Abstract
Numerous studies have described the role of the microbiome-gut-brain axis in depression. However, the molecular mechanisms underlying the involvement of gut microbiota in the development of prenatal depression are limited. In this study, fecal microbiota from women with prenatal depression was transplanted into germ-free mice to investigate the potential causal relationships between the gut microbiota and depressive phenotypes. Shotgun metagenomic sequencing and untargeted metabolomics approaches were used to investigate the characteristics of gut microbiota and microbial metabolites. The levels of neuroinflammation in the brain were detected using immunofluorescence and real-time quantitative PCR. We found significant changes in gut microbiota composition and metabolites in mice with fecal microbiota transplantation (FMT) from women with prenatal depression, including decreased Ligilactobacillus, increased Akkermansia, and abnormal glycerophospholipid metabolism. Besides, significant increase in plasma lipopolysaccharide (LPS) levels and significant proliferation of microglia in the hippocampus were observed in mice receiving FMT from women with prenatal depression, accompanied by a significant increase in the expression of nuclear factor-κB (NF-κB) p65, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) mRNA. The gut microbiota and its metabolites were strongly associated with depressive-like behaviors, plasma LPS and neuroinflammation. Our study collectively demonstrates that dysbiosis of the gut microbiota may play a causal relationship in the development of prenatal depression. This process potentially involves the activation of neuroinflammation through the LPS-NF-κB signaling pathway.
Similar content being viewed by others
Introduction
Prenatal depression is one of the most common disorders during pregnancy [1]. The overall incidence of prenatal depression is about 20.7% worldwide, with a higher incidence of 20–30% in low- and middle-income countries, including China [2]. Prenatal depression could lead to self-injury, suicidal attempts, and adverse perinatal outcomes [3]. The prevailing perspective is that susceptibility to depression and other psychiatric disorders is influenced by both genetic and environmental factors [4]. Several pathogenic mechanisms have been proposed, including abnormal function of the hypothalamic-pituitary-adrenal axis, inflammatory cytokines, neurotrophic factors, sex hormones, and metabolic changes. These alterations in neurotransmitters, intracellular signaling, and gene transcription can contribute to short- and long-term imbalances of neuronal function and behaviors [5]. Nonetheless, the definitive pathogenesis of prenatal depression remains largely elusive.
Recently, the role of the gut microbiota in depression is emerging. There are well-characterized bidirectional communication channels between the gut and the brain involving neural, endocrine, and inflammatory mechanisms, which collectively constitute the microbiome-gut-brain (MGB) axis [6, 7]. Animal studies have shown that the gut microbiota of patients with major depressive disorder can contribute to depressive-like behaviors in mice through altering host metabolism [8, 9]. During a normal, healthy pregnancy, the body undergoes significant hormonal, immunological, and metabolic changes. Simultaneously, the gut microbiota undergoes dramatic changes from the first to the third trimesters of pregnancy, particularly characterized by a substantial increase in β-diversity, an overall rise in Proteobacteria and Actinobacteria, and a decrease in richness [10, 11]. The MGB axis are key players in the prenatal period when the maternal microbiota is particularly sensitive, and any negative interference with the MGB axis is associated with a higher maternal risk for neuropsychiatric disorders [12]. Therefore, there is a pressing need to identify the pathophysiologic mechanisms underlying the development of prenatal depression under the support of the MGB axis.
Our previous work showed significant differences in gut microbiota between pregnant women with and without prenatal depression [13]. The development of depression is associated with systemic immune activation, comprising abnormalities in inflammatory markers, immune cell numbers, and antibody titers [14,15,16]. The molecular mechanisms involved may include central nervous system inflammatory responses induced by NLRP3 activation [17], increased permeability of the blood-brain barrier (BBB) [18], and abnormalities in tryptophan metabolism [19]. Notably, changes in microbial levels in the host can lead to dysregulation of the peripheral immune response, further affecting host behaviors [20]. The germ-free (GF) mice has made it possible to study the role of the gut microbiota on prenatal depression. Additionally, the development of fecal microbiome transplant (FMT) has opened new opportunities to understand the effects of microbiota alteration on behaviors and provided strong evidence for establishing causal relationships. Therefore, based on the previous population studies, we transplanted the gut microbiota from women with prenatal depression into GF mice. This approach allowed us to investigate the mechanisms by which the gut microbiota influences local and systemic inflammation, leading to behavioral changes, possibly mediated by bacterial metabolites. Our findings provide considerable strategies for the intervention of prenatal depression.
Methods and materials
Pregnant women recruitment and fecal sample collection
The clinical experimentation protocol was reviewed and approved by the Research Ethics Boards of Medical School of Wuhan University (2019YF2019). All methods were performed in accordance with the relevant guidelines and regulations. The informed consent was obtained from all the participants. Our previous study has reported detailed recruitment of women with prenatal depression and healthy controls [13]. Prenatal depression screening in the third trimester were carried out using the Edinburgh Postnatal Depression Scale (EPDS) of the mainland Chinese version [21]. An EPDS score equal to or greater than 10 indicates the presence of depressive symptoms in pregnant women. All participants were healthy women who had not used antibiotics or prebiotics during pregnancy and had no pregnancy complications. Fecal samples were collected within 1 week of prenatal depression screening and stored in the refrigerator at −80 °C. The methods for collecting fecal samples are described in Supplementary information: Supplementary methods.
Mice and FMT
The animal experimentation protocol was reviewed and approved by the Laboratory Animal Centre of Huazhong Agriculture University (HZAUMO-2024-0088). All methods were performed in accordance with the relevant guidelines and regulations. Female GF C57BL/6 mice at 5 weeks were obtained from the GF Animal Platform of Huazhong Agricultural University. Mice were housed in sterile isolators (temperature 25 ± 2 °C; relative humidity 45–60%; lighting/dark cycle, 12/12 h; light hours 06:30-18:30) with free access to sterile food and water. The sample size for this study was determined based on previous literature [22], experimental feasibility, and the requirement to minimize animal use while ensuring sufficient data for biological relevance.
Fecal samples for FMT were obtained from randomly selected healthy controls (n = 6, mean age 32.17 years) or women with prenatal depression (n = 6, mean age 31.33 years). The characteristics of the human donors were listed in Table S1, with no significant differences in demographic information other than EPDS score. Aliquots of thawed fecal samples were mechanically homogenized in equal amounts under anaerobic condition. The mixed feces were added into sterile normal saline and glycerol mixed solution (ratio of saline: glycerol of 85:15, per 100 ml) at a ratio of 10% (w/v), roughly filtered through sterile gauze, and then filtered through a sterile 100μm filter to prepare fecal bacteria suspension, then immediately dispensed to cryotubes, and stored at −80 °C [23]. An investigator not involved in data collection used www.randomizer.org to generate a random-assignment sequence, allocating fourteen female 5 weeks GF C57BL/6 mice into two groups (7 mice per group). These mice were then transplanted with mixed fecal bacterial suspensions from donors with healthy control and prenatal depression, respectively. GF mice were inoculated orally with 0.2 mL of fecal bacterial suspension daily for 6 days, and then every other day for 4 more times [8]. Behavioral tests were conducted three weeks after the last gavage, and then mice were sacrificed to collect the samples. FMT experiments as well as feeding without any intervention for three weeks were performed in sterile isolators (Fig. 1A).
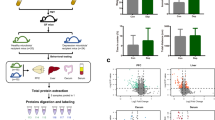
A FMT experimental design. FMT experiments as well as feeding without any intervention for three weeks were performed in sterile isolators. B The α-diversity of microbiota of two groups using alpha diversity measures Ace, Chao, Sobs and Shannon. C Principal coordinate analysis (PCoA) of bray_curtis distances showed significant difference in β-diversity between two groups. D At the phylum level, the composition and statistical tests of the gut microbiota from two groups. E At the genus levels, the composition and linear discriminant analysis (LDA score > 4) of the gut microbiota in two groups. F Kyoto Encyclopedia of Genes and Genomes (KEGG) functional pathways of gut microbiota between two groups. HC-FMT, mice receiving FMT from women with healthy control (n = 7); PD-FMT, mice receiving FMT from women with prenatal depression (n = 7); data are shown as mean ± SEM; ****, p < 0.0001; differences are assessed by Student’s t-test.
Behavioral tests in mice
Behavioral tests were conducted by two skilled technicians who were blinded for the group assignment. Mice were acclimated to the test environment for at least 1 h prior to testing. Behavioral test data were videotaped and analyzed by the Ethovision XT 17.5 software (Noldus Information Technology, The Netherlands). Details of open-field test (OFT), forced swimming test (FST) and tail suspension test (TST) were included in the Supplementary information: Supplementary methods.
Shotgun metagenomic sequencing of cecal contents
DNA of cecal contents was extracted using the PF Mag-Bind Stool DNA Kit (Omega Bio-tek, Norcross, GA, USA). The sequencing libraries were prepared by using a NEXTFLEX® Rapid DNA-Seq (Bioo Scientific, Austin, TX, USA) following the manufacturer’s instructions. Paired-end sequencing of DNA fragments with an average size of approximately 400 bp was performed on an Illumina NovaSeq 6000 platform (Illumina Inc., San Diego, CA, USA). The processing of metagenomic reads was detailed in the Supplementary information: Supplementary methods.
Metabolomics analysis and other experiments
LC-MC untargeted metabolomics analysis, luminex liquid suspension micro arrays, enzyme-linked immunosorbent assay (ELISA), hematoxylin and eosin (H&E) and histopathological evaluation, immunofluorescence staining, real-time quantitative PCR (qPCR) were performed as described in the Supplementary information: Supplementary methods.
Statistical analysis
The experiment data were presented as the mean ± standard error of the mean (SEM). Prior to statistical analysis, the normality of all data was verified with the Shapiro-Wilk test, and homogeneity of variance was assessed using Levene’s test. Both assumptions were met (p > 0.05), justifying the use of Student’s t-test. Comparison of categorical variables between two groups was performed using the Fisher’s exact test. The gut microbiota and metabolomics data analysis was performed on the platform of Majorbio Co. Ltd. (Shanghai, China) [24]. Spearman correlation analysis was employed for associations between gut microbiota and its metabolites, blood cytokines and LPS, inflammatory markers in hippocampus, and behavioral tests. All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, USA) and SPSS 26.0 (IBM, Chicago, IL, USA).
Results
Alterations of gut microbiota and metabolites
We first analyzed the differences of the microbiota between healthy control and prenatal depression donors. Being consistent with our previous findings [13], there were significant differences in microbial diversity and taxa between healthy control and prenatal depression donors (Fig. S1).
We transplanted fecal samples from healthy control and prenatal depression donors to GF mice. Metagenomic sequencing revealed significant differences in the diversity and composition of the gut microbiota in mice (Fig. 1B-E). Mice receiving FMT from women with prenatal depression showed an increased richness in gut microbiota, which was similar to that in donors (Fig. 1B and Fig. S1A). Moreover, Principal Coordinates Analysis (PcoA) showed dissimilarities between mice receiving FMT from healthy women versus women with prenatal depression (Fig. 1C). The differential phylum between the two mice groups was Verrucomicrobia (p < 0.0001) (Fig. 1D); the relative abundances of genera Ligilactobacillus, Mediterraneibacter, Anaerostipes, Hungatella, Bacteroides and Collinsella were significantly decreased while Akkermansia, unclassified_o__Eubacteriales, unclassified_f__Oscillospiraceae, Ruminococcus, Faecalibacterium were significantly more abundant in the mice gut microbiota transplanted with fecal microbiota from women with prenatal depression (Fig. 1E). Table S2 provides taxonomic details and functional traits of these bacteria based on relevant literature. Mediterraneibacter and Anaerostipes belonging to family Lachnospiraceae. While unclassified_f__Oscillospiraceae, Ruminococcus, and Faecalibacterium belonging to family Oscillospiraceae. The gut microbiota of recipient mice receiving FMT from pregnant women with prenatal depression was predominantly composed of conditionally pathogenic bacteria.
Kyoto Encyclopedia of Genes and Genomes (KEGG) functional pathway enrichment analysis showed a significant increase of LPS biosynthesis in the gut microbiota of mice receiving FMT from women with prenatal depression (Fig. 1F).
Untargeted metabolomics analysis of cecal contents from mice that received FMT from both healthy controls and women with prenatal depression. Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) both showed a significant separation of metabolites between the two mice groups (Fig. 2A). Differential metabolites were screened with FDR < 0.05, VIP_PLS-DA > 1, and fold change > 2. For mice receiving FMT from women with prenatal depression, 44 metabolites were significantly altered compared with control mice (Fig. 2B and Table S3).
A Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) of fecal metabolic profiles in two groups. B Volcano plot showed differential metabolites in the gut microbiota between two groups. C Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differential metabolites. D Spearman correlation analysis of differential genera with differential metabolites. HC-FMT, mice receiving FMT from women with healthy control (n = 7); PD-FMT, mice receiving FMT from women with prenatal depression (n = 7); metab_4091, PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)); metab_5356, PS(20:3(8Z,11Z,14Z)/18:3(9Z,12Z,15Z)); data are shown as mean ± SEM; *, p < 0.05; **, p < 0.01; differences are assessed by Student’s t-test.
KEGG enrichment analysis of differential metabolites revealed upregulation of immune- and infection-related disease signaling pathways (Fig. 2C), suggesting potentially abnormal immune-inflammatory responses in mice receiving FMT from women with prenatal depression. The metabolites enriched to these three signaling pathways are phosphatidylserine (PS) in glycerophospholipid metabolism, including PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) (metab_4091) and PS(20:3(8Z,11Z,14Z)/18:3(9Z,12Z,15Z)) (metab_5356). Further analysis of the associations between differential genera and metabolites found that PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) (metab_4091) and PS(20:3(8Z,11Z,14Z)/18:3(9Z,12Z,15Z)) (metab_5356) were negatively associated with Ligilactobacillus, Mediterraneibacter, Hungatella and Collinsella, and positively associated with Akkermansia, unclassified_o__Eubacteriales, unclassified_f__Oscillospiraceae, Ruminococcus and Faecalibacterium (Fig. 2D).
Disruptions of intestinal barrier and immune responses in mice receiving FMT from women with prenatal depression
H&E staining revealed that the mice receiving FMT from women with prenatal depression had a higher pathology rating (Fig. 3A and Table S4) and markedly shorter colon (p = 0.0159, Fig. 3A) compared to control group. Moreover, we detected tight junction protein zonula occludens (ZO-1) and CD3+ T cells in colon tissues by using immunofluorescence and qPCR was used to detect the expression of T helper 17 (Th17) and T regulatory (Treg) cells. Compared to control group, mice receiving FMT from women with prenatal depression showed an decreased expression of ZO-1 in colon tissue and an inreased expression of CD3+ and mRNA level of Th17/ Treg (RORγT+ /FOXP3+) ratio (Fig. 3B).
A Representative microscopic images of colon H&E staining (magnification 200) and comparison of colon length in recipient mice (yellow arrowhead indicate moderately abnormal intestinal tissue structure with epithelial cell detachment and degeneration in the mucosal layer; red arrowhead indicate a decrease in the number of crypts in the mucosal layer with a large number of inflammatory cells). B Representative images of the tight junction proteins ZO-1 and CD3+ T cells in the colon, and relative mRNA levels of RORγT+ and FOXP3+ in the colon tissue of recipient mice. ZO-1 staining (green), CD3 staining (red) and nuclear staining (DIPA, blue). Scale bar = 500 μm. C Plasma cytokine and lipopolysaccharide (LPS) concentrations. HC-FMT, mice receiving FMT from women with healthy control (n = 7); PD-FMT, mice receiving FMT from women with prenatal depression (n = 7); data are shown as mean ± SEM; *, p < 0.05; ****, p < 0.0001; differences are assessed by Student’s t-test.
To further evaluate the peripheral inflammatory response in recipient mice, we assayed plasma cytokines by Luminex. The results showed that the plasma G-CSF (p < 0.0001), IL-17 (p = 0.0242), MIP-1α (p = 0.0189) and MIP-1β (p = 0.0353) of mice receiving FMT from women with prenatal depression were significantly lower than that of healthy control mice. Furthermore, plasma LPS (p < 0.0001) concentrations were significantly increased in mice receiving FMT from women with prenatal depression (Fig. 3C). LPS could enter the bloodstream via the leaky gut, causing host inflammation. Thus, the blood LPS concentration could indirectly reflect the intestinal permeability. These results suggest that FMT from women with prenatal depression disrupts the intestinal barrier and immune homeostasis in mice.
Depressive-like behaviors and hippocampal neuroinflammation in GF mice receiving FMT from women with prenatal depression
To investigate whether transferring microbiota from women with prenatal depression induces depressive-like behaviors in GF mice, we conducted OFT, FST, and TST. GF mice that received FMT from women with prenatal depression were compared with GF mice that received FMT from controls. Mice receiving FMT from women with prenatal depression showed significantly lower movement distance (p = 0.0044), entries (p = 0.0066), and duration (p = 0.0432) in the central zone in the OFT (Fig. 4A), and increased duration of immobility in the FST (p = 0.0259) and TST (p = 0.6050) (Fig. 4B), as compared with those in healthy control FMT mice. Critically, comparable total distance traveled in the OFT (p = 0.0698) (Fig. S2) ruled out locomotor impairment as a potential confounder. Collectively, these findings suggest that depressive symptoms in women with prenatal depression can be transmitted via the FMT.
A Numbers of entries, time spent, travel distance in central zone and representative tracks of recipient mice in the open-field test (OFT). B Immobility time of recipient mice in the forced swimming test (FST) and tail suspension test (TST). C Representative images of the Iba1+ cells (magnification 1000) in the dentate gyrus (DG) of two groups. Iba1 staining (red) and nuclear staining (DIPA, blue). D The expression of inflammatory markers in the hippocampus of recipient mice. E Relative mRNA expression of synaptic proteins and neurotrophic proteins in the hippocampus of recipient mice. HC-FMT, mice receiving FMT from women with healthy control (n = 7); PD-FMT, mice receiving FMT from women with prenatal depression (n = 7); data are shown as mean ± SEM; *, p < 0.05; **, p < 0.01; differences are assessed by Student’s t-test.
In addition, we found no significant differences in the expression of ZO-1 mRNA in the hippocampus of recipient mice (Fig. S3). However, FMT from women with prenatal depression induced a significant increase in the number of hippocampal Iba1+ in mice (p = 0.0373), accompanied by elevated expression of pro-inflammatory markers nuclear factor-κB (NF-κB) p65 (p = 0.0169), tumor necrosis factor-α (TNF-α) (p = 0.0128), and interleukin-6 (IL-6) (p = 0.0392) (Fig. 4C, D). These findings collectively demonstrate that FMT from women with prenatal depression triggers neuroinflammation in recipient mice.
We further examined the postsynaptic density protein 95 (PSD-95), α-amino-3-hydroxy-5-methyl-4-isoxazole-propionicacid receptor (AMPAR), brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinase B (TrkB), and nerve growth factor (NGF), and did not find any significant difference between the two groups (Fig. 4E).
Associations between the MGB axis and the development of prenatal depression
We separately analyzed the associations between the abundance of gut differential bacterial genera and their metabolites and the results of other experiments to demonstrate the effect of microbiota on depressed mice (Figs. S4, S5). Furthermore, we selected the relative abundances of Ligilactobacillus and Akkermansia, as well as the PS (16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) (metab_4091) represented microbiota and its metabolites changes in two groups, respectively. The levels of plasma LPS as the representative features of intestinal permeability. The movement distance in the central zone in the OFT, the Iba1+ cell numbers, the expression of NF-κB p65, TNF-α and IL-6 in the hippocampus were chosen to represent the alterations in the brain. We performed correlation analysis of these experimental results.
The results demonstrated that plasma LPS levels were significantly negatively associated with the relative abundance of Ligilactobacillus (r = −0.635, p = 0.017), and significantly positively associated with the relative abundance of Akkermansia (r = 0.688, p = 0.008) and the PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) (metab_4091) (r = 0.692, p = 0.008). We also witnessed a significantly negative association between the relative abundance of Ligilactobacillus and IL-6 (r = −0.688, p = 0.008), and a significantly positive association between the relative abundance of Akkermansia and IL-6 (r = 0.749, p = 0.003). In addition, the plasma LPS levels showed significantly positive association with the expression of NF-κB p65 (r = 0.675, p = 0.010) and IL-6 (r = 0.587, p = 0.030), and significantly negative association with the movement distance in the central zone in the OFT (r = −0.903, adj p < 0.001) (Fig. 5). Collectively, these results support the involvement of the MGB axis in the development of prenatal depression.
g__Ligilacto., g__Ligilactobacillus; g__Akk., g__Akkermansia; metab_4091, PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)); Distance(OFT), movement distance in the central zone in the open-field test; *, p < 0.05; **, p < 0.01; ***, p < 0.001; associations among different results are assessed by Spearman correlation analysis.
Discussion
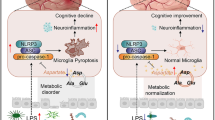
Bidirectional communication between gut microbiota and the brain has an important role in the development of depression. However, the microbial species and molecular mechanisms that lead to prenatal depression have not been studied. Our present study confirms that symptoms in women with prenatal depression can be transferred to GF mice via the fecal microbiota, accompanied by alterations in gut microbial metabolites, blood cytokines and LPS, and neuroinflammatory signaling pathways in the hippocampus. Our findings contributed to identification of causal microbiota that may predispose to prenatal depression through modulating the microbiota-immune-brain axis pathway.
Dysbiosis of the gut microbiota has been reported in women with depression [13, 25, 26]. Intervention strategies targeting the gut microbiota, such as probiotic supplementation, have also shown benefits in reducing prenatal depression [27]. This study added to the literature that diversity and composition of the gut microbiota of mice receiving FMT from women with prenatal depression was altered compared to mice receiving FMT from healthy controls, including decreased Ligilactobacillus and increased Akkermansia. In a clinical study, metagenomic sequencing also found Akkermansia to be enriched in fecal samples from depressed women and Lactobacillus to be enriched in the control group [26]. Similarly, in patients with atypical major depressive disorder, there was an increase in the Verrucomicrobiota phylum, in which Akkermansia is the predominant bacterial genus [28]. Moreover, the relative abundance of Lactobacillus was also found to be lower in an animal model of chronic unpredictable mild stress. Inconsistent with our findings, the relative abundance of Akkermansia was also lower [29]. Variability in microbiota community structure between patients and animal models may result from differences in hosts, gut microbiota sampling sites, and sequencing technologies, which explain the discrepant results in different studies.
“Core functional bacterial groups” are defined as non-redundant microbial consortia that perform critical biochemical functions essential for host physiological homeostasis, wherein functional loss cannot be fully compensated by other taxa, leading to dysbiosis-associated pathologies when disrupted [30]. It is well known that Ligilactobacillus are probiotics involved in the production of short-chain fatty acids (SCFAs), which can compete with pathogenic bacteria for growth, attenuate mucosal inflammatory responses, and enhance the intestinal barrier function [31, 32]. In animal studies, semen trigonellae (dried mature seeds of Trigonella foenum-graecum L.) alleviated LPS-induced depression-like behaviors by reversing hippocampal declines in 5-hydroxytryptamine (5-HT) and BDNF levels while reducing serum concentrations of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6. Gut microbiota analysis revealed that semen trigonellae intervention dose-dependently reshaped the microbial community structure and substantially increased the relative abundance of Ligilactobacillus murinus and Ligilactobacillus animalis. Subsequent experiments confirmed that oral gavage with L. murinus and L. animalis in LPS-challenged mice replicated fenugreek’s antidepressant-like effects, evidenced by elevated 5-HT/BDNF levels and reduced pro-inflammatory cytokines [33].
However, the effects of Akkermansia on host health are controversial [34]. Some studies observed that Akkermansia may improve the symptoms of neuropsychiatric disorders by regulating gut microbiota and metabolites [35, 36], while others have reported that Akkermansia may be involved in the development of neuropsychiatric disorders by exacerbating inflammatory responses and degrading mucus oligosaccharides [37, 38]. Crocetin intake exacerbates dextran sulfate sodium (DSS)-induced colitis in mice, potentially through aberrant elevation of Akkermansia abundance. This dysbiosis disrupts intestinal homeostasis and metabolomic profiles while simultaneously delaying colitis resolution [38]. Furthermore, in experimental models where high-fat diets accelerate inflammatory bowel disease (IBD) progression, omega-6 polyunsaturated fatty acid (PUFA)-enriched interventions aggravated mucus layer destruction and colitis severity in Muc2-/- mice. This effect was attributed to significant enrichment of Akkermansia muciniphila, a recognized mucin-degrading bacterium, which exacerbates mucosal damage via proteolytic degradation of protective mucins [39]. Bae et al. found that the cell membrane phospholipid a15:0-i15:0 PE (a diacyl PE containing 2 branched chains) of Akkermansia induced the release of TNF-α and IL-6 from dendritic cells, and low doses of a15:0-i15:0 PE reset the activation threshold of dendritic cells, reducing the response to subsequent immune stimulation [40]. However, in our study, the abundance of Akkermansia was significantly increased in the gut of mice receiving FMT from women with prenatal depression, possibly leading to abnormal elevations of its metabolite a15:0-i15:0 PE beyond the dose threshold for immunomodulatory effects, thereby exacerbating the host inflammatory response.
In addition, the relative abundances of genera Mediterraneibacter and Anaerostipes (belonging to family Lachnospiraceae) were significantly decreased in the mice receiving FMT from women with prenatal depression. Lachnospiraceae are also the main species involved in SCFAs production [41]. In putative probiotic fermented milk containing buriti pulp or passion fruit pulp, Mediterraneibacter constituted the dominant microbiota. When administered into a simulator of the human intestinal microbial ecosystem, this fermented milk formulation elicited beneficial restructuring of intestinal microbial communities and enhanced SCFA biosynthesis [42]. While the significantly enriched unclassified_f__Oscillospiraceae, Ruminococcus, and Faecalibacterium belonging to family Oscillospiraceae. Oscillospiraceae, strictly anaerobic gut bacteria, function as primary inflammation-associated intestinal pathogens in colitis pathogenesis [43]. Their pro-inflammatory properties may stem from metabolites such as hydrogen sulfide (H₂S)—produced by select family members—which induces oxidative stress and mucosal damage by inhibiting mitochondrial respiratory chain complex IV in colonic epithelial cells [44]. Furthermore, pro-inflammatory activity of Oscillospiraceae correlates with obstructive sleep apnea (OSA) pathogenesis [45]. In OSA patients, intermittent hypoxia upregulates hypoxia-inducible factor-1α (HIF-1α), promoting Oscillospiraceae proliferation. This expansion subsequently enhances T helper 17 (Th17) cell differentiation and drives systemic inflammation [46, 47].
These findings suggest that FMT from women with prenatal depression induces gut microbiota dysbiosis in recipient mice, characterized by a significant decrease in SCFAs-producing bacteria and a significant increase in pro-inflammatory bacteria. Wu et al. identified two core microbiota clusters critical to human health: the “beneficial guild” and “detrimental counterpart”. These two core microbial clusters reciprocally modulate human health through dynamic fluctuations in their abundance. The “beneficial guild” primarily comprises SCFAs-producing bacteria. These bacteria degrade dietary fibers to generate SCFAs, which play vital roles in human nutrition, immune regulation, and mental health. Critically, these bacteria harbor virtually no antibiotic resistance genes or virulence factor genes, rendering them safe for human hosts. In contrast, the “detrimental counterpart” is dominated by opportunistic pathogenic bacteria. Research indicates that when maintained at controlled levels, these opportunistic pathogenic bacteria can train the neonatal immune system to develop properly and sustain a state of moderate vigilance in adult immunity. However, excessive overgrowth of such pathogenic bacteria may trigger systemic inflammation, thereby promoting the initiation and progression of multiple diseases. Therefore, the dominance of this guild over the beneficial one could contribute to a pro-inflammatory state detrimental to host health [30]. Collectively, we suggest that it may be dysbiosis of the gut microbiota associated with prenatal depression rather than a single bacterium that leads to depression-like behavior in recipient mice.
Recent studies have demonstrated that gut microbiota disturbances may contribute to the onset of depressive symptoms or depressive-like behaviors by regulating glycerophospholipid metabolism [48,49,50]. In this study, the immune- and infection-related disease signaling pathways of gut microbiota metabolites were significantly upregulated in mice receiving FMT from women with prenatal depression. PS was identified as the metabolite enriched in these pathways. PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) (metab_4091) showed a significant positive correlation with depressive-like behaviors and central neuroinflammation (Fig. S4). PS(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) (metab_4091) is a specific PS molecule containing palmitic acid (16:0) and docosahexaenoic acid (DHA, 22:6(4Z,7Z,10Z,13Z,16Z,19Z)) as its fatty acid chains. DHA is an ω-3 polyunsaturated fatty acid, which is generally considered to have anti-inflammatory properties [51, 52]. However, under certain specific conditions, such as oxidative stress, PS(16:0/22:6) may be involved in pro-inflammatory responses. For example, DHA may generate pro-inflammatory metabolites like lipid peroxides, thereby triggering inflammatory response [53]. In our study, the upregulated PE (20:5(7Z,9Z,11E,13E,17Z)-3OH(5,6,15)/18:4(6Z,9Z,12Z,15Z)) (metab_2717) (Table S3) in mice receiving FMT from women with prenatal depression is one of the oxidized lipids. It inhibits autophagy via stimulating the delipidation of oxidized LC3-PE [54]. Autophagy can prevent the release of mitochondrial reactive oxygen species into the cytoplasm and restrict the formation of NLRP1 inflammasome [55]. Therefore, the inhibitory effect of oxidized PE on autophagy may aggravate the inflammatory response. Combined with our results, disturbance of glycerophospholipid metabolism induced by altered gut microbiota may contribute to the development of depressive phenotype through activation of inflammatory responses.
The integrity of the intestinal barrier plays an important role in communication between the gut and the brain [56]. In our results, mice receiving FMT from women with prenatal depression had increased colon histopathology scores, decreased ZO-1 protein expression, significantly lower plasma IL-17A and G-CSF concentrations, and significantly increased plasma LPS. Since IL-17A is involved in the development of mucosal immunity, it induces the expression of chemokines and G-CSF in intestinal epithelial cells, which then enhances the expression of antimicrobial peptides to play a protective effect in intestinal mucosa [57]. LPS is a major component of the outer membrane of gram-negative bacteria and can enter the bloodstream through the leaky gut and cause host inflammation. The concentration of LPS in the blood reflects the permeability of the gut [56]. Moreover, LPS biosynthesis was significantly increased in the gut microbiota of mice receiving FMT from women with prenatal depression, suggesting that mice receiving FMT from women with prenatal depression have a higher abundance of gram-negative bacteria in the gut and may produce more LPS. It has been reported that Akkermansia can cause the thinning of intestinal mucus layer and increase intestinal permeability in mice by degrading mucus oligosaccharides [37]. Therefore, dysbiosis of the gut microbiota associated with prenatal depression may leads to colonic mucosal damage and increased intestinal permeability in recipient mice, allowing more LPS in the gut to leak into the peripheral circulation.
Central neuroinflammation is important factor promoting to the development of perinatal depression [58]. Sustained intestinal barrier damage associated with gut microbiota dysbiosis could leak microbes and LPS into the peripheral circulation. LPS binding to toll-like receptor 4 (TLR4) activates the myeloid differentiation factor 88 (MyD88)-dependent signaling pathway in brain microglia. This cascade triggers phosphorylation of the IκB kinase (IKK) complex, leading to proteasomal degradation of NF-κB inhibitor alpha (IκBα). Consequently, nuclear translocation of the NF-κB p65 subunit occurs, driving transcriptional upregulation of pro-inflammatory cytokines such as TNF-α and IL-6. These molecular events ultimately induce neuroinflammation and associated behavioral alterations [27, 59,60,61,62]. In this study, mice receiving FMT from women with prenatal depression exhibited significantly elevated plasma LPS concentrations. This increase was associated with markedly upregulated NF-κB mRNA expression in the hippocampus, suggesting transcriptional activation of this pathway. Furthermore, mRNA levels of IL-6 and TNF-α (direct transcriptional targets of NF-κB) were concurrently elevated. This coordinated gene expression pattern reveals NF-κB’s central role in transducing upstream signals (e.g., LPS-TLR4 interaction) into production of inflammatory mediators through transcriptional regulation. It has been shown that there is a significant correlation between the expression level of NF-κB mRNA and its protein activity, and the target genes of NF-κB include various pro-inflammatory cytokines (e.g., IL-6, TNF-α), and the expression level of these genes is directly regulated by the transcriptional activity of NF-κB [63, 64]. Therefore, the simultaneous elevation of NF-κB p65, TNF-α and IL-6 mRNA expression can indirectly reflect the activity status of NF-κB. We observed coordinated upregulation and statistically significant correlations among plasma LPS concentrations, hippocampal NF-κB mRNA levels, and effector molecules (IL-6 and TNF-α mRNA). These findings are entirely consistent with the canonical “LPS-TLR4-NF-κB activation-pro-inflammatory cytokines (e.g., IL-6, TNF-α)-neuroinflammation-behavioral deficits” pathway. Our findings are consistent with Wang et al.’s report that elevated serum LPS levels in mice with chronic unpredictable mild stress activate hippocampal TLR4/NF-κB/mitogen-activated protein kinase (MAPK) signaling, promoting pro-inflammatory cytokine production and inducing depression-like behaviors [65]. This mechanistic convergence underscores the fundamental role of gut-derived LPS-triggered neuroinflammation via NF-κB signaling in depression pathogenesis, irrespective of induction method (stress or dysbiosis transfer). Although direct p65 phosphorylation data are lacking, the integrated evidence from significant alterations in downstream effector molecules and established biological principles of LPS-TLR4-NF-κB signaling compellingly demonstrates NF-κB’s pivotal role in mediating the effects of LPS. Importantly, correlation analysis revealed that plasma LPS levels, hippocampal pro-inflammatory cytokines were strongly associated with microbiota and metabolites. The strong associations indicate that microbial community structure influences the communication between the gut and the brain. Collectively, the results of this study support that the gut microbiota dysbiosis associated with prenatal depression contributes to the depressive phenotype via the activation of central neuroinflammatory responses by its metabolites. LPS has a systemic immune activating effect as evidenced by an increase in spleen weight and blood inflammatory factors [66]. However, we did not find significant changes in other inflammatory markers (e.g., TNF-α, IL-6) in the blood of recipient mice. IL-6 and TNF-α were significantly increased in the hippocampus of mice receiving FMT from women with prenatal depression, suggesting that LPS may directly activate central neuroinflammatory via the MGB axis, and may not necessarily depend on the response of systemic immune organs such as the spleen [67, 68]. Our results suggest that the direct effects of gut microbiota dysbiosis on the central nervous system may be independent of classical systemic immune pathways. This finding is highly relevant to the neuroinflammatory hypothesis of clinical perinatal depression [58].
The vagus nerve plays a crucial role in the MGB axis. Ma et al. showed that celiac vagotomy attenuated depressive and anxiety-like behaviors and neuroinflammation induced by extracellular vesicles from Escherichia fergusonii culture administered via oral gavage, but had no effect on the elevation of blood LPS and TNF-α levels, colonic TNF-α expression, NF-κB-positive cell number, or fecal LPS levels [69]. Similarly, Celiac vagotomy significantly decreased Escherichia fergusonii- or Veillonella infantium-induced cognitive impairment-like behavior and hippocampal Iba1+ cell population and TNF-α expression and increased Escherichia fergusonii- or Veillonella infantium-suppressed hippocampal BDNF expression. However, Enterococcus faecium-induced cognitive impairment-like behavior and hippocampal Iba1+ cell population and TNF-α expression were partially, but not significantly, attenuated by celiac vagotomy. Furthermore, celiac vagotomy did not affect Escherichia fergusonii-, Veillonella infantium-, or Enterococcus faecium-induced LPS levels in the blood and feces and TNF-α expression and NF-κB-positive cell population in the colon [70]. These results suggest that LPS-producing gut microbiota may induce NF-κB-mediated neuroinflammation through the translocation of LPS into the brain through gut/blood-vagus nerve-brain pathway. In this study, we found that FMT from women with prenatal depression to mice induced significant increases in plasma LPS levels and hippocampal neuroinflammation, but did not significantly alter BBB permeability. These observations suggest that the vagus nerve may play a key role in mediating gut-brain communication. Future studies should employ celiac vagotomy in FMT models of women with prenatal depression to further elucidate their mechanistic relationships.
Since patterns of synaptic activity are the closest physical representation of mood, emotion, and conscience that we can conceptualize, and activation of inflammatory pathways can modulate neuronal function by affecting synaptic activity [5]. We examined the relative mRNA expression of synaptic proteins and neurotrophic maker proteins in the hippocampus of recipient mice to clarify whether FMT from women with prenatal depression lead to alterations in neuronal function via activation of inflammatory responses. The results showed no significant differences between the two groups. Similar to our findings FMT from patients with major depression also did not alter the mRNA expression levels of synaptic and neurotrophic proteins in the hippocampal tissue of recipient mice [9]. The dissociation between depressive phenotype transmission and the expression levels of synaptic plasticity-associated proteins suggests that the “microbiome-gut-brain axis” may influence behavior via non-classical synaptic remodeling pathways (e.g., neuroinflammation-mediated alterations in synaptic transmission efficiency or synaptic pruning imbalance), rather than directly regulating the transcription of synaptic structural proteins. For instance, pro-inflammatory cytokines (e.g., TNF) can enhance the expression/activity of L-type voltage sensitive Ca(2+) channels in neurons, leading to Ca(2+) dyshomeostasis, hyperactivated calcineurin activity, and synaptic suppression [71]. TNF-α mediated changes in excitatory and inhibitory neurotransmission in a concentration-dependent manner, where low concentration strengthened glutamatergic neurotransmission via synaptic accumulation of GluA1-only-containing AMPA receptors and higher concentration increased inhibition [72]. Therefore, whether neuroinflammation induced by gut microbiota and their metabolites affects synaptic plasticity still requires further validation through analysis of protein post-translational modifications (PTMs, e.g., phosphorylation, ubiquitination), observation of synaptic ultrastructure (e.g., synaptic density via electron microscopy), and assessment of functional plasticity (e.g., action potential recording).
Current research findings on the link between neuroinflammation and synapses exhibit heterogeneity. It was shown that LPS-induced central neuroinflammatory and depressive-like behaviors were associated with reduced hippocampal PSD-95 and synaptophysin expression, and that levomilnacipran upregulated BDNF/TrkB mediated phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt)/ mammalian target of rapamycin (mTOR) signaling pathway, enhanced the expression of PSD-95 and synaptophysin, and ameliorated LPS-induced impairment of neuronal synaptic plasticity [73]. Wang et al. showed a significant decrease in the expression of synaptic proteins PSD-95 and GluA1 in the prefrontal lobe of a mouse model of chronic social frustration stress [74], whereas another study showed a significant increase in IL-6 and PSD-95 in the brain tissues of mice after stimulation with chronic social frustration stress [75]. In this study, the observed increase in PSD-95 expression may be related to pseudoelevation of detection signals caused by microglial phagocytosis of postsynaptic density remnants containing PSD-95 fragments. Notably, variations in stress duration and detection time windows across studies could capture distinct phases of PSD-95 dynamics, such as compensatory upregulation in acute stages versus sustained downregulation in chronic phases. Furthermore, methodological differences, including detection approaches (e.g., whole-brain homogenization potentially masking region-specific changes), antibody specificity, and sample processing protocols (e.g., fixation methods affecting epitope accessibility) may contribute to inter-study heterogeneity. Critically, these seemingly paradoxical findings suggest that stress-induced alterations in PSD-95 expression exhibit spatiotemporal- and pathology-dependent regulation. This bidirectional modulation likely reflects a balancing act between compensatory neuroplasticity and synaptic injury, necessitating future mechanistic dissection via spatial omics technologies and temporally stratified experimental designs. Studies of brain postmortem from patients with bipolar and major depression found that PSD-95 protein levels were decreased in the frontal cortex, suggesting that PSD-95 protein may have a role in the cognitive function of mood disorders [76]. However, no studies to date have demonstrated impaired neuronal synaptic plasticity in pregnant women with prenatal depression. Therefore, the relationship between depression-like behavior induced by gut microbiota and alterations in synaptic plasticity requires further validation through additional clinical and/or preclinical studies.
This study had several limitations. Firstly, using female mice for FMT may not fully recapitulate pregnancy-associated physiology, limiting mechanistic insights into prenatal depression development. Secondly, mice from the same group were co-housed to prevent microbial transfer between different groups. However, the tendency for co-housed mice to share more similar microbiota may have potentially masked or exaggerated the results. Thirdly, the implementation of sucrose preference testing (SPT) was precluded by maintenance of recipient mice on sterilized feed and sterile water throughout behavioral experiments, thereby limiting direct assessment of anhedonia, a core depressive feature. Fourthly, due to sample limitations, the assessment of synaptic plasticity in recipient mice lacked multimodal validation, including electrophysiological techniques, ultrastructural examination, and quantitative analysis of synaptic plasticity-related proteins. Lastly, we did not directly assay protein levels of NF-κB as well as measure spleen size due to limitations in research resources and sample preservation. In future studies, we recommend assessing spleen weight and histological changes following FMT, combined with single-cell sequencing technology, to investigate the differential regulation of gut microbiota-LPS signaling on central and peripheral immune organs.
In conclusion, our study highlights the significance of gut microbiota dysbiosis in the development of prenatal depression and elucidates the underlying mechanisms of the MGB axis in this process. Our findings demonstrate that mice receiving FMT from women with prenatal depression exhibit depressive-like behaviors, gut microbiota dysbiosis, and central neuroinflammation. Specifically, FMT of “depression-related microbiota” from these women induced depressive phenotypes by activating central neuroinflammation through its metabolite LPS. Further in-depth studies are necessary to validate and expand upon our findings.
Data availability
The metagenomic sequencing data have been deposited to the NCBI Sequence Read Archive and are available at the accession number PRJNA1230565.
References
The Lancet. Perinatal depression: a neglected aspect of maternal health. Lancet. 2023;402:667.
Yin X, Sun N, Jiang N, Xu X, Gan Y, Zhang J, et al. Prevalence and associated factors of antenatal depression: systematic reviews and meta-analyses. Clin Psychol Rev. 2021;83:101932.
Zhang ZY, Yu JJ, Zeng WT, Zhou MC, Duan CC, Zhu LL. Association between antenatal depression and adverse perinatal outcomes: a prospective cohort study. J Affect Disord. 2023;323:490–5.
Samuelsen K, Ystrom E, Gjerde LC, Eilertsen EM. Kind of blue - an evaluation of etiologies for prenatal versus postnatal depression symptoms. J Affect Disord. 2023;335:305–12.
Fries GR, Saldana VA, Finnstein J, Rein T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2023;28:284–97.
Deng W, Yi P, Xiong Y, Ying J, Lin Y, Dong Y, et al. Gut metabolites acting on the gut-brain axis: regulating the functional state of microglia. Aging Dis. 2024;15:480–502.
Macpherson AJ, Pachnis V, Prinz M. Boundaries and integration between microbiota, the nervous system, and immunity. Immunity. 2023;56:1712–26.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–96.
Zhang Y, Fan Q, Hou Y, Zhang X, Yin Z, Cai X, et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav Immun. 2022;102:11–22.
Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–80.
Nuriel-Ohayon M, Neuman H, Ziv O, Belogolovski A, Barsheshet Y, Bloch N, et al. Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 2019;27:730–6.e1-e3.
Edwards SM, Cunningham SA, Dunlop AL, Corwin EJ. The maternal gut microbiome during pregnancy. MCN Am J Matern Child Nurs. 2017;42:310–7.
Xie T, Fan X, Pang H, Zang T, Wu N, Liu J, et al. Association between gut microbiota and its functional metabolites with prenatal depression in women. Neurobiol Stress. 2023;28:100592.
Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87.
Köhler-Forsberg O, Lydholm CN, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404–19.
Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–56.
Yao H, Zhang D, Yu H, Yuan H, Shen H, Lan X, et al. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation. Mol Psychiatry. 2023;28:919–30.
Sun ZW, Wang X, Zhao Y, Sun ZX, Wu YH, Hu H, et al. Blood-brain barrier dysfunction mediated by the EZH2-Claudin-5 axis drives stress-induced TNF-α infiltration and depression-like behaviors. Brain Behav Immun. 2024;115:143–56.
Butler MI, Long-Smith C, Moloney GM, Morkl S, O’Mahony SM, Cryan JF, et al. The immune-kynurenine pathway in social anxiety disorder. Brain Behav Immun. 2022;99:317–26.
Yuan C, He Y, Xie K, Feng L, Gao S, Cai L. Review of microbiota gut brain axis and innate immunity in inflammatory and infective diseases. Front Cell Infect Microbiol. 2023;13:1282431.
Wang Y, Guo X, Lau Y, Chan KS, Yin L, Chen J. Psychometric evaluation of the mainland Chinese version of the Edinburgh postnatal depression scale. Int J Nurs Stud. 2009;46:813–23.
Pu Y, Zhang Q, Tang Z, Lu C, Wu L, Zhong Y, et al. Fecal microbiota transplantation from patients with rheumatoid arthritis causes depression-like behaviors in mice through abnormal T cells activation. Transl Psychiatry. 2022;12:223.
Yang Y, Zheng X, Wang Y, Tan X, Zou H, Feng S, et al. Human fecal microbiota transplantation reduces the susceptibility to dextran sulfate sodium-induced germ-free mouse colitis. Front Immunol. 2022;13:836542.
Ren Y, Yu G, Shi C, Liu L, Guo Q, Han C, et al. Majorbio cloud: a one-stop, comprehensive bioinformatic platform for multiomics analyses. Imeta. 2022;1:e12.
Fang Q, Tu Y, Fan X, Zang T, Wu N, Qiu T, et al. Inflammatory cytokines and prenatal depression: is there a mediating role of maternal gut microbiota? J Psychiatr Res. 2023;164:458–67.
Chen YH, Xue F, Yu SF, Li XS, Liu L, Jia YY, et al. Gut microbiota dysbiosis in depressed women: the association of symptom severity and microbiota function. J Affect Disord. 2021;282:391–400.
Song J, Zhou B, Kan J, Liu G, Zhang S, Si L, et al. Gut microbiota: linking nutrition and perinatal depression. Front Cell Infect Microbiol. 2022;12:932309.
Busch A, Roy S, Helbing DL, Colic L, Opel N, Besteher B, et al. Gut microbiome in atypical depression. J Affect Disord. 2024;349:277–85.
He H, He H, Mo L, You Z, Zhang J. Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav Immun. 2024;115:280–94.
Wu G, Xu T, Zhao N, Lam YY, Ding X, Wei D, et al. A core microbiome signature as an indicator of health. Cell. 2024;187:6550–65.e1-e4.
Chen YY, Fei F, Ding LL, Wen SY, Ren CF, Gong AH. Integrated gut microbiome and metabolome analysis reveals the inhibition effect of Lactobacillus plantarum CBT against colorectal cancer. Food Funct. 2024;15:853–65.
Sobrino OJ, Alba C, Arroyo R, Pérez I, Sariego L, Delgado S, et al. Replacement of metaphylactic antimicrobial therapy by oral administration of Ligilactobacillus salivarius MP100 in a pig farm. Front Vet Sci. 2021;8:666887.
Chang W, Guo J, Yang Y, Zou L, Fu Y, Li M, et al. Semen trigonellae alleviates LPS-induced depressive behavior via enhancing the abundance of Ligilactobacillus spp. Food Sci Nutr. 2024;12:9414–27.
Lei W, Cheng Y, Gao J, Liu X, Shao L, Kong Q, et al. Akkermansia muciniphila in neuropsychiatric disorders: friend or foe? Front Cell Infect Microbiol. 2023;13:1224155.
Li N, Tan S, Wang Y, Deng J, Wang N, Zhu S, et al. Akkermansia muciniphila supplementation prevents cognitive impairment in sleep-deprived mice by modulating microglial engulfment of synapses. Gut Microbes. 2023;15:2252764.
Ding Y, Bu F, Chen T, Shi G, Yuan X, Feng Z, et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 2021;105:8411–26.
Yoshihara T, Oikawa Y, Kato T, Kessoku T, Kobayashi T, Kato S, et al. The protective effect of Bifidobacterium bifidum G9-1 against mucus degradation by Akkermansia muciniphila following small intestine injury caused by a proton pump inhibitor and aspirin. Gut Microbes. 2020;11:1385–404.
Feng P, Li Q, Liu L, Wang S, Wu Z, Tao Y, et al. Crocetin prolongs recovery period of DSS-induced colitis via altering intestinal microbiome and increasing intestinal permeability. Int J Mol Sci. 2022;23:3832.
Haskey N, Ye J, Estaki M, Verdugo Meza AA, Barnett JA, Yousefi M, et al. A mediterranean-like fat blend protects against the development of severe colitis in the mucin-2 deficient murine model. Gut Microbes. 2022;14:2055441.
Bae M, Cassilly CD, Liu X, Park SM, Tusi BK, Chen X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature. 2022;608:168–73.
Fusco W, Lorenzo MB, Cintoni M, Porcari S, Rinninella E, Kaitsas F, et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15:2211.
Borgonovi TF, Salgaço MK, Oliveira GLV, Carvalho LAL, Pinheiro DG, Todorov SD, et al. Functional fermented milk with fruit pulp modulates the in vitro intestinal microbiota. Foods. 2022;11:4113.
Yang JY, Chen SY, Wu YH, Liao YL, Yen GC. Ameliorative effect of buckwheat polysaccharides on colitis via regulation of the gut microbiota. Int J Biol Macromol. 2023;227:872–83.
Han S, Li Y, Gao H. Generation and physiology of hydrogen sulfide and reactive sulfur species in bacteria. Antioxidants. 2022;11:2487.
Valentini F, Evangelisti M, Arpinelli M, Di Nardo G, Borro M, Simmaco M, et al. Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 2020;76:140–47.
Arias C, Sepúlveda P, Castillo RL, Salazar LA. Relationship between hypoxic and immune pathways activation in the progression of neuroinflammation: role of HIF-1α and Th17 cells. Int J Mol Sci. 2023;24:3073.
Shi Y, Zhang H, Miao C. Metabolic reprogram and T cell differentiation in inflammation: current evidence and future perspectives. Cell Death Discov. 2025;11:123.
Xu K, Ren Y, Zhao S, Feng J, Wu Q, Gong X, et al. Oral D-ribose causes depressive-like behavior by altering glycerophospholipid metabolism via the gut-brain axis. Commun Biol. 2024;7:69.
Zu X, Xin J, Xie H, Xu X, Shen Y, Wang J, et al. Characteristics of gut microbiota and metabolic phenotype in patients with major depressive disorder based on multi-omics analysis. J Affect Disord. 2024;344:563–76.
Li Q, Sun H, Guo J, Zhao X, Bai R, Zhang M, et al. The effect of prenatal stress on offspring depression susceptibility in relation to the gut microbiome and metabolome. J Affect Disord. 2023;339:531–7.
Raza ML, Hassan ST, Jamil S, Fatima W, Fatima M. Nutritional interventions in depression: the role of vitamin D and omega-3 fatty acids in neuropsychiatric health. Clin Nutr. 2025;45:270–80.
Zhang Z, Liu Y, Feng W, Mao P, Yang J, Zhao Z, et al. Omega-3 polyunsaturated fatty acids protect against cisplatin-induced nephrotoxicity by activating the Nrf2 signaling pathway. Int J Biol Macromol. 2024;282:137457.
Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–43.
Li W, Luo LX, Zhou QQ, Gong HB, Fu YY, Yan CY, et al. Phospholipid peroxidation inhibits autophagy via stimulating the delipidation of oxidized LC3-PE. Redox Biol. 2022;55:102421.
Hao T, Fang W, Xu D, Chen Q, Liu Q, Cui K, et al. Phosphatidylethanolamine alleviates OX-LDL-induced macrophage inflammation by upregulating autophagy and inhibiting NLRP1 inflammasome activation. Free Radic Biol Med. 2023;208:402–17.
Gong X, Ma Y, Deng X, Li A, Li X, Kong X, et al. Intestinal dysbiosis exacerbates susceptibility to the anti-NMDA receptor encephalitis-like phenotype by changing blood brain barrier permeability and immune homeostasis. Brain Behav Immun. 2024;116:34–51.
McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906.
Miller ES, Sakowicz A, Roy A, Yang A, Sullivan JT, Grobman WA, et al. Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. Am J Obstet Gynecol. 2019;220:271.e1–10.
Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G, et al. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 2021;9:226.
Solarz A, Majcher-Maślanka I, Kryst J, Chocyk A. Early-life stress affects peripheral, blood-brain barrier, and brain responses to immune challenge in juvenile and adult rats. Brain Behav Immun. 2023;108:1–15.
Jamar G, Ribeiro DA, Pisani LP. High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit Rev Food Sci Nutr. 2021;61:836–54.
Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut. 2019;68:829–43.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023.
Giridharan S, Srinivasan M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J Inflamm Res. 2018;11:407–19.
Wang M, Sun P, Li Z, Li J, Lv X, Chen S, et al. Eucommiae cortex polysaccharides attenuate gut microbiota dysbiosis and neuroinflammation in mice exposed to chronic unpredictable mild stress: beneficial in ameliorating depressive-like behaviors. J Affect Disord. 2023;334:278–92.
Rewell SSJ, Shad A, Chen L, Macowan M, Chu E, Gandasasmita N, et al. A post-injury immune challenge with lipopolysaccharide following adult traumatic brain injury alters neuroinflammation and the gut microbiome acutely, but has little effect on chronic outcomes. Exp Neurol. 2025;386:115150.
Xu Y, Shen B, Pan X, Liu C, Wang Y, Chen X, et al. Palmatine ameliorated lipopolysaccharide-induced sepsis-associated encephalopathy mice by regulating the microbiota-gut-brain axis. Phytomedicine. 2024;124:155307.
Yu Z, Chen W, Zhang L, Chen Y, Chen W, Meng S, et al. Gut-derived bacterial LPS attenuates incubation of methamphetamine craving via modulating microglia. Brain Behav Immun. 2023;111:101–15.
Ma X, Park HS, Shin YJ, Kim JK, Hong JK, Han SW, et al. The extracellular vesicle of depressive patient-derived Escherichia fergusonii induces vagus nerve-mediated neuroinflammation in mice. J Neuroinflammation. 2024;21:224.
Ma X, Kim JK, Shin YJ, Park HS, Lee DY, Yim SV, et al. Lipopolysaccharide-producing Veillonella infantium and Escherichia fergusonii cause vagus nerve-mediated cognitive impairment in mice. Brain Behav Immun. 2024;118:136–48.
Sama DM, Norris CM. Calcium dysregulation and neuroinflammation: discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res Rev. 2013;12:982–95.
Kleidonas D, Kirsch M, Andrieux G, Pfeifer D, Boerries M, Vlachos A. Microglia modulate TNFα-mediated synaptic plasticity. Glia. 2023;71:2117–36.
Wu Y, Zhu Z, Lan T, Li S, Li Y, Wang C, et al. Levomilnacipran improves lipopolysaccharide-induced dysregulation of synaptic plasticity and depression-like behaviors via activating BDNF/TrkB mediated PI3K/Akt/mTOR signaling pathway. Mol Neurobiol. 2024;61:4102–15.
Wang S, Qu Y, Chang L, Pu Y, Zhang K, Hashimoto K. Antibiotic-induced microbiome depletion is associated with resilience in mice after chronic social defeat stress. J Affect Disord. 2020;260:448–57.
Bai Z, Gao T, Zhang R, Lu Y, Tian J, Wang T, et al. Inhibition of IL-6 methylation by saikosaponin C regulates neuroinflammation to alleviate depression. Int Immunopharmacol. 2023;118:110043.
Leung E, Lau EW, Liang A, de Dios C, Suchting R, Östlundh L, et al. Alterations in brain synaptic proteins and mRNAs in mood disorders: a systematic review and meta-analysis of postmortem brain studies. Mol Psychiatry. 2022;27:1362–72.
Acknowledgements
The study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Hubei and Fundamental Research Funds for the Central Universities (Grant No. 82473647, 2023AFB710, 2042023kf0189).
Author information
Authors and Affiliations
Contributions
YC, XF, TZ, and YL designed the experiment. YC, XF. and TZ performed the experiments and wrote the manuscript. TQ and QF analyzed the data. JB. and YL revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, Y., Fan, X., Zang, T. et al. Prenatal depression-associated gut microbiota induces depressive-like behaviors and hippocampal neuroinflammation in germ-free mice. Transl Psychiatry 15, 383 (2025). https://doi.org/10.1038/s41398-025-03606-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03606-x