Abstract
Background
The standard treatment for unresectable non-small cell lung cancer (NSCLC) is anti-PD-L1 therapy combined with chemoradiotherapy (anti-PD-L1-CRT). Although some patients achieve complete cancer eradication and cure, more than half of patients retain persistent cancer cells. Our research aimed to unravel the nuanced mechanisms involved in both immune attack and evasion induced by anti-PD-L1-CRT with single cell spatial transcriptome.
Methods
Xenium is a cutting-edge single-cell spatial analysis tool that enables pathology-based and single-cell analyses while preserving spatial information. In our study, we used Xenium to identify the tumour microenvironment (TME), immune dynamics, and residual cancer cells at the single-cell level following treatment with anti-PD-L1-CRT.
Results
Posttreatment alterations included a significant increase in CXCL9+ cells and CXCL13 + T cells, particularly around tumour cells. Additionally, we discovered that CXCL13 + T cells directly impact cancer cells in the posttreatment environment. Moreover, we identified clusters of immune-cold cancer cells posttreatment, revealing their activation of DNA repair pathways and high proliferative capacity. The novel spatial analysis tool Xenium enabled identification of the immune environment at the single-cell level following treatment with anti-PD-L1-CRT, elucidating its characteristics.
Conclusions
These findings suggest potential advancements in developing new treatments to improve posttreatment immune responses and address resistance challenges.

Similar content being viewed by others
Introduction
Radiotherapy (RT) is an important component of cancer treatment and is administered to approximately 50–60% of all cancer patients. For the past century, the major antitumour effect of RT has been thought to occur through induction of lethal DNA damage. Recently, accumulating evidence has revealed that immune responses and immunological cell death also contribute to the antitumour effect of RT [1, 2]. Exploitation of the immune response to improve cancer treatment has led to development of immune checkpoint inhibitors (ICIs), and many clinical trials of RT and chemoradiotherapy (CRT) combined with ICIs have been undertaken. Phase I and II trials of anti-PD-1 or anti-PD-L1 therapy combined with CRT have shown promising responses in some cancer types [3,4,5]. Notably, addition of anti-PD-L1 therapy significantly improved the effect of CRT on non-small cell lung cancer (NSCLC) in a phase III study [6, 7]. Furthermore, phase I and II trials of anti-PD-1 or anti-PD-L1 therapy combined with CRT have shown promising responses in other cancer types, such as head and neck, pancreatic and colorectal cancer [3,4,5]. However, several trials of anti-PD-1/PD-L1 combined with CRT have reported negative or inconclusive results [8,9,10,11,12,13], and that concurrent anti-PD-L1 therapy does not provide a survival benefit [14]. Furthermore, more than half of patients experience recurrence in NSCLC patients despite adjuvant anti-PD-L1 therapy [8]. These findings suggest the inadequate effect of the simple combination of anti-PD-1/PD-L1 therapy and CRT, and it is necessary to optimize ICI therapy combined with CRT. Moreover, the underlying mechanisms involved after treatment with an anti-PD-1/PD-L1 antibody combined with CRT need to be determined to establish a diagnostic tool for biomarker selection and/or integrate therapies targeting additional ICI targets.
Reports on immune environment changes after CRT with anti-PD-L1 therapy in patient tissues are still limited. Recent spatial analyses in esophageal cancer have shown infiltration of PD-L1 and IDO1-expressing cells post-treatment [15]. However, many findings on post-radiotherapy changes rely on preclinical models, and definitive conclusions remain elusive [16,17,18,19,20,21,22,23]. In contrast, immune changes following anti-PD-1/PD-L1 therapy have been extensively analyzed in patient tissues, highlighting the roles of regulatory T cells and CXCL13 + T cells, though their precise localization and function within the tumor microenvironment (TME) remain unclear [24,25,26,27,28,29].
Xenium, a novel technology allowing for multi-in situ hybridization, preserves spatial information for single-cell analysis [30, 31], enabling integrated analysis with haematoxylin and eosin (HE) staining and straightforward identification of treatment-responsive or treatment-resistant areas.
In this study, we performed novel single-cell spatial analysis using tissues following treatment with concurrent CRT and anti-PD-L1 therapy (anti-PD-L1-CRT) for the identification of treatment-induced changes in the immune environment. Initially, we performed technical and histological assessment using Xenium on surgically resected specimens following anti-PD-L1-CRT. Subsequently, we aimed to elucidate alterations in the immune environment after anti-PD-L1-CRT and to unravel the mechanisms driving treatment efficacy and resistance.
Methods
Tissue
A total of 12 biopsies of resected specimens were collected from 8 patients (surgery alone [32]: 1, preoperative CRT: 2, preoperative ICI-CRT: 5). Biopsy or surgical specimens were taken from previous patients [33]. This study was approved by the National Comprehensive Cancer Study Board (NCCHE) Institutional Review Board (Protocol Number 2022-407). Preoperative CRT was performed by platinum-based chemotherapy combined with RT (45 Gy in 25 fractions), and preoperative ICI-RT was performed according to the predefined protocols [33] (https://jrct.niph.go.jp/en-latest-detail/jRCT2080224981), carboplatin, paclitaxel and durvalumab concurrent with RT (50 Gy in 25 fractions). All patients who received preoperative CRT/ICI-CRT were at UICC 8th Stage IIIA.
Experimental protocol for Xenium analysis
In situ expression analysis with Xenium was performed using Xenium Slides & Sample Prep Reagents (PN-1000460, 10x Genomics). FFPE tissue blocks were sectioned at 5 μm thickness and placed on a Xenium slide (10x Genomics) according to the manufacturer’s protocol (CG000578, Rev A, 10x Genomics). Deparaffinization and decrosslinking were performed according to the manufacturer’s protocol (CG000580, Rev A, 10x Genomics). Probe hybridization, ligation and amplification were also performed according to the Xenium In Situ Gene Expression user guide (CG000582, Rev A, 10x Genomics). After that, autofluorescence quenching and nuclear staining were performed, and the slides were kept in the dark. The Xenium Analyser (10x Genomics) was run for the prepared Xenium slides. The Lung panel was kindly provided by Ayako S. The Immune panel was created using custom-ordered components [34]. The genes in the panels are listed in Supplementary Tables S1 and S2.
Cell count analysis using Xenium data
The Python library stLearn (v0.4.12) [35] was used to extract information on gene expression per cell and area per cell from Xenium In situ data. We compared cell counts for n ≥ 1 and n ≥ 2 and concluded that n ≥ 2 allows for a more specific evaluation of cell numbers. Positive (n ≥ 2) and negative (n = 0) results were defined for each cell for the 302 genes in the panel for each sample.
Gene expression analysis
Tumour regions
The tumour region was delineated based on expression of KRT and CDH1, confirmed through HE staining patterns, and validated by a pathologist. Gene expression was assessed using gene density (gene expression/area) calculated by Xenium Explorer ver. 1.2. The analysis was conducted over the entire field.
Field analysis
The delineated tumour was subdivided into distances of 20 μm, 70 μm, and 120 μm and segmented using Xenium Explorer, after which the gene density was analysed in each region. Clustering was performed by unweighted pair-group using centroids (UPGMC) using the Euclidian distance. The analysis was conducted only for Patient 7, which exhibited sufficient stromal content and allowed for distance analysis. The results represent the cumulative values from three locations.
Calculation of the distance from the tumour surface
The “tl.cci.grid” function of stLearn was used for gridding Xenium data at 10 μm intervals in the posttreatment sample of Patient 7. To extract grids containing tumour tissue, CDH1 + , SFTPB + , SFTPC + , and SFTPD− grids were extracted. Next, automatic contour extraction was performed using the Python library opencv (v4.8.1) to delineate the tumour surface based on grids containing the tumour. Specifically, median filtering (3 * 3 kernel) was performed using the “medianBlur” function, followed by smoothing (3 * 3 kernel) using the “blur” function to clarify the tumour boundaries. The “findContours” function was subsequently used to contour and extract only the first contour in the hierarchy.
Finally, to calculate the distance of each grid from the tumour surface, the grids were transformed into a graph structure (weighted by Euclidean distance), and the “multi_source_dijkstra” function in the Python library network (v3.1) was used to calculate the shortest distance from the grids on the tumour surface to the other grids.
Hot and cold comparison analysis
Comparison of hot and cold regions was conducted at both the single-cell and region levels. For Patient 7, cancer cell regions were segmented at the single-cell level using Xenium Explorer. Segmentation was verified using HE staining, and poorly segmented cells were excluded. Segmentation was defined as either hot (CD274 + ) or cold (CD274 − ) cells. For region-level analysis, areas with high CD274 expression were manually delineated. The validity of each cell and region was confirmed by checking CD274 expression using Python. Hot/Cold cells consisted of 100/74 cells in Patient 7 and 35/101 in Patient 12, and the regions were defined using five distinct areas for each.
Pathway enrichment analysis
Samples from the custom panel involving Patient 7 were used; the ‘pp.filter_genes’ and ‘pp.filter_cells’ functions in the Python library scanpy (v 1.9.3) (Wolf et al. Genome Biology, 2018) were used to filter out genes with less than 10 total counts and cells with fewer than 10 counts of mRNA in the cell. Scanpy’s ‘pp.normalize_total’ function was used to determine gene expression levels. The “tl.cci.grid” function in stLearn (v0.4.12) was subsequently used to grid the entire field of view into 125*125 = 15625 spots, and genes with expression levels in the top 5% of all these spots were defined as DEGs. GO enrichment analysis was then performed using the ‘enrichr’ function of the Python library gseapy (v1.0.6), with a background of 302 genes in the whole panel of Xenium In situ, for genes with differential expression in each spot.
Comparative analysis
Welch’s t test was used for significance difference tests (RRID:SCR_008058); the Benjamini–Hochberg method was used to correct for multiple testing; Cohen’s d was used for effect size; the effect size was calculated using a standardized pair group using the centroid method.
Gene expression analysis of CXCL9+ and CXCL13+ cells
To identify cell types among CXCL13+ and CXCL9+ cells, lymphocyte markers (CD3E, CD4, CD8A, GZMB, and CD19), macrophage markers (MSR1, CD68, and CD163), dendritic cell markers (MS4A4A, ITGAX, and HLA-DQB2), epithelial markers (KRT8 and CDH1) and ICI targets (CD274, CTLA4, IDO1, LAG3, and TIGIT) were used. For each marker, the z scores of average expression in CXCL13+ and CXCL9+ cells were calculated.
Similarly, to determine the role of CXCL13 − CD8 + , CXCL13 − CD8 + , CXCL9 − CD68 + , CXCL9 + CD68 + , CD274 − EPCAM+ and CD274 + EPCAM+ cells in the immune response, receptor genes (CCR1, CCR2, CCR3, CCR4, CCR6, CCR7, CCR8, CXCR2, CXCR3, CXCR4, CXCR6, IL10RB, IL12RB1, IL23R, IL3RA, IL6R, IL7R, IFNAR1, IFNAR2, IFNGR1, IFNGR2, and IFNLR1), humoral immune genes (CCL2, CCL3, CCL3L1, CXCL9, CXCL10, CXCL13, CXCL16, IFNG, IL18, and IL33), regulator genes (TLR2, TLR4, TMEM173, CGAS, DDX58, IRF1, IRF9, NFKB1, NFKB1, STAT1, and CIITA), the percentage of positive cells and the z score of the average expression levels were calculated.
Availability of data and materials
The clinical specimen data, including Xenium data produced during this research, are archived in the DDBJ BioProject. The accession number is currently in the process of being requested. All the data necessary for assessing the conclusions presented in the paper can be found within the paper itself and/or in the Supplementary Materials.
All the code used for the analysis has been uploaded to the web server of the Human Genome Center, University of Tokyo (https://www.hgc.jp/~ssakai/publication/Lung/code.zip).
Results
Study objectives and design
The aim of this study was to elucidate alterations in the immune environment after treatment with anti-PD-L1-CRT and to investigate the underlying mechanisms at the single-cell level via use of Xenium. First, we evaluated tissue conditions using HE staining and performed technical evaluation of the results by comparing marker gene expression via HE staining. The study included two pairs of tissue specimens isolated before and after treatment with anti-PD-L1-CRT. In addition, the analysis included one patient with pretreatment only and two patients with posttreatment only. In these patients, the pretreatment tissue quantity was limited, and an adequate number of cells could not be obtained with Xenium. The primary focus of this study was pre-post analysis of anti-PD-L1-CRT. However, for comparative purposes, the analysis also included two patients with pre-post pairs of CRT alone and one patient with resection alone (Fig. 1a and Tables 1, 2).
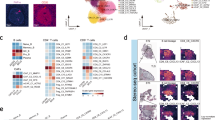
a Characteristics of the tissue samples used in this study. The cell number indicates the number of cells that can be analysed with Xenium. Posttreatment histology revealed stable disease (SD), major pathological response (MPR), and complete response (CR). b Representative HE staining image of pre- and posttreatment tumours. Cancer cells were delineated via HE staining (yellow line). c, d Representative images of HE staining, cell clustering and gene expression. After each cell line was distinguished and utilizing 302 gene expression patterns, pretreatment cells were categorized into 11 clusters, and posttreatment cells were classified into 22 clusters. Each cluster was classified as epithelial (KRT5 + , CDH1 + ), CAF (COL1A1 + ), myeloid (CD68 + , CD163 + ), LYM (lymphocyte, CD3E + , CD4 + , CD8 + ), plasma (POU2AF1 + ), or U.C. (dead cells or unclassified cells).
Tissue quality assessment and Xenium validation
Initially, we conducted material evaluation of posttreatment tissues, with HE staining and Xenium staining for whole biopsy tissues and 5 mm core samples of resected tissues, respectively. The cell count for each tissue was assessed using Xenium Explorer, confirming an ample quantity of cells. As part of the standard pathological diagnostic process, pathologists identify cancer cells and immune cells by evaluating the morphology and size of nuclei and cells through HE staining, thereby assessing the efficacy of treatment. In pretreatment tissue, we found densely packed cancer cell clusters with relatively uniform nuclei and cytoplasm (Fig. 1b and Supplementary Figs. S1A-G). In contrast, in posttreatment tissues, we observed increased immune cell infiltration into tumours and diverse tissue patterns, such as regions where cancer cells were attacked by immune cells, areas where cancer cells were replaced by immune cells, and sites where viable cancer cells coexisted with immune cells (Fig. 1b and Supplementary Figs. S1A-F).
Next, to evaluate Xenium and ensure that we had enough cells, we performed Xenium analysis, which was used to assess expression of 302 genes (Table S1) in samples obtained pre- and post-PD-L1-CRT treatment. Sufficient cell count information was obtained for all samples (Fig. 1a). Based on the expression patterns of the 302 genes, each cell was clustered into 11 groups in the pretreatment phase and 22 groups in the posttreatment phase by Xenium Analyzer (10X Genomics) (Fig. 1c, d). The data obtained by gene expression and clustering analyses were merged with HE staining images using Xenium Explorer version 1.2. By using this approach, the cell types identified by HE staining and morphological classification were confirmed and shown to correspond to cells in which genetic markers of epithelial cells (CDH1), monocytes/macrophages (CD68 and CD163), lymphocytes (CD4 and CD8), and B lymphocytes (CD19) were expressed (Fig. 1c, d and Supplementary Fig. S1H). Additionally, we assessed tissue structures, including lymphatic vessels (PDPN), smooth muscle (LMOD1), and vascular endothelium (PECAM1), by comparing HE staining with marker gene expression.
Expression of each gene, the groups based on which it was expressed, and location of the genes obtained by these Xenium analyses were consistent with the HE staining findings (Supplementary Fig. S1H). In this manner, we showed that Xenium analysis following treatment with anti-PD-L1-CRT yielded both adequate material quality and technical reliability, validating its suitability for our study. Moreover, we found that tumour cells and immune cells were morphologically altered by anti-PD-L1-CRT, and we next analysed gene expression in detail using Xenium.
Characterization of cells and gene expression post-PD-L1-CRT
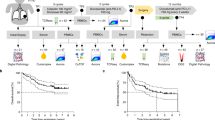
We analysed gene expression data obtained by Xenium using the following workflow (Fig. 2a). First, we systematically examined alterations following anti-PD-L1-CRT. We analysed the entire tissue (total field) and the delineated cancer cell region (field-based) separately and evaluated the alterations in cell and gene expression after treatment. Second, we examined the localization and function of cells through pathology-based analysis (“Cell Biology”), focusing in particular on histological images of immune cells interacting with cancer cells. Finally, we analysed CXCL13 + CD8 T cells, which are effector cells, and immune-cold cancer cells on a cell-by-cell basis via “single-cell analysis”.
a Workflow of the analysis in this study. b, c The number of cells in the total field analysed before (Patients 1, 2, and 7) and after (Patients 1, 3, 7 and 8) surgery. Positivity was defined as ≥2 or above. In all pre-post comparisons, a statistically significant difference was observed (P < 0.05, Student’s t-test). d The number of white blood cells (WBCs) and lymphocytes in the plasma. Pre: plasma within 2 weeks before treatment; Post: plasma within 2 weeks after resection. e Bar plot illustrating differences in the density of mRNA expression per cell area between pretreatment (Patient 1: 1029 cells, Patient 3: 1248 cells, and Patient 7: 340 cells) and posttreatment (Patient 1: 1502 cells, Patient 2: 1059 cells, and Patient 7: 1049). The x-axis indicates the effect size (Cohen’s d), and the y-axis indicates each gene with significant changes in expression (Welch’s t test, P < 0.05). f Confirmation of gene expression through Xenium Explorer. g Heatmap with dendrogram illustrating differences in the density of gene expression between manually delineated intertumoural, ≤20 μm, ≤70 μm, and ≤120 μm regions. The x-axis indicates each delineated region, and the y-axis indicates 302 genes in the Xenium I lung panel. h Merged images of gene expression and HE staining.
To investigate changes in the TME after treatment with anti-PD-L1-CRT, we first explored alterations in epithelial and immune cell numbers in the total field. We assessed cell type based on expression of marker genes: KRT5, KRT8, KRT14, and CDH1 for epithelial cells; COL1A1 for fibroblasts; and a panel of markers (T cells: CD3E, CD4, CD8A, IL2RG, and CXCR6; B cells: CD19, and POU2AF1; monocyte/macrophages: CD68, and CD163; and dendritic cells: ITGAX, CYTIP, and MSR1) for immune cells. We then compared the number of the cell counts per area for each type in tissue samples obtained pre- and posttreatment (n = 3 and 4, respectively) using t tests. We evaluated immune cells by HE staining and evaluated marker gene expression; cells with a score of 2 or higher were defined as positive (Supplementary Fig. S2). We observed a general decrease in epithelial cells after treatment and a particularly marked reduction in KRT5− and KRT14+ cells, which were almost undetectable in posttreatment samples (Fig. 2b). The remaining epithelial cells in posttreatment samples were largely KRT8+ and CDH1+ cells. While the number of COL1A1+ cancer-associated fibroblasts (CAFs) tended to increase in posttreatment samples, significant differences were not observed. The numbers of immune cells, including T lymphocytes (TLYMs), B lymphocytes (BLYMs), natural killer cells (NKs), monocytes/macrophages (Mo/Mφ), and dendritic cells (DCs), were significantly greater in posttreatment samples than in pretreatment samples (Fig. 2c).
We confirmed an increase in a broad range of immune cells, including lymphocytes, in tissues after CRT alone and anti-PD-L1-CRT (Fig. 2b, Supplementary Fig. S3). The increase in immune cells was particularly prominent in the anti-PD-L1 treatment group. To confirm that these increases were not simply caused by an increase in white blood cells (WBCs), including lymphocytes (LYMs), in peripheral blood, we examined the WBC and LYM counts in the blood over a 12-week period from just before the initiation of treatment to surgery. Unlike within tissues after anti-PD-L1-CRT, WBC counts in peripheral blood decreased after the initiation of treatment compared to before the initiation of treatment (Fig. 2d). The observed discrepancy in lymphocyte counts between cancer tissue and peripheral blood posttreatment indicates that a specific and local immune response can be induced in cancer tissue.
To reveal changes in gene expression in the tumour region induced by anti-PD-L1-CRT, we manually delineated tumour regions via HE staining and compared gene expression levels via Xenium between pretreatment (Patients 1, 2, and 7) and posttreatment (Patients 1, 3, 7, and 8). Expression of FOXA1, SOX2, TSTD1, CDH1, and PTP4A1 increased significantly (Welch’s t test, P < 0.05) in posttreatment, while that of TMEM45A, KRT14, EGFR, TP63, FSCN1, SLC2A1, and KRT5 decreased significantly (P < 0.05) (Fig. 2e). Moreover, nuclear enlargement and chromatin condensation were observed posttreatment, along with obvious decreases in KRT5 and KRT14 and increases in CDH1 expression (Fig. 2f). Taken together, these findings suggest that anti-PD-L1-CRT therapy dramatically alters expression of cytoskeletal genes, such as KRT5 and CDH1, in tumours, along with changes in cell morphology.
As no common targetable pathways were identified in integrated analysis of pretreatment (3 patients) and posttreatment (4 patients), we conducted a targeted exploration in the pair analysis of Patient 1 and 7. These 2 patients presented with significant activation or inhibition of several genes; these genes included genes in targetable pathways such as VEGFA, KIT, ERBB2, and EGFR (Supplementary Fig. S4). According to our gene expression analysis focused on cancer tissues, activation of such targetable pathways is not common but rather individualized, and our findings suggest the need for individualized therapeutic strategies targeting treatments directed at these genes to discern the activated state following treatment with anti-PD-L1-CRT (Supplementary Fig. S5). Although treatment clearly changes cell numbers and gene expression, it is not known where these changes occur, i.e., whether specifically within the tumour or stroma. To clarify this point and screen more crucial cells and genes, spatial analysis was conducted for Patient 7, whose tissue had all the characteristics of posttreatment tissues (i.e., regions where cancer cells were attacked by immune cells, areas where cancer cells were replaced by immune cells, and sites where viable cancer cells coexisted with immune cells). Regions close to the tumour were segmented into the tumour and regions outside the tumour according to the distance from the tumour surface, namely, peritumour (peri, <20 μm), mid-peritumour (mid, 20-70 μm) and distal peritumour (distal, 70-120 μm), using Xenium Explorer; the cells within these regions and gene expression pattern were evaluated for clustering analysis. The peri cluster included immune-related genes such as CXCL9 and CXCL13, the mid cluster included cytotoxic T lymphocyte (CTL) and macrophage markers such as CD8 and CD68, and the distal cluster included B-cell markers and M2 macrophage markers such as CD19 and CD163 (Fig. 2g). The location of the genes was reconfirmed by Xenium Explorer analysis, and our analysis enabled us to distinguish the cells localized within or at the periphery of the tumour from those located further from the tumour (Fig. 2h). In our analysis of cancer cells, we found limited common upregulation of genes or pathways, making identification challenging. However, in our analysis of immune cells, we observed a broad increase in most cell types, posing difficulties in mechanism analysis. Through our spatial analysis, we identified CXCL13 + , CD274 + , CTLA4+ and CXCL9+ immune cells, which directly interact with the tumour, hypothesizing their pivotal role. Subsequent analyses were undertaken with a specific focus on this identified cluster.
Single-cell spatial transcriptome analysis revealed the characteristics of CXCL9+ cells and CXCL13+ cells that accumulate near cancer cells after treatment with anti-PD-L1-CRT
Spatial analysis of Patient 7 suggested interactions of CD274 + , CTLA4 + , CXCL9 + , and CXCL13+ cells with cancer cells. Recent reports have shown that CXCL9+ and CXCL13+ cells are strongly associated with the effect of anti-PD-1/PD-L1 therapy without RT [24,25,26,27]. We investigated CXCL9+ and CXCL13+ cells among all anti-PD-L1-CRT pre-post samples and CRT alone pre-post samples (Fig. 3a and Supplementary Fig. S5). As confirmed in Patient 7, upregulation of CXCL9+ and CXCL13+ cells and localization within the tumour were confirmed in all post anti-PD-L1-CRT tissues (Fig. 3a). In contrast, the number of CXCL9+ and CXCL13+ cells moderately increased in post-CRT alone tissue. In addition, we investigated expression of CTLA4, ICOS, IDO1, and LAG3, which are reported to be expressed in CXCL13+ cells, and the chemokines CXCL5 and CXCL14. As for CXCL13+ cells, CTLA4 + , ICOS + , IDO1 + , and LAG3+ cells were increased in all tissues after anti-PD-L1-CRT, and no correlation with treatment was confirmed for CXCL5 or CXCL14 (Fig. 3a). In Patient 7, which exhibited high levels of cellular heterogeneity, large clusters of cancer cells were observed in some regions (Fig. 3b, Post 1, 2); in other areas, immune cell-mediated tumour elimination was observed (Post 3,4). Additionally, complete eradication of cancer cells was observed in some regions (Post 5). By using Xenium Explorer, we confirmed strong expression of CXCL9 and CXCL13 in almost all areas around the tumour, including at sites of tumour eradication (Fig. 3b). The same findings were obtained for another patient with complete response (Patient 8), while CXCL9 and CXCL13 expression was low in pretreatment tissues and tissues from patients after surgical resection alone (Fig. 3c), indicating that their expression was induced by anti-PD-L1-CRT.
a Treatment, tissue, genotype, response, and number of cells expressing CXCL9, CXCL13, CD274, CTLA4, ICOS, IDO1, LAG3, CXCL5, and CXCL14. b Peripheral tumour gene expression of CXCL13 and CXCL9 in Patient 7. Posts 1-4 show residual cancer cells, and post 5 shows only accumulated lymphocytes. c CXCL13 and CXCL9 expression in Patient 7 pre, Patient 8 post, and Patient 11 posttissue. d Bubble chart with a colour scale showing the expression level and proportion of cell marker genes and ICI target genes in CXCL13+ and CXCL9+ cells using in situ Xenium data for Patient 7. The x-axis indicates the cells, the y-axis indicates each marker gene, the colour scale indicates the z score of the mean number of positive cells for each marker gene, and the circle size indicates the proportion of positive cells for each marker gene. e CXCL13 + CD8 + T cells coexpressing exhaustion marker genes on tumour cells. f CD8 + T cells coexpressing exhaustion marker genes on tumour cells. f CD68− and CD163+ myeloid cells coexpressing CXCL9.
Given that CXCL9 and CXCL13 expression appears to be induced by anti-PD-L1-CRT and that these cells localize to the vicinity of the tumour, we next sought to identify these cell types through the expression of genes that identify cell types. The analysis revealed that CXCL13+ cells were CD8+ lymphocytes and that CXCL9+ cells were double positive for macrophage markers (MSR1, CD68, and CD163) and DC markers (MS4A4A, ITGAX, and HLA-DQB2) (Fig. 3d). An analysis of all CD8+ cells revealed that CXCL13 + CD8+ cells accumulated inner- and peri-region of the tumour, indicating that they may directly interact with cancer cells. Moreover, these cells exhibited elevated expression levels of exhaustion markers, including TIGIT, LAG3, and IDO1. (Fig. 3e). While we did not observe an increase in expression of specific CXCL9+ cell markers, which was previously reported [28] after anti-PD-L1-CRT (Fig. 3f), we did observe double-positive macrophages/DCs, a characteristic feature of CXCL9+ cells localized around the tumour after anti-PD-L1-CRT (Fig. 3f). Moreover, we found that CXCL9 was expressed not only by macrophages/DCs but also by some tumour cells and lymphocytes (Fig. 3d). Thus, we confirmed the increase in the number of CXCL9+ cells and CXCL13+ cells and their concentration within and peripheral to the tumour in all four patients following post-PD-L1-CRT. Additionally, we found that CXCL13+ cells were enriched in exhaustion marker-positive CD8+ lymphocytes.
CXCL13 + CD8 + T cells accumulate in cancer tissue after treatment and are in close proximity to cancer cells
Although the CXCL13+ cells we identified reportedly correlate with the therapeutic efficacy of anti-PD-L1 therapy, the localization and mechanism of these cells are unclear. We investigated this cellular function using spatial information and pathology-based analysis. In the cancer immune response, activated cytotoxic T lymphocytes (CTLs) directly contact and release perforin and granzymes, causing the cancer cell membrane to rupture and leading to apoptosis [36]. According to our pathology-based analysis, CXCL13 + CD8+ cells directly engage with cancer cells, and most CXCL13 − CD8+ cells were ubiquitously distributed in Patient 7 (Fig. 4a). We quantified the spatial localization of CXCL13 + CD8+ and CXCL13 − CD8+ cells inner-, peri-, and outer-region of the tumour (Fig. 4b, c). This trend of CXCL13 + CD8+ cells engaging directly with cancer cells, as opposed to CXCL13 − CD8+ cells, was further validated through region stratification analysis [37] across all examined fields. Furthermore, in all three post-PD-L1-CRT tissue samples, we confirmed that CXCL13 + CD8+ lymphocytes expressed GZMB and directly interacted with cancer cells at the single-cell resolution. (Fig. 4d). Based on these results, we concluded that CXCL13 + CD8 + T cells are the main type of CTL that directly attacks cancer cells in the environment after treatment with anti-PD-L1-CRT.
a Localization of CXCL13 − CD8 + T cells and CXCL13 + CD8 + T cells in Patient 7. The regions enclosed by the yellow rectangle in the upper row are shown in the lower row. The green dashed lines are the tumour surface, and the yellow dashed lines are the contour lines at 30 µm intervals. b Image shows the tumour surfaces (green) and contours (red) at 30 µm intervals (−60 to 150 μm) in two areas in Patient 7. c Bar plot shows the proportions of CXCL13 + CD8 + T cells and CXCL13 − CD8 + T cells in each region. d CXCL13 + CD8 + T cells expressing GZMB were near or inside the tumour in Patients 1, 2, and 7.
CXCL13 + CD8 + T cells are associated with the CXCL16-CXCR6 axis and linked to IFNG pathway activation
We identified distinct tumour areas in Patient 7 by HE staining; the presence of strong immune cell infiltration indicated active immune attack on cancer cells (“hot” pattern), and the absence of immune cells suggested reduced immune activation in surviving cancer cells (“cold” pattern). This dichotomy provides insights into the mechanisms of induced immune activation and evasion in the context of anti-PD-L1-CRT. Further analysis of this tissue was performed to unravel these complex immunological dynamics. We performed additional Xenium analyses of tissues from Patient 7 using a newly developed panel of 300 genes that specifically target the immune response, including DNA damage response genes (n = 19), nucleic acid sensor genes (n = 15), transcription factor genes (n = 21), chemokine/interleukin/interferon ligand receptor genes (n = 66), haematopoietic marker genes (n = 45), and immune checkpoint inhibitor target genes (n = 35) (Supplementary Table S2).
Our initial focus was on understanding the mechanisms through which CXCL9+ cells and CXCL13+ cells are attracted to the tumour vicinity and evaluating chemokines, interleukins, and IFN receptors. We investigated expression of all of these receptors in tissues and found that CXCR6 is a distinctive receptor on CXCL13+ cells by examining gene expression in these cells (Fig. 5a; Supplementary Fig. S6A). Expression of CXCL16, a ligand of CXCR6 [38, 39], was expressed in CD68+ cells, including CXCL9+ cells, and partially induced in EPCAM+ cells (Fig. 4b and Supplementary Fig. S6B). CXCR6 expression in CXCL13 + CD8+ cells and CXCL16 expression near CXCL13+ cells were visually confirmed by Xenium Explorer analysis (Fig. 5d and Supplementary Fig. S6D). In CXCL9+ cells, we identified distinctive candidate receptors, such as CXCR2, IL10RB, and TLR4 (Fig. 5a-c and Supplementary Figs. S6A-C), which are involved in chemotaxis [40,41,42]; however, we could not confirm their association with specific ligands. We found that CXCL9+ cells produced CCL18 (Supplementary Fig. S6B), a strong lymphocyte attractant, suggesting that CXCL9+ cells attract lymphocytes to the tumour vicinity.
a Bubble chart with a colour scale showing the average expression level and proportion of genes associated with receptors in CXCL13 − CD8 + , CXCL13 + CD8 + , CXCL9 − CD68 + , CXCL9 + CD68+ and CXCL9 − CD68+ cells according to gene expression data for Patient 7. The x-axis indicates the cell type, the y-axis indicates the marker gene, the colour scale indicates the z score of the mean number of positive cells for each marker gene, and the circle size indicates the proportion of positive cells for each marker gene for each type of cell. b, c Bubble chart of genes associated with humoral immunity and regulators in CXCL13 − CD8 + , CXCL13 + CD8 + , CXCL9- CD68 + , CXCL9 + CD68 + , CXCL9 − CD68 + , CD274 − EPCAM + , and CD274 + EPCAM+ cells. d CXCL13 + CD8 + T cells coexpress IFNG, and CXCL9 and CXCL10 expression is found near these cells. e Heatmaps indicating spatial enrichment of the IFN-mediated signalling pathway (log-adjusted P value) and expression levels of CXCL13, CXCL9, and CXCL10 (normalized count). The values below the heatmaps are the Pearson correlation coefficients between expression levels and enrichment of IFN-mediated signalling pathways. f Models of cell‒cell interactions in the tumour environment.
Furthermore, we focused on the concordance between CXCL13+ cells and IFNG-expressing cells (Fig. 5b, orange arrow). We confirmed that CXCL13 + CD8+ cells expressed IFNG (Fig. 5d), and we statistically verified the activation of CXCL13 + CD8+ cells and the IFNG pathway (Fig. 5e). Furthermore, Xenium exploration confirmed that the majority of cells in the TME expressed IFNGR (Supplementary Fig. S6D). CXCL9, CXCL10, and CD274 are reportedly downstream of IFNG [43]. We also statistically verified the correlation between the IFNG pathway and CXCL9 and CXCL10 expression (Fig. 5e). IFNG pathway total field analysis of CXCL13 + , CXCL9+ and CXCL10+ cells using stLearn (v0.4.12) and GSEApy (v1.0.6) revealed correlation coefficients of 0.74, 0.74, and 0.72, respectively (Pearson, respectively). Thus, we created a hypothetical model of CXCR6-CXCL16-dependent chemotaxis of CXCL13 + CD8+ cells and IFNG-dependent CXCL9 and CXCL10 expression (Fig. 5f).
Characterization of hot and cold cancer cells after anti-PD-L1-CRT treatment
Our attention is now directed towards detailed investigation of the immune-cold state following anti-PD-L1-CRT, with subsequent comparative analysis against the immune-hot state. We examined gene expression within a representative one field of a pathological image that includes hot and cold region with gene expression profile, and we observed significant heterogeneity in CD274 expression (Fig. 6a). Regions with high levels of CD274 aligned with areas of high CXCL9 and CXCL10 expression. Conversely, in regions with low CD274 expression, we detected MKI67 expression, indicating high proliferative potential.
a Image of HE staining of mixed hot and cold tumours. b, c Violin plot of genes whose expression significantly differed (p < 0.05; Welch’s t test) between hot and cold tumours in Patient 7. The y-axis shows the density of gene expression per cell area (μm2). Genes are shown separately based on their categorization as hot, cold, or ICI-targetable genes. d Confirmation of gene expression through Xenium Explorer.
Given that states categorized as “cold” characterized by low immune cell infiltration coupled with high cellular proliferation can lead to treatment resistance, we conducted detailed gene expression analyses to identify features of immune-cold cancer cells. We also conducted both single-cell-based analyses (Fig. 6b-d) and region-based comparative analyses (Supplementary Fig. S7A) in the same field to identify features of immune-cold cancer cells. In single-cell analysis, we manually identified and precisely segmented hot and cold cancer cells within the regions with reference to the HE images. We defined CD274 expression ≥ 2 within cells as immune-hot (Fig. 6a, red arrow) and 1 or 0 as immune-cold (Fig. 6a, blue arrow). Some cancer cells formed aggregates, and those with poor segmentation were excluded from the analysis. To mitigate the impact of segmentation errors during single-cell analysis, we concurrently conducted region-based gene expression analysis (Supplementary Fig. S7A). Welch’s t test was used for statistical analysis (Supplementary Table S3). Initially, we confirmed expression of CD274 and CTNNB1, which are known markers of hot and cold regions, respectively [44].
In hot cells (100 cells), expression of genes such as KRT17, OAS2, IRF1, IFIH1, CXCL9, and CXCL10 was significantly upregulated. In contrast, expression of such genes as CDH1, MKI67, MYC, SOX2, RAD23, RAD21, HMGB1, VEGFB, and CXCR4 was significantly greater in cold cells (74 cells, Fig. 6c). Notably, a high proportion of the genes expressed in hot cells, such as IFIH1, IRF1, and OAS2, are related to the nucleic acid sensor pathway necessary for the RT-induced immune response [45,46,47,48]. In cold cells, expression of genes involved in cell proliferation, such as MKI67 and MYC, as well as genes associated with DNA repair, including SOX2, RAD21 and RAD23 [39, 49,50,51,52], was increased. Additionally, to assess whether the features of the cold state are specific to post-PD-L1-CRT, we performed hot vs. cold single-cell analysis in Patient 12 post-CRT alone (Supplementary Fig. S7B). We observed a similar trend in single-cell analysis of gene expression in hot and cold cells isolated from tissue samples obtained from Patient 12. We identified potential therapeutic targets for both hot and cold cells, including IDO1, VEGFB, and CXCR4.
In this manner, even within the same cancer tissue, expression of CD274 and hot/cold markers exhibited clear localization biases. Interestingly, hot and cold cell populations were observed to form a mosaic pattern, whereby clusters of several dozen hot or cold cells were observed within the same cancer cell mass (Supplementary Fig. S8). Attempts to convert cancer cells from cold to hot have attracted increased interest in cancer therapy. Therefore, this mosaicism represents a significant discovery, indicating that there are crucial factors (in addition to known factors such as the microbiome and driver genes) influencing hot/cold transitions.
Discussion
This study utilized the single-cell spatial transcriptomics platform Xenium to explore immune environment alterations in NSCLC patient tissues following anti-PD-L1-CRT. One significant finding was that, even when immune cell levels decreased in peripheral blood, most immune cells within the tissue increased, and we identified distinct localized variations in immune activity. Moreover, we identified immune cells that directly act on cancer cells after treatment and elucidated their gene expression profiles and the surrounding immune environment. We identified CXCL13 + CD8 + T cells expressing GMZB and IFNG—cytotoxic T lymphocytes (CTLs)—as the cells that attack cancer cells following PD-L1-CRT. Previous studies have indicated that CXCL13, CXCL9, and CXCL10 are essential for the effectiveness of anti-PD-1/PD-L1 therapy [24,25,26,27,28] (without radiotherapy) and have been reported as potential biomarkers. Our findings elucidate that these cells directly engage cytotoxic T lymphocytes (CTLs) against cancer cells following PD-L1-CRT, suggesting their potential as biomarkers. Another important finding is that major pathological response (MPR), defined by the presence of less than 10% viable tumour cells, exhibited two distinct patterns of residual cancer cells. The residual tumour tissue could be classified into immunologically ‘hot’ and ‘cold’ regions. Notably, in certain ‘cold’ areas, we observed strong cellular proliferation, suggesting that immune suppression within these regions may contribute to continued tumour progression. These results, demonstrating that the residual tumour can be divided into immunologically ‘hot’ responsive regions and ‘cold’ resistant regions, underscore the importance of separately analysing these areas. Such an approach is critical for uncovering the mechanisms underlying immune-mediated tumour eradication and the immune evasion strategies that support tumour cell survival.
Subsequent analysis revealed that ‘immune-cold’ cells exhibited activated DNA repair mechanisms and enhanced proliferation. The cell-killing effects of radiation are induced through DNA damage, and radiation-induced immune responses are also triggered by DNA damage [48]. Therefore, increased DNA repair activity serves as a reasonable mechanism for both radiation resistance and suppression of CRT-induced immune responses. Furthermore, increased cellular proliferation was observed in cold cancer cells after treatment, suggesting their potential role as contributors to posttreatment recurrence and highlighting their role as crucial targets for new therapeutic strategies. Considering that DNA repair becomes activated in these cells, the efficacy of radiation dose escalation or additional DNA damage agents would be limited. Hence, our findings suggest the importance of exploring combinatory approaches involving cell proliferation pathways such as CXCR4 and VEGF, which operate independently of DNA damage, to improve therapeutic outcomes.
On the other hand, in hot regions, CXCL13 + CD8 + T cells expressed exhaustion markers such as TIGIT, LAG3, and CTLA4. Since targeted therapies against these markers have been reported to further activate the immune response, they show promise as potential therapeutic targets to enhance the efficacy of PD-L1-CRT. Thus, our pathology-based single-cell spatial analysis is valuable, providing detailed insights into the TME alteration after PD-L1-CRT. This approach deepens our understanding of treatment responses and resistance. Notably, many of the genes identified in this study are confirmed clinical drug targets, such as anti-VEGF, PD-1/PD-L1 and anti-CTLA-4 antibodies, which have FDA approval. Also, antibodies targeting IDO1, LAG3 and TIGIT are in late stage clinical development and may be approved in the near future. Hence, our insights would contribute to future clinical trials and inform standard therapeutic approaches soon.
The limitation of this study is the sample size, as we analysed only five cases of PD-L1-CRT, and no prognostic analyses were conducted. Therefore, the current analysis primarily offers pathology-based biological insights, identifying potential marker candidates. The SQUAT trial involving 31 patients will allow for a correlation analysis with prognostic data once it becomes available, facilitating the validation of prognostic markers. Both anti-PD-L1 therapy and CRT act on nearly all solid tumours. Induction of CXCL13+ cells after ICI treatment has been reported in various solid cancers. Hence, the CXCL13–CD8-based immune mechanism proposed in our study may apply not only to ICI-CRT in NSCLC but also to other cancers and treatments.
In summary, single-cell spatial transcriptome analysis is a very effective tool for investigating the immune environment post-ICI-CRT at the single-cell level. Identification of CXCL13 + CD8 + -centred immune responses and immune-cold cancer cells after anti-PD-L1-CRT therapy holds promise for future developments in treatment strategies.
Data availability
The clinical specimen data, including Xenium data produced during this research, are archived in the DDBJ BioProject. The accession number is currently in the process of being requested. All the data necessary for assessing the conclusions presented in the paper can be found within the paper itself and/or in the Supplementary Materials.
References
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71.
Bando H, Tsukada Y, Ito M, Yoshino T. Novel immunological approaches in the treatment of locally advanced rectal cancer. Clin Colorectal Cancer. 2022;21:3–9.
Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2021;22:1093–102.
Weiss J, Sheth S, Deal AM, Grilley Olson JE, Patel S, Hackman TG, et al. Concurrent definitive immunoradiotherapy for patients with stage III-IV head and neck cancer and cisplatin contraindication. Clin Cancer Res. 2020;26:4260–7.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends in Cancer. 2022;8:9–20.
Lim M, Weller M, Idbaih A, Steinbach J, Finocchiaro G, Raval RR, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24:1935–49.
Monk BJ, Toita T, Wu X, Vázquez Limón JC, Tarnawski R, Mandai M, et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1334–48.
Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–62.
Machiels JP, Tao Y, Licitra L, Burtness B, Tahara M, Rischin D, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2024;25:572–87.
Tao Y, Aupérin A, Sun X, Sire C, Martin L, Coutte A, et al. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J Cancer. 2020;141:21–29.
Bradley JD, Sugawara S, Lee, KHH, Ostoros G, Demirkazik A, Zemanova M, et al. Durvalumab in combination with chemoradiotherapy for patients with unresectable stage III NSCLC: Final results from PACIFIC-2. In: Vol. 9 1-12 (2024).
Oyoshi H, Du J, Sakai SA, Yamashita R, Okumura M, Motegi A, et al. Comprehensive single-cell analysis demonstrates radiotherapy-induced infiltration of macrophages expressing immunosuppressive genes into tumor in esophageal squamous cell carcinoma. Sci Adv. 2023;9:eadh9069.
Brown JM, Thomas R, Nagpal S, Recht L. Macrophage exclusion after radiation therapy (MERT): A new and effective way to increase the therapeutic ratio of radiotherapy. Radiother Oncol. 2020;144:159–64.
Hsieh RC, Krishnan S, Wu RC, Boda AR, Liu A, Winkler M, et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci Immunol. 2022;7:eabl9330.
Walle T, Kraske JA, Liao B, Lenoir B, Timke C, von Bohlen Und Halbach E, et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci Adv. 2022;8:eabh4050.
Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8:198.
Takamochi K, Suzuki K, Tsuboi M, Niho S, Ishikura S, Oyamada S, et al. Randomized phase II trial of pemetrexed-cisplatin plus bevacizumab or thoracic radiotherapy followed by surgery for stage IIIA (N2) nonsquamous non-small cell lung cancer. J Thorac Cardiovasc Surg. 2022;164:661–671.e664.
Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38:706–14.
Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–99.
Król K, Mazur A, Stachyra-Strawa P, Grzybowska-Szatkowska L. Non-Small Cell Lung Cancer Treatment with Molecularly Targeted Therapy and Concurrent Radiotherapy-A Review. Int J Mol Sci 24 (2023).
Yin J, Yuan J, Li Y, Fang Y, Wang R, Jiao H, et al. Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial. Nat Med. 2023;29:2068–78.
Thibaudin M, Fumet JD, Chibaudel B, Bennouna J, Borg C, Martin-Babau J, et al. First-line durvalumab and tremelimumab with chemotherapy in RAS-mutated metastatic colorectal cancer: a phase 1b/2 trial. Nat Med. 2023;29:2087–98.
Liu B, Zhang Y, Wang D, Hu X, Zhang Z. Single-cell meta-analyses reveal responses of tumor-reactive CXCL13(+) T cells to immune-checkpoint blockade. Nat Cancer. 2022;3:1123–36.
Liu B, Hu X, Feng K, Gao R, Xue Z, Zhang S, et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat Cancer. 2022;3:108–21.
Zhang Y, Chen H, Mo H, Hu X, Gao R, Zhao Y, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39:1578–1593.e1578.
Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–50.
Zhang Q, Jiang S, Schroeder A, Hu J, Li K, Zhang B, et al. Leveraging spatial transcriptomics data to recover cell locations in single-cell RNA-seq with CeLEry. Nat Commun. 2023;14:4050.
Fatemi MY, Lu, Y, Sharma C, Feng E, Azher ZL, Diallo AB, et al. Feasibility of Inferring Spatial Transcriptomics from Single-Cell Histological Patterns for Studying Colon Cancer Tumor Heterogeneity. medRxiv https://doi.org/10.1101/2023.10.09.23296701 (2023).
Takano Y, Suzuki J, Nomura K, Fujii G, Zenkoh J, Kawai H, et al. Spatially resolved gene expression profiling of tumor microenvironment reveals key steps of lung adenocarcinoma development. Nature Communications. 2024;15:10637.
Hamada A, Soh J, Hata A, Nakamatsu K, Shimokawa M, Yatabe Y, et al. Phase II study of neoadjuvant concurrent chemo-immuno-radiation therapy followed by surgery and adjuvant immunotherapy for resectable stage IIIA-B (Discrete N2) Non-small-cell Lung Cancer: SQUAT trial (WJOG 12119L). Clin Lung Cancer. 2021;22:596–600.
Haga Y, Sakamoto Y, Kajiya K, Kawai H, Oka M, Motoi N, et al. Whole-genome sequencing reveals the molecular implications of the stepwise progression of lung adenocarcinoma. Nat Commun. 2023;14:8375.
Pham D, Tan X, Balderson B, Xu J, Grice LF, Yoon S, et al. Robust mapping of spatiotemporal trajectories and cell–cell interactions in healthy and diseased tissues. Nature Communications. 2023;14:7739.
Prakash MD, Munoz MA, Jain R, Tong PL, Koskinen A, Regner M, et al. Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity. 2014;41:960–72.
Sakai SA, Nomura R, Nagasawa S, Chi S, Suzuki A, Suzuki Y, et al. SpatialKNifeY (SKNY): Extending from spatial domain to surrounding area to identify microenvironment features with single-cell spatial omics data. PLOS Computational Biology. 2025;21:e1012854.
Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107.
Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304.
Wu Y, Wang S, Farooq SM, Castelvetere MP, Hou Y, Gao JL, et al. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J Biol Chem. 2012;287:5744–55.
Liu Z, Jiang Y, Li Y, Wang J, Fan L, Scott MJ, et al. TLR4 Signaling augments monocyte chemotaxis by regulating G protein-coupled receptor kinase 2 translocation. J Immunol. 2013;191:857–64.
Jang GY, Lee JW, Kim YS, Lee SE, Han HD, Hong KJ, et al. Interactions between tumor-derived proteins and Toll-like receptors. Exp Mol Med. 2020;52:1926–35.
Gocher AM, Workman CJ, Vignali DAA. Interferon-γ: teammate or opponent in the tumour microenvironment? Nat Rev Immunol. 2022;22:158–72.
Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol. 2019;10:379.
Du J, Kageyama SI, Yamashita R, Tanaka K, Okumura M, Motegi A, et al. Transposable elements potentiate radiotherapy-induced cellular immune reactions via RIG-I-mediated virus-sensing pathways. Commun Biol. 2023;6:818.
Petroni G, Cantley LC, Santambrogio L, Formenti SC, Galluzzi L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat Rev Clin Oncol. 2022;19:114–31.
Du J, Kageyama SI, Yamashita R, Hirata H, Hakozaki Y, Okumura M, et al. Impacts of the STING-IFNAR1-STAT1-IRF1 pathway on the cellular immune reaction induced by fractionated irradiation. Cancer Sci. 2022;113:1352–61.
McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203–17.
Fong YW, Inouye C, Yamaguchi T, Cattoglio C, Grubisic I, Tjian R. A DNA repair complex functions as an Oct4/Sox2 coactivator in embryonic stem cells. Cell. 2011;147:120–31.
Schauber C, Chen L, Tongaonkar P, Vega I, Lambertson D, Potts W, et al. Rad23 links DNA repair to the ubiquitin/proteasome pathway. Nature. 1998;391:715–8.
Pardo J, Wallich R, Martin P, Urban C, Rongvaux A, Flavell RA, et al. Granzyme B-induced cell death exerted by ex vivo CTL: discriminating requirements for cell death and some of its signs. Cell Death Differ. 2008;15:567–79.
Birkenbihl RP, Subramani S. Cloning and characterization of rad21 an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res. 1992;20:6605–11.
Acknowledgements
We thank Dr. SungGi Chi, Mr. Yuichi Sakamoto, and Ryosuke Nomura for valuable comments on this study. We also thank the personnel at National Cancer Center Hospital East for their assistance in collecting clinical samples and providing valuable advice. Additionally, we extend our appreciation to all the patients and their families who generously contributed to this study.
Funding
This work was supported by Grants-in-Aid for Scientific Research KAKENHI 21K07582 and JST SPRING JPMJSP2108.
Author information
Authors and Affiliations
Contributions
MN, MT, NK, HO, and SK contributed to conceptualization and sample management. Data curation, investigation, methodology, resource allocation, validation, and visualization were conducted by SAS, SK, AS, YS, JZ, and RY. SAS, HO, and SK were responsible for writing the original draft, as well as reviewing and editing. Gene expression analysis for Xenium was performed by SAS and SK, while the investigation, methodology, resource allocation, validation, and visualization of Xenium results were carried out by JZ, AS, and YS. Pathological diagnosis and sample preparation were handled by MT, YN, KM, TT, TK, and GI. Radiotherapy was provided by OH, MN, HH, HH, AM, and TA. The summarization of all data for figures and tables was done by SAS, SK, and HO. Study supervision was provided by RY, YS, TA, TK, AH, TM, JS, and KT. All members approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.N. reports personal fees from AstraZeneca and a research grant from illumina, outside the submitted work. T.K. received research grant from Roche Diagnostics K.K. outside of this work. M.T. has received honorarium and lecture fees from Johnson & Johnson Japan, AstraZeneca KK, Eli Lilly Japan, Chugai Pharmaceutical CO., LTD, Taiho Pharma, Medtronic Japan, Ono Pharmaceutical CO., LTD, MSD, Bristol-Myers Squibb KK, Novartis, Amgen KK, and Daiichi-Sankyo. Additionally, M.T. has been involved in consulting or advisory roles with AstraZeneca KK, Chugai Pharmaceutical CO., LTD, MSD, and Novartis, and has received research grants and engaged in commissioned research (e.g., clinical trials) from Boehringer-Ingelheim Japan, MSD, AstraZeneca KK, Ono Pharmaceutical CO., LTD, MSD, Bristol-Myers Squibb KK, Novartis, Eli Lilly Japan, and MiRXES. T.M. has received honorarium and lecture fees from AstraZeneca KK, Chugai Pharmaceutical CO., LTD, Ono Pharmaceutical CO., LTD, and Bristol-Myers Squibb KK, MSD, Boehringer Ingelheim, Taiho, Eli-Lilly, Novartis, Amgen, Takeda, Kyorin and receieved research grant from AstraZeneca, Chugai, Boeringer Ingelheim, Pfizer, Taiho, MSD, Ono, Bridge Biopharma and Natera. A.H. has received honorarium and lecture fees from AstraZeneca KK, Chugai Pharmaceutical CO., LTD, Ono Pharmaceutical CO., LTD, and Bristol-Myers Squibb KK. Additionally, A.H. has been involved in consulting or advisory roles with AstraZeneca KK and has received research grants and engaged in commissioned research (e.g., clinical trials) from AstraZeneca KK. J.S. received honorarium and lecture fees from Johnson & Johnson Japan, Chugai Pharmaceutical CO., LTD, Taiho Pharma, Medtronic Japan, and Intuitive Japan.
Ethics approval and consent to participate
Ethical approval was obtained from the ethics committee of National Cancer Center Japan (Protocol Number: 2022-407). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakai, S.A., Oyoshi, H., Nakamura, M. et al. Single-cell spatial analysis with Xenium reveals anti-tumour responses of CXCL13 + T and CXCL9+ cells after radiotherapy combined with anti-PD-L1 therapy. Br J Cancer 133, 795–808 (2025). https://doi.org/10.1038/s41416-025-03088-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03088-0
This article is cited by
-
RT–ICI therapy induces a distal immunometabolic axis that shapes systemic macrophage polarization and enhances local T cell immunity
Cell Communication and Signaling (2026)