Abstract
Background
Objective biomarkers of diet, such as metabolomics, may improve dietary assessment and provide additional insight into how diet influences disease risk. The portfolio diet, a cholesterol-lowering plant-based diet, is recommended for lowering low-density lipoprotein cholesterol (LDL-C). This diet is low in saturated fat and includes nuts, plant protein (legumes), viscous fiber, and phytosterols.
Objective
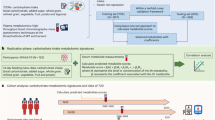
We examined metabolomic profiles in response to the portfolio diet in two randomized controlled trials (RCTs), where all foods were provided to the participants, compared to a control vegetarian diet and the same control diet with a statin.
Methods
The first RCT included 34 adults (age 58.4 ± 8.6 y) and the second RCT included 25 adults (age 61.0 ± 9.6 y), all with high LDL-C (>4.1 mmol/L). Plasma samples were obtained at baseline, week 2, and week 4 in both RCTs for metabolomics analysis using liquid chromatography–tandem mass spectrometry. Linear mixed models were used to examine effects of the interventions on the metabolites in each RCT, applying a Bonferroni correction.
Results
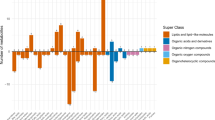
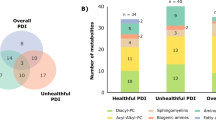
Of 496 known metabolites, 145 and 63 metabolites significantly changed within the portfolio diet interventions in the first and second RCT, respectively. The majority were glycerophosphocholines (32%), triacylglycerols (20%), glycerophosphoethanolamines (14%), sphingomyelins (8%), and amino acids and peptides (8%) in the first RCT, and glycerophosphocholines (48%), glycerophosphoethanolamines (17%), and amino acids and peptides (8%) in the second RCT. Fifty-two metabolites were consistently changed in the same direction with the portfolio diet intervention across both RCTs, after Bonferroni correction.
Conclusions
Many of these metabolites likely reflect the plant-based nature, low saturated fat content, and cholesterol-lowering effects of the diet, such as increased N2-acetylornithine, L-pipecolic acid, lenticin, and decreased C18:0 lipids and cholesteryl esters. Further research is needed to validate these metabolites as biomarkers of a plant-based dietary pattern.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Because of participant confidentiality and privacy concerns, data cannot be shared publicly, and requests to access the study data, code book, and analytic code must be submitted to the corresponding authors in writing.
References
Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL. Nutrition therapy. Can J Diabetes. 2018;42:S64–s79. https://doi.org/10.1016/j.jcjd.2017.10.009.
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–50. https://doi.org/10.1016/j.cjca.2021.03.016.
Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–82. https://doi.org/10.1016/j.cjca.2016.07.510.
Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–22. https://doi.org/10.1093/eurheartj/ehv043.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1046–e1081. https://doi.org/10.1161/CIR.0000000000000624.
Jain R, Stone JA, Agarwal G, Andrade JG, Bacon SL, Bajaj HS, et al. Canadian cardiovascular harmonized national guideline endeavour (C-CHANGE) guideline for the prevention and management of cardiovascular disease in primary care: 2022 update. CMAJ. 2022;194:E1460–E1480. https://doi.org/10.1503/cmaj.220138.
Chiavaroli L, Nishi SK, Khan TA, Braunstein CR, Glenn AJ, Mejia SB, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61:43–53. https://doi.org/10.1016/j.pcad.2018.05.004.
Glenn AJ, Hernandez-Alonso P, Kendall CWC, Martinez-Gonzalez MA, Corella D, Fito M, et al. Longitudinal changes in adherence to the portfolio and DASH dietary patterns and cardiometabolic risk factors in the PREDIMED-Plus study. Clin Nutr. 2021;40:2825–36. https://doi.org/10.1016/j.clnu.2021.03.016.
Glenn AJ, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, Kendall CWC, et al. Relationship between a plant-based dietary portfolio and risk of cardiovascular disease: findings from the women’s health initiative prospective cohort study. J Am Heart Assoc. 2021;10:e021515 https://doi.org/10.1161/JAHA.121.021515. e-pub ahead of print 20210804.
Glenn AJ, Li J, Lo K, Jenkins DJA, Boucher BA, Hanley AJ, et al. The portfolio diet and incident type 2 diabetes: findings from the women’s health initiative prospective cohort study. Diabetes Care. 2023;46:28–37. https://doi.org/10.2337/dc22-1029.
Glenn AJ, Guasch-Ferre M, Malik VS, Kendall CWC, Manson JE, Rimm EB, et al. Portfolio diet score and risk of cardiovascular disease: findings from 3 prospective cohort studies. Circulation. 2023;148:1750–63. https://doi.org/10.1161/CIRCULATIONAHA.123.065551.
Lo K, Glenn AJ, Yeung S, Kendall CWC, Sievenpiper JL, Jenkins DJA. et al. Prospective Association ofthe Portfolio Diet with All-Cause and Cause-Specific Mortality Risk in the Mr. OS and Ms. OS Study. Nutrients. 2021;13:4360. https://doi.org/10.1093/aje/kwg091.
Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158:14–21. https://doi.org/10.1093/aje/kwg091.
Guasch-Ferre M, Bhupathiraju SN, Hu FB. Use of metabolomics in improving assessment of dietary intake. Clin Chem. 2018;64:82–98. https://doi.org/10.1373/clinchem.2017.272344.
Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, et al. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond). 2015;39:1109–13. https://doi.org/10.1038/ijo.2014.199.
Archer E, Lavie CJ, Hill JO. The failure to measure dietary intake engendered a fictional discourse on diet-disease relations. Front Nutr. 2018;5:105 https://doi.org/10.3389/fnut.2018.00105.
Hall KD. Challenges of human nutrition research. Science. 2020;367:1298–1300. https://doi.org/10.1126/science.aba3807.
Brennan L, de Roos B. Role of metabolomics in the delivery of precision nutrition. Redox Biol. 2023;65:102808 https://doi.org/10.1016/j.redox.2023.102808.
Li J, Guasch-Ferre M, Chung W, Ruiz-Canela M, Toledo E, Corella D, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41:2645–56. https://doi.org/10.1093/eurheartj/ehaa209.
Rebholz CM, Lichtenstein AH, Zheng Z, Appel LJ, Coresh J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr. 2018;108:243–55. https://doi.org/10.1093/ajcn/nqy099.
Kim H, Lichtenstein AH, White K, Wong KE, Miller ER 3rd, Coresh J, et al. Plasma metabolites associated with a protein-rich dietary pattern: results from the omniheart trial. Mol Nutr Food Res. 2022;66:e2100890 https://doi.org/10.1002/mnfr.202100890.
Esko T, Hirschhorn JN, Feldman HA, Hsu YH, Deik AA, Clish CB, et al. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr. 2017;105:547–54. https://doi.org/10.3945/ajcn.116.144428.
Landberg R, Hanhineva K. Biomarkers of a healthy nordic diet-from dietary exposure biomarkers to microbiota signatures in the metabolome. Nutrients. 2019;12:27. https://doi.org/10.3390/nu12010027.
Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–10. https://doi.org/10.1001/jama.290.4.502.
Jenkins DJ, Kendall CW, Marchie A, Faulkner D, Vidgen E, Lapsley KG, et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism. 2003;52:1478–83. https://doi.org/10.1016/s0026-0495(03)00260-9.
Kavanagh ME, Glenn AJ, Chiavaroli L, Morgan GA, Josse RG, Malik VS, et al. Simulation model to assess the validity of the clinical portfolio diet score used in the PortfolioDiet.app for dietary self-tracking: a secondary analysis of a randomized controlled trial in hyperlipidemic adults. Front Nutr. 2024;11:1398450 https://doi.org/10.3389/fnut.2024.1398450.
Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137:841–53. https://doi.org/10.1161/CIRCULATIONAHA.117.029468.
Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38:52–54. https://doi.org/10.5395/rde.2013.38.1.52.
Wei R, Wang J, Su M, Jia E, Chen S, Chen T, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep. 2018;8:663 https://doi.org/10.1038/s41598-017-19120-0.
Heart N, Institute L. Manual of Laboratory Operations Lipid Research Clinics Program Volume 1 Lipid and Lipoprotein Analysis, U.S. Government Printing Office, (1974).
Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Fink PC, Römer M, Haeckel R, Fateh-Moghadam A, Delanghe J, Gressner AM, et al. Measurement of proteins with the Behring Nephelometer. A multicentre evaluation. J Clin Chem Clin Biochem. 1989;27:261–76.
Kris-Etherton PM, Griel AE, Psota TL, Gebauer SK, Zhang J, Etherton TD. Dietary stearic acid and risk of cardiovascular disease: intake, sources, digestion, and absorption. Lipids. 2005;40:1193–1200. https://doi.org/10.1007/s11745-005-1485-y.
Bonanome A, Grundy SM. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N. Engl J Med. 1988;318:1244–8. https://doi.org/10.1056/NEJM198805123181905.
Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–80. https://doi.org/10.1016/j.plipres.2008.03.003.
King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83:227–36. https://doi.org/10.1093/ajcn/83.2.227.
Liang S, Nasir RF, Bell-Anderson KS, Toniutti CA, O’Leary FM, Skilton MR. Biomarkers of dietary patterns: a systematic review of randomized controlled trials. Nutr Rev. 2022;80:1856–95. https://doi.org/10.1093/nutrit/nuac009.
Landberg R, Karra P, Hoobler R, Loftfield E, Huybrechts I, Rattner JI, et al. Dietary biomarkers-an update on their validity and applicability in epidemiological studies. Nutr Rev. 2024;82:1260–80. https://doi.org/10.1093/nutrit/nuad119.
Cuparencu C, Bulmus-Tuccar T, Stanstrup J, La Barbera G, Roager HM, Dragsted LO. Towards nutrition with precision: unlocking biomarkers as dietary assessment tools. Nat Metab. 2024;6:1438–53. https://doi.org/10.1038/s42255-024-01067-y.
Lepine G, Fouillet H, Remond D, Huneau JF, Mariotti F, Polakof S. A scoping review: metabolomics signatures associated with animal and plant protein intake and their potential relation with cardiometabolic risk. Adv Nutr. 2021;12:2112–31. https://doi.org/10.1093/advances/nmab073.
Margara-Escudero HJ, Paz-Graniel I, Garcia-Gavilan J, Ruiz-Canela M, Sun Q, Clish CB, et al. Plasma metabolite profile of legume consumption and future risk of type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2024;23:38 https://doi.org/10.1186/s12933-023-02111-z.
Bownik A, Stepniewska Z. Ectoine as a promising protective agent in humans and animals. Arh Hig Rada Toksikol. 2016;67:260–5. https://doi.org/10.1515/aiht-2016-67-2837.
Garcia-Gavilan JF, Babio N, Toledo E, Semnani-Azad Z, Razquin C, Dennis C, et al. Olive oil consumption, plasma metabolites, and risk of type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2023;22:340 https://doi.org/10.1186/s12933-023-02066-1.
Evans M, Dai L, Avesani CM, Kublickiene K, Stenvinkel P. The dietary source of trimethylamine N-oxide and clinical outcomes: an unexpected liaison. Clin Kidney J. 2023;16:1804–12. https://doi.org/10.1093/ckj/sfad095.
Shi W, Huang X, Schooling CM, Zhao JV. Red meat consumption, cardiovascular diseases, and diabetes: a systematic review and meta-analysis. Eur Heart J. 2023;44:2626–35. https://doi.org/10.1093/eurheartj/ehad336.
Gu X, Drouin-Chartier J-P, Sacks FM, Hu FB, Rosner B, Willett WC. Red meat intake and risk of type 2 diabetes in a prospective cohort study of United States females and males. Am J Clin Nutr. 2023;118:1153–63. https://doi.org/10.1016/j.ajcnut.2023.08.021.
Giosuè A, Calabrese I, Lupoli R, Riccardi G, Vaccaro O, Vitale M. Relations between the consumption of fatty or lean fish and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Adv Nutr. 2022;13:1554–65. https://doi.org/10.1093/advances/nmac006.
Costabile G, Vetrani C, Bozzetto L, Giacco R, Bresciani L, Del Rio D, et al. Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am J Clin Nutr. 2021;114:1342–50. https://doi.org/10.1093/ajcn/nqab188.
O’Connor LE, Hall KD, Herrick KA, Reedy J, Chung ST, Stagliano M, et al. Metabolomic profiling of an ultraprocessed dietary pattern in a domiciled randomized controlled crossover feeding trial. J Nutr. 2023;153:2181–92. https://doi.org/10.1016/j.tjnut.2023.06.003.
Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103:703–11. https://doi.org/10.3945/ajcn.115.121269.
Pranger IG, Joustra ML, Corpeleijn E, Muskiet FAJ, Kema IP, Oude Elferink S, et al. Fatty acids as biomarkers of total dairy and dairy fat intakes: a systematic review and meta-analysis. Nutr Rev. 2019;77:46–63. https://doi.org/10.1093/nutrit/nuy048.
Weitkunat K, Schumann S, Nickel D, Hornemann S, Petzke KJ, Schulze MB, et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017;105:1544–51. https://doi.org/10.3945/ajcn.117.152702.
Weitkunat K, Bishop CA, Wittmüss M, Machate T, Schifelbein T, Schulze MB. et al. Effect of microbialstatus on hepatic odd-chain fatty acids is diet-dependent. Nutrients. 2021;13:1546. https://doi.org/10.3390/nu13051546.
Morgan G, Glenn A, Kavanagh ME, Chiavaroli L, Paquette M, Mornin K, et al. Adherence to the portfolio diet in the PortfolioEx Trial. Can J Dietetic Pract Res. 2024;85:280–1.
Chiavaroli L, Kavanagh M, Glenn A, Chen V, Back S, Khan T, et al. P22-010-23 coronary heart effectiveness assessment of the portfolio diet in primary care (CHEAP) trial: protocol for a randomized controlled trial. Curr Dev Nutr. 2023;7:101714 https://doi.org/10.1016/j.cdnut.2023.101714.
Jenkins DJ, Chiavaroli L, Wong JM, Kendall C, Lewis GF, Vidgen E, et al. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. CMAJ. 2010;182:1961–7. https://doi.org/10.1503/cmaj.092128.
Ge L, Sadeghirad B, Ball GDC, da Costa BR, Hitchcock CL, Svendrovski A, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. Bmj. 2020;369:m696 https://doi.org/10.1136/bmj.m696.
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72. https://doi.org/10.1093/eurheartj/ehx144.
Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2:810–8. https://doi.org/10.1016/S2213-8587(14)70146-9.
Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. 2012;9:e1001255 https://doi.org/10.1371/journal.pmed.1001255.
Li Z, Lei H, Jiang H, Fan Y, Shi J, Li C, et al. Saturated fatty acid biomarkers and risk of cardiometabolic diseases: A meta-analysis of prospective studies. Front Nutr. 2022;9:963471 https://doi.org/10.3389/fnut.2022.963471.
Schwab US, Maliranta HM, Sarkkinen ES, Savolainen MJ, Kesaniemi YA, Uusitupa MI. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. 1996;45:143–9. https://doi.org/10.1016/s0026-0495(96)90044-x.
Shi F, Chowdhury R, Sofianopoulou E, Koulman A, Sun L, Steur M, et al. Association of circulating fatty acids with cardiovascular disease risk: analysis of individual-level data in three large prospective cohorts and updated meta-analysis. Eur J Preventive Cardiol. 2024;32:233–46. https://doi.org/10.1093/eurjpc/zwae315.
Zong G, Li Y, Sampson L, Dougherty LW, Willett WC, Wanders AJ, et al. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr. 2018;107:445–53. https://doi.org/10.1093/ajcn/nqx004.
Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obes (Silver Spring). 2010;18:1695–1700. https://doi.org/10.1038/oby.2009.510.
Guasch-Ferre M, Ruiz-Canela M, Li J, Zheng Y, Bullo M, Wang DD, et al. Plasma acylcarnitines and risk of type 2 diabetes in a mediterranean population at high cardiovascular risk. J Clin Endocrinol Metab. 2019;104:1508–19. https://doi.org/10.1210/jc.2018-01000.
Guasch-Ferre M, Zheng Y, Ruiz-Canela M, Hruby A, Martinez-Gonzalez MA, Clish CB, et al. Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr. 2016;103:1408–16. https://doi.org/10.3945/ajcn.116.130492.
Cheng T, Yun Z, Fan S, Wang H, Xue W, Zhang X, et al. Causal association between blood metabolites and risk of hypertension: a Mendelian randomization study. Front Cardiovasc Med. 2024;11:1373480 https://doi.org/10.3389/fcvm.2024.1373480.
Luan G, Tie F, Yuan Z, Li G, He J, Wang Z. et al. Hypaphorine, an Indole Alkaloid Isolated from Caragana korshinskii Kom., Inhibites 3T3-L1 adipocyte differentiation and improves insulin sensitivity in vitro. Chem. Biodivers. 2017;14:1–9. https://doi.org/10.1002/cbdv.201700038.
Wang F, Baden MY, Guasch-Ferre M, Wittenbecher C, Li J, Li Y, et al. Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes. Diabetologia. 2022;65:1119–32. https://doi.org/10.1007/s00125-022-05692-8.
Acknowledgements
Aspects of this work were presented in abstract form at the Precision Nutrition Forum in Copenhagen, Denmark (April 2024) and Canadian Nutrition Society Annual Conference in Edmonton, Canada (May 2024).
Funding
This work was supported by a Diabetes Canada End Diabetes 100 Award (2021-2024). The Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund provided the infrastructure for the conduct of this work. AJG was supported by a Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship and Toronto 3D Postdoctoral Fellowship Top-up Award. AJT was supported by a CIHR Postdoctoral Fellowship. MEK was funded by a CIHR Canada Graduate Scholarship and Toronto 3D Postdoctoral Fellowship Award. DJAJ was funded by the Government of Canada through the Canada Research Chair Endowment. VSM has received funding from the Canada Research Chairs Program; Connaught New Researcher Award, University of Toronto; The Joannah & Brian Lawson Centre for Child Nutrition, University of Toronto; Temerty Faculty of Medicine Pathway Grant, University of Toronto; Canada Foundation for Innovation; Ontario Research Fund, CIHR, and National Institutes of Health.
Author information
Authors and Affiliations
Contributions
AJG and JLS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: AJG, DJAJ, CWCK, JLS. Acquisition, analysis, or interpretation of data: AJG, AJT, MEG, GAM, CWCK, CBC, DJAJ, FBH, JLS. Drafting of the manuscript: AJG. Critical revision of the manuscript for important intellectual content: AJG, AJT, MEG, GAM, JSS, VSM, AJH, RPB, EMC, AES, SL, BAB, CWCK, CBC, DJAJ, FBH, JLS.
Corresponding authors
Ethics declarations
Competing interests
AJG has received travel support and/or honoraria from the Lawson Centre Nutrition Digital Series (University of Toronto), the Good Food Institute, Vinasoy, and the British Nutrition Society. AJH has received funding from the Dairy Farmers of Canada. EMC has received research support from Ocean Spray Cranberries and Lallemand Health Solutions (outside of this study). CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Lantmannen, Loblaw Brands Ltd., Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials. Foundation. DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Re-search Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit Council (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI), and the Ontario Research Fund (ORF). He has received in-kind supplies formtrials as research support from the Almond Board of California,Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Lawson Centre Nutrition Digital Series, 19th Annual Stare-Hegsted Lecture, 2024 Diabetes Canada Conference, Nutritional Fundamentals for Health (NFH)-Nutramedica, Saint Barnabas Medical Center, The University of Chicago, 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter&Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbo-hydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular RiskmReduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8) and his sister, Caroline Brydson, received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. He is also a vegan. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of Health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), International Nut and Dried Fruit Council (INC) Foundation, National Honey Board (U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS; formerly ILSI North America), Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (USDA soy “Checkoff” program), Protein Industries Canada (a Government of Canada Global Innovation Clusters), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF), and The Nutrition Trialists Network Research Fund at the University of Toronto (a fund which has received donations from the Calorie Control Council, Physicians Committee for Responsible Medicine, and vegan grants through the Karuna Foundation). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, Danone, Nutrartis, Soylent, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from ASN, Danone, Dairy Farmers of Canada, FoodMinds LLC, Nestlse, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, International Sweeteners Association, International Glutamate Technical Committee, Arab Beverages Association, and Phynova. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Inquis Clinical Research, Ingredion, and Brightseed. He is a former member of the European Fruit Juice Association Scientific Expert Panel and a former member of the Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves as an unpaid member of the Board of Trustees of IAFNS and formerly served as an unpaid scientific advisor for the Carbohydrates Committee of IAFNS. He is a Director at Large of the Canadian Nutrition Society (CNS), a founding member of the International Carbohydrate Quality Consortium (ICQC), an Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and a director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glenn, A.J., Tessier, AJ., Kavanagh, M.E. et al. Metabolomic profiling of a cholesterol lowering plant-based diet from two randomized controlled feeding trials. Eur J Clin Nutr 79, 863–875 (2025). https://doi.org/10.1038/s41430-025-01625-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-025-01625-x