Abstract
Li-based all-solid-state batteries (ASSBs) are considered feasible candidates for the development of the next generation of high-energy rechargeable batteries. However, ASSBs are detrimentally affected by a limited rate capability and inadequate performance at high currents. To circumvent these issues, here we propose the use of Nb1.60Ti0.32W0.08O5-δ (NTWO) as negative electrode active material. NTWO is capable of overcoming the limitation of lithium metal as the negative electrode, offering fast-charging capabilities and cycle stability. Physicochemical and electrochemical characterizations of NTWO in combination with the Li6PS5Cl (LPSCl) solid-state electrolyte demonstrate that the formation of LiWS2 at the electrode|electrolyte interphase is the main responsible for the improved battery performance. Indeed, when an NTWO-based negative electrode and LPSCl are coupled with a LiNbO3-coated LiNi0.8Mn0.1Co0.1O2-based positive electrode, the lab-scale cell is capable of maintaining 80% of discharge capacity retention after 5000 cycles at 45 mA cm−2 at 60 °C and 60 MPa.
Similar content being viewed by others
Introduction
All-solid-state batteries (ASSB), emerging as the next generation of batteries, aim to address the safety and energy density concerns associated with conventional lithium-ion batteries (LIBs)1,2. Research has explored various types of solid electrolytes, such as sulfides and oxides. However, garnet-type solid electrolytes based on Li7La3Zr2O123,4 face challenges in large-scale production due to the necessity of high-temperature sintering in their manufacturing process5. In contrast, sulfide solid electrolytes are considered most suitable for ASSB, owing to their high lithium-ion conductivity, which typically ranges from 1 to 25 mS cm−1 at 25 °C6. The widely used lithium metal negative electrode is under active research to overcome its limitations7,8. While significant progress has been made, key challenges are yet to be fully resolved, such as cost, the instability of lithium dendrite formation9,10, and issues with fast charging11. To overcome these challenges, various negative electrodes have been explored, including silicon-based materials12,13,14 and initially anode-free ASSBs15,16. However, concerns regarding fast charging and cycle lifespan remain unresolved. Recently, Nb-oxide has gained attention as a promising electrode material in LIBs, notably for its fast-charging capability and durability17,18. Defect-induced Nb2O5 phases19 have shown enhanced fast-charging characteristics and cycle stability. In this study, we introduced Ti and W into the Nb2O5 structure to create Nb1.60Ti0.32W0.08O5−δ (NTWO) and applied it as the negative electrode in ASSBs. Compared to conventional Li-metal electrodes, NTWO exhibited improved fast-charging ability and cycle stability. Notably, during operation, the reaction between NTWO and the Li6PS5Cl (LPSCl) solid electrolyte led to the self-construction of a thin film of LiWS2, which improved cycle stability of the cell during long-term cycling Previous studies have demonstrated that a positive electrode coating of tungsten dichalcogenides (such as WS2 and WSe2) enhances the stability of the positive electrode|electrolyte interface, thereby effectively enhancing the cycling performance. Specifically, the interface between the LPSCl electrolyte and the LiNixMnyCozO2 (NMC) electrode is stabilized when the NMC surface is coated with WS2, leading to improved cycling performance in ASSBs20. Similarly, the introduction of WSe2 on the positive electrode surface in conventional battery systems utilizing non-aqueous liquid electrolyte solutions has been shown to protect the electrode against the dissolution of transition metal and confer structural stability, thus contributing to enhanced stability21. In contrast, our study demonstrates that a thin film of lithiated WS2 is formed in situ at the NTWO|LPSCl interphase without any additional surface treatment, enhancing the electrochemical stability of ASSBs. The use of an NTWO negative electrode suppressed the formation of problematic Li2S phases, which is typically observed when a Li-metal negative electrode is used22, but promoted the formation of a favorable LiWS2 phase, as confirmed through simulation. The NTWO negative electrode tested in combination with LPSCl solid electrolyte and LiNbO3-coated LiNi0.8Mn0.1Co0.1O2 (NMC811) positive electrode enables a discharge/charge current density of 45 mA cm−2 and an areal capacity of 1 mAh cm−2 under the stacking pressure of 60 MPa when operated at 60 °C. After 5000 cycles, it retained 80% of its cycle retention.
Results
Physicochemical characterization of the NTWO
Scanning electron microscopy (SEM) images reveal that the prepared NTWO powder samples consist of primary particles measuring 80–150 nm. These primary particles aggregate to form spherical secondary particles with a size range of 800–1000 nm (Fig. 1a, b). The scanning transmission electron microscopy (STEM) image (Fig. 1b) and energy-dispersive X-ray spectroscopy (EDS) image (Fig. 1c) clearly indicate that the elements Nb, Ti, and W are uniformly distributed within the structure. To observe the physicochemical characteristics of NTWO, we performed X-ray diffraction (XRD) and Raman spectroscopy analyses. As shown in Fig. 1d, the XRD pattern of NTWO is different from that of Ti2Nb10O29 (TNO) and H-Nb2O5 annealed under the same condition. This suggests that the introduction of W into the structure of TNO resulted in the creation of a defected structure, as revealed by high-angle annular dark-field (HAADF) STEM images (Supplementary Fig. 1)23. The broadening of peaks in the Raman spectra (Fig. 1e) is attributed likely to mixed-phase effects. Particularly, the intensity of the peaks within the 450–550 and 800–900 cm−1 range, corresponding to the corner-sharing transition metal (Nb,Ti,W)/O polyhedrons, is markedly lower compared to other materials. This suggests an improved lithium transport ability24,25,26. As shown in Fig. 1f, NTWO primarily comprises Nb5+, Ti4+, and W6+. A marginal reduction in these elements is observed as Ti and W are incorporated into the Nb2O5 structure. This incorporation gives rise to diverse block structures within the NTWO framework and contributes to the formation of defect structures27 (Supplementary Fig. 1).
Electrochemical characterizations of the NTWO-based electrodes
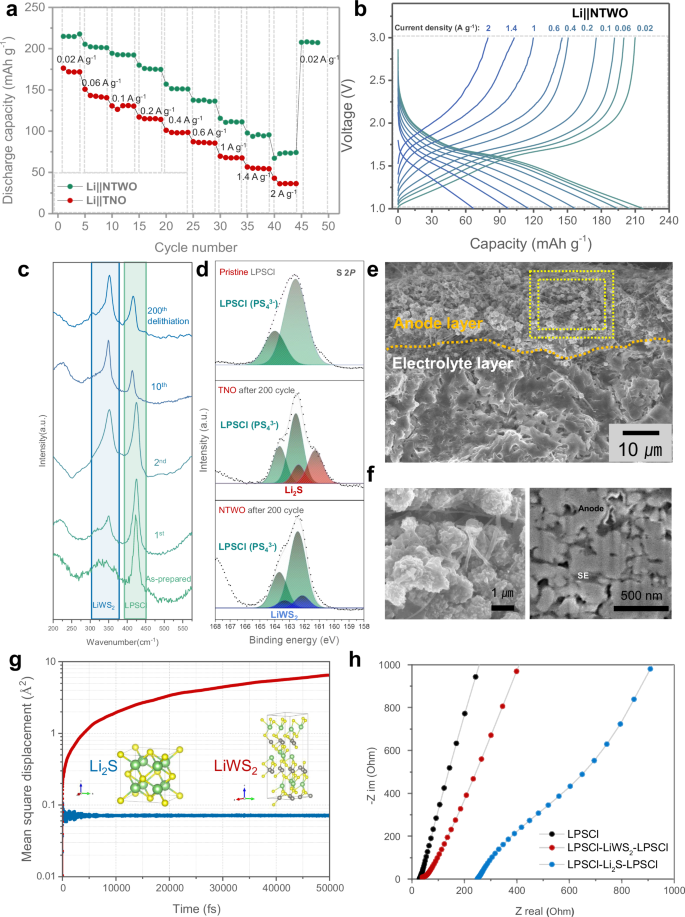
The NTWO-based electrodes with a loading level of 1.8 mg cm−2 were tested in combination with LPSCl solid electrolyte and Li metal counter electrode. The operation at 2 A g−1 (10 C) was feasible (Fig. 2a) at 25 °C under a stack pressure of 60 MPa. The performance variations of NTWO materials with temperature are described in Supplementary Note 1. The voltage profile shows stable behavior under current conditions ranging from 0.02 A g−1 (0.1 C) to 2 A g−1 (10 C) (Fig. 2b). The theoretical capacity of Nb₂O₅ is 200 mAh g−1 28, and considering the variation in actual capacity depending on the dopant and phase, it is evident that a reasonable specific capacity was achieved in the Li|LPSCl|NTWO cell test. Furthermore, subsequent operation at 0.02 A g−1 post a 2 A g−1 operation resulted in a specific capacity of 96.97% compared to the initial operation at 0.02 A g−1. When compared to TNO, Li metal cells with NTWO-based electrodes show improved capacity development across all rate spectrums. Notably, cells employing TNO experienced failure after high-output operations at 2 A g−1 under a stack pressure of 60 MPa. This suggests that the presence of tungsten (W) in NTWO might be pivotal in contributing to its distinguished performance. In order to observe long-cycle stability at 25 °C, we tested the NTWO electrode under the current density of 1.08 mA cm−2 (Supplementary Fig. 3). The result revealed Li|LPSCl|NTWO cell capacity retentions of 92.33% at 100 cycles and 70.28% at 4000 cycles. The average Coulombic efficiency throughout these 4000 cycles was 99.91%, and a consistent operational mechanism was observed across cycles, as evidenced by dQ dV−1 analysis (Supplementary Fig. 4).
a Evaluation of rate capability at 25 oC for Li||NTWO and Li||TNO cells with LPSCl solid-state electrolyte under the stack pressure of 60 MPa. b The voltage profile for the Li|LPSCl|NTWO cell rate capability test at 25 oC under the stack pressure of 60 MPa. c Raman analysis results for the as-prepared NTWO negative electrode composite and the negative electrode composite after discharge following the 1st, 2nd, 10th, and 200th cycles at a current density of 1.08 mA cm⁻². d XPS analysis of S 2p after discharge following 200 cycles for cells using TNO and NTWO as respective negative electrode composites as well as pristine LPSCl. e Cross-sectional SEM images of the negative electrode layer after the operation, and f high-magnification images of NTWO particles before and after FIB milling. g Mean square displacement from MD simulations of Li2S and LiWS2. h EIS measurement results for LPSCl pellet, LPSCl|LiWS2|LPSCl pellet, and LPSCl|Li2S|LPSCl pellet.
In the general ASSBs operation process, it has already been confirmed that the LPSCl on the negative electrode side decomposes into components such as Li2S, LiCl, and Li3P29. This decomposition, particularly of Li2S, known for its poor ionic and electronic conductivities, increases interfacial resistance between the negative electrode and the LPSCl electrolyte30. This phenomenon critically hampers stable cell operation. We have employed Raman spectroscopy analysis to observe the changes in the negative electrode composite containing NTWO during operation (Fig. 2c). In our analysis of the negative electrode composite phases across operating cycles, no peak related to Li2S was detected in NTWO. Instead, a peak corresponding to the LiWS2 phase31,32 was identified starting from the second cycle. The intensity of the LiWS2 peak progressively increased and saturated by the 10th cycle. Thereafter, the LiWS2 peak remained consistent up to the 200th cycle. To further understand the correlation between the formation of LiWS2 and the generation of Li2S due to solid electrolyte decomposition, we analyzed the NTWO negative electrode composite after 200 cycles of operation in detail using XPS. For comparison, the TNO negative electrode composite, operated identically for 200 cycles, was also analyzed (Fig. 2d). After 200 cycles, the TNO negative electrode composite clearly showed the formation of Li2S, while the NTWO negative electrode composite only exhibited LiWS2 peaks33,34 along with PS43− peaks, without any trace of Li2S. The results of the Raman and XPS analysis suggest the possibility that the LiWS2 formed in the NTWO negative electrode composite suppresses the formation of Li2S. Figure 2e presents SEM images of the cross-section and the morphology of the NTWO negative electrode material after the operation. Upon observing the negative electrode composite, NTWO, LPSCl, and vapor-grown carbon fibers (VGCFs) are appropriately mixed and blended (Fig. 2f), and they maintain uniform distribution after the operation. Notably, there were no deformations, such as the collapse of spheres or size changes observed after operation. Examination of the cross-section using FIB revealed no internal defects in NTWO particles, confirming the maintenance of composite formation with the solid electrolyte. Cyclic voltammetry (CV) results further confirm that changes occur due to the formation of LiWS2 (Supplementary Fig. 5). Details regarding the CV results are described in Supplementary Note 2.
To investigate the formation of the interphase layer, we systematically constructed bulk structures for NTWO, LPSCl, Li2S, LiWS2, then conducted density functional theory (DFT) calculations35 (see the supplementary Figs. 6–9) and molecular dynamics-Monte Carlo (MD-MC) simulations were conducted using a supercell of Nb180Ti36W8O552, employing the m3gnet interatomic potential36. It was observed that Ti atoms tend to occupy the center of 3*4 blocks while W atoms are situated at the corners of 4*4 and 3*4 blocks. Detailed descriptions of the construction of the NTWO structure and the formation reactions of Li2S and LiWS2 are provided in Supplementary Note 3.
Initially, we investigated reaction 1 (Eq. (1) in Supplementary Note 3), which elucidates the generating of Li2S from LPSCl while preserving its original crystal structure. The computed energy difference for this reaction was determined to be 0.77 eV. Subsequently, upon the introduction of NTWO into the system, we observed a more energetically favorable formation of LiWS2. As delineated in Reaction 2 (Eq. (2) in Supplementary Note 3), the generation of LiWS2, involving both LPSCl and NTWO, while preserving their crystal structures in the resulting products, displayed a notable energy difference of −2.40 eV. Following these investigations, we conducted ab initio molecular dynamics (AIMD) simulations for the two interphase layers, Li2S and LiWS2, with a focus on the trajectory of Li ions within these structures. Machine learning interatomic potentials (ML-IAP) tailored for Li2S (Supplementary Fig. 10) and LiWS2 (Supplementary Fig. 11) were developed. Subsequently, we performed 50 ps of AIMD simulations at a temperature of 300 K and calculated the mean squared displacement (MSD) of Li ions within both structures. Our analysis of the resulting graphs (Fig. 2g) reveals that the MSD of Li ions in Li2S remains nearly constant over time, indicating the anchoring of Li ions in their original positions. In contrast, the MSD of Li ions in LiWS2 exhibits a linear increase over time, revealing a distinctive pattern of Li-ion mobility within this structure.
To compare the conductivity of Li2S and LiWS2, special pellets were designed with LPSCl|interphase material (LiWS2 or Li2S)|LPSCl symmetric cell configuration and conducted electrochemical impedance spectroscopy (EIS) measurements and analyses (Fig. 2h). Specifications of the cell are described in Supplementary Note 4. The pellet with the inserted Li2S layer showed an increased total impedance value compared to the pure LPSCl pellet. In contrast, the pellet with the inserted LiWS2 layer in the middle showed a relatively moderate increase in total impedance. Furthermore, the comparison of electronic conductivity revealed that LiWS₂ has higher electronic conductivity than Li₂S at 25 °C (Supplementary Fig. 13). To compare the electronic conductivity of LiWS2 and Li2S, each material was prepared as a pellet, and descriptions of the experimental process and results are provided in Supplementary Note 5. These analysis results experimentally demonstrate that the formation of the LiWS2 phase at the interphase between the negative electrode and solid electrolyte, compared to the formation of Li2S, has lower resistance to Li-ion transport, aligning with the simulation results.
Assembly and testing of symmetric and asymmetric cells containing NTWO-based electrodes
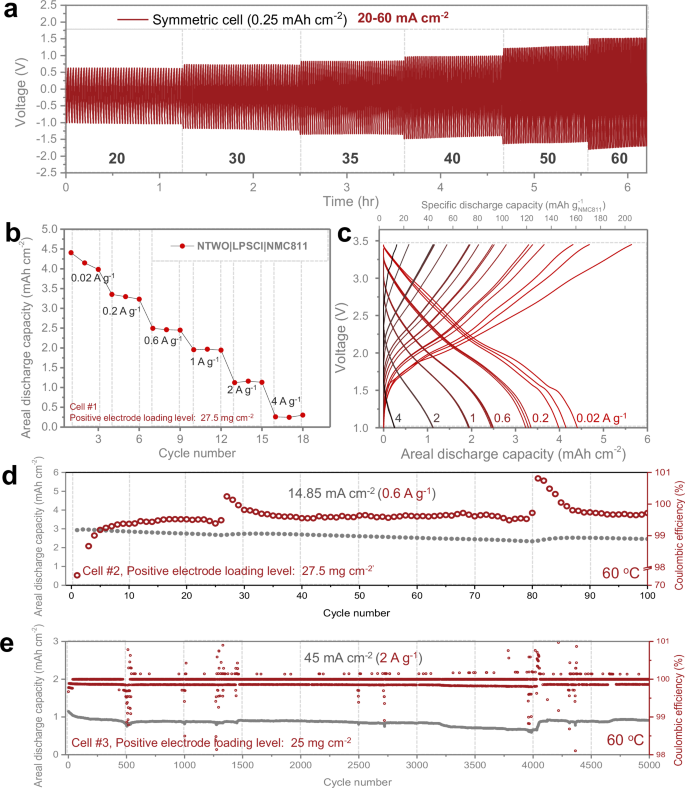
To determine the maximum current density that the NTWO negative electrode can sustain without being influenced by the performance of other electrode materials, we evaluated a symmetric cell configuration consisting of lithiated NTWO|LPSCl|NTWO configuration. Previous research on similar sulfide-type solid electrolyte systems has shown that symmetric cells utilizing a lithium metal negative electrode can operate up to a current density of 20 mA cm−237. Our results show the possibility of NTWO materials operating efficiently up to the current density of 20 mA cm−2 (Supplementary Fig. 14), and even at a current density as high as 60 mA cm−2 with an overpotential around 1.5 V (Fig. 3a). When operated continuously at 20 mA cm−2, the cell showed stable performance over 2000 cycles without a significant increase in overpotential (Supplementary Fig. 15). Even at a current density of 70 mA cm−2, degradation was minimal, with the initial overpotential of 1.9 V increasing to only 2.0 V after 100 h (Supplementary Fig. 16).
a The measurement results of the overpotential according to the current density ranging from 20 to 60 mA cm−2 for the lithiated NTWO|LPSCl|NTWO symmetric cell at 25 °C. The areal capacity was maintained at a fixed value of 0.25 mAh cm−2 throughout the test. b Rate capability at 60 °C for NTWO||NCM811 cell (positive electrode loading level = 27.5 mg cm−2), and c corresponding voltage profile. d Assessment of long cycle stability at a current density of 14.85 mA cm−2 (positive electrode loading level = 27.5 mg cm−2), and e at a current density of 45 mA cm−2 at 60 °C (positive electrode loading level = 25 mg cm−2). Stacked pressure during cycling is 60 MPa.
When paired with an NMC811 positive electrode, several noteworthy observations emerged. The NTWO|LPSCl|NMC811 cell was initially assessed at 25 °C (Supplementary Fig. 17) under the stack pressure of 60 MPa. In this evaluation, a cell constructed with a positive electrode loading of 13.12 mg cm−2 exhibited an initial areal capacity of about 1.4 mAh cm−2 when operated at a current density of 1.6 mA cm−2. Upon elevating the temperature to 60 °C, the evaluation at a higher loading level and higher operational current density further underscored the potential of NTWO (Fig. 3b). For the evaluation of rate capability at 60 °C, Cell#1 was prepared with a positive electrode loading level of 27.5 mg cm−2. As evident from the voltage profile (Fig. 3c), stable behavior was observed at all operating currents. In particular, the NTWO|LPSCl|NMC811 cell achieved an areal capacity of 1 mAh cm−2 even at 50 mA cm−2. Moreover, operations remained steady even at 100 mA cm−2. The cell with varied positive electrode loading configurations was tested for the long cycle stability of the NTWO|LPSCl|NMC811 cell. In the case of Cell#2, featuring a positive electrode loading level of 27.5 mg cm−2, the cell was operated at a current density of 14.85 mA cm−2 at 60 °C, as shown in Fig. 3d. Notably, Cell#2 demonstrated a high areal capacity of 2.96 mAh cm−2, and exhibited a capacity retention of approximately 84% after 100 cycles. These results demonstrate that the NTWO|LPSCl|NMC811 cell can maintain an areal capacity of around 3 mAh cm−2, even under the current density of 14.85 mA cm−2. The Cell#3, as shown in Fig. 3e, was fabricated with a positive electrode loading of 25 mg cm−2. Despite the high current density of 45 mA cm−2 at 60 °C, Cell#3 still exhibited an initial areal capacity of about 1 mAh cm−2, with around 80% capacity retention observed after 5000 cycles. Additional results could be achieved with different operational conditions. Detailed descriptions of Cells #4, #5, #6, and #7, evaluated under various conditions, are provided in Supplementary Notes 6–8. When comparing the Nb2O5|LPSCl|NCM811 cell without the LiWS2 layer under similar conditions (Supplementary Fig. 21), it initially showed electrochemical performance similar to that of the NTWO-based cell. However, the cell with Nb₂O₅ exhibited severe capacity fading after 1300 cycles. This result demonstrates the impact of LiWS₂ on stability due to the suppression of Li2S formation.
The negative electrode layer and positive electrode layer were further examined after operation after 5000 cycles under 45 mA cm−2 at 60 °C under the stack pressure of 60 MPa via ex-situ measurements (Supplementary Figs. 22 and 23). The EDS analysis of the negative electrode composite is described in Supplementary Note 9, and details regarding the structure of the positive electrode are provided in Supplementary Note 10. After operation at harsh conditions (5000 cycles under 45 mA cm−2 at 60 °C under the stack pressure of 60 MPa), the positive electrode layer exhibited deterioration (Supplementary Fig. 23d). The deformed structure of the positive electrode composite suggests the possibility that the main cause of capacity fading could lie on the positive electrode side.
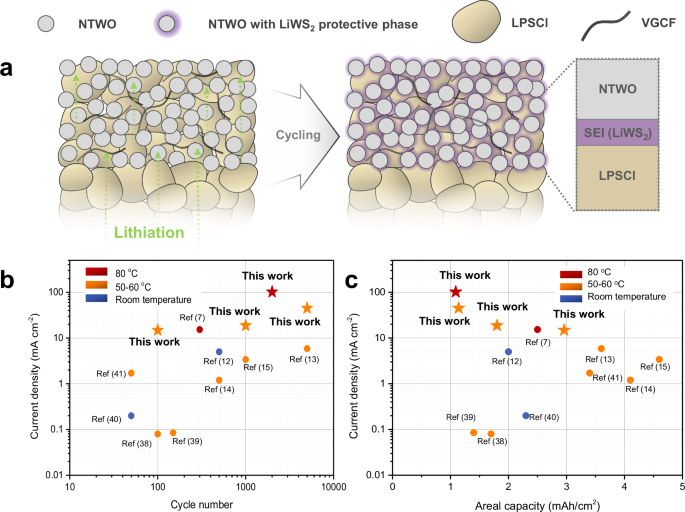
Figure 4a presents a schematic illustration depicting the interphase formation during the operation of the ASSB. The NTWO negative electrode undergoes lithiation and delithiation processes, leading to the formation of thermodynamically more favorable LiWS2 over the decomposition of LPSCl, specifically the formation of Li2S. This process results in the formation of a solid electrolyte interphase (SEI) at the interface. This process has been observed to occur consistently over 10 charge–discharge cycles, reaching saturation thereafter, as demonstrated by Raman analyses. LiWS2 interphase effectively prevents performance degradation during long-term operation by suppressing the formation of Li2S, which has inadequate electrical conductivity. Instead, the LiWS2 interphase mitigates the degradation of the electrochemical performance of the cell even under harsh long-term operating conditions (5000 cycles under 45 mA cm−2 at 60 °C under the stack pressure of 60 MPa). The cell, utilizing the NTWO negative electrode and NMC811 positive electrode, has exhibited stable operation under various temperature conditions and operational current densities based on electrochemical evaluation.
a Schematic illustration of the forming LiWS2 protective phase at the interface between the NTWO negative electrode and LPSCl during operation. b Comparison based on current density and cycle number and c current density and areal capacity with reported studies demonstrating long-term operational stability in recent sulfide-type all-solid-state batteries, specifically those exhibiting an areal capacity of more than 1 mAh cm−2 and operating under stack pressures ranging from 2.5 to 265 MPa.
In Fig. 4b, c, materials and results from state-of-the-art studies38,39,40,41 reporting practical areal capacities of over 1 mAh cm−2 in sulfide-type ASSBs have been reorganized and compared in terms of cycle number, areal capacity, and current density. The results presented in this study demonstrate the possibility of operation at higher current densities compared to other studies, even proving long-term operation. In particular, operation at 45 mA cm−2 under 60 °C with a stack pressure of 60 MPa and at 101.66 mA cm−2 under 80 °C with a stack pressure of 60 MPa demonstrated higher current operation compared to other systems shown in this graph. While several previously reported studies have shown high values in terms of areal capacity compared to our results, the system utilizing NTWO as the negative electrode material in this study also demonstrated commendable performance, yielding an areal capacity of approximately 2.96 mAh cm−2 at 60 °C It is crucial to note, however, that this areal capacity of 2.96 mAh cm−2 was achieved under a higher current density of 14.85 mA cm−2, which is higher than that used in previous studies (Table 1). We also compared our results with those of applying the well-known metal oxide negative electrode material, Li4Ti5O12 (LTO), in ASSBs (Supplementary Table. 1). Although LTO has often been used as a negative electrode in fast-charging systems due to its excellent rate capability, it could only operate at very low currents in ASSBs because of unstable interfaces between the electrode and solid electrolyte, and the formation of electrochemically detrimental decomposition products like Li2S. Consequently, the LTO negative electrode has not been able to fully utilize its advantages. However, the NTWO material discussed in this study demonstrated stable operation at higher current densities, proving its strong potential as a material for practical fast-charging ASSB systems.
In summary, the NTWO negative electrode is investigated as a promising solution enabling stable long-term operation under high current densities in ASSBs. Extensive electrochemical measurements have demonstrated that the NTWO negative electrode maintains stable performance even at high rates exceeding 2 A g−1. Furthermore, symmetric cell tests have confirmed its ability to operate without cell failure even under a high current density of 70 mA cm−2 at 25 °C under the stack pressure of 60 MPa. These results underscore the robustness and reliability of NTWO negative electrode as a promising electrode material for ASSBs. In NTWO|LPSCl|NMC811 cell configurations, the performance of the NTWO negative electrode coupled with NMC811 positive electrode exhibited a commendable areal capacity of 2.96 mAh cm−2 at a current density of 14.85 mA cm−2 at 60 °C under the stack pressure of 60 MPa, maintaining ~1 mAh cm−2 under the high current density of 45 mA cm−2 over 5000 cycles under the stack pressure of 60 MPa. At 80 °C, the NTWO|LPSCl|NMC811 cell sustained an areal capacity of around 1 mAh cm−2 over 2000 cycles, even at a high current density of 101 mA cm−2 under the stack pressure of 60 MPa. Through Raman, XPS, and simulation studies, we have analyzed the critical role played by the LiWS2 formed at the LPSCl|NTWO negative electrode interphase. The LiWS2 effectively inhibits the formation of Li2S, which is typically observed when a Li-metal negative electrode is used, enhancing the long-term operational stability of the ASSBs with an NTWO negative electrode. This LiWS2 phase, formed in situ at the NTWO|LPSCl interface during operation without any pretreatment, enhances the stability of the electrode–electrolyte interphase. As a result, our ASSB system with the NTWO negative electrode exhibits improved durability at high current density.
Methods
Material preparation
The Li6PS5Cl solid electrolyte was synthesized through a solid-state reaction. Lithium sulfide (Li2S, Sigma-Aldrich, 99.98%), phosphorus pentasulfide (P2S5, Sigma-Aldrich, 99.9%), and lithium chloride (LiCl, Sigma-Aldrich, 99%) were prepared in a molar ratio of 5:1:2 and then mixed. Subsequently, the mixture was ball-milled at 500 rpm for 10 h using a high-energy planetary ball mill (Pulverisette 7, Fritsch) under the argon atmosphere. The powder after ball-milling was heat-treated at 550 °C for 10 h in an argon-filled environment to prepare the solid electrolyte. To obtain NTWO, 900 mg of NbCl5 (Sigma-Aldrich, anhydrous, powder, 99.995% trace metals basis), 178 µL of titanium isopropoxide (Sigma-Aldrich, 99.999% trace metals basis), and 66 mg of WCl6 (Sigma-Aldrich, ≥99.9% trace metals basis) were dissolved into 60 ml of ethanol and mixed by using magnetic stir bar for 1 h. The solution was transferred to the 100 ml solvothermal kettle. The kettle was placed in the autoclave vessel for solvothermal synthesis and heated at 200 °C for 12 h. Then, the obtained powder was rinsed with laboratory-grade deionized water (resistance of 18 MΩ cm) and ethanol (95%) three times before drying in the oven overnight. The obtained precursor powder was calcined at the desired temperature, 900 °C for 5 h with a ramp rate of 5 °C min−1 in a box furnace in the air. The synthesized powder was stored in a drying oven at 70 °C.
Electrode preparation
The negative electrode composite was prepared by quantitatively mixing NTWO, LPSCl, and vapor-grown carbon fibers (VGCF) (Sigma-Aldrich, pyrolytically stripped, platelets(conical), >98% carbon basis, D × L 100 nm × 20−200 μm) in a weight ratio of 6:3:1. For the preparation of the positive electrode composite, LiNbO3 coated Li(Ni0.8Mn0.1Co0.1)O2 (NMC811), LPSCl, VGCF, and polytetrafluoroethylene (PTFE) were quantified in a weight ratio of 75:22:2:1. This mixture was then processed through a dry process to form a film. NMC811 and LPSCl were mixed in a mortar for 10 min, followed by the addition of VGCF and PTFE, which were then kneaded together. Subsequently, a cylindrical rod was used to roll out the mixture to prepare the film. For evaluation purposes, the film was punched into discs with a diameter of 12 mm. The average thickness of the positive electrode is 70 µm, while the thickness of the negative electrode is 30 µm.
Physicochemical characterizations
Raman spectroscopy (Renishaw RM1000 microspectroscopic system) was utilized to further investigate the chemical structure and phase of the NTWO negative electrode. Raman spectroscopy was also employed to analyze phase evolution within the negative electrode composite during operation. For ex-situ analysis using Raman spectroscopy, Li|LPSCl|NTWO cell was prepared and operated up to a specified cycle at a specific current of 0.6 A g−1. Subsequently, the discharged sample was placed inside an air-tight holder, one side of which was made of sapphire glass, fabricated in-house. This assembly was conducted within a glovebox. The surface of the NTWO layer was then measured. X-ray photoelectron spectroscopy (XPS, Thermo K-Alpha XPS) was conducted to investigate the electronic states of the cations constituting the NTWO negative electrode. XPS also demonstrates the changes in the electronic states of the negative electrode composite before and after operation. For ex-situ analysis using XPS, Li|LPSCl|NTWO cell was prepared and operated up to a specific cycle at a specific current of 0.6 A g−1. After discharging, the sample was hermetically sealed within an air-tight container inside a glovebox and swiftly transferred to the sample mount, maintaining a vacuum state during measurement. The sample was exposed to air for no more than five seconds during transfer, which could lead to surface oxidation. To remove this oxidation, the surface was etched with Ar plasma for 20 min before proceeding with the measurements. Scanning electron microscopy (SEM, Hitachi SU8230) was applied to examine the microstructure of the NTWO negative electrode, as well as observe the microstructural changes in the electrode layer cross-sections before and after operation. Energy-dispersive X-ray spectroscopy (EDS, Hitachi SU8230) was employed alongside SEM to ascertain the distribution of cations within the negative electrode. For SEM analysis, a sample was hermetically sealed in an air-tight container within a glovebox and swiftly transferred to the sample mount of the equipment, maintaining a vacuum state during measurement. During the transfer process, the sample was exposed to air for no more than five seconds. Transmission Electron Microscopy (TEM, Hitachi HD−2700) analysis was performed to gain detailed microscopic information into the atomic structure of NTWO.
Cell preparation
The optical photograph of the cell is shown in Supplementary Fig. 24, and it consists of a mold for pelletizing, an insulator, plunges, and a case. The part in direct contact with the cell is made of polyether ether ketone (PEEK), and the pressure during operation is determined by the torque applied along three axes. The diameter of the die is 13 mm. All cell assembly processes were conducted inside an argon-filled glovebox, where both oxygen and moisture levels were controlled to be below 1 ppm. For the assembly of the Li||NTWO cell, 150 mg of LPSCl was first placed in a die and pressed using the plunge at 0.1 MPa. Subsequently, the prepared negative electrode composite was quantified to achieve a loading level of 1.8 mg cm−2 and spread on top of the electrolyte, followed by compression at 430 MPa for 2 min. Lithium metal with a thickness of 500 µm was then attached to the opposite side of the electrolyte and pressed at 50 MPa. The final thickness of the LPSCl pellet was 500 µm, and the NTWO electrodes were 5 µm thick, with a diameter of 13 mm. For the preparation of symmetric cells, a negative electrode composite containing fully lithiated NTWO active material was prepared. To fully lithiate NTWO for symmetric cell measurements, an NTWO|LPSCl|Li cell was prepared, and NTWO was lithiated to 1.0 V at 10 mA g−1. Lithiation was performed with only a single charging cycle. After lithiation, the NTWO|LPSCl|Li cell was disassembled inside a glovebox, exposing the plunge on the NTWO side. By applying impact to the NTWO layer with a metal rod and a rubber hammer, the NTWO layer was separated. The separated NTWO layer was then ground using a mortar and reused. To form an LPSCl pellet, 150 mg of LPSCl was placed in a 13 mm die and lightly pressed manually. The negative electrode composite with a loading level of 25 mg cm−2 was then placed on one side of the pellet. On the other side, a pristine NTWO-containing negative electrode composite was prepared and similarly added at a loading level of 25 mg cm−2, followed by compression at 430 MPa for 2 min. For the assembly of the NTWO|LPSCl|NMC811, 150 mg of LPSCl was similarly placed in a 13 mm die to prepare a pellet. A positive electrode film was attached to the prepared pellet, and a negative electrode composite was placed on the opposite side to achieve an N/P ratio of 1.05. The N/P ratio of 1.05 represents the ratio of the capacity of the negative electrode (N) to the positive electrode (P) in a battery or electrochemical cell. A ratio of 1.05 indicates that the negative electrode has a slightly higher capacity (5% excess) compared to the positive electrode. This value is selected to ensure that the positive electrode fully utilizes its capacity without the negative electrode becoming a limiting factor. A slightly higher N/P ratio helps prevent lithium plating on the negative electrode, which can occur when the negative electrode becomes overcharged due to insufficient capacity. This balance enhances the stability, safety, and lifespan of the cell. The loading level varied according to the cell evaluation, and the positive electrode loading level for each NTWO||NCM811 cell evaluation was separately indicated in the respective figure. The cell was then prepared by compressing at 430 MPa for 2 min. The cell was subjected to a constant pressure of 60 MPa during cycling.
Electrochemical evaluation
The Li|LPSCl|NTWO cell testing was conducted by setting the cutoff voltage to 1.0–3.0 V, reflecting the potential for lithium insertion/extraction in the NTWO negative electrode material. Measurements were carried out using a potentiostat (Neware) by applying currents corresponding to each current density. To create various operational temperature environments, the cell kit was placed in a temperature-controlled oven for measurement. For the evaluation of symmetric cells, the operational areal capacity for each cycle was fixed at 0.25 mAh cm−2, and the potential was measured at 25 °C while the current was applied through the potentiostat (Neware). The cutoff voltage for the NTWO||NCM811 cell was set at 1.0–3.45 V, considering the operational potentials of the NMC811 positive electrode active material and the NTWO negative electrode active material. The potential was measured by applying the current through the potentiostat (Neware). Temperature control for these measurements was also conducted by setting the ambient temperature in the oven and then placing the cell kit inside for evaluation. For electrochemical impedance spectroscopy (EIS) measurements, a potentiostatic signal was used, with an amplitude of 20 mV applied over a frequency range from 1 MHz to 10 Hz. 10 points per decade of frequency have been recorded, including the value at the final frequency of 10 Hz, resulting in a total of 51 data points. The reproducibility of each electrochemical experiment was verified by evaluating two cells simultaneously. The mass for specific capacity was determined by considering only the weight of the active material, such as NMC811 or NTWO.
Calculation
All spin-polarized calculations were performed with the density functional theory (DFT) method using the Vienna ab initio simulation package (VASP)42,43. The projector augment wave (PAW) method was applied with W([Xe]4f145d46s2), Nb([Ar]4s24p64d45s1), Ti([Ne]3p63d24s2), Cl([Ne]3s23p5), S([Ne]3s23p4), P([Ne]3s23p3), O([He]2s22p4), Li(1s22s1), to solve the interaction between ionic core electrons and valence electrons. The generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) function was used to take the exchange correlations into consideration in the Kohn–Sham equations44. The energy cutoff and convergence criteria were set as 520 and 10−5 eV, respectively. The structures were relaxed until the force on each atom was less than 0.02 eV Å−1. The RMM-DIIS algorithm and the conjugate gradient were used during the electronic and ionic optimization, respectively. The dispersion correction, DFT-D3, is also considered. For the ab initio molecular dynamics (AIMD) simulation of Li2S and WS2, their machine learning force fields (MLFFs) were developed in advance at 300 K. In the case of WS2, Li atoms were deliberately introduced into the tetrahedral interstitial sites formed by sulfur atoms. Subsequently, AIMD simulations were carried out at 300 K using the Nose-Hoover thermostat in the NVT ensemble for Li2S and WS2, each for 100 ps and 50 ps, respectively. The mean squared displacement (MSD) of Li atoms was computed using vaspkit45.
Data availability
All data are available in the main text or the supplementary materials.
References
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 1–16 (2017).
Bachman, J. C. et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Thangadurai, V., Narayanan, S. & Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem. Soc. Rev. 43, 4714–4727 (2014).
Yamada, H. et al. High cathode loading and low‐temperature operating garnet‐based all‐solid‐state lithium batteries—material/process/architecture optimization and understanding of cell failure. Small 19, 2301904 (2023).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 1–7 (2016).
Wan, H., Wang, Z., Zhang, W., He, X. & Wang, C. Interface design for all-solid-state lithium batteries. Nature 623, 739–744 (2023).
Wan, H. et al. Critical interphase overpotential as a lithium dendrite-suppression criterion for all-solid-state lithium battery design. Nat. Energy 8, 473–481 (2023).
Kim, C. et al. Ion‐conducting channel implanted anode matrix for all‐solid‐state batteries with high rate capability and stable anode/solid electrolyte interface. Adv. Energy Mater. 11, 2102045 (2021).
Kasemchainan, J. et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019).
Banerjee, A., Wang, X., Fang, C., Wu, E. A. & Meng, Y. S. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem. Rev. 120, 6878–6933 (2020).
Tan, D. H. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Yan, W. et al. Hard-carbon-stabilized Li–Si anodes for high-performance all-solid-state Li-ion batteries. Nat. Energy 8, 800–813 (2023).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Lee, Y.-G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020).
Sandoval, S. E. et al. Structural and electrochemical evolution of alloy interfacial layers in anode-free solid-state batteries. Joule 7, 2054–2073 (2023).
Chen, D. et al. Unraveling the nature of anomalously fast energy storage in T-Nb2O5. J. Am. Chem. Soc. 139, 7071–7081 (2017).
Jing, P. et al. Engineering the architecture and oxygen deficiency of T-Nb2O5–carbon–graphene composite for high-rate lithium-ion batteries. Nano Energy 89, 106398 (2021).
Li, T. et al. A niobium oxide with a shear structure and planar defects for high-power lithium ion batteries. Energy Environ. Sci. 15, 254–264 (2022).
Karger, L. et al. Protective nanosheet coatings for thiophosphate‐based all‐solid‐state batteries. Adv. Mater. Interfaces 11, 2301067 (2024).
Maiti, S. et al. Stabilizing high-voltage lithium-ion battery cathodes using functional coatings of 2D tungsten diselenide. ACS Energy Lett. 7, 1383–1391 (2022).
Lau, J. et al. Sulfide solid electrolytes for lithium battery applications. Adv. Energy Mater. 8, 1800933 (2018).
Ahn, Y. et al. Mixed‐phase niobium oxide as a durable and ultra‐fast charging anode for high‐power lithium‐ion batteries. Adv. Funct. Mater. 34, 2310853 (2024).
Li, T. et al. Operando Raman and DFT analysis of (de) lithiation in fast-charging, shear-phase H-Nb2O5. ACS Energy Lett. 8, 3131–3140 (2023).
Guo, C. et al. Nano‐sized niobium tungsten oxide anode for advanced fast‐charge lithium‐ion batteries. Small 18, 2107365 (2022).
Lv, Z. et al. Cation mixing in Wadsley–Roth phase anode of lithium-ion battery improves cycling stability and fast Li+ storage. Appl. Phys. Rev. 8, 031404 (2021).
Li, Q. et al. Improving the oxygen redox reversibility of Li-rich battery cathode materials via Coulombic repulsive interactions strategy. Nat. Commun. 13, 1123 (2022).
Griffith, K. J., Forse, A. C., Griffin, J. M. & Grey, C. P. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J. Am. Chem. Soc. 138, 8888–8899 (2016).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Wenzel, S., Sedlmaier, S. J., Dietrich, C., Zeier, W. G. & Janek, J. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 318, 102–112 (2018).
Huang, F., Jian, J. & Wu, R. Few-layer thick WS2 nanosheets produced by intercalation/exfoliation route. J. Mater. Sci. 51, 10160–10165 (2016).
Liu, H.-L. et al. Deep-ultraviolet Raman scattering spectroscopy of monolayer WS2. Sci. Rep. 8, 11398 (2018).
Lu, S. et al. A novel honeycomb-like WS2−x/CoS@C composite as anode for lithium ion batteries. J. Mater. Sci. 57, 5118–5129 (2022).
Wang, H., Huang, C.-C. & Polcar, T. Triboelectrification of two-dimensional chemical vapor deposited WS2 at nanoscale. Sci. Rep. 9, 12570 (2019).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Chen, C. & Ong, S. P. A universal graph deep learning interatomic potential for the periodic table. Nat. Comput. Sci. 2, 718–728 (2022).
Ye, L. & Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021).
Li, X. et al. High‐performance Li–SeSx all‐solid‐state lithium batteries. Adv. Mater. 31, 1808100 (2019).
Zhang, Z., Wu, L., Zhou, D., Weng, W. & Yao, X. Flexible sulfide electrolyte thin membrane with ultrahigh ionic conductivity for all-solid-state lithium batteries. Nano Lett. 21, 5233–5239 (2021).
Poetke, S. et al. Nanostructured Si−C composites as high‐capacity anode material for all‐solid‐state lithium‐ion batteries. Batter. Supercaps 4, 1323–1334 (2021).
Kraft, M. A. et al. Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1–xGexS5I for all-solid-state batteries. J. Am. Chem. Soc. 140, 16330–16339 (2018).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Acknowledgements
This work was supported by CBMM and by a Hightower Endowment to the Georgia Tech Foundation.
Author information
Authors and Affiliations
Contributions
C.K., G.N., Y.A., and M.L. conceived the ideas for the study and designed the experiments. C.K. designed the ASSB cell configuration and performed electrochemical tests. Y.A. synthesized negative electrodes for the experiments and performed XRD, Raman, and XPS. G.N. and Y.A. performed the FIB-SEM and SEM. C.K., G.N., Y.A., and M.L. participated in the scientific discussion and data analysis. X.H. performed a simulation. C.K., G.N., and M.L. wrote the manuscript. All authors have discussed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, C., Nam, G., Ahn, Y. et al. Nb1.60Ti0.32W0.08O5−δ as negative electrode active material for durable and fast-charging all-solid-state Li-ion batteries. Nat Commun 15, 8832 (2024). https://doi.org/10.1038/s41467-024-52767-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52767-8