using this model, we classified POMA-treated patients as well as SOC-treated patients as responders vs. non-responders, and computed sensitivity, specificity, and p values. P values were calculated using a one-sided test to see if model accuracy was greater than the no-information rate. We took the no-information rate to be the percentage prevalence of the largest class in the training set [44]. Model statistical tests were conducted using the R package caret, version 6.0–94.
Positive and negative symptom outcomes
To identify responders based on improvement in positive symptoms, the statistical steps and model tuning process detailed above for cognitive symptoms were carried out, with the following exceptions. We took as the outcome measure percentage decrease in the PANSS Positive Symptom subscale. We used a decrease in symptoms of 30% or more as the definition of treatment response. We chose this cutoff as it is a commonly used threshold to define response in antipsychotic drug trials e.g., [45]. We set a slightly higher cutoff to define response in terms of improvement in cognitive symptoms as we wished to use a threshold that identified a similar percentage of patients in each instance. This is also consistent with the literature on response to glutamatergic agents in schizophrenia. There have been a range of findings [46] in these studies, but improvements in cognitive function of 40% or more have been seen in responders [47]. For negative symptoms, we took as outcome measures (i) percentage decrease in the NSA-16, and (ii) percentage decrease in the PANSS Negative Symptom subscale. We took response to be an average of (i) and (ii) above, and again defined responder as one who showed a 30% or greater decrease in symptoms.
Using the above operationalization of treatment response, we saw that there was a degree of overlap between responders in the three categories; this is quantified in Table 1A. How symptomatic profiles differed among these groups in terms of particular patterns of clinical response is shown in Table 1B.
Results
Association between individual EEG biomarkers and improvements in measures of cognitive performance
Statistical analysis to determine whether a correlation existed between any of our calculated EEG metrics at any electrode in the pre-treatment condition and clinical improvement on POMA in terms of cognitive outcome measures produced a number of predictor-outcome pairs with high statistical significance and medium to large effect size, as measured by correlation coefficients (Pearson’s r) (Table 2). As an example, Fig. 2A shows the correlation between PLE and improvement in working memory performance among patients who were treated with POMA. Patients with greater PLEs in frontal lobes showed greater improvement in working memory tasks during treatment, compared with those with lower PLEs. There was a medium to large effect size, with a maximum r of 0.41 at right frontal lead F4. Additional examples are shown in Fig. 2B, C. All-in-all for Trial 1, 14 effects were identified. These metrics embodied a range of EEG predictors and were not restricted to one specific cortical area; taken together, they form a complex “spectral fingerprint”, which can be used to identify a patient subgroup that is uniquely responsive to POMA in terms of improvements in cognitive functioning.
Here, correlation coefficient is expressed as Pearsons’ r; value of r is indicated on the color bar in each panel. r = 1 represents perfect positive correlation, r = −1 represents perfect negative correlation, r = 0 represents no correlation. If the r value did not reach statistical significance at a particular electrode, it was treated as 0 for display purposes. MCCB, MATRICS Consensus Cognitive Battery. A Correlation coefficient between pre-treatment power law exponent in the resting state and treatment response, where treatment response is measured as percentage improvement in the working memory domain score of the MCCB. B Correlation coefficient between pre-treatment resting beta activity and treatment response, where treatment response is measured as percentage improvement in the attention-vigilance domain score of the MCCB. C Correlation coefficient between pre-treatment β/γ in the photically stimulated state and treatment response, where treatment response is measured as percentage improvement in the speed of processing domain score of the MCCB.
Machine learning algorithm predicts cognitive responders to POMA
We employed a gradient boosted decision tree machine learning algorithm to predict POMA responders as compared to non-responders. For Trial 1, as features (i.e., inputs to ML algorithm) we used the 14 EEG metrics shown in Table 2. EEG metrics predicted responders to POMA with an accuracy of 92% (misidentification rate of 8%); AUC was 0.963. The predictive model had a sensitivity and specificity of 0.769 and 0.929, respectively (P = 0.004). The comparator group for this trial was treated with standard-of-care antipsychotics since there was not a placebo group, per se. However, we wished to understand whether the model selected those patients with a preferential response to POMA or identified responders to antipsychotics generically. We therefore applied the predictive model that we generated to the SOC group. We found that it did not yield a significant aggregate of “responders” (P = 0.9996), indicating that the method selects for POMA responders specifically.
The above methodology was also used to predict responders in Trial 2. EEG metrics predicted responders to POMA with an accuracy of 91% (misidentification rate of 0.09); AUC as 0.917. (The specific set of predictors in this case was not identical to that of Trial 1; predictors for Trial 2 are shown in Supplementary Table S1). Sensitivity and specificity of the predictive model were 0.778 and 0.938, respectively (P = 0.041). For SOC patients in Trial 2, the method also did not yield a significant aggregate of “responders” (P = 0.9998).
Predicting patient response to POMA as measured by positive and negative symptoms
For positive symptom outcomes, we found that many of our EEG markers positively correlated with treatment response, with robust statistical significance (Supplementary Table S2), although at slightly lower effect sizes when compared with cognitive outcomes. When used as features in ML model, these also had good predictive ability. For Trial 1 it produced accuracy of 87.9% (P = 0.011) and an AUC of 0.986, and for Trial 2, it produced an AUC of 0.963 and an accuracy of 88.2% (P = 0.004). Interestingly, predictive patterns of EEG activity were different for positive symptom outcomes in comparison with negative and cognitive ones. For example, increased pre-treatment gamma band activity in the fronto-temporal area in the photically driven condition correlated with post-treatment reductions in positive symptoms in POMA-treated patients, a pattern not seen in the other outcome groups (Fig. 3A).
The correlation coefficient is expressed as Pearson’s r; the value of r is indicated on the color bar in each panel. If the r value did not reach statistical significance at a particular electrode, it was treated as 0 for display purposes. A Correlation coefficient, r, between pre-treatment photically driven gamma activity and post-treatment improvement in positive symptoms among POMA treated patients in Trial 1. Here, improvement is measured as percentage decrease in the PANSS Positive Subscale. B, C Example of similarity of predictive EEG patterns between negative symptom responders and cognitive symptom responders among POMA treated patients. Data are from Trial 2. In (B), the correlation coefficient between pre-treatment resting beta activity and post-treatment improvement in negative symptoms. Here, improvement is measured as percentage decrease in the NSA-16. In (C), the correlation coefficient between pre-treatment resting beta activity and post-treatment improvement in cognitive functioning is shown. Here, improvement is measured as percentage increase in the MCCB social cognition domain score.
For negative symptoms, we also found that many of our EEG markers showed a positive correlation with individual treatment responses. While effect sizes of each individual marker, as operationalized by Pearson’s r, again tended to be slightly smaller than those associated with cognitive outcomes, several markers were identified with high statistical significance (Supplementary Table S3). When used as features in ML model, we found that the model was able to identify responders with accuracy of 96.5% (P = 0.011) and an AUC of 0.998 for Trial 1 and 88.4% (P = 0.045) for Trial 2, with an AUC of 0.981. For negative symptoms and cognitive outcomes notable overlap existed between the EEG predictive patterns. Examples are shown in Fig. 3B, C. Negative symptom responders tended to show enhanced resting beta activity in the posterior (e.g., occipital and temporo-occipital) leads as shown in Fig. 3B. This is similar to that seen in cognitive symptom responders, an example of which is shown in Fig. 3C for comparison. Broadly, for the particular biomarkers for response in terms of cognitive improvement, there was greater overlap with negative symptom response biomarkers than with positive symptoms response ones. This is depicted graphically in Fig. 4.
A Information of Table 2 and Supplementary Table S1 (for Trials 1 and 2 respectively) are depicted in blue, with cortical location on the X-axis and calculated EEG metric on the Y-axis. The predictive “strength” (that is, the quantitative correlation between the metric and improvement in cognitive outcome) is indicated by the size of the circle, as shown in the legend. Similarly, biomarkers for response in terms of negative symptom improvement (From Supplementary Table S3) are shown in red. Areas of overlap are shown in shades of purple. B Response pattern for cognitive responders is again depicted in blue. Biomarkers for response in terms of positive symptoms (from Supplementary Table S2) are shown in green. Note the lower degree of overlap, as compared with (A) above. X-axis abbreviations: Fp2, right frontal pole; F8, right prefrontal; T4, right temporal; Or, right temporo-occipital (electrodes T6, P4, and O2); C, central (electrodes, C3, Cz, C4); Ol, left temporo-occipital (electrodes T5, P3, and O1); T3, (left temporal); F7, left prefrontal, Fz, frontal (electrodes F3, Fz, F4). EEG metrics: ratio, β/γ; alpha, 5–14 Hz; lo beta, 14–24 Hz; hi beta, 24–30 Hz; lo gamma, 30–39 Hz; high gamma, 39–50 Hz, PLE, power law exponent.
Discussion
Schizophrenia is a devastating psychiatric disorder that affects one percent of the population worldwide and is the seventh most costly medical illness, with an estimated economic burden in the US alone of 330 billion dollars per year [48]. The failure of currently used antipsychotics as well as recent research that has implicated the glutamate system in the illness has given rise to the development of several experimental glutamatergic drugs to treat SZ [24]. Using the results of two failed clinical trials with the experimental mGluR2/3 agonist POMA [26, 28, 29], we identified pre-treatment EEG patterns that predicted responders to the drug with high significance. These robust effects were observed with cognitive deficits as well as positive and negative symptoms in patients diagnosed with SZ; these comprise the three major symptomatic domains of the disorder. These markers identified POMA responders prior to treatment and did so compared to SOC treatment, indicating they are specific to POMA and do not represent a marker for response to antipsychotic treatment generically. The results have face validity as the predictive EEG patterns for negative symptoms exhibit some overlap with those for cognitive symptoms but not with positive symptoms [49], whereas the predictive EEG patterns for positive symptoms exhibit a unique prominence of gamma rhythms, consistent with the research showing a correlation between positive symptoms of SZ and aberrations in gamma synchrony [50]. Importantly, the method revealed that POMA significantly reduced cognitive and negative symptoms, domains unaffected by traditional antipsychotic drugs, with the exception of clozapine [15, 16], while also mitigating positive symptoms in the responsive subgroup. The responsive subgroup represents approximately fifteen percent of those satisfying the DSM-5 diagnostic criteria for SZ, which is obscured by the eighty-five percent of non-responders in the Phase III clinical trials.
Past attempts to use EEG patterns to predict response to traditional dopaminergic antipsychotic medications have not been successful [30]. Researchers have not yet used EEG approaches to predict patient response to the experimental glutamatergic medications, a relatively new class of drugs. Glutamate is an excitatory neurotransmitter that directly drives neuronal activity, whereas the effects of the modulatory neurotransmitter dopamine are more subtle and complex. Moreover, glutamate is the most prevalent neurotransmitter in the human cortex, and dopamine exerts its effects primarily in subcortical structures such as the basal ganglia. Thus, the EEG, which preferentially measures cortical electrical activity rather than that of deep structures, is an ideal modality for the study of POMA. The GWAS results do not point to a particular molecular mechanism of glutamatergic dysfunction in SZ. Rather, they suggest disrupted glutamatergic signaling, regardless of the molecular lesion, underlies the SZ phenotype or a particular subtype [21, 51, 52]. Based on our research, we speculate that any of these may lead to similar system level behaviors, as measured by EEG activity. That is, these behaviors may represent a final common pathway by which various genetically encoded deficits are manifested and lead to psychopathology—i.e., an endophenotype.
This hypothesis suggests that the method that we have described is not limited to POMA but could be applied to identify responders to other experimental glutamatergic agents. These agents act through a variety of direct and indirect mechanisms but all have the ultimate effect of altering signaling at the glutamatergic synapse. In addition to the metabotropic glutamate receptor type 2/3 (mGluR2/3) agonist, other examples include glycine modulatory site (GMS) agonists, which act to enhance NMDA receptor functioning, as glycine is a co-agonist of glutamate at the NMDA receptor; glycine transporter type 1 (GlyT-1) inhibitors, which inhibit glycine uptake (clearance), and thus increase the availability of glycine; and D-amino-oxidase (DAAO) inhibitors, that are effective because DAAO is the primary enzyme responsible for breaking down D-serine, which is a glycine modulatory site agonist in the forebrain [53].
While the focus of the current study was a glutamatergic agent targeting SZ, our method can generalize to other neurotransmitter systems and to other psychiatric disorders associated with abnormal EEGs. A prime example is autism spectrum disorder (ASD). ASD has an estimated prevalence of 2.5% of the US population and no known cure. ASD has a clear genetic basis, with an estimated heritability of 81% [54]. As GWAS on ASD increase in size and statistical power, increasing numbers of risk genes for ASD are being identified, supporting the etiologic heterogeneity of the disorder. Not surprisingly, risk genes that affect brain development—specifically neural connectivity—are thought to play an important role [55]. Consistent with this, EEG abnormalities, though not proving to be diagnostic of the disorder, frequently occur, with nearly 40% of patients with ASD having a diagnosed seizure disorder [56]. A recent trial using the GABA-B receptor agonist arbaclofen did not show an across-the-board response as compared to placebo but did reveal a subgroup (13% of subjects) with very robust improvement [57], reminiscent of our findings with POMA. Given the considerable heterogeneity of ASD and its likely neuropathological basis, our method could be utilized to identify such subgroups.
We found that PLE, calculated in the resting pre-treatment condition, had a significant positive correlation with improvement in treatment response on the Working Memory domain score of the MCCB. Notably, this was seen in the frontal lobes, structures critical to this type of memory [58]. For Trial 1, we found medium to high effect size both at right frontal lead F4 (r = 0.41) and left frontal lead F3 (r = 0.36). A similar distribution was seen in Trial 2. While the precise physical interpretation of the PLE is not clear, it likely reflects some emergent property of complex systems [59]. Power law behavior has been seen at many spatial scales—in fMRI signals [60], local field potentials, neuronal spike trains [61], and the dynamics of neurotransmitter release [62]. Power law exponents of fMRI signals have been shown to be affected by task performance and mental state [63]. There is at least one study that has looked at this metric in subjects with SZ [64], but none that we are aware of has attempted to use this to predict treatment response. Given its possible functional significance, and both its relatively higher value over the electrodes of the frontal lobe and its predictive power on the working memory outcome measure, its potential as a biomarker has clear face validity.
In addition to cognitive outcomes, we also identified EEG patterns that predicted improvement in positive and negative symptoms. Of note, for negative symptom and cognitive symptom outcomes, there was a good deal of overlap between the EEG predictive patterns. This overlap is consistent with the clinical findings, which indicate a significant correlation between negative and cognitive symptoms in patients with SZ but not between these and positive symptoms [49, 65, 66]. Interestingly, predictive patterns of EEG activity were different for positive symptom outcomes, in comparison with negative and cognitive ones. For example, increased pre-treatment gamma band activity during the photically driven condition correlated with post-treatment improvements in positive symptoms, which was particularly prominent in the fronto-temporal area (Fig. 3A). This pattern was dissimilar from those occurring in patients exhibiting cognitive and negative symptom improvement. This disconnect is noteworthy given the substantial evidence of abnormalities in gamma band response to sensory stimulation in patients with SZ [50, 67, 68], as well as the substantial clinical literature showing a lack of correlation between positive symptoms and negative symptoms [69], or between positive symptoms and cognitive symptoms in schizophrenia [70].
We believe that the practical implications of this research are twofold. The EEG “spectral fingerprints” identified by the method that we have described represent biomarkers that can prospectively identify responders to glutamatergic, and potentially other agents. With a positive response rate of 15–20%, consistent with the genetic heterogeneity in SZ, the EEG patterns associated with responders could achieve significance with as few as one hundred subjects, thus allowing it to be used in a typical Phase II study. This would allow those conducting Phase III clinical trials on novel neuropsychiatric drugs to stratify patients and thereby design trials for more specific and biologically homogeneous patient populations. In addition to facilitating FDA approval of novel psychotropic drugs, the method could be used in a clinical care to select likely responders to glutamatergic or other drugs. This would decrease time and resources devoted to psychopharmacologic trial and error and help to prevent the adverse events associated with multiple medication trials.
There are some limitations to our study and areas in which future research is indicated. To develop our predictive model, we used machine learning with a standard cross-validation procedure, in which the classifier was tested on cases that were not used in the training set. We therefore believe that the method would have good generalizability to new cases (e.g., in a future trial of POMA on patients with SZ). It should be noted that recent research [71] has indicated that well-validated AI models, when applied in new clinical settings—even with similar patient populations and outcome measures—may underperform [10]. Therefore, it would be important to confirm the reliability of our method by using it to predict responders in a clinical trial of POMA that was not part of the current study.
Data availability
Data and code are available from the corresponding author upon reasonable request.
References
Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–94.
Fusar-Poli L, Rutten BPF, van Os J, Aguglia E, Guloksuz S. Polygenic risk scores for predicting outcomes and treatment response in psychiatry: hope or hype? Int Rev Psychiatry. 2022;34:663–75.
Colomar L, San José Cáceres A, Álvarez-Linera J, González-Peñas J, Huertas Patón A, Martín de Blas D, et al. Role of cortical excitatory/inhibitory imbalance in autism spectrum disorders from a symptom severity trajectories framework: a study protocol. BMC Psychiatry. 2023;23:213.
Babiloni C, Blinowska K, Bonanni L, Cichocki A, De Haan W, Del Percio C, et al. What electrophysiology tells us about Alzheimer’s disease: a window into the synchronization and connectivity of brain neurons. Neurobiol Aging. 2020;85:58–73.
Kamondi A, Grigg-Damberger M, Löscher W, Tanila H, Horvath AA. Epilepsy and epileptiform activity in late-onset Alzheimer disease: clinical and pathophysiological advances, gaps and conundrums. Nat Rev Neurol. 2024;20:162–82.
Vellante F, Ferri F, Baroni G, Croce P, Migliorati D, Pettoruso M, et al. Euthymic bipolar disorder patients and EEG microstates: a neural signature of their abnormal self experience? J Affect Disord. 2020;272:326–34.
Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr Bull. 2011;37:659–63.
Hunt MJ, Kopell NJ, Traub RD, Whittington MA. Aberrant network activity in schizophrenia. Trends Neurosci. 2017;40:371–82.
Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci. 1999;11:190–208.
Rajpurkar P, Chen E, Banerjee O, Topol EJ. AI in health and medicine. Nat Med. 2022;28:31–38.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. 5th edition, text revision. Washington, DC: American Psychiatric Association Publishing; 2022.
McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023;28:1902–18.
Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff’? Schizophr Bull. 2000;26:119–36.
Baldessarini RJ. Chemotherapy in psychiatry: pharmacologic basis of treatments for major mental illness. 3rd ed. Springer New York: Imprint: New York, NY: Springer; 2013.
Lee MA, Thompson PA, Meltzer HY. Effects of clozapine on cognitive function in schizophrenia. J Clin Psychiatry. 1994;55:82–87.
Wagner E, Siafis S, Fernando P, Falkai P, Honer WG, Röh A, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11:487.
Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174:927–42.
Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172:1703–11.
Coyle JT, Ruzicka WB, Balu DT. Fifty years of research on schizophrenia: the ascendance of the glutamatergic synapse. Am J Psychiatry. 2020;177:1119–28.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42.
Sonnenschein SF, Grace AA. Insights on current and novel antipsychotic mechanisms from the MAM model of schizophrenia. Neuropharmacology. 2020;163:107632.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7.
Wu Q, Huang J, Wu R. Drugs based on NMDAR hypofunction hypothesis in schizophrenia. Front Neurosci. 2021;15. https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2021.641047 (accessed 15 Feb2024). https://doi.org/10.3389/fnins.2021.641047.
Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78:754–62.
Adams DH, Kinon BJ, Baygani S, Millen BA, Velona I, Kollack-Walker S, et al. A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry. 2013;13:143.
Siekmeier P, Lowen S. Novel EEG biomarkers for the a priori identification of responders to the experimental antipsychotic agent pomaglumetad methionil [abstract]. Neuropsychopharmacology. 2019;44:S486.
A Safety Study Comparing LY2140023 to Atypical Antipsychotic Standard Treatment in Schizophrenic Patients, https://clinicaltrials.gov/study/NCT00845026 (2022).
A Study of LY2140023 in Schizophrenia Patients With Prominent Negative Symptoms, https://clinicaltrials.gov/study/NCT01052103 (2022).
Galderisi S, Mucci A, Volpe U, Boutros N. Evidence-based medicine and electrophysiology in schizophrenia. Clin EEG Neurosci. 2009;40:62–77.
Cannon J, McCarthy MM, Lee S, Lee J, Börgers C, Whittington MA, et al. Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci. 2014;39:705–19.
Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99:2656–71.
Lowen SB, Teich MC. Fractal-based point processes. Hoboken, N.J.: Wiley-Interscience; 2005.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Alphs LD, Summerfelt A, Lann H, Muller RJ. The negative symptom assessment: a new instrument to assess negative symptoms of schizophrenia. Psychopharmacol Bull. 1989;25:159–63.
Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9.
Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13.
Chen T, Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. New York, NY, USA: Association for Computing Machinery; 2016, pp 785-94.
XGBoost Parameters, https://xgboost.readthedocs.io/en/latest/parameter.html (2025).
Chen T, He T, Benesty M, Khotilovich V, Tang Y, Cho H et al, xgboost: Extreme Gradient Boosting, https://cran.r-project.org/web/packages/xgboost/index.html (2024).
Visual XGBoost Tuning with caret, https://kaggle.com/code/pelkoja/visual-xgboost-tuning-with-caret (2018).
Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28:1–26.
Hastie, T, Tibshirani, R, Friedman, J. Chapter 7: model assessment and selection. In: The elements of statistical learning: data mining, inference, and prediction. New York, NY: Springer; 2017, pp 219-59.
Kuhn, M, Johnson, K. Chapter 11: measuring performance in classification models. In: Applied predictive modeling. New York, NY: Springer; 2013, pp 247-66.
Kaul I, Sawchak S, Claxton A, Sauder C, Hassman HH, Kakar R, et al. Efficacy of xanomeline and trospium chloride in schizophrenia: pooled results from three 5-week, randomized, double-blind, placebo-controlled, EMERGENT trials. Schizophr. 2024;10:102.
Iwata Y, Nakajima S, Suzuki T, Keefe RSE, Plitman E, Chung JK, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2015;20:1151–60.
Lin C-Y, Liang S-Y, Chang Y-C, Ting S-Y, Kao C-L, Wu Y-H, et al. Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: a randomised, double-blind, placebo-controlled trial. World J Biol Psychiatry. 2017;18:357–68.
Kadakia A, Fan A, Marden J, Dembek C, Catillon M, Anderson A, et al. The economic burden of schizophrenia in the United States in 2019. CNS Spectr. 2022;27:227–.
Lim J, Lee S-A, Lam M, Rapisarda A, Kraus M, Keefe RSE, et al. The relationship between negative symptom subdomains and cognition. Psychol Med. 2016;46:2169–77.
Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry. 2015;77:1001–9.
Uliana DL, Lisboa JRF, Gomes FV, Grace AA. The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia. Biochem Pharmacol. 2024;228:116298.
Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–6.
Tsai GE. Ultimate translation: developing therapeutics targeting on N-Methyl-d-aspartate receptor. Adv Pharmacol. 2016;76:257–309.
Bai D, Yip BHK, Windham GC, Sourander A, Francis R, Yoffe R, et al. Association of genetic and environmental factors with autism in a 5-Country cohort. JAMA Psychiatry. 2019;76:1035–43.
Klei L, McClain LL, Mahjani B, Panayidou K, De Rubeis S, Grahnat A-CS, et al. How rare and common risk variation jointly affect liability for autism spectrum disorder. Mol Autism. 2021;12:66.
Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–8.
Veenstra-VanderWeele J, Cook EH, King BH, Zarevics P, Cherubini M, Walton-Bowen K, et al. Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology. 2017;42:1390–8.
Funahashi S. Working memory in the prefrontal cortex. Brain Sci. 2017;7:49.
Bak P. How nature works: the science of self-organized criticality. New York, NY, USA: Copernicus; 1996.
Fadili MJ, Bullmore ET. Wavelet-generalized least squares: a new BLU estimator of linear regression models with 1/f errors. Neuroimage. 2002;15:217–32.
Lowen SB, Ozaki T, Kaplan E, Saleh BE, Teich MC. Fractal features of dark, maintained, and driven neural discharges in the cat visual system. Methods. 2001;24:377–94.
Lowen SB, Cash SS, Poo M, Teich MC. Quantal neurotransmitter secretion rate exhibits fractal behavior. J Neurosci. 1997;17:5666–77.
Tolkunov D, Rubin D, Mujica-Parodi L. Power spectrum scale invariance quantifies limbic dysregulation in trait anxious adults using fMRI: adapting methods optimized for characterizing autonomic dysregulation to neural dynamic timeseries. Neuroimage. 2010;50:72.
Radulescu AR, Rubin D, Strey HH, Mujica-Parodi LR. Power spectrum scale invariance identifies prefrontal dysregulation in paranoid schizophrenia. Hum Brain Mapp. 2012;33:1582–93.
Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 2006;32:250–8.
Huang M, Huang Y, Yu L, Hu J, Chen J, Jin P, et al. Relationship between negative symptoms and neurocognitive functions in adolescent and adult patients with first-episode schizophrenia. BMC Psychiatry. 2016;16:344.
Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–18.
Thuné H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry. 2016;73:1145–53.
Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–94.
Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res. 1991;5:123–34.
Chekroud AM, Hawrilenko M, Loho H, Bondar J, Gueorguieva R, Hasan A, et al. Illusory generalizability of clinical prediction models. Science. 2024;383:164–7.
Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6.
Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol. 2009;5:e1000609.
Acknowledgements
We thank Steven B. Lowen, PhD for his contributions to this project.
Author information
Authors and Affiliations
Contributions
PJS and JTC conceptualized the study, developed the methodology, interpreted results, wrote the original draft, reviewed and edited the manuscript, and secured funding. PJS curated the data, developed software, carried out analysis, and performed data visualization. JTC provided supervision for this work. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
JTC is a co-founder of Waveform Biosciences (Cambridge, MA), which proposes using EEG patterns to predict responders to novel psychiatric drugs, and is a member of the Scientific Advisory Board of SyneuRx (New Taipei City, Taiwan), which is developing potential psychiatric drugs from nutraceuticals. PJS is a co-founder of Waveform Biosciences. JTC and PJS are co-inventors on patent applications PCT/US2020/49194, “Apparatuses and methods for identification and treatment of patients responsive to antipsychotic agent therapy” and PCT/US2020/49283, “Method of increasing cognitive function with glutamate receptor agonist”, which are related to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Siekmeier, P.J., Coyle, J.T. Novel electroencephalographic biomarkers for the prediction of responders to an experimental glutamatergic agent in patients with schizophrenia. Transl Psychiatry 15, 390 (2025). https://doi.org/10.1038/s41398-025-03604-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03604-z
This article is cited by
-
Novel drug treatments for schizophrenia
Nature Reviews Drug Discovery (2026)