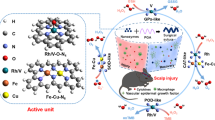
Abstract
Electrochemical methods with tissue-implantable microelectrodes provide an excellent platform for real-time monitoring the neurochemical dynamics in vivo due to their superior spatiotemporal resolution and high selectivity and sensitivity. Nevertheless, electrode implantation inevitably damages the brain tissue, upregulates reactive oxygen species level, and triggers neuroinflammatory response, resulting in unreliable quantification of neurochemical events. Herein, we report a multifunctional sensing platform for inflammation-free in vivo analysis with atomic-level engineered Fe single-atom catalyst that functions as both single-atom nanozyme with antioxidative activity and electrode material for dopamine oxidation. Through high-temperature pyrolysis and catalytic performance screening, we fabricate a series of Fe single-atom nanozymes with different coordination configurations and find that the Fe single-atom nanozyme with FeN4 exhibits the highest activity toward mimicking catalase and superoxide dismutase as well as eliminating hydroxyl radical, while also featuring high electrode reactivity toward dopamine oxidation. These dual functions endow the single-atom nanozyme-based sensor with anti-inflammatory capabilities, enabling accurate dopamine sensing in living male rat brain. This study provides an avenue for designing inflammation-free electrochemical sensing platforms with atomic-precision engineered single-atom catalysts.
Similar content being viewed by others
Introduction
Synergistic interactions between various neurochemicals are crucial for maintaining brain homeostasis1,2,3,4. Precise quantification of neurochemical variations under physiological or pathological conditions is essential for unravelling the molecular mechanisms underlying brain function5,6,7,8. Electrochemical sensors based on implantable microelectrodes provide an effective platform for in vivo tracking of neurochemicals, owing to their exceptional spatiotemporal resolution, high selectivity and sensitivity9,10,11,12,13,14. However, the implantation of microelectrodes into brain tissue inevitably triggers the inflammatory response, leading to the excessive generation of reactive oxygen species (ROS)15,16,17,18. Elevated ROS induces secondary injury and exacerbates the inflammatory response, and thereby disrupts the neurochemical microenvironment around the microelectrode, leading to electrode fouling and further inaccuracies and unreliability of long-term neurochemical sensing19. Therefore, it is imperative to develop an in vivo sensing platform capable of eliminating ROS to ensure precise monitoring of neurochemicals.
To address the challenge mentioned above, we turn to leveraging the properties of antioxidative enzymes or their mimics that can mitigate the negative effects of ROS and inflammation induced by implanted devices, presenting a viable strategy for maintaining sensor performance in vivo. Natural antioxidative enzymes, such as catalase (CAT), peroxidase, and superoxide dismutase (SOD), are highly efficient and versatile biocatalysts that help balance oxidative stress20. However, their practical applications are significantly hindered by poor stability, weak environmental tolerance and high costs. As an alternative, nanomaterials with intrinsic enzyme-like activities, known as nanozymes, has emerged as promising candidates for regulating ROS in biological system due to their high stability, customizable structure, and adjustable performance21,22,23. Recently, single-atom catalysts (SACs), which feature coordination-unsaturated single active metal sites and atomic-precision designability, have been identified as a new type of nanozymes with superior enzyme-mimicking performances24,25,26,27. To date, a diverse array of single-atom nanozymes (SAzymes) have been successfully employed in anti-inflammatory treatments, cancer therapy and antibacterial applications28,29,30,31. Notably, most of these SAzymes are produced through high-temperature carbonization, resulting in carbon supports with inherent high electrode reactivity, which shows great promise in electrochemical sensing32,33,34. Taken together, the implementation of carbon-based antioxidative SAzymes in developing electrochemical sensing platforms with anti-inflammatory capacity would provide an avenue to in vivo neurochemical sensing.
Herein, we report the atomic-level modulation of metal active site using a temperature-controlled pyrolysis strategy to fabricate Fe SACs with dual functions: mimicking antioxidative enzyme and acting as electrode material for electrochemical in vivo sensing (Fig. 1). We employ iron phthalocyanine (FePc)-encapsulated metal-organic framework (MOF) MET-6 (FePc@MET-6) as the precursor to prepare a series of hierarchical porous nitrogen-dopped carbon-supported Fe SACs (i.e., Fe1/NC SACs) with different coordination environments (e.g., FeN5, FeN4, and FeN3C) by varying the calcination temperatures. We find that the Fe1/NC SAC with FeN4 active sites shows excellent CAT- and SOD-mimicking property and hydroxyl radical (•OH) eliminating ability for ROS scavenging as well as electrode reactivity for the oxidation of neurochemicals, e.g., dopamine (DA). This finding offers great possibilities in developing platforms with improved reliability for long-term in vivo sensing of neurochemicals.
Results
Synthesis and characterization of Fe1/NC SACs
In our previous study, we proposed a concept of SAzyme and reported an iron-based antioxidative SAzyme35. Since Fe is the active metal center in most natural CATs for catalytic decomposition of H2O2, we aimed to develop Fe-based SACs with similar catalytic functions by mimicking the active site structure of CAT. In addition, Fe is known to exhibit versatile redox chemistry, allowing it to participate in various reduction-oxidation reactions. This redox activity of Fe is crucial for enabling CAT-like activity and other antioxidative activities in scavenging ROS. Fe1/NC SACs were synthesized using an in-situ trapping-pyrolysis method with FePc@MET-6 as precursor at different temperatures, i.e., 800, 900, and 1000 °C, referred to as Fe1/NC-T (T represents the specific pyrolysis temperature) (Fig. 2a). MET-6, composed of Zn clusters and triazolate ligands, was chosen because the volatilization of Zn and decomposition of high-energy triazoles during high-temperature pyrolysis facilitate the generation of gas, forming hierarchical porous carbon network structure that enhances mass transfer and offers accessible active sites for catalysis36. Control samples denoted as NC-T were also synthesized with the same procedure but without FePc. Both MET-6 and FePc@MET-6 show similar octahedral morphology and X-ray diffraction (XRD) peaks (Supplementary Figs. 1 and 2), indicating that FePc does not significantly affect the structure and morphology of MET-6 or the microporous structure (Supplementary Fig. 3 and Supplementary Table 1). However, after high-temperature calcination, all precursors transformed from an octahedral morphology to a hierarchical porous structure. The obtained Fe1/NC SACs show no observable particles in scanning electron microscopy (SEM) and transmission electron microscope (TEM) images (Fig. 2b, c and Supplementary Figs. 4 and 5). Element mapping images demonstrate the uniform distribution of Fe, N, and C in Fe1/NC SACs (Fig. 2d and Supplementary Fig. 6). Aberration-corrected high-angle annular dark-field scanning TEM (AC HAADF-STEM) images show that Fe exists as single atoms for all three Fe1/NC SACs (Fig. 2e–g).
a Scheme of the synthesis of Fe1/NC SACs. SEM (b) and TEM (c) images of Fe1/NC-900. d HAADF-STEM image and corresponding EDS elemental mapping images of Fe1/NC-900. AC HAADF-STEM images of Fe1/NC-800 (e), Fe1/NC-900 (f) and Fe1/NC-1000 (g). Each experiment was repeated independently three times with similar results. Representative images are shown.
In addition, the XRD patterns of Fe1/NC SACs and NC samples show a diffraction peak near 23.3°, corresponding to the (002) crystal plane of graphitic carbon, with no other peaks associated with nanoparticles, further indicating the absence of metal particles in Fe1/NC SACs (Supplementary Fig. 7)37. Raman spectra demonstrate that all the catalysts display two peaks around 1335.7 cm−1 and 1575.4 cm−1, assigned to the D band and G band, respectively (Supplementary Fig. 8)38. Among the NC samples, NC-900 possesses the lowest intensity ratio of ID/IG, which represents the extent of graphitization and is closely related to the calcination temperature, demonstrating the highest degree of graphitization for NC-900 (Supplementary Table 2)39. Fe1/NC SACs show a similar trend with NC catalysts, indicating that the introduction of Fe has little effect on the graphitization of the catalysts. X-ray photoelectron spectroscopy (XPS) and inductively coupled plasma mass spectrometry (ICP-MS) characterizations show similar Fe content in all three Fe1/NC SACs (Supplementary Fig. 9 and Supplementary Tables 3 and 4), while the carbon and nitrogen contents of Fe1/NC SACs depend on the pyrolysis temperature. With increasing the pyrolysis temperature (i.e., 800, 900, and 1000 °C), the carbon content gradually increases (78.18%, 82.07%, and 83.13%, respectively), and the nitrogen content gradually decreases (9.48%, 6.14%, and 5.54%, respectively). In addition, the binding energy of Fe shifts negatively with increasing temperature, indicating a decrease in the valence state of Fe and the attenuation of the interaction between Fe and N (Supplementary Fig. 9c)40. Moreover, the high-resolution N 1s XPS spectra of Fe1/NC can be deconvoluted into three peaks located at around 398.5 eV, 400.0 eV, and 401.1 eV, corresponding to pyridinic nitrogen, pyrrolic nitrogen, and graphitic nitrogen, respectively (Supplementary Fig. 9d)41. Furthermore, the content of pyridinic nitrogen in Fe1/NC SACs gradually decreases with the increase of pyrolysis temperature. These results suggest that the Fe-N coordination environment is highly dependent on the pyrolysis temperature.
To further analyze the atomic configuration of the catalysts, we carried out X-ray absorption spectroscopy (XAS) characterization. The Fe K-edge X-ray absorption near-edge structure (XANES) spectra show that the valence states of Fe in Fe1/NC SACs range between 0 (Fe foil) and +3 (Fe2O3) (Fig. 3a), and the energy shifts negetively as the pyrolysis temperature increases, implying a decrease in the oxidation state of Fe, consistent with the XPS results42. Additionally, the extended X-ray absorption fine structure (EXAFS) spectra of Fe1/NC SACs display only one major peak at 1.4 Å in the R space, corresponding to the Fe-N(C) coordination shell, with no Fe-Fe peak similar to that of Fe foil which corresponds to the Fe-Fe coordination shell being observed (Fig. 3b, c), further confirming that Fe in Fe1/NC SACs exists as single atoms. Moreover, the intensity of the Fe-N shell gradually decreases with increasing the pyrolysis temperature, verifying a decrease in the coordination number of N around the Fe center41. To obtain the structural parameters of Fe atoms, we performed EXAFS fitting of Fe1/NC SACs. According to the fitting curves and parameters (Fig. 3d–f and Supplementary Table 5), the coordination numbers of the Fe-N first shell in Fe1/NC-800, Fe1/NC-900, and Fe1/NC-1000 were calculated to be 4.96, 4.09, and 3.26, respectively. In addition to the Fe-N coordination shell, a Fe-C coordination shell with a coordination number of 1.08 was observed in Fe1/NC-1000. Taken together, the atomic configurations of the active centers in Fe1/NC-800, Fe1/NC-900, and Fe1/NC-1000 were depicted as FeN5, FeN4, and FeN3C, respectively, as displayed in the insets of Fig. 3d–f.
Fe K-edge XANES (a) and FT EXAFS (b) spectra of Fe1/NC SACs, Fe foil and Fe2O3. c EXAFS spectra of Fe1/NC SACs, Fe foil and Fe2O3 in k space. Fitting plots of EXAFS spectra for Fe1/NC-800 (d), Fe1/NC-900 (e) and Fe1/NC-1000 (f) in R space. Insets: structural models of Fe1/NC-800, Fe1/NC-900, and Fe1/NC-1000, respectively (N, blue; C, gray; Fe, deep red). Source data are provided with the paper.
Antioxidative performance of Fe1/NC SACs
Having demonstrated the coordination environment of Fe active sites can be atomically modulated via high-temperature pyrolysis, we moved forward to investigate the relationship between the CAT-mimicking activity of Fe1/NC SACs and the atomic configuration of their active centers. The CAT-mimicking activity was evaluated by determining O2 generation from the catalytic disproportionation of H2O243,44,45. As shown in Fig. 4a, the as-synthesized Fe1/NC SACs exhibit distinct CAT-like activity, among which, Fe1/NC-900 with the atomic configuration of FeN4 shows the highest activity. In contrast, NC controls without Fe show negligible activity, showing the dominating role of Fe single atoms in mimicking CAT. Moreover, the O2 generation capacities of Fe1/NC SAzymes linearly depend on the concentration of the catalysts (Fig. 4b and Supplementary Fig. 10), implying that the catalytic reaction of the SAzymes follows first order reaction kinetics like natural enzymes46. We next quantitatively determined the specific activities of these three SAzymes, and found that Fe1/NC-900 shows much higher activity (28.9 U·mg−1), nearly three times that of Fe1/NC-800 (10.4 U·mg−1) and Fe1/NC-1000 (11.4 U·mg−1).
a Time-dependent O2 generation in Britton–Robison (BR) buffer containing 5 mM H2O2 and 5 μg·mL−1 catalysts. b Specific activities (SA, U·mg−1) of Fe1/NC SACs in BR buffer (pH 7.0) containing 5 mM H2O2. c Kinetics for CAT-like activity of Fe1/NC SACs (5 μg·mL−1) with different concentrations of H2O2. EPR spectra of 25 mM BMPO in methanol containing 5 mM H2O2 without or with 5 μg·mL−1 Fe1/NC-800 (d), Fe1/NC-900 (e), Fe1/NC-1000 (f) and their controls. Blank: no catalyst. g Schematic structure of Fe1/NC-900 with intermediates during the catalytic H2O2 disproportionation reaction. N, blue; C, gray; O, red; H, light gray; Fe, deep red. h Free energy diagrams of H2O2 disproportionation on Fe1/NC SAzymes. Each experiment was repeated independently three times with similar results (a–f). Representative plots are shown. Source data are provided with the paper.
Subsequently, we studied the steady-state kinetics of the three SAzymes in mimicking CAT (Supplementary Fig. 11). As exhibited in Fig. 4c and Supplementary Fig. 12, the decomposition reaction catalyzed by Fe1/NC SAzymes conforms to the typical Michaelis–Menten kinetics. The kinetic parameters of the SAzymes were determined and listed in Supplementary Table 6. Fe1/NC-900 exhibits a maximum reaction rate (Vm) of 0.51 mM/min and a reaction rate constant kcat of 2.05 × 103 min−1, which are much higher than those of Fe1/NC-800 and Fe1/NC-1000 SAzymes, revealing that the highest catalytic activity of Fe1/NC-900. The higher Michaelis constant (Km) value of Fe1/NC-900 indicates a lower binding affinity. In addition, the CAT-like activity of the SAzymes shows an obvious dependence on the pH value of the reaction solution (Supplementary Fig. 13), similar to the natural enzymes47.
To evaluate the stability of the Fe1/NC-900 SAzyme, we conducted post-reaction characterizations. As shown in Supplementary Fig. 14a, b, the used Fe1/NC-900 catalyst exhibits a morphology similar to that observed before the catalytic tests. The HAADF-STEM image indicates that no Fe or FexOy nanoparticles were formed in Fe1/NC-900 after catalysis (Supplementary Fig. 14c). The corresponding elemental mapping images confirmed the homogeneous distribution of C, N and Fe elements (Supplementary Fig. 14c). In addition, the XRD spectrum of the used Fe1/NC-900 demonstrates the absence of Fe-related nanoparticles (Supplementary Fig. 15), showing the high stability of the single-atom configuration.
In addition to the CAT-like activity, we also explored the SOD-mimicking activity of Fe1/NC-900. SOD is an antioxidative enzyme for catalyzing the disproportionation of O2− into O2 and H2O2. To assess the SOD-like activity of Fe1/NC-900, we employed SOD assay kits with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (monosodium salt) (WST-1), a selective probe that reacts with O2− to produce a water-soluble formazan dye, to monitor the disproportionation of O2−48,49. The SOD-mimicking ability of Fe1/NC-900 was evaluated and quantified by the inhibition of formazan formation. As shown in Supplementary Fig. 16, the inhibition of formazan formation increases rapidly with increasing the concentration of Fe1/NC-900, indicating that Fe1/NC-900 can mimic SOD and displays a concentration-dependent manner. In comparison, NC-900 without FeN4 active site shows relatively lower SOD-like activity, demonstrating the single Fe atoms in Fe1/NC-900 are responsible for the catalytic disproportionation of O2−.
Furthermore, we investigated the activity of Fe1/NC-900 toward eliminating hydroxyl radicals (•OH), another crucial reactive oxygen species. The •OH elimination capacity of Fe1/NC-900 was evaluated and quantified by using terephthalic acid (TA) as a selective fluorescent probe50. TA can be oxidized by •OH to generate 2-hydroxyterephthalic acid (TA-OH), which can emit a fluorescent signal at 435 nm51. As shown in Supplementary Fig. 17, no •OH was detected with TA and H2O2 alone. However, upon the addition of Fe2+, an obvious fluorescence peak representing the TA-OH appears, indicating the generation of •OH through the Fenton reaction. When Fe1/NC-900 was added, the fluorescence intensity of TA-OH significantly decreases, demonstrating that •OH generated from the Fenton reaction can be effectively scavenged by Fe1/NC-900.
Given that Fe-based SACs also exhibit peroxidase-like and oxidase-like activities, which may cause cytotoxicity, we systematically evaluated these activities of Fe1/NC-900. We used the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) to blue oxidized TMB (ox-TMB) with a characteristic adsorption at ca. 652 nm as a catalytic model reaction. As shown in Supplementary Fig. 18a, Fe1/NC-900 SAC exhibits peroxidase-like activity in BR buffer with a pH value of 4.00. However, under physiological conditions (pH 7.00), Fe1/NC-900 SAC shows negligible peroxidase-like activity, even in the presence of 5 mM H2O2. Similarly, the negligible absorbance of ox-TMB in O2-saturated BR buffer (pH 7.00) indicates that Fe1/NC-900 possesses low oxidase-like activity under a physiological pH (Supplementary Fig. 18b). Therefore, Fe1/NC-900 is unable to cause undesirable oxidative damage due to the low peroxidase-like and oxidase-like activities under the physiological pH.
Mechanism study of CAT-mimicking activity of Fe1/NC SACs
To investigate the CAT-mimicking catalytic process of Fe1/NC SAzymes, we conducted electron paramagnetic resonance (EPR) characterization. As shown in Supplementary Fig. 19, the EPR spectra of 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) and 2,2,6,6-tetramethyl-4-piperidinone hydrochloride (4-oxo-TEMP·HCl), specific probes for •OH and singlet oxygen (1O2), repectively, show no significant difference with or without Fe1/NC SAzymes, indicating that •OH and 1O2 were not generated during the catalytic process52,53,54. Subsequently, we employed BMPO to capture hydroperoxyl radical (•OOH) in methanol and found that •OOH was generated during the disproportionation reaction of H2O2 catalyzed by the three SAzymes (Fig. 4d–f), which aligns closely with the mechanism of natural enzymes55,56. In addition, the EPR intensity for •OOH correlates well with the CAT-mimicking activity of Fe1/NC SAzymes, indicating that Fe1/NC SAzymes catalyze the decomposition of H2O2 through the generation of the •OOH intermediate.
To further elucidate the catalytic mechanism and the difference in CAT-mimicking activity of Fe1/NC SAzymes, we carried out density functional theory (DFT) calculations. Based on the EPR and DFT results, we proposed the reaction pathways for CAT-like activity with Fe1/NC SACs (Fig. 4g), which involve six processes, including a significant •OOH generation step. Whereafter, we calculated the free energies of the reaction steps on the Fe active sites in Fe1/NC to identify the rate-determining step (RDS). As displayed in Fig. 4h and Supplementary Table 7, the adsorption energies of the reactant H2O2 on FeNx (x = 5, 4, and 3) sites in Fe1/NC-800, Fe1/NC-900, and Fe1/NC-1000 are -0.54 eV, 0.11 eV and 0.06 eV, respectively, demonstrating Fe1/NC-800 exhibits the highest binding affinity for H2O2, which is consistent with the result obtained from Km value. Furthermore, by analyzing the Gibbs free energy (ΔG) of the involved steps, we identified the H2O desorption step with a ΔG of 0.41 eV, the H2O2 adsorption step with a ΔG of 0.11 eV, and the •OOH generation step with a ΔG of 0.28 eV as the RDSs for Fe1/NC-800, Fe1/NC-900, and Fe1/NC-1000, respectively. Comparatively, Fe1/NC-900 possesses the lowest ΔG value of RDS, suggesting it has the highest CAT-mimicking activity. These findings demonstrate that the CAT-mimicking activity of SACs can be tuned by modulating the adsorption energy of the reactants and intermediates through atomic engineering of local coordination environment of active sites using high-temperature pyrolysis.
Inflammation-free electrochemical sensing with Fe1/NC-900
Encouraged by the excellent antioxidative activity of Fe1/NC-900 SAC, we further explored its electrode reactivity toward the oxidation of electroactive neurochemicals. We firstly employed K3Fe(CN)6, a widely used redox probe, to investigate the electrode reactivity of Fe1/NC SACs. Supplementary Fig. 20 shows typical cyclic voltammogram (CV) obtained at Fe1/NC SACs-modified glassy carbon electrodes (GCEs) in artificial cerebrospinal fluid (aCSF) containing 1 mM K3Fe(CN)6. Almost no current ascribed to the redox process of Fe(CN)64-/3- was recorded at Fe1/NC-800-modified GCE, suggesting the poor electrode activity of Fe1/NC-800. In contrast, Fe1/NC-900-modified GCE shows obvious redox peaks with an anodic/cathodic peak-to-peak separation (ΔEp) of about 150 mV, which is smaller than that at Fe1/NC-1000-modified GCE (170 mV), revealing the good electrode activity of Fe1/NC-900.
The good electrode activity of Fe1/NC-900 enabled us to develop an in vivo sensing platform for neurochemicals. DA is an important monoamine neurotransmitter that regulates a wide variety of complex neurochemical processes, such as motion, reward, and attention57,58. We chose DA as the target molecule to evaluate the electrochemical sensing ability of Fe1/NC SACs. To do this, we conducted CV measurements of DA at the Fe1/NC SACs-modified GCEs. As shown in Fig. 5a, the electrochemical oxidation of DA at Fe1/NC-900 commences at a potential of ca. −0.05 V (vs. Ag/AgCl) and shows an oxidation peak at ca. + 0.12 V (vs. Ag/AgCl). The onset potential at Fe1/NC-800 was more positive than 0.05 V with a tailed current response (Supplementary Fig. 21a). Fe1/NC-1000 and NC-900-modified GCEs show similar onset potentials compared to the Fe1/NC-900-modified GCE, but with significantly lower oxidation current responses (Supplementary Fig. 21b, c). Moreover, the current responses toward DA oxidation were also recorded with different catalysts under a constant potential (+0.20 V vs. Ag/AgCl). As displayed in Fig. 5b and Supplementary Figs. 22 and 23, the response sensitivity toward DA at Fe1/NC-900 was calculated to be 85 nA/μM, which is much higher than those obtained at Fe1/NC-800 (22 nA/μM), Fe1/NC-1000 (75 nA/μM) and NC-900 (54 nA/μM), suggesting a higher electrode activity of Fe1/NC-900 toward DA oxidation.
a CVs obtained at Fe1/NC-900-modified GCE in aCSF in the absence (gray) and presence (red) of 1 mM DA. Scan rate, 50 mV/s. b Plot of current response vs. DA concentration obtained with Fe1/NCs-modified GCEs. Applied potential, +0.20 V vs. Ag/AgCl. c CVs obtained at Fe1/NC-900-modified CFE in aCSF in the absence (gray) and presence (red) of 20 μM DA. Scan rate, 50 mV/s. d Amperometric response recorded at Fe1/NC-900-modified CFE toward successive additions of 5 μM DA in aCSF. Applied potential, +0.20 V vs. Ag/AgCl. Each experiment was repeated independently three times with similar results (a–d). Representative plots are shown. e Amperometric i-t curve recorded with Fe1/NC-900-modified CFE in rat NAc. f Typical amperometric response of Fe1/NC-900-modified CFE in rat NAc upon electrical stimulation of rat VTA (3 s at 60 Hz, ±300 μA) before (gray) and 25 min after (red) the rat were injected i.p. with a DA uptake inhibitor (NOM, 12 mg/kg). Applied potential, 0.20 V vs. Ag/AgCl. The in vivo experiments were repeated with 3 animals. Source data are provided with the paper.
Before in vivo analysis, we conducted in vitro experiments on Fe1/NC SACs using SH-SY5Y cell line. The SH-SY5Y cell line was chosen due to its neuronal-like properties, making it a commonly used model system to study various aspects of neurobiology, such as evaluating the effects of neurotoxins and the neuroprotective property of different agents59,60,61. First, we conducted the cytotoxicity experiments of Fe1/NC SACs. As shown in Supplementary Fig. 24, the cell viability assay indicates that the Fe1/NC SACs exhibit minimal cytotoxicity even at a concentration of 50 μg/mL, demonstrating their high biocompatibility. The negligible cytotoxicity of the Fe1/NC SACs suggests their potential application for safe and reliable in vivo neurochemical sensing.
We subsequently compared the efficiency of Fe1/NC SACs in protecting cells against oxidative stress using SH-SY5Y cell line. As displayed in Supplementary Fig. 25, H2O2 can induce obvious cytotoxicity on SH-SY5Y cells. Compared with the NC-900 control, the addition of 10 μg mL−1 Fe1/NC SACs to the cell-culture medium effectively attenuates H2O2-mediated oxidative damage and maintains cell viability. Among the SACs tested, Fe1/NC-900 exhibits the highest capability to eliminate H2O2. Furthermore, we used the xanthine oxidation reaction catalyzed by xanthine oxidase (XOD) to produce O2− in situ. After 12-hour exposure to Fe1/NC-900, the damage of SH-SY5Y cells caused by O2− is mostly reduced when compared with the NC-900 control and other Fe1/NC SACs (Supplementary Fig. 26), demonstrating Fe1/NC-900 also possesses the highest SOD-like activity for scavenging O2−. Additionally, we employed the Fenton reaction to produces •OH from the reaction between H2O2 and Fe2+. As shown in Supplementary Fig. 27, Fe1/NC SACs reduce apparent Fenton reagent-induced apoptosis, indicating their specific capability to eliminate •OH. All these results validated that the Fe1/NC-900 exhibits optimal capability to effectively mitigate oxidative stress and protect cells from ROS induced cytotoxicity.
Combining the excellent electrode activity with the high antioxidative properties of Fe1/NC-900, we prepared Fe1/NC-900-based carbon fiber electrodes (CFEs) to develop a platform for in vivo DA sensing. As shown in Fig. 5c, d and Supplementary Fig. 28, the Fe1/NC-900-modified CFE exhibits high performance toward DA oxidation with a good linearity between current response and DA concentrations. We implanted the Fe1/NC-900-modified CFE into nucleus accumbens (NAc) to test the stability of the microsensor. As displayed in Fig. 5e, the current response keeps constant over 3600-s measurement, demonstrating the high stability of Fe1/NC-900-based CFE in in vivo sensing. We further used the microsensor to in vivo record DA release in NAc triggered by electrical stimulation of ventral tegmental area (VTA)18. As shown in Fig. 5f, upon electrical stimulation, the current signal ascribed to DA release rises rapidly, reaches its maximal value within few seconds, and quickly decreases to the basal level. To verify the signal comes from DA release, we employed a DA uptake inhibitor, nomifensine (NOM), to block the DA uptake sites, thereby increasing DA overflow62. As illustrated in Fig. 5f, upon intraperitoneal injection of NOM, the current signal significantly increases, showing that the signals detected are indeed attributable to DA release. In addition, we assessed the stability of Fe1/NC-900 under in vivo conditions through TEM characterization. As displayed in Supplementary Fig. 29, the TEM images and corresponding elemental mapping images of Fe1/NC-900 after in vivo experiments show no significant changes, confirming the high stability of the catalyst under physiological environments. These results demonstrate the reliability of Fe1/NC-900 modified CFE for in vivo monitoring of DA dynamics with excellent spatial and temporal resolution.
As reported previously, the implantation of CFE into brain tissue inevitably results in a progressive inflammatory tissue response, including the activation of nearby microglia cells and astrocytes, which migrate to the electrode interface. In addition, the increase of hemoglobin can directly upregulate ROS level, further aggravating the inflammatory response15,16,17,18,19. To demonstrate the Fe1/NC-900, with a high antioxidative performance, can potentially endow the microsensor with anti-inflammatory properties, we performed immunohistochemical analysis of brain slices. We used specific markers for activated microglia (ionized calcium-binding adaptor molecule-1, Iba-1) and astrocyte (glial fibrillary acidic protein, GFAP), as well as diamidio-2-phenylindole (DAPI) to stain nuclei63,64.
To this end, after the implantation of the microsensor into rat brain for 8 h, brain tissues surrounding Fe1/NC-900-modified and bare (i.e., without surface modification with Fe1/NC-900) CFEs were collected, sliced, and stained for confocal laser scanning microscopy (CLSM) imaging. As displayed in Fig. 6a, Fe1/NC-900 modified CFE elicited a significantly lesser GFAP response toward astrocytes compared to the bare CFE. The markedly reduced intensity and spread of GFAP staining around the Fe1/NC-900 modified CFE indicates a lower level of astrocytic activation, suggesting that the Fe1/NC-900 effectively minimizes the astrocytic inflammatory response (Fig. 6b). Furthermore, more microglia were observed adjacent to the bare CFE compared to the Fe1/NC-900 modified CFE, as indicated by Iba-1 staining (Fig. 6a). The higher density of Iba-1 positive cells around the bare CFE signifies a robust microglial activation and clustering. In contrast, the Fe1/NC-900 modified CFE shows a significantly reduced microglial presence, suggesting that Fe1/NC-900 helps in mitigating microglial activation and the associated inflammatory processes (Fig. 6c). These histological data collectively demonstrate the high efficacy of Fe1/NC-900 in mitigating inflammation, thereby creating an improved microenvironment at neural interfaces adjacent to the CFE.
a Histological comparison of brain tissues from untreated rats and rats after 8-hour acute implantation of bare or Fe1/NC-900-modified CFE in NAc (n = 3, for each group). Tissues are labeled for astrocytes (green), microglia (magenta) and nuclei (blue). Scale bar, 50 µm. Fluorescence intensities of astrocytes (b) and microglia (c) in brain tissues from untreated rats (blue line) and rats after 8-h acute implantation of bare CFE (gray line) and Fe1/NC-900-modified CFE (red line) (n = 3, for each group). The distance was measured from the image center for the control group and from the implantation center for the bare and Fe1/NC-900-modified CFE groups. The data were presented as mean ± SEM. Quantitative analysis of ELISA for IL-6 (d), TNF-α (e), and IL-1β (f) in brain tissues from untreated rats (blue column) and rats after 8-hour acute implantation of bare (gray column) and Fe1/NC-900-modified (red column) CFE (n = 3, for each group). The data were presented as mean ± SEM and p values were provided in the figures using one-way analysis of variance (ANOVA). Source data are provided with the paper.
In addition, we investigated the expression of typical inflammatory cytokines using enzyme-linked immunosorbent assay (ELISA). Specifically, we measured the levels of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) in the brain tissue surrounding the implant site64,65. The IL-1β level indicates the activation of the inflammatory cascade, while the levels of IL-6 and TNF-α are known to mediate and amplify inflammatory processes. As shown in Fig. 6d–f, the insertion of the bare CFE dramatically upregulated inflammatory cytokines, indicating a pronounced inflammatory response. However, the introduction of Fe1/NC-900 gradually restored the levels of IL-1β, IL-6, and TNF-α to near-normal levels, suggesting an effective suppression of the inflammatory response. This anti-inflammatory effect was attributed to the antioxidative properties of the Fe1/NC-900, which mitigates the oxidative stress and subsequent cytokine production.
Overall, Fe1/NC-900 can simultaneously function both as SAzymes for scavenging ROS and as electrode material for DA oxidation, paving an avenue for accurately probing of neurochemical events in vivo with optimized biocompatibility and reduced local acute neuroinflammatory responses.
Discussion
To sum up, we developed a multifunctional sensing platform with antioxidative SAzyme for in vivo electrochemical sensing free from inflammation. By modulating the coordination environment through varying the pyrolysis temperature, we obtained Fe1/NC-900 SAC with FeN4 active sites, exhibiting the highest antioxidative activity and optimal electrochemical DA oxidation performance. The atomic-precision engineering of active sites enables the optimization of the adsorption energy of reactants and products, as well as the free energy of the reaction processes, thereby achieving the modulation of antioxidative ability and electrocatalytic activity. By integrating the dual functions of Fe1/NC-900 SAzyme, we successfully developed an implantable microsensor for DA sensing with anti-inflammatory capacity, enabling precise quantitative tracking of DA dynamics in vivo.
This study underscores the potential of SACs in biological applications, particularly in the development of implantable devices for reliable neurochemical sensing in vivo. The ability to effectively manage oxidative stress and inflammation presents approaches for enhancing the performance and reliability of neural interfaces, significantly advancing the understanding of brain function and disease mechanisms. Moreover, the successful integration of antioxidative SACs with CFEs highlights the feasibility of using multifunctional SACs in conjunction with in vivo electrochemistry. This integration will not only improve the biocompatibility of the sensing devices but also extend their functional lifespan, reducing the need for frequent replacements and minimizing the risk of complications associated with chronic implantation. Additionally, the highly designable SACs with atomic-level engineered active sites offer the potential to creating new SACs with tailored properties for specific biomedical applications.
Methods
Ethics declaration
Our research complies with all relevant ethical regulations: All animal experiments were conducted in accordance with the guidelines of the Animal Advisory Committee at the State Key Laboratory of Cognitive Neuroscience and Learning and were approved by the Animal Care and Use Committee at Beijing Normal University [IACUC(BNU)-NKLCNL 2021-09].
Materials
H2O2 (30%) was purchased from Sinopharm. FePc was obtained from Alfa Aesar. Zinc chloride was bought from Acros. 1H-1,2,3-triazole was purchased from Ark Pharm, Inc. Ammonia (25%), phosphoric acid, sodium hydroxide, and ferrous sulfate were purchased from Beijing Chemical Plant. Ethanol, glacial acetic acid, N, N-dimethylformamide and methanol were purchased from Tianjin Concord Technology Co., Ltd. SOD assay kits with WST-1 as a selective O2− probe and BMPO were purchased from DOJINDO Molecular Technologies. 4-Oxo-TEMP·HCl was purchased from Sigma-Aldrich. Boric acid was purchased from Beijing Yili Fine Chemicals Co., Ltd. Terephthalic acid, xanthine, and XOD were obtained from Sigma Aldrich. The BR buffer was formulated by mixing 0.04 M phosphoric acid, acetic acid and boric acid, and adjusting the pH to 7.0 by 0.2 M NaOH solution. Electrolyte used for electrochemical measurements was an aCSF prepared by mixing KCl (2.4 mM), NaCl (126 mM), KH2PO4 (0.5 mM), NaHCO3 (27.5 mM), MgCl2 (0.85 mM), CaCl2 (1.1 mM), and Na2SO4 (0.5 mM), with the pH adjusted to 7.4. The experimental water was Mili-Q pure water (18.2 MΩ·cm).
Synthesis of FePc@MET-6
Typically, a mixture of 1.25 g zinc chloride and 5 mg FePc was dissolved into a solution containing 12.5 mL ethanol, 12.5 mL N, N-dimethylformamide, and 18.75 mL water using ultrasonication. Then, 5 mL of ammonia was added to the solution and stirred uniformly, followed by the dropwise addition of 1.565 mL 1H-1,2,3-triazole. Subsequently, the mixture was stirred slowly at room temperature for 24 hours. The precipitate was centrifuged, washed three times with ethanol, and vacuum-dried to obtain FePc@MET-6. MET-6 was synthesized using the same procedure without FePc.
Synthesis of Fe1/NC SACs
The prepared FePc@MET-6 was ground into powder with a mortar and calcined in a tube furnace under Ar atmosphere at certain temperatures (800 °C for Fe1/NC-800, 900 °C for Fe1/NC-900) for 2 h, with a heating rate of 5 °C·min−1. Fe1/NC-1000 was obtained by calcining at 900 °C for 1 h followed by 1000 °C for an additional hour. Control catalysts (NCs) were obtained using similar procedures, but with MET-6 synthesized without FePc as the precursors.
Characterization
SEM images were recorded using a Hitachi S-4800. TEM images were acquired with a Hitachi-7700. XPS spectra were recorded using a PHI Quantum 2000 photoelectron spectrometer with monochromatic Al-Kα radiation, produced by an electron beam operating at 15 kV. XRD patterns were acquired using a Rigaku RU-200b X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). Nitrogen adsorption/desorption experiments were performed on a Quantachrome Autosorb iQ one-stop adsorption apparatus (77 K). Raman spectra were acquired using a LabRAM HR Raman Microscope at an excitation wavelength of 633 nm. Fe contents were measured using ICP-MS (Thermo Scientific™ iCAP™ RQ). Briefly, the catalyst was dissolved in aqua regia (HCl/HNO3 = 3:1, v/v) at 180 °C for 40 min using a microwave digestion system (ETHOS 1, LabTech), followed by ICP-MS analysis. HAADF-STEM images and elemental mapping images were acquired using a Titan G2-600 TEM (FEI) with a spherical aberration corrector. XAS spectra of Fe K edge were gained at BL14W1 station of the Shanghai Synchrotron Radiation Facility. The XAS data were obtained in fluorescence mode and monochromatized by a Si (111) double-crystal, with the energy calibrated using Fe foil.
Tests for CAT-like activity of the catalysts
CAT-like activity was assessed by measuring the O2 generation during the decomposition of H2O2 using a portable dissolved O2 meter (Seven2GoTM DO, Mettler Toledo). Typically, O2 generation was detected every 30 s in BR buffer (pH 7.0) containing 5 mM H2O2 and 5 μg·mL−1 catalyst. The CAT-mimicking kinetic analysis of the catalysts was carried out by using different H2O2 concentration (0.5–5.0 mM) while maintaining a fixed catalyst concentration of 5 μg·mL−1. Michaelis kinetic curves were obtained by plotting the initial rate of O2 generation against the H2O2 concentration. The maximum velocity (Vm) and the Michaelis constant (Km) were calculated by using the double reciprocal plot of the Michaelis–Menten curves.
Tests for SOD-like activity of Fe1/NC-900
The superoxide scavenging ability of Fe1/NC-900 and its comparison with NC-900 was determined using SOD assay kits following the manufacturer’s instructions.
Tests for •OH eliminating activity of Fe1/NC-900
The •OH radicals were produced through a Fenton reaction using 2 mM FeSO4 and 5 mM H2O2 for 10 min. After the reaction, 20 μg·mL−1 of enzyme mimics were added to the mixture. The amount of scavenged •OH radicals was subsequently assessed by measuring the fluorescence of 2-hydroxyterephthalic acid generated through the reaction of 0.5 mM terephthalic acid with •OH radicals at an excitation wavelength of 320 nm.
Theoretical calculation
DFT calculations were performed using the Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation and the projected enhanced wave method, as implemented in the Vienna Ab initio Stimulation Package (VASP). The cut-off energy of the plane wave fundamental set was set to 450 eV. Ultrasoft pseudopotentials were used to describe the interaction between valence electron and ionic nuclei. A special k-point grid of 3 × 3 × 1 Monkhorst–Pack was used for geometric optimization and electronic structure calculations. During geometry optimization, all atoms were freely released until the convergence thresholds for the maximum force and energy were less than 0.01 eV/Å and 1.0 × 10−5 eV/atom, respectively.
The free energy change (∆G) of every elementary step was given by the following equation:
∆E: the reaction energy
∆EZPE: zero-point energy difference
T: temperature
∆S: entropy difference
U: potential
q: charge
kB: Boltzmann constant.
Cell culture
The SH-SY5Y cell line purchased from Peking Union Medical College Hospital was used for in vitro experiments. SH-SY5Y cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere. The culture medium was partially renewed every 2 days.
Cell viability assay
The cytotoxicity of Fe1/NC SACs or NC controls were evaluated using the MTT assay (Beyotime Biotechnology, Shanghai, China). Briefly, SH-SY5Y cells were seeded into each well of 96-well plates at a density of 1 × 104 cells and incubated in the culture medium overnight to allow cell attachment. Afterward, the old culture medium was replaced with fresh medium containing Fe1/NC SACs or NC controls at final concentrations ranging from 0 to 50 μg·mL−1. Following an additional 12-h incubation, the cells were treated with MTT assay according to the manufacturer’s protocol for 2 h. Subsequently, relative cell viability was determined by measuring the absorbance of MTT at 490 nm using a Synergy H1M microplate reader (n = 4, for each group).
Protecting cells from H2O2 or •OH-induced oxidative damage
SH-SY5Y cells were seeded into 96-well plates at a density of 1 × 104 cells per well and incubated overnight. The old culture medium was then replaced with fresh medium containing 10 μg·mL−1 Fe1/NC SACs or NC controls. After an additional 12-h incubation, the old medium was discarded, and the cells were exposed to fresh medium containing 1 mM H2O2 or Fenton’s reagent (1 mM H2O2 and 400 μM FeSO4) for another 4-h incubation. Finally, the cell viability for each group was detected using MTT assay, respectively (n = 3, for each group).
Protecting cells from O2 −-induced oxidative damage
SH-SY5Y cell were seeded into 96-well plates at a density of 1 × 104 cells per well and incubated overnight. The old culture medium was replaced with fresh medium containing 10 μg·mL−1 Fe1/NC SACs or NC controls for a 12-hour incubation. Afterward, the old medium was discarded, and the cells were exposed to fresh medium containing XOD (20 mU·mL−1) and 250 μM xanthine for another 3-hour incubation. Finally, cell viability for each group was assessed using MTT assay, respectively (n = 3, for each group).
Electrochemical measurements
Electrochemical measurements were conducted on the CHI 660E electrochemical workstation using GCE (diameter: 3 mm) modified with catalyst, Pt electrode, and Ag/AgCl electrode, as the working electrode, auxiliary electrode, and reference electrode, respectively. For CV measurements of K3Fe(CN)6, GCE was modified with Fe1/NC SACs or NC controls (2.5 μL of 1 mg·mL−1 aqueous ink) and dried. The potential window was from −0.2 V to 0.6 V for 5 cycles, which was initiated at ca. 0.3 V cathodically and ended at 0.6 V. For CV measurement of DA, GCE was modified with Fe1/NC SACs or NC controls (7 μL of 2 mg·mL−1 aqueous ink) and dried. The potential window was from −0.2 V to 0.6 V for 3 cycles, which was initiated at −0.2 V and ended at 0.6 V. For amperometric measurement of DA, the Fe1/NC SACs or NC modified GCE were polarized at 0.2 V and staircase response of anodic current were acquired during DA addition.
Fabrication of Fe1/NC-900 modified CFE
CFEs were fabricated as follows33. Briefly, the exposed carbon fiber was cut to a length of 200–300 μm using a surgery scalpel under a microscope. The CFEs were then electrochemically activated in 1.0 M NaOH at +1.5 V for 80 s and scanned with CV from 0 to 1.0 V at a scan rate of 0.1 V·s−1 until a stable CV curve was obtained. To modify the CFEs with Fe1/NC-900, one drop of the Fe1/NC-900 aqueous dispersion (1 mg·mL−1) was dropped onto a clean glass slide and the CFE was carefully immersed and rolled in the droplet under a microscopy. The Fe1/NC-900-modified CFE was then allowed to dry at ambient temperature.
Electrical stimulation experiment for DA sensing
In vivo analysis was performed using adult male SD rats (300 ± 50 g), which were housed on a 12:12 h light-dark schedule with food and water ad libitum. The animals were anesthetized with isoflurane (4% induction, 2% maintenance) using a gas pump (RWD R520, Shenzhen, China) and positioned on a stereotaxic frame. The Fe1/NC-900 modified CFE was carefully implanted into NAc (Anteroposterior (AP) = +2.0 mm, mediolateral (ML) = +1.5 mm, and dorsoventral (DV) = − 6.0 mm from dura) for in vivo DA monitoring. An Ag/AgCl reference microelectrode was positioned into the brain tissue, and a platinum wire served as the auxiliary electrode. The Fe1/NC-900-modified CFE was polarized at 0.20 V vs. Ag/AgCl for in vivo measurement of DA. A bipolar stimulating electrode was implanted in VTA (AP = −4.9 mm, ML = +1.0 mm, DV = −8.2 mm from dura). A 180-pulse stimulation at 60 Hz ( ± 300 μA, 2 ms per phase) was applied to trigger DA release in the NAc. Pharmacological investigation of DA release was performed by intraperitoneal injection of a DA uptake inhibitor (nomifensine, NOM, 12 mg/kg), followed by another electrical stimulation 25 min after drug injection. The NOM was dissolved in saline.
Immunofluorescence staining and its quantitative analysis
The rats were perfused transcardially with saline followed by cold 4% paraformaldehyde (PFA) after the removal of bare or Fe1/NC-900 modified CFEs (n = 3, for each group). Dissected brains were block-fixed in 4% PFA overnight, then incubated overnight in 20% sucrose solution, and subsequently in 30% sucrose solution until the brains sank. The embedded brains were sectioned at a thickness of 30 μm perpendicular to the implantation path of CFEs using a freezing microtome. The sections underwent the following treatments: (1) three rinses (5 min each) with 0.01 M phosphate buffer saline (PBS) containing 0.2% Triton X-100 (Sigma-Aldrich, T8787), (2) blocking with normal Donkey serum for 30 min, (3) incubation with primary antibodies against Iba-1 (1:200, ab178846, Abcam) and GFAP (1:200, ab302644, Abcam) overnight at 4 °C. (4) three rinses (2 min each) with PBS, (5) incubation with secondary antibodies-Donkey Anti-Goat IgG H&L (Alexa Fluor® 488) (1:400, ab150133, Abcam) and Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 568) (1:400, ab175470, Abcam) for 1 h at room temperature. (6) three rinses (2 min each) with PBS, (7) incubation in DAPI for 5 min, (8) three rinses (2 min each) with PBS, and (9) final mounting onto glass slides with coverslips using Prolong Gold mounting media. Confocal images were collected using a Nikon single-particle microscopy equipped with a 40× objective lens. Immunomarkers were quantified using fluorescent intensity as a function of distance from the implantation site with the ImageJ software. The center of the implantation site was set as x = 0 μm. To obtain the fluorescence intensity curve, the adjacent region around the implantation center at 160 μm (assigned as d = 160 μm) was split into the circular contours of 20 μm increments, and the average intensities of these 20 μm circular contours were calculated. For normal tissue slices, the center of the image was set at x = 0 μm.
Measurement of inflammatory cytokines
The rats were divided into three groups of control, bare CFE, Fe1/NC-900-modified CFE (n = 3, for each group). Brain tissues surrounding the implants were harvested, homogenized on ice, and centrifuged at 8000 g for 10 minutes after 8-h insertion. The supernatant was stored at −80 °C for future use. Inflammatory cytokines were quantified by ELISA kits for IL-6 (Solarbio, SEKR-0005), IL-1β (Solarbio, SEKR-0002), and TNF-α (Solarbio, SEKR-0009). The experimental procedures were conducted according to the manufacturer’s instructions. Each sample was tested in duplicate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are present in the main text and Supplementary Information files. The raw data sets are available from the corresponding authors upon request. Source data are provided with this paper.
References
Chantranupong, L. et al. Dopamine and glutamate regulate striatal acetylcholine in decision-making. Nature 621, 577–585 (2023).
Lezmy, J. et al. Astrocyte Ca2+-evoked ATP release regulates myelinated axon excitability and conduction speed. Science 374, eabh2858 (2021).
Knaus, L. S. et al. Large neutral amino acid levels tune perinatal neuronal excitability and survival. Cell 186, 1950–1967 (2023).
Krok, A. C. et al. Intrinsic dopamine and acetylcholine dynamics in the striatum of mice. Nature 621, 543–549 (2023).
Zong, W. et al. Large-scale two-photon calcium imaging in freely moving mice. Cell 185, 1240–1256 (2022).
Wu, Z., Lin, D. & Li, Y. Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators. Nat. Rev. Neurosci. 23, 257–274 (2022).
Bednarik, P. et al. 1H magnetic resonance spectroscopic imaging of deuterated glucose and of neurotransmitter metabolism at 7 T in the human brain. Nat. Biomed. Eng. 7, 1001–1013 (2023).
Zhang, Y., Jiang, N. & Yetisen, A. K. Brain neurochemical monitoring. Biosen. Bioelectron. 189, 113351 (2021).
Robinson, D. L., Hermans, A., Seipel, A. T. & Wightman, R. M. Monitoring rapid chemical communication in the brain. Chem. Rev. 108, 2554–2584 (2008).
Wightman, R. M. Probing Cellular chemistry in biological systems with microelectrodes. Science 311, 1570–1574 (2006).
Schwerdt, H. N. et al. Long-term dopamine neurochemical monitoring in primates. Proc. Nat. Acad. Sci. USA. 114, 13260–13265 (2017).
Shao, Z., Wilson, L., Chang, Y. & Venton, B. J. MPCVD-grown nanodiamond microelectrodes with oxygen plasma activation for neurochemical applications. ACS Sens. 7, 3192–3200 (2022).
Forderhase, A. G., Ligons, L. A., Norwood, E., McCarty, G. S. & Sombers, L. A. Optimized fabrication of carbon-fiber microbiosensors for codetection of glucose and dopamine in brain tissue. ACS Sens. 9, 2662–2672 (2024).
Zhao, C. et al. Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 7, eabj7422 (2021).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Wellman, S. M. & Kozai, T. D. Y. Understanding the inflammatory tissue reaction to brain implants to improve neurochemical sensing performance. ACS Chem. Neurosci. 8, 2578–2582 (2017).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Clark, J. J. et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7, 126–129 (2010).
Kozai, T. D. Y., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C. & Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 6, 48–67 (2015).
Forman, H. J. & Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nature Rev. Drug Discov. 20, 689–709 (2021).
Huang, Y., Ren, J. & Qu, X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119, 4357–4412 (2019).
Wu, J. et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076 (2019).
Jiang, W. et al. Chiral metal-organic frameworks incorporating nanozymes as neuroinflammation inhibitors for managing Parkinson’s disease. Nat. Commun. 14, 8137 (2023).
Xu, B. et al. A single-atom nanozyme for wound disinfection applications. Angew. Chem. Int. Ed. 58, 4911–4916 (2019).
Kraushofer, F. & Parkinson, G. S. Single-atom catalysis: insights from model systems. Chem. Rev. 122, 14911–14939 (2022).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Huang, L., Chen, J., Gan, L., Wang, J. & Dong, S. Single-atom nanozymes. Sci. Adv. 5, eaav5490 (2019).
Li, G. et al. Dimensionality engineering of single-atom nanozyme for efficient peroxidase-mimicking. J. Am. Chem. Soc. 145, 16835–16842 (2023).
Ji, S. et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 4, 407–417 (2021).
Lu, X. et al. Single-atom catalysts-based catalytic ROS clearance for efficient psoriasis treatment and relapse prevention via restoring ESR1. Nat. Commun. 14, 6767 (2023).
Cao, F. et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nat. Nanotech. 18, 617–627 (2023).
Fei, H. et al. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 48, 5207–5241 (2019).
Gao, X. et al. A single-atom Cu–N2 catalyst eliminates oxygen interference for electrochemical sensing of hydrogen peroxide in a living animal brain. Chem. Sci. 12, 15045–15053 (2021).
Zhou, M. et al. Single-atom Ni-N4 provides a robust cellular NO sensor. Nat. Commun. 11, 3188 (2020).
Ma, W. et al. A single-atom Fe–N4 catalytic site mimicking bifunctional antioxidative enzymes for oxidative stress cytoprotection. Chem. Commun. 55, 159–162 (2019).
Gándara, F. et al. Porous, Conductive metal-triazolates and their structural elucidation by the charge-flipping method. Chem. Eur. J. 18, 10595–10601 (2012).
Chen, D. et al. Bioinspired porous three-coordinated single-atom Fe nanozyme with oxidase-like activity for tumor visual identification via glutathione. Nat. Commun. 14, 7127 (2023).
Wei, S. et al. Self-carbon-thermal-reduction strategy for boosting the Fenton-like activity of single Fe-N4 sites by carbon-defect engineering. Nat. Commun. 14, 7549 (2023).
Barrio, J. et al. FeNC oxygen reduction electrocatalyst with high utilization penta-coordinated sites. Adv. Mater. 35, 2211022 (2023).
Fu, H. et al. Machine-learning-assisted optimization of a single-atom coordination environment for accelerated Fenton catalysis. ACS Nano 17, 13851–13860 (2023).
Xiong, Y. et al. Single-atom Fe catalysts for Fenton-like reactions: Roles of different N species. Adv. Mater. 34, 2110653 (2022).
Xue, D. et al. Altering the spin state of Fe-N-C through ligand field modulation of single-atom sites boosts the oxygen reduction reaction. Nano Energy 105, 108020 (2023).
Chen, Y. et al. Atomic-level regulation of cobalt single-atom nanozymes: Engineering high-efficiency catalase mimics. Angew. Chem. Int. Ed. 62, e202301879 (2023).
Zhou, J. et al. Coordination-driven self-assembly strategy-activated Cu single-atom nanozymes for catalytic tumor-specific therapy. J. Am. Chem. Soc. 145, 4279–4293 (2023).
Jiang, P. et al. Tuning oxidant and antioxidant activities of ceria by anchoring copper single-site for antibacterial application. Nat. Commun. 15, 1010 (2024).
Singh, N., Savanur, M. A., Srivastava, S., D’Silva, P. & Mugesh, G. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. Int. Ed. 56, 14267–14271 (2017).
Feng, K., et al. Breaking the pH limitation of nanozymes: Mechanisms, methods, and applications. Adv. Mater. 36, 2401619 (2024).
Ma, L. et al. A natural biogenic nanozyme for scavenging superoxide radicals. Nat. Commun. 15, 233 (2024).
Gao, W. et al. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 14, 160 (2023).
Wang, Z. et al. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 9, 3334 (2018).
Wang, B. et al. Atomization-induced high intrinsic activity of a biocompatible MgAl-LDH supported Ru single-atom nanozyme for efficient radicals scavenging. Angew. Chem. Int. Ed. 62, e202307133 (2023).
Liu, Q. et al. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat. Mater. 20, 395–402 (2021).
Teng, K.-X., Niu, L.-Y., Xie, N. & Yang, Q.-Z. Supramolecular photodynamic agents for simultaneous oxidation of NADH and generation of superoxide radical. Nat. Commun. 13, 6179 (2022).
Wen, D., Li, K., Deng, R., Feng, J. & Zhang, H. Defect-rich glassy IrTe2 with dual enzyme-mimic activities for sono-photosynergistic-enhanced oncotherapy. J. Am. Chem. Soc. 145, 3952–3960 (2023).
Alfonso-Prieto, M., Biarnés, X., Vidossich, P. & Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 131, 11751–11761 (2009).
Campomanes, P., Rothlisberger, U., Alfonso-Prieto, M. & Rovira, C. The molecular mechanism of the catalase-like activity in horseradish peroxidase. J. Am. Chem. Soc. 137, 11170–11178 (2015).
Garritsen, O., van Battum, E. Y., Grossouw, L. M. & Pasterkamp, R. J. Development, wiring and function of dopamine neuron subtypes. Nat. Rev. Neurosci. 24, 134–152 (2023).
Liu, C., Goel, P. & Kaeser, P. S. Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci. 22, 345–358 (2021).
Kovalevich, J. & Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. In Neuronal cell culture: Methods and protocols (eds. Amini, S. & White, M. K.) 9−21 (Humana Press, 2013).
Xicoy, H., Wieringa, B. & Martens, G. J. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol. Neurodegener. 12, 10 (2017).
Wu, D. et al. Pathogenesis-adaptive polydopamine nanosystem for sequential therapy of ischemic stroke. Nat. Commun. 14, 7147 (2023).
Condon, M. D. et al. Plasticity in striatal dopamine release is governed by release-independent depression and the dopamine transporter. Nat. Commun. 10, 4263 (2019).
Xu, T. et al. Synergistic charge percolation in conducting polymers enables high-performance in vivo sensing of neurochemical and neuroelectrical signals. Angew. Chem. Int. Ed. 61, e202204344 (2022).
Liu, S. et al. A nanozyme-based electrode for high-performance neural recording. Adv. Mater. 36, 2304297 (2024).
Wei, S. et al. Shape-changing electrode array for minimally invasive large-scale intracranial brain activity mapping. Nat. Commun. 15, 715 (2024).
Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (Grant Nos. 22134002 for L.M., 22125406 and 22074149 for P.Y.), the Natural Science Foundation of Beijing (2242028 for W.M. and Z230022 for P.Y.), the National Basic Research Program of China (2018YFA0703501, 2018YFA1204503 and 2022YFA1204500 for P.Y.). We are grateful to the photoemission end station beamline BL14W1 in the Shanghai Synchrotron Radiation Facility for XAS characterizations.
Author information
Authors and Affiliations
Contributions
L.M. and W.M. conceived the idea. X.G. carried out the synthesis, characterization and performance measurements. H.W. and W.J. conducted the in vivo analysis. J.M. performed the XAS characterization. W.W. analyze the XAS data. P.Y. participated in the discussion of the study. X.G., W.M. and H.W. wrote the manuscript, P.Y. and L.M. revised and finalized it. All authors contributed comments on this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhimin Tian, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, X., Wei, H., Ma, W. et al. Inflammation-free electrochemical in vivo sensing of dopamine with atomic-level engineered antioxidative single-atom catalyst. Nat Commun 15, 7915 (2024). https://doi.org/10.1038/s41467-024-52279-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52279-5
This article is cited by
-
Preparation and biomedical applications of single-metal atom catalysts
Nature Protocols (2026)
-
Scalable synthesis of sustainable single-atom catalysts for vascular healing
Nature Sustainability (2025)
-
In vivo multimodal neurochemical interfaces for real-time decoding of brain circuit
Nature Reviews Neuroscience (2025)