Abstract
RNA modifications are widely distributed in almost all types of RNA, including mRNA, rRNA, miRNA, circRNA, and lncRNA, which are deeply involved in disease initiation and progression and are emerging therapeutic targets in diseases such as cancer, among which N6-methyladenosine (m6A) is the most abundant mRNA modification. Accumulating studies have demonstrated the critical role of m6A during cancer progression and its therapeutic potential in prostate cancer, which is one of the most common malignancies in men worldwide. Here, we reviewed the emerging roles of m6A regulators, including readers, writers, and erasers, and the downstream m6A-modified mRNA and noncoding RNA in prostate cancer. We also discussed the therapeutic potential of targeting m6A in prostate cancer and summarized the emerging agents and technologies, such as the cutting-edge CRISPR-Cas13 in prostate cancer treatment by targeting m6A regulatory pathways. At last, we elucidated the perspective of developing efficient and specific RNA targeting agents and technological platforms to provide new strategies for treating prostate cancer by targeting RNA modifications.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed cancers in men worldwide [1]. Surgical removal of the prostate, radiation, chemotherapy, or hormone therapy are the main treatments for the vast majority of patients. Despite the improvement of these therapies, approximately 10% of patients are diagnosed with metastatic prostate cancer, which carries a 5-year survival rate of only 37% [2]. The prognosis of early-stage PCa is good with surgery and androgen deprivation therapy (ADT), while most patients eventually develop metastatic castration-resistant prostate cancer after ADT [3]. Metastasis is one of the most important factors responsible for death, and the survival rate decreases significantly if the tumor has metastasized since diagnosis [4]. In addition to surgical treatment and ADT, most chemotherapy drugs have narrow therapeutic indexes, poor pharmacokinetics, and non-selective distribution in vivo, leading to high drug toxicity and side effects. Therefore, it is urgent to better understand the molecular mechanism that regulates the metastasis of PCa and find new targets for PCa cells with reduced side effects.
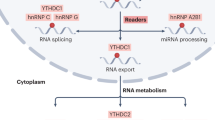
Post-transcriptional modifications are widely distributed across mRNA, rRNA, miRNA, and lncRNA, which are deeply involved in the occurrence and development of tumors and are emerging therapeutic targets, among which N6-methyladenosine (m6A) is the most abundant mRNA modification [5, 6]. In molecular mechanisms, m6A is involved in almost all steps of RNA metabolism, such as RNA degradation, splicing, export, folding, mRNA translation, etc.[7]. m6A has emerged as a multifaceted controller for gene expression regulation, mediated through its regulatory proteins, including writers, readers, and erasers [8]. It plays an important role in gene expression regulation [9]8, animal development [10], and human diseases [11]. The regulators in m6A pathways include “writers” and “erasers” that respectively install and remove the methylation, and “readers” that recognize methylation status [8, 12]. The methyltransferase complex contains two catalytic components, METTL3 (Methyltransferase-like 3, also known as MTA70, METTL14) and the accessory WTAP (Wilm’s tumor 1-associated protein) [13]. As demethylases, ALKBH5 and FTO can reverse m6A methylation, assisted with m6A-binding proteins, including members of the YTH domain family and the heterogeneous nuclear ribonucleoprotein (HNRNP) family [1, 14]. During prostate progression, some writer proteins can inhibit the degradation of relevant mRNA by targeting m6A, thus affecting cancer progression [15]. Moreover, m6A-modified ncRNAs, such as lncRNA, circRNA, miRNA, and siRNA, in PCa can participate in the formation process of RNA complexes, thereby affecting the progression of prostate cancer [14].
Given the critical implications of m6A in cancer progression, emerging efforts are being made to develop agents and technologies for targeting the oncogenic m6A regulators and events. Notably, small molecules, especially agents derived from traditional medicines, are being used as m6A-targeted anticancer drugs, which treat the disease by targeting prostate cancer-related m6A. In addition, emerging technologies such as CRISPR-Cas9 and CRISPR-13 are also being applied with anti-tumor diagnostics and therapies, which have achieved great advantages and effectiveness, especially by specifically targeting RNA modifications such as m6A. Studies have shown that CRISPR-Cas13 can target m6A for the treatment of m6A dysregulation-related diseases, and it is reasonable to expect that this technology can be better integrated with m6A-targeted treatment of prostate cancer in the future.
In this review, we summarized the roles and mechanisms of m6A regulators and m6A-modified oncogenic or tumor-suppressor RNAs in PCa and discussed and prospected the therapeutic potential of the agents and CRISPR-Cas13 platforms for the treatment of prostate cancer by targeting m6A.
M6A regulators in prostate cancer
M6A is a dynamic and reversible RNA modification and is one of the most frequently reported RNA modifications in cancer. The m6A is added, removed, or recognized by the corresponding writer, eraser, and reader, respectively. Dysregulated expression or activity of “writers” and “erasers” determines the abnormal level of m6A in cancer, and “readers” are mainly responsible for the recognition of the targeted RNA m6A modification. Briefly, the “writers” mainly include METTL3, METTL14, and cofactor WTAP [9, 16,17,18]. The YTH domain-containing proteins including YTHDF1, YTHDF2, and YTHDF3 are the “readers”[8]. In addition, ALKBH5 and FTO are the main “erasers”, which is now clear that they demethylate m6A. The basic process of m6A modification is that it is installed by m6A methyltransferase, removed by m6A demethylase, and recognized by m6A reading molecules, thereby regulating RNA metabolism. Many studies have found that m6A is widely involved in PCa initiation, progression, and resistance through various molecular mechanisms [16, 19] (Fig. 1).
The m6A Reader protein YTHDF2 promotes prostate cell proliferation by modulating the phosphorylation of downstream AKT signaling. YTHDF1 enhances anti-tumor immunity in prostate cancer by regulating mRNA stability and thereby affecting protein expression in T cells. The Eraser proteins FTO and ALKBH5 modulate the m6A methylation level of TSPAN1, leading to the suppression of autophagy in prostate cancer cells. The Writer protein METTL14 increases m6A abundance, thereby promoting T cell infiltration into the tumor microenvironment. In contrast, downregulation of METTL3 has been shown to inhibit prostate cancer progression.
m6A “writers” in prostate cancer
The m6A “writers” function to promote the methylation of targeted RNAs. There are seven “writers” reported, including METTL3, METTL5, METTL14, WTAP, RBM15, ZC3H13, and VIRMA [17]. Diverse writers have disparate effects on different types of cancer by regulating the status of target RNA m6A [18]. Dysregulation of METTL3 can promote or inhibit cancer progression through distinctive mechanisms in different cancers. It has been demonstrated in pancreatic cancer that cigarette smoke condensation induces hypomethylation of the METTL3 promoter, followed by recruitment of the transcription factor NFIC to induce METTL3 overexpression and promote cancer progression [20]. The gut microbial metabolite butyrate is reported to downregulate METTL3 expression and inhibit the development of colorectal cancer [21]. Cui et al. reported that METTL3 knockdown in liver cancer significantly inhibits HB cell proliferation, migration, and invasion by regulating Wnt/β-catenin pathway-related proteins [22]. Mechanistic studies revealed that METTL3 may exert an oncogenic role by targeting and regulating the m6A modification of oncogenes such as Myc and cyclin D1 in PCa [23]. Specifically, the oncogenic function of METTL3 depends on its methyltransferase catalytic activity, as a mutant METTL3 without catalytic activity cannot methylate the Myc mRNA and lose its tumorigenesis-promoting role [23]. Cai et al. found that the reduction of METTL3 inhibited the SHH-GLI1 signaling axis, decreased mRNA levels of the downstream targets, such as c-Myc and cyclin D1, and significantly decreased PCa cell survival and colony formation capacity [24]. By lowering the amounts of ubiquitin-specific protease 4 (USP4) protein, METTL3 causes the ELAVL1 (embryonic lethal, abnormal vision, Drosophila-like 1) protein to degrade and increases the expression of Rho GDP dissociation inhibitor (GDI) alpha protein (ARHGDIA), thus promoting PCa cells migration and invasion [25, 26]. The highly expressed METTL3 reduces the ELAVL1 protein level by inducing the degradation of ELAVL1 protein in PCa cells and increases the expression of downstream invasion and migration-related protein ARHGDIA, and significantly upregulates the mRNA level, thus promoting the proliferation and migration of prostate cancer [26]. METTL3 can upregulate plasmacytoma variant translocation 1 (PVT1) and regulate the miR-27b-3p/bloom syndrome protein (BLM) axis to promote the progression of PCa [27]. Li et al. found that castration of m6A and METTL3 activated the ERK pathway and promoted ADT resistance in prostate cancer [28]. These studies collectively indicated the oncogenic role of METTL3 in cancers.
Another m6A writer, METTL14, is both a tumor suppressor and an oncogene in different conditions. The dysregulation of METTL14 is related to tumorigenesis, proliferation, metastasis, and invasion [29]. Knockdown of METTL14 in macrophages promotes tumor development in colorectal cancer [30], and knockdown of miR-126, the downstream target gene of METTL14, can also promote tumor metastasis in metastatic liver cancer [29]. In contrast, upregulation of METTL14 was correlated with poor prognosis in PCa patients, and knockdown of METTL14 inhibited tumor proliferation both in vitro and in vivo by regulating the expression of Thrombospondin 1 (THBS1) in an m6A-dependent manner, resulting in the recruitment of YTHDF2 to recognize and degrade THBS1 mRNA [31]. Besides, recent studies demonstrated that WTAP, a regulatory subunit of methyltransferase, is an oncogene that closely influences tumor progression, whose absence reduces the RNA-binding capacity of methyltransferase [32]. In general, WTAP is upregulated in a variety of cancers, including liver cancer, esophageal cancer, AML, and osteosarcoma [33,34,35,36,37,38,39]. circPDE5A interacts with WTAP to inhibit prostate cancer metastasis and block certain activities of m6A [40]. Other “writers” are also reported to correlate with PCa tumorigenesis or progression. For example, ZC3H13 demonstrated a marked decrease in PCa, while RBM15B and RBM15 showed high expression in PCa tissues [41].
m6A “readers” in prostate cancer
Numerous m6A “readers” have been reported so far, including YTHDF1, YTHDF2, YTHDF3, YTHDC1, YTHDC2, RBMX, LRPPRC, IGF2BP3, IGF2BP2, IGF2BP1, HNRNPC, HNRNPA2B1, FMR1, and ELAVL1 [15]. As an important m6A reader, YTHDF2 was previously demonstrated to induce targeted mRNA decay [42, 43]. YTHDF2 mediated the mRNA degradation of LHPP and NKX3-1 by reading m6A-modified sites in m6A-dependent manners [44]. Recent research has established that m6A readers impact the incidence and progression of PCa. Upregulated YTHDF2 was involved in PCa proliferation and migration by regulating m6A levels and phosphorylated AKT signal pathways [44]. Another m6A reader, YTHDF1, also acts as an oncogene in PCa by regulating several downstream m6A-modified targets. YTHDF1 boosts the expression of m6A-modified polo-like kinase 1 (PLK1) by the PI3K/AKT signaling pathway to drive the progression of PCa [45]. YTHDF1 can also promote prostate cancer progression by regulating TRIM44 m6A modification and degradation [46]. At the same time, YTHDF1 can also repress the CD8+ T cell-mediated anti-cancer immunity and ferroptosis in prostate cancer in an m6A/PD-L1 manner [47]. SLC12A5 interacts with YTHDC1 and upregulates the transcription factor HOXB13, thereby promoting prostate cancer progression [48].
In addition to the oncogenic roles of YTHDF1 and YTHDF2, knocking down HnRNPL can reduce the stability of YY1 mRNA in CRPC and inhibit the migration of tumor cells [49]. It can also induce autophagy through the cirCCSPP1-mir-520h-EGR1 axis to promote the development of prostate tumors [50]. HnRNPH/F ablation leads to G2/M cell cycle arrest, induces apoptosis of prostate cancer cell lines, and inhibits the proliferation and migration of cancer cells [50, 51]. Other readers such as ELAVL1, HNRNPA2B1, HNRNPC, RBMX, YTHDC2, YTHDF1, and YTHDF2 were also expressed at a high level in PCa tissues, but FMR1, IGF2BP2 were expressed at low levels in PCa tissues [52]. Studies have found that ELAVL1 interacts with YTHDC1 and IGF2BP1, which play a synergistic regulatory role in tumors [53, 54]. However, the functions of these readers in PCa need to be further explored.
m6A erasers in prostate cancer
Demethylases, also known as “erasers”, include FTO and ALKBH5, which function to remove the m6A methyl group from RNA and are also reported to be implicated in prostate cancer progression. FTO, as a well-studied m6A demethylase, is associated with a greater risk of endometrial cancer, breast cancer, thyroid cancer, and pancreatic cancer [55,56,57,58]. The sequences of FTO were analyzed, and the results showed the distant homology of FTO and ALKB dioxygenase families [59]. FTO is a nucleic acid demethylase that can act on both DNA and RNA [60]. The expression of FTO in tumor cells is lower than that in normal cells. Downregulation of FTO can promote the progression of PCa by targeting MCR4 in the way of m6A modification, while overexpression of MCR4 can promote the malignant progression of prostate cancer cells [61]. FTO knockout increases m6A methylation of p53 transcriptional targets, including FAS, TP53INP1, and SESN2, and induces cycle arrest and apoptosis in PCa [62]. Recent studies also suggested that FTO suppresses PCa growth and migration by lowering the decay of CLIC4 mRNA and that CLIC4 is a functional target of FTO-mediated m6A modification [63].
Furthermore, ALKBH5 is an endogenous m6A demethylase, whose knockdown or overexpression led to an increase or decrease of several critical oncogenic RNA m6A in cancers. ALKBH and most AlkB homologs have α-Kg conservative asparagine residue with hydrogen bonds, but ALKBH7 has no such structure [64]. Unlike FTO, ALKBH is not active against m6A [65]. ALKBH5 was demonstrated to be upregulated in some cancers, such as glioblastoma stem cells [66, 67], breast cancer stem cells [68], colorectal cancer, esophageal cancer, thyroid cancer, gastric cancer, acute myeloid leukemia, and prostate adenocarcinomas [68,69,70]. Some studies have found that most of the targets of FTO may not be mRNAs, but snRNA [71]. Future research should focus on identifying the exact roles and mechanisms of these downstream effectors in cancers.
Dual roles of mRNA m6A modifications in cancers
Increasing evidence shows that m6A of different downstream RNAs plays distinct roles in cancer [72, 73]. On the one hand, m6A modification enhances oncogenes’ stability or translation efficiency or downregulates the tumor suppressors’ mRNA activity to promote cancer initiation and progression [74, 75]. On the other hand, m6A events may result in the corresponding oncogenic mRNA degradation, which thereby inhibits cancer proliferation, migration, and invasion [24, 44, 76,77,78,79].
Tumor promotion roles of mRNA m6A modifications
In breast cancer, upregulation of m6A modifications in MYB, BCL2, and PTEN can enhance the protein-binding ability and translation efficiency of RNA, leading to tumorigenesis [80]. Similarly, in hepatocellular carcinoma, excessive m6A modification of the tumor suppressor gene SOCS2 reduces mRNA stability and accelerates its degradation, leading to tumor progression [81]. In addition, upregulated m6A modification on HBXIP and MAGT3 in breast cancer also promotes cancer development [82]. In PCa, PCAT6 directly interacts with IGF2BP2 and upregulates the stability of IGF1R mRNA. Overexpression of PCAT6 or IGFBP2 increased the stability of IGF1R mRNA, and IGF1R overexpression in turn enabled the inhibitory role of PCAT6 on the proliferation of PCa [74]. Some studies indicated that METTL3 influences the activity of the Wnt/β-catenin pathway through m6A methylation of lymphoid enhancer-binding factor 1 (LEF1) mRNA, thereby promoting the progression of PCa [75]. In summary, RNA hyper-m6A of several mRNAs accelerates tumor progression in PCa.
m6A functions as a tumor suppressor
On the other hand, mRNA m6A may also suppress tumor growth in PCa through several targets. NKX3-1 has been reported as a tumor suppressor whose functions are closely linked to its mRNA m6A modification status, and loss of NKX3-1 contributed to prostate carcinogenesis and tumor progression [76,77,78]. Knocking down both YTHDF2 and METTL3 induced NKX3-1 expression and inhibited NKX3-1 m6A-dependent AKT phosphorylation in PCa [44]. Besides, m6A modification mediates the biogenesis of circDDIT4, which binds to ELAVL1 and acts as an RBP sponge to downregulate the expression of ELAVL1 target mRNAs, including ANO7, thus exerting a tumor suppressor effect on the progression of prostate cancer [79]. METTL3 stabilizes ARHGDIA mRNA by regulating the expression of mRNA-binding protein ELAVL1, thereby alleviating the decay of ARHGDIA mRNA [83]. Cai et al. found that the reduction in METTL3 inhibited the SHH-GLI1 signaling axis, decreased mRNA levels of the downstream targets, such as c-Myc and cyclin D1, and significantly decreased PCa cell survival, and inhibited the development of tumors [24].
Non-coding RNA m6A modifications in prostate cancer
In addition to m6A modifications of mRNA that are functionally critical during prostate cancer initiation, progression, and treatment, other noncoding RNAs’ m6A modifications, such as miRNA, lncRNA, and circRNA are also emerging and reported to be involved in various aspects of prostate cancer (Fig. 2).
MiRNA m6A modification in cancer
MiRNA is a group of ncRNA that has been proven to be functionally critical during PCa development and progression. Recent accumulating studies have demonstrated the indispensable roles of m6A in miRNAs for their function execution in cancer. MiR-96/182/183 cluster regulated the patient survival and tumor proliferation in melanoma and breast cancer [84, 85]. The upregulation of miR-182 significantly enhanced the migration and invasion of PCa by regulating the expression of FOXO1 or activating the Wnt/β-catenin signaling pathway [86, 87]. Further studies indicated that METTL3 knockdown reduced the expression of miR-182 in PCa in a m6A-dependent manner. Additionally, miR-182 inhibitors can reduce cell proliferation promoted by METTL3 overexpression to a certain extent, and METTL3 is required for DGCR8-regulated pri-miRNA in PCa [88], as the reader protein complex recognizes and processes pri-miRNA, thereby producing mature miRNA that affects cellular processes [89]. Recent studies also reported that MiR-330-3p plays an anti-tumor role in many cancers, including PCa [90,91,92]. Knocking out HNRNPA2B1 inhibited the interaction between DGCR8 and pri-miR-93, while knocking down METTL3 reduced the level of miR-93-5p and promoted the accumulation of pro-mir-93. In general, HNRNPA2B1 facilitated the processing of pri-miR-93 by recruiting DGCR8 in an m6A-dependent manner [83]. Overexpression of METTL3 can significantly improve the level of miR-148a-3p by promoting m6a modification of pri-miR-148-3p [93]. Additionally, other miRNAs, including miR-320d, miR-495, and miR-139-5p, are also demonstrated to be regulated by m6A and involved in prostate cancer progression. For instance, the overexpression of miR-320d downregulates the mRNA expression of METTL3 and also reduces the expression level of KIF3C, thus delaying the proliferation, invasion, and migration of PCa [94]. KDM5A can promote the proliferation, invasion, and migration of PCa cells and reduce cell apoptosis by down-regulating the expression of miR-495 [95]. METTL1 leads to improved mRNA stability of CDK14 by adding m7G modifications inside its mRNA, finally promoting CRPC progression [96]. Azhati et al. found that in FTO overexpressing PCa cells, inhibition of miR-139-5p and overexpression of ZNF217 could inhibit cell proliferation, mitosis, and EMT [97]. Moreover, FTO inhibited the expression of ZNF217 in a mir-139-5p-dependent manner in PCa cells [97]. In summary, accumulating studies emphasize that m6A modification of miRNAs is closely involved in PCa, which may serve as potential therapeutic targets in the future with the development of RNA targeting technologies.
LncRNA m6A modification in cancer
More and more studies have shown that lncRNAs can be used as cancer prognostic and diagnostic markers, providing new strategies for cancer prevention and treatment. Abnormal changes of lncRNA have been found in many types of cancer, such as lung cancer, stomach cancer, colon cancer, and PCa [98,99,100,101]. Liu et al reported that m6A induced upregulation of lncRNA small nucleolar RNA host gene 7 (SNHG7), which was significantly upregulated in PCa tissues and cells, and accelerated glycolysis through the serine/arginine-rich splice factor 1 (SRSF1)/c-Myc axis and promoted the progression of PCa [102]. In addition, SNHG7 expression was higher in AR inactive PC-3 and DU-145 cells compared to AR-active LNCaP and VCaP cells, and the upregulation of METTL3 increases the stability of SNHG7 in PCa [103]. Besides, METTL3 could upregulate the m6A level of LncRNA-MALAT1, which activates the PI3K/AKT signaling pathway and promotes the growth and invasion of PCa [104]. The enhanced expression of lncRNA (HOXD-AS1) in CRPC cell lines can regulate chemotherapy resistance and proliferation by interacting with the WDR5/MLL1 complex, thereby promoting CRPC transformation [105]. Furthermore, lncRNA SNHG11 expression level is also upregulated in PCa tissues and cells and promotes PCa progression through modification of miR-184 and upregulation of IGF-1R expression in an m6A-dependent manner [106]. LncRNA can also act as a miRNA sponge, inhibit the degradation of target genes mediated by relevant miRNAs, reshape chromatin to regulate the activity of transcription regulators, and bind to RNA-binding proteins to control RNA stability in m6A-dependent manners [107,108,109,110].
CircRNA m6A modification in cancer
As competitive endogenous RNAs, circRNA has been shown to perform multiple functions, especially in cancer, such as displaying a specific spatial and temporal expression pattern [111]. The structure of circRNA lacks free 5 and 3 ends, making it more stable than linear RNA, which is more suitable for serving as a biomarker, compared with other types of RNA [112]. Recent studies have confirmed that some circRNAs play a crucial role in the progression of PCa and have uncovered the potential molecular mechanism of circRNAs with m6A modifications in PCa [103, 113]. Ding et al. reported that circRNA midline-1 (circ-MID1) was overexpressed and functioned to sponge miR-330-3p in PCa cells and promoted the progression of PCa by regulating the miR-330-3p/YTHDC2/IGF1R/AKT axis [114]. Yu et al. reported that overexpressed circ-0003258 could bind to IGF2BP3, increase HDAC4 mRNA stability, and activate the ERK signaling pathway, thereby accelerating PCa metastasis [115]. CircABCC4 interacted with IGF2BP2 protein to upregulate CCAR1 and subsequently repressed the expression of target genes in the Wnt/β-catenin signaling pathway [116]. Additionally, data showed that circRBM33 was m6A-modified and highly expressed in PCa than in normal cells/tissues, and enhanced circRBM33 m6A modification promoted tumor growth and invasion, and decreasing the m6A level rescued the tumor-promoting effect of circRBM33 [117]. Growing evidence shows that circRNA plays an important role in the occurrence and progression of PCa, especially in driving the progression of CRPC [118]. Both lncRNA and circRNA can prevent the degradation of targeted mRNA by sponging miRNA [119, 120]. For example, circ-TRPS1 inhibits the growth, invasion, and migration of CRPC cells by sponging miR-124-3p to enhance EZH2 expression [121]. Further studies are needed to reveal the interaction network and regulation of mRNA and ncRNA, as well as the implications of m6A modification in cancer.
Strategies to target cancer therapy through m6A
Given the close relation of m6A dysregulation with tumorigenesis and malignancy progression [18, 122,123,124], targeting m6A regulators or the oncogenic m6A events holds huge potential for PCa treatment. Over the past few decades, there have been several advances in targeted cancer therapy, including many anti-cancer drugs and emerging technologies based on transcription and post-transcription levels by targeting m6A modifications in cancer.
Small molecules targeting m6A regulators hold therapeutic potential for prostate cancer
Given that the dysregulation of m6A in cancer can promote the development of malignant tumors, more and more small molecules have proven to be effective in suppressing prostate cancer by targeting m6A (Table 1). Specifically, Feng et al. found that β-elemive can also reduce the m6A modification level of PTEN mRNA by down-regulating the expression of METTL3, leading to increased expression of PTEN protein, and ultimately inhibiting the growth of tumor cells and promoting cell apoptosis [125]. Patients with metastatic prostate cancer exhibiting PTEN loss tend to have longer imaging-based progression-free survival and are more frequently associated with advanced AJCC stages [28, 48]. Another small molecule, STM2457, a METTL3 inhibitor, suppresses the proliferation, migration, and invasion of prostate cancer cells by reducing m6A methylation levels. It also exhibits synergistic effects with the PARP inhibitor Olaparib to further inhibit prostate cancer progression [126]. FB23-2 is a promising inhibitor by targeting FTO, which mediates the demethylation of m6A of FB23-2 and induces inhibition of cell proliferation and activation of apoptosis in AML cells [127]. IGF2BP2, an m6A binding protein that enhances the stability and translation of mRNA, is highly expressed in acute myeloid leukemia AML cells and promotes leukemia progression by regulating the expression of MYC. Its expression is upregulated in prostate cancer tissues with distant metastasis, indicating its potential as a therapeutic target [128, 129]. ALKBH5 deficiency in macrophages increases m6A modification on IL-11 mRNA, which results in decreased IL-11 stability [130]. Susanne M. et al. found that long-term consumption of quercetin and green tea extract in prostate cancer patients enhanced the bioavailability of polyphenols and reduced methylation activity [131]. A potent METTL3 selective inhibitor (UZH2, METTL3 IC50 = 5 nM) has certain inhibitory activity in the MOLM-13 cells (EC50 = 0.7 μM) and prostate cancer cells (EC50 = 2.5 μM) [132]. FTO signal transduction promotes UUO/ TGF-β1-induced rise of RUNX1 in vivo and in vitro by demethylating RUNX1 mRNA and improving its stability [133]. TGF-β expression was downregulated and bone morphogenetic protein (BMP-2) was enhanced in castrated SCID mice xenografted with C4-2B cells injected into the tibia and treated with curcumin, but downstream signaling was not affected [134]. Treatment of LNCaP cells with curcumin can induce a change in their methylation status and upregulate the expression of HDAC4 in the cells [135]. After curcumin treatment of 22Rv1 cells, the expression of MIR-141 and MIR-183 was enhanced, but the expression of the androgen receptor (AR) was decreased [136]. Previous studies confirmed that baicalin could also be used to treat various disorders by regulating methylation in key genes [137,138,139]. Baicalin improved mouse embryo development in vitro by improving DNA methylation, inhibiting cellular apoptosis, and modulating HSP70 expression [138]. Moreover, baicalin promoted suv39h1 splicing by enhancing RNA m6A methylation, resulting in anti-nasopharyngeal carcinoma (NPC) behavior [137]. The expression of FOXM1 in LNCaP and PC3 cells of the baicalin-treated group was significantly lower than that of the control group [140]. Baicalin treatment decreased the expression level of NF-κB p-P65 protein in xenograft tumors [141].
Natural products derived from traditional medicine for targeting m6A
Accumulating evidence indicates the emerging role of natural products in anti-tumors, especially by targeting m6A (Table 2), such as phenols [142,143,144], flavonoids [137, 145,146,147], alkaloids [148], and anthraquinones [149,150,151]. For example, Curcumin, a phenolic compound, inhibited adipogenesis by reducing the expression of ALKHB5 and led to higher m6A modification of TNF receptor-associated factor 4 (TRAF4) mRNA [142]. Epigallocatechin gallate (EGCG) is a polyphenol present in green tea (Camellia sinensis), which has revealed anti-cancer effects toward a variety of cancers in vitro and indicated protective potential against neurodegenerative diseases such as Alzheimer’s and Parkinson’s [152]. EGCG decreases FTO protein expression, which leads to increased m6A-modified CCNA2 and CDK2 mRNA and has a significant effect on inhibiting obesity and adipogenesis [147]. Ren et al found that the camptothecin (CPT) analogs caused the fluorescence quenching of FTO through a static quenching procedure [153]. Given the oncogenic role of FTO in cancers, these agents may also exert anti-tumor efficacy, though further experiments are required. Recent studies confirmed that baicalin hydrate(BH), a natural product derived from traditional Chinese medicines (TCM), could also be used to treat various disorders by regulating methylation in key genes’ mRNA [137,138,139]. Baicalin promoted suv39h1 splicing by enhancing RNA m6A methylation, resulting in anti-nasopharyngeal carcinoma (NPC) behavior [137]. Elemene, a natural hemiterpenoid compound, is an extract of turmeric and is a mixture of β-, γ-, δ-elemene, with β-elemene as the main component. β-emolene could directly inhibit the expression of METTL3 and lead to the downregulation of autophagy-related proteins LC3B, ATG5, and ATG7, thereby inhibiting autophagy, tumor cell proliferation, and promoting cell apoptosis [154]. Although most of these compounds lack direct evidence for anti-tumor effects in prostate cancer specifically (despite efficacy in other cancers), they hold great promise for prostate cancer therapy. This potential stems from their ability to target m6A regulators and m6A-modified oncogenes known to drive prostate cancer, highlighting the need for focused future research.
CRISPR-Cas systems targeting m6A regulators and oncogenic m6A events
In addition to the small agents modulating the m6A for the treatment of cancer, emerging technologies such as CRISPR-Cas9 and CRISPR-Cas13 [155,156,157] are being used in targeting m6A for tumor therapies. For example, unlike traditional chemotherapy drugs that damage normal cells, gRNA-guided RNA-targeting CRISPR-Cas13 is of great help in cancer diagnosis, treatment, and research due to its high specificity and efficiency in targeting RNA m6A (Fig. 3) [158]. CRISPR-Cas13-based biosensors have been used to efficiently and specifically detect cancer markers such as circulating tumor DNA, tumor-derived RNA, and as well as m6A-modified RNAs from liquid biopsy samples [159, 160]. CRISPR-Cas13 has also been used to efficiently and specifically target a variety of cancer-associated RNA and RNA modifications, such as m6A in vitro and in vivo [161]. Unlike the DNA-targeting activity of CRISPR-Cas9 and CRISPR-Cas12, CRISPR-Cas13 systems are the first and only RNA-targeting CRISPR-Cas systems that exclusively cleave ssRNA to date [162]. A-to-I editing is a significant post-transcriptional modification mediated by the ADAR family of enzymes. ADAR1 has recently been identified as a downstream target of METTL3 and has been shown to promote the progression of glioblastoma [163]. Moreover, elevated ADAR1 expression is associated with the advancement of castration-resistant prostate cancer (CRPC), highlighting its potential as a therapeutic target[171]. To enable programmable RNA editing, Feng Zhang’s team developed the REPAIR system, which fuses catalytically inactive Cas13 with the deaminase domain of ADAR [164]. This RNA-targeting platform not only facilitates precise A-to-I editing but also holds promise for identifying novel therapeutic targets in prostate cancer. In addition, several RNA manipulation tools based on CRISPR-Cas13 have been developed [160, 161, 165]. Lekha Nair et al. have employed dCas13b-FTO and dCas13b-ALKBH5 to demethylate the m6A modification on SμGLT and found that m6A can catalyze the function of CSR [162]. Zheng et al. speculated that the CRISPR-Cas13-mediated m6A editing platform may also be an effective tool to improve the antiviral ability of crops [166].
In various cancer models—including lung cancer, acute myeloid leukemia (AML), and chronic kidney disease (CKD)—the expression of downstream molecules is influenced by targeting m6A regulators or m6A modifications. It is therefore reasonable to hypothesize that related epigenetic changes, both in vivo and in vitro, can be detected using CRISPR-Cas13–based systems.
Sigitas et al. developed the meCLICK-Seq technique to capture extensive methylation of low-abundance transcriptome regions at high resolution and low RNA initiation in introns and intergenic regions, identifying RNA-modified substrates. M6A is widely present in intergenic regions of lncRNA and introns, mainly deposited by METTL16. This discovery provides new ideas for the development of new technologies that target the degradation ability of nucleic acids [166]. Jie et al. found that PAMECR can provide an efficient and precise spatiotemporal control of m6A editing and manipulate cell differentiation. It also provided a basis for the development of improved gene and non-coding RNA manipulation tools and control of cells at the surface transcriptome level [166]. Zhao et al. engineered an optogenetic system, PAMECR, based on CRISPR-Cas13 and light-inducible heterodimers (CIBN-dCas13/CRY2PHR-m6A effector), enabling spatiotemporally controlled m6A editing via blue light and allowing multiplexed editing and near-infrared operation via upconversion nanoparticles for therapeutic epitranscriptome engineering [159]. These studies collectively demonstrated great potential for m6A-targeted therapy with CRISPR-Cas-based platforms.
Discussion and perspective
Epigenetic modifications include DNA methylation, histone modifications, and RNA modifications, which have unique roles and mechanisms in the development of PCa. m6A is the most prevalent epigenetic modification in various RNA types and plays an important role in promoting the development of PCa. m6A-based epigenetic events in PCa have emerged as a key player in PCa development, progression, and treatment resistance [167]. Two kinds of molecules may serve as anti-tumor targets in the m6A process in PCa, the m6A regulators including “readers”, “erasers”, and “readers”, which regulate the stability, metabolism, and maturation of cancer-related mRNA and ncRNA, and the oncogenic m6A modification events including mRNA and ncRNAs such as miRNA, circRNA and lncRNA. Future studies are needed to further reveal the roles and mechanisms of m6A regulators and their downstream m6A modification-regulated targets in PCa for helping to screen the specific targets in different circumstances for cancer treatment [168]. It is worth noting that, natural products derived from traditional medicine, as well as the emerging RNA editing technologies, especially CRISPR-Cas13/dCas13, hold great promise for PCa treatment by targeting m6A regulators and m6A events.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Raychaudhuri R, Lin DW, Montgomery RB. Prostate cancer: a review. JAMA. 2025;333:1433–46.
Onishi K, Nakai Y, Maesaka F, Tomizawa M, Shimizu T, Hori S, et al. Impact of androgen deprivation therapy on sexual health in patients who underwent brachytherapy for prostate cancer. Andrology. 2025. https://doi.org/10.1111/andr.70066.
Chang AJ, Autio KA, Roach M III, Scher HI, et al. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11:308–23.
He L, Li H, Wu A, Peng Y, Shu G, Yin G, et al. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176.
Zhou X, Zhu H, Luo C, Yan Z, Zheng G, Zou X, et al. The role of RNA modification in urological cancers: mechanisms and clinical potential. Discov Oncol. 2023;14:235.
Liu Q, Gregory RI. RNAmod: an integrated system for the annotation of mRNA modifications. Nucleic Acids Res. 2019;47:W548–w555.
Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–24.
Roundtree IA, Evans ME, Pan T, He C, et al. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200.
Frye M, Harada BT, Behm M, He C, et al. RNA modifications modulate gene expression during development. Science. 2018;361:1346–9.
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–27.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–50.
Li J, Zhang H, Wang H. N(1)-methyladenosine modification in cancer biology: Current status and future perspectives. Comput Struct Biotechnol J. 2022;20:6578–85.
Jiang Y, Liang X, Sun H, Yin P, Zhou J, Yu C, et al. Immunomodulatory role of RNA modifications in sex hormone-dependent cancers. Front Immunol. 2025;16:1513037.
Han Z, Yi X, Li J, Zhang T, Liao D, You J, Ai J, et al. RNA m(6)A modification in prostate cancer: A new weapon for its diagnosis and therapy. Biochim Biophys Acta Rev Cancer. 2023;1878:188961.
An Y, Duan H. The role of m6A RNA methylation in cancer metabolism. Mol Cancer. 2022;21:14.
Fang Z, Mei W, Qu C, Lu J, Shang L, Cao F, et al. Role of m6A writers, erasers and readers in cancer. Exp Hematol Oncol. 2022;11:45.
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74.
Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613.
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858.
Zhu W, Si Y, Xu J, Lin Y, Wang JZ, Cao M, et al. Methyltransferase like 3 promotes colorectal cancer proliferation by stabilizing CCNE1 mRNA in an m6A-dependent manner. J Cell Mol Med. 2020;24:3521–33.
Cui X, Wang Z, Li J, Zhu J, Ren Z, Zhang D, et al. Cross talk between RNA N6-methyladenosine methyltransferase-like 3 and miR-186 regulates hepatoblastoma progression through Wnt/β-catenin signalling pathway. Cell Prolif. 2020;53:e12768.
Yuan Y, Du Y, Wang L, Liu X, et al. The M6A methyltransferase METTL3 promotes the development and progression of prostate carcinoma via mediating MYC methylation. J Cancer. 2020;11:3588–95.
Cai J, Yang F, Zhan H, Situ J, Li W, Mao Y, et al. RNA m(6)A methyltransferase METTL3 promotes the growth of prostate cancer by regulating hedgehog pathway. Onco Targets Ther. 2019;12:9143–52.
Chen Y, Pan C, Wang X, Xu D, Ma Y, Hu J, et al. Silencing of METTL3 effectively hinders invasion and metastasis of prostate cancer cells. Theranostics. 2021;11:7640–57.
Cai Z, Xu H, Bai G, Hu H, Wang D, Li H, et al. ELAVL1 promotes prostate cancer progression by interacting with other m6A regulators. Front Oncol. 2022;12:939784.
Chen, B, Liu C, Long H, Bai G, Zhu Y, Xu H, et al. N(6)-methyladenosine-induced long non-coding RNA PVT1 regulates the miR-27b-3p/BLM axis to promote prostate cancer progression. Int J Oncol. 2023;62:16.
Handa N, Li EV, Michael J, Proudfoot JA, Weiner AB, Alam R, et al. Prevalence of potential candidates for targeted therapies according to treatment-related transcriptomic signatures among 140 548 patients with nonmetastatic prostate cancer. Eur Urol Oncol. 2025. https://doi.org/10.1016/j.euo.2025.04.022.
Guan Q, Lin H, Miao L, Guo H, Chen Y, Zhuo Z, et al. Functions, mechanisms, and therapeutic implications of METTL14 in human cancer. J Hematol Oncol. 2022;15:13.
Dong L, Chen C, Zhang Y, Guo P, Wang Z, Li J, et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–57.e10.
Wang Y, Chen J, Gao WQ, Yang R, et al. METTL14 promotes prostate tumorigenesis by inhibiting THBS1 via an m6A-YTHDF2-dependent mechanism. Cell Death Discov. 2022;8:143.
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–89.
Chen Y, Peng C, Chen J, Chen D, Yang B, He B, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127.
Naren D, Yan T, Gong Y, Huang J, Zhang D, Sang L, et al. High Wilms’ tumor 1 associating protein expression predicts poor prognosis in acute myeloid leukemia and regulates m(6)A methylation of MYC mRNA. J Cancer Res Clin Oncol. 2021;147:33–47.
Zhao H, Xu Y, Xie Y, Zhang L, Gao M, Li S, et al. m6A regulators is differently expressed and correlated with immune response of esophageal cancer. Front Cell Dev Biol. 2021;9:650023.
Chen S, Li Y, Zhi S, Ding Z, Wang W, Peng Y, et al. WTAP promotes osteosarcoma tumorigenesis by repressing HMBOX1 expression in an m(6)A-dependent manner. Cell Death Dis. 2020;11:659.
Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang K, et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut. 2016;65:1482–93.
Chen L, Wang X. Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis. Oncol Lett. 2018;16:6966–70.
Yu HL, Ma XD, Tong JF, Li JQ, Guan XJ, Yang JH, et al. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Ther. 2019;12:6191–201.
Ding L, Wang R, Zheng Q, Shen D, Wang H, Lu Z, et al. circPDE5A regulates prostate cancer metastasis via controlling WTAP-dependent N6-methyladenisine methylation of EIF3C mRNA. J Exp Clin Cancer Res. 2022;41:187.
Barros-Silva, D, Lobo J, Guimarães-Teixeira C, Carneiro I, Oliveira J, Martens-Uzunova ES, et al. VIRMA-dependent N6-methyladenosine modifications regulate the expression of long non-coding RNAs CCAT1 and CCAT2 in prostate cancer. Cancers. 2020;12:771.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20.
Zhu T, Roundtree IA, Wang P, Wang X, Wang L, Sun C, et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–6.
Li J, Xie H, Ying Y, Chen H, Yan H, He L, et al. YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer. 2020;19:152.
Li P, Shi Y, Gao D, Xu H, Zou Y, Wang Z, et al. ELK1-mediated YTHDF1 drives prostate cancer progression by facilitating the translation of Polo-like kinase 1 in an m6A dependent manner. Int J Biol Sci. 2022;18:6145–62.
Li W, Chen G, Feng Z, Zhu B, Zhou L, Zhang Y, et al. YTHDF1 promotes the proliferation, migration, and invasion of prostate cancer cells by regulating TRIM44. Genes Genomics. 2021;43:1413–21.
Wang Y, Jin P, Wang X. N(6)-methyladenosine regulator YTHDF1 represses the CD8 + T cell-mediated antitumor immunity and ferroptosis in prostate cancer via m(6)A/PD-L1 manner. Apoptosis. 2024;29:142–53.
Weng WC, Lin YW, Lai CH, Lin CY, Wen YC, Chang LC, et al. Genetic variants of IGF2BP2 as potential predictors for perineural invasion of prostate cancer in a Taiwanese population. Int J Med Sci. 2025;22:1269–77.
Zhou X, Zou L, Liao H, Luo J, Yang T, Wu J, et al. Abrogation of HnRNP L enhances anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8(+) T cell-mediated ferroptosis in castration-resistant prostate cancer. Acta Pharm Sin B. 2022;12:692–707.
Cheng Y, Li L, Wei X, Xu F, Huang X, Qi F, et al. HNRNPC suppresses tumor immune microenvironment by activating Treg cells promoting the progression of prostate cancer. Cancer Sci. 2023;114:1830–45.
Chen X, Yang HT, Zhang B, Phillips JW, Cheng D, Rigo F, et al. The RNA-binding proteins hnRNP H and F regulate splicing of a MYC-dependent HRAS exon in prostate cancer cells. Proc Natl Acad Sci USA. 2023;120:e2220190120.
Liu Z, Zhong J, Zeng J, Duan X, Lu J, Sun X, et al. Characterization of the m6A-associated tumor immune microenvironment in prostate cancer to aid immunotherapy. Front Immunol. 2021;12:735170.
Liang D, Lin WJ, Ren M, Qiu J, Yang C, Wang X, et al. mA reader YTHDC1 modulates autophagy by targeting SQSTM1 in diabetic skin. Autophagy. 2022;18:1318–37.
Chen F, Chen Z, Guan T, Zhou Y, Ge L, Zhang H, et al. N(6)-methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Cancer Res. 2021;81:2847–60.
Azzam, SK, Alsafar H, Sajini AA. FTO m6A demethylase in obesity and cancer: implications and underlying molecular mechanisms. Int J Mol Sci. 2022;23:3800.
Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11.
Huang J, Sun W, Wang Z, Lv C, Zhang T, Zhang D, et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J Exp Clin Cancer Res. 2022;41:42.
Ou B, Liu Y, Gao Z, Xu J, Yan Y, Li Y, et al. Senescent neutrophils-derived exosomal piRNA-17560 promotes chemoresistance and EMT of breast cancer via FTO-mediated m6A demethylation. Cell Death Dis. 2022;13:905.
Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–72.
Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. The AlkB family of Fe(II)/α-ketoglutarate-dependent dioxygenases: repairing nucleic acid alkylation damage and beyond. J Biol Chem. 2015;290:20734–42.
Li S, Cao L. Demethyltransferase FTO alpha-ketoglutarate dependent dioxygenase (FTO) regulates the proliferation, migration, invasion and tumor growth of prostate cancer by modulating the expression of melanocortin 4 receptor (MC4R). Bioengineered. 2022;13:5598–612.
Zhang J, Wei J, Sun R, Sheng H, Yin K, Pan Y, et al. A lncRNA from the FTO locus acts as a suppressor of the m(6)A writer complex and p53 tumor suppression signaling. Mol Cell. 2023;83:2692–2708.e7.
Zou L, Chen W, Zhou X, Yang T, Luo J, Long Z, et al. N6-methyladenosine demethylase FTO suppressed prostate cancer progression by maintaining CLIC4 mRNA stability. Cell Death Discov. 2022;8:184.
Walker AR, Silvestrov P, Müller TA, Podolsky RH, Dyson G, Hausinger RP, et al. ALKBH7 variant related to prostate cancer exhibits altered substrate binding. PLoS Comput Biol. 2017;13:e1005345.
Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, et al. Reversible methylation of m(6)A(m) in the 5’ cap controls mRNA stability. Nature. 2017;541:371–5.
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606.e6.
Dixit D, Xie Q, Rich JN, Zhao JC. Messenger RNA methylation regulates glioblastoma tumorigenesis. Cancer Cell. 2017;31:474–5.
Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m⁶A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:E2047–56.
Wei C, Wang B, Peng D, Zhang X, Li Z, Luo L, et al. Pan-cancer analysis shows that ALKBH5 is a potential prognostic and immunotherapeutic biomarker for multiple cancer types including gliomas. Front Immunol. 2022;13:849592.
Shen C, Sheng Y, Zhu AC, Robinson S, Jiang X, Dong L, et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell. 2020;27:64–80.e9.
Zhai J, Chen H, Wong CC, Peng Y, Gou H, Zhang J, Pan Y, et al. ALKBH5 drives immune suppression via targeting AXIN2 to promote colorectal cancer and is a target for boosting immunotherapy. Gastroenterology. 2023;165:445–62.
Wang S, Chai P, Jia R, Jia R. Novel insights on m(6)A RNA methylation in tumorigenesis: a double-edged sword. Mol Cancer. 2018;17:101.
He L, Li J, Wang X, Ying Y, Xie H, Yan H, et al. The dual role of N6-methyladenosine modification of RNAs is involved in human cancers. J Cell Mol Med. 2018;22:4630–9.
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, et al. m (6) A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11:e426.
Ma XX, Cao ZG, Zhao SL. m6A methyltransferase METTL3 promotes the progression of prostate cancer via m6A-modified LEF1. Eur Rev Med Pharmacol Sci. 2020;24:3565–71.
Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM, et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA. 2002;99:2884–9.
Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA, et al. Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol. 2002;22:1495–503.
Bowen C, Bubendorf L, Voeller HJ, Slack R, Willi N, Sauter G, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000;60:6111–5.
Kong Z, Lu Y, Yang Y, Chang K, Lin Y, Huang Y, et al. m6A-mediated biogenesis of circDDIT4 inhibits prostate cancer progression by sequestrating ELAVL1/HuR. Mol Cancer Res. 2023;21:1342–55.
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–41.
Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–70.
Ni TK, Elman JS, Jin DX, Gupta PB, Kuperwasser C, et al. Premature polyadenylation of MAGI3 is associated with diminished N(6)-methyladenosine in its large internal exon. Sci Rep. 2018;8:1415.
Sun M, Shen Y, Jia G, Deng Z, Shi F, Jing Y, et al. Activation of the HNRNPA2B1/miR-93-5p/FRMD6 axis facilitates prostate cancer progression in an m6A-dependent manner. J Cancer. 2023;14:1242–56.
Segura MF, Hanniford D, Menendez S, Reavie L, Zou X, Alvarez-Diaz S, et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc Natl Acad Sci USA. 2009;106:1814–9.
Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–16.
Wallis CJ, Gordanpour A, Bendavid JS, Sugar L, Nam RK, Seth A, et al. MiR-182 is associated with growth, migration and invasion in prostate cancer via suppression of FOXO1. J Cancer. 2015;6:1295–305.
Wang D, Lu G, Shao Y, Xu D. MiR-182 promotes prostate cancer progression through activating Wnt/β-catenin signal pathway. Biomed Pharmacother. 2018;99:334–9.
Wang D, Wang X, Huang B, Zhao Y, Tu W, Jin X, et al. METTL3 promotes prostate cancer progression by regulating miR-182 maturation in m6A-dependent manner. Andrologia. 2022;54:1581–91.
Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–308.
Jin Z, Jia B, Tan L, Liu Y. miR-330-3p suppresses liver cancer cell migration by targeting MAP2K1. Oncol Lett. 2019;18:314–20.
Huang Y, Sun H, Ma X, Zeng Y, Pan Y, Yu D, et al. HLA-F-AS1/miR-330-3p/PFN1 axis promotes colorectal cancer progression. Life Sci. 2020;254:117180.
Li Q, Wang W, Zhang M, Sun W, Shi W, Li F, et al. Circular RNA circ-0016068 promotes the growth, migration, and invasion of prostate cancer cells by regulating the miR-330-3p/BMI-1 axis as a competing endogenous RNA. Front Cell Dev Biol. 2020;8:827.
Li G, Liu J, Wang Y, Liu H, Fu J, Zhao Y, et al. METTL3-mediated m6A modification of pri-miR-148a-3p affects prostate cancer progression by regulating TXNIP. Environ Toxicol. 2023;38:2377–90.
Ma H, Zhang F, Zhong Q, Hou J. METTL3-mediated m6A modification of KIF3C-mRNA promotes prostate cancer progression and is negatively regulated by miR-320d. Aging. 2021;13:22332–44.
Du C, Lv C, Feng Y, Yu S. Activation of the KDM5A/miRNA-495/YTHDF2/m6A-MOB3B axis facilitates prostate cancer progression. J Exp Clin Cancer Res. 2020;39:223.
Zhang M, Kan D, Zhang B, Chen X, Wang C, Chen S, et al. P300/SP1 complex mediating elevated METTL1 regulates CDK14 mRNA stability via internal m7G modification in CRPC. J Exp Clin Cancer Res. 2023;42:215.
Azhati B, Reheman A, Dilixiati D, Rexiati M. FTO-stabilized miR-139-5p targets ZNF217 to suppress prostate cancer cell malignancies by inactivating the PI3K/Akt/mTOR signal pathway. Arch Biochem Biophys. 2023;741:109604.
Yu W, Ding J, He M, Chen Y, Wang R, Han Z, et al. Estrogen receptor β promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene. 2019;38:1225–38.
Parolia A, Venalainen E, Xue H, Mather R, Lin D, Wu R, et al. The long noncoding RNA HORAS5 mediates castration-resistant prostate cancer survival by activating the androgen receptor transcriptional program. Mol Oncol. 2019;13:1121–36.
Zhang E, He X, Zhang C, Su J, Lu X, Si X, et al. A novel long noncoding RNA HOXC-AS3 mediates tumorigenesis of gastric cancer by binding to YBX1. Genome Biol. 2018;19:154.
Yue B, Liu C, Sun H, Liu M, Song C, Cui R, et al. A positive feed-forward loop between LncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol Ther. 2018;26:1287–98.
Liu J, Yuan JF, Wang YZ. METTL3-stabilized lncRNA SNHG7 accelerates glycolysis in prostate cancer via SRSF1/c-Myc axis. Exp Cell Res. 2022;416:113149.
Xiang Z, Xu C, Wu G, Liu B, Wu D. CircRNA-UCK2 increased TET1 inhibits proliferation and invasion of prostate cancer cells via sponge MiRNA-767-5p. Open Med. 2019;14:833–42.
Mao Y, Li W, Weng Y, Hua B, Gu X, Lu C, et al. METTL3-mediated m(6)A modification of lncRNA MALAT1 facilitates prostate cancer growth by activation of PI3K/AKT signaling. Cell Transpl. 2022;31:9636897221122997.
Gu P, Chen X, Xie R, Han J, Xie W, Wang B, et al. lncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting WDR5. Mol Ther. 2017;25:1959–73.
Xie Q, Zhao S, Kang R, Wang X. lncRNA SNHG11 facilitates prostate cancer progression through the upregulation of IGF-1R expression and by sponging miR-184. Int J Mol Med. 2021;48:182.
Cui S, Peng Q, Ma Q, Xu X, Zhang W, Jiang X, et al. Crosstalk between RNA-binding proteins and non-coding RNAs in tumors: molecular mechanisms, and clinical significance. Int J Biol Sci. 2025;21:2991–3010.
Wu Y, Ye Q, Chen D, Huang L, Mo R, Cai X. METTL14-mediated lncRNA NEAT1 promotes asthma progression by regulating the miR-302a-3p/March5 axis. Int Immunopharmacol. 2025;158:114850.
Ding Z, Fu L, Zhu Q, Bian S, Cui M, Li Y, et al. AC074117.1/miR-193a-3p axis regulates the malignant progression of uterine corpus endometrial carcinoma via the m6A-related gene ALKBH5. Am J Med Sci. 2025;369:726–38.
Wang Y, Ling S, Feng H, Hua J, Han Z, Chai R. Recent advances in the mutual regulation of m6A modification and non-coding RNAs in atherosclerosis. Int J Gen Med. 2025;18:1047–73.
Salzman J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016;32:309–16.
Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol. 2019;16:661–74.
Kong Z, Wan X, Lu Y, Zhang Y, Huang Y, Xu Y, et al. Circular RNA circFOXO3 promotes prostate cancer progression through sponging miR-29a-3p. J Cell Mol Med. 2020;24:799–813.
Ding Y, Wang M, Yang J. Circular RNA midline-1 (circMID1) promotes proliferation, migration, invasion and glycolysis in prostate cancer. Bioengineered. 2022;13:6293–308.
Yu YZ, Lv DJ, Wang C, Song XL, Xie T, Wang T, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer. 2022;21:12.
Huang C, Xu R, Zhu X, Jiang H. m6A-modified circABCC4 promotes stemness and metastasis of prostate cancer by recruiting IGF2BP2 to increase stability of CCAR1. Cancer Gene Ther. 2023;30:1426–40.
Zhong C, Long Z, Yang T, Wang S, Zhong W, Hu F, et al. M6A-modified circRBM33 promotes prostate cancer progression via PDHA1-mediated mitochondrial respiration regulation and presents a potential target for ARSI therapy. Int J Biol Sci. 2023;19:1543–63.
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, et al. The landscape of circular RNA in cancer. Cell. 2019;176:869–81.e13.
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, et al. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38.
Brown CJ, Lafreniere RG, Powers VE, Sebastio G, Ballabio A, Pettigrew AL, et al. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature. 1991;349:82–4.
Sha J, Xia L, Han Q, Chi C, Zhu Y, Pan J, et al. Downregulation of circ-TRPS1 suppressed prostatic cancer prognoses by regulating miR-124-3p/EZH2 axis-mediated stemness. Am J Cancer Res. 2020;10:4372–85.
Wiener D, Schwartz S. The epitranscriptome beyond m(6)A. Nat Rev Genet. 2021;22:119–31.
Wang T, Kong S, Tao M, Ju S, et al. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. 2020;19:88.
Zhang Y, Chen W, Zheng X, Guo Y, Cao J, Zhang Y, et al. Regulatory role and mechanism of m(6)A RNA modification in human metabolic diseases. Mol Ther Oncolytics. 2021;22:52–63.
Feng Y, Li C, Liu S, Yan F, Teng Y, Li X, et al. β-elemene restrains PTEN mRNA degradation to restrain the growth of lung cancer cells via METTL3-mediated N(6) methyladenosine modification. J Oncol. 2022;2022:3472745.
Chen X, Wang M, Wang H, Yang J, Li X, Zhang R, et al. METTL3 inhibitor suppresses the progression of prostate cancer via IGFBP3/AKT pathway and synergizes with PARP inhibitor. Biomed Pharmacother. 2024;179:117366.
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677–91.e10.
Weng H, Huang F, Yu Z, Chen Z, Prince E, Kang Y, et al. The m(6)A reader IGF2BP2 regulates glutamine metabolism and represents a therapeutic target in acute myeloid leukemia. Cancer Cell. 2022;40:1566–82.e10.
Fu W, Ding J, You X, Li Q, Pei X, Qin G, et al. Four types of RNA modification writers predict the prognosis of prostate cancer. Andrologia. 2022;54:e14552.
Zhuang T, Chen MH, Wu RX, Wang J, Hu XD, Meng T, et al. ALKBH5-mediated m6A modification of IL-11 drives macrophage-to-myofibroblast transition and pathological cardiac fibrosis in mice. Nat Commun. 2024;15:1995.
Henning SM, Wang P, Lee RP, Trang A, Husari G, Yang J, et al. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food Funct. 2020;11:4114–22.
Feng G, Wu Y, Hu Y, Shuai W, Yang X, Li Y, et al. Small molecule inhibitors targeting m(6)A regulators. J Hematol Oncol. 2024;17:30.
Wang DX, Bao SY, Song NN, Chen WZ, Ding XQ, Walker RJ, et al. FTO-mediated m6A mRNA demethylation aggravates renal fibrosis by targeting RUNX1 and further enhancing PI3K/AKT pathway. FASEB J. 2024;38:e23436.
Dorai T, Diouri J, O’Shea O, Doty SB. Curcumin inhibits prostate cancer bone metastasis by up-regulating bone morphogenic protein-7 in vivo. J Cancer Ther. 2014;5:369–86.
Shu L, Khor TO, Lee JH, Boyanapalli SS, Huang Y, Wu TY, et al. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011;13:606–14.
Teiten MH, Gaigneaux A, Chateauvieux S, Billing AM, Planchon S, Fack F, et al. Identification of differentially expressed proteins in curcumin-treated prostate cancer cell lines. Omics. 2012;16:289–300.
Lai W, Jia J, Yan B, Jiang Y, Shi Y, Chen L, et al. Baicalin hydrate inhibits cancer progression in nasopharyngeal carcinoma by affecting genome instability and splicing. Oncotarget. 2018;9:901–14.
Qi X, Li H, Cong X, Wang X, Jiang Z, Cao R, et al. Baicalin increases developmental competence of mouse embryos in vitro by inhibiting cellular apoptosis and modulating HSP70 and DNMT expression. J Reprod Dev. 2016;62:561–9.
Zhang XT, Wang G, Ye LF, Pu Y, Li RT, Liang J, et al. Baicalin reversal of DNA hypermethylation-associated Klotho suppression ameliorates renal injury in type 1 diabetic mouse model. Cell Cycle. 2020;19:3329–47.
Yu Z, Zhan C, Du H, Zhang L, Liang C, Zhang L. Baicalin suppresses the cell cycle progression and proliferation of prostate cancer cells through the CDK6/FOXM1 axis. Mol Cell Biochem. 2020;469:169–78.
Wu MH, Wu K, Zhu YB, Li DC, Yang H, Zeng H, et al. Baicalin antagonizes prostate cancer stemness via inhibiting Notch1/NF-κB signaling pathway. Chin J Integr Med. 2023;29:914–23.
Chen Y, Wu R, Chen W, Liu Y, Liao X, Zeng B, et al. Curcumin prevents obesity by targeting TRAF4-induced ubiquitylation in m(6) A-dependent manner. EMBO Rep. 2021;22:e52146.
Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, et al. Health benefits of resveratrol: Evidence from clinical studies. Med Res Rev. 2019;39:1851–91.
Gan Z, Wei W, Wu J, Zhao Y, Zhang L, Wang T, et al. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m(6)A RNA methylation in the intestine of weaning piglets. ACS Omega. 2019;4:17438–46.
Xu W, Xie S, Chen X, Pan S, Qian H, Zhu X. Effects of quercetin on the efficacy of various chemotherapeutic drugs in cervical cancer cells. Drug Des Devel Ther. 2021;15:577–88.
Singh S, Meena A, Luqman S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol Res. 2021;164:105387.
Wu R, Yao Y, Jiang Q, Cai M, Liu Q, Wang Y, et al. Epigallocatechin gallate targets FTO and inhibits adipogenesis in an mRNA m(6)A-YTHDF2-dependent manner. Int J Obes. 2018;42:1378–88.
Zhang L, Qi Y, ALuo Z, Liu S, Zhang Z, Zhou L, et al. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct. 2019;10:216–23.
Zhuang S, Yu R, Zhong J, Liu P, Liu Z, et al. Rhein from Rheum rhabarbarum inhibits hydrogen-peroxide-induced oxidative stress in intestinal epithelial cells partly through PI3K/Akt-mediated Nrf2/HO-1 pathways. J Agric Food Chem. 2019;67:2519–29.
Chen B, Ye F, Yu L, Jia G, Huang X, Zhang X, et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134:17963–71.
Yu J, Chen M, Huang H, Zhu J, Song H, Zhu J, et al. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2018;46:1412–23.
Bakun P, Mlynarczyk DT, Koczorowski T, Cerbin-Koczorowska M, Piwowarczyk L, Kolasiński E, et al. Tea-break with epigallocatechin gallate derivatives - Powerful polyphenols of great potential for medicine. Eur J Med Chem. 2023;261:115820 p.
Ren T, Wang Z, Zhang L, Wang N, Han X, Wang R, et al. Study of the binding between camptothecin analogs and FTO by spectroscopy and molecular docking. J Fluoresc. 2017;27:1467–77.
Song N, Cui K, Zhang K, Yang J, Liu J, Miao Z, et al. The role of m6A RNA methylation in cancer: implication for nature products anti-cancer research. Front Pharmacol. 2022;13:933332.
Song N, Cui K, Zhang K, Yang J, Liu J, Miao Z, et al. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–27.
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–4.
Kordyś M, Sen R, Warkocki Z. Applications of the versatile CRISPR-Cas13 RNA targeting system. Wiley Interdiscip Rev RNA. 2022;13:e1694.
Shi P, Wu X. Programmable RNA targeting with CRISPR-Cas13. RNA Biol. 2024;21:1–9.
He C, Li Y, Liu J, Li Z, Li X, Choi JW, et al. Application of CRISPR-Cas system in human papillomavirus detection using biosensor devices and point-of-care technologies. BME Front. 2025;6:0114.
Ma Y, Tan Y, Li J, Xiang Q, Liu S, Jin X, et al. High-sensitivity enzyme-free fluorescence probe based on CRISPR/Cas13 and the isothermal amplification strategy for Axl sensing. Anal Chem. 2024;96:16269–79.
He Q, Chen Q, Lian L, Qu J, Yuan X, Wang C, et al. Unraveling the influence of CRISPR/Cas13a reaction components on enhancing trans-cleavage activity for ultrasensitive on-chip RNA detection. Mikrochim Acta. 2024;191:466.
Sun X, Wang DO, Wang J. Targeted manipulation of m(6)A RNA modification through CRISPR-Cas-based strategies. Methods. 2022;203:56–61.
Paz N, Levanon EY, Amariglio N, Heimberger AB, Ram Z, Constantini S, et al. Altered adenosine-to-inosine RNA editing in human cancer. Genome Res. 2007;17:1586–95.
Li Y, Zhu S, Chen Y, Ma Q, Kan D, Yu W, et al. Post-transcriptional modification of m6A methylase METTL3 regulates ERK-induced androgen-deprived treatment resistance prostate cancer. Cell Death Dis. 2023;14:289.
Yang H, Patel DJ. Structures, mechanisms and applications of RNA-centric CRISPR-Cas13. Nat Chem Biol. 2024;20:673–88.
Zheng HX, Sun X, Zhang XS, Sui N, et al. m(6)A editing: new tool to improve crop quality?. Trends Plant Sci. 2020;25:859–67.
Kumaraswamy A, Welker Leng KR, Westbrook TC, Yates JA, Zhao SG, Evans CP, et al. Recent advances in epigenetic biomarkers and epigenetic targeting in prostate cancer. Eur Urol. 2021;80:71–81.
Fei X, Liu J, Xu J, Jing H, Cai Z, Yan J, et al. Integrating spatial transcriptomics and single-cell RNA-sequencing reveals the alterations in epithelial cells during nodular formation in benign prostatic hyperplasia. J Transl Med. 2024;22:380.
Tassinari V, Cesarini V, Tomaselli S, Ianniello Z, Silvestris DA, Ginistrelli LC, et al. ADAR1 is a new target of METTL3 and plays a pro-oncogenic role in glioblastoma by an editing-independent mechanism. Genome Biol. 2021;22:51.
Funding
This study was supported by the National Natural Science Foundation of China (82202922 and 82473185 to FW-Y), the National Natural Science Foundation of China (62101319 to YT-S), the Healthcare Talents Youth Program of Shanghai Pudong New Area (2024PDWSYCQN-04), Key Discipline Development Initiative of the Shanghai Health System (2024ZDXK0043).
Author information
Authors and Affiliations
Contributions
YT-S, Z-W, and FW-Y have full access to all of the data in the study and take responsibility for the integrity of the data, the accuracy of the data analysis, and the critical revision of the manuscript for important intellectual content. JY-X and DJ-G took responsibility for the concept, design, picture, and paper written. CJ-R completed the literature search, summary, and arrangement of the work. All authors contributed to the article and approved its final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, J., Gao, D., Ren, C. et al. Emerging implications of N6-methyladenosine in prostate cancer progression and treatment. Cell Death Discov. 11, 391 (2025). https://doi.org/10.1038/s41420-025-02680-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-025-02680-w