Abstract
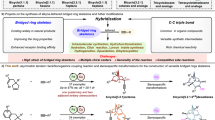
Bridged chiral biaryls are axially chiral compounds with a medium-sized ring connecting the two arenes. Compared with plentiful methods for the enantioselective synthesis of biaryl compounds, synthetic approaches for this subclass of bridged atropisomers are limited. Here we show an atroposelective synthesis of 1,3-diaxial bridged eight-membered terphenyl atropisomers through an Co/SPDO (spirocyclic pyrrolidine oxazoline)-catalyzed aerobic oxidative coupling/desymmetrization reaction of prochiral phenols. This catalytic desymmetric process is enabled by combination of an earth-abundant Co(OAc)2 and a unique SPDO ligand in the presence of DABCO (1,4-diaza[2.2.2]bicyclooctane). An array of diaxial bridged terphenyls embedded in an azocane can be accessed in high yields (up to 99%) with excellent enantio- (>99% ee) and diastereoselectivities (>20:1 dr).
Similar content being viewed by others
Introduction
Biaryl compounds with an axial chirality are valuable architectures that present in bioactive natural products and promising pharmaceuticals1,2,3. The axial frameworks of these biaryl atropisomers also serve as privileged scaffolds of important chiral catalysts and ligands4,5. It is because of the widely recognized functions of these enantioenriched molecules that a prevailing research focus has centered on the atropenantioselective synthesis of axially chiral biaryls6,7,8. Despite recent achievements that continuously enrich the research field, relatively less methodologies are developed for the construction of bridged biaryls, a subclass of these compounds that bear a tether to connect the two arenes and form a medium-sized ring9,10. As such bridged biaryls, especially ones with a 7, 8, or 9 membered aza-ring linkage, are core structures of bioactive agents from either natural or medicinally relevant molecules (Fig. 1a), developing effective catalytic enantioselective approaches for their assembly would be highly desirable. However, compared with other biaryls, construction of axially chiral bridged biaryls would encounter synthetic challenges associated with not only stereogenic formation of aryl–aryl axis but also assembling the medium-sized ring with torsional strain9,10. Simultaneously tackling those geometrical factors in a highly efficient and stereoselective manner would be nontrivial from the aspect of asymmetric catalysis. In this regard, several inspiring strategies have been developed, most involving the construction of the medium-sized ring from an achiral biaryl precursor (Fig. 1b, top left) or through an aromatization reaction with concurrent formation of an axial chirality (Fig. 1b, top right)11,12,13,14,15,16,17,18,19. In comparison, intramolecular coupling of tethered aryls was less studied with only a notable example taking advantage of Pd-catalyzed aryl-aryl coupling (Fig. 1b, bottom left)20,21. Direct oxidative coupling to access axially chiral bridged biaryls, to the best of our knowledge, has not been reported (Fig. 1b, bottom right), and synthesis of such biaryls bearing multiple axial stereochemical elements is also unprecedented22. Compared with a number of studies on the enantioselective formation of a single axis, development of effective methods to access chiral diaxial atropisomers is still in its infancy22,23,24,25,26,27,28,29,30,31,32,33,34, probably due to the added challenges associated with controlling both diastereo- and atropenantioselectivities.
Considering the synthetic challenges of chiral diaxial bridged atropisomers and in continuation with our research interest in the synthesis of axially chiral compounds such as unsymmetrically substituted BINOLs and NOBINs via Cu/SPDO-catalyzed aerobic oxidative cross-coupling reactions35,36, herein, we design and develop a Co/SPDO-catalyzed aerobic oxidative coupling/desymmetrization sequence of prochiral phenols for the enantioselective synthesis of biaxial bridged m-terphenyls embedded in an azocane (Fig. 1c). Notably, despite continued advances of earth-abundant metal-catalyzed oxidative coupling of phenols such as Cu, Co, Cr, Fe, or V37, an asymmetric variant reaction catalyzed by cobalt, to the best of our knowledge, has not yet been achieved before our work38,39.
Results
Reaction conditions optimization
To begin with, readily available dopamine derivative 1a was chosen as a model substrate (Table 1). Since our previous studies have shown that variation of substitution or configuration of the oxazoline of SPDO ligand has a strong influence on its catalytic capability, we started by the preparation of a series of ligands, mainly by varying C4 substitution of oxazoline (L1-L4)35,36,40. Screening of these ligands was carried out under the catalysis of Co(OAc)2 in acetonitrile at −30 °C with air as the terminal oxidant. Delightfully, the desired axially chiral terphenyl product 2a was formed with excellent enantioselectivity after 58–80 h, and the reaction performed with phenyl-substituted ligand L1 would give the best result (entries 1-4). Notably, changing the C4 stereochemistry of oxazoline (L2-L4) would result in the complete reversion of the enantioselectivity, while modification of the pyrrolidine to a lactam would be detrimental to the reaction (entry 5). Cobalt salts were next screened (entries 6-8), and it turns out that inferior results were obtained when other cobalt salts were used either at −30 or 0 °C. Further investigation of solvents revealed acetone to be a better choice with respect to substrate solubility, product yield, and enantioselectivity. Next, we investigated some bases as the additive and found that addition of DABCO can significantly accelerate the reaction rate (12 h) and improve both the diastereo- and enantioselectivity (entry 13). Finally, the yield and enantioselectivity could be further improved when the concentration of the reaction system was diluted using acetone/alcohol (3 mL/1 mL) as a mixed solvent (entry 15).
Scope of sulfonyl substitution on nitrogen
With the above-optimized conditions identified, the scope of triaryl phenols was then explored (Fig. 2). Alternation of the sulfonyl protecting group was well-tolerated, and the reactions could deliver the desired products 2 in good yields with excellent enantioselectivities (92–95% ee) (2a–2f). Notably, the steric hindrance and electronic property of the sulfonyl benzene has a great influence on the diastereoselectivity (5/1–10/1 dr), with bulkier and electron-rich aryl group giving better results (2c–2f).
aReaction conditions: unless otherwise noted, the reactions were conducted with 1 (0.1 mmol), Co(OAc)2 (10 mol%), L1 (12 mol%), and DABCO (40 mol%), in acetone/EtOH (3 mL/1 mL) at −30 °C. Isolated yields. The d.r. was determined by 1H NMR analysis of the crude mixture of products. The ee values were determined by UPC2 or HPLC.
Scope of aryl, heteroaryl, and naphthyl substitution
Next, we examined substrates with diverse substituents on the phenyl group (Fig. 3). A series of mono and polysubstituted phenyls were well-tolerated. The reactions took place smoothly to form the desired products in good yields with excellent dr and ee values regardless of either electron-donating or withdrawing substituent on arene (2g-2w). Interestingly, introduction of heteroatom and bulkier substituent on ortho position of the aryl group significantly improved the diastereoselectivity of the reaction (2g-2n). while strong electron-withdrawing substituents such as nitro-, cyano-, and carbonyl groups (2u, 2w, 2ab) would be detrimental to the diastereoselectivity. In addition, a range of heteroarenes that widely present in functional organic molecules, such as benzofuran, thianthrene, 9-fluorenone, dibenzofuran, and quinoline, were also amenable, affording products in 82–99% yields with 78–96% ee (2x-2ac). It is worth noting that a methyl-substituted substrate (2ad) is also capable of producing the expected product, albeit with only moderate enantioselectivity. The absolute configurations of 2ad and 3a (derivatization from 2a) were assigned by single crystal X-ray diffraction analysis.
aReaction conditions: unless otherwise noted, the reactions were conducted with 1 (0.1 mmol), Co(OAc)2 (10 mol%), L1 (12 mol%), and DABCO (40 mol%) in acetone/EtOH (3 mL/1 mL) at −30 °C. bR6 = Mesitylene-2-sulfonyl. cR6 = Tosyl. Isolated yields. The d.r. was determined by 1H NMR analysis of the crude mixture of products. The ee values were determined by UPC2 or HPLC.
To further demonstrate the generality of the method, we studied triaryls with a naphthyl group (Fig. 3). In general, all substrates gave the desired products in good yields with good to excellent diastereo- and enantioselectivities. Similar to the above results, the introduction of polar functional groups on the ortho position of naphthalene could evidently improve the diastereoselectivity of the reaction (2af, 2ah), while a C4-substituent on naphthyl (2ai-2ak, 2am-2ao) would greatly reduce the diastereoselectivity. Substrates containing acenaphthene, fluoranthene, phenanthrene, and benzo[b]naphtho[1,2-d]thiophene moieties were also assessed, and the corresponding products were obtained with high yields and enantioselectivities (2am-2ap). Finally, for structural diversity, biaryls with a longer tether or a carbon linkage instead of a nitrogen were also prepared and investigated. However, these substrates (1au-1ax) failed to provide the coupling products (please see Supplementary Fig. 16 for details).
Preparation of bridged atropisomers with a single stereogenic axis
Furthermore, a range of bridged eight-membered biaryl atropisomers with a single axial chirality was also interrogated (Fig. 4). Generally, good reaction outcomes were obtained with respect to yields and enantioselectivities. Notably, methyl protection of one of the hydroxyl group of resorcinol or replacement of N-sulfonyl protection with benzyl was allowed. The corresponding products were obtained with similar yields and enantioselectivities (2as, 2at). In addition, swapping the position of resorcinol and catechol group does not affect the viability of substrate. In this case, 2az was obtained in high yield and enantioselectivity.
Synthetic applications
The oxidative coupling of phenol 1a could be carried out in gram-scale under the catalysis of 5% Co(II)/SPDO (Fig. 5). A slight decrease of the product yield and enantioselectivity was observed with maintained diastereoselectivity. Silyl protection of 2a as its TBS ether was also performed to provide compound 3a (please see Supplementary Fig. 17 for details).
Proposed reaction mechanism
On the basis of previous literature reports and some mechanistic studies (please see Supplementary Figs. 12–15 for details), we proposed a possible reaction mechanism as depicted in Fig. 6. Specifically, the catalytic cycle begins with the formation of cobalt(III)/SPDO−superoxide species I39,41,42. Abstraction of a hydrogen atom (HAT) and an ensuing proton-coupled-electron transfer (PCET) process from catechol by cobalt-superoxide complex I generated a highly active o-benzoquinone II and a cobalt(II)/SPDO/H2O2 species39,41, completing the oxidation process in the catalytic cycle. The following base-assisted 8-exo-trig cycloaddition catalyzed by cobalt(II)/SPDO serving as a Lewis acid proceeds from the Re face of the o-benzoquinone that is not shielded by the phenyl group of ligand and thus affords intermediate III with both central and axial chiralities40,43,44. Finally, the desired product 2 was produced after aromatization that renders a chirality transfer from central to axial, completing the catalytic process.
Discussion
In summary, we have successfully developed a novel method for the atroposelective synthesis of unprecedented biaxial bridged eight-membered terphenyl atropisomers through the Co(II)/SPDO-catalyzed aerobic oxidative coupling/desymmetrization sequence of phenols, featuring a broad substrate scope, good yields, excellent stereoselectivities, and environmentally benign conditions. This work also represents the first asymmetric oxidative coupling of phenols with earth-abundant cobalt catalyst. Further applications of this catalytic system in other conversions are currently ongoing in our laboratory.
Methods
General information
All reactions were performed using oven-dried or flame-dried glassware equipped with a magnetic stir bar before used. All reagents were purchased from commercial suppliers and used without further purification. All solvents were purified by standard method. Toluene and tetrahydrofuran were distilled from sodium; dichloromethane (DCM) was distilled from calcium hydride; acetone and ethanol were purchased from commercial suppliers and used without further purification. Thin-layer chromatography was performed with EMD silica gel 60 F254 plates eluting with solvents indicated, visualized by a 254 nm UV lamp and stained with phosphomolybdic acid. 1H NMR, 13C NMR and 19F NMR spectra were obtained on Bruker AM-400, Bruker AM-500. Chemical shifts (δ) were quoted in ppm relative to tetramethylsilane or residual solvent as internal standard CDCl3: 7.26 ppm for 1H NMR, 77.0 ppm for 13C NMR, D4-CD3OD: 3.31 ppm for 1H NMR, 49.00 ppm for 13C NMR; D6-Acetone: 2.05 ppm for 1H NMR, 206.68 ppm and 29.92 ppm for 13C NMR; multiplicities are as indicated: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. High-resolution mass spectral analysis (HRMS) data was measured on a Bruker impact II (Q-TOF) mass spectrum by means of the ESI technique. Crystallographic data were obtained from a Bruker D8 VENTURE diffractometer. Optical rotations were detected on RUDOLPH A21202-J APTV/GW. The enantiomeric excesses (ee) of the products were determined by high-performance liquid chromatography (HPLC) analysis or UPC2.
General procedure for the oxidative coupling/desymmetrization reaction of prochiral phenols
Unless otherwise noted, reactions were performed: Co(OAc)2 (1.7 mg, 10 mol%) and L1 (3.2 mg, 12 mol%) were added to acetone (3.0 mL) at room temperature, and stirred for 10 min. Then DABCO (4.48 mg, 40 mol%) was added and stirred for 1.5 h, then 1 mL EtOH was added to the system. The system was placed in a bath of ethanol at −30 °C 10 min later, and substrates (0.1 mmol) were added followed by stirring the reaction for 5–10 h. The reaction was quenched by addition of 40 μl HCl (2 mol/L in EtOAc), Then the product was purified by flash chromatography (DCM: Acetone = 8:1) on silica gel directly.
Data availability
The data generated in this study are provided in the Supplementary Information file. The experimental procedures, data of NMR, and HRMS have been deposited in Supplementary Information file. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2244054 (2ad) and 2244056 (3a). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.” All other data are available in the main text or the Supplementary Information, or from the corresponding author upon request.
References
Bringmann, G. & Menche, D. Stereoselective total synthesis of axially chiral natural products via biaryl lactones. Acc. Chem. Res. 34, 615–624 (2001).
Kozlowski, M. C., Morgan, B. J. & Linton, E. C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 38, 3193–3207 (2009).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
Zhang, D. & Wang, Q. Palladium catalyzed asymmetric Suzuki–Miyaura coupling reactions to axially chiral biaryl compounds: chiral ligands and recent advances. Coord. Chem. Rev. 286, 1–16 (2015).
Kumar, A., Sasai, H. & Takizawa, S. Atroposelective synthesis of C-C axially chiral compounds via mono- and dinuclear vanadium catalysis. Acc. Chem. Res. 55, 2949–2965 (2022).
Wang, Y. B. & Tan, B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 51, 534–547 (2018).
Cheng, J. K., Xiang, S. H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Cheng, J. K., Xiang, S. H. & Tan, B. Organocatalytic enantioselective synthesis of axially chiral molecules: development of strategies and skeletons. Acc. Chem. Res. 55, 2920–2937 (2022).
Kotwal, N., Tamanna & Chauhan, P. Catalytic asymmetric synthesis of medium-sized bridged biaryls. Chem. Commun. 58, 11031–11044 (2022).
Qu, B., He, L., Shi, J., Lu, L. & Xiao, W. Advances in the construction of axially chiral biaryl-contained medium-sized rings via asymmetric catalysis. Sci. Sin. Chim. 53, 402–409 (2023).
Zhang, S., Chen, F., He, Y. M. & Fan, Q. H. Asymmetric hydrogenation of dibenzo[c,e]azepine derivatives with chiral cationic ruthenium diamine catalysts. Org. Lett. 21, 5538–5541 (2019).
Yang, T., Guo, X., Yin, Q. & Zhang, X. Intramolecular asymmetric reductive amination: synthesis of enantioenriched dibenz[c,e]azepines. Chem. Sci. 10, 2473–2477 (2019).
Lu, S. et al. Diastereo- and atroposelective synthesis of bridged biaryls bearing an eight-membered lactone through an organocatalytic cascade. J. Am. Chem. Soc. 141, 17062–17067 (2019).
Zhang, Y., Liu, Y. Q., Hu, L., Zhang, X. & Yin, Q. Asymmetric reductive amination/ring-closing cascade: direct synthesis of enantioenriched biaryl-bridged NH lactams. Org. Lett. 22, 6479–6483 (2020).
Hu, H. et al. Palladium-catalyzed enantioselective 7-exo-trig carbopalladation/carbonylation: cascade reactions to achieve atropisomeric dibenzo[b,d]azepin-6-ones. Org. Lett. 23, 3636–3640 (2021).
Rodriguez-Salamanca, P. et al. Asymmetric synthesis of dibenzo[b,d]azepines by Cu-catalyzed reductive or borylative cyclization. Chem. Sci. 12, 15291–15297 (2021).
Du, J. Y., Balan, T., Claridge, T. D. W. & Smith, M. D. Counterion-mediated enantioconvergent synthesis of axially chiral medium rings. J. Am. Chem. Soc. 144, 14790–14797 (2022).
Jia, S. et al. Atroposelective construction of nine-membered carbonate-bridged biaryls. Angew. Chem. Int. Ed. 61, e202206501 (2022).
Yang, X. et al. Atroposelective access to 1,3-oxazepine-containing bridged biaryls via carbene-catalyzed desymmetrization of imines. Angew. Chem. Int. Ed. 62, e202211977 (2023).
Saget, T. & Cramer, N. Enantioselective C-H arylation strategy for functionalized dibenzazepinones with quaternary stereocenters. Angew. Chem. Int Ed. 52, 7865–7868 (2013).
Newton, C. G., Braconi, E., Kuziola, J., Wodrich, M. D. & Cramer, N. Axially chiral dibenzazepinones by a Palladium(0)-catalyzed atropo-enantioselective C-H arylation. Angew. Chem. Int Ed. 57, 11040–11044 (2018).
Bao, X., Rodriguez, J. & Bonne, D. Enantioselective synthesis of atropisomers with multiple stereogenic axes. Angew. Chem. Int. Ed. 59, 12623–12634 (2020).
Shibata, T., Fujimoto, T., Yokota, K. & Takagi, K. Iridium complex-catalyzed highly enantio- and diastereoselective [2+2+2] cycloaddition for the synthesis of axially chiral teraryl compounds. J. Am. Chem. Soc. 126, 8382–8383 (2004).
Tan, Y. et al. Enantioselective construction of vicinal diaxial styrenes and multiaxis system via organocatalysis. J. Am. Chem. Soc. 140, 16893–16898 (2018).
Lotter, D., Castrogiovanni, A., Neuburger, M. & Sparr, C. Catalyst-controlled stereodivergent synthesis of atropisomeric multiaxis systems. Acs. Cent. Sci. 4, 656–660 (2018).
Dherbassy, Q., Djukic, J. P., Wencel-Delord, J. & Colobert, F. Two stereoinduction events in one C-H activation step: a route towards terphenyl ligands with two atropisomeric axes. Angew. Chem. Int. Ed. 57, 4668–4672 (2018).
Jia, S., Li, S., Liu, Y., Qin, W. & Yan, H. Enantioselective control of both helical and axial stereogenic elements through an organocatalytic approach. Angew. Chem. Int. Ed. 58, 18496–18501 (2019).
Beleh, O. M., Miller, E., Toste, F. D. & Miller, S. J. Catalytic dynamic kinetic resolutions in tandem to construct two-axis terphenyl atropisomers. J. Am. Chem. Soc. 142, 16461–16470 (2020).
Gao, Q. et al. Catalytic synthesis of atropisomeric o-terphenyls with 1,2-diaxes via axial-to-axial diastereoinduction. J. Am. Chem. Soc. 143, 7253–7260 (2021).
Zhang, P.-C. et al. Simultaneous construction of axial and planar chirality by gold/TY-Phos-catalyzed asymmetric hydroarylation. Nat. Commun. 12, 4609 (2021).
Si, X.-J. et al. Atroposelective isoquinolinone synthesis through cobalt-catalysed C–H activation and annulation. Nat. Synth. 1, 709–718 (2022).
Wang, B. J. et al. Single‐step synthesis of atropisomers with vicinal C−C and C−N diaxes by cobalt‐catalyzed atroposelective C−H annulation. Angew. Chem. Int Ed. 61, e202208912 (2022).
Ye, J. et al. Enantioselective assembly of ferrocenes with axial and planar chiralities via palladium/chiral norbornene cooperative catalysis. JACS Au 3, 384–390 (2023).
Moser, D. & Sparr, C. Synthesis of atropisomeric two-axis systems by the catalyst-controlled syn- and anti-selective arene-forming aldol condensation. Angew. Chem. Int. Ed. 61, e202202548 (2022).
Tian, J. M. et al. Copper-complex-catalyzed asymmetric aerobic oxidative cross-coupling of 2-naphthols: enantioselective synthesis of 3,3’-substituted C(1) -symmetric BINOLs. Angew. Chem. Int. Ed. 58, 11023–11027 (2019).
Zhao, X. J. et al. Enantioselective synthesis of 3,3’-disubstituted 2-amino-2’-hydroxy-1,1’-binaphthyls by copper-catalyzed aerobic oxidative cross-coupling. Angew. Chem. Int. Ed. 60, 7061–7065 (2021).
Bansal, S., Shabade, A. B. & Punji, B. Advances in C(sp2)−H/C(sp2)−H oxidative coupling of (hetero)arenes using 3d transition metal catalysts. Adv. Synth. Catal. 363, 1998–2022 (2021).
Jiang, Q., Sheng, W., Tian, M., Tang, J. & Guo, C. Cobalt(II)-porphyrin-catalyzed aerobic oxidation: oxidative coupling of phenols. Eur. J. Org. Chem. 2013, 1861–1866 (2013).
Reiss, H. et al. Cobalt(II)[salen]-catalyzed selective aerobic oxidative cross-coupling between electron-rich phenols and 2-naphthols. J. Org. Chem. 84, 7950–7960 (2019).
Xi, C. C. et al. Atroposelective synthesis of axially chiral 3-arylindoles by copper-catalyzed asymmetric cross-coupling of indoles with quinones and naphthoquinones. Org. Lett. 22, 4995–5000 (2020).
Anson, C. W., Ghosh, S., Hammes-Schiffer, S. & Stahl, S. S. Co(salophen)-catalyzed aerobic oxidation of p-hydroquinone: mechanism and implications for aerobic oxidation catalysis. J. Am. Chem. Soc. 138, 4186–4193 (2016).
Wang, Y. H. et al. Kinetic and mechanistic characterization of low-overpotential, H2O2-selective reduction of O2 catalyzed by N2O2-ligated cobalt complexes. J. Am. Chem. Soc. 140, 10890–10899 (2018).
Chen, Y. H. et al. Atroposelective synthesis of axially chiral biaryldiols via organocatalytic arylation of 2-naphthols. J. Am. Chem. Soc. 137, 15062–15065 (2015).
Zhu, S. et al. Organocatalytic atroposelective construction of axially chiral arylquinones. Nat. Commun. 10, 4268 (2019).
Acknowledgements
We thank Prof. S.H. Hou (Shanghai Jiao Tong University) and B.M. Yang (Joint School of the National University of Singapore and Tianjin University) for valuable discussions. We thank Instrumental Analysis Center of SJTU for helpful spectral measurements. We acknowledge the NSFC (Nos. 21871117, YQT; 91956203, YQT; 22071147, YQT and 92256303, YQT), the “111” Program of MOE, Beijing National Laboratory for Molecular Sciences (BNLMS202109, YQT) for financial supports.
Author information
Authors and Affiliations
Contributions
The project was conceived and directed by Y.-Q.T., S.-H.W. designed the experiments and analyzed the data. S.-H.W., S.-Q.W., Y.Z., K.L., K.-L.D. and T.-M.D. performed the experiments. S.-H.W., X.-M.Z. and S.-Y.Z. prepared the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, SH., Wei, SQ., Zhang, Y. et al. Atroposelective synthesis of biaxial bridged eight-membered terphenyls via a Co/SPDO-catalyzed aerobic oxidative coupling/desymmetrization of phenols. Nat Commun 15, 4591 (2024). https://doi.org/10.1038/s41467-024-48858-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-48858-1
This article is cited by
-
Chiral medium-sized rings beyond central chirality
Nature Reviews Chemistry (2025)
-
SPA-PNN ligand for the kinetic resolution of carbocyclic and heterocyclic spiranes by asymmetric hydrogenation
Nature Communications (2025)
-
Atroposelective construction of axially chiral alkenylindole-fused nine-membered rings via catalytic asymmetric formal (4 + 5) cycloaddition
Nature Communications (2025)
-
De novo synthesis of atropisomeric benzofurans via Cu/SPDO complex catalyzed asymmetric formal [3 + 2] annulation
Science China Chemistry (2025)
-
Catalytic atroposelective synthesis of indolyl quinazolinones bearing N–N/C–C diaxes
Science China Chemistry (2025)