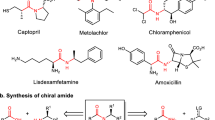
Abstract
Chiral amides are common and effective structural motifs found in many pharmaceuticals and biologically active molecules. Despite their importance, existing synthetic methods are predominantly employed for the synthesis of α-amides and β-amides. The synthesis of remote chiral amides, characterized by distal stereocenters, typically requires intricate synthetic steps conducted under demanding conditions. Here, we present a general procedure for the copper-catalyzed enantioselective synthesis of γ-chiral amides, employing a reductive relay hydroaminocarbonylation strategy with trisubstituted allylic benzoates and hydroxylamine electrophiles. This approach demonstrates a wide substrate scope with excellent enantioselectivity and regioselectivity, thus providing access to challenging enantioenriched γ-chiral amides.
Similar content being viewed by others
Introduction
The field of chemical science includes various areas of study, from the synthesis of new compounds to the investigation of their properties and applications. Among these areas, amides have become significant compounds, attracting the attention of scientists and researchers1,2,3. Amides, owing to their importance in natural products, pharmaceuticals, and materials, serve as essential building blocks in organic chemistry4,5,6,7. Amides stand out as one of the most common functional groups, constituting approximately 40% of a dataset with 420,000 bioactive molecules, as well as constituting two-thirds of the population of drug candidates. Additionally, surveys indicate that amides account for 25% of all pharmaceuticals currently accessible on the market3,8,9,10. Among these active molecules, chiral amides play a crucial role, demonstrating significant importance in the therapeutic intervention of specific diseases. Given the biological activities of stereoisomers may vary11,12, the development of methods for synthesizing amides with high stereochemical purity is particularly valuable.
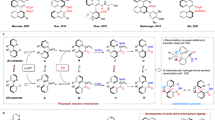
The transition-metal-catalyzed hydroaminocarbonylation of readily available alkenes has emerged as an ideal approach to accessing amides13,14. Over the past few decades, significant advances have been made in ligand-controlled, metal-catalyzed hydroaminocarbonylation of alkenes15. A diverse array of valuable linear or branched amides has been selectively synthesized through Markovnikov or anti-Markovnikov hydroaminocarbonylation processes16,17,18,19,20. Notwithstanding the advancements made, achieving catalytic asymmetric hydroaminocarbonylation of alkenes to synthesize chiral amides with high enantioselectivity remains a major challenge. In 2020, the Guan group reported an effective Pd-catalyzed enantioselective Markovnikov hydroaminocarbonylation of alkenes with anilines by using a phosphoramidite ligand (Fig. 1a)21. A variety of amides bearing an α-stereocenter were obtained straightforwardly in high yields and enantioselectivities. In the same year, our group developed a copper-catalyzed enantioselective hydroaminocarbonylation of styrenes with electrophilic hydroxylamines22,23. The method exhibits broad functional group tolerance and operates under mild conditions, yielding α-chiral amides with high efficiency. Very recently, a general process for the anti-Markovnikov hydroaminocarbonylation of 1,1-disubstituted alkenes to β-chiral amides was realized by utilizing a bulky bidentate phosphine ligand (R)-DTBM-Segphos-based copper catalyst (Fig. 1a)24. While these strategies have been developed for the synthesis of chiral amides, they currently only provide control over the stereocenters at the α or β positions of the incorporated amide bonds. Despite the identification of γ-chiral amide moieties in numerous drugs and biologically active molecules (Fig. 1b), there is currently no documented direct asymmetric carbonylation for the synthesis of γ-chiral amide motifs. Moreover, simultaneously constructing well-defined stereocenters at sites distant from a newly introduced functional group continues to be a formidable challenge for synthetic organic chemists25,26,27,28.
a State-of-the-art of asymmetric hydroaminocarbonylation of alkenes to α- and β-chiral amides. b Selected examples of biologically active molecules and pharmaceuticals featuring γ-chiral amide motifs. c This work: synthesis of γ-chiral amides via Cu-catalyzed reductive relay hydroaminocarbonylation.
In light of the rapid rise of Cu-catalyzed enantioselective hydrofunctionalization and allylic substitution reactions in recent years29,30,31,32,33,34,35,36, we anticipate that a trisubstituted allylic substrate could potentially deliver the amide products with a stereocenter at the γ position. Under our previously reported hydroaminocarbonylation conditions, trisubstituted olefins exhibited general unreactivity due to the unfavorable nature of the insertion reaction involving highly substituted alkenes37,38. Herein, we report an efficient strategy to synthesize enantioenriched γ-chiral amides through the reductive relay process, in which a single cooper catalyst mediates sequential reduction and anti-Markovnikov hydroaminocarbonylation of trisubstituted allylic benzoates (Fig. 1c). This reaction is compatible with achiral allylic benzoates with both aliphatic and aromatic substituents, as well as cyclic and acyclic hydroxylamine electrophiles. The transformation offers a modular and versatile approach towards the synthesis of chiral amides containing minimally differentiated alkyl substituents at the γ-position with excellent enantioselectivity.
Results and discussion
Reaction development and optimization
Our investigation commenced with an exploration of the reaction between geraniol derived alkene 1a and hydroxylamine ester 2a (refer to Table 1 and Supplementary Tables S1 for additional details). After extensive experimentation, we identified that the best conditions were achieved using Cu(OAc)2 (5 mol%), (R)-DTBM-Segphos (7 mol%), PMHS (4 equiv.), and CsF (3 equiv.) in tetrahydrofuran (THF) at 60 °C under 10 bar CO atmosphere. Under these conditions, the reaction afforded γ-chiral amide 3a in 80% isolated yield with 98% enantiomeric excess (Table 1, entry 1). The yield slightly decreased with the use of 1,4-dioxane as solvent, and when 1,2-dichloroethane (DCE) was employed, only trace amounts of desired product 3a were generated (Table 1, entries 2 and 3). Using other copper sources, such as CuCl and CuOAc, did not lead to improved results (Table 1, entries 4 and 5). The use of LiOtBu instead of CsF resulted in trace amounts of 3a, with the majority of 1a being recovered (Table 1, entry 6). No or <10% yield of desired product was observed when replacing (R)-DTBM-Segphos (L1) with L2-L839,40 as ligands (Table 1, entries 7 and 8). The utilization of alternative electrophilic aminating reagents bearing a benzoyl group (2a-1) or a 4-(N,N-dimethylamino)benzoyl group (2a-2) resulted in moderate yields of 3a (Table 1, entries 9 and 10).
Substrate scope
A variety of leaving groups were examined, revealing that the reactivity is influenced by the nature of the leaving group but does not correlate with enantioselectivity. (Fig. 2). Reactions involving substrates 1 with alkoxy(1a-3) and silyloxy (1a-4) led to very low conversion, predominantly resulting in the recovery of 1a-3 and 1a-4. Improved yield of 3a with 96% ee was achieved when using carbonate 1a-6. Product yields ranging from 19% to 73% were obtained with a high level of enantioselectivity by employing carboxylates with different substituents (1a-5, 1a-7-11).
As shown in Fig. 3, under our optimal conditions, we explored the scope of allylic benzoates that could undergo the transformation. Various 3,3-dialkyl substituted allylic benzoates were initially tested. The opposite enantiomer 3a’ can be obtained in 77% yield with −99% ee when using (S)-DTBM-Segphos instead of (R)-DTBM-Segphos as the ligand. Substrates derived from (E,E)-Farnesol, Solanesol, Phytol, and Monocyclofarnesol all furnished the corresponding γ-chiral amides in moderate to good yields and exceptionally high enantioselectivities (3b-3d, 3r). Diverse functional groups were readily accommodated, including aryl groups (3e,3f,3j), ethers (3k, 3m, 3n), an alkyl chloride (3l), esters (3p, 3q). The presence of bulky groups at the 3-position of the allylic substrates was well-tolerated, resulting in the formation of the desired γ-chiral amides 3h and 3i with yields of 68% with 99% ee and 53% with 99% ee, respectively. In the presence of a substrate containing an aldehyde, the reduction product 3o was obtained in 42% yield with 99% ee, as expected. The substrate containing an achiral epoxide functional group was also tolerated, affording a mixture of diastereomers (1:1) in 69% yield (3s), both diastereomers exhibited high enantioselectivities (99% ee). Furthermore, this reductive relay hydroaminocarbonylation was successfully extended to include allylic benzoates with 3-aryl substitution. Substrates with both electron-rich aryl substituent (3u) and electron-poor aryl substituents (3v-3w) were effectively tolerated under the catalytic system. Notably, the reaction conditions were also compatible with both a 2-naphthyl-substituted substrate (3x) and a 3-thienyl-substituted substrate (3y).
Reactions conditions: 1 (0.36 mmol), 2 (0.2 mmol), Cu(OAc)2 (5 mol%), (R)-DTBM-Segphos (7 mol%), CsF (3 equiv.), PMHS (4 equiv.), CO (10 bar), 60 °C and THF (1.0 mL). Isolated yields, ees were determined by chiral HPLC, and drs were determined by crude 1H NMR or chiral HPLC. aUse (S)-DTBM-Segphos (7 mol%) as the ligand. bFrom aldehyde 1o. cMorpholino 4-(diethylamino)benzoate as the substrate.
The versatility of this catalytic system was further demonstrated by investigating various hydroxylamine electrophiles (Fig. 4). Cyclic hydroxylamine derivatives such as piperidine (4a), morpholine (4b), protected piperidone (4c), piperazine containing a carbamate protecting group (4d), and tetrahydroisoquinoline (4e) were introduced into the reaction, resulting in the desired γ-chiral amides in 51%-87% yields with 84%-99% ees. Additionally, acyclic dialkyl-substituted hydroxylamine electrophiles (4f–4i) proved to be suitable partners, providing the corresponding products in good yields and excellent enantioselectivities as well. Aniline-based hydroxylamine derivatives were also compatible substrates, generating products 4j and 4j–1 in 51%-61% yields with high enantioselectivities. Moreover, to showcase potential late-stage functionalization, hydroxylamines derived from drug fragments or natural products were introduced into this reductive relay hydroaminocarbonylation reaction, leading to their successful transformation into the corresponding drugs containing γ-chiral amide groups (4k–4o’). Employing 1aa and 1ab as the substrates also afforded liner amides 4q and 4r in 69%-78% yields.
Reactions conditions: 1a (0.36 mmol), 2 (0.2 mmol), Cu(OAc)2 (5 mol%), (R)-DTBM-Segphos (7 mol%), CsF (3 equiv.), PMHS (4 equiv.), CO (10 bar), 60 °C and THF (1.0 mL). Isolated yields, ees were determined by chiral HPLC, and drs were determined by crude 1H NMR or chiral HPLC. aUse corresponding O-benzoylhydroxylamine as the electrophile. bUse corresponding O-pivaloylhydroxylamine as the electrophile. cUse (S)-DTBM-Segphos (7 mol%) as the ligand.
Synthetic utility
To gain insight into the effects of substrate structure on reaction activity and enantioselectivity, we conducted reactions using substrates that vary in the geometry of the allylic benzoates. (Fig. 5a). When the isomeric nerol-derived benzoate (Z-1a), containing a (Z)-configured allylic double bond, was subjected to the standard conditions, the enantiomer with the opposite configuration 3a′ was obtained with a dramatically decreased yield and slightly lower enantioselectivity compared to 3a. Then, isomeric allylic benzoate 1z, with an E/Z ratio of 7/3, was exposed to the reaction under optimized conditions, the product 3z was obtained 41% yield with 95% ee, while 42% of the remaining starting material 1z was recovered with an E/Z ratio of 4.5/5.5. Gratifyingly, the reaction could be performed on 1 mmol scale with a 2.5 mol% catalyst loading, delivering the product 3a in good yield (72%, 191 mg) with almost complete enantioselectivity (Fig. 5b). Subsequently, the γ-chiral amides can undergo further reduction using Nickel catalysis41, resulting in δ-chiral amides with high yields (Fig. 5c). Unfortunately, the ee values for compounds 8 and 9 could not be determined under the current chiral HPLC conditions in our laboratory.
Mechanistic investigations
To elucidate the mechanism underlying the reductive relay hydroaminocarbonylation, a reaction employing 1.5 equivalents of PMHS with 1i was conducted, yielding terminal alkene 7 in 81% yield with 98% ee (Fig. 6a). A deuterium-labelling experiment, utilizing Ph2SiD2 with 1i, resulted in the isolation of alkene 7-D with 74% yield and 70% deuterium incorporation at the α position of the C-C double bond. Subsequently, the terminal alkene 7 was reacted with 2a under hydroaminocarbonylation conditions using racemic DTBM-Segphos. The γ-chiral amide product 3i was obtained in a 59% yield with >99% ee (Fig. 6b). These results indicate that the terminal alkene 7 serves as a competent intermediate, and the enantioselectivity of the product (γ-chiral amide) is established in the initial reduction cycle, unrelated to the hydroaminocarbonylation cycle. In the non-linear effect experiments, a linear relationship was observed, suggesting that one ligand may coordinate with one copper atom to form the active catalyst (Fig. 6c).
Based on the experimental results and literature reports42,43,44,45,46, we tentatively propose a plausible reaction mechanism depicted in Fig. 6d. Initially, copper salts coordinate with the ligand, and subsequent interaction with the base and hydrosilane leads to the formation of the starting LCuH species. The following step involves LCuH species reacting with allylic benzoate to generate alkylcopper intermediate I. This intermediate I then undergoes β-OBz elimination, resulting in the transient formation of enantioenriched terminal alkene II and ligated LCuOBz species. The terminal alkene II enters the hydroaminocarbonylation cycle through an anti-Markovnikov hydrocupration, transforming into alkylcopper species III. The interception of alkylcopper species III by the hydroxylamine electrophile and CO leads to the formation of intermediate IV. Finally, reductive elimination occurs to deliver the desired γ-chiral amide and ligated LCuOR’. Both LCuOBz species and LCuOR’ could undergo transmetallation with hydrosilane to regenerate LCuH species for the next catalytic cycle.
Discussion
In summary, we have reported an enantioselective Cu-catalyzed reductive relay hydroaminocarbonylation for the synthesis of optically active γ-chiral amides from allylic benzoates, CO, and electrophilic aminating reagents. Employing the Cu/(R)-DTBM-Segphos catalytic system facilitates the challenging establishment of chiral centers at a remote distance from the amide bond, specifically at the γ position. Notably, this method also showcases a broad substrate scope with excellent enantioselectivity and regioselectivity, providing access to valuable enantioenriched γ-chiral amides that are often challenging to obtain using alternative approaches.
Methods
General procedure for the carbonylation-triggered migration
A vial (4 mL) was charged with (R)-DTBM-Segphos (7.0 mol%), Cu(OAc)2 (5.0 mol%), 2 (0.2 mmol, 1.0 equiv), CsF (91 mg, 3 equiv.), and a stirring bar. The vial was closed by PTFE/white rubber septum (Wheaton 13 mm Septa) and phenolic cap and connected with atmosphere with a needle. The vial was evacuated under vacuum and recharged with argon for three times. Then, THF (1.0 mL) was injected under argon by using a syringe. After that PMHS (0.8 mmol, 4 equiv.) and allylic benzoate 1 (0.36 mmol, 1.8 equiv.) were added, and the vial (or several vials) was placed in an alloy plate, which was transferred into a 300 mL autoclave of the 4560 series from Parr Instruments. After flushing the autoclave three times with CO, a pressure of 10 bar of CO was adjusted at ambient temperature. Then, the reaction was performed for 16 h at 60 oC. After the reaction was complete, the autoclave was cooled down with ice water to room temperature, and the pressure was released carefully. The reaction was diluted with EA (ethyl acetate) and filtered through a pad of silica gel (a pipette with about 3 cm silica gel). The filtrate was concentrated under reduced pressure and the residue was directly purified by column chromatography to afford the corresponding products. The enantiomeric excesses (% ee) were determined by HPLC analysis using chiral stationary phases. Note: Because of the high toxicity of carbon monoxide, all the reactions should be performed in an autoclave. The laboratory should be well-equipped with a CO detector and alarm system.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information or from the authors upon request.
References
Larock, R. C. Comprehensive organic transformations (Wiley-VCH, 1999).
Benz, G. Synthesis of amides and related compounds: comprehensive organic synthesis (Pergamon, 1991).
Lundberg, H., Tinnis, F., Selander, N. & Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 43, 2714–2742 (2014).
Humphrey, J. M. & Chamberlin, A. R. Chemical synthesis of natural product peptides: coupling methods for the incorporation of noncoded amino acids into peptides. Chem. Rev. 97, 2243–2266 (1997).
Bray, B. L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. 2, 587–593 (2003).
Ge, Y. et al. Nat. Synth https://doi.org/10.1038/s44160-023-00411-6 (2023).
de Figueiredo, R. M., Suppo, J.-S. & Campagne, J.-M. Nonclassical routes for amide bond formation. Chem. Rev. 116, 12029–12122 (2016).
Ghose, A. K., Viswanadhan, V. N. & Wendoloski, J. J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1, 55–68 (1999).
Carey, J. S., Laffan, D., Thomson, C. & Williams, M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 4, 2337–2347 (2006).
Ertl, P., Altmann, E. & McKenna, J. M. The most common functional groups in bioactive molecules and how their popularity has evolved over time. J. Med. Chem. 63, 8408–8418 (2020).
Evidente, A., Cimmino, A. & Andolfi, A. The effect of stereochemistry on the biological activity of natural phytotoxins, fungicides, insecticides and herbicides. Chirality 25, 59–78 (2013).
Kumar, R., Hassan, M. & Pahuja, K. Effects of stereoisomers on drug activity. Am. J. Biomed. Sci. Res 13, 220–222 (2021).
Cai, S., Zhang, H. & Huang, H. Transition-metal-catalyzed Hydroaminocarbonylations of alkenes and alkynes. Trends Chem. 3, 218–230 (2021).
Wu, X.-F., Han, B., Ding, K., Liu Z. eds. The chemical transformations of C1 compounds (Wiley-VCH Weinheim) (2022).
Wu, X.-F. et al. Transition-metal-catalyzed carbonylation reactions of Olefins and Alkynes: A personal account. Acc. Chem. Res. 47, 1041–1053 (2014).
Jiménez-Rodriguez, C. et al. Selective formation of α,ω-ester amides from the aminocarbonylation of castor oil derived methyl 10-undecenoate and other unsaturated substrates. Catal. Sci. Technol. 4, 2332–2339 (2014).
Dong, K. et al. Rh(I)-catalyzed hydroamidation of Olefins via selective activation of N–H bonds. Aliphatic Amines J. Am. Chem. Soc. 137, 6053–6058 (2015).
Zhang, G., Gao, B. & Huang, H. Palladium-catalyzed hydroaminocarbonylation of alkenes with amines: a strategy to overcome the basicity barrier imparted by aliphatic amines. Angew. Chem. Int. Ed. 54, 7657–7661 (2015).
Gao, B., Zhang, G., Zhou, X. & Huang, H. Palladium-catalyzed regiodivergent hydroaminocarbonylation of alkenes to primary amides with ammonium chloride. Chem. Sci. 9, 380–386 (2018).
Yang, H.-Y., Yao, Y.-H., Chen, M., Ren, Z.-H. & Guan, Z.-H. Palladium-catalyzed Markovnikov Hydroaminocarbonylation of 1,1-Disubstituted and 1,1,2-Trisubstituted Alkenes for formation of amides. Quat. Carbon J. Am. Chem. Soc. 143, 7298–7305 (2021).
Yao, Y.-H. et al. Asymmetric Markovnikov Hydroaminocarbonylation of alkenes enabled by palladium-monodentate phosphoramidite catalysis. J. Am. Chem. Soc. 143, 85–91 (2021).
Yuan, Y. et al. Copper-catalyzed carbonylative hydroamidation of styrenes to branched amides. Angew. Chem. Int. Ed. 59, 22441–22445 (2020).
Yuan, Y., Zhao, F. & Wu, X.-F. Copper-catalyzed enantioselective carbonylation toward α-chiral secondary amides. Chem. Sci. 12, 12676–12681 (2021).
Yuan, Y., Zhang, Y., Li, W., Zhao, Y. & Wu, X.-F. Regioselective and Enantioselective copper-catalyzed hydroaminocarbonylation of unactivated alkenes and alkynes. Angew. Chem. Int. Ed. e202309993 (2023)
Mei, T.-S., Patel, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014).
Wang, Z.-X., Bai, X.-Y., Yao, H.-C. & Li, B.-J. Synthesis of amides with remote stereocenters by catalytic asymmetric γ-Alkynylation of α,β-unsaturated amides. J. Am. Chem. Soc. 138, 14872–14875 (2016).
Zhao, W., Chen, K.-Z., Li, A.-Z. & Li, B.-J. Remote stereocenter through amide-directed, rhodium-catalyzed enantioselective hydroboration of unactivated. Intern. Alkenes. J. Am. Chem. Soc. 144, 13071–13078 (2022).
Zultanski, S. L. & Fu, G. C. Catalytic asymmetric γ-Alkylation of carbonyl compounds via stereoconvergent Suzuki cross-couplings. J. Am. Chem. Soc. 133, 15362–15364 (2011).
Yorimitsu, H. & Oshima, K. Recent progress in asymmetric allylic substitutions catalyzed by chiral copper complexes. Angew. Chem. Int. Ed. 44, 4435–4439 (2005).
Ito, H., Ito, S., Sasaki, Y., Matsuura, K. & Sawamura, M. Copper-catalyzed enantioselective substitution of allylic carbonates with Diboron: An efficient route to optically active α-Chiral Allylboronates. J. Am. Chem. Soc. 129, 14856–14857 (2007).
Guzman-Martinez, A. & Hoveyda, A. H. Enantioselective synthesis of allylboronates bearing a tertiary or quaternary B-substituted stereogenic carbon by NHC-Cu-catalyzed substitution reactions. J. Am. Chem. Soc. 132, 10634–10637 (2010).
Ito, H., Kunii, S. & Sawamura, M. Direct enantio-convergent transformation of racemic substrates without racemization or symmetrization. Nat. Chem. 2, 972–976 (2010).
Zhu, S., Niljianskul, N. & Buchwald, S. L. A direct approach to amines with remote stereocentres by enantioselective CuH-catalysed reductive relay hydroamination. Nat. Chem. 8, 144–150 (2016).
Han, J. T., Lee, J. Y. & Yun, J. Asymmetric synthesis of γ-chiral borylalkanes via sequential reduction/hydroboration using a single copper catalyst. Chem. Sci. 11, 8961–8965 (2020).
Ito, H., Kosaka, Y., Nonoyama, K., Sasaki, Y. & Sawamura, M. Synthesis of optically active boron–silicon bifunctional cyclopropane derivatives through enantioselective Copper(I)-catalyzed reaction of allylic carbonates with a diboron derivative. Angew. Chem. Int. Ed. 47, 7424–7427 (2008).
Jia, T. et al. A Cu/Pd cooperative catalysis for enantioselective allylboration alkenes. J. Am. Chem. Soc. 137, 13760–13763 (2015).
Pirnot, M. T., Wang, Y.-M. & Buchwald, S. L. Copper hydride catalyzed hydroamination of alkenes and alkynes. Angew. Chem. Int. Ed. 55, 48–57 (2016).
Liu, R. Y. & Buchwald, S. L. CuH-Catalyzed Olefin functionalization: from hydroamination to carbonyl addition. Acc. Chem. Res. 53, 1229–1243 (2020).
Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019).
Wang, L.-L. et al. A general copper-catalysed enantioconvergent radical Michaelis–Becker-type C(sp3)–P cross-coupling. Nat. Synth. 2, 430–438 (2023).
Simmons, B. J., Hoffmann, M., Hwang, J., Jackl, M. K. & Garg, N. K. Nickel-catalyzed reduction of secondary and tertiary amides. Org. Lett. 19, 1910–1913 (2017).
Tsuda, T., Hashimoto, T. & Saegusa, T. Cuprous tert-butoxide. New and useful metalation reagent. J. Am. Chem. Soc. 94, 658–659 (1972).
Deutsch, C. & Krause, N. CuH-catalyzed reactions. Chem. Rev. 108, 2916–2927 (2008).
Noh, D., Chea, H., Ju, J. & Yun, J. Highly Regio- and enantioselective copper-catalyzed hydroboration of styrenes. Angew. Chem. Int. Ed. 48, 6062–6064 (2009).
Miki, Y., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew. Chem. Int. Ed. 52, 10830–10834 (2013).
Zhu, S., Niljianskul, N. & Buchwald, S. L. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 135, 15746–15749 (2013).
Acknowledgements
We appreciate the financial support provided by the National Key R&D Program of China (2023YFA1507500), the National Natural Science Foundation of China (22302198), the China Postdoctoral Science Foundation (2022M723087), the International Partnership Program of the Chinese Academy of Sciences (Grant No.028GJHZ2023045FN).
Author information
Authors and Affiliations
Contributions
Y. Y. designed and carried out the reactions and analyzed the data. Y. Z. carried out the reactions and provided raw material support. Y. Y. and Y. Z. contributed equally to this work. X.-F. W designed and supervised the project. Y. Y. wrote the manuscript and X.-F. W revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bi-Jie Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, Y., Zhang, Y. & Wu, XF. Enantioselective synthesis of γ-chiral amides via copper-catalyzed reductive relay hydroaminocarbonylation. Nat Commun 15, 6705 (2024). https://doi.org/10.1038/s41467-024-51048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51048-8