Abstract
Metabolic functioning in nearly all eukaryotes relies on molecular machinery dual-encoded by mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) genomes. The two genomes have sustained an extraordinary degree of cooperation across evolutionary time, preserving the capacity for indispensable processes including oxidative phosphorylation and ATP production, which in turn influence many fitness-related traits. How this cooperation is maintained when one member of the pair is debilitated by deleterious mutation is poorly understood, as is the influence of mutation location (mtDNA or nDNA), mating system, or the potentially compensatory effects of mtDNA copy number changes on the process. We asked whether and to what extent populations experiencing mitonuclear mismatch can recover ancestral levels of fitness by allowing C. elegans nematodes containing either mitochondrial or nuclear mutations of electron transport chain (ETC) genes to evolve under three mating systems–facultatively outcrossing (wildtype), obligately selfing, and obligately outcrossing–for 60 generations. In alignment with evolutionary theory, we observed an inverse relationship between the magnitude of fitness recovery and the ancestral fitness level of strains with the latter outweighing any effect of mating system. We interpret these findings in light of previously reported male frequency evolution in the same mutant lines. The relationship between the amount of fitness evolution and change in mtDNA copy number was influenced by strains’ ETC mutant background and its interaction with mating system. To our knowledge, this work provides the first direct test of the effects of reproductive mode and evolution under mitonuclear mismatch on the population dynamics of mtDNA genomes.
Similar content being viewed by others
Introduction
Mitochondria are multifunctional organelles crucial for many cellular processes, but are most well-known for their central role in generating bioenergy in the form of ATP through the electron transport chain (ETC). In addition to nuclear DNA (nDNA) genomes, typical eukaryotic cells contain many mitochondria, each of which contains multiple copies of their mitochondrial DNA (mtDNA) genome. In eukaryotes, most energy is generated through oxidative phosphorylation (OXPHOS), a process requiring the coordinated expression of both nDNA- and mtDNA-encoded genes. Of the five ETC protein complexes, four are dual-encoded by the two genomes. Therefore, tight mitonuclear coordination and ongoing intergenomic coevolution are essential for proper ETC function and, consequently, organismal survival (Ellison and Burton 2008).
How such coevolution is maintained given the radically different population biologies experienced by nDNA and mtDNA is not well understood. For instance, even under typical conditions in non-hybrid lineages, several features of mitochondria are expected to promote higher rates of deleterious mutation accumulation as compared to the nuclear genome such as limited DNA repair and lack of recombination (Denver et al. 2000; Howe and Denver 2008; Neiman and Taylor 2009; Konrad et al. 2017). The accumulation of detrimental mtDNA mutations—mtDNA decay—is expected to lead to a reduction in mitochondrial function (Ballard and Whitlock 2004). The “nuclear compensatory hypothesis” suggests that adaptive evolution in nDNA-encoded ETC (and other mitochondria-targeted) genes has been accelerated to compensate for degradation of their mtDNA counterparts (Barreto and Burton 2013; Havird et al. 2015b; Hill 2020). This hypothesis therefore proposes that mitonuclear matching is maintained to a large extent by the nDNA genome (Havird and Sloan 2016). For example, Osada and Akashi (2012) inferred that the rapid evolution observed in primate cox genes was driven by adaptive evolution at nDNA-encoded mitochondrial proteins to counteract slightly deleterious mtDNA substitutions. If true, this might also imply that the effects of fixed deleterious ETC mutations of nDNA origin could be more difficult to compensate via fixation of secondary mutations than those of mtDNA origin–or simply that they are more likely to be compensated by other nDNA-encoded mutations. However, other studies do not support the nuclear compensatory hypothesis (Zhang and Broughton 2013; Piccinini et al. 2021; Weaver et al. 2022). For instance, another analysis found no difference in the efficacy of purifying selection between mtDNA and nDNA genomes of flies and humans (Cooper et al. 1994). This result adds to a growing body of evidence for efficient purifying selection in mtDNA—particularly on genes encoding core ETC subunits (Rand et al. 2004; Bazin et al. 2006; Ellison and Burton 2006; Meiklejohn et al. 2007; Burton et al. 2013). Theory also shows that uniparental inheritance may prevent mtDNA decay under most circumstances (Radzvilavicius et al. 2017). If true, this may imply that deleterious ETC mutations of nDNA and mtDNA origin are equally likely to be compensated via fixation of secondary ETC mutations regardless of their genomic source.
Alterations of mitochondrial DNA copy number (mtDNA-CN)–the average number of mtDNA genomes per cell–may also allow for compensation of mitonuclear genetic mismatch. Because mitochondrial life cycles, which are characterized by genome replication and series of organelle fission, fusion and mitophagy events within host generations (Twig et al. 2008), this process could feasibly occur on both short, within-generation timescales as well as across host generations. MtDNA-CN, where higher values typically reflect greater numbers of mitochondria, plays a critical role in regulating cellular energy production. Being positively associated with mitochondrial enzyme activity and ATP production, it is often considered a biomarker of mitochondrial function (Castellani et al. 2020; Filograna et al. 2021). Although the molecular mechanisms are not fully understood, mtDNA-CN is regulated in tissue- and developmental stage-specific ways critical to meeting organisms’ metabolic demands. Given that mtDNA-CN can be upregulated as a homeostatic response to sustain cellular bioenergetics in the face of deleterious mutation (e.g., Gitschlag et al. 2016; Filograna et al. 2019), one might expect a positive relationship to emerge between mtDNA-CN and evolutionary fitness during evolution under mitonuclear genetic mismatch. Such compensation could be vital for maintaining metabolic health despite mitochondrial dysfunction as has been suggested in some but not all correlative studies of human mitochondrial disease (reviewed in Filograna et al. 2021), as well as laboratory mutation accumulation (MA) studies in C. elegans (Wernick et al. 2016, 2019; Dubie et al. 2020, 2024; Sequeira et al. 2024) and Daphnia (Paquin et al. 2025) showing elevated mtDNA-CN across generations under drift conditions. Conversely, the opposite pattern was observed among a smaller set of C. briggsae MA lines, some of which contained naturally-occurring selfish mtDNA deletions (Wagner et al. 2020), suggesting the relationship between mtDNA-CN and fitness may be less straightforward.
Here we extend our previous work (Wernick et al. 2019; Bever et al. 2022; reviewed in Estes et al. 2023), employing laboratory adaptation with C. elegans mitonuclear mutants to understand the consequences of mitonuclear mismatch on baseline levels of fitness and mtDNA-CN, as well as their patterns of change under competitive conditions promoting efficient natural selection and adaptive evolution. Wernick et al. (2019) reported the first direct study of adaptive evolution of the mitochondrial ETC using experimental evolution with a nuclear-encoded mutant strain of C. elegans, gas-1. Results indicated rapid adaptation that was at least partially driven by fixation of compensatory mitochondrial mutations that likely restored mitonuclear cooperation. We also found that lineages evolving high male frequency and rates of outcrossing tended to achieve greater fitness gains in alignment with evolutionary theory (Felsenstein 1974; Barton 1995). The study of Bever et al. (2022) expanded upon this work by utilizing a variety of both nuclear- and mitochondrial-encoded ETC mutants experiencing evolution within three different mating systems (selfing, facultatively outcrossing, and outcrossing). Our findings further suggested that mitonuclear mismatch may occasionally favor outcrossing as predicted by the mitonuclear sex hypothesis (Havird et al. 2015a). Here we further extend this work by reporting patterns of fitness and mtDNA-CN evolution, and addressing three key questions: 1) How does the genomic location of a mitonuclear mutation influence patterns of fitness and mtDNA-CN evolution? 2) Does the mating system or the frequency of outcrossing affect patterns of evolution in mitonuclear mutant populations? 3) Is there a correlation between mtDNA-CN and fitness with evolution from states of mitonuclear mismatch?
To address these questions, we utilize the same experimental lines as Wernick et al. (2019) and Bever et al. (2022), which were generated by allowing replicate lineages of C. elegans nematodes containing either mitochondrial or nuclear mutations of electron transport chain (ETC) genes to evolve under three mating systems—facultatively outcrossing (wildtype), obligately selfing, and obligately outcrossing—for 60 generations. The ETC genes included the nuclear gas-1 and isp-1, mitochondrial cox-1 and ctb-1, and an isp-1 IV; ctb-1 M mitonuclear double mutant. Critically for this study, C. elegans–a primarily selfing androdioecious species (self-fertile hermaphrodites and rare males with whom they can outcross)–is unique among metazoan experimental systems in that sex determination can be genetically manipulated. Populations with variable ratios of males, females, and hermaphrodites—and thus different degrees of selfing and outcrossing—can be obtained allowing for direct tests of the role of recombination in evolution (Teotónio et al. 2017).
Materials and methods
ETC mutant strains
As described in Bever et al. (2022), C. elegans strains containing missense mutations in ETC genes listed in Table 1 were utilized for all experiments. Both the nuclear-encoded (gas-1 and isp-1) and mitochondrial-encoded (ctb-1 and cox-1) ETC mutant alleles are single base pair substitutions that were integrated onto the N2 nuclear background via 10 rounds of backcrossing of ctb-1 or cox-1 hermaphrodites with N2 males. The ctb-1(qm189) mutant was obtained from the Caenorhabditis Genetics Center (CGC; University of Minnesota); the cox-1 mutant was kindly provided by Dr. Marni Falk (Children’s Hospital of Philadelphia). Finally, we utilized a previously characterized isp-1(qm150) IV; ctb-1(189)M double mutant, also obtained from the CGC and integrated onto our N2 wildtype nuclear strain background. Following standard C. elegans nomenclature, IV refers to the nuclear chromosome number and M refers to the mitochondrial genome in the double mutant name. The mutant strains exhibited deleterious phenotypes as detailed in Bever et al. (2022) and below.
The nuclear-encoded gas-1 gene, so named for its general anaesthetic hyper-sensitive response, is located on the X chromosome and encodes GAS-1, a core 51 kDa protein subunit (orthologue of NDUFS2 in human and mouse and the bovine 49 kDa subunit) of mitochondrial ETC complex I that is required for oxidative phosphorylation (Kayser et al. 1999). Mutations of this gene, including the fc21 allele used here, are associated with severe phenotypic consequences including reduced progeny production, reduced complex I-dependent metabolism, hypersensitivity to oxidative stress owing to increased ROS production, and low ATP levels relative to wildtype (reviewed in Van der Bliek et al. 2017).
The nuclear isp-1 gene encodes the Rieske iron sulfur protein of ETC complex III (cytochrome bc1 complex), which transfers electrons from ubiquinol to cytochrome c and, like complex I, simultaneously pumps protons across the mitochondrial inner membrane thereby helping to establish the proton gradient. The isp-1(qm150) and other mutations have been characterized by slowed metabolism and extended lifespan (Rea 2005; Ventura et al. 2006). The slow-living phenotype of isp-1 appears to result from an increased reliance upon alternate, and less efficient, metabolic pathways for energy production and a concomitant reduction in mitochondrial respiration (Rea 2005; Jafari et al. 2015).
In addition to isp-1(qm150), we recreated a previously characterized isp-1 IV; ctb-1 M double missense mutant (Feng et al. 2001). The mutant screen of Feng et al. (2001) identified isp-1(qm150) and also yielded an isp-1(qm150) IV; ctb-1(189) M double mutant. For this study, we first isolated the nuclear isp-1 mutant allele onto our laboratory wildtype N2 strain via 10 rounds of backcrossing; males were then mated to hermaphrodites of the mitochondrial ctb-1 mutant strain prior to use in the current experiments. ISP-1 and the mtDNA-encoded cytochrome b, CTB-1, physically interact within ETC complex III. This isp-1 mutation replaces a conserved proline with a serine in the head domain of ISP-1, which functions to transfer reducing equivalents within complex III to cytochrome c1 through a series of conformational changes (Iwata et al. 1998). Homology modeling indicates that this mutation distorts the structure and alters the redox potential of ISP-1 (Feng et al. 2001; Jafari et al. 2015). ctb-1(qm189) is a homoplasmic (fixed within and across individuals in a population) allele that substitutes a valine for a conserved alanine in CTB-1 near the binding site of the ISP head domain (Feng et al. 2001). The isp-1 and ctb-1 mutant locations are not predicted to directly interact (Iwata et al. 1998). However, ctb-1(qm189) partially suppresses the isp-1(qm150) phenotype via beneficial allosteric effects on complex I (Suthammarak et al. 2009) This finding makes sense in light of the fact that ETC complexes I, III and IV form stable supercomplexes that improve ETC functionality (Acín-Pérez et al. 2008). isp-1(qm150) weakens the association of this supercomplex and reduces the amount and activity of complex I. The ctb-1(qm189) mutation exhibits sign epistasis (Weinreich et al. 2005) as it is beneficial within the context of isp-1(qm150), but by itself causes slightly deleterious effects on fitness and complex III activity (Suthammarak et al. 2009).
The cox-1 gene encodes cytochrome c oxidase I (COX-1), the main catalytic subunit of cytochrome c oxidase of ETC complex IV. The three mtDNA-encoded subunits (COX-I, II, III) form the functional core of the complexes; the nDNA-encoded subunits are essential for complex assembly and function (Barrientos et al. 2002; Li et al. 2006). Many additional nDNA genes are essential for biogenesis of the functional complexes (Barrientos et al. 2002), and reduce oxygen to water. It is not believed to be a major contributor to ROS production. However, its structural/functional state may indirectly affect ROS generation via other members of the I:III:IV supercomplex (Greggio et al. 2017). Cytochrome c oxidase deficiency is a leading cause of human mitochondrial disorders (Shoubridge 2001), and interpopulation (Rawson and Burton 2002) and interspecies (Sackton et al. 2003) hybrid incompatibilities are documented to result from breakup of coadapted gene complexes involving this enzyme. We used a cox-1 mutation isolated from a wild C. elegans strain, CB4856 (Hawaii) (Dingley et al. 2014). The mtDNA of this strain differs from that of the laboratory wildtype N2 by a substitution that replaces an alanine with a serine in the N-terminus of COX-1 within the matrix side of the complex IV catalytic core. Interestingly, the variant was found to be beneficial (i.e., cause increased mitochondrial membrane potential) to CB4856 worms cultured at their native temperature of 25 °C, but exerted a variety of deleterious effects at the standard laboratory temperature of 20 °C (i.e., reduced lifespan, elevated mitochondrial matrix oxidant burden, and oxidative stress). A transmitochondrial cybrid strain containing a CB4856 mtDNA genome (homoplasmic for the cox-1 variant) on a N2 nDNA background exhibited similar deleterious phenotypes at 20 °C (Dingley et al. 2014). Later studies including that by Zhu et al. (2019) found that CB4856 mitochondria were also associated with reduced fecundity in the presence of an N2 nuclear background.
Manipulating the mating system
Toward understanding the impact of mating system on mitonuclear evolutionary rates and patterns, strains containing mutations in ETC genes listed in Table 1 were generated on Katju laboratory wild-type N2 (facultatively outcrossing), and on xol-1 (selfing) and fog-2 (outcrossing) deletion-mutant genetic backgrounds. The xol-1 and fog-2 deletion alleles were generated via CRISPR-Cas9 editing (NemaMetrix/InVivo Biosystems, Eugene, OR). XOL-1 is the master sex-determination switch that functions in X-chromosome dosage compensation; disruption of this function in xol-1 mutants causes male (XO) lethality and results in obligate selfing (Miller et al. 1988). FOG-2 is an F-box protein that helps to initiate sperm production in hermaphrodites; fog-2 mutant populations consist of equal proportions of feminized hermaphrodites and males that reproduce via obligate outcrossing (Clifford et al. 2000).
Culture conditions
Each ETC ancestral mutant strain on each of the three genetic backgrounds (henceforth referred to as “G0” for generation 0) was divided into either 24 replicates (for gas-1) or eight replicates (all other strains) and subjected to laboratory evolution as described in Wernick et al. (2019) and Bever et al. (2022), respectively, wherein replicate “recovery lines” (RC lines; cf., Estes and Lynch 2003) were maintained under standard laboratory conditions but in large population sizes (i.e., bottleneck sizes of 1000) for at least 60 generations, resulting in sets of “G60 RC lines”. Eight replicates each of the N2, fog-2, and xol-1 control strains underwent the same treatment. Populations were maintained at 20 °C on 100 mm Petri plates containing Nematode Growth Medium Light (NGML), 1 ml of 200 mg/ml streptomycin, and streptomycin-resistant OP50-1 Escherichia coli as a food source. For each RC line, standardized bleach treatment was used to maintain evolving populations in non-overlapping generations. Each generation, worms were rinsed from crowded plates using M9 buffer into 15 ml conical tubes. Conical tubes were centrifuged at 800 rpm for 30 s and excess M9 poured off. A mixture of three parts diluted commercial bleach (final concentration = 2.75% bleach in di H2O) and one part 5 M NaOH was then added to the conical tubes. Tubes were inverted every 2 mins until the worms were dissolved, releasing viable embryos. Once dissolved, the conical tubes were centrifuged again until an embryo pellet formed. The bleach, NaOH and M9 were poured off and fresh M9 added to rinse the embryo pellet. This rinse process was repeated three times. The embryo pellet was then transferred to a 1 ml microtube and vortexed, after which 1 ul was transferred onto an eight-well slide. Embryo counts were used to calculate the amount needed to transfer 1000 individuals onto new large plates to initiate the next generation. Strains were transferred when the majority of hermaphrodites or females reached peak gravidity and began laying embryos with a few hatched larvae; plates were typically well-starved by this point. The time in days between transfers was tracked for all lines across the experiment.
Fitness assays
Following standard protocols, we assayed fitness-related traits for all lines alongside the wildtype N2 control and the appropriate ancestral mutant. For all facultatively outcrossing (N2 background) and obligately selfing (xol-1 background) lines, we assayed daily production of selfed progeny following established methods (e.g., Wernick et al. 2019). These assays were initiated by allowing 10–15 adult hermaphrodites from a line to lay embryos for 5 h. Single embryos were then transferred to individual 60 mm Petri plates containing NGML, 1 ml of streptomycin, and OP50-1 E. coli food source, and allowed to develop. Once hatched, the number of plates was reduced to 20 for the N2 control, 10 for each ancestral mutant, and five for all G60 RC lines. At the same time each day, hermaphrodite parents were transferred to a fresh plate. Offspring were allowed to develop to the L3/L4 larval stage and then killed with a drop of 0.5 M sodium azide and stored at −4 °C to be counted. Offspring were counted by counterstaining plates with toluidine blue dye.
Outcrossed progeny production was assayed for the obligately outcrossing fog-2 lines. These assays were initiated by picking individual L4 larval stage male and female pairs onto fresh plates–20 pairs for the ancestral mutant and 10 pairs for all G60 RC lines. The focal pairs were transferred together every 24 h and offspring were counted as described above.
Offspring counts from both selfed- and outcrossed-fitness assays were used to generate reproductive schedules and calculate total reproductive output and relative fitness of the mutants compared with N2, and with the relevant G0 strain following Christy et al. (2017). Relative fitness of each individual was computed as: ω = Σe -rx l(x)m(x), where l(x) is the number of worms surviving to day x, m(x) is the productivity (number of surviving progeny) at day x, and r is the mean intrinsic population growth rate of the assay-specific N2 or ancestral mutant G0 control as appropriate. The latter was calculated by solving Euler’s equation for r from ω = Σe -rx l(x) m(x) = 1 using an average value of l(x) m(x) for each block-specific control. We used x = 4.75 on the first reproductive day (cf., Vassilieva et al. 2000).
Male frequency
Male frequency counts were conducted for fog-2 and xol-1 control strains, and for each RC line at G5, G10 and every ten generations up to 60 exactly as described in Bever et al. (2022). The number of males and hermaphrodites (or females for fog-2 lineages) on each plate were counted to calculate an average ratio of males for each RC line.
L1 larvae tissue collection and genomic DNA extraction for mtDNA copy number estimation
A total of 144 C. elegans lines were assayed for mtDNA copy number (mtDNA-CN henceforth) including 128 G60 RC lines and 16 G0 ancestral controls. All lines were retrieved by thawing cryopreserved stocks and establishing populations comprising 25–75 live worms on 30 mm NGM plates seeded with a lawn of E. coli OP50 and incubated at 20 °C. For each line, nematode populations were allowed to grow for an additional two to three generations and then collected with M9 solution and transferred to two large 90 mm NGM plates seeded with E. coli OP50 to enable further population expansion.
The number of mitochondrial genome copies and mtDNA genomes per cell shows substantial variation depending on the developmental stage of C. elegans, with ~25,000 copies (40–45 copies per cell) through the first three larval stages, L1 to L3 (Lemire 2005). This is followed by a five-fold increase in mtDNA-CN at the fourth larval stage (L4) and another six-fold increase in adult hermaphrodites (Lemire 2005). Furthermore, it remains to be determined if adult C. elegans males vary in mtDNA-CN relative to hermaphrodites. Hence, to generate standardized mtDNA-CN measurements without the influence of developmental stage or sex of the adult, we probed mtDNA for all evolved and ancestral nematodes lines at the first larval (L1) developmental stage. To prepare synchronous populations of L1 worms, the expanded worm populations on large NGM/OP50 plates with abundant gravid hermaphrodites or females were bleached using standard protocols (Brenner 1974). The resultant egg pellet was transferred to an unseeded NGM plate and incubated overnight. Synchronized L1 larvae emerging from the hatched eggs were collected in 0.5 ml TEN solution and the L1 tissue was frozen at −20 °C for subsequent DNA extraction. Genomic DNA (gDNA) for each line was isolated from the frozen L1 tissue using the PureGene Genomic DNA Tissue Kit (Qiagen no. 158622) as per a previously described protocol (Konrad et al. 2017) followed by DNA quantification using a Thermo Fisher Nanodrop spectrophotometer.
Quantification of mtDNA-CN using digital PCR (dPCR)
Primers and probes for dPCR were designed using the PrimerQuest™ Tool (Integrated DNA Technologies) and targeted the mitochondrial gene cox-1 and a single-copy nuclear gene, daf-3, on the X chromosome.
Primer-probes for mtDNA gene cox-1:
Forward Primer: 5′− GTT GTG GGA CTA GGT GAA CAG −3′
Reverse Primer: 5′− ACC CTG CTG CAT GTA AAC TAA −3′
Probe: 5′− /56-FAM/AA ATC TAC TCT ACT TCC AGG GTG CCC /3BHQ_1/ −3′
Primer-probes for nuclear gene daf-3:
Forward Primer: 5′− GAA ATC GAT TAT CGT TGA ACT TGG T −3′
Reverse Primer: 5′− CAG GTT CTG GAG ATC ATT CTT CTT A −3′
Probe: 5′− /5HEX/AA AGC TTG CGA GTC CCT GGT GAA A/3BHQ_1/ −3′
The average mtDNA-CN for each nematode line at the L1 larval stage was measured using the QIAcuity Digital PCR (dPCR) System, Nanoplate 26 K 24-well plates (Qiagen) and QIAcuity Probe PCR Kit (Qiagen, catalog # 250102). Each 40 μl dPCR reaction mix comprised 4 μl gDNA template (0.05 ng/μl), 10 μl Probe PCR Master Mix (4X), 4 μl custom dPCR copy number assay with a FAM fluorophore targeting the mitochondrial gene cox-1 (4 μM), 4 μl custom dPCR copy number assay with a HEX fluorophore targeting the nuclear gene daf-3 (4 μM), and 18 μl molecular-grade water. Digital PCR assays for each line were performed in duplicate using the following profile: 2 min at 95 °C, followed by 35 cycles of 15 s at 95 °C and 30 s at 60 °C. The QIAcuity Software Suite 2.1 was used to analyze the dPCR results and generate mtDNA-CN per sample. This approach provides a measurement of the relative mtDNA copy number normalized by line-specific nuclear DNA content or in other words, the average number of mtDNA copies per haploid nuclear genome per cell at the L1 stage.
Statistical analysis
Figures 1–3 and Supplementary Fig. S2 were created with R v. 4.4.1 (R Core Team 2024) utilizing RStudio IDE v. 2024.4.2.764 (Posit team 2024), and the packages ggplot2 (Wickham et al. 2007), ggbreak (Xu et al. 2021), gridExtra (Auguie and Antonov 2017) and cowplot (Wilke 2024). We first assessed whether fitness of the N2 control differed across assay blocks, and whether control strains (N2, xol-1, and fog-2) differed from one another using a one-way ANOVA. We then assessed whether these control strains exhibited any fitness changes across 60 generations of evolution by testing the model: ω relative to N2 = μ + generation + line + ε for each strain separately, and with generation (G0 ancestor versus G60 RC) as a fixed effect and line as a random effect using JMP Pro v. 18 (JMP Statistical Discovery LLC 2025).
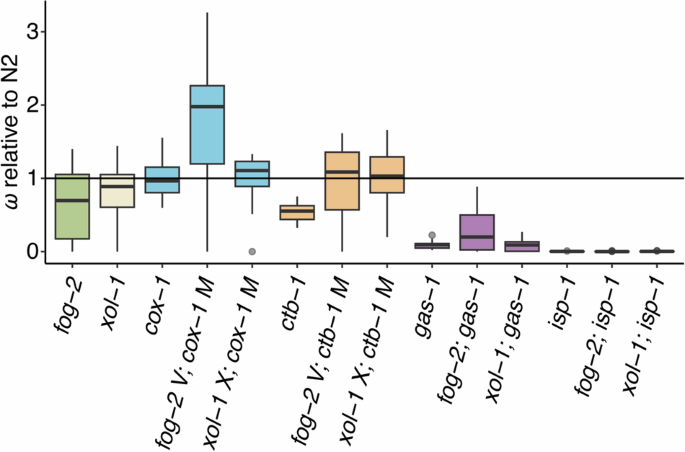
Box plots of fitness data for the ancestral (G0) fog-2 and xol-1 control strains and the G0 ETC mutants relative to wildtype N2. Fitness is a composite measure of average daily survival and offspring production, taking into account the mean intrinsic population growth rate of the assay-specific N2 control. Each plot represents an average of 5–20 independently measured replicates. Boxes encompass the upper and lower quartiles of the data with the central line denoting the median, and upper and lower whiskers denoting the maximum and minimum excluding outliers, respectively. Single points denote statistical outliers. The horizontal line at 1.0 represents wildtype N2 fitness. From left, data for the obligately outcrossing fog-2 (green) and obligately selfing xol-1 (cream) G0 ancestral controls are shown. These are followed by groups of G0 ancestral mutants organized by ETC mutant type (left to right): the mitochondrial-encoded cox-1 (blue) and ctb-1 (orange), and the nuclear-encoded gas-1 (purple) and isp-1 (not visible). Each group encompasses three mating systems (left to right): facultatively outcrossing, obligately outcrossing (“fog-2”) and obligately selfing (“xol-1”).
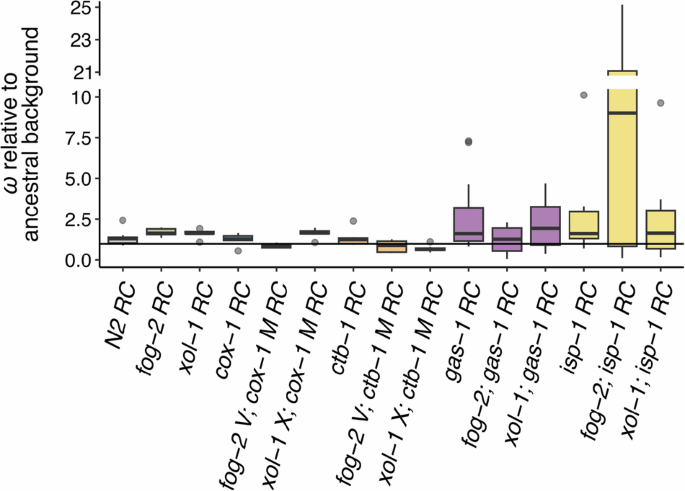
Box plots of fitness data for G60 RC lines relative to that of their respective G0 ancestors. Fitness is a composite measure of average daily survival and offspring production, taking into account the mean intrinsic population growth rate of the relevant assay-specific G0 control. Each plot was created using average relative fitnesses from eight independently evolving replicate lines (each with n = 5–20 technical replicates). The horizontal line at 1.0 represents a strain’s G0 ancestral fitness. Note the broken y-axis and change in scale relative to Fig. 1. RC lines are grouped by ETC mutant type as in Fig. 1 with the addition of the N2 RC lines. From left, data for the facultatively outcrossing N2, obligately outcrossing fog-2, and obligately selfing xol-1 G60 evolved controls are shown. These are followed by groups of G60 RC lines organized by ETC mutant type (left to right): the mitochondrial-encoded cox-1 (blue, although not visible) and ctb-1 (orange), and the nuclear-encoded gas-1 (purple) and isp-1 (yellow). Each group encompasses three mating systems (left to right): facultatively outcrossing, obligately outcrossing (“fog-2”) and obligately selfing (“xol-1”).
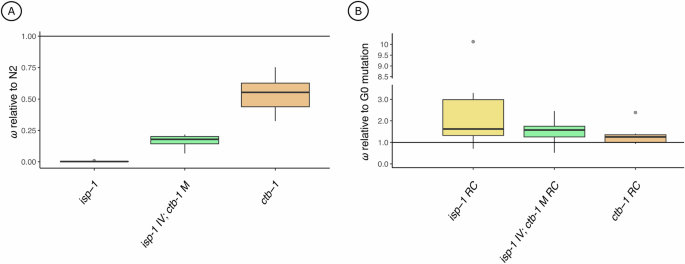
A Box plots of fitness data for G0 isp-1 (yellow) and ctb-1 (orange) single mutants and the isp-1 IV; ctb-1 M double mutant (green) relative to N2 (same data as in Fig. 1). B A box plot of G60 isp-1 and ctb-1 single mutant RC lines and isp-1 IV; ctb-1 M double mutant RC lines relative to their respective G0 ancestral background (same data as in Fig. 2). Note the broken y-axis and change in scale relative to (A). Each plot was created using average relative fitnesses from eight independently evolving replicate lines. Single points denote statistical outliers. All data in panels A and B are from animals experiencing the wildtype facultatively outcrossing mating system.
Because distributions of relative fitness tended to be right-skewed (owing to scores of 0) for the ETC mutants, these data were square-root transformed prior to analysis. The transformation did not achieve normality for all strains, but did improve model fits. First, to examine differences in fitness among the ancestral ETC mutant strains, a two-factor ANOVA testing the model: ω relative to N2 = μ + ETC background + mating system + (ETC background x mating system) + ε, where ETC background (cox-1, ctb-1, gas-1, or isp-1) and mating system (N2, fog-2, or xol-1, corresponding to wildtype facultatively outcrossing, outcrossing, and selfing) are fixed effects.
Second, to further investigate the impact of ETC mutation type and mating system on fitness evolution of mutant strains, we evaluated the model: ω relative to the appropriate G0 background = μ + ETC mutation + mating system + generation + their full-factorial interactions + line(ETC mutant) + ε, where generation (G0 ancestor versus G60 RC) is a fixed effects and line(strain) is a random effect, using restricted maximum likelihood (REML) with the mixed procedure of JMP Pro v. 18.0 (JMP Statistical Discovery LLC 2025). These analyses excluded control strains (both G0 and G60 N2, xol-1 and fog-2). We also performed select one-way ANOVAs to test the effects of ETC background or RC line on G60 fitness data separately, followed by Tukey’s HSD comparisons.
Third, we tested the model: y = μ + ETC mutation + generation + (ETC mutation × generation) + line(ETC mutant) + ε for two traits (ω relative to the appropriate G0 background and early productivity) to explore the impacts of these factors on evolution of the nDNA/mtDNA double mutant, isp-1 IV; ctb-1 M, and its two corresponding single mutants using the same procedure as above.
To determine whether the evolution of high male frequency within strains may have contributed to fitness evolution as suggested to be the case with the gas-1 RC lines (Wernick et al. 2019), we calculated Spearman’s rank correlation coefficients to evaluate relationships between RC-line specific mean relative fitness measures and their G60 male frequency for the other facultatively outcrossing strains. These included the G60 N2 RC lines and those of cox-1, ctb-1, isp-1 mutants, and the isp-1 IV; ctb-1 M double mutant.
The distribution of mtDNA-CN data was fairly normally distributed excepting a small group of high mtDNA-CN outliers. As no transformation could achieve normality, we performed Van der Waerden tests followed by non-parametric Steel-Dwass comparisons of mtDNA-CN to assess differences among various groups of lines.
Finally, we used a MANOVA to evaluate the influence of a strain’s ETC mutant background, mating system, and their interaction on the bivariate relationship between fitness and mtDNA-CN. Both traits were measured relative to the appropriate G0 ancestor. Raw data were used for this analysis and we employed the Pillai’s Trace test statistic which is robust to violations of assumptions including, in our case, homogeneity of variance-covariance matrices across strains.
Results
Supplementary Table S1 presents mean relative fitness, change in fitness over 60 generations of experimental evolution and mean relative mtDNA copy number of all the experimental lines in this study. Supplementary Table S2 reports the mtDNA copy number data for all ancestral and evolved experimental C. elegans lines. Supplementary Table S3 reports productivity (i.e., total number of surviving offspring), relative fitness, and average male frequency where relevant for all experimental C. elegans lines. Note that fitness and male frequency data for the gas-1 line set were previously reported in Wernick et al. (2019) and Bever et al. (2022), respectively.
No evolution of fitness within control strains
As in our previous studies (Christy et al. 2017; Bever et al. 2022), there was no significant effect of assay block on mean relative fitness of the wildtype N2 control. Similarly, RC lines initiated from N2, fog-2 or xol-1 strains exhibited no significant evolution of relative fitness on average across 60 generations.
Higher starting fitness of mtDNA-encoded ETC mutants
Comparison of the 12 ancestral ETC strains (4 ETC mutant backgrounds x 3 mating systems) revealed significant differences in fitness relative to the N2 background (F11,153) = 34.54, p < 0.0001) such that the ETC mutant background (F3 = 110.11, p < 0.0001) was the primary contributor followed by mating system (F2 = 3.31, p = 0.04). The interaction effect of these two factors was not quite statistically significant (F6 = 2.10, p = 0.057). Figure 1 illustrates the fitness of these strains alongside the fog-2 and xol-1 controls, and reveals an overall tendency of mtDNA-encoded ETC mutant strains to have higher fitness than nDNA-encoded ETC mutants regardless of the mating system. Indeed, a test of the same model replacing ETC mutant background (cox-1, ctb-1, gas-1, or isp-1) with mutant location (simply mtDNA or nDNA) as a factor revealed that only mutant location significantly impacted G0 strain fitness (F1 = 240.00, p < 0.0001) such that mtDNA-encoded ETC mutant strains exhibited higher fitness than nuclear-encoded ETC mutants. Note that, because each assay block was conducted with a selfing N2 control only (and not an outcrossing fog-2-containing control), the fog-2 strains in Fig. 1 should technically only be compared with one another since their biological capacity for progeny production is greater than that of selfing strains.
Larger fitness gains for nDNA-encoded ETC mutants
Only the random effect of line within ETC mutant—but not ETC mutant background, mating system, generation (G0 or G60), or their interactions—significantly impacted relative fitness. Considering G60 data alone, however, we tested the model: ω relative to the appropriate G0 background = μ + ETC background (i.e., mtDNA or nDNA) + mating system (i.e., facultatively outcrossing, obligately selfing, or obligately outcrossing) + (ETC background × line) + line(ETC background) + ε, where line is a random effect. Only ETC background (F1 = 8.70, p = 0.0039) and the random effect of line were suggested to influence G60 patterns, with nDNA-encoded mutants exhibiting greater fitness gains across the experiment than mtDNA-encoded ETC mutants (Fig. 2). Of all RC line sets, fog-2; isp-1 exhibited the most impressive fitness gain, which was driven by extreme, statistically significant fitness increases for four of the eight RC lines within this set (F7 = 24.94, p < 0.0001; Tukey’s HSD, α = 0.05). Raw values of ω ± 95% CI for these four lines were: 16.94 [11.22, 22.65], 20.44 [14.42, 26.47], 22.97 [16.95, 29.00], and 25.15 [19.44, 30.86], whilst the other four RC lines were not significantly different than 1 (i.e., no different than fitness of their G0 ancestor). Supplementary Fig. S1 shows the reproductive schedules for the fog-2; isp-1 RC line set and reveals that these fitness gains were driven by increased total progeny production and a shift to earlier progeny production, which has an outsized impact on ω.
Fitness of double mutant compared to single mutants
Fitness of the G0 nDNA/mtDNA double mutant, isp-1 IV; ctb-1 M, was intermediate to that of the corresponding single mutants. Figure 3A compares fitness of the G0 isp-1 IV; ctb-1 M double mutant alongside that of each G0 single mutant. A one-way ANOVA revealed significant differences among these strains (F2,28 = 251.86, p < 0.0001) such that the double mutant is significantly less fit than ctb-1 and significantly more fit than isp-1 (Tukey’s HSD, α = 0.05).
Among the same set of strains evolved for 60 generations, the fitness of isp-1 RC lines compared to their G0 ancestor trended higher than that of the ctb-1 RC line set and that of the double mutant (Fig. 3B), but the difference among strains was not significant. The slightly higher mean ω for the isp-1 RC line set was largely driven by a single line, H8, which exhibited an extremely large raw ω ± 95% CI of 10.12 [8.91, 11.32].
No relationship between evolution of higher male frequency and fitness
As previously reported, at least one RC line from each strain evolved higher male frequency at some point during the experiment (Fig. 1 and Supplementary Table S1 in Bever et al. 2022). Evolution of higher male frequency was most common in cox-1 and isp-1 IV; ctb-1 M lines and, for all lines, confirmed not to result from increased X-chromosome nondisjunction. Wernick et al. (2019) showed that gas-1 RC lines with highest evolved fitness at G60 tended to have high male frequency, but that this was not a prerequisite for large fitness gains. However, we find no general tendency for G60 fitness to be associated with evolution of higher male frequency in analyses of other strains on the wildtype mating system background; i.e., the evolved N2 control, cox-1, ctb-1, isp-1, or the isp-1 IV; ctb-1 M double mutant (Spearman’s ⍴).
MtDNA copy number and its relationship with fitness
Estimates of mtDNA-CN were obtained for all control strains, G0 ancestral mutants, and G60 RC lines (Table 2, Supplementary Tables S1, S2, Fig. 4). Variance in mtDNA-CN estimates among technical replicates was very low for all lines (Supplementary Table S2). We estimated that our wildtype N2 G0 worms contained on average 33.9 mtDNA genome copies per haploid nuclear genome at the L1 stage. Among the G0 ancestral ETC mutants, mtDNA-CN ranged from 20.5 copies for cox-1 to 60 copies for xol-1 X; cox-1 M (Fig. 4). With the caveat of small sample size, we detected no differences in mtDNA-CN when these G0 strains were grouped by ETC background (including wildtype) or mating system (Van der Waerden tests, df = 4 and 2, respectively). There was also no relationship between G0 strains’ mtDNA-CN and ancestral level of fitness (not shown).
Comparison of mitochondrial DNA copy number (mtDNA-CN) across G0 ancestral strains. The horizontal bar represents the mean mtDNA-CN for all strains. Error bars represent the range of variation among technical replicates for each strain. From left to right, data for five independent facultatively outcrossing N2 control lines, followed by single lines of the other control strains (obligately outcrossing fog-2 and obligately selfing xol-1), followed by groups of G0 ancestral mutants organized by ETC mutant type: the mitochondrial-encoded cox-1 and ctb-1, and the nuclear-encoded gas-1 and isp-1. Each group encompasses three mating systems (left to right): facultatively outcrossing, obligately outcrossing (“fog-2”) and obligately selfing (“xol-1”). The datum for the G0 isp-1 IV; ctb-1 M double mutant (on the wildtype, facultatively outcrossing background) appears at the far right. Apart from the N2 controls, each line had two technical replicates. Line N2-1 had 17 technical replicates, and lines N2-2 through N2-5 each had five technical replicates.
Absolute values of mtDNA-CN measured for the G60 recovery lines ranged from 19.2 copies for a ctb-1 line to 225 copies for a xol-1; isp-1 line. With respect to relative mtDNA-CN (capturing the difference between a G60 line and its G0 ancestor), this range spanned from 0.34 for a xol-1 X; cox-1 M line to 5.10 for the aforementioned xol-1; isp-1 line. No significant differences in relative mtDNA-CN with respect to ETC background or mating system emerged among the full set of G60 RC lines. However, among the G60 control line set, the average relative mtDNA-CN for xol-1 control lines (1.83) was significantly greater than that for N2 (0.69) or fog-2 (0.74) control lines (Steel Dwass test, ɑ = 0.05).
Some differences between G0 and G60 RC line mtDNA-CN emerged when lines were grouped by mating system. The average mtDNA-CN in the N2 evolved control lines was significantly lower than that of the N2 ancestor (G60 N2 lines: mean mtDNA-CN = 23.5, n = 8; G0 N2 lines: mean mtDNA-CN = 33.9, n = 5; t-test statistic = 4.19, p = 0.0015, Supplementary Table S1). Similarly, the average mtDNA-CN in the evolved fog-2 lines (mean mtDNA-CN = 49.7, n = 8) appears to have been reduced compared to the fog-2 ancestor (mean mtDNA-CN = 67.5), but a lack of biological replicates precluded further statistical testing. However, average mtDNA-CN for the evolved fog-2 lines was significantly higher than that for N2 lines (t-test statistic = 3.65, p = 0.0026). In contrast to the evolved N2 and fog-2 lines, the evolved xol-1 lines appear to have increased their mean mtDNA-CN (46.1) relative to their xol-1 ancestor (average of technical replicates = 25.1). There was no relationship between G60 control strain fitness and mtDNA-CN relative to their appropriate G0 ancestors (not shown).
There existed three clear outliers within the G60 xol-1 group (Supplementary Table S1; Supplementary Fig. S2)–one xol-1; isp-1 line and two xol-1 X; ctb-1 M lines with mtDNA copies ranging between 88.8 and 225, and with relative fitnesses ranging from 0.61 to 9.63. The line with an estimated 225 mtDNA copies was of the xol-1; isp-1 genotype and evolved very high fitness relative to its G0 ancestor (ω = 9.63). However, neither this nor the other xol-1-containing lines were among the most fit RC lines when considering the entire group. There was no obvious relationship between either relative or absolute fitness and mtDNA-CN when the full G60 RC dataset was considered (Supplementary Figs. S3 and S4, respectively). However, a MANOVA revealed a significant impact of both a strain’s ETC background (Pillai’s Trace V = 0.33, F6, 168 = 5.53, p < 0.0001) and the interaction between ETC background and mating system (Pillai’s Trace V = 0.46, F12, 168 = 4.21, p < 0.0001) on the bivariate relationship between relative fitness and relative mtDNA-CN. For example, only among RC lines bearing the cox-1 mutation, there was a highly significant inverse relationship between the line-specific change in fitness and mtDNA-CN relative to the ancestor (R2 = 0.5454, r = -0.7385, df = 22, p < 0.0001); however, this relationship is driven entirely by differences in these traits for the xol-1 X; cox1 M line group compared to the other mating systems. No such correlation exists for RC lines bearing the other ETC mutations. Post-hoc contrasts and visual analysis of canonical centroid plots (not shown) revealed three G60 strains–(i) gas-1, (ii) xol-1 X; ctb-1 M, and especially (iii) fog-2; isp-1–to be most divergent from others with respect to the relationship between relative fitness and relative mtDNA-CN. In particular, the gas-1 RC line group exhibited comparatively lower mean relative mtDNA-CN (0.67 ± 0.11) and higher relative fitness; the xol-1 X; ctb-1 M RC line group had higher mean relative mtDNA-CN (1.93 ± 1.17) and lower fitness; and the fog-1; isp-1 RC line group had middling relative mtDNA-CN (1.32 ± 0.45) and very high fitness with a few extreme outliers.
Considering the ETC double mutant, isp-1 IV; ctb-1 M, and its single-mutant counterparts separately, Fig. 4 shows that the G0 ctb-1 ancestral mutant was estimated to have greater absolute mtDNA-CN than either isp-1 or the double mutant for the single samples measured. The G60 RC lines generated from these strains all exhibited similar, minor amounts of upward evolution of mtDNA-CN from their respective G0 ancestor (nonsignificant Van der Waerden test), but the ctb-1 RC line set evolved high variance such that high- and low-mtDNA-CN groups emerged (with higher and lower mtDNA-CN than the G0 ancestor). However, there was no hint of a relationship between mtDNA-CN and fitness for this subset of lines (Supplementary Table S1).
Discussion
We aimed to determine if the genomic location of a mitonuclear mutation influences patterns of fitness and mtDNA-CN evolution. Consistent with previous studies (Christy et al. 2017; Bever et al. 2022), our control strains (N2, fog-2, xol-1) exhibited no significant fitness evolution across 60 generations of large-population size treatment. This stability in fitness suggests that any observed fitness changes in the mitonuclear mutant strains were not artifacts of the experimental design or assay block but rather a direct result of evolutionary processes acting on the specific mitonuclear mismatched genotypes.
Evolutionary gains may depend more on ancestral fitness than ETC mutation location (mtDNA vs. nDNA)
With the caveat that our study included only four ETC mutant backgrounds, our findings showed that nDNA-encoded ETC mutants exhibited larger fitness gains compared to mtDNA-encoded mutant strains irrespective of mating system. While this pattern could be due to the difference in mutation location, because both of our nDNA-encoded G0 mutants were less fit than their mtDNA-encoded counterparts (Fig. 1), we cannot provide a fair comparison of their adaptive journeys. This weakness of the experimental design is difficult to avoid as C. elegans strains available for experimental study rarely, if ever, harbor homoplasmic (fixed) mtDNA mutant alleles that match the deleterious fitness levels of nDNA-encoded ETC mutants since the former are often lethal at the cell, tissue, and organismal levels (Rossignol et al. 2003). Nonetheless, we believe that the greater adaptive gains exhibited by nDNA-encoded ETC strains more likely results from their lower starting fitnesses compared to the mtDNA-encoded mutants rather than from their ETC mutant genome location. Such a pattern is expected from adaptive landscape theory, which predicts that the probability of adaptive improvement increases with the distance of a population’s mean fitness from a theoretical optimum (Fisher 1930; Whitlock and Otto 1999; Poon and Otto 2000; Martin and Lenormand 2006, 2015; Chevin et al. 2010). This concept has been demonstrated in previous laboratory experimental evolution studies (e.g., Barrick et al. 2010; Kryazhimskiy et al. 2014) and described by Couce and Tenaillon (2015) as the “rule of declining adaptability”. This rule states that, in a given environment, lower-fitness genotypes adapt faster than their more fit counterparts because they have more mutational opportunities available to them; i.e., the distribution of fitness effects of beneficial mutations depends upon how well-adapted a population is (Couce and Tenaillon 2015).
It is important to note that the amount of adaptation achieved by our mtDNA mutant strains may have been influenced by synergistic epistases between the mtDNA-encoded ETC mutant alleles, cox-1 and ctb-1, and the fog-2 and xol-1 mutant alleles affecting mating system. Compared to fitnesses of the four mutants in isolation, relative fitness of G0 strains trended higher when either mtDNA mutation was paired with either mating system mutation (Fig. 1 and Bever et al. 2022). The biological mechanism(s) underlying such potential interactions remains unclear but, if present, they could influence the pathways available along a strain’s adaptive walk beyond the impact of a change in mating system alone (de Visser et al. 2009; Greene and Crona 2014).
No consistent benefit of males for mitonuclear evolution
Our ETC mutant strains evolving in the context of the wildtype facultatively outcrossing mating system–and thus able to evolve higher frequencies of males and associated rates of outcrossing–provided an opportunity to study the relationship between evolved male frequency and the amount of fitness evolution achieved. While a previous study (Wernick et al. 2019) noted that gas-1 RC lines with higher evolved fitness tended to have elevated male frequencies, we failed to detect a consistent relationship between G60 fitness and evolved male frequency in this expanded study. While sexual recombination is expected to facilitate adaptation under certain conditions (Fisher 1930; Muller 1932; Christiansen et al. 1998), it is also predicted to slow adaptation by breaking up beneficial multilocus genotypes (Otto 2009) and hindering a population’s ability to fix beneficial alleles in the most advantageous order (Kondrashov and Kondrashov 2001). Furthermore, the relative advantage of outcrossing vs. selfing in fixing beneficial mutations can also depend on their degree of dominance. For instance, recessive mutations (h < 0.5) are more likely to fix in self-fertilizing populations than in outcrossing ones (Hartfield et al. 2017). One might expect that the higher starting fitness of the mitochondrial ETC mutants positioned them too close to a fitness optimum to favor the evolution of higher male frequency and outcrossing rates; however, the cox-1 RC lines experienced the most consistently elevated male frequencies among all strains (Supplementary Table S3 and Fig. 1 in Bever et al. 2022). Likewise, the lower starting fitness of nuclear ETC mutants did not equate with a general increase in male frequency across all strains, and there was no association between evolved fitness and male frequency in any (nDNA or mtDNA) ETC mutant lines other than gas-1. It is, however, worth noting that the lines experiencing the greatest adaptive gains were members of the obligately outcrossing fog-2; isp-1 group (Fig. 2). In any case, our findings suggest that increased male frequency and outcrossing is not a prerequisite for adaptive evolution in cases of mitonuclear mismatch and fails to provide clear support for the idea that experimentally induced mitonuclear mismatches will promote elevated rates of outcrossing in facultatively outcrossing species (Havird et al. 2015a).
Mitochondrial copy number in C. elegans ETC mutants
We provide the first estimates of relative mtDNA-CN for an array of C. elegans ETC mutant strains. With the caveat of small sample sizes, we found that our wildtype L1-stage worms contain an average of 33.9 mtDNA genomes relative to the haploid nuclear DNA genome, which is in line with previous estimates (Lemire 2005). This number fell near the middle of the range measured for the G0 mitonuclear mutants studied here, suggesting no general tendency for mitonuclear mutants to have elevated mtDNA-CN as found for C. elegans mtDNA deletion mutants (Gitschlag et al. 2016; Dubie et al. 2020; Sequeira et al. 2024) and nDNA-encoded clk-1 mutants, which experience reduced mitochondrial functioning as result of disrupted ubiquinone biosynthesis (Kirby and Patel 2021). Interestingly, the cox-1 ancestor was at the low end of the range with 20.5 copies, while the xol-1 X; cox-1 M ancestor fell at the high end with 60 copies. While it is extremely unlikely that XOL-1 directly influences mtDNA-CN regulation, it may be that the presence of xol-1 and cox-1 mutations in combination causes metabolic stress and subsequent elevation of mtDNA-CN as a homeostatic response (cf., Filograna et al. 2019).
Complex relationship between fitness and mtDNA-CN
Studies of mitochondrial diseases of nuclear genome origin; i.e., mtDNA depletion syndromes, have established a link between decreased mtDNA-CN and disease phenotypes (Filograna et al. 2021). Namely, mutations of nuclear genes involved in mtDNA repair, replication, maintenance, and quality control lead to tissue-specific reductions of absolute mtDNA-CN and reduced mtDNA expression. However, mitochondrial diseases of mitochondrial genome origin often exhibit either increased or unchanged mtDNA-CN (reviewed in Filograna et al. 2021). Increased mtDNA-CN has been associated with mitochondrial deletions (Gitschlag et al. 2016; Dubie et al. 2020, 2024; Sequeira et al. 2024), drift decay in mutation accumulation experiments (e.g., Paquin et al. 2025, but see Wagner et al. 2020), and responses to oxidative (Hori et al. 2009; Crane et al. 2013; Long et al. 2024) and exogenous stressors (Fetterman et al. 2017). Thus, some but not all previous studies indicate that organisms respond to various endogenous and exogenous stressors by increasing mtDNA-CN to maintain mitochondrial functioning, and that the direction of change in mtDNA-CN can depend on whether the mtDNA or nDNA genome has experienced mutation damage. In contrast, the induction of mitophagy, an important process in mitochondrial quality control, has also been shown to reduce mtDNA-CN (Wang et al. 2021).
We found no relationship between the fitness and mtDNA-CN of our G0 ancestral strains. However, after 60 generations, the N2 lines had converged on a relatively small number of mtDNA-CN (mean 23.5). In contrast, the mtDNA-CN in evolved populations containing the reproductive mutations fog-2 and xol-1 without any ETC mutations was double that found in N2 at G60. The mean mtDNA-CN at G60 was 49.6 and 46.1 per haploid genome in the fog-2 and xol-1 lines, respectively. This suggests that either the reproductive mode or the specific reproductive mutations may influence mitochondrial regulation. We observed no across-the-board increase in mtDNA-CN in evolved RC lines, nor any relationship with respect to ETC mutant genome location (Table 2; Supplementary Fig. S2) as might have been expected based on aforementioned human mitochondrial disease findings (Filograna et al. 2021). Indeed, mtDNA-CN ranged from only 19.2 in a ctb-1 line to 225 for a xol-1; isp-1. It is notable that G60 lines exhibiting the highest mtDNA-CN levels contained the xol-1 mutation (i.e., xol-1, isp-1 and xol-1 X; ctb-1 M RC lines), once again indicating that an unknown feature of the xol-1 knockout–as opposed to the effect of evolution under obligate selfing per se–may promote elevated mtDNA-CN.
One of the key observations from our study was the absence of a consistent relationship between mtDNA-CN and fitness. Based on previous findings of elevated mtDNA-CN among MA lines of C. elegans (Wernick et al. 2016, 2019; Dubie et al. 2020, 2024; Sequeira et al. 2024) and Daphnia (Paquin et al. 2025), we might have anticipated a similar pattern of increased mtDNA-CN in either or both of our G0 ancestral and G60 evolved lines if worms were employing increased mtDNA-CN to partially compensate for the deleterious effects of mitochondrial dysregulation caused by the ETC mutations. We instead found no pattern between these traits among G0 mutants and a complex relationship influenced by both ETC background and its interaction with mating system in the G60 RC lines.
This lack of consistency in the relationship between mtDNA-CN and fitness raises several possible mechanistic explanations. First, while elevated mtDNA-CN could serve to maintain OXPHOS capacity in the face of mitonuclear mismatch as previously discussed, this will not necessarily translate into improved cellular energy efficiency or fitness, the latter of which relies on organisms having sufficient energy stores to survive to maturity and successfully reproduce. For instance, if a large proportion of mtDNA genomes carry deleterious mutations (i.e., mutation heteroplasmy levels are high), increasing mtDNA-CN may not yield improved energy metabolism or fitness benefits. The inherent metabolic costs associated with maintaining higher mtDNA-CN might outweigh the benefits in this case since overall mitochondrial genome quality would remain poor. Second, there likely exist physiological tipping points caused by extreme endogenous stress (e.g., deleterious mutation accumulation or the presence of excess reactive oxygen species) or exogenous stress beyond which mtDNA-CN cannot compensate for mitochondrial dysfunction. For example, Paquin et al. (2025) observed that Daphnia MA lines assayed under lower temperature experienced elevated mtDNA-CN alongside a maintenance of control levels of fecundity, suggesting a compensatory effect, while the same lines assayed under high temperature had reduced fecundity but no increase in mtDNA-CN. Because our ancestral mutant lines contained either large-effect nDNA mutations or homoplasmic mtDNA mutations, it is possible that they began the experiment beyond such a tipping point, thus obscuring any relationship between mtDNA-CN and fitness that may otherwise have emerged during laboratory adaptation. Third, as Paquin et al. (2025) acknowledge, it is also possible that selection under the lower temperature treatment in their study favored expansion of mtDNA haplotypes with a replicative advantage. Perhaps certain physiological conditions, environments and/or genetic backgrounds facilitate the proliferation of the mtDNA haplotypes with replicative advantage that can lead to an increase in total mtDNA-CN per cell.
Complexity in the relationship between mtDNA-CN and fitness is to be expected since the maintenance of bioenergetics involves a complex mitochondrial life cycle and quality control mechanisms–including mtDNA replication and co-occurring cycles of fission, fusion, and mitophagy (Twig et al. 2008). We do not know how such dynamics or potential dysregulation of the mitochondrial life cycle may have affected mtDNA-CN and fitness in our various ETC mutant lines. Finally, as previously noted, C. elegans and C. briggsae MA lines have revealed species-specific differences in mtDNA-CN evolution. MtDNA-CN tended to increase under drift conditions in C. elegans but decreased in C. briggsae lines (Wagner et al. 2020). Some C. briggsae isolates including those studied by Wagner et al. (2020) contain large, selfishly replicating mtDNA deletions affecting the nduo-5 gene (Clark et al. 2012). Whether the presence of this element underlies the difference in mtDNA-CN evolution is unknown, but the interspecies difference suggests that the relationship between mtDNA-CN and fitness may be highly context-dependent and influenced by species-specific biology and/or genetic background.
Future work will include analysis of whole-genome sequences for the evolved RC lines to disentangle the relative contributions of intra- versus inter-genomic mutations to fitness evolution. Such analyses could reveal whether adaptive evolution observed in our study is likely to be driven by mitochondrial mutations, nuclear mutations, or interactions between the two genomes–thus providing a direct test of the nuclear compensatory hypothesis (Barreto and Burton 2013). Understanding these dynamics will be critical for interpreting the evolutionary potential of mitochondrial and nuclear genomes in different environmental and genetic contexts. Future work could also consider the prevalence of mtDNA mutation (heteroplasmy frequency) and the variant impact, as both may play a role in determining when mtDNA-CN reaches a tipping point beyond which increasing or maintaining mtDNA-CN is no longer beneficial.
References
Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32(4):529–539. https://doi.org/10.1016/j.molcel.2008.10.021
Auguie B, Antonov A (2017) gridExtra: miscellaneous functions for “grid” graphics. R Package Version 2.3. https://CRAN.R-project.org/package=gridExtra
Ballard JWO, Whitlock MC (2004) The incomplete natural history of mitochondria. Mol Ecol 13(4):729–744. https://doi.org/10.1046/j.1365-294x.2003.02063.x
Barreto FS, Burton RS (2013) Evidence for compensatory evolution of ribosomal proteins in response to rapid divergence of mitochondrial rRNA. Mol Biol Evol 30(2):310–314. https://doi.org/10.1093/molbev/mss228
Barrick JE, Kauth MR, Strelioff CC, Lenski RE (2010) Escherichia coli rpoB mutants have increased evolvability in proportion to their fitness defects. Mol Biol Evol 27(6):1338–1347. https://doi.org/10.1093/molbev/msq024
Barrientos A, Barros MH, Valnot I, Rötig A, Rustin P, Tzagoloff A (2002) Cytochrome oxidase in health and disease. Gene 286(1):53–63. https://doi.org/10.1016/S0378-1119(01)00803-4
Barton NH (1995) Linkage and the limits to natural selection. Genetics 140(2):821–841. https://doi.org/10.1093/genetics/140.2.821
Bazin E, Glémin S, Galtier N (2006) Population size does not influence mitochondrial genetic diversity in animals. Science 312(5773):570–572. https://doi.org/10.1126/science.1122033
Bever BW, Dietz ZP, Sullins JA, Montoya AM, Bergthorsson U, Katju V et al. (2022) Mitonuclear mismatch is associated with increased male frequency, outcrossing, and male sperm size in experimentally-evolved C. elegans. Front Genet 13:742272. https://doi.org/10.3389/fgene.2022.742272/full
Van der Bliek AM, Sedensky MM, Morgan PG (2017) Cell biology of the mitochondrion. Genetics 207(3):843–871. https://doi.org/10.1534/genetics.117.300262
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94. https://doi.org/10.1093/genetics/77.1.71
Burton RS, Pereira RJ, Barreto FS (2013) Cytonuclear genomic interactions and hybrid breakdown. Annu Rev Ecol Evol Syst 44(1):281–302. https://doi.org/10.1146/annurev-ecolsys-110512-135758
Castellani CA, Longchamps RJ, Sun J, Guallar E, Arking DE (2020) Thinking outside the nucleus: mitochondrial DNA copy number in health and disease. Mitochondrion 53:214–223. https://doi.org/10.1016/j.mito.2020.06.004
Chevin L-M, Martin G, Lenormand T (2010) Fisher’s model and the genomics of adaptation: restricted pleiotropy, heterogenous mutation, and parallel evolution. Evolution 64(11):3213–3231. https://doi.org/10.1111/j.1558-5646.2010.01058.x
Christiansen FB, Otto SP, Bergman A, Feldman MW (1998) Waiting with and without recombination: the time to production of a double mutant. Theor Popul Biol 53(3):199–215. https://doi.org/10.1006/tpbi.1997.1358
Christy SF, Wernick RI, Lue MJ, Velasco G, Howe DK, Denver DR et al. (2017) Adaptive evolution under extreme genetic drift in oxidatively stressed Caenorhabditis elegans. Genome Biol Evol 9(11):3008–3022. https://doi.org/10.1093/gbe/evx222
Clark KA, Howe DK, Gafner K, Kusuma D, Ping S, Estes S et al. (2012) Selfish little circles: Transmission bias and evolution of large deletion-bearing mitochondrial DNA in Caenorhabditis briggsae nematodes. PLoS One 7(7):e41433. https://doi.org/10.1371/journal.pone.0041433
Clifford R, Lee MH, Nayak S, Ohmachi M, Giorgini F, Schedl T (2000) FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development 127(24):5265–5276. https://doi.org/10.1242/dev.127.24.5265
Cooper BS, Burrus CR, Ji C, Hahn MW, Montooth KL (1994) Similar efficacies of selection shape mitochondrial and nuclear genes in both Drosophila melanogaster and Homo sapiens. G3 Bethesda 5(10):2165–2176. https://doi.org/10.1534/g3.114.016493
Couce A, Tenaillon OA (2015) The rule of declining adaptability in microbial evolution experiments. Front Genet 6:99. https://doi.org/10.3389/fgene.2015.00099
Crane JD, Abadi A, Hettinga BP, Ogborn DI, MacNeil LG, Steinberg GR et al. (2013) Elevated mitochondrial oxidative stress impairs metabolic adaptations to exercise in skeletal muscle. PLoS One 8(12):e81879. https://doi.org/10.1371/journal.pone.0081879
de Visser JAGM, Park S, Krug J (2009) Exploring the effect of sex on empirical fitness landscapes. Am Nat 174(S1):S15–S30. https://doi.org/10.1086/599081
Denver DR, Morris K, Lynch M, Vassilieva LL, Thomas WK (2000) High direct estimate of the mutation rate in the mitochondrial genome of Caenorhabditis elegans. Science 289(5488):2342–2344. https://doi.org/10.1126/science.289.5488.2342
Dingley SD, Polyak E, Ostrovsky J, Srinivasan S, Lee I, Rosenfeld AB et al. (2014) Mitochondrial DNA variant in COX1 subunit significantly alters energy metabolism of geographically divergent wild isolates in Caenorhabditis elegans. J Mol Biol 426(11):2199–2216. https://doi.org/10.1016/j.jmb.2014.02.009
Dubie JJ, Katju V, Bergthorsson U (2024) Dissecting the sequential evolution of a selfish mitochondrial genome in Caenorhabditis elegans. Heredity 133(3):186–197. https://doi.org/10.1038/s41437-024-00704-2
Dubie JJ, Caraway AR, Stout MM, Katju V, Bergthorsson U (2020) The conflict within: origin, proliferation and persistence of a spontaneously arising selfish mitochondrial genome. Philos Trans R Soc Lond B Biol Sci 375(1790):20190174. https://doi.org/10.1098/rstb.2019.0174
Ellison CK, Burton RS (2006) Disruption of mitochondrial function in interpopulation hybrids of Tigriopus californicus. Evolution 60(7):1382–1391. https://doi.org/10.1111/j.0014-3820.2006.tb01217.x
Ellison CK, Burton RS (2008) Interpopulation hybrid breakdown maps to the mitochondrial genome. Evolution 62(3):631–638. https://doi.org/10.1111/J.1558-5646.2007.00305.x
Estes S, Lynch M (2003) Rapid fitness recovery in mutationally degraded lines of Caenorhabditis elegans. Evolution 57(5):1022–1030. https://doi.org/10.1111/j.0014-3820.2003.tb00313.x
Estes S, Dietz ZP, Katju V, Bergthorsson U (2023) Evolutionary codependency: insights into the mitonuclear interaction landscape from experimental and wild Caenorhabditis nematodes. Curr Opin Genet Dev 81:102081. https://doi.org/10.1016/j.gde.2023.102081
Felsenstein J (1974) The evolutionary advantage of recombination. Genetics 78(2):737–756. https://doi.org/10.1093/genetics/78.2.737
Feng J, Bussière F, Hekimi S (2001) Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell 1(5):633–644. https://doi.org/10.1016/S1534-5807(01)00071-5
Fetterman JL, Sammy MJ, Ballinger SW (2017) Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology 391:18–33. https://doi.org/10.1016/j.tox.2017.08.002
Filograna R, Mennuni M, Alsina D, Larsson N-G (2021) Mitochondrial DNA copy number in human disease: the more the better?. FEBS Lett 595(8):976–1002. https://doi.org/10.1002/1873-3468.14021
Filograna R, Koolmeister C, Upadhyay M, Pajak A, Clemente P, Wibom R et al. (2019) Modulation of mtDNA copy number ameliorates the pathological consequences of a heteroplasmic mtDNA mutation in the mouse. Sci Adv 5(4):eaav9824. https://doi.org/10.1126/sciadv.aav9824
Fisher, RA, 1930. The genetical theory of natural selection: a complete variorum edition. Oxford University Press, Oxford, Oxford
Gitschlag BL, Kirby CS, Samuels DC, Gangula RD, Mallal SA, Patel MR (2016) Homeostatic responses regulate selfish mitochondrial genome dynamics in C. elegans. Cell Metab 24(1):91–103. https://doi.org/10.1016/j.cmet.2016.06.008
Greene D, Crona K (2014) The changing geometry of a fitness landscape along an adaptive walk. PLoS Comput Biol 10(5):e1003520. https://doi.org/10.1371/journal.pcbi.1003520
Greggio C, Jha P, Kulkarni SS, Lagarrigue S, Broskey NT, Boutant M et al. (2017) Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 25(2):301–311. https://doi.org/10.1016/j.cmet.2016.11.004
Hartfield M, Bataillon T, Glémin S (2017) The evolutionary interplay between adaptation and self-fertilization. Trends Genet 33(6):420–431. https://doi.org/10.1016/j.tig.2017.04.002
Havird JC, Sloan DB (2016) The roles of mutation, selection, and expression in determining relative rates of evolution in mitochondrial versus nuclear genomes. Mol Biol Evol 33(12):3042–3053. https://doi.org/10.1093/molbev/msw185
Havird JC, Hall MD, Dowling DK (2015a) The evolution of sex: a new hypothesis based on mitochondrial mutational erosion. Bioessays 37(9):951–8. https://doi.org/10.1002/bies.201500057
Havird JC, Whitehill NS, Snow CD, Sloan DB (2015b) Conservative and compensatory evolution in oxidative phosphorylation complexes of angiosperms with highly divergent rates of mitochondrial genome evolution. Evolution 69(12):3069–3081. https://doi.org/10.1111/evo.12808
Hill GE (2020) Mitonuclear compensatory coevolution. Trends Genet 36(6):403–414. https://doi.org/10.1016/j.tig.2020.03.002
Hori A, Yoshida M, Shibata T, Ling F (2009) Reactive oxygen species regulate DNA copy number in isolated yeast mitochondria by triggering recombination-mediated replication. Nucleic Acids Res 37(3):749–761. https://doi.org/10.1093/nar/gkn993
Howe DK, Denver DR (2008) Muller’s Ratchet and compensatory mutation in Caenorhabditis briggsae mitochondrial genome evolution. BMC Evol Biol 8(1):62. https://doi.org/10.1186/1471-2148-8-62
Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B et al. (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281(5373):64–71. https://doi.org/10.1126/science.281.5373.64
Jafari G, Wasko BM, Tonge A, Schurman N, Dong C, Li Z et al. (2015) Tether mutations that restore function and suppress pleiotropic phenotypes of the C. elegans ISP-1(qm150) Rieske iron-sulfur protein. Proc Natl Acad Sci USA 112(45):E6148–E6157. https://doi.org/10.1073/pnas.1509416112
JMP Pro Version 18 (2025) JMP Statistical Discovery LLC, Cary, NC
Kayser EB, Morgan PG, Sedensky MM (1999) GAS-1: a mitochondrial protein controls sensitivity to volatile anesthetics in the nematode Caenorhabditis elegans. Anesthesiology 90(2):545–554. https://doi.org/10.1097/00000542-199902000-00031
Kirby CS, Patel MR (2021) Elevated mitochondrial DNA copy number found in ubiquinone-deficient clk-1 mutants is not rescued by ubiquinone precursor 2-4-dihydroxybenzoate. Mitochondrion 58:38–48. https://doi.org/10.1016/j.mito.2021.02.001
Kondrashov FA, Kondrashov AS (2001) Multidimensional epistasis and the disadvantage of sex. Proc Natl Acad Sci USA 98(21):12089–12092. https://doi.org/10.1073/pnas.211214298
Konrad A, Thompson O, Waterston RH, Moerman DG, Keightley PD, Bergthorsson U et al. (2017) Mitochondrial mutation rate, spectrum and heteroplasmy in Caenorhabditis elegans spontaneous mutation accumulation lines of differing population size. Mol Biol Evol 34(6):1319–1334. https://doi.org/10.1093/molbev/msx051
Kryazhimskiy S, Rice DP, Jerison ER, Desai MM (2014) Global epistasis makes adaptation predictable despite sequence-level stochasticity. Science 344(6191):1519–1522. https://doi.org/10.1126/science.1250939
Lemire B (2005) Mitochondrial genetics. WormBook Sep 14:1–10. https://doi.org/10.1895/wormbook.1.25.1
Li Y, Park JS, Deng JH, Bai Y (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr 38(5–6):283–291. https://doi.org/10.1007/s10863-006-9052-z
Long S, Zheng Y, Deng X, Guo J, Xu Z, Scharffetter-Kochanek K et al. (2024) Maintaining mitochondrial DNA copy number mitigates ROS-induced oocyte decline and female reproductive aging. Commun Biol 7(1):1–19. https://doi.org/10.1038/s42003-024-06888-x
Martin G, Lenormand T (2006) A general multivariate extension of Fisher’s geometrical model and the distribution of mutation fitness effects across species. Evolution 60(5):893–907. https://doi.org/10.1111/j.0014-3820.2006.tb01169.x
Martin G, Lenormand T (2015) The fitness effect of mutations across environments: Fisher’s geometrical model with multiple optima. Evolution 69(6):1433–1447. https://doi.org/10.1111/evo.12671
Meiklejohn CD, Montooth KL, Rand DM (2007) Positive and negative selection on the mitochondrial genome. Trends Genet 23(6):259–263. https://doi.org/10.1016/j.tig.2007.03.008
Miller LM, Plenefisch JD, Casson LP, Meyer BJ (1988) xol-1: a gene that controls the male modes of both sex determination and X chromosome dosage compensation in C. elegans. Cell 55(1):167–183. https://doi.org/10.1016/0092-8674(88)90019-0
Muller HJ (1932) Some genetic aspects of sex. Am Nat 66(703):118–138. https://doi.org/10.1086/280418
Neiman M, Taylor DR (2009) The causes of mutation accumulation in mitochondrial genomes. Proc R Soc B Biol Sci 276(1660):1201–1209. https://doi.org/10.1098/rspb.2008.1758
Osada N, Akashi H (2012) Mitochondrial-nuclear interactions and accelerated compensatory evolution: evidence from the primate cytochrome c oxidase complex. Mol Biol Evol 29(1):337–346. https://doi.org/10.1093/molbev/msr211
Otto SP (2009) The evolutionary enigma of sex. Am Nat 174:S1–S14. https://doi.org/10.1086/599084
Paquin F, Cristescu ME, Blier PU, Lemieux H, Dufresne F (2025) Cumulative effects of mutation accumulation on mitochondrial function and fitness. Mitochondrion 80:101976. https://doi.org/10.1016/j.mito.2024.101976
Piccinini G, Iannello M, Puccio G, Plazzi F, Havird JC, Ghiselli F (2021) Mitonuclear coevolution, but not nuclear compensation, drives evolution of OXPHOS complexes in bivalves. Mol Biol Evol 38(6):2597–2614. https://doi.org/10.1093/molbev/msab054
Poon A, Otto SP (2000) Compensating for our load of mutations: freezing the meltdown of small populations. Evolution 54(5):1467–1479. https://doi.org/10.1111/j.0014-3820.2000.tb00693.x
Posit Team (2024) RStudio: integrated development environment for R. Posit Software, PBC, Boston, MA. http://www.posit.co/
R Core Team (2024) R: a language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Radzvilavicius AL, Kokko H, Christie JR (2017) Mitigating mitochondrial genome erosion without recombination. Genetics 207(3):1079–1088. https://doi.org/10.1534/genetics.117.300273
Rand DM, Haney RA, Fry AJ (2004) Cytonuclear coevolution: the genomics of cooperation. Trends Ecol Evol 19(12):645–653. https://doi.org/10.1016/j.tree.2004.10.003
Rawson PD, Burton RS (2002) Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc Natl Acad Sci USA 99(20):12955–12958. https://doi.org/10.1073/pnas.202335899
Rea SL (2005) Metabolism in the Caenorhabditis elegans Mit mutants. Exp Gerontol 40(11):841–849. https://doi.org/10.1016/j.exger.2005.06.015
Rossignol R, Faustin B, Rocher C, Malgat M, Mazat JP, Letellier T (2003) Mitochondrial threshold effects. Biochem J 370(3):751–762. https://doi.org/10.1042/bj20021594
Sackton TB, Haney RA, Rand DM (2003) Cytonuclear coadaptation in Drosophila: disruption of cytochrome c oxidase activity in backcross genotypes. Evolution 57(10):2315–2325. https://doi.org/10.1111/j.0014-3820.2003.tb00243.x
Sequeira AN, O’Keefe IP, Katju V, Bergthorsson U (2024) Friend turned foe: selfish behavior of a spontaneously arising mitochondrial deletion in an experimentally evolved Caenorhabditis elegans population. G3 Bethesda 14(4):jkae018. https://doi.org/10.1093/g3journal/jkae018
Shoubridge EA (2001) Cytochrome c oxidase deficiency. Am J Med Genet 106(1):46–52. https://doi.org/10.1002/ajmg.1378
Suthammarak W, Yang YY, Morgan PG, Sedensky MM (2009) Complex I function is defective in complex IV-deficient Caenorhabditis elegans. J Biol Chem 284(10):6425–6435. https://doi.org/10.1074/jbc.M805733200
Teotónio H, Estes S, Phillips PC, Baer CF (2017) Experimental evolution with Caenorhabditis nematodes. Genetics 206(2):691–716. https://doi.org/10.1534/genetics.115.186288
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777(9):1092–1097. https://doi.org/10.1016/j.bbabio.2008.05.001
Vassilieva LL, Hook AM, Lynch M (2000) The fitness effects of spontaneous mutations in Caenorhabditis elegans. Evolution 54(4):1234–1246. https://doi.org/10.1111/J.0014-3820.2000.tb00557.x
Ventura N, Rea SL, Testi R (2006) Long-lived C. elegans mitochondrial mutants as a model for human mitochondrial-associated diseases. Exp Gerontol 41(10):974–991. https://doi.org/10.1016/j.exger.2006.06.060
Wagner JT, Howe DK, Estes S, Denver DR (2020) Mitochondrial DNA variation and selfish propagation following experimental bottlenecking in two distantly related Caenorhabditis briggsae isolates. Genes 11(1):77. https://doi.org/10.3390/genes11010077
Wang H, Chen H, Han S, Fu Y, Tian Y, Liu Y et al. (2021) Decreased mitochondrial DNA copy number in nerve cells and the hippocampus during nicotine exposure is mediated by autophagy. Ecotoxicol Environ Saf 226:112831. https://doi.org/10.1016/j.ecoenv.2021.112831
Weaver RJ, Rabinowitz S, Thueson K, Havird JC (2022) Genomic signatures of mitonuclear coevolution in mammals. Mol Biol Evol 39(11):msac233. https://doi.org/10.1093/molbev/msac233
Weinreich DM, Watson RA, Chao L (2005) Perspective: sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59(6):1165–1174. https://doi.org/10.1111/j.0014-3820.2005.tb01768.x
Wernick RI, Christy SF, Howe DK, Sullins JA, Ramirez JF, Sare M et al. (2019) Sex and mitonuclear adaptation in experimental Caenorhabditis elegans populations. Genetics 211(3):1045–1058. https://doi.org/10.1534/genetics.119.301935
Wernick RI, Estes S, Howe DK, Denver DR (2016) Paths of heritable mitochondrial DNA mutation and heteroplasmy in reference and gas-1 strains of Caenorhabditis elegans. Front Genet 7. https://doi.org/10.3389/fgene.2016.00051
Whitlock MC, Otto SP (1999) The panda and the phage: compensatory mutations and the persistence of small populations. Trends Ecol Evol 14(8):295–296. https://doi.org/10.1016/S0169-5347(99)01662-6
Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C et al. (2007) ggplot2: Create elegant data visualisations using the grammar of graphics. https://ggplot2.tidyverse.org
Wilke CO (2024) cowplot: streamlined plot theme and plot annotations for “ggplot2”. R package version 4.4.1. CRAN. https://cran.r-project.org/web/packages/cowplot/index.html
Xu S, Chen M, Feng T, Zhan L, Zhou L, Yu G (2021) Use ggbreak to effectively utilize plotting space to deal with large datasets and outliers. Front Genet 12:774846. https://doi.org/10.3389/fgene.2021.774846
Zhang F, Broughton RE (2013) Mitochondrial–nuclear interactions: compensatory evolution or variable functional constraint among vertebrate oxidative phosphorylation genes. Genome Biol Evol 5(10):1781–1791. https://doi.org/10.1093/gbe/evt129
Zhu Z, Han X, Wang Y, Liu W, Lu Y, Xu C et al. (2019) Identification of specific nuclear genetic loci and genes that interact with the mitochondrial genome and contribute to fecundity in Caenorhabditis elegans. Front Genet 10:28. https://doi.org/10.3389/fgene.2019.00028
Acknowledgements
This manuscript benefitted greatly from comments provided by two anonymous reviewers and the Associate Editor. This work was funded by National Science Foundation awards MCB-2232413 to SE, MCB-1817762 to VK and UB, and MCB-1817993 to VK, UB and SE, as well as an American Heart Association predoctoral fellowship (23PRE899291) and Portland State University Forbes-Lea research grant to ZPD. We thank Anne Walraven for providing assistance in the use of the digital PCR machine.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
SE, VK, UB and ZPD were responsible for overall experimental design, with VK and DB leading on design and execution of mitochondrial DNA copy number assays. ZPD led on execution, analysis and interpretation of fitness assays as well as manuscript preparation including creation of figures and tables. JAS, BWB and SFC were primarily responsible for conducting all laboratory experimental evolution with support from ZPD and SE.
Corresponding authors
Ethics declarations
COMPETING INTERESTS
The authors declare no competing interests.
Ethics
The research involved laboratory-evolved lines of C. elegans, a model invertebrate system. The culturing of C. elegans is not associated with any research ethics constraints.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Alper Akay.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dietz, Z.P., Banerji, D., Sullins, J.A. et al. Effects of mating system and adaptedness on the evolution of fitness and mtDNA copy number in mitonuclear mismatched C. elegans. Heredity (2025). https://doi.org/10.1038/s41437-025-00786-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41437-025-00786-6