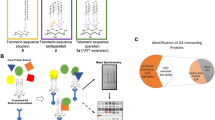
Abstract
G-quadruplex (G4) structures are found in eukaryotic cell replication origins, but their role in origin function remains unclear. In this study G4 motifs are found in the lytic DNA replication origin (oriLyt) of human cytomegalovirus (HCMV) and recombinant viruses show that a G4 motif in oriLyt essential region I (ER-I) is necessary for viral growth. Replication assays of oriLyt-containing plasmids and biochemical/biophysical analyses show that G4 formation in ER-I is crucial for viral DNA replication. G4 pull-down analysis identifies viral DNA replication factors, such as IE2, UL84, and UL44, as G4-binding proteins. In enzyme-linked immunosorbent assays, specific G4-binding ligands inhibit G4 binding by the viral proteins. The Epstein-Barr virus oriLyt core element also forms a stable G4 that could substitute for the oriLyt ER-I G4 in HCMV. These results demonstrate that viral G4s in replication origins represent an essential structural element in recruiting replication factors and might be a therapeutic target against viral infections.
Similar content being viewed by others
Introduction
G-quadruplex (G4) is formed by repetitive G-rich sequences in single-stranded DNA or RNA. In a G4, four guanine bases are connected by a Hoogsteen base pair to form a G-tetrad, and multiple G-tetrads can stack on top of each other. G4s are stabilized by monovalent or divalent cations1. G4s are involved in diverse cellular processes such as transcription, translation, DNA replication, recombination, and genome instability2,3. During DNA replication, G4s can interfere with the progression of replication forks if not adequately resolved, for example, by helicases. However, evidence has emerged that G4s can play a positive role in initiating DNA replication4,5. Genome-wide mapping studies revealed that most metazoan replication origins contain G-rich sequences containing G4-forming sequences (G4 motifs) of the replication initiation sites6,7,8,9. Indeed, G4s in chicken replication origins promoted the associated origin activity10. Recently, it has been suggested that human replication origins are divided into two categories: those positively regulated by G4 formation and those activated by transcription without G411. The mechanisms by which G4s activate replication origin have yet to be clarified.
Human cytomegalovirus (HCMV) belongs to the beta-herpesvirus subfamily and contains a large double-stranded DNA genome. Primary infection of HCMV in healthy people usually causes persistent or latent infection. However, reactivation of the virus in immunocompromised individuals, such as AIDS patients and bone marrow or organ transplant patients, causes several diseases, such as pneumonia, hepatitis, and retinitis and, in some cases, death. HCMV is also the most frequent causative virus of congenital infection in fetuses and can lead to neurologic disorders12. DNA replication of HCMV is a very complicated process involving several viral proteins. During lytic HCMV infection, viral DNA replication begins with one replication origin (called oriLyt) located in the genome’s unique long (UL) region13. In transient transfection replication assays, which measure replication of oriLyt-containing plasmids in transfected cells, 11 viral genetic loci are needed for efficient oriLyt-dependent DNA replication14. These include genes encoding six core proteins acting on DNA replication forks: UL54 (DNA polymerase), UL44 (polymerase process factor), UL57 (single-stranded DNA-binding protein), UL105 (helicase), UL70 (primase), and UL102 (primase-associated factor), which are well preserved in the genome of all herpesviruses15,16. The others are UL36-38, IRS1/TRS1, UL122 (IE2-p86)/123 (IE1-p72), UL84, and UL112-11314,17,18. Although UL36-38, IRS1/TRS1, IE1, and UL112-113 are not essential for oriLyt plasmid replication, IE2 and UL84, which interact with each other, act on oriLyt directly19 and play an essential role in oriLyt-dependent DNA replication, probably by acting as initiator proteins or origin binding proteins20,21.
HCMV oriLyt is about 2 kb long and has two essential regions, ER-I and II22. ER-I contains the oriLyt promoter, the smallest replicator transcript (SRT) region, and the pyrimidine-rich sequence called “Y-block,” while ER-II contains RNA4.9 promoter, the vRNA-2 transcript region, a DNA/RNA hybrid site, and an RNA stem-loop region that binds UL8419,23. HCMV oriLyt also contains several transcription control elements. The C/EBPα/β binding sites within oriLyt are essential for oriLyt-dependent DNA replication24. hnRNP K also promotes DNA replication in transient-transfection replication assays by interacting with UL84 and associating with oriLyt25. The oriLyt promoter is a bi-directional promoter containing IE2-binding sites, and its activation is regulated by UL84 and IE220. SRT is a small non-polyadenylated transcript whose transcription is thought to be controlled by cellular and viral transcription factors26. Since oriLyt-dependent DNA replication is inhibited without activation of oriLyt or SRT promoters22, it is thought that the transcripts in oriLyt may regulate replication. UL84 is not essential for the replication of some HCMV strains (such as TB40E and TR) containing a single amino acid change in IE2, highlighting the importance of the functional interaction between IE2 and UL84 in initiating DNA replication27,28.
In this study, we investigated whether the G4 motifs found in HCMV oriLyt form a G4 structure that regulates oriLyt activity. By analyzing recombinant viruses and performing replication assays of oriLyt-containing plasmids and several biochemical/biophysical analyses, we show that the G4 formation in HCMV oriLyt ER-I plays a crucial role in viral DNA replication by recruiting viral replication factors, and that G4-binding ligands can inhibit G4 binding of viral factors. Furthermore, we show that G4 formation is conserved in the oriLyt core element essential for replication of Epstein-Barr virus (EBV), a gamma-herpesvirus. Our study demonstrates that viral G4 structures formed in lytic DNA replication origins are essential for origin activity and can be a therapeutic target to control viral infections.
Results
Identification of G4 motifs in HCMV oriLyt
In silico prediction of the G4 motifs in the entire HCMV genome indicated an average of one motif per kb genome29. We found several G4 motifs in the HCMV oriLyt region and studied their role in the oriLyt function. G4s can be divided into conventional, long-loop, and bulged categories based on the schema used for prediction30 (see Methods). HCMV oriLyt contained six non-overlapping sequences for conventional G4s and several additional sequences for long-loop or bulged G4s. Since conventional G4s are generally more stable than the others31, we focused on the motifs for conventional G4s (oriG4-1 and oriG4-3 to −7). oriG4-1 was found in the “Y-block” in ER-I, while oriG4-3 to −7 were found in ER-II (Fig. 1a; Supplementary Fig. 1). We also included oriG4-2 in ER-II for analysis as a control since this G4 motif was previously shown to form a bulged G4 that can regulate RNA4.9 promoter activity29. The G4 motif showed relatively high G-scores ranging from 38 to 61, suggesting high potential for G4 formation (Fig. 1a).
a Schematic diagram of HCMV Toledo (Genbank GU937742.1) oriLyt and the locations of the G4 motifs tested. The positions of ER-I and ER-II; oriLyt promoter; and open-reading frames for SRT, vRNA-2, and RNA4.9 are indicated. The location of Y-block; the binding sites for C/EBPα, C/EBPβ, and IE2; the regions including RNA-DNA hybrid and stem-loop RNA (SL-RNA) structures; and restriction sites are also indicated. Nucleotide (nt) numbers shown are with reference to the Toledo strain DNA sequence. The sequences for oriG4-1 to −7 and their G-scores are indicated. b CD spectra of wild-type and mutant oriG4 ODNs. Each spectrum was an average of three scans in the wavelength range between 220 and 320 nm. The spectra were blanked with buffer only. c HF cells transfected with bacmids via electroporation at 2 weeks after transfection. The scale bar corresponds to 500 μm. d HF cells were infected with recombinant HCMV [wild-type (Wt) or revertant of oriG4-1m (oriG4-1m-R)] at an MOI of 2 for 5 days (n = 3 biological replicates). Virus titers in the culture supernatants were determined by infectious center assays. The data are shown as the mean ± SD. e Scheme of transient transfection replication assays. HF cells were co-transfected with plasmids containing the HCMV oriLyt (wild-type or mutant) and plasmids expressing six core replication proteins and three auxiliary proteins. At 5 days, total cellular DNAs were isolated and digested with DpnI, and the amount of newly replicated oriLyt plasmid DNA was determined by qPCR. f Comparison of the copy numbers of oriLyt plasmids in HF cells co-transfected with wild-type or oriG4-1m oriLyt plasmids in transient transfection replication assays. In the control (Cont.), the wild-type oriLyt plasmid was used and the IE2 and UL84 plasmids were not included. The data obtained from three biological replicates are shown as the mean ± SD. Statistical significance is indicated by p < 0.001 (***). Source data are provided as a Source Data file.
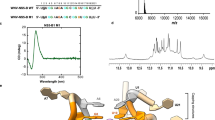
Circular dichroism (CD) analysis of oligodeoxynucleotides (ODNs) corresponding to oriG4-1 to −7 and their mutant sequences, in which two to six G-to-T or G-to-A changes were introduced in G-runs to disrupt G4 formation (Supplementary Table 1), suggested that oriG4-1, 2, 3, 5, 6, and 7 form parallel G4s, which displayed a prominent peak at ~265 nm and a trough at ~240 nm in the presence of 100 mM KCl. In contrast, oriG4-4 forms an anti-parallel G4 with a peak at ~290 nm and a trough at ~260 under the same conditions32,33,34 (Fig. 1b). The CD spectra of all mutant sequences were significantly changed from those of wild-type sequences (Fig. 1b).
The G4 motif in oriLyt ER-I is necessary for viral DNA replication
To investigate whether the G4 motifs in oriLyt regulate viral growth, we produced recombinant viruses with mutations in G4 motifs using HCMV bacmid mutagenesis (Supplementary Fig. 2a; Supplementary Tables 2 and 3). When the mutant bacmids were introduced into human fibroblast (HF) cells, the oriG4-1m virus did not grow (Fig. 1c), whereas its revertant virus (oriG4-1m-R) grew like the wild-type virus (Fig. 1d). The oriG4-2m, -3m, and -5m viruses showed slightly increased viral growth, while the oriG4-4m and -7m viruses showed reduced viral growth (Supplementary Fig. 2b). Therefore, among the G4 motifs tested, that in oriLyt ER-I was essential for viral growth in cultured fibroblast cells. In transient transfection replication assays, the replication of oriLyt plasmids was markedly suppressed by mutations in oriG4-1 in HF (Fig. 1e, f) and HeLa cells (Supplementary Fig. 2c), demonstrating that the G4 motif in ER-I is necessary for oriLyt-dependent DNA replication. The replication levels of the wild-type and oriG4-1m plasmids were not affected by SRT expression in trans (Supplementary Fig. 2d), indicating that a significant decrease in replication efficiency of the oriG4-1m plasmid is not due to the loss of an unknown role of SRT.
Evidence for G4 formation in oriG4-1
We investigated G4 formation in oriG4-1 using several biophysical and biochemical assays. CD spectra of a parallel G4 structure were observed at concentrations as low as 5 μM ODNs or 5 mM KCl (Supplementary Fig. 3a). NMM is a specific ligand binding to a parallel G435 (Supplementary Fig. 3b) and produces characteristic fluorescence upon G4 binding36. NMM displayed its characteristic fluorescence with the G4 from oriG4-1 under KCl buffer conditions (where G4 structures are favored) but not under LiCl buffer conditions (where G4s are not formed), supporting the formation of a G4 structure in oriG4-1 in vitro (Fig. 2a).
a The preformed oriG4-1, oriG4-1m, or poly-A ODNs were incubated with NMM (4 μM) in 100 mM KCl or LiCl for 20 min, and the NMM-specific fluorescence was measured. b oriG4-1 and oriG4-1m ODNs (15 μM) were annealed in K+ buffer and subjected to native PAGE analysis (15%) at room temperature. c–e oriG4-1 and oriG4-1m ODNs (15 μM) were annealed in 100 mM K+ buffer and subjected to native PAGE as in (b). The oriG4-1 and oriG4-1m bands (a–c) on native PAGE were eluted, and their purity was shown (c). The eluted ODNs (2 μM) were incubated with NMM, and their fluorescence was shown. Poly-A was used as a control (d). TDS of the eluted (a–c) bands and poly-A (15 µM) in 10 mM Tris-HCl pH 7.5 and 100 mM KCl (e). f Double-stranded DNA fragments (122-bp) containing wild-type or mutant oriG4-1 (oriG4-1 and oriG4-1m) sequences (top) were PCR-amplified from oriLyt-containing plasmids using the primer set (LMV2909/2910) (Supplementary Table 3). The PCR fragments were denatured by heating and annealed in the presence of Li or K ions, followed by native PAGE (12%) analysis (bottom) at 4 °C. g The denatured and annealed double-stranded DNA in (f) was incubated with increasing amounts (0.15 and 0.5 μM) of BG4 antibody or control IgG and subjected to native PAGE (8%) analysis. A super-shifted DNA band is indicated (arrowhead). A representative result of at least three independent experiments is shown in (b, c, f, g). h, i HF cells were mock-infected or infected with HCMV at an MOI of 1 for 72 h, and G4 ChIP assays were performed with BG4 antibody or with control IgG (n = 3 biological replicates). The ChIP samples were subjected to PCR with primer sets that amplify four distinct areas (A, B, C, and D) in oriLyt (h) and the remote region in RL11 as a control. The fold enrichment of PCR products (over the PCR products with IgG in mock infection) is shown in (i). The data are shown as the mean ± SD. Source data are provided as a Source Data file.
In native polyacrylamide gel electrophoresis (PAGE), G4-structured oriG4-1 ODNs migrated faster than unstructured mutant ODNs (oriG4-1m), suggesting the formation of intramolecular G4s, and also showed slowly migrating ladders that may represent intermolecular G4s (Fig. 2b; Supplementary Fig. 3c). When eluted from the gel, G4 structures from oriG4-1 (a and b in Fig. 2c) but not control DNA from oriG4-1m (c in Fig. 2c) showed G4-bound NMM fluorescence (Fig. 2d). The eluted intramolecular G4s from ori-G4-1 also showed a characteristic thermal difference spectra (TDS) with a negative peak at ~295 nm and positive peaks at ~240 and ~270 nm37 (Fig. 2e). These native PAGE, CD, and TDS analyses demonstrated that oriG4-1 forms an intramolecular G4 structure.
To investigate G4 formation within a duplex DNA, the oriG4-1-containing double-stranded DNA fragments (122 or 322-bp) were PCR-amplified from the oriLyt (wild-type or oriG4-1m)-containing plasmids, heat denatured, and then annealed in solutions containing LiCl or KCl under molecular crowding conditions containing 40% (w/v) polyethylene glycol (PEG) 200, and G4 formation was analyzed by native PAGE38. PEG 200 is a widely used crowding agent to mimic intracellular conditions and stabilizes G4 formation with less other effects, such as viscosity increase and excluded volume effect, compared to large-sized PEGs39. Only wild-type fragments showed a slowly migrating DNA band with KCl but not with LiCl at 4 °C or room temperature (Fig. 2f; Supplementary Fig. 3d, e), suggesting that the slowly migrating bands contain a G4 structure. In the absence of PEG 200, this slowly migrating band was not produced (Supplementary Fig. 3f). Indeed, treatment with BG4, a G4-specific antibody, resulted in a band shift, but control IgG did not (Fig. 2g). These results showed that G4 can be produced in the oriG4-1-containing oriLyt region when the oriG4-1 sequence is exposed on single-stranded DNA.
We also evaluated G4 formation in HCMV-infected cells. When virus-infected cells were stained with G4-specific 1H6 antibody, G4s were detected in the nuclei of uninfected and in IE1-positive virus-infected cells at 24 h (Supplementary Fig. 4). At 72 h after infection, G4s were enriched in viral replication compartments (vRCs) within the nucleus, where viral UL112-113 replication proteins also accumulated (Supplementary Fig. 4, arrows). This G4 enrichment in vRCs was also observed when cells were stained with BG4 antibody and were not observed after DNase treatment (Supplementary Fig. 5). These results indicated that G4s are formed in replicating viral DNAs. Furthermore, in G4 chromatin immunoprecipitation (ChIP) assays with BG4, G4s were formed in the oriLyt region but not in the control RL11 region (Fig. 2h, i). These results with G4-specific antibodies demonstrated that G4s are formed in replicating viral DNA and oriLyt during HCMV infection.
HCMV DNA replication factors interact with oriLyt ER-I G4
We investigated whether viral or cellular proteins bind to oriLyt ER-I G4 by performing G4 pull-down assays and spectrometry analysis (Fig. 3a). Six viral proteins, UL84, UL44, UL89, UL122 (IE2), US22, and UL148, and 14 cellular proteins were identified to bind more effectively to G4-structured ODNs than unstructured ODNs in two independent experiments (Table 1). IE2, UL84, and UL44 are directly involved in HCMV DNA replication. Therefore, their association with ER-I G4 was further studied. For G4 pull-down assays, single-stranded RNA ODNs corresponding to the LANA mRNA G4 motif mutant sequence40 were used as an RNA control. The complementary C-rich sequences of G4 motifs often form a distinct structure called an i-motif41,42,43. The complementary sequence of oriG4-1 (oriG4-1C) formed an i-motif at pH 4.2 to 7.0 (Supplementary Fig. 6). Therefore, oriG4-1C and the complementary sequences of oriG4-1m (oriG4-1Cm) were also used. When HCMV-infected cell lysates were pulled down and the binding proteins were determined by immunoblotting, IE2 (p86), UL84, and UL44 (p52 and smaller isoforms) effectively bound to the G4 (oriG4-1) but not to single-stranded DNA (oriG4-1m) or RNA (ssRNA). They also did not bind to the i-motif (oriG4-1C), although UL84 weakly associated with oriG4-1Cm (Fig. 3b, c). As controls, IE1-p72 did not bind to any sequences, and hnRNP K bound to the i-motif and its mutant single-stranded sequence but not to the G4 or its single-stranded mutant sequence (Fig. 3b, c).
a Scheme for the pull-down assay to identify viral and cellular proteins binding to ER-I G4 (see Methods). b, c HF cells were infected with HCMV at an MOI of 1. Cell lysates were prepared at 120 h and used for pull-down assays. Biotinylated ODNs (0.5 μg), pre-incubated to form G4 or i-motif structures (see the Methods), were incubated with 50 μg of cell lysates and immobilized on streptavidin beads. After washing, the proteins were eluted and analyzed by immunoblotting with specific antibodies for hnRNP K, IE1/IE2, p52 (encoded by UL44), and UL84. Input (1/10), 5 μg of cell lysates. (-), no biotinylated ODNs added (b). The relative binding efficiency of proteins [input (1/10)%] to different ODNs in three independent experiments is also indicated in (c). The data are shown as the mean ± SD. Source data are provided as a Source Data file.
To investigate whether IE2, UL84, and UL44 directly bind to G4, proteins of IE2(87-580), UL84, and UL44(1-299) with a 6xHis tag were prepared in bacterial cells (Fig. 4a) and were used in pull-down assays. These proteins bound to ER-I G4 but not to single-stranded DNA or RNA, and these interactions were stronger than those with the well-studied G4 found in the cMyc promoter (Fig. 4b). These results demonstrated that IE2, UL84, and UL44 independently bind to ER-I G4 with higher affinities than those toward cMyc-G4. In addition, IE2, UL84, and UL44 bound to both intra- and intermolecular G4s (Fig. 4c), although it is unlikely that intermolecular G4s are formed in oriLyt in the context of viral replication in cells.
a 6xHis-tagged proteins (500 ng) (Coomassie brilliant blue staining) purified from E. coli. b Proteins were incubated with biotinylated ODNs pre-incubated to form structures. The bound proteins were determined by immunoblotting with specific antibodies. His-UL44(1-290), which lacked epitopes for the antibody used, was detected by Coomassie brilliant blue. Input (1/10), 50 ng of purified hnRNP K, IE2(87-579), and UL84 and 100 ng of UL44(1-290). (-), no ODNs. The relative binding efficiency of proteins to ODNs is indicated as graphs (n = 3). c Inter and intramolecular G4 structures from oriG4-1 (in Fig. 2) were incubated with viral proteins, and the bound proteins were determined by immunoblotting or Coomassie brilliant blue staining as in (b). d Biotinylated ODNs (17 μM), which were pre-incubated to form a G4 (oriG4-1 and ori-G4-1m) or duplex DNA (ds oriG4-1m and ds crs), were incubated with 150 μg of cell lysates and immobilized on streptavidin beads. The proteins bound were analyzed by immunoblotting with anti-IE1/IE2 antibody. Input (1/30), 5 μg of cell lysates. (-), no biotinylated ODNs. The binding efficiency of proteins to ODNs is indicated (n = 3). e UL84-6xHis (500 ng) was incubated with biotinylated ODNs (500 ng) that were pre-incubated to form a G4 (oriG4-1 and oriG4-1m) or a stem-loop (SL DNA and SL RNA) structure or duplex DNA (ds oriG4-1m). The bound proteins were determined by immunoblotting. Input (1/10), 50 ng of UL84-6xHis. (-), no ODNs. The binding efficiency of proteins to ODNs is indicated (n = 3). HF cells co-transfected as in Fig. 1e, f were subjected to ChIP assays with anti-UL84 (f) or anti-IE1/IE2 (g) antibodies or with control IgG (n = 3 biological replicates). The ChIP samples were subjected to PCR to amplify ER-I (the A region in Fig. 2h). The fold enrichment of PCR products (over that with IgG) is shown. h HF cells were mock-infected or infected with HCMV at an MOI of 1 for 3 days, followed by ChIP assays as in (f and g). The data are shown as the mean ± SD. Statistical significance is indicated by p < 0.05(*), <0.01 (**), and <0.001 (***). Source data are provided as a Source Data file.
IE2 binds to the 14-bp double-stranded DNA element known as the cis-repression signal (crs) in the major immediate-early promoter to downregulate its transcription44,45,46. When the abilities of IE2 to bind to ER-I G4 and the crs duplex were compared, IE2 more effectively bound to the G4 than did the crs duplex DNA (Fig. 4d). UL84 has been reported to bind to a stem-loop RNA (SL-RNA) structure found in ER-II in oriLyt23. However, under conditions showing G4 binding, no binding of UL84 to SL-RNA or SL-DNA was detected, demonstrating that the binding of UL84 to G4 in ER-I is much stronger than its binding to SL-RNA in ER-II (Fig. 4e).
Binding of IE2 and UL84 to ER-I in oriLyt-containing plasmids was further examined by ChIP assays. The newly replicated oriLyt-containing plasmid DNAs were prepared by co-transfection replication reactions (as in Fig. 1e), followed by ChIP assays with control IgG or anti-UL84 or anti-IE1/IE2 antibodies. The qPCR assays showed that the ER-I-containing DNA fragments were effectively immunoprecipitated with anti-IE1/IE2 or anti-UL84 antibodies but not with control IgG, indicating that IE2 and UL84 interact with ER-I of oriLyt in cells (Fig. 4f, g). The association of UL44 with oriLyt ER-I could not be tested using the anti-UL44 antibody we used. In similar ChIP assays using virus-infected cells, UL84 and IE2 bound to the oriG4-1 region in ER-I (Fig. 4h).
Inhibition of oriLyt ER-I G4 binding of UL84 and IE2 by G4 ligands
To study the binding affinity of IE2, UL44, and UL84 to ER-I G4, an enzyme-linked immunosorbent assay (ELISA) was established in which G4-structured 3′-biotin-labeled ODNs were immobilized on streptavidin-coated plates. Then, the specific binding of viral proteins to G4 was measured by an enzyme linked to a specific antibody. We found that UL84 and UL44 effectively bound to G4 with dissociation constant (Kd) values of 14.17 nM and 25.46, respectively, whereas IE2 bound to G4 with a Kd of 123.1 nM (Fig. 5a). To obtain information about G4 recognition by the viral proteins, we produced mutant oriG4-1 sequences (L1 to L5) that retain the minimal four G-runs necessary for G4 formation but contain the G-to-A substitutions at different positions in G-runs or TGA-to-AAT substitution at the center of G4 motif and used for G4 pull-down assays (Fig. 5b, c). We found that the TGA-to-AAT substitution at the center of the G4 motif (in L2) did not affect the G4 structure in CD and NMM fluorescence analyses and the binding of viral proteins in G4 pull-down assays. The G-to-A substitutions in G-runs (in L1 and L3) slightly reduced the propensity to form a parallel G4 in CD and NMM fluorescence analyses. However, they still did not significantly affect the binding to viral proteins. The G-to-A substitutions in both L1 and L3 resulted in changes in G4 topology from parallel G4 to a mixture of parallel and anti-parallel G4s (in L4 and L5) and showed a reduced binding by all viral proteins. These results showed that the number of consecutive G residues in oriG4-1 is important for forming parallel G4 and that UL84, UL44, and IE2 bind to parallel G4.
a 6xHis-tagged HCMV proteins were incubated with biotinylated ODNs (5 nM) pre-incubated to form a structure and bound to streptavidin-coated plates. The G4-binding affinity of HCMV proteins was determined by ELISA (error bars display standard deviation, N = 3). b CD spectra and NMM fluorescence (under KCl conditions) of wild-type and mutant oriG4-1 sequences were measured as in Figs. 1b and 2a, respectively. c G4 structures of oriG4-1 sequences in Fig. 5b were incubated with purified viral proteins, and the bound UL84, UL44, and IE2 proteins were eluted and determined by immunoblotting with anti-UL84, anti-His, anti-IE2 antibodies, respectively. d The G4-binding affinity of UL84, UL44, and IE2 was determined by ELISA (error bars display standard deviation, N = 3) as in (a) in the presence of increasing amounts of G4 ligands. The relative amounts of G4 bound proteins at different concentrations are shown as graphs. The relatively low IC50 values (less than 5 μM) of G4 ligands on G4 binding by UL84, UL44, and IE2 are also shown. e Co-transfection replication assays were performed in HF cells as in Fig. 1f with or without treatment of G4 ligands (5 μM). f, g Co-transfection replication assays and ChIP assays were performed in HF cells with wild-type or mutant oriLyt plasmid and TMPyP4 (5 μM). The data obtained from three biological replicates are shown as the mean ± SD. Statistical significance is indicated by p < 0.05(*) and <0.001 (***). Source data are provided as a Source Data file.
G4s can be binding sites for transcriptional factors, and G4-binding ligands can compete with transcription factors at G4 binding sites47. Treatment with G-quartet-binding ligands, such as NMM, TMPyP4, BRACO19, and PDS, did not affect G4 topology formed by oriG4-1 ODNs (Supplementary Fig. 7a). When the CD thermal melting curves were determined, ER-I G4 was stable even at high temperature, and treatment with NMM, BRACO19, and PDS slightly increased the melting temperature (Tm), indicating that the ODNs form a G4 structure that can be targeted by G4-binding ligands (Supplementary Fig. 7b). In ELISA, TMPyP4, BRICO19, and PDS inhibited the G4 binding by UL84, showing inhibitory concentrations (IC50) of 1.33, 1.38, and 0.30 μM, respectively. For UL44 and IE2, TMPyP4 effectively inhibited G4 binding with an IC50 of 0.03 and 4.31 μM, but NMM, BRACO19, and PDS did not exhibit a robust inhibitory effect (Fig. 5d). A G4 groove binder Peimine did not inhibit the G4 binding by UL84, UL44, and IE2 (Fig. 5d). These results suggest that G-quartet binding may be more important than groove binding in recognition of ER-I G4 by UL84, UL44, and IE2.
Next, we determined the effect of G4 ligands on the replication of oriLyt plasmids using co-transfection replication assays. TMPyP4, BRACO19, and PDS effectively suppressed the replication of oriLyt plasmids, whereas NMM only marginally did so, demonstrating that the oriLyt activation during HCMV infection can be regulated by specific G4-binding ligands (Fig. 5e). Furthermore, the oriLyt containing L4 mutations was less effectively replicated in co-transfection replication assays and showed reduced UL84 and IE2 binding in ChIP assays; on the contrary, the oriLyt containing L1 mutations showed a similar replication efficacy and UL84 and IE2 binding as the wild-type oriLyt plasmid (Fig. 5f, g). These results showed that the G4 formation at oriG4-1 well correlates with the binding by viral replication factors and the replication level of the oriLyt plasmid.
G4 formation in Epstein-Barr virus oriLyt
Epstein-Barr virus (EBV) uses two replication origins: oriP during latent infection and oriLyt for lytic infection. The oriLyt spans about 7.7 kb, including the promoters of BHLF1 and BHRF1 genes, and the oriLyt core consists of upstream and downstream components48. The 40-bp TD sequence in the downstream components is essential for oriLyt function49,50. We found that the TD sequence contains a G4 motif that is similar to that of oriG4-1 (Fig. 6a). CD analysis with wild-type and mutant TD ODNs showed parallel G4 formation by the TD sequence (Fig. 6b). The NMM treatment did not change the topology of TD-G4 or the melting curve, suggesting a stable structure (Fig. 6c). TD-G4 also showed the characteristic fluorescence under KCl buffer conditions but not under LiCl conditions, where the pattern was similar to the NMM fluorescence pattern of cMyc-G4 (Fig. 6d). In native PAGE, G4-structured TD ODNs showed migration patterns of both intra- and intermolecular G4s (Fig. 6e). Replacement of oriG4-1 with TD-G4, but not with TD-G4m, in HCMV oriLyt-containing plasmid allowed replication of the plasmid in co-transfection replication assays (Fig. 6f). Consistent with this, UL84, IE2, and UL44 interacted with TD-G4 in pull-down assays (Fig. 6g). Therefore, the TD sequence in EBV oriLyt forms a stable G4 that can replace oriG4-1 for HCMV oriLyt activity.
a Schematic diagram of EBV oriLyt. The G4 motifs of HCMV oriG4-1 and EBV TD-G4 are compared. b Sequences of wild-type TD-G4 and its mutant (TD-G4m) ODNs and their CD spectra (15 μM). c CD spectra of TD-G4 ODNs annealed in 10 mM Tris-HCl (pH 7.5) and 100 mM KCl buffer with DW or 30 μM NMM (DNA to chemical ratio 1:2) are shown on the left. CD thermal melting curve analysis of TD-G4 with or without NMM. d Preformed wild-type or mutant TD-G4 (TD-G4 or TD-G4m) or poly-A ODNs were incubated with NMM (4 μM) in 100 mM KCl or LiCl for 20 min, and the NMM-specific fluorescence was measured. The NMM fluorescence of cMyc-G4 was measured under 100 mM KCl or LiCl conditions. The ODN sequence used for cMyc-G4 is shown. e TD-G4 and TD-G4m ODNs (5 to 15 μM) were annealed in 100 mM K+ buffer and subjected to native PAGE (15%) analysis. f Co-transfection replication assays were performed in HF cells with the HCMV oriLyt plasmids containing wild-type or mutant oriG4-1 (oriG4-1 or oriG4-1m) or TD-G4 (TD-G4 or TD-G4m) (n = 3 biological replicates). The data are shown as the mean ± SD. Statistical significance is indicated by p < 0.05(*) and <0.001 (***). g Pull-down assays of purified IE2(87-579), UL84, and UL44(1-290) were performed with preformed G4s (oriG4-1, TD-G4, or TD-G4m). The bound IE2 and UL84 were identified by immunoblotting with specific antibodies, and the bound UL44 was detected by Coomassie brilliant blue staining. Input (1/5) is 25 ng of purified proteins. (-), no biotinylated ODNs were added. A representative result of at least three independent experiments is shown in (e, g). Source data are provided as a Source Data file.
Discussion
In this study, we show that G4s are formed in several motifs in ER-I and ER-II of the HCMV DNA replication origin, oriLyt, and that the G4 formed in ER-I plays a critical role in viral growth by promoting DNA replication. We also show that ER-I G4 is a recognition site for viral replication proteins UL84, IE2, and UL44. Importantly, our results show that viral replication initiators recognize the G4 formed in a viral replication origin. As HCMV replication initiators, UL84 and IE2 associated with oriLyt. However, the mechanism by which they recognize replication origin was unclear. By performing several G4 binding assays in vitro and in virus-infected cells, we demonstrate that UL84 and IE2 directly bind to the ER-I G4. G4 has been suggested to play a positive role in initiating DNA replication by aiding helix opening through preventing nucleosome formation51 or recruiting specific replication factors. Supporting the latter, G4s are targeted by the origin recognition complex (ORC) that determines the initiation sites of DNA replication52 or the MDM two binding protein (MTBP) that is necessary for assembly of the CDC45-MCM-GINS complex53,54. Our results support the hypothesis that G4 is a recognition site for specific replication initiators.
The G4 motif found in ER-I was previously recognized as a “Y-block” whose sequence was essential for replication of oriLyt-containing plasmid in co-transfection replication assays26. However, the involvement of the sequence in DNA replication was unclear. Our study showed that the G4 motif in Y-block forms a stable G4 structure that recruits viral replication initiators. Similar sequences are preserved in simian and murine CMV replication origins and in the oriLyt of gammaherpesviruses EBV and herpesvirus papio55. Of note, this conserved element within the “downstream component” in EBV oriLyt was also essential for origin functions in co-transfection replication assays49,50. We also found in CD and thermal melting curve analysis that the G4 motif within the downstream component in EBV oriLyt forms a stable G4. Therefore, the role of G4 appears to be conserved in at least some beta- and gammaherpesvirus replication origins.
For G4 formation in oriLyt ER-I, the G4 motif sequence needs to be present on single-stranded DNA. G4 formation is favored by transient opening of the double helix during transcription. The G4 motif in ER-I exists on the sense strand of the SRT open reading frame (ORF). Therefore, active transcription of SRT appears to provide an environment favorable for G4 formation in ER-I. Furthermore, SRT transcription has been reported to terminate immediately after the G4 motif26. Therefore, RNA G4 formed in SRT may affect the termination of SRT transcription. Interestingly, the expression of SRT was necessary for replicating the oriLyt-containing plasmid in co-transection replication assays22. This further supports that active transcription of SRT promotes G4 formation in ER-I. However, other SRT-specific functions not yet known in DNA replication cannot be excluded.
The G4 binding assays with purified viral proteins showed that UL84 most strongly bound to ER-I G4, followed by UL44 and then IE2. Their G4 binding does not appear to be confined to the virus genome. These viral proteins interacted with the well-known G4 structure in the cMyc promoter, although their affinity to cMyc G4 was weaker than that to ER-I G4. IE2 binds to a duplex DNA sequence known as crs, and the ER-I G4 binding strength of IE2 was 2.4-fold stronger than that of crs. A study showed that UL84 bound to a stem-and-loop (S-L) RNA sequence found in oriLyt ER-II23. In our assays, the RNA S-L sequence binding of UL84 was not clear; even if there is binding, UL84 binds to G4 much more strongly than to S-L RNA. Although the crystal structure of UL44 has been solved, the structures of IE2 and UL84 are unknown. When we examined the highly reliable regions of the AlphaFold-predicted structures of IE2 and UL84 and the structure of UL44, we could not identify a region with notable structural similarities. Bovine and Drosophila DHX36 structures bound to the cMyc G4 have been reported. In these structures, the N-terminal DNA-binding-induced alpha-helix and the oligonucleotide and oligosaccharide-binding (OB)-fold-like domain participate in binding parallel G4. Notably, the N-terminal alpha-helical structure of DHX36 contains non-polar amino acids that contact the non-canonical G-quartet56,57. However, no sequences in the viral proteins were found to be homologous to this DHX36 alpha-helix region. If the viral proteins similarly interact with the G4 to DHX36, both sequence differences of viral proteins involved in the G-quartet binding and the formation of a positively charged pocket for the G4 entry may contribute to the differences in Kd values.
Although the G4 motifs in oriLyt ER-II were not essential for viral growth, further research is needed to determine whether G4 binding by virus replication factors in ER-II directly affects DNA replication. Interestingly, the G4 motifs observed in ER-II exist in the ORF of RNA 4.9, which encodes a non-coding RNA that plays a role in viral replication58. Notably, four of the G4 formed in ER-II are present on the template strand of the RNA4.9 ORF. We have recently shown that G4s in the template strand of the ORF14 gene of Varicella-Zoster virus can suppress the transcription of ORF1459. Therefore, ER-II G4s may indirectly affect viral replication by regulating RNA4.9 transcription.
We showed that some G4 ligands can inhibit the binding of UL84, UL44, and IE2 to ER-I G4. Our analyses with mutant oriG4-1 sequences and different G4 ligands showed that the viral proteins bind to parallel G4 and that G-quartet binding may be more important than groove binding for all IE2, UL84, and UL44. Our results demonstrate that the G4 ligands can be developed as an anti-HCMV drug. In our previous study, treatment of G4 ligands effectively inhibited the activation of viral promoters containing G4 motifs, suppressing the production of progeny virions29. The results of the present study show that specific G4 ligands can inhibit the initiation of DNA replication. The G4 ligand can also affect the G4s formed in the host genome. However, the use of G4 ligands can be a good antiviral strategy, considering that the number of viral genomes in virus-infected cells is much higher than the number in host genomes. Notably, CX-5461, a G4 ligand in phase I clinical trials for patients with BRCA1/2-deficient tumors60, efficiently inhibits HCMV growth61.
G4s on the viral genome are detrimental to the movement of the DNA replication machinery and should be resolved for successful DNA replication. In this regard, notable cellular proteins bound to ER-I G4 are G-rich sequence factor 1 (GRSF1) and DHX9. GRSF1 binds to G4 and unfolds it62, and several DEAD or DEAH-box helicases such as DHX36, Pif1, and FANCJ resolve G4 structures63,64. RecQ1 helicase binds to the lytic replication origin of Kaposi’s sarcoma-associated herpesvirus (KSHV)65. Recently, it has been reported that G4s are formed in the KSHV lytic replication origin and that mutations impairing G4 formation reduce RecQ1 binding and viral DNA replication66. It would be intriguing to examine whether GRSF1 and DHX9 play a role in HCMV DNA replication.
Methods
Cell culture, virus, and electroporation
Primary HF cells (American Type Culture Collection; ATCC PCS-201-010) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 µg/mL) in a 5% CO2 humidified incubator at 37 °C. The recombinant HCMV (Toledo strain) was prepared from the bacmid as previously described67. HF cells were transiently transfected via electroporation at 1300 V for 40 ms using a Microporator MP-100 (Digital Bio) according to the manufacturer’s instructions.
Infectious center assays
Virus titers were determined by infectious center assays. HF cells (1 × 105) were seeded into 24-well plates and incubated for 24 h before infection. Ten-fold serial diluted viral stocks (200 μL) were added to each well and incubated for 1 h. The inoculum was removed, and fresh complete medium (2 mL) was added. After 24 h incubation, cells were fixed with 500 µL of methanol at 4 °C for 10 min. Cells were washed with 500 µL cold phosphate-buffered saline (PBS) three times and incubated with 200 µL anti-IE1 rabbit polyclonal antibody (PAb) in PBS at 37 °C for 1 h, followed by incubation with phosphatase-labeled anti-rabbit immunoglobulin (IgG) in PBS at 37 °C for 1 h. Finally, the cells were treated with 200 µL of AP buffer (100 mM Tris-HCl, 100 mM NaCl, and 5 mM MgCl2) mixed with 5-bromo-4-chloro-3-indolyl phosphatase/nitro blue tetrazolium (BCIP/NBT, Millipore) at a 1:1 ratio. The IE1-positive cells were counted in five fields per well under a light microscope (200X).
Chemicals
NMM (N-methyl mesoporphyrin IX) and PDS (Pyridostatin) were purchased from Santa Cruz Biotechnology and Cayman Chemical, respectively. TMPyP4 (meso-5,10,15,20-Tetrakis-(N-methyl-4-pyridyl)porphine, Tetratosylate) and BRACO19 (N,N′-(9-(4-(Dimethylamino)phenylamino)acridine-3,6-diyl)bis(3-(pyrrolidin-1-yl)propanamide)) were purchased from GLPBIO. Peimine was purchased from MedChemExpress (MCE).
Prediction of G4 motifs in oriLyt
The oriLyt region in the HCMV Toledo genome (GU937742.1) was previously mined for potential G4 motifs using the Quadparser program29,68. Three types of G4s were considered for the prediction. Conventional G4s were predicted using the schema G3-6N1−7G3-6N1-7G3-6N1-7G3-6, and these represent the conventional three-stack G4s. Long-loop G4s were restricted to a single long loop and were predicted using the schema G3-6N8-50G3-6N1-2G3-6N1-2G3-6 (and two other combinations of different loops), while bulged G4s were predicted using the schema G3-6N1-7G3-6N1-7G3-6N1-7G2(A/T/C)G (and three other combinations for the remaining three G runs).
CD spectroscopy and thermal melting curve analysis
ODNs used for CD spectroscopy are described in Supplementary Table 1. For CD spectroscopy, the ODNs were dissolved at a concentration of 15 μM in a buffer containing 10 mM Tris-HCl (pH 7.5, room temperature) and 100 mM KCl, followed by denaturation at 95 °C for 5 min and annealing at room temperature overnight. CD spectra were obtained using Jasco J-810 spectroscopy with a Peltier temperature controller. For studies with G4-binding ligands, preformed G4s were treated with 30 μM G4 ligands for a DNA-to-ligand ratio of 1:2. CD spectra were measured at 25 °C as the average of three accumulations between 220 and 320 nm, with a response time of 1 s, scanning speed of 100 nm/min, and data pitch of 1 nm. CD melting curves were recorded between 25 and 100 °C at a wavelength of 262 nm for all nucleotides. After subtracting the buffer spectrum from all samples, the data were normalized to the maximum ellipticity. The first derivative of the melting curve was plotted and fit using existing functions in Sigma-Plot 12.5.
Thermal difference spectra (TDS)
The UV spectra were measured on a Jasco V-750 UV spectrophotometer fitted with a Peltier temperature controller (ETCS-761, Jasco, Japan). The ODNs were dissolved at a concentration of 15 μM in a buffer containing 10 mM Tris-HCl (pH 7.5, room temperature) and 100 mM KCl, followed by denaturation at 95 °C for 5 min and annealing at room temperature for 2 h. The UV spectra were measured using a 1-mm-path length quartz cuvette (Hellma, Germany) at 20 °C and 90 °C, following which the spectra were blanked using buffer-only spectra. Finally, the TDS was measured as the difference between absorbance at 20 °C and 90 °C. The data were normalized to the maximum TDS value.
Bacmid mutagenesis
The Toledo-bacmid that contains mutations in oriG4 sequences was generated using a bacterial artificial chromosome (BAC) modification kit (Gene Bridges). Briefly, the rpsL-neo cassettes flanked by homology arms (Hm) with 100 nucleotides of the regions upstream and downstream of the target site were amplified using primer sets (for example, LMV2666 and LMV2667 for oriG4-1 mutation; Supplementary Table 2). The amplified rpsL-neo fragments were purified and introduced into E. coli DH10B containing wild-type Toledo-bacmids for recombination via electroporation using a Gene Pulser II (Bio-Rad). The intermediate Toledo-bacmid construct containing the rpsL-neo cassette was selected on Luria Bertani (LB) agar plates containing kanamycin. Next, the fragments containing oriG4 mutations for replacing the rpsL-neo cassette were generated by annealing two single-stranded ODNs (for example, LMV2688 and LMV2669 for oriG4-1m; Supplementary Table 2). The annealed ODNs were recombined into the Toledo-bacmid DNAs containing the rpsL-neo cassette, and E. coli cells containing the oriG4 mutant Toledo-bacmid were selected on LB plates containing streptomycin. The mutated regions were amplified by PCR with primer sets (LMV2776/2777 for oriG4-1; LMV2058/2059 for oriG4-2, 3, 4, and 5; LMV3082/3085 for oriG4-6; LMV2060/2000 for oriG4-7; Supplementary Table 3) and sequenced to verify the desired mutations. To generate the revertant Toledo-bacmid from the mutant containing oriG4-1m, the rpsL-neo cassette was inserted into the target site to generate the intermediate bacmid, and then the wild-type G4 fragments generated by annealing single-stranded ODNs (for example, LMV2761 and LMV2762 for oriG4-1; Supplementary Table 2) were inserted by homologous recombination as described above.
Plasmids
The plasmid (pSP38) harboring HCMV replication-origin DNA and plasmids expressing the six replication core proteins (UL54, UL44, UL57, UL105, UL70, and UL102) and auxiliary proteins (IE2 and UL84) have been previously described 21, 69. A two-step PCR approach constructed the pSP38 with the oriG4-1 mutant sequence. In the first PCR step, head and tail sequences were amplified from the pSP38 plasmid by the primer sets LMV2831/LMV2834 and LMV2833/LMV2832, respectively. In the second PCR step, the first head and tail PCR products were mixed and then used as a template for PCR amplification by the primer set LMV2831/LMV2832. The final PCR product was used to replace the AsiSI-NotI fragment in pSP38. A similar two-step PCR approach constructed the pSP38 with the TD-G4 sequence. In the first PCR step, head and tail sequences were amplified from the pSP38 plasmid by the primer sets LMV2831/LMV3172 and LMV3171/LMV2832, respectively. The second PCR step and the following cloning step were described above. The pSP38 plasmid with the L1 or L4 sequence was constructed by site-directed mutagenesis PCR. pSP38 was PCR-amplified by the primer set LMV3247/LMV3248 (for L1) or LMV3253/LMV3254 (for L4). The products were digested with DpnI to remove the template DNA, heat-inactivated (56 °C, 30 min), and then transformed into DH5α cells to obtain colonies containing the desired constructs. The pSP38 with the TD-G4m sequence was constructed by site-directed mutagenesis PCR using pSP38-TD-G4 as the template using the primer set LMV3235/LMV3236. The primer sequences are listed in Supplementary Table 3.
Transient transfection replication assays
HF cells (2 × 106) were co-transfected with the replication protein plasmids (1 μg of each) expressing the six replication core proteins, plus IE2, UL84, and UL112-113 and a plasmid containing the HCMV replication origin (oriLyt) by electroporation and were plated on 100-mm dishes. Total cellular DNAs were harvested five days post-transfection using QIAamp DNA Mini Kits (QIAGEN). DNAs (10 μg) were digested with DpnI and eluted in 100 µL. One microliter of eluate was used for qPCR to measure the amount of replicated oriLyt plasmid DNA using the Power SYBR Green PCR Master Mix and QuantStudioTM Real-Time PCR System (Applied Biosystems). The primers to amplify a specific oriLyt region were 5′-CTGCCTATATACATATTTAG-3′ and 5′-GATATAGACGATACACGTC-3′. Real-time PCR with SYBR Green was performed at 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s and 60 °C for 15 s.
Native polyacrylamide gel electrophoresis (PAGE)
ODNs (1 μM) in 10 mM Tris-HCl (pH 7.5, room temperature) buffer were heated to 95 °C for 5 min and then cooled to room temperature overnight and subjected to native polyacrylamide gel (15%) electrophoresis. For double-stranded DNA fragments, wild-type and mutant dsDNAs obtained by PCR were diluted to the final concentration of 1 μM in 10 mM Tris-HCl (pH 7.5, room temperature) buffer containing 40% (w/v) PEG 200 and 100 mM KCl or 100 mM LiCl. The dsDNA samples were heated to 95 °C for 5 min and then cooled to room temperature overnight. The samples were loaded on native polyacrylamide gels (12%) containing 100 mM KCl and 40% (w/v) PEG 200 and electrophoresed at 4 °C. The gel was then stained with SYBR Gold and scanned using a GelDoc XR+ System (BIO-RAD). For the super-shift assay with double-stranded DNAs, preformed dsDNAs were incubated with BG4 antibody for 1 h, and the samples were loaded for native polyacrylamide gel (8%) electrophoresis as described above.
Antibodies
G4-specific mouse monoclonal antibodies (MAbs) BG4 (MABE1126) and 1H6 (Ab00174-1.1) were purchased from Sigma-Aldrich and Absolute, respectively. Rabbit polyclonal antibodies (PAbs) specific to IE1/2 and UL112-113 p84 were previously described69,70,71. Anti-UL44 (p52) and anti-UL84 mouse MAbs were purchased from Virusys. Anti-IE2 mouse MAb (12E2) was purchased from Santa Cruz. Anti-hnRNP K rabbit PAb was purchased from Cell Signaling Technology. Horseradish peroxidase (HRP)-conjugated anti-His rabbit PAb was purchased from Abcam. Secondary antibodies such as fluorescein isothiocyanate (FITC)-labeled donkey anti-rabbit immunoglobulin G (IgG) and rhodamine/redX-coupled donkey anti-mouse IgG were obtained from Jackson ImmunoResearch Laboratories, Inc.
Indirect immunofluorescence assay (IFA)
Cells were washed in PBS, fixed with 2% paraformaldehyde in PBS at room temperature for 5 min, rewashed three times with PBS for 3 min each, and then permeabilized on ice with 0.2% Triton X-100 at 4 °C for 20 min. Cells were incubated with mouse MAb BG4 (at 1:200 dilution), rabbit PAb for IE1 (at 1:100 dilution), or UL112-113 (at 1:100 dilution) at 37 °C for 1 h and washed three times with PBS containing 0.2% Triton X-100 for 5 min each. This was followed by incubation with FITC-conjugated donkey anti-rabbit IgG and Rhod/Red-labeled donkey anti-mouse IgG at 1:100-fold dilutions at 37 °C for 1 h. Then, cells were washed with PBS containing 0.2% Triton-X100 for 3 min, 3 times. The cell nuclei were stained by Hoechst at 1:200-fold dilution in mounting solution. All slides were examined and photographed with a Carl Zeiss Axiophot microscope.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the ChIP Assay Kit (Millipore#17-295). For this, HCMV-infected HF cells (2 × 106) were harvested at 3 days post-infection, fixed with 1% formaldehyde for 10 min, and then lysed with a lysis buffer provided with the kit. The cell extracts were sheared by sonication. ChIP assays were performed with BG4 antibodies, antibodies for UL84 or IE1/IE2, or control IgG. For detection of the target region, DNAs purified from immunocomplexes were amplified by PCR using primers: oriG4-1 (5′-GAACGGGAACCACCGTAAC-3′ and 5′-CACTCGAGTCACCATCCCAT-3′), oriG4-2 (5′-GGAAAATTACCGCTCCGCCC-3′ and 5′-GAACCCTGCCGCGGACTGC-3′), oriG4-6 (5’-GCACCGGGGTCCCGGTTC-3’ and 5’-GAGGGACCCAAAAGCCAGC-3’), oriG4−7 (5′-GGTTCCTAGGCTCGTTCCG-3′ and 5′-CACCTACCGTCGTCGTCGG-3′), and RL11 (5′-CTCACG CTTGTCATAGTCAC-3′ and 5′-ATGGCTAGTGTTAAGCCTGG-3′). The PCR program was 95 °C for 5 min, with 50 amplification cycles of 95 °C for 30 s and 60 °C for 30 s.
Pull-down assays with 3′ biotin-tagged ODNs
Several dishes of HF cells (2 × 106 cells in a 150-mm dish) were infected with HCMV (Toledo) at an MOI of 1 for 120 h. Cells were harvested, resuspended in PBS containing protease inhibitor cocktails, and disrupted by sonication on ice. Cellular debris was cleared from the lysates using centrifugation, and the amount of protein was determined by a Bradford Assay (Bio-Rad). Immobilized DNA ODNs were prepared by incubating preformed biotin-tagged ODNs (11.3 μM) with 30 µg of streptavidin agarose beads (Sigma, S1638) in 300 µL of buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 1 M NaCl, 0.003% NP40) for 30 min at room temperature with constant rotation. The immobilized ODNs were collected by centrifugation and incubated in 300 µL of blocking buffer (2.5 mg/mL of BSA in 10 mM HEPES, pH 7.6, 100 mM potassium glutamate, 2.5 mM dithiothreitol (DTT), 10 mM magnesium acetate, 5 mM EGTA, 3.5% glycerol with 0.003% NP40, and 5 mg/mL of polyvinylpyrrolidone) for 30 min at room temperature to minimize non-specific interactions. The immobilized ODNs were collected using centrifugation and incubated with cell lysates prepared from virus-infected cells (100 µg of protein) or purified proteins (1 µg) in 400 µL of binding buffer (10 mM HEPES, pH 7.6, 100 mM potassium glutamate, 80 mM KCl, 2.5 mM DTT, 10 mM magnesium acetate, 5 mM EGTA, 3.5% glycerol with 0.002% NP40, and 1 µg of non-specific carrier DNA) for 1 h at 4 °C with constant rotation. After 1 h incubation, the immobilized ODN/protein complexes were washed three times with 500 µL of washing buffer (10 mM HEPES, pH 7.6 at room temperature, 100 mM potassium glutamate, 2.5 mM DTT, 10 mM magnesium acetate, 5 mM EGTA, 3.5% glycerol, 0.5 mg/mL BSA, and 0.2% NP40). Twenty µL of SDS–polyacrylamide gel electrophoresis (PAGE) sample buffer was added to the immobilized ODN/protein complexes and incubated at 37 °C for 15 min. After centrifugation, the supernatants (eluents) were boiled at 97 °C for 7 min and stored at −70 °C for further analysis using mass spectrometry and SDS-PAGE.
Mass spectrometry
The LC-MS/MS analysis was performed as a custom service by e-biogen Inc. The prepared samples were resuspended in 0.1% formic acid in water and analyzed using a Q-Exactive Orbitrap hybrid mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) along with an Ultimate 3000 system (Thermo Fisher Scientific, Waltham, MA, USA). We used a 2 cm × 75 μm ID trap column packed with 3 μm C18 resin and a 50 cm × 75 μm ID analytical column packed with 2 μm C18 resin to peptides. The mobile phase solvents consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in 90% acetonitrile, and the flow rate was fixed at 300 nL/min. The gradient of mobile phase was as follows: 4% solvent B in 10 min, 4–22% solvent B in 30 min, 22–50% solvent B in 12 min, 50–96% solvent B in 0.1 min, holding at 96% of solvent B in 7.9 min, 96–4% solvent B in 0.1 min, and 4% solvent B for 14.9 min. A data-dependent acquisition method was adopted, and the top 10 precursor peaks were selected and isolated for fragmentation. Ions were scanned in high resolution (70,000 in MS1, 17,500 in MS2 at m/z 400), and the MS scan range was 400–2000 m/z at both the MS1 and MS2 levels. Precursor ions were fragmented with Normalized Collisional Energy 27%. Dynamic exclusion was set to 30 s. For proteome data analysis, Thermo MS/MS raw files of each analysis were searched by using Proteome Discoverer™ software (ver. 2.5), and the Homo sapiens and HCMV Toledo databases were downloaded from Uniprot. The appropriate consensus workflow included a peptide-spectrum match (PSM) validation step and SEQUEST HT process for detection as a database search algorithm. The search parameters were set up as follows: 10 ppm of tolerances of precursor ion masses, 0.02 Da fragment ion mass, maximum of two missed cleavages with trypsin enzyme. The dynamic modification on peptide sequence was as follows: static carbamidomethylation of cysteine (+57.012 Da), variable modifications of methionine oxidation (+15.995 Da), acetylation of protein N-term (+42.011 Da), and carbamylation of protein in N-term (+43.0006 Da). After searching, the data results below 1% of FDR were selected and filtered at least 6 more peptides length.
Purification of 6xHis-tagged proteins in bacterial cells
E. coli BL21(DE3) cells harboring plasmids expressing hnRNP K, IE2(87-579), UL44(1-290), and UL84 were cultured in LB broth at 37 °C until the optical density at 600 nm (OD600) reached 0.4–0.6. The culture was cooled to 18 °C, and the expression of hnRNP K, IE2(87-579), and UL44(1-290) was induced by adding 0.1 mM IPTG for 24 h. The expression of UL84 was induced by the addition of 0.5 mM IPTG for 4 h. Cells were harvested by centrifugation at 6000 × g for 7 min at 4 °C, resuspended in buffer A (50 mM Tris-HCl pH 8.0, 0.5 M NaCl, and 40 mM imidazole), and disrupted by sonication on ice. After centrifugation at 48,000 × g for 1 h at 4 °C, the cell-free extracts were loaded onto a 5-mL Ni-NTA column (GE Healthcare, USA), and bound proteins were eluted by a gradient of 0.04–1.0 M imidazole in buffer A. The elution fractions containing hnRNP K, IE2(87-579), UL44(1-290), and UL84 were buffer exchanged with PBS by dialysis. Proteins were then concentrated and stored at −70 °C until use.
Immunoblot analysis
Cells were washed with PBS, and total cell lysates were prepared by boiling the cell pellets in sodium dodecyl sulfate (SDS) loading buffer. Equal amounts of clarified cell extracts were separated on an SDS-polyacrylamide gel and electroblotted onto nitrocellulose membranes. Blots were blocked with PBS plus 0.1% Tween 20 (PBST) containing 5% nonfat dry milk for 1 h at room temperature. After three washes with PBST, blots were incubated with the appropriate antibodies in PBST for 1 h at room temperature or overnight at 4 °C. After three 5-min washes with PBST, blots were incubated with HRP-conjugated goat anti-mouse or anti-rabbit IgG secondary antibody (Ab Frontier) for 1 h at room temperature. For 6xHis-tagged proteins, HRP-conjugated anti-His antibody was used. Blots were then washed three times with PBST, and the protein bands were visualized with an enhanced chemiluminescence system (Amersham).
Enzyme-linked immunosorbent assay (ELISA)
For ELISA, 3′ biotin-tagged ODNs were bound to Pierce Streptavidin-Coated High-Capacity Plates (ThermoFisher), followed by blocking with 10% BSA (for IE2), 5% skim milk (for UL84), or 5% BSA (for UL44) and incubation with 6xHis-tagged HCMV proteins in the binding buffer used in pull-down assays. After washes with washing buffer used in pull-down assays, the bound proteins were detected with HRP-conjugated anti-His antibody (ab1187, Abcam) and 3,3′,5,5′-tetramethylbenzidine (TMB) ELISA substrate (Slow Kinetic Rate, ab171525, Abcam). Signal intensity was measured at 450 nm on an xMarkTM microplate absorbance spectrophotometer (Bio-Rad). The dissociation constant (Kd) was calculated from binding curves derived using Prism (GraphPad Software Inc.). Standard errors of means were calculated from three replicates.
Statistical analysis
Samples were compared using Student’s t test, and p < 0.05 (*p < 0.05, **p < 0.01, or ***p < 0.001) were considered to indicate significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data generated in this study have been deposited to the ProteomeXchange Consortium via the PRIDE repository with dataset identifier PXD050439. Source data are provided with this paper.
References
Varshney, D., Spiegel, J., Zyner, K., Tannahill, D. & Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 21, 459–474 (2020).
Rhodes, D. & Lipps, H. J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 43, 8627–8637 (2015).
Hansel-Hertsch, R., Antonio, M. D. & Balasubramanian, S. DNA G-quadruplexes in the human genome: detection, functions and therapeutic potential. Nat. Rev. Mol. Cell Biol. 18, 279–284 (2017).
Valton, A. L. & Prioleau, M. N. G-Quadruplexes in DNA replication: a problem or a necessity? Trends Genet. 32, 697–706 (2016).
Bryan, T. M. Mechanisms of DNA replication and repair: insights from the study of G-Quadruplexes. Molecules 24, 3439 (2019).
Cayrou, C. et al. New insights into replication origin characteristics in metazoans. Cell Cycle 11, 658–667 (2012).
Besnard, E. et al. Unraveling cell type-specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat. Struct. Mol. Biol. 19, 837–844 (2012).
Cayrou, C. et al. The chromatin environment shapes DNA replication origin organization and defines origin classes. Genome Res. 25, 1873–1885 (2015).
Langley, A. R., Gräf, S., Smith, J. C. & Krude, T. Genome-wide identification and characterisation of human DNA replication origins by initiation site sequencing (ini-seq). Nucleic Acids Res. 44, 10230–10247 (2016).
Valton, A. L. et al. G4 motifs affect origin positioning and efficiency in two vertebrate replicators. EMBO J. 33, 732–746 (2014).
Prorok, P. et al. Involvement of G-quadruplex regions in mammalian replication origin activity. Nat. Commun. 10, 3274 (2019).
Mocarski, E. S., Shenk, T., Griffiths, P. D. & Pass, R. F. Cytomegaloviruses. In Fields virology (eds. D. M. Knipe, P. M. Howley, J. I. Cohen, D. E. Griffin, R. A. Lamb, M. A. Martin, V. R. Racaniello, & B. Roizman) (Lippincott Williams & Wilkins, Philadelphia, PA, 2013).
Anders, D. G. & Punturieri, S. M. Multicomponent origin of cytomegalovirus lytic-phase DNA replication. J. Virol. 65, 931–937 (1991).
Pari, G. S. & Anders, D. G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67, 6979–6988 (1993).
Chee, M. S. et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154, 125–169 (1990).
Davison, A. J. et al. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84, 17–28 (2003).
Pari, G. S., Kacica, M. A. & Anders, D. G. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67, 2575–2582 (1993).
Smith, J. A. & Pari, G. S. Expression of human cytomegalovirus UL36 and UL37 genes is required for viral DNA replication. J. Virol. 69, 1925–1931 (1995).
Pari, G. S. Nuts and bolts of human cytomegalovirus lytic DNA replication. Curr. Top. Microbiol. Immunol. 325, 153–166 (2008).
Xu, Y., Cei, S. A., Rodriguez Huete, A., Colletti, K. S. & Pari, G. S. Human cytomegalovirus DNA replication requires transcriptional activation via an IE2- and UL84-responsive bidirectional promoter element within oriLyt. J. Virol. 78, 11664–11677 (2004).
Sarisky, R. T. & Hayward, G. S. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J. Virol. 70, 7398–7413 (1996).
Zhu, Y., Huang, L. & Anders, D. G. Human cytomegalovirus oriLyt sequence requirements. J. Virol. 72, 4989–4996 (1998).
Colletti, K. S., Smallenburg, K. E., Xu, Y. & Pari, G. S. Human cytomegalovirus UL84 interacts with an RNA stem-loop sequence found within the RNA/DNA hybrid region of oriLyt. J. Virol. 81, 7077–7085 (2007).
Kagele, D., Gao, Y., Smallenburg, K. & Pari, G. S. Interaction of HCMV UL84 with C/EBPalpha transcription factor binding sites within oriLyt is essential for lytic DNA replication. Virology 392, 16–23 (2009).
Kagele, D., Rossetto, C. C., Tarrant, M. T. & Pari, G. S. Analysis of the interactions of viral and cellular factors with human cytomegalovirus lytic origin of replication, oriLyt. Virology 424, 106–114 (2012).
Huang, L., Zhu, Y. & Anders, D. G. The variable 3’ ends of a human cytomegalovirus oriLyt transcript (SRT) overlap an essential, conserved replicator element. J. Virol. 70, 5272–5281 (1996).
Spector, D. J. UL84-independent replication of human cytomegalovirus strains conferred by a single codon change in UL122. Virology 476, 345–354 (2015).
Manska, S. & Rossetto, C. C. Characteristics of Immediate-Early 2 (IE2) and UL84 Proteins in UL84-Independent Strains of Human Cytomegalovirus (HCMV). Microbiol. Spectr. 9, e0053921 (2021).
Ravichandran, S. et al. Genome-wide analysis of regulatory G-quadruplexes affecting gene expression in human cytomegalovirus. PLoS Pathog. 14, e1007334 (2018).
Chambers, V. S. et al. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 33, 877–881 (2015).
Ravichandran, S., Razzaq, M., Parveen, N., Ghosh, A. & Kim, K. K. The effect of hairpin loop on the structure and gene expression activity of the long-loop G-quadruplex. Nucleic Acids Res. 49, 10689–10706 (2021).
Burge, S., Parkinson, G. N., Hazel, P., Todd, A. K. & Neidle, S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 34, 5402–5415 (2006).
Bochman, M. L., Paeschke, K. & Zakian, V. A. DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet 13, 770–780 (2012).
Davis, J. T. G-quartets 40 years later: from 5’-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. Engl. 43, 668–698 (2004).
Ren, J. & Chaires, J. B. Sequence and structural selectivity of nucleic acid binding ligands. Biochemistry 38, 16067–16075 (1999).
Georgakopoulos-Soares, I. et al. Alternative splicing modulation by G-quadruplexes. Nat. Commun. 13, 2404 (2022).
Mergny, J. L., Li, J., Lacroix, L., Amrane, S. & Chaires, J. B. Thermal difference spectra: a specific signature for nucleic acid structures. Nucleic Acids Res. 33, e138 (2005).
Zheng, K. W., Chen, Z., Hao, Y. H. & Tan, Z. Molecular crowding creates an essential environment for the formation of stable G-quadruplexes in long double-stranded DNA. Nucleic Acids Res. 38, 327–338 (2010).
Nakano, S., Miyoshi, D. & Sugimoto, N. Effects of molecular crowding on the structures, interactions, and functions of nucleic acids. Chem. Rev. 114, 2733–2758 (2014).
Dabral, P., Babu, J., Zareie, A. & Verma, S. C. LANA and hnRNP A1 regulate the translation of LANA mRNA through G-Quadruplexes. J. Virol. 94, e01508–e01519 (2020).
Day, H. A., Pavlou, P. & Waller, Z. A. i-Motif DNA: structure, stability and targeting with ligands. Bioorg. Med. Chem. 22, 4407–4418 (2014).
Abou Assi, H., Garavís, M., González, C. & Damha, M. J. i-Motif DNA: structural features and significance to cell biology. Nucleic Acids Res. 46, 8038–8056 (2018).
Zeraati, M. et al. I-motif DNA structures are formed in the nuclei of human cells. Nat. Chem. 10, 631–637 (2018).
Pizzorno, M. C., O’Hare, P., Sha, L., LaFemina, R. L. & Hayward, G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J. Virol. 62, 1167–1179 (1988).
Cherrington, J. M., Khoury, E. L. & Mocarski, E. S. Human cytomegalovirus ie2 negatively regulates alpha gene expression via a short target sequence near the transcription start site. J. Virol. 65, 887–896 (1991).
Liu, B., Hermiston, T. W. & Stinski, M. F. A cis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J. Virol. 65, 897–903 (1991).
Spiegel, J. et al. G-quadruplexes are transcription factor binding hubs in human chromatin. Genome Biol. 22, 117 (2021).
Hammerschmidt, W. & Sugden, B. Replication of Epstein-Barr viral DNA. Cold Spring Harb. Perspect. Biol. 5, a013029 (2013).
Zhang, Q., Holley-Guthrie, E., Ge, J. Q., Dorsky, D. & Kenney, S. The Epstein-Barr virus (EBV) DNA polymerase accessory protein, BMRF1, activates the essential downstream component of the EBV oriLyt. Virology 230, 22–34 (1997).
Gruffat, H., Renner, O., Pich, D. & Hammerschmidt, W. Cellular proteins bind to the downstream component of the lytic origin of DNA replication of Epstein-Barr virus. J. Virol. 69, 1878–1886 (1995).
Fenouil, R. et al. CpG islands and GC content dictate nucleosome depletion in a transcription-independent manner at mammalian promoters. Genome Res. 22, 2399–2408 (2012).
Hoshina, S. et al. Human origin recognition complex binds preferentially to G-quadruplex-preferable RNA and single-stranded DNA. J. Biol. Chem. 288, 30161–30171 (2013).
Boos, D., Yekezare, M. & Diffley, J. F. Identification of a heteromeric complex that promotes DNA replication origin firing in human cells. Science 340, 981–984 (2013).
Kumagai, A. & Dunphy, W. G. MTBP, the partner of Treslin, contains a novel DNA-binding domain that is essential for proper initiation of DNA replication. Mol. Biol. Cell 28, 2998–3012 (2017).
Ryon, J. J. et al. The lytic origin of herpesvirus papio is highly homologous to Epstein-Barr virus ori-Lyt: evolutionary conservation of transcriptional activation and replication signals. J. Virol. 67, 4006–4016 (1993).
Chen, W. F. et al. Molecular mechanistic insights into drosophila DHX36-Mediated G-quadruplex unfolding: a structure-based model. Structure 26, 403–415.e404 (2018).
Chen, M. C. et al. Structural basis of G-quadruplex unfolding by the DEAH/RHA helicase DHX36. Nature 558, 465–469 (2018).
Tai-Schmiedel, J. et al. Human cytomegalovirus long noncoding RNA4.9 regulates viral DNA replication. PLoS Pathog. 16, e1008390 (2020).
Chung, W. C. et al. G-quadruplexes formed by Varicella-Zoster virus reiteration sequences suppress expression of glycoprotein C and regulate viral cell-to-cell spread. PLoS Pathog. 19, e1011095 (2023).
Xu, H. et al. CX-5461 is a DNA G-quadruplex stabilizer with selective lethality in BRCA1/2 deficient tumours. Nat. Commun. 8, 14432 (2017).
Westdorp, K. N. & Terhune, S. S. Impact of RNA polymerase I inhibitor CX-5461 on viral kinase-dependent and -independent cytomegalovirus replication. Antivir. Res. 153, 33–38 (2018).
Pietras, Z. et al. Dedicated surveillance mechanism controls G-quadruplex forming non-coding RNAs in human mitochondria. Nat. Commun. 9, 2558 (2018).
Estep, K. N., Butler, T. J., Ding, J. & Brosh, R. M. G4-Interacting DNA helicases and polymerases: potential therapeutic targets. Curr. Med. Chem. 26, 2881–2897 (2019).
Mendoza, O., Bourdoncle, A., Boule, J. B., Brosh, R. M. Jr. & Mergny, J. L. G-quadruplexes and helicases. Nucleic Acids Res. 44, 1989–2006 (2016).
Wang, Y., Li, H., Tang, Q., Maul, G. G. & Yuan, Y. Kaposi’s sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: involvement of host cellular factors. J. Virol. 82, 2867–2882 (2008).
Dabral, P., Uppal, T. & Verma, S. C. G-quadruplexes of KSHV oriLyt play important roles in promoting lytic DNA replication. Microbiol. Spectr. 11, e0531622 (2023).
Kwon, K. M., Oh, S. E., Kim, Y. E., Han, T. H. & Ahn, J. H. Cooperative inhibition of RIP1-mediated NF-kappaB signaling by cytomegalovirus-encoded deubiquitinase and inactive homolog of cellular ribonucleotide reductase large subunit. PLoS Pathog. 13, e1006423 (2017).
Huppert, J. L. & Balasubramanian, S. Prevalence of quadruplexes in the human genome. Nucleic Acids Res. 33, 2908–2916 (2005).
Kim, Y. E. & Ahn, J. H. Role of the specific interaction of UL112-113 p84 with UL44 DNA polymerase processivity factor in promoting DNA replication of human cytomegalovirus. J. Virol. 84, 8409–8421 (2010).
Kim, E. T. et al. Analysis of human cytomegalovirus-encoded SUMO targets and temporal regulation of SUMOylation of the immediate-early proteins IE1 and IE2 during Infection. PLoS One 9, e103308 (2014).
Park, M. Y. et al. Interactions among four proteins encoded by the human cytomegalovirus UL112-113 region regulate their intranuclear targeting and the recruitment of UL44 to prereplication foci. J. Virol. 80, 2718–2727 (2006).
Acknowledgements
We thank Gary S. Hayward for providing HCMV replication plasmids and Hua Zhu for bacmids. We also thank Jae-Hyun Park for helpful discussion. This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2020R1A4A1018019, 2021M3A9I2080488, and 2022R1A2C1006748).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.P., W.-C.C., and J.-H.A. Bioinformatics analysis: D.P. and S.R. Investigation: D.P., W.-C.C., S.G., G.M.L., and M.H. Data analysis and curation: D.P., W.-C.C., K.K.K., and J.-H.A. Funding acquisition: J.-H.A. Supervision: J.-H.A. Writing – original draft: D.P. and W.-C.C.; Writing – review and editing: J.-H.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, D., Chung, WC., Gong, S. et al. G-quadruplex as an essential structural element in cytomegalovirus replication origin. Nat Commun 15, 7353 (2024). https://doi.org/10.1038/s41467-024-51797-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51797-6