Abstract
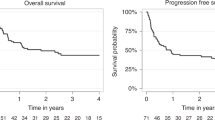
The impact of the number of induction cycles required to achieve first complete remission (CR1) on transplant outcomes in adult acute lymphoblastic leukemia (ALL) patients remains unknown. We conducted a retrospective EBMT registry analysis (2000–2022) of ALL patients who underwent transplantation in CR1 after one (n = 2038), two (n = 296), or three or more (n = 110) induction cycles. Median age was 40 years (range 18–73); 79% had B-ALL. At 2 years, relapse incidence was 23%, 31%, and 32%, while non-relapse mortality was 17%, 18%, and 16%, for those achieving CR1 after one, two, and ≥3 cycles, respectively. Multivariable analysis showed that requiring ≥2 cycles was associated with increased relapse risk. Leukemia‐free survival (LFS) at 2 years was 60%, 51%, and 52%, and overall survival (OS) was 68%, 61%, and 60%, for patients needing one, two, and ≥3 cycles, respectively. Multivariable analysis confirmed significantly worse LFS and OS in patients requiring multiple cycles versus one. These findings suggest that the number of induction cycles to achieve CR1 is a key prognostic factor for post-transplant outcomes in adult ALL and support the development of risk-adapted strategies in this setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data can be obtained from corresponding author for appropriated reasons.
References
DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: updated 2019 evidence-based review from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25:2113–23.
Giebel S, Marks DI, Boissel N, Baron F, Chiaretti S, Ciceri F, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2019;54:798–809.
Marcoux C, Kebriaei P. Transplant in ALL: who, when, and how? Hematol Am Soc Hematol Educ Program. 2024;2024:93–101.
Shen Z, Gu X, Mao W, Yin L, Yang L, Zhang Z, et al. Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer. 2018;18:755.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from hl-a-matched sibling donor,s. Transplantation. 1974;18:295–304.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51:610–1.
Sanz J, Galimard J-E, Labopin M, Afanasyev B, Sergeevich MI, Angelucci E, et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J Hematol Oncol. 2021;14:84.
Boyiadzis M, Zhang M-J, Chen K, Abdel-Azim H, Abid MB, Aljurf M, et al. Impact of pre-transplant induction and consolidation cycles on AML allogeneic transplant outcomes: a CIBMTR analysis in 3113 AML patients. Leukemia. 2022;37:1–12.
Walter RB, Sandmaier BM, Storer BE, Godwin CD, Buckley SA, Pagel JM, et al. Number of courses of induction therapy independently predicts outcome after allogeneic transplantation for acute myeloid leukemia in first morphological remission. Biol Blood Marrow Transplant. 2015;21:373–8.
Giebel S, Labopin M, Socié G, Beelen D, Browne P, Volin L, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2016;102:139–49.
Acknowledgements
The authors are particularly thankful to all centers from the Acute Leukemia Working Party of the EBMT, who kindly agreed to participate in this study.
Author information
Authors and Affiliations
Contributions
Conception and design: JM and JS. Data analysis and interpretation: JM, ATF, and JS. Manuscript writing: JM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montoro, J., Ferhat, AT., Dhedin, N. et al. Impact of pre-transplant induction cycles on post-transplant outcomes in patients with ALL: a study from the ALWP EBMT. Bone Marrow Transplant 60, 1309–1315 (2025). https://doi.org/10.1038/s41409-025-02669-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02669-0